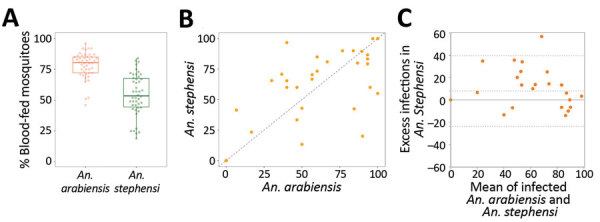
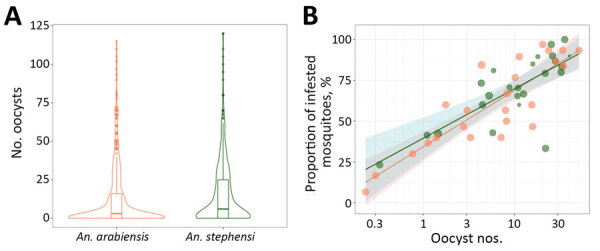
Fitsum G Tadesse
Fitsum G Tadesse
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,✉,
Temesgen Ashine
Temesgen Ashine
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Hiwot Teka
Hiwot Teka
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Endashaw Esayas
Endashaw Esayas
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Louisa A Messenger
Louisa A Messenger
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Wakweya Chali
Wakweya Chali
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Lisette Meerstein-Kessel
Lisette Meerstein-Kessel
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Thomas Walker
Thomas Walker
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Sinknesh Wolde Behaksra
Sinknesh Wolde Behaksra
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Kjerstin Lanke
Kjerstin Lanke
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Roel Heutink
Roel Heutink
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Claire L Jeffries
Claire L Jeffries
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Daniel Abebe Mekonnen
Daniel Abebe Mekonnen
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Elifaged Hailemeskel
Elifaged Hailemeskel
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Surafel K Tebeje
Surafel K Tebeje
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Temesgen Tafesse
Temesgen Tafesse
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Abrham Gashaw
Abrham Gashaw
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Tizita Tsegaye
Tizita Tsegaye
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Tadele Emiru
Tadele Emiru
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Kigozi Simon
Kigozi Simon
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Eyuel Asemahegn Bogale
Eyuel Asemahegn Bogale
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Gedeon Yohannes
Gedeon Yohannes
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Soriya Kedir
Soriya Kedir
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Girma Shumie
Girma Shumie
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Senya Asfer Sabir
Senya Asfer Sabir
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Peter Mumba
Peter Mumba
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Dereje Dengela
Dereje Dengela
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Jan H Kolaczinski
Jan H Kolaczinski
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Anne Wilson
Anne Wilson
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Thomas S Churcher
Thomas S Churcher
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Sheleme Chibsa
Sheleme Chibsa
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Matthew Murphy
Matthew Murphy
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Meshesha Balkew
Meshesha Balkew
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Seth Irish
Seth Irish
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Chris Drakeley
Chris Drakeley
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Endalamaw Gadisa
Endalamaw Gadisa
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13,
Teun Bousema
Teun Bousema
1Armauer Hansen Research Institute, Addis Ababa, Ethiopia (F.G. Tadesse, T. Ashine, H. Teka, E. Esayas, W. Chali, S.W. Behaksra, D.A. Mekonnen, E. Hailemeskel, S.K. Tebeje, T. Tafesse, A. Gashaw, T. Tsegaye, T. Emiru, E.A. Bogale, G. Shumie, S.A. Sabir, E. Gadisa);
2Radboud University Medical Center, Nijmegen, the Netherlands (F.G. Tadesse, L. Meerstein-Kessel, K. Lanke, R. Heutink, E. Hailemeskel, S.K. Tebeje, T. Bousema);
3Addis Ababa University, Addis Ababa (F.G. Tadesse, D.A. Mekonnen, E. Hailemeskel);
4United States Agency for International Development, Addis Ababa (H. Teka, S. Chibsa, M. Murphy);
5London School of Hygiene and Tropical Medicine, London, UK (L.A. Messenger, T. Walker, C.L. Jeffries, K. Simon, C. Drakeley, T. Bousema);
6President’s Malaria Initiative VectorLink Ethiopia Project, Addis Ababa (G. Yohannes, P. Mumba, M. Balkew);
7Oromia Regional Health Bureau, Adama, Ethiopia (S. Kedir);
8President’s Malaria Initiative VectorLink Project, Rockville, Maryland, USA (D. Dengela);
9World Health Organization, Geneva, Switzerland (J.H. Kolaczinski);
10Liverpool School of Tropical Medicine, Liverpool, UK; (A. Wilson);
11Imperial College London, London (T.S. Churcher);
12United States President’s Malaria Initiative, Atlanta, Georgia, USA (S. Chibsa, M. Murphy, S. Irish);
13Centers for Disease Control and Prevention, Atlanta (S. Irish)
1,2,3,4,5,6,7,8,9,10,11,12,13