Abstract
Animals coexist in commensal, pathogenic or mutualistic relationships with complex communities of diverse organisms including microbes1. Some bacteria produce bioactive neurotransmitters which have been proposed to modulate host nervous system activity and behaviors2,3. However, the mechanistic basis of this microbiota-brain signaling and its physiological relevance is largely unknown. Here we show that in C. elegans, the neuromodulator tyramine produced by gut-colonizing commensal Providencia bacteria bypasses the requirement for host tyramine biosynthesis to manipulate a host sensory decision. Bacterially-produced tyramine is likely converted to octopamine by the host tyramine β-hydroxylase enzyme. Octopamine in turn targets the OCTR-1 octopamine receptor on the ASH nociceptive neurons to modulate an aversive olfactory response. We identify genes required for tyramine biosynthesis in Providencia, and show that these genes are necessary for modulation of host behavior. We further find that C. elegans colonized by Providencia preferentially select these bacteria in food choice assays, and that this selection bias requires bacterially produced tyramine and host octopamine signaling. Our results demonstrate that a neurotransmitter produced by gut microbiota mimics the functions of the cognate host molecule to override host control of a sensory decision, thereby promoting fitness of both host and microbe.
The pathways mediating chemical communication between gut-colonizing bacteria and host nervous systems are largely undescribed3. Recently, the nematode C. elegans has emerged as a powerful experimental system in which to study host-microbe chemical communication4. Diverse populations of pathogenic and non-pathogenic bacteria both colonize the C. elegans intestine and serve as its primary food source in the wild5. Exposure to pathogenic bacteria alters C. elegans olfactory behaviors6, but whether commensal gut bacteria also modulate host behaviors is unknown.
Gut bacteria alter olfactory behavior
To identify novel modes of microbial influence on host behaviors, we screened non-pathogenic bacterial strains typically associated with wild nematodes7 for their ability to influence C. elegans olfactory responses. In long-range population chemotaxis assays8, adult hermaphrodites co-cultivated on these bacterial strains exhibited robust attraction to a panel of attractive volatile odorants similar to the behaviors of animals grown on the standard E. coli food source OP50 (Extended Data Fig. 1a). However, co-cultivation with the Providencia alcalifaciens strain (JUb39)7,9 resulted in decreased avoidance of the volatile repellent 1-octanol, but not of the repellents 2-nonanone and 8M glycerol, as compared to OP50-grown animals (Fig. 1a, Extended Data Fig. 1b). This decreased avoidance is henceforth referred to as octanol modulation. Animals grown on a distantly-related Providencia rettgeri strain isolated from nematodes in compost (PYb007, Extended Data Fig. 1c) exhibited similar octanol modulation (Fig. 1b). These observations indicate that upon co-culture, multiple Providencia strains modulate octanol aversion in C. elegans.
Fig. 1. Providencia colonizes the C. elegans intestine and modulates octanol avoidance.
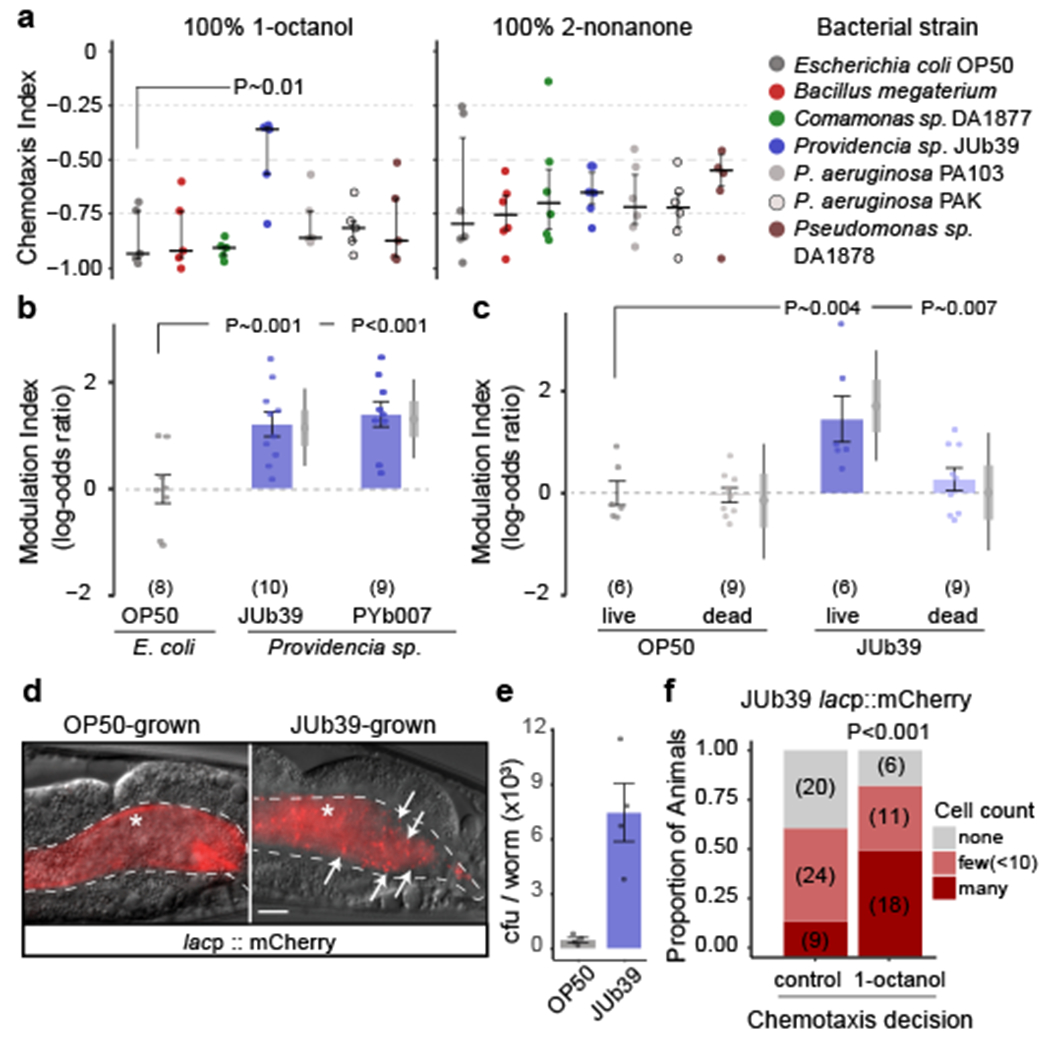
a, Long-range chemotaxis assays (Extended Data Fig. 1a) of C. elegans grown on the indicated bacterial strains to aversive odors. Chemotaxis index (CI) = (animals at odorant – animals at control)/total animals. Dots, CI from single assays of approximately 100 animals. Horizontal line, median; errors, 1st and 3rd quartiles. P-value is derived from z-statistic from a binomial general linearized mixed-effects model (GLMM) with random intercepts for assay plate and date and with false discovery rate (FDR) for post-hoc comparisons (two-sided test). n = 5 and 6 independent experiments for octanol and nonanone assays, respectively, over 3 days. b-c, Modulation index of worms in response to 100% octanol. ‘Dead’ (c), bacteria pre-treated with gentamicin. Modulation index, the log odds-ratio of the proportion of worms at octanol vs control of each condition normalized to the OP50-grown condition per independent day (gray dashed line). Positive numbers, reduced octanol avoidance. Errors are SEM. Gray vertical bars, Bayesian 95% and 66% credible intervals, respectively. P-values, two-sided GLMM with Dunnett-type (b), and Tukey-type multivariate-t adjustment (c). Numbers in parentheses, independent experiments over 3-5 days with approximately 100 animals each (also see Extended Data Fig. 9a). d, mCherry-expressing bacteria in the posterior intestines of young adults. Arrows, intact cells, asterisk, diffuse intestinal fluorescence, dashed lines, intestinal boundary. Anterior at left. Scale bar, 10 μm. e, Intestinal bacterial load in animals grown on the indicated bacterial strains. Dots, estimation of bacterial load in colony forming units (cfu) of 10 worms; n = 4 independent samples (see Methods). Data are mean ± SEM. f, Proportion of animals that migrated to 100% octanol or control in chemotaxis assays with indicated distribution of mCherry-expressing JUb39 cells. Numbers in parentheses, number of animals; 3 independent assays. P-value is derived from an ordinal regression z-statistic, using number of animals.
Under specific conditions, food deprivation reduces octanol avoidance10. JUb39 has been categorized as a ‘beneficial’ bacterium that supports robust C. elegans growth and does not induce stress responses7, suggesting that JUb39-fed animals are unlikely to be nutrition-deprived. Consistently, growth on JUb39 did not alter expression of a tph-1p::gfp fusion gene, a reporter of feeding state11,12 (Extended Data Fig. 1d). Moreover, growth of C. elegans on the poor bacterial food Bacillus megaterium13 did not alter octanol avoidance (Fig. 1a). We infer that the observed octanol modulation by Providencia is unlikely to be solely due to changes in feeding state.
While OP50 is typically crushed by the pharyngeal grinder in young adult C. elegans14, a subset of bacterial strains can bypass the grinder and survive in the worm intestine7,15,16. Feeding C. elegans with JUb39 pre-treated with the antibiotic gentamicin eliminated octanol modulation (Fig. 1c). In addition, neither exposure of OP50-grown animals to JUb39-derived odors, nor pre-incubation of OP50-grown animals with JUb39-conditioned media altered octanol responses (Extended Data Fig. 1e–f), suggesting that C. elegans must ingest live JUb39 to induce octanol modulation.
To test whether colonization of the worm gut drives octanol modulation, we transformed OP50 and JUb39 with a plasmid encoding a constitutively-expressed mCherry fluorescent reporter. While the guts of OP50-fed adult worms displayed only diffuse intestinal fluorescence consistent with these bacteria being lysed, the guts of JUb39-fed worms contained typically large numbers of intact rod-shaped cells expressing mCherry (Fig. 1d). Large numbers of bacterial colonies could be isolated after crushing JUb39-fed, but not OP50-fed worms following surface bleaching (Fig. 1e), indicating that JUb39 is likely alive in the nematode intestine. JUb39 cells tended to be enriched in the posterior intestine (Fig. 1d), unlike the reported localization pattern of severely pathogenic bacteria17. Moreover, nematodes colonized by JUb39 did not exhibit phenotypes characteristic of pathogenic infection such as anal swelling18 (Fig. 1d), and had a similar lifespan to OP50-fed worms (Extended Data Fig. 1g), further confirming that JUb39 is largely non-pathogenic to C. elegans. We performed additional chemotaxis assays with animals fed mCherry-labeled JUb39, and quantified intestinal bacterial cells in animals that had navigated either toward octanol or the control. We found that animals navigating toward octanol consistently contained more gut bacteria (Fig. 1f). We conclude that JUb39 colonizes the worm gut, and the extent of colonization is correlated with decision-making in response to octanol.
Providencia bacteria produce tyramine
We next investigated the mechanistic basis for Providencia-mediated octanol modulation. Octanol avoidance is subject to extensive modulation directly and indirectly via multiple biogenic amines including tyramine (TA) and octopamine (OA), as well as neuropeptides10,19–23. TA is produced from Tyrosine (L-Tyr) via the activity of a tyrosine decarboxylase (TDC; encoded by tdc-1 in C. elegans); TA is subsequently converted to OA via tyramine beta hydroxylase (encoded by tbh-1)24 (Fig. 2a). Consequently, all tbh-1 mutant phenotypes resulting from lack of OA are expected to be shared by tdc-1 mutants24. Unexpectedly, we found that while tdc-1 mutants grown on JUb39 continued to exhibit octanol modulation, the modulation exhibited by tbh-1 mutants was reduced (Fig. 2b). Mutations in the cat-2 tyrosine hydroxylase25 and tph-1 tryptophan hydroxylase26 enzymes required for the production of dopamine and serotonin in C. elegans, respectively, did not affect octanol modulation (Fig. 2b). These results raise the possibility that C. elegans-produced OA, but not TA, is partly necessary for JUb39-mediated octanol modulation.
Fig. 2. Providencia-produced TA compensates for loss of C. elegans tdc-1.
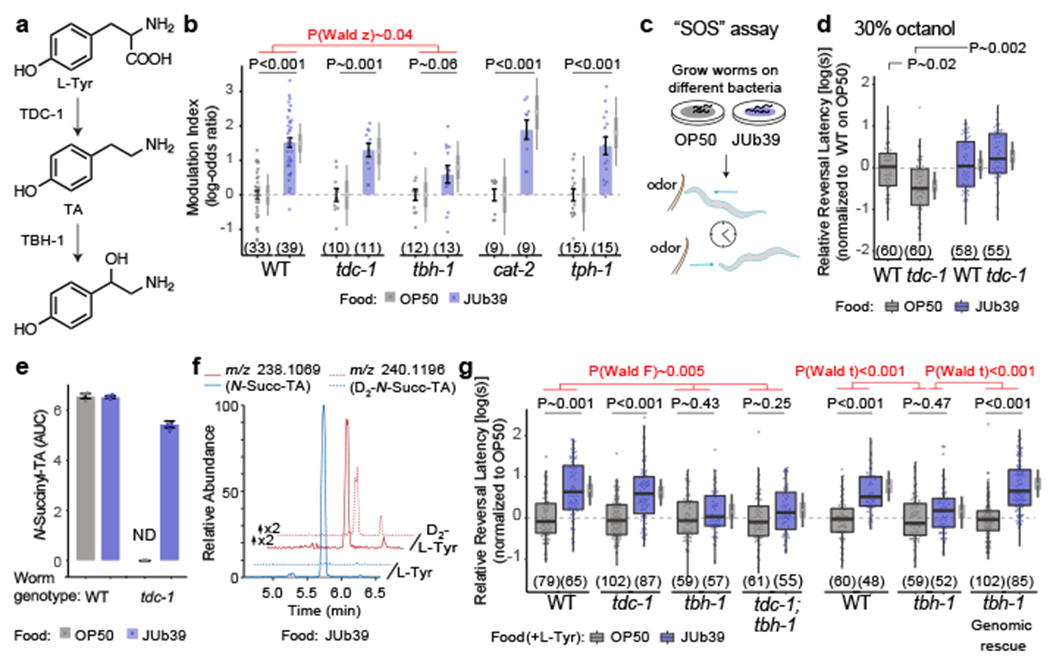
a, Biosynthesis of TA and OA in C. elegans24. b, Modulation index of animals in response to 100% octanol. Dots, individual chemotaxis assays with approximately 100 animals each. Numbers in parentheses, number of independent assays over at least 3 independent days. Y-axis is log-odds (logit) scale, normalized to OP50 for each day (gray dashed lines). Errors are SEM. Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals, respectively. P-values, GLMM with Dunnett-type multivariate-t adjustment. P-value in red, Wald z-statistic for the magnitude of the JUb39 effect in tbh-1 compared to wild-type. c, Cartoon of SOS assay 10,27. d, g, Reversal response latency of animals on NGM without (d) or with 0.5% L-Tyr (g) in response to 30% (d) or 100% octanol (g) in SOS assays. Dots, response time of single animals. Y-axis is log10-scaled, normalized to the indicated control group for each experimental day. Numbers in parentheses, number of worms tested in assays over at least 3 independent days. Boxplot, median and quartiles, whiskers, data range (excluding outliers). Gray vertical bars, Bayesian 95% and 66% credible intervals for the difference of means, respectively. P-values, linear-mixed effects regression on log-transformed data (LMM). P-values in red (g), Wald F-statistic (left) or Wald t-statistic (right) for the effect of the indicated genotypes on the magnitude of the JUb39 effect. Data from wild type in (g, right) are repeated in Extended Data Fig. 7b. Alleles: tbh-1(ok1196) (g, left), tbh-1(n3247) (g, right). e, Quantification of N-succinyl TA. Dots, area under the curve (AUC) from three biologically independent experiments. Data are mean ± SEM. ND, not detected. f, HPLC-MS (ESI+) ion chromatograms for N-succinyl TA or D2-N-succinyl TA in worms grown on JUb39 with the indicated amino-acid supplementation. Chromatograms for D2-L-Tyr fed worms are scaled two-fold relative to L-Tyr feeding. Statistical comparisons are two-sided.
To account for these observations, we hypothesized that JUb39 may produce TA that functionally compensates for the host tdc-1 mutation. tdc-1 mutants grown on OP50 have been reported to display more rapid aversive responses to dilute (30%) octanol, which is suppressed by exogenous TA19. Monoaminergic modulation of octanol responses has typically been assessed via short-range acute avoidance assays [the “smell-on-a-stick” (SOS) assay]10,27 performed on individual animals (Fig. 2c). In this assay, the strength of avoidance is inversely correlated with reversal latency when the animal encounters the repellent as it is moving forward. To more easily permit comparisons with data from previously published work, and to gain insight into the behavioral subprograms altered by JUb39, we performed all subsequent octanol behavioral experiments using SOS assays. As expected, tdc-1 mutants grown on OP50 responded more rapidly to 30% octanol than wild-type animals in SOS assays (Fig. 2d). This enhanced aversion was suppressed upon growth on JUb39 (Fig. 2d). These results are consistent with the notion that bacterially-produced TA functionally complements for the loss of host-derived TA in driving a sensory behavioral decision.
We performed metabolomic analyses to test whether growth on JUb39 compensates for the loss of TA and its metabolites in tdc-1 worms. Using HPLC-HRMS28 we found that tdc-1 worms grown on OP50 were deficient in N-succinyl TA as reported previously24, as well as additional metabolites that appear to be TA-derived based on MS2 analysis (Fig. 2e, Extended Data Figs. 2–5). Metabolism of serotonin was largely unperturbed (Extended Data Fig. 6). Production of these TA-derived metabolites was restored when tdc-1 mutant worms were grown on JUb39 (Fig. 2e, Extended Data Figs. 3, 4d, 5d). Metabolomic analysis of tdc-1 worms grown on JUb39 supplemented with stable isotope labeled D2-L-tyrosine confirmed de novo biosynthesis of the TA moiety (Fig. 2f). We did not detect either free TA or TA conjugates in the absence of C. elegans. We conclude that JUb39 in association with C. elegans produces TA which can compensate for the lack of endogenous TA production in tdc-1 mutant worms.
Although TA biosynthesis in bacteria has been demonstrated in some Gram-positive genera, production appears to be uncommon in Gram-negative bacteria which include Providencia29,30. TA production in Gram-positive strains is induced upon supplementation with L-Tyr31. We found that growth on L-Tyr-supplemented media enhanced octanol modulation by JUb39 in SOS assays (Extended Data Fig. 7a). Mutations in tbh-1 fully suppressed octanol modulation in SOS assays, whereas tdc-1 mutants continued to exhibit robust octanol modulation (Fig. 2g), consistent with our observations in long-range chemotaxis assays (Fig. 2b). Moreover, octanol avoidance behaviors of tdc-1; tbh-1 double mutants were similar to those of tbh-1 mutants alone (Fig. 2g), indicating that the lack of host-derived OA, and not accumulation of TA due to loss of TBH-124,28 accounts for the reduced octanol modulation in tbh-1 mutants. Expression of wild-type genomic tbh-1 sequences rescued octanol modulation in tbh-1 mutants (Fig. 2g). Consistent with a role for neuronal OA release in octanol modulation, animals mutant for the neuron-specific cat-1 vesicular monoamine transporter32 were also deficient in octanol modulation (Extended Data Fig 7b).
Bacterial decarboxylases generate tyramine
Biogenic amines are typically generated from aromatic amino acids and L-glutamate by pyridoxyl phosphate (PLP)-dependent group II aromatic acid decarboxylase enzymes (AADCs) in both eukaryotes and bacteria33. In Gram-positive Enterococcus and Lactobacillus (Lb), TA production is mediated by the TDC-encoding (tyrDC) AADC and tyrosine permease/transporter (tyrP) genes present in an operon; this operon is inducible by L-Tyr (Fig. 3a, Extended Fig. 8a–c, SI Table 1)34,35. Although genes related to Enterococcus tyrDC and tyrP were largely absent in Gammaproteobacteria (Extended Fig. 8c), we confirmed the presence of homologous operons containing tyrDC and tyrP in JUb39 and PYb007 in de novo genome assemblies via whole genome sequencing (Fig. 3a, SI Table 1). tyrDC homologs were also identified in the genomes of additional members of the Morganellaceae family, although the operon structure was conserved in only a subset of these genomes (Fig. 3a–b, SI Table 1).
Fig. 3. Two Providencia AADC enzymes act redundantly to modulate octanol avoidance.
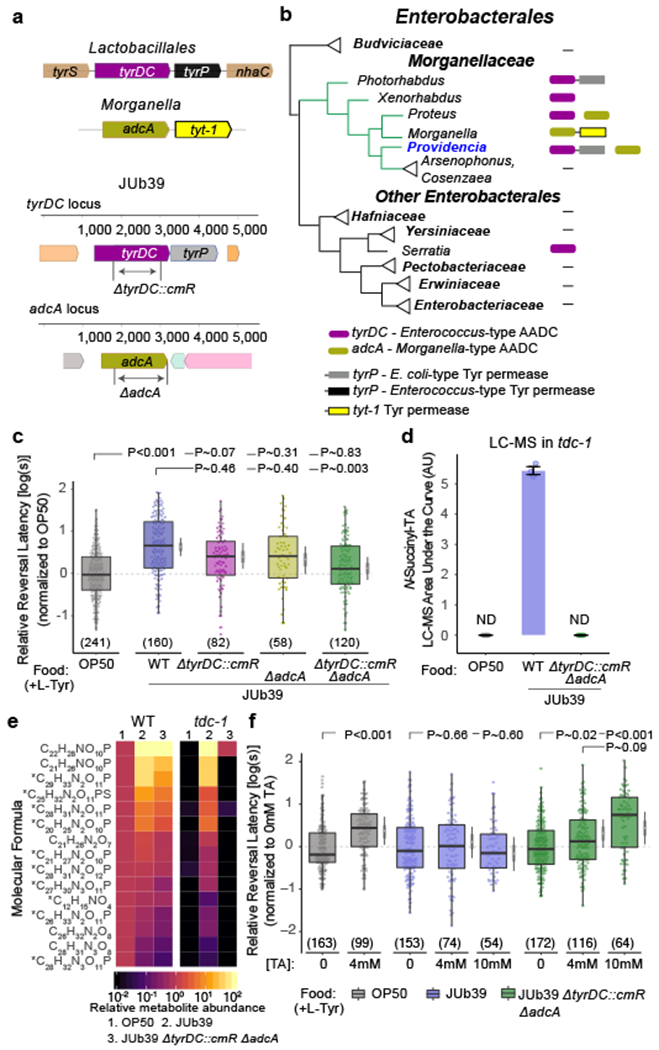
a, Cartoons depicting the tyrDC locus in Lactobacillales, the adcA locus in Morganella (top), and corresponding loci including engineered muations in JUb39 (bottom). b, Presence of tyrDC, adcA, E.coli-type tyrP and Morganella-type tyt-1 at the family and genus level among Enterobacterales. Linked boxes indicate organization in an operon. c, f, Reversal response latency of wild-type C. elegans grown on the indicated bacterial genotypes in NGM + 0.5% L-Tyr (c) or supplemented with the indicated concentrations of TA (f) to 100% octanol using SOS assays. Dots, response time of single worms. Y-axis is log10-scaled and normalized to the indicated control group for each experimental day (gray dashed line). Numbers in parentheses, number of worms tested in assays over at least 3 independent days. Boxplot, median and quartiles, whiskers, data range (excluding outliers). Gray thin and thick vertical bars at right, Bayesian 95% and 66% credible intervals for the difference of means, respectively. P-values between indicated conditions are from a LMM with Tukey-type multivariate-t adjustment. d, Quantification of N-succinyl TA in tdc-1 mutant animals grown on the indicated bacterial strains. OP50 and JUb39 data are repeated from Fig. 2e. Dots, area under the curve (AUC) from each of 3 biologically independent experiments. Data are mean ± SEM. ND, not detected. e, Heatmap showing mean abundance of TA-derived compounds of indicated molecular formula detected in N2 wild-type and tdc-1 mutant worms grown on the indicated bacterial strains, relative to N2 worms grown on OP50. Means are calculated from 3 independent experiments. Asterisks indicate D2-containing compounds confirmed using D2-L-tyrosine supplementation (Fig. 2f).
Providencia TyrDC is highly homologous to the Lb enzyme, which has been well characterized with respect to substrate specificity36. Protein modeling using the crystal structure of Lb-TyrDC36 as a guide (see Methods) indicated that JUb39 TyrDC shares most known catalytic sites with Lb-TyrDC (Extended Data Fig. 8d). Interestingly, JUb39 TyrDC contains a substitution at A600 (S586 in Lb-TyrDC; Extended Data Fig. 8d), a variant demonstrated to enhance specific catalytic activity of Lb-TyrDC for tyrosine36. We infer that JUb39 TyrDC likely generates TA from tyrosine.
Morganella strains (Morganellaceae family) have been reported to produce TA under certain conditions29, despite having no discernible tyrDC orthologs (Fig. 3b, Extended Data Fig. 8a–c, SI Table 1). Instead in Morganella, we identified an AADC-encoding gene (hereafter adcA) with ~29% and 27% sequence identity to Enterococcus TyrDC and human GAD67, respectively, in an operon upstream of a gene encoding a TYT-1 family putative tyrosine permease (Fig. 3a–b)37. An adcA homolog is also present in Providencia genomes, including in JUb39, but is not adjacent to a tyrosine transporter (Fig. 3a–b, Extended Data Fig. 8a–c, SI Table 1). We conclude that Providencia encodes at least two AADCs with the potential to generate TA, and the phylogenetic incongruence suggests that both tyrDC and adcA genes in the Morganellaceae family may have either been lost or acquired via horizontal gene transfer.
To test whether one or both JUb39 AADCs are necessary for octanol modulation, we engineered deletions in JUb39 tyrDC and adcA (ΔtyrDC::cmR and ΔadcA, respectively; Fig. 3a). Whereas cultivation on each of the bacterial single mutants weakly decreased octanol modulation, growth of wild-type C. elegans on the JUb39 ΔtyrDC::cmR ΔadcA double knockout bacteria abolished octanol modulation (Fig. 3c). We confirmed that JUb39 ΔtyrDC::cmR ΔadcA colonizes the C. elegans gut (Extended Data Fig. 9a). In contrast to wild-type JUb39, comparative metabolomics analyses showed that tdc-1 worms grown on the JUb39 ΔtyrDC::cmR ΔadcA double mutant bacterial strain did not produce N-succinyl TA or other TA-derived metabolites (Fig. 3d–e, Extended Data Figs. 3, 4d, 5d). Octanol modulation was restored in wild-type C. elegans grown on JUb39 ΔtyrDC::cmR ΔadcA strains upon supplementation with TA (Fig. 3f). Moreover, while exogenous TA did not further increase octanol avoidance in wild-type JUb39-grown animals, TA supplementation was sufficient to induce octanol modulation in OP50-grown animals (Fig. 3f). Together, these results indicate that TA produced by multiple AADC enzymes in Providencia is both necessary and sufficient to modulate octanol avoidance by wild-type C. elegans.
TA regulates behaviors such as egg-laying in C. elegans24,38. We asked whether TA produced by AADC enzymes in Providencia is sufficient to suppress egg-laying defects of tdc-1 mutants, in addition to modulating octanol avoidance indirectly via OA. We found that while as reported, tdc-1 mutants grown on OP50 contained on average younger eggs in utero than wild-type animals24, growth on wild-type JUb39, but not on JUb39 ΔtyrDC::cmR ΔadcA, suppressed this egg-laying defect of tdc-1 mutants (Extended Data Fig. 10). These results indicate that TA produced by JUb39 in worms complements multiple tdc-1 dependent phenotypes.
Bacterial tyramine targets sensory neurons
We next sought to identify the molecular targets of Providencia-mediated octanol modulation in the host. The bilateral ASH nociceptive neurons in the head amphid organs of C. elegans have been implicated in sensing octanol10,20,27. These neurons express multiple TA and OA receptors, a subset of which is required for octanol modulation by these monoamines19,39. Among ASH-expressed OA receptors, mutations in octr-1, but not ser-3, abolished JUb39-mediated octanol modulation, without altering the extent of gut colonization (Fig. 4a, Extended Data Fig. 9). We also observed an effect on octanol modulation in tyra-2 TA receptor mutants, primarily due to decreased octanol avoidance upon growth on OP50 (4.3 ± 0.25 sec for tyra-2 vs. 2.9 ± 0.13 sec for WT) (Extended Data Fig. 9b). Expression of octr-1 cDNA in the ASH but not ASI sensory neurons fully restored octanol modulation (Fig. 4a). octr-1 mutants also lacked octanol modulation when grown on JUb39 ΔtyrDC::cmR ΔadcA supplemented with TA (Fig. 4b). OCTR-1 has been shown to inhibit innate immune responses to pathogens in C. elegans via suppression of an unfolded protein response pathway40. However, the observations that JUb39 does not induce expression of virulence, stress or unfolded protein response genes7, and only minimally affects C. elegans longevity (Extended Data Fig. 1g), together with the well-established role of ASH in OA-modulated octanol avoidance10,19–21, suggest that the observed octanol behavioral phenotypes are unlikely to arise from changes in innate immune responses in JUb39-grown animals.
Fig. 4. Modulation of octanol avoidance by Providencia requires the OCTR-1 OA receptor in the ASH sensory neurons.
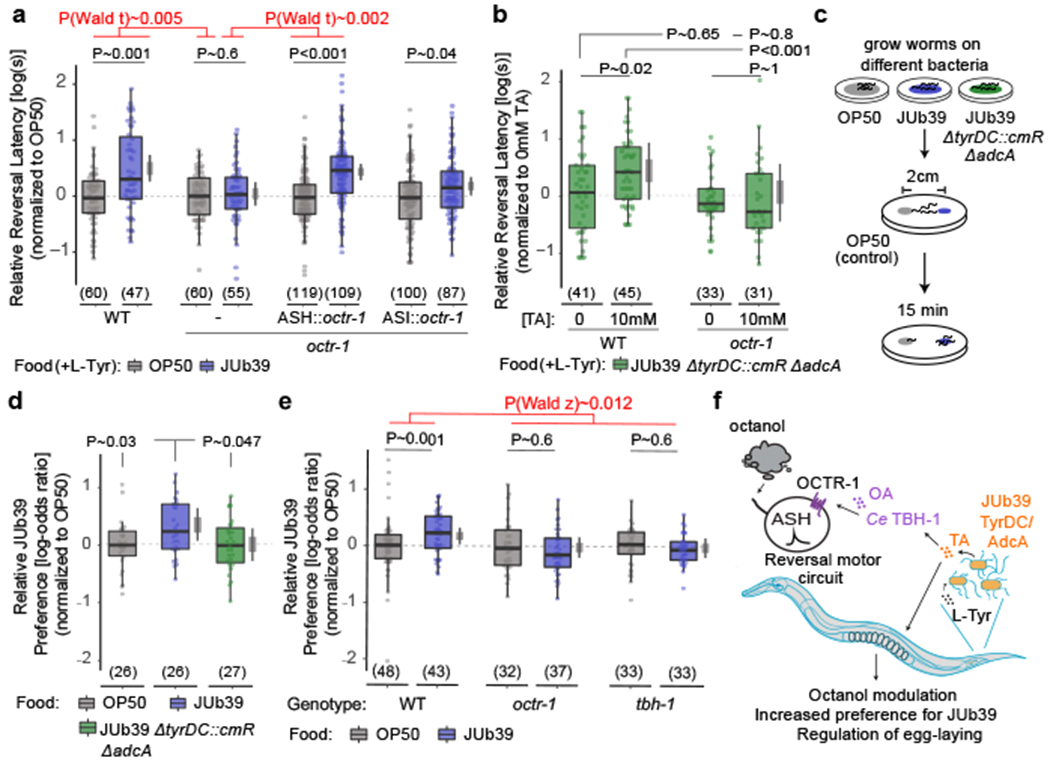
a-b, Reversal response latency of animals of the indicated worm genotypes grown on the indicated bacteria on NGM + 0.5% L-Tyr (a) and supplemented with the indicated concentrations of TA (b) to 100% octanol using SOS assays. Dots, response time of single worms. Y-axis is log10-scaled and normalized to the indicated control group (a) or to the wild-type control (b) for each experimental day. Numbers in parentheses, number of worms tested in assays over at least 3 independent days. Wild-type octr-1 sequences were expressed in ASH and ASI under the srv-11 and srg-47 promoters, respectively. Boxplot, median and quartiles, whiskers, data range (excluding outliers). Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals for the difference of means. P-values between indicated conditions are from a LMM with Tukey-type multivariate-t adjustment. P-values in red, Wald t-statistic representing the genotype x food interaction effect relative to WT. c, Cartoon depicting short-range bacterial choice assay. d-e, Relative preference index of wild-type or mutant animals grown on the indicated bacteria for the test bacteria JUb39. Data are normalized to the OP50 control condition for each genotype. Dots, single assays of at least 10 animals. Numbers in parentheses, number of assays over at least 5 independent days. Y-axis is on log-odds ratio (logit) scale. Errors are SEM. Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals, respectively. P-values represent difference of means relative to JUb39-grown animals from a GLMM with Dunnett-type multivariate-t adjustment. P-value in red, Wald z-statistic representing the genotype x food interaction effect relative to WT (e). Allele used: tbh-1(n3247) (e). f, Cartoon of working model.
Bacterial tyramine biases feeding choices
We next investigated the biological relevance of the JUb39-directed decrease in aversive olfactory responses by C. elegans. While many Gram-negative enteric bacteria produce long-chain alcohols including octanol41, whether Providencia produces this chemical is unknown. However, JUb39 and other Providencia strains produce the branched alcohol isoamyl alcohol (IAA), which is aversive to C. elegans when concentrated42,43. Similar to octanol, avoidance of high IAA concentrations is also mediated by the ASH sensory neurons43. We hypothesized that reduced avoidance of JUb39-produced aversive alcohols or other odorants may preferentially bias JUb39-grown C. elegans to select these bacteria in food choice assays (Fig. 4c). Indeed, animals grown on JUb39 preferred JUb39 as compared to OP50-grown worms, which showed only a slight preference for JUb39 in a short-range food choice assay (Fig. 4d). The bias towards JUb39 was largely eliminated in animals grown on JUb39 ΔtyrDC::cmR ΔadcA (Fig. 4d), as well as in octr-1 and tbh-1 mutants (Fig. 4e) suggesting that both bacterial TA production and host OA signaling are necessary for this food preference. Together, these results imply that TA produced by JUb39 in the worm intestine acts via host OA and the OCTR-1 OA receptor to reduce ASH-mediated avoidance of JUb39-produced aversive cues such as concentrated alcohols and allow preferential selection of these bacteria (Fig. 4f).
Discussion
Our observations support a model in which the neurotransmitter TA produced by intestinal Providencia bacteria can subvert host-dependent TA production to modulate multiple monoaminergic pathways and alter behavior and physiology in C. elegans. Bacterially-produced TA is converted to OA by C. elegans TBH-1; OA subsequently acts on the ASH neurons via the OCTR-1 OA receptor to decrease aversion of enteric bacterially-produced volatiles such as octanol, and alter food choices (Fig. 4f). We speculate that the preference for Providencia upon colonization of C. elegans by these bacteria promotes increased consumption leading to stable association5,44 and bacterial dispersal. As Providencia is a rich food source for C. elegans7, this association may be mutually beneficial. Other metabolites from commensal bacteria have been shown to have a beneficial effect on feeding decisions as well as maintenance and dispersal of these bacteria in Drosophila45–47, and intestinal bacteria can modulate satiety responses in mammals via largely uncharacterized mechanisms48,49. Our results describe a pathway by which neurotransmitters produced by natural commensal bacteria direct host sensory behavioral decisions by supplementing or compensating for the activity of key host biosynthetic enzymes, thereby altering fitness of both host and microbe.
METHODS
Strains
C. elegans:
All C. elegans strains were maintained on nematode growth medium (NGM) at 20°C, and hermaphrodites were used for all experiments. srv-11p::octr-1 (pMOD110) and srg-47p::octr-1 (pMOD111) plasmids were injected at 10 ng/μL, tbh-1p::tbh-1a::SL2::mcherry (pMOD115) was injected at 20ng/μL together with the unc-122p::mcherry coinjection marker at 30 ng/μL to generate transgenic strains. At least two independent lines were examined for all rescue experiments. Strain genotypes are listed in SI Table 2.
Bacteria:
For all experiments, bacterial strains were streaked from glycerol stocks prior to use and grown to saturation in LB media at 37°C. For conditioned media, bacteria were grown to saturation in NGM media overnight at 37°C, then cleared by centrifugation at 14,000g for 3 mins. Prior to use, conditioned media or NGM was supplemented with 5x concentrated OP50 from a saturated LB culture to prevent starvation. To expose animals to bacterial odors, worms were grown on seeded NGM plates whose lids were replaced with NGM plates containing the test bacteria; these were sealed with parafilm. For L-Tyr and TA supplementation experiments, 0.5% (~28mM) L-Tyr (Sigma T3754) or 4mM or 10mM TA (Sigma T2879) were added to the NGM media and agar prior to pouring plates. To generate antibiotic-killed bacteria, saturated cultures of OP50 or JUb39 were concentrated 5x by centrifugation followed by resuspension in LB media containing 200 μg/mL gentamicin (Sigma G1397) for 4 hours at 37° C. Four 100 μL spots of this treated bacteria suspensions were added to 10 cm NGM plates and allowed to dry before adding L4 stage worms.
Plasmids were transformed into JUb39 and OP50 via electroporation. Deletions in JUb39 were induced using homologous recombination with the temperature-sensitive pSC101 replicon at 42°C, and sacB-sucrose counter-selection at 30°C, in the absence of NaCl as described50, with the exception that bacteria were incubated for 1 hr at room temperature in the presence of 10mM arabinose for lambda Red induction prior to selection at 42°C. Deletions were confirmed by sucrose resistance and kanamycin sensitivity, followed by PCR and sequencing of deleted intervals.
Molecular biology
The octr-1 cDNA was a gift from Dr. Richard Komuniecki. The cDNA was amplified by PCR and cloned using Gibson homology cloning. The 1940bp srv-11 promoter and 978bp srg-47 promoter sequences were cloned from genomic DNA. tbh-1a cDNA was amplified from a mixed-stage cDNA pool by PCR. The 1357bp tbh-1 promoter plasmid was a gift from Mark Alkema. Vector maps are available on Github (https://github.com/SenguptaLab/ProvidenciaChemo.git). For introduction of deletions via homologous recombination in JUb39, pKD46-derivative plasmids containing a lambda Red cassette and deletion homology arms for JUb39 tyrDC and adcA were constructed (denoted pMOD102 and pMOD107, respectively). Briefly, the cas9 coding region and sgRNA regions of pDK46-derivative pCAS51 were deleted and replaced with the sacB sequence from pCM43352 via PCR and Gibson homology cloning. For pMOD102, 5’ and 3’ homology arms were approximately 400bp each flanking a 1233bp deletion of the tyrDC coding sequence which was replaced with a chloramphenicol resistance cassette. For pMOD107, 5’ and 3’ homology arms were 701 and 422bp, respectively, flanking a 1398bp deletion of the adcA CDS. For expression of mCherry in OP50 and JUb39, a pUCP20T-mCherry plasmid53 was modified to replace bla(ampR) with aph(kanR).
Microscopy
All fluorescence and DIC microscopy was performed using animals anesthetized with 100mM levamisole (Sigma Aldrich). Animals were imaged on 2% agarose pads using an upright Zeiss Axio Imager with a 63X oil immersion objective.
Quantification of intestinal bacterial cell numbers:
All rod-shaped punctae in the intestines of young adult worms of approximately 1-2μm were included in the quantification. Each animal was recorded in one of three categories containing 0, <10, or >10 cells per animal. Exact numbers in animals with > 10 cells were not recorded, but rarely exceeded approximately 100 cells.
Fluorescence intensity measurements:
All images were collected in z-stacks of 0.5 μm through the heads of young adult worms. Quantification was performed using ImageJ (NIH). Fluorescence was quantified by identifying the focal plane in which the cell soma was visible, followed by manually drawing an ROI around the soma. Mean pixel intensity was recorded for each neuron pair per animal and the average of fluorescence in each animal is shown.
Quantification of intestinal colony forming units (cfu)
To estimate intestinal cell number, surface sterilization was performed as reported54 with modifications. N2 young adult worms cultured for a single generation on OP50 or JUb39 were washed off a culture plate with M9 buffer, allowed to settle for 1–2 minutes followed by aspiration of supernatant. Animals were rinsed 4 more times with 1mL M9 buffer, followed by one rinse with M9 + 25mM levamisole (Sigma Aldrich) to block pharyngeal pumping. Animals were then incubated with 1% bleach in M9 + 25mM levamisole for 5 min, followed by 5 more rinses with M9 buffer. 100μl of the final supernatant was plated on LB media as a wash control. Animals were then placed on an NGM plate, after which 10 young adult worms per condition were picked into separate tubes containing 100μL M9 buffer and were crushed using a pestle (UAS Scientific 1415–5390). The resulting samples were serially diluted and plated on LB media. CFU per 10 worms were normalized by subtracting the number of colonies observed in plates from the wash control.
C. elegans assays
Long-range chemotaxis:
Long-range chemotaxis assays were performed essentially as described8,55. Worms were cultured for 1 generation with the relevant bacteria prior to the assay. Assays were performed using 10cm square NGM plates. The number of worms in two horizontal rows adjacent to the odor and ethanol spots were quantified.
SOS assays:
Smell-on-a-stick (SOS) assays in response to 1-octanol or 2-nonanone were performed as described10,27. NGM plates were pre-dried for 1 hour prior to assays. Age-matched young adult animals were picked from food to a clean transfer plate and allowed to briefly crawl away from food for approximately 1 min. Animals were then transferred to another clean NGM plate for 15 mins prior to assaying responses to 100% octanol (Sigma O4500) and 100% 2-nonanone (Sigma 108731), or 20 mins for 30% octanol assays. 30% octanol was prepared immediately before the assay by dilution in 200-proof ethanol (Acros Organics 61509–0010).
Short-range bacterial choice assay:
Animals were raised and prepared identically to those used in long-range chemotaxis assays, with the exception that the final wash with water was omitted. NGM plates containing two 15μL spots separated by 2 cm of overnight-grown bacterial food concentrated to OD600 = 10 were allowed to dry, then incubated with a closed lid for 5 hrs at room temperature. Approximately 30 animals were placed between the two spots, and excess liquid was removed. Animals were allowed to navigate for 15 mins following which 2μL of sodium azide was applied to each spot to anesthetize worms. Minimal lawn-leaving behavior was observed during this short time period. Adult animals on the control spot and test spot were counted.
Osmotic avoidance assay:
Animals off the bacterial food on the cultivation plate were picked using a 10% methyl cellulose polymer solution and placed in the center of an NGM plate with a ring of 8M glycerol containing bromophenol blue (Sigma B0126). The number of worms inside and outside of the ring were counted after 10 mins.
Quantification of eggs in utero:
N2 or tdc-1 animals were grown from egg to the L4 stage on NGM plates containing either OP50, JUb39 or JUb39 ΔtyrDC::cmR ΔadcA. Age-matched L4 larvae were transferred to new plates 18h prior to the assay. The number of eggs younger or older than the 4-cell stage were recorded over 3 independent days of assays.
Lifespan assays:
N2 animals were grown on 6cm NGM plates containing 100μL OP50, JUb39 or PA14. 20 L4 animals were placed on each plate at the beginning of the experiment. 4 replicate plates were analyzed for each condition per day over 3 independent days of experiments. Animals were transferred to new plates at least once every two days until they ceased to lay eggs. Animals were censored if they crawled off of the plate, exhibited in utero larval hatching (“bagging”), or if they were injured during transfers. Animals were scored as dead if they failed to respond to being prodded in the head.
Tyrosine supplement for metabolomics assay:
tdc-1 animals were grown on plates containing 0.5% L-Tyr (w/v) or at the same concentration containing a 1:1 mixture of L-Tyr and 3,3-D2-L-Tyr, purchased from Cambridge Isotope Laboratories (DLM-2317-.5).
Bacteria genome sequencing
Sequencing was performed by the Broad Technology Labs at the Broad Institute. Resulting PacBio reads for JUb39 and PYb007 were assembled using Canu v1.8 (https://github.com/marbl/canu.git)56. Assemblies were trimmed, oriented and circularized using Circlator v1.5.5 (https://sanger-pathogens.github.io/circlator/)57.
Phylogenetic analysis of group II pyridoxal-dependent decarboxylase genes
JUb39 TyrDC and AdcA were initially identified as the only significant hits via a tblastn search of the draft JUb39 genome assembly using Enterococcus faecalis TyrDC as a query sequence. An initial BLASTP screen of the nr sequence database restricted to bacteria was performed using the P. alcalifaciens JUb39 TyrDC and AdcA coding regions. Searches were performed hierarchically, limited initially to Enterobacteriaceae, followed by Enterobacterales, Gammaproteobacteria, Proteobacteria and finally all Eubacteria. With the exception of members of Morganellaceae (Providencia, Proteus, Morganella, Xenorhabdus, Photorhabdus, Arsenophonus and Moellerella), only two protein sequences per genus were retained for subsequent phylogenetic analysis. Representative group II decarboxylase enzymes with known substrate specificity from Eukaryota and Archaea as well as glutamate decarboxylase (gadA/B) and histidine decarboxylase sequences were also included.
Multiple sequence alignments were produced using the Phylomizer workflow (https://github.com/Gabaldonlab/phylomizer), which used the MUSCLE v3.8.31 (http://www.drive5.com/muscle)58, MAFFT v7.407 (https://mafft.cbrc.jp/alignment/software)59 and Kalign v2.04 (http://msa.sbc.su.se/cgi-bin/msa.cgi)60 multiple sequence aligners; these were trimmed to produce a consensus alignment using trimAL v1.4rev15 (https://github.com/scapella/trimal)61. An initial phylogenetic tree was produced using PhyML v3.3.20180621 (http://www.atgc-montpellier.fr/phyml/)62 using the NNI algorithm with an LG substitution model. This tree showed three major, well-supported clusters containing: (1) Enterococcus and Providencia TyrDCs - denoted “Enterococcus-type TDC”, (2) Eukaryotic AADCs denoted “Eukaryotic-type AADC”, and (3) Morganella AdcA and Providencia AdcA.
Based on this initial tree, a second tblastn search was used to determine the presence or absence of homologous genes among complete Gammaproteobacteria genomes. Enterococcus faecalis TyrDC and C. elegans TDC-1 were used as tblastn search query sequences. Hierarchical search was performed as described above, limited to an e-value cutoff of 10−5. A maximum of 2 highly similar sequences were retained per genus for phylogenetic analysis as listed in SI Table 1.
A final phylogenetic tree was constructed using the amino acid sequences derived from these tblastn queries. These were assembled into a consensus alignment using the Phylomizer workflow as described above. ProtTest (https://github.com/ddarriba/prottest3)63 was used to identify the optimal model for likelihood estimation, using Aikake Information Criterion (AIC) values for selection. The model selected and subject to PhyML analysis was an LG model with discrete gamma distribution, an estimated proportion of invariant sites (+I), empirical frequencies of amino acids (+F), estimated gamma shape parameter (+G) for rate variation among sites with the default 4 substitution rate categories, and the subtree pruning and regrafting (SPR) algorithm. 100 bootstrap pseudoreplicates were analyzed. Representatives from the resulting phylogeny were used to categorize and compile the cladogram in Fig. 3b. Adjacent genomic sequences, up to 3 CDS 5’ or 3’, were examined for genes encoding amino-acid permeases or transporters in an apparent operon as defined by close proximity and same orientation with respect to each tblastn hit (SI Table 1).
Molecular modeling
The putative amino acid sequence for JUb39 TyrDC was used to model active site residues using the Lb-TyrDC crystal structure in complex with PLP (5hsj.136) as a template guide using SWISS-MODEL (https://swissmodel.expasy.org)64. This resulted in a Qmean Z-score of 0.33, indicative of good agreement between structures. This process was also attempted with AdcA, and modeling was performed with the top 6 available structures based on sequence homology. The maximum QMean of AdcA was found with Lb-TyrDC, but with a value −5.71, indicative of low quality. Resulting models were visualized using Chimera v1.13.1 (https://www.cgl.ucsf.edu/chimera/)65. For Extended Data Fig. 8d, L-Tyrosine was manually docked according to the reported docking position36 for illustrative purposes only.
Statistical analyses
All statistical analyses were performed in R (https://www.R-project.org/)66 and RStudio (http://www.rstudio.com)67. Sample sizes were chosen according to conventional estimates of power for all assays. No randomization or blinding was performed. For modulation index and relative latency figures, data were normalized to the relevant control group mean value for each experimental day on the log scale via subtraction. Outliers in boxplots were defined as greater than 1.5 x interquartile range, but were included for analysis. All statistical analyses were performed on raw, non-normalized data. To avoid inflated P-values and to account for non-independence of observations, we employed mixed-effects regression analysis in lieu of simple ANOVA and t-tests. For behavioral assays, frequentist statistical comparisons were performed using a binomial generalized linear mixed-effects model (GLMM) with a logit link function for chemotaxis, food choice and egg-in-utero assays, while a linear mixed-effects model (LMM) on log10-transformed data was used to analyze SOS assays using the ‘lme4’ package v1.1–2168. In all cases, a random intercept term for assay plate was used to account for non-independence of animals on each assay plate and random intercept for date was used to account for day-to-day variability. In the presence of interactions, for example, the effects of bacterial strains across different odorants in Fig. 1a and Extended Data Fig. 1a, a random slope term per date was also used when appropriate. Estimated P-values for pairwise comparison of fixed effects were determined using Kenward-Roger approximated degrees of freedom as implemented in the ‘emmeans’ v1.4.569 and ‘pbkrtest’ v0.4–8670 packages. In nearly all cases, inclusion of random effects model terms resulted in conservative P-value estimates compared to a simple ANOVA. In the event of singular model fit, any random slope term, followed by random date effect terms were removed to allow convergence. For Wald statistics of model terms, packages ‘lmerTest’ v3.1-171 or ‘car’ v3.0-672 were used.
Additionally, for each dataset, a maximal Bayesian model was fit using the ‘rstanarm’ v2.19.373 package. Data presented are posterior credible intervals for fixed effect levels derived from posterior fitted values of the MCMC chains as implemented by the ‘emmeans’, ‘coda’ v0.19-374, ‘bayesplot’ v1.7.175 and ‘tidybayes’ v2.0.176 packages. Post-hoc corrections for multiple comparisons and type-I error were implemented using the ‘emmeans’ package. For comparison of intestinal bacterial cell numbers, an ordinal logistic regression was performed using the ‘MASS’ v7.3-51.477 package and ‘polr’ function. Categories of cell numbers were considered ordered factors of ‘none’, ‘some’ or ‘many’ cells. All statistical analysis code and raw data are available (https://github.com/SenguptaLab/ProvidenciaChemo.git).
For survival analysis data were fit to a Gompertz-Makeham function using ‘nls’ function in R using the following equation: S(t) = e^(−(G/γ)*(e^(γt)−1)). Bootstrap confidence intervals were derived from 1000 bootstrap samples with replacement, followed by nls fit. To calculate bootstrap median survival, using each nls fit and solving for t at S = 0.5 yielded the equation: t = (1/γ)*ln(1−(γ/G)*ln(0.5)).
Sample preparation for HPLC-MS
Approximately 10,000 mixed-staged worms in 1.5mL microfuge tubes were lyophilized for 18-24 hrs using a VirTis BenchTop 4K Freeze Dryer. After the addition of two stainless steel grinding balls and 1mL of 80% methanol, samples were sonicated for 5 min (2 sec on/off pulse cycle at 90 A) using a Qsonica Q700 Ultrasonic Processor with a water bath cup horn adaptor (Model 431C2). Following sonication, microfuge tubes were centrifuged at 10,000 RCF for 5 min in an Eppendorf 5417R centrifuge. 800μL of the resulting supernatant was transferred to a clean 4mL glass vial, and 800μL of fresh methanol added to the sample. The sample was sonicated and centrifuged as described, and the resulting supernatant was transferred to the same receiver vial and concentrated to dryness in an SC250EXP Speedvac Concentrator coupled to an RVT5105 Refrigerated Vapor Trap (Thermo Scientific). The resulting powder was suspended in 120μL of 100% methanol, followed by vigorous vortex and brief sonication. This solution was transferred to a clean microfuge tube and subjected to centrifugation at 20,000 RCF for 10 min in an Eppendorf 5417R centrifuge to remove precipitate. The resulting supernatant was transferred to an HPLC vial and analyzed by HPLC-MS.
HPLC-MS analyses
Reversed-phase chromatography was performed using a Vanquish LC system controlled by Chromeleon Software (ThermoFisher Scientific) and coupled to an Orbitrap Q-Exactive High Field mass spectrometer controlled by Xcalibur software (ThermoFisher Scientific). Methanolic extracts prepared as described above were separated on an Agilent Zorbax Eclipse XDB-C18 column (150 mm x 2.1 mm, particle size 1.8 μm) maintained at 40 °C with a flow rate of 0.5 mL/min. Solvent A: 0.1% formic acid in water; solvent B: 0.1% formic acid in acetonitrile. A/B gradient started at 5% B for 3 min after injection and increased linearly to 98% B at 20 min, followed by 5 min at 98% B, then back to 5% B over 0.1 min and finally at 5% B held for an additional 2.9 min to re-equilibrate the column. Mass spectrometer parameters: spray voltage, −2.9 kV/+3.5 kV; capillary temperature 380 °C; probe heater temperature 400 °C; sheath, auxiliary, and sweep gas, 60, 20, and 2 AU, respectively; S-Lens RF level, 50, resolution 240,000 at m/z 200; AGC target, 3E6. Each sample was analyzed in negative (ESI-) and positive (ESI+) electrospray ionization modes with m/z range 150–800. Parameters for MS/MS (dd-MS2): MS1 resolution, 60,000; AGC Target, 1E6. MS2 resolution, 30,000; AGC Target, 2E5; maximum injection time, 50 msec; isolation window, 1.0 m/z; stepped normalized collision energy (NCE), 10, 30; dynamic exclusion, 5 seconds; top 10 masses selected for MS/MS per scan. LC-MS data were analyzed using Metaboseek software (see below) and quantification performed via integration in Excalibur Quan Browser (ThermoFisher Scientific).
Metabolic network analysis
MS2 networking was performed using Metaboseek MS analysis software (doi: 10.5281/zenodo.3360087)78. Documentation and source code are available in the Metaboseek R package on GitHub: https://mjhelf.github.com/Metaboseek. For additional information, installation instructions and a user guide, please visit https://metaboseek.com. The xcms package79 within Metaboseek was used with “Metaboseek_default” settings, suitable for HRMS instruments, followed by MS2 matching using the following parameters: ppm window, 3; RT window, 5 seconds; ‘unique assignments’ on. From roughly 200,000 features detected in ESI+ HPLC-MS, approximately 10,000 features were matched to MS2 spectra, which was further culled to 4,563 features using the ‘Peak Shapes’ functionality to calculate peak quality scores, applying a threshold of 0.97. Networking was performed using the Compare MS2 function in Metaboseek with the following parameters: m/z tolerance, 0.002; ppm tolerance, 3; min. number of peaks in common, 4; noise level, 2%; with ‘parent masses’ turned on and ‘ignore small fragments’ (m/z < 100) turned on. The network was modified by removing edges below similarity score (cosine) = 0.7, and further simplified by restricting the maximum number of edges per node to the top 5 ranked by similarity score.
Extended Data
Extended Data Fig. 1. Octanol modulation by Providencia requires ingestion of bacteria and is not mediated by nutritive cues.
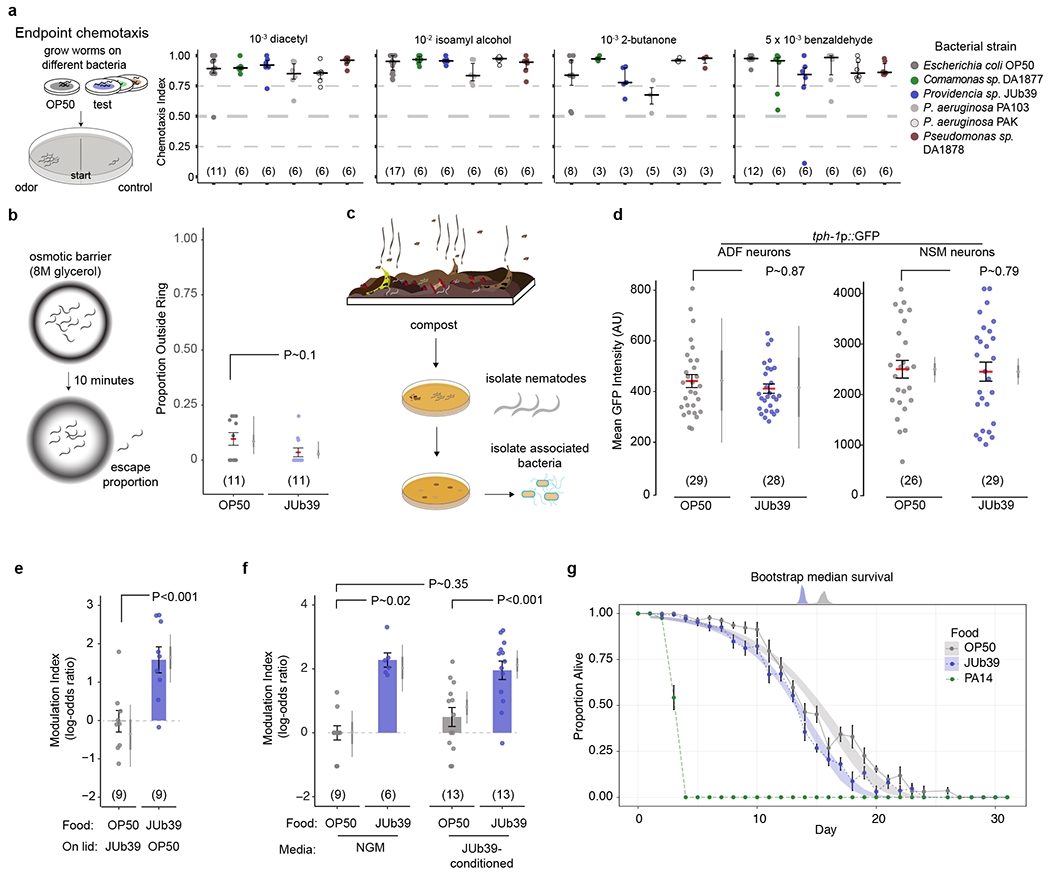
a, Cartoon and data from long-range chemotaxis assays of C. elegans grown on the indicated bacterial strains to the indicated attractive odors. Chemotaxis index (CI) = (animals at odorant – animals at control)/total animals. Dots, CI from single assays of approximately 100 animals. Horizontal line, median; errors, 1st and 3rd quartiles. Numbers in parentheses, number of assays performed over at least 3 days. b, Cartoon and data from osmotic ring avoidance assay. Dots, single assays of 10 animals. Numbers in parentheses, number of assays over at least 3 independent days. Y-axis is proportion of animals leaving an osmotic ring barrier of 8M glycerol after 10 minutes. P-value represents difference of means relative to JUb39-grown animals from a GLMM. Errors are SEM. Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals, respectively. c, Isolation of nematode-associated bacteria. Nematodes were isolated from residential compost in Massachusetts. Worms were allowed to crawl onto NGM plates from which they were picked to clean plates. Resulting bacterial colonies were isolated, grown on LB media and characterized via 16S rRNA sequencing. d, Expression of a tph-1p::gfp fluorescent reporter in indicated head neurons of young adult animals grown on either OP50 or JUb39. Dots, mean fluorescence of the soma of single neurons. Horizontal bar is mean; errors are SEM. Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals, respectively. P-values are from two-way ANOVA. e-f, Modulation index of worms grown on the indicated bacterial strains to 100% octanol, under the shown conditions. Animals were exposed to the indicated bacteria on the plate lid (e) for one generation, or to NGM control or bacteria-conditioned NGM (f) for 2 hours prior to the assay. Numbers in parentheses, independent experiments over 2 days with approximately 100 animals each. Values are shown on a log-odds (logit) scale and are normalized to the values of wild-type animals grown on OP50 for each day, indicated with a gray dashed line. Positive numbers indicate reduced avoidance of octanol. Errors are SEM. Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals, respectively. P-values between the indicated conditions are post-hoc comparisons from a GLMM, with Tukey-type multivariate-t adjustment for (f). g, Survival analysis of worms grown on the indicated strains. Dots, average proportion of surviving worms from 12 plates of 20 worms each on the indicated days. Error bars are SEM. Shaded blue and gray curves indicate 95% confidence intervals derived from 1000 bootstrap Gompertz function non-linear least squares (nls) fits of the indicated data. Top blue and gray distributions show bootstrapped median survival of the indicated strains.
Extended Data Fig. 2. Detection of N-succinyl TA.
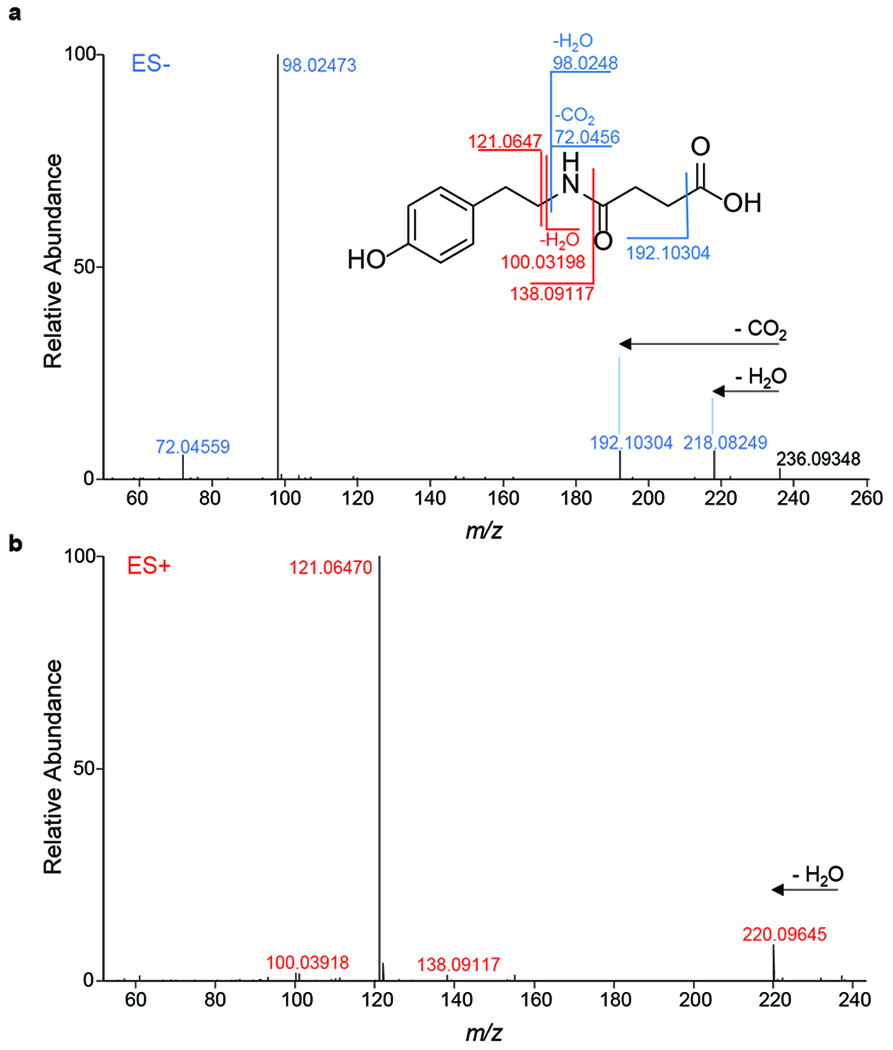
Major fragmentation reactions and resulting fragment ions are indicated in mass spectra of N-succinyl TA obtained in ESI− (a) and ESI+ (b) HPLC-MS2. Representative data are shown from at least three biologically independent experiments.
Extended Data Fig. 3. Complementation of TA-containing metabolites in tdc-1 mutants via Providencia TDC-encoding genes.
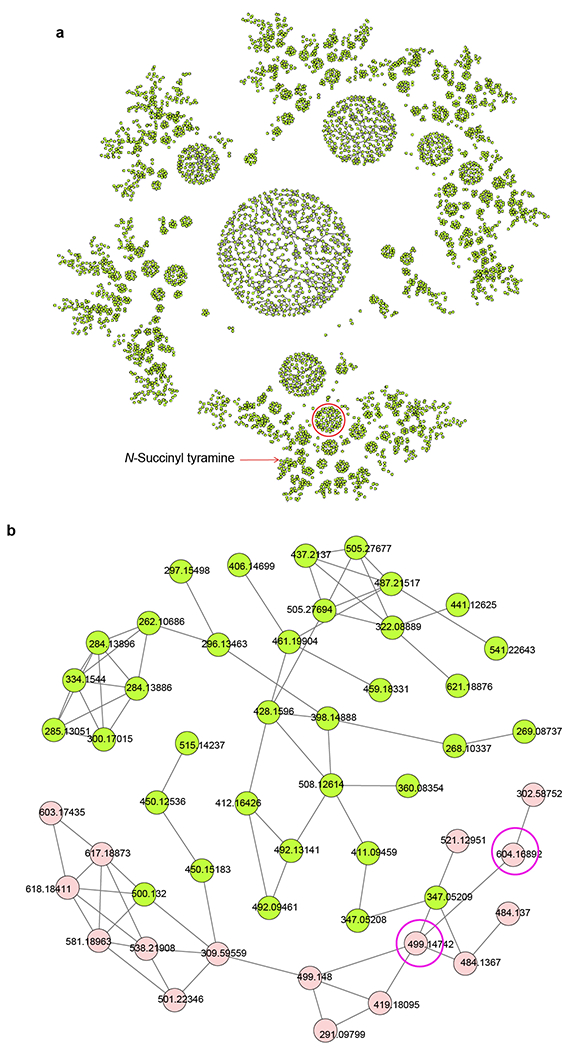
a, Overview of MS2 network obtained in positive ion mode. Each node represents a unique feature, and edges between nodes indicate similarity between MS2 spectra. Red circle highlights the sub-network containing the majority (16 out of 24 features, representing 10 out of 15 differential compounds) of differential TA-containing metabolites. This sub-network contains glycosylated TA-derivatives, N-succinyl-TA is represented by a node in a different sub-network (red arrow). b, Magnified view of sub-network highlighted in (a). The TA-containing features restored upon growth of tdc-1 worms on wild-type JUb39, and abolished when grown on JUb39 ΔtyrDC::cmR ΔadcA, are highlighted in light red. MS2 spectra for two example compounds at m/z 499.1474 and m/z 604.1689 (circled in magenta) are shown in Extended Data Figs. 4 and 5.
Extended Data Fig. 4. MS2 analysis and quantitation of TA-containing metabolite m/z 499.1474.
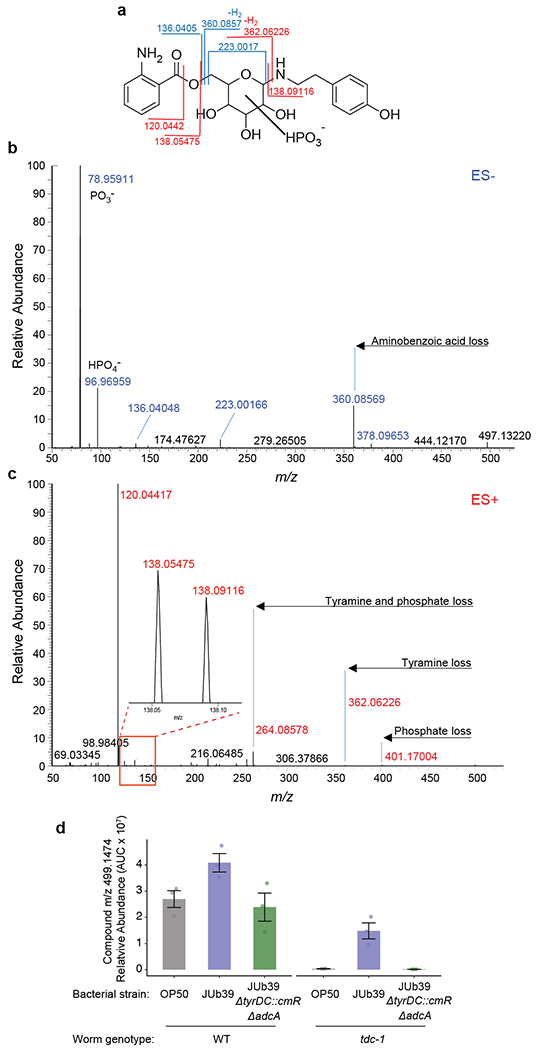
a, Major fragmentation reactions of m/z 499.1474 and resulting fragment ions. The shown putative structure is based on fragmentation and stable isotope incorporation. Stereochemistry and exact substitution pattern are unknown. b-c, MS2 spectra obtained in (b) ESI- and (c) ESI+ mode. Representative data are shown from at least three biologically independent experiments. d, Quantification of m/z 499.1474 in wild-type and tdc-1 worms fed the indicated bacterial strains as determined by positive-ion ESI+ HPLC-MS. Dots, independent samples from n=3 experiments. Errors are SEM.
Extended Data Fig. 5. MS2 analysis and quantitation of TA-containing metabolite m/z 604.1689.
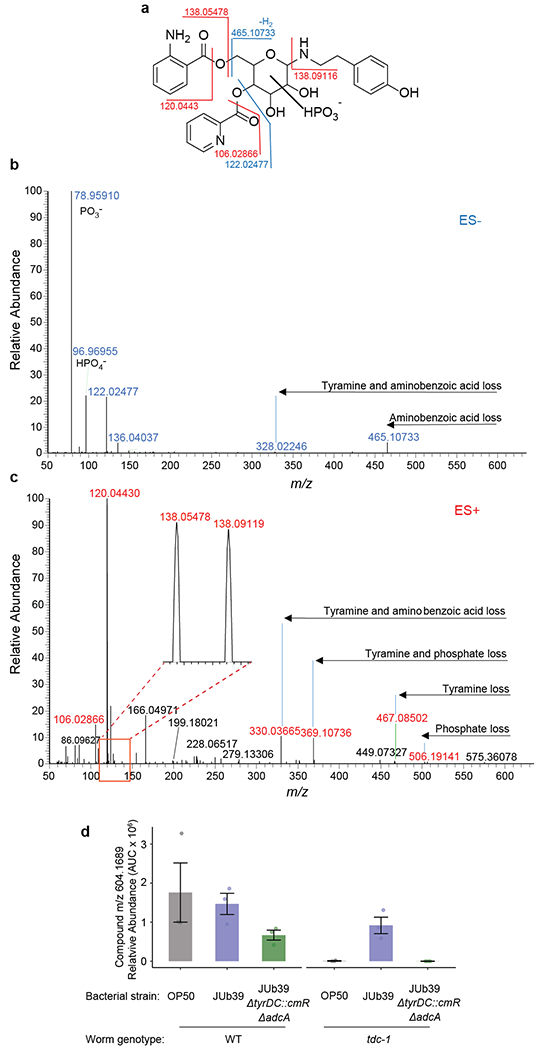
a, Major fragmentation reactions of m/z 604.1689 and resulting fragment ions. Stereochemistry and exact substitution pattern are unknown. The shown structure is based on fragmentation and stable isotope incorporation. b-c, MS2 spectra obtained in (b) negative- and (c) positive-ion mode. Representative data are shown from at least three biologically independent experiments. d, Quantification of m/z 604.1689 in wild-type and tdc-1 worms fed the indicated bacterial strains as determined by positive-ion ESI HPLC-MS. Dots, independent samples from n=3 experiments. Errors are SEM.
Extended Data Fig. 6.
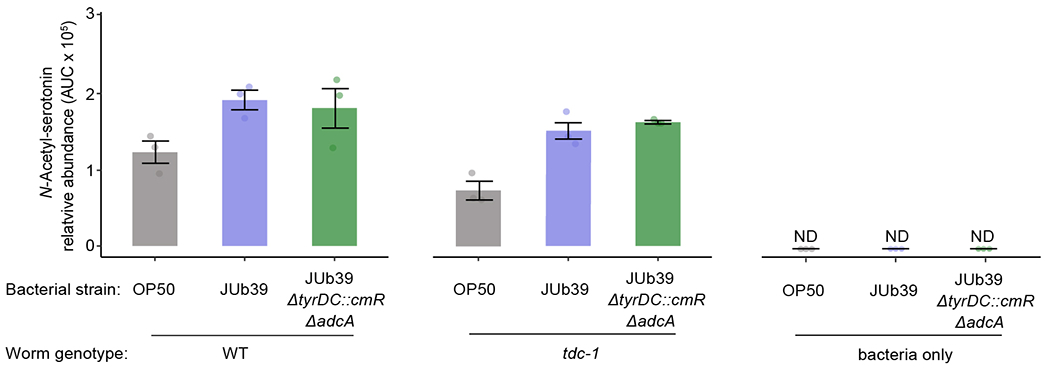
Quantification of N-acetyl serotonin in worms fed the indicated bacteria, or in bacterial cultures alone, as determined by ESI+ HPLC-MS. Dots, independent samples from n=3 experiments. Errors are SEM.
Extended Data Fig. 7. L-Tyr supplementation enhances octanol modulation.
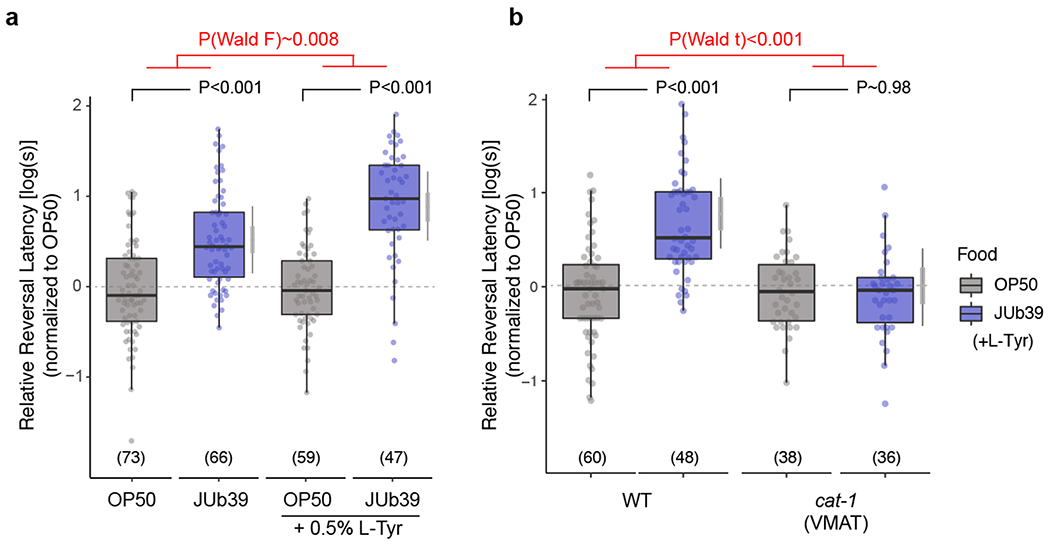
a-b, Reversal response times of animals of the indicated genotypes grown on the indicated bacteria in control conditions (a) or supplemented with 0.5% L-Tyr (a,b) to 100% octanol using SOS assays. Dots, response time of single worms. Y-axis is log10-scaled for these log-normal distributed data, and normalized to the indicated control group for each experimental day. Numbers in parentheses, number of worms tested in assays over at least 3 independent days. Boxplot, median and quartiles, whiskers, data range (excluding outliers). Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals for the difference of means, respectively. P-values indicating comparisons of means relative to the OP50 control for each conditions are from a LMM with Tukey-type multivariate-t adjustment. P-value in red indicates Wald F-statistic (a) or Wald t-statistic (b) for the effect of L-Tyr supplementation (a) or genotype (b) on the magnitude of the JUb39 effect. Wild-type data in (b) are also shown in Fig. 2g.
Extended Data Fig. 8. Phylogenetic analysis of group II decarboxylase genes in Gammaproteobacteria.
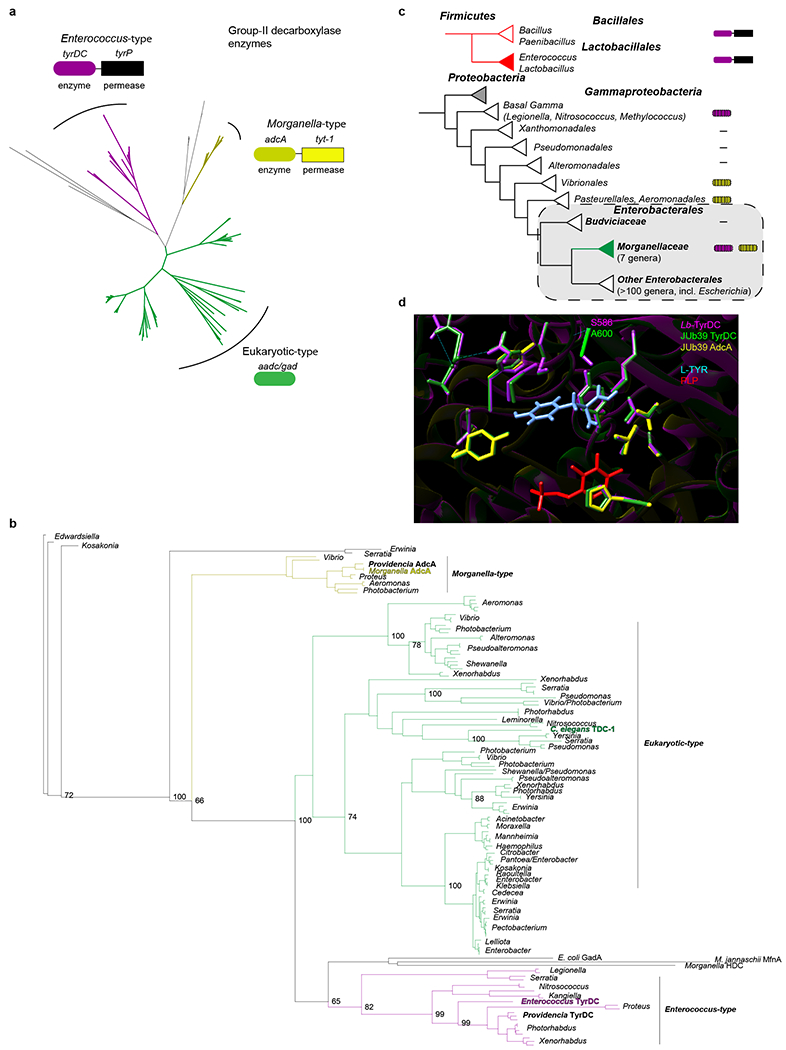
a, Neighbor-joining unrooted tree based on sequences identified via a BLAST search using Enterococcus faecalis TyrDC and C. elegans TDC-1. Initial tree indicates 3 major groups. Representative enzymes and operon structures for each group are indicated by colored boxes. b, Bootstrapped maximum likelihood phylogeny using PhyML and Phylomizer pipeline. Maximum of two highly similar sequences per genus were included after each BLAST search. Genera are indicated to the right. Numbers on branch-points matching this tree out of 100 bootstrap replicates are indicated at values >60. Group representatives from (a) are indicated in corresponding colors. Providencia and C. elegans sequences discussed in this work are indicated in bold. Accession numbers and BLAST metrics are listed in SI Table 1. c, Presence of tyrDC and adcA among complete genomes in Gammaproteobacteria. Linked boxes indicate organization in an operon. Hatched shading indicates variable presence among genera. Colored triangles indicate taxa of interest. d, Homology-based model of the TyrDC catalytic domain in Providencia based on the Lb-TyrDC crystal structure36 using SWISS-MODEL (https://swissmodel.expasy.org). Residues in magenta, green and yellow are from Lb-TyrDC, JUb39-TyrDC, and JUb39-AdcA, respectively. PLP is depicted in red and L-Tyr (manually docked for illustration) is indicated in light blue. Position of A600/S58636 in JUb39 TyrDC and Lb-TyrDC are indicated.
Extended Data Fig. 9. Disruption of JUb39 TA production or host OA receptor signaling affects octanol modulation without altering intestinal bacterial cell numbers.
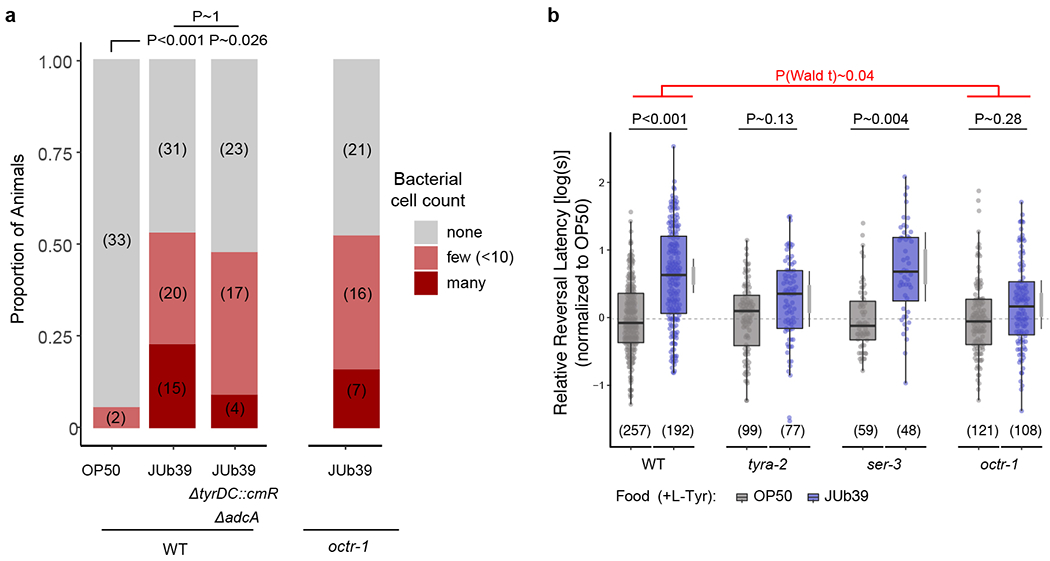
a, Presence of mCherry-expressing bacteria in the posterior intestines of young adult wild-type or octr-1 mutant animals. Bars show proportion of animals with the indicated distribution of bacterial cells present in animals grown on shown bacteria. Numbers in parentheses, number of animals. P-value is derived from an ordinal regression. b, Reversal response latency of animals of the indicated genotypes grown on the shown bacteria in control conditions of NGM + 0.5% L-Tyr to 100% octanol using SOS assays. Dots, response time of single worms. Y-axis is log10-scaled for these log-normal distributed data, and normalized to the indicated control group for each experimental day. Numbers in parentheses, number of worms tested in assays over at least 3 independent days. Boxplot, median and quartiles, whiskers, data range (excluding outliers). Gray thin and thick vertical bars, Bayesian 95% and 66% credible intervals for the difference of means, respectively. P-values between indicated conditions are from a LMM with Tukey-type multivariate-t adjustment. P-values in red indicate Wald t-statistic representing the genotype x food interaction effect relative to WT.
Extended Data Fig. 10. Providencia-derived TA complements the egg-laying defects of C. elegans tdc-1 mutants.
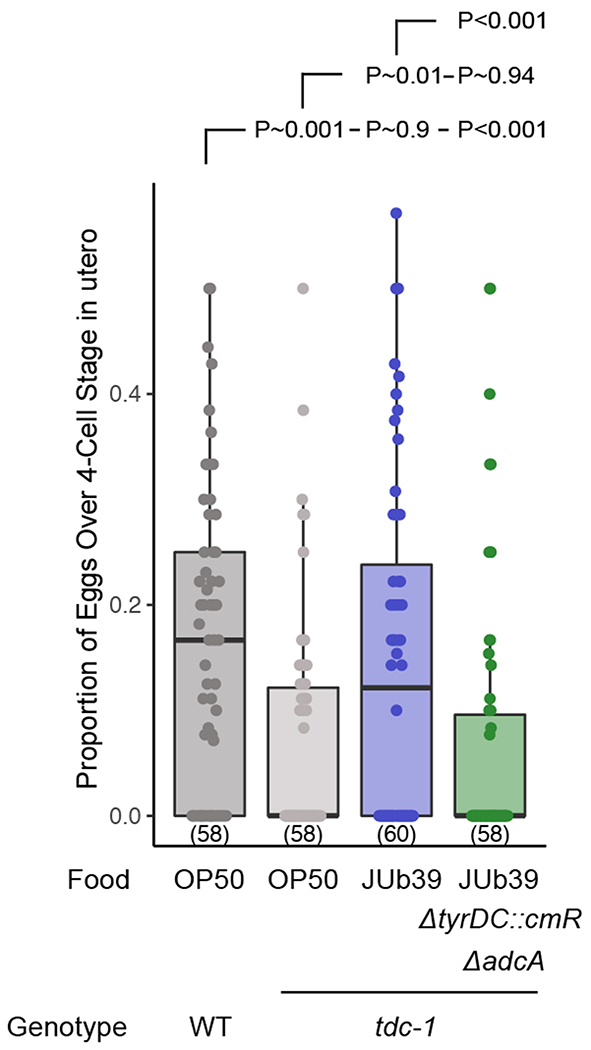
Quantification of the age of eggs in utero of animals of the indicated genotypes grown on the shown bacterial strains. Dots, proportion of eggs at or older than the 4-cell stage in individual animals. Numbers in parentheses, total number of animals scored in three independent experiments. Boxplot, median and quartiles, whiskers, data range (excluding outliers). P-values between the indicated conditions are from a binomial generalized linear mixed effects regression using a logit link function, with a post-hoc Tukey correction for multiple comparisons.
Supplementary Material
Acknowledgements
We thank Richard Komuniecki for the octr-1 cDNA, Mark Alkema for the tbh-1 promoter, multiple members of the C. elegans community for bacterial strains (listed in SI Table 2), and the Caenorhabditis Genetics Center for C. elegans strains. We are grateful to the Broad Institute for bacterial genome sequencing. We thank Max Helf for assistance with the Metaboseek software and Jingfang Yu and Henry Le for synthesis of N-succinyl neurotransmitter standards. We thank Sue Lovett, Laura Laranjo, Mark Alkema and the Sengupta lab for advice. We acknowledge the Sengupta lab, Cori Bargmann and Oliver Hobert for comments on the manuscript. This work was partly supported by the NIH (T32 NS007292 and F32 DC013711 – M.P.O., R01 GM088290 and R35 GM131877 – F.C.S., and R35 GM122463 and R21 NS101702 – P.S.), and the NSF (IOS 1655118 – P.S.).
Footnotes
Competing Interest Statement
The authors declare no competing interests.
Supplementary Information is available for this paper.
Data Availability Statement
All statistical analysis code and data necessary to reproduce these analyses are available as uploaded Source Data and/or at https://github.com/SenguptaLab/ProvidenciaChemo.git. Raw data from HPLC-MS experiments are available upon request due to large file sizes.
References
- 1.Douglas AE Fundamentals of Microbiome Science: How Microbes Shape Animal Biology. (Princeton University Press, 2018). [Google Scholar]
- 2.Guo R, Chen L-H, Xing C & Liu T Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth 123, 637–654 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Strandwitz P Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Holdorf AD & Walhout AJ C. elegans and its bacterial diet as a model for systems-level understanding of host-microbiota interactions. Curr. Opin. Biotechnol 46, 74–80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulenburg H & Félix M-A The natural biotic environment of Caenorhabditis elegans. Genetics 206, 55–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisel JD & Kim DH Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 35, 465–470 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Samuel BS, Rowedder H, Braendle C, Félix M-A & Ruvkun G Caenorhabditis elegans responses to bacteria from its natural habitats. Proc. Natl. Acad. Sci. USA 113, E3941–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bargmann CI, Hartwieg E & Horvitz HR Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 74, 515–527 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Song B-M, Faumont S, Lockery S & Avery L Recognition of familiar food activates feeding via an endocrine serotonin signal in Caenorhabditis elegans. Elife 2, e00329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao MY, Komatsu H, Fukuto HS, Dionne HM & Hart AC Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. USA 101, 15512–15517 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang B, Moussaif M, Kuan C-J, Gargus JJ & Sze JY Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 4, 429–440 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Entchev EV et al. A gene-expression-based neural code for food abundance that modulates lifespan. Elife 4, e06259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery L & Shtonda BB Food transport in the C. elegans pharynx. J. Exp. Biol 206, 2441–2457 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery L The genetics of feeding in Caenorhabditis elegans. Genetics 133, 897–917 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg M et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME J. 10, 1998–2009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirksen P et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biol. 14, 38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan M-W, Mahajan-Miklos S & Ausubel FM Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96, 715–720 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irazoqui JE et al. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6, e1000982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wragg RT et al. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J. Neurosci. 27, 13402–13412 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills H et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J. 31, 667–678 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris GP et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J. Neurosci. 30, 7889–7899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezak MJ & Ferkey DM The C. elegans D2-Like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS ONE 5, e9487 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezcurra M, Tanizawa Y, Swoboda P & Schafer WR Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J. 30, 1110–1122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkema MJ, Hunter-Ensor M, Ringstad N & Horvitz HR Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46, 247–260 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lints R & Emmons SW Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development 126, 5819–5831 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Sze JY, Victor M, Loer C, Shi Y & Ruvkun G Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403, 560–564 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Troemel ER, Chou JH, Dwyer ND, Colbert HA & Bargmann CI Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Artyukhin AB et al. Succinylated octopamine ascarosides and a new pathway of biogenic amine metabolism in Caenorhabditis elegans. J. Biol. Chem 288, 18778–18783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugin B et al. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis 28, 1353881 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbieri F, Montanari C, Gardini F & Tabanelli G Biogenic amine production by lactic acid bacteria: a review. Food 8, 17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcobal Á, Martín-Álvarez PJ, Moreno-Arribas MV & Muñoz R A multifactorial design for studying factors influencing growth and tyramine production of the lactic acid bacteria Lactobacillus brevis CECT 4669 and Enterococcus faecium BIFI-58. Res. Microbiol 157, 417–424 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Duerr JS et al. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J. Neurosci 19, 72–84 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandmeier E, Hale TI & Christen P Multiple evolutionary origin of pyridoxal-5’-phosphate-dependent amino acid decarboxylases. Eur. J. Biochem 221, 997–1002 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Connil N et al. Identification of the Enterococcus faecalis Tyrosine decarboxylase operon involved in tyramine production. Appl. Environ. Microb 68, 3537–3544 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linares DM, Fernández M, Martín MC & Alvarez MA Tyramine biosynthesis in Enterococcus durans is transcriptionally regulated by the extracellular pH and tyrosine concentration. Microb. Biotechnol 2, 625–633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H et al. Crystal structure of tyrosine decarboxylase and identification of key residues involved in conformational swing and substrate binding. Sci. Rep 6, 27779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quick M, et al. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum. J. Biol. Chem 281, 26444–26454 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Collins KM et al. Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition. Elife 5, e21126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rex E et al. TYRA-2 (F01E11.5): A Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J. Neurochem 94, 181–191 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Singh V, Kajino-Sakamoto R & Aballay A Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332, 729–732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elgaali H et al. Comparison of long-chain alcohols and other volatile compounds emitted from food-borne and related Gram positive and Gram negative bacteria. J. Basic Microbiol 42, 373–380 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Worthy SE et al. Identification of attractive odorants released by preferred bacterial food found in the natural habitats of C. elegans. PLoS ONE 13, e0201158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida K et al. Odour concentration-dependent olfactory preference change in C. elegans. Nat. Commun 3, 739 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Zhang F et al. Caenorhabditis elegans as a model for microbiome research. Front. Microbiol 8, 485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leitão-Gonçalves R et al. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 15, e2000862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pais IS, Valente RS, Sporniak M & Teixeira L Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol. 16, e2005710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henriques SF et al. Metabolic cross-feeding allows a gut microbial community to overcome detrimental diets and alter host behaviour. BioRxiv doi: 10.1101/821892 (2019). [DOI] [Google Scholar]
- 48.Breton J et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 23, 324–334 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Fetissov SO Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol 13, 11–25 (2017). [DOI] [PubMed] [Google Scholar]
Methods References
- 50.Blomfield IC, Vaughn V, Rest RF & Eisenstein BI Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol 5, 1447–1457 (1991). [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microb 81, 2506–2514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marx CJ Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res. Notes 1, 1 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbier M & Damron FH Rainbow Vectors for broad-range bacterial fluorescence labeling. PLoS ONE 11, e0146827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alegado RA & Tan M-W Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol. 10, 1259–1273 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Troemel ER, Kimmel BE & Bargmann CI Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Koren S, Walenz BP, Berlin K, Miller JR & Phillippy AM Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt M et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 16, 294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgar RC MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 35, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katoh K, Misawa K, Kuma K & Miyata T MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lassmann T & Sonnhammer ELL, Kalign – an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6, 298 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capella-Gutierrez S, Silla-Martinez JM & Gabaldon T Bioinformatics 25, 1972–1973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guindon S et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol 59, 307–321 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Darriba D, Taboada GL, Doallo R & Posada D ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waterhouse A et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen EF et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 66.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 67.RStudio Team. RStudio: integrated development for R. RStudio Inc., Boston, MA. [Google Scholar]
- 68.Bates D, Maechler M, Bolker B & Walker S Fitting linear mixed-effects models using lme4. J. Stat. Softw 67, 1–48 (2015). [Google Scholar]
- 69.Lenth R, emmeans: estimated marginal means, aka least-squares means. R package version 1.4.5. (2020) https://CRAN.R-project.org/package=emmeans [Google Scholar]
- 70.Halekoh U & Højsgaard S A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models - the R package pbkrtest. J. Stat. Softw 59, 1–30 (2014).26917999 [Google Scholar]
- 71.Kuznetsova A, Brockhoff PB & Christensen RHB lmerTest package: tests in linear mixed effects models. J. Stat Softw 82, 1–26 (2017). [Google Scholar]
- 72.Fox J & Weisberg S An R Companion to Applied Regression, Third Edition (Sage Press, 2019). [Google Scholar]
- 73.Goodrich B, Gabry J, Ali I & Brilleman S rstanarm: Bayesian applied regression modeling via Stan. (2020). [Google Scholar]
- 74.Plummer M, Best N, Cowles K & Vines K CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11 (2006). [Google Scholar]
- 75.Gabry J, Simpson D, Vehtari A, Betancourt M & Gelman A Visualization in Bayesian workflow. J. R. Stat. Soc. A 182, 389–402 (2019). [Google Scholar]
- 76.Kay M tidybayes: tidy data and geoms for Bayesian models. (2020). doi: 10.5281/zenodo.1308151 (URL: 10.5281/zenodo.1308151). [DOI] [Google Scholar]
- 77.Venables WN & Ripley BD Modern Applied Statistics with S. Fourth Edition (Springer, 2002) ISBN 0-387-95457-0. [Google Scholar]
- 78.Helf M Metaboseek: an interactive, browser-based tool to analyze your mass spectrometry data. (2019). doi: 10.5281/zenodo.3360087 (URL: 10.5281/zenodo.3360087). [DOI] [Google Scholar]
- 79.Smith CA, Want EJ, O’Maille G, Abagyan R & Siuzdak G XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem 78, 779–787 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


