Abstract
Sweat is a promising, yet relatively unexplored biofluid containing biochemical information that offers broad insights into the underlying dynamic metabolic activity of the human body. The rich composition of electrolytes, metabolites, hormones, proteins, nucleic acids, micronutrients, and exogenous agents found in sweat dynamically vary in response to the state of health, stress, and diet. Emerging classes of skin-interfaced wearable sensors offer powerful capabilities for the real-time, continuous analysis of sweat produced by the eccrine glands in a manner suitable for use in athletics, consumer wellness, military, and healthcare industries. This perspective examines the rapid and continuous progress of wearable sweat sensors through the most advanced embodiments that address the fundamental challenges currently restricting widespread deployment. It concludes with a discussion of efforts to expand the overall utility of wearable sweat sensors and opportunities for commercialization, in which advances in biochemical sensor technologies will be critically important.
Keywords: microfluidics, sweat, wearables, sensors, lab-on-chip
1. Introduction:
Emerging classes of skin-interfaced wearable sensors offer a powerful set of capabilities for the non-invasive assessment of the biochemical, biophysical, and kinematic signals arising from the natural physiological processes of the human body. Conventional wearable biosensors, designed in wrist-worn, chest-strapped, and apparel-integrated formats, offer quantitative assessment of physiological parameters in continuous modes of operation, by leveraging the integration of multimodal sensors. While existing classes of wearable technologies[1] have enabled remote monitoring of health, performance, environmental safety, and activities of daily living, they lack the ability to characterize important metabolic processes, which are essential for creating a comprehensive understanding of health, nutrition, and wellness status.
Standard approaches to monitoring body chemistry require invasive blood-based analysis using bulky laboratory equipment. Alternative biofluid targets to blood, such as saliva, tears, and sweat, represent attractive noninvasive media for biomarker analytics with the potential for remote health monitoring outside of controlled laboratory settings[2]. Sweat is of particular interest owing to the rich composition of electrolytes, metabolites, hormones, proteins, nucleic acids, micronutrients, and exogenous agents[3–7]. Laboratory-based sweat analysis techniques have utilized a combination of strap-based flexible tubes, absorbent pads, wicking materials, and adhesive tapes for collection with evaluation to follow on separate laboratory instrumentation. Such an approach is sufficient for medical diagnostics or controlled sports performance monitoring; however, the need for trained personnel, utilization of costly equipment, and complex sampling procedures restrict utility to highly controlled laboratory environments. Recent advances in material science, biochemical sensors, and flexible/stretchable electronics form the foundations for a new class of skin-interfaced wearable platforms that could assess the biomolecular composition and dynamics of sweat in either a continuous or intermittent fashion, unencumbered by a reliance on external instrumentation[3,8–10]. The consequences are significant in terms of enhanced measurement accuracy, modes of deployment, and integration with other body-worn sensors. Real-time measurement of sweat constituents, either singularly or in tandem, along with sweat dynamics (e.g. total sweat volume and rate variations) offers broad insights into the underlying dynamic metabolic activity of the human body.
This perspective highlights the latest key advances in wearable sweat biosensors, with particular emphasis on the biosensing concepts key to shaping the directions of future platform developments. Recent reviews contextualize wearable sweat sensors within the scope of skin-interfaced devices[1,3,6,10–12] or examine progress within the context of sensing technologies[9,11,13–19], specific application[5,7,8,14,19–22], material systems[23,24], and fabrication methods[25]. By contrast, this perspective examines the rapid and continuous progress of wearable sweat sensors through the most advanced embodiments that address the fundamental challenges hindering widespread deployment. A short introductory section summarizes key sensing schemes and fabrication constructs underpinning these wearable sweat systems. The second part broadly classifies the technical challenges according to needs in fluid handling, analytical performance, and power management with representative examples of the current approaches. The perspective concludes with a discussion of efforts to expand overall utility and opportunities for commercialization, in which advances in biochemical sensor technologies will be critically important.
2. Enabling Technologies for Wearable Sweat Sensors
Wearable, sweat-based biochemical analytical systems must operate across a broad range of applications ranging from passive modes of sweat collection to intense exercise. Environmental conditions could also vary from dry, arid hot temperatures to high humidity and wet settings (e.g. aquatic sporting activities). These demanding use cases necessitate careful designs that establish and maintain a robust conformal interface with the epidermis, in order to support continuous sampling and collection of sweat. Such demands also apply to the design of chemical sensors with key performance requirements, including operational stability, precision, sensitivity, selectivity, power efficiency, and methodology of data transfer. Emerging classes of wearable sweat sensing platforms, classified as either colorimetric, electrochemical, or hybrid sweat sensors, highlight key strategies to address these operational parameters.
2.1. Colorimetric Sweat Sensors
Epidermal microfluidic (‘epifluidic’) devices exploit low-modulus, biocompatible elastomeric substrates with embedded microfluidic channels for the capture and storage of sweat. Integration of colorimetric[3,26] (Fig. 1A) or fluorescent[27,28] (Fig. 1B) assays enable the quantitative analysis of sweat constituents of interest. Such devices utilize the natural pressure generated by sweat glands to characterize sweat rate and concurrently route sweat to individual chambers where sweat components interact with the specific chemical reagents that develop a distinct optical signal corresponding to a target analyte concentration. Smartphone-based image capture and color-based analysis offer a simple, inexpensive analytical pathway with recent reports demonstrating quantification of targets including sweat chloride, pH, glucose, urea, creatinine, and lactate[26,29–32].
Figure 1. Sensing methods for sweat analysis.
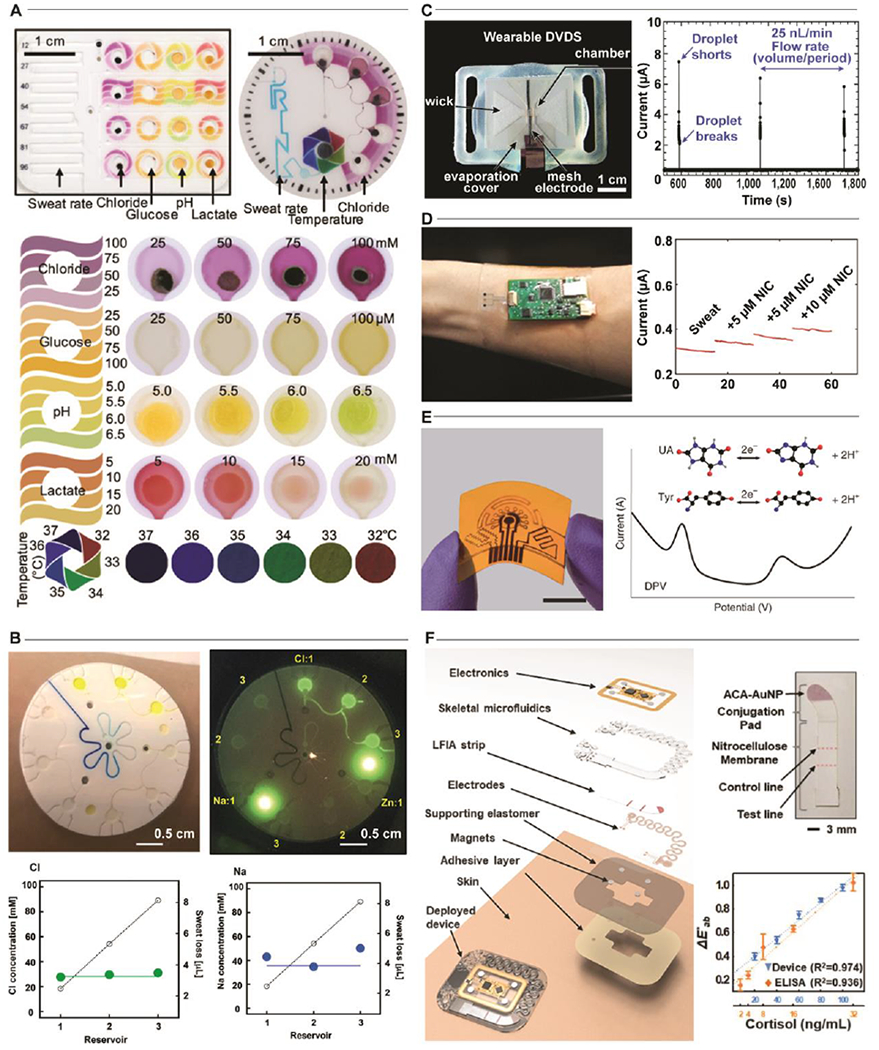
Colorimetric Sweat Sensors. A. Representative example of integration of multiple colorimetric assays in an epidermal microfluidic device for quantifying sweat constituents. Adapted with permission from Ref. [3]. Copyright 2018 American Association for the Advancement of Science. B. An image of a fluorescent-assay based epidermal microfluidic device for the measurement of sodium and chloride in sweat. Adapted with permission from Ref. [27]. Copyright 2018 The Royal Society of Chemistry. Electrochemical Sweat Sensors. C. Utilization of sweat conductivity measurements enables the real-time measurement of sweat rate. Adapted with permission from Ref. [34]. Copyright 2019 The Royal Society of Chemistry. D. Functionalization of electrodes permit amperiometric measurements of nicotine in sweat for monitoring of secondhand smoke exposure. Adapted with permission from Ref. [35]. Copyright 2020 American Chemical Society. E. Voltimetric measurements using functionalized laser-engraved graphene electrodes enable biochemical monitoring of signals associated with gout and metabolic disorders (uric acid, UA; tyronsine, Tyr) in a wearable device. Adapted with permission from Ref. [36]. Copyright 2020 Springer Nature. Hybrid Sweat Sensors. F. Demonstration of a multimodal epidermal microfluidic platform that integrates electrochemical and colorimetric sensors with a lateral flow assay for the detection of stress-related parameters. Adapted with permission from Ref. [37].
2.2. Electrochemical Sweat Sensors
Real-time monitoring of sweat biomarkers is critical as concentrations of sweat constituents have been shown to dynamically vary in response to the state of health, stress, and diet[33]. Skin-interfaced electrochemical sensors leverage conductometric, amperometric, potentiometric, and voltimetric measurement techniques (Fig. 1C–E) to provide continuous monitoring of target analytes in sweat[34–36]. These methods generate electrical signals proportional to analyte concentrations with high sensitivity and specificity, demonstrate rapid response times, and require minimal power capacity, thereby enabling miniaturized sensor designs suitable for integration into wearable platforms, which utilize onboard memory modules and wireless communication to transmit data to an external personal device assistant (i.e. smartphone or smartwatch) for real-time sweat analysis.
2.3. Hybrid Sweat Sensors
Recent developments[37] in wearable sensors highlight a hybrid strategy that integrates both electrochemical and optical sensing technologies into a single analytical platform for the wireless, battery-free, multimodal analysis of biomarkers in continuous and spot check modes of operation (e.g. cortisol, ascorbic acid, glucose, and sweat rate) (Fig 1F). This dual sensing approach utilizes colorimetric lateral flow immunoassay for monitoring cortisol and fluorescent assays for ascorbic acid and glucose, while exploiting impedance-based sensors to capture sweat rate and galvanic skin response for assessing sweat gland activity. Field trials demonstrate these capabilities in multi-day physiological tracking of parameters relevant to physical and mental stresses. The modes of deployment that result from this type of hybrid approach offer significant promise for long-term continuous and intermittent monitoring of physiological parameters and conditions.
3. Key Technical Challenges
Continued progress in the field of wearable sweat sensing requires further advances in manufacturing processes, biosensor development, testing, and validation in the uncontrolled field settings, miniaturization of sensor and fluidics packaging to address the remaining technical challenges, which can be broadly classified according to the following categories: (1) rapid collection, sampling, and storage of sweat in remote environments (fluid handling); (2) high sensitivity biosensing across various environmental conditions (analytical performance); and (3) adequate power and memory capacity for continuous operation (energy requirements).
3.1. Fluid Handling
Wearable sweat sensors that harness the natural pressure of activated eccrine sweat glands can passively route sweat to biochemical sensors via networks of microfluidic channels. These devices must support operation under a wide range of flow conditions, including anatomical variations in sweat gland density, physiological changes (e.g., stress, diet), and differing environmental conditions (humidity, temperature effects). These external factors could significantly influence the rate of sweat production and biocomposition. Continuous monitoring of sweat using electrochemical sensing approaches requires a constant flux of newly excreted sweat to permit a constant reaction/analysis process on the sensor surface. Conversely, colorimetric and fluorescence-based assays require the collection of defined volumes of sweat to ensure a complete reaction and evolution of optical signals. These competing needs, as well as additional constraints from operating conditions and body-interfacing locations, requires sophisticated and nuanced strategies for device designs.
Research efforts seek to adapt well-established microfluidic-handling techniques, developed using hard, rigid device constructions, into soft, stretchable microfluidics suitable for epidermal interfaces. Initial device demonstrations utilized simple networks of microchannels and reservoirs to capture sweat directly from the epidermis. More recent embodiments impart more complex valves (Fig. 2A,B) and fluid mixing (Fig. 2C) strategies. Passive valve constructs utilize geometry-defined pressure variations (capillary bursting valves[38]), surface functionalization (hydrophobic/hydrophilic[39]), or one-time chemical reactions[40] to route sweat to specific isolated sensing regions. Combinations of such valves enable microfluidic designs that permit the “chronosampling” of sweat in a time (or volume) sequenced manner. Powerful in the simplicity of implementation, passive valves exhibit two key drawbacks: they are irreversible once activated and prone to failure when utilized for high-intensity applications. Active valving represents a potential means for addressing these limitations by enabling dynamic control of fluid transport. Although many variations exist for lab-on-chip/lab-on-CD systems[41], few examples exist for wearable sweat analytical platforms. The most recent demonstration[42] utilizes thermo-responsive hydrogels and wireless heating elements as fast response valves integrated in a microfluidic network for dynamic fluid control. The control enabled by this embodiment suggests significant potential programmatic, system-level control of sweat routing and subsequent sensor interaction.
Figure 2. Fluid Handling.
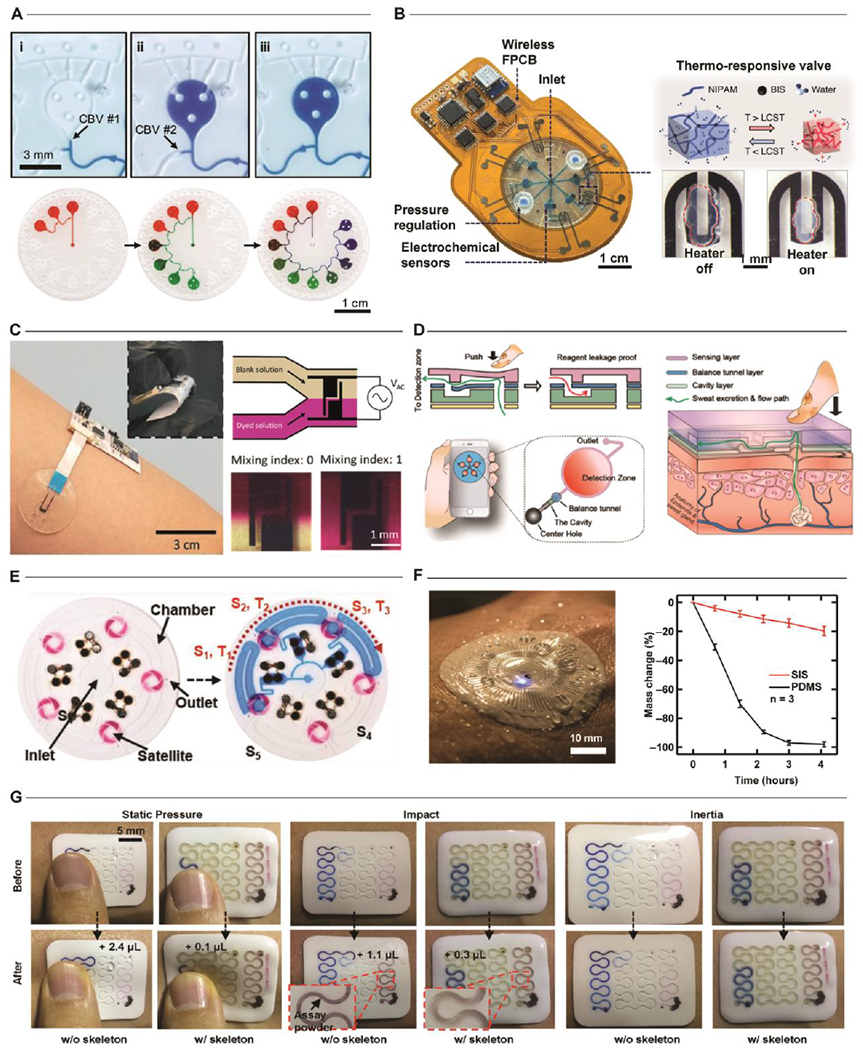
A. Passive Valves. Series of optical images highlighting the use of capillary burst valves (CBV) to enable the time/volume sequenced sampling of sweat. Adapted with permission from Ref. [38]. Copyright 2017 The Royal Society of Chemistry. B. Active Valves. Example of a wearable platform utilizing a thermo-responsive hydrogel with miniature heaters as valves to programmatically route sweat when activated by localized heating. Adapted with permission from Ref. [42]. Copyright 2020 Springer Nature. C. Mixing. Demonstration of a wearable sensor that utilizes electrical-field induced thermal gradients for in situ mixing. Adapted with permission from Ref. [44]. Copyright 2019 The Royal Society of Chemistry. D. Activation. A battery-free approach combines manual (finger-press) activation with micro-molded flaps for on-demand sample mixing. Adapted with permission from Ref. [45]. Copyright 2019 American Chemical Society. E. Timing. Ingress of sweat activates periodically-spaced galvanic cells to enable temporal, battery-free sweat analysis via colorimetric assays. Adapted with permission from Ref. [47]. Copyright 2019 WILEY-VCH Verlad GmbH & Co. F. Evaporation. Control of fluid permeability is necessary for enabling deployment of sensors in demanding environments or to ensure accurate measurements in clinical settings by eliminating evaporation-induced sample degradation. Adapted with permission from Ref. [32]. Copyright 2019 American Association for the Advancement of Science. G. Deformation. Integration of hard/soft materials enables optimization of device geometries to resist mechanical deformations from physical impact. Adapted with permission from Ref. [48]. Copyright 2020 WILEY-VCH Verlad GmbH & Co.
A logical extension of valve-based fluidic controls is the integration of active mixing in epidermal microfluidic constructs to facilitate complex sample processing and fluid analysis. As sweat-based devices operate in a low-Reynolds number regime, the resulting laminar flow profiles preclude non-diffusive passive mixing strategies. Moreover, most designs seek to avoid diffusive sample interaction to reduce assay-to-assay cross contamination. Traditional microfluidic devices offer insight into an alternative approach by which the application of external fields (electrophoretic, acoustophoretic, magnetophoretic) or physical structures (flaps, valves) mix fluids[43]. Such methods often utilize form factors that preclude utilization in wearable formats; however, a recent demonstration[44] (Fig. 2C) utilizes local temperature gradients induced by the application of a non-uniform oscillating electric field to facilitate on-demand sample mixing. Another approach[45] combines air chambers and flap valves to permit mixing through manual pumping (Fig. 2D). Continued expansion of the library of active mixing approaches, when paired with programmatic fluidic control, will enable further development of high-precision sensing approaches for low concentration biomarkers targets of interest.
Field deployment of complex epidermal microfluidic sensors requires careful assessment of both operating conditions and the sweat-based biomarkers of interest. Some sweat constituents tend to change dynamically as a function of sweat rate (e.g. sodium chloride concentrations) and physiological or mental state (e.g. cortisol)[46]. Continuous electrochemical sensing and colorimetric technologies have enabled accurate correlation of sweat biomarkers with sample time. One recent colorimetric device platform[47] employs a simple timing strategy that incorporates sweat-activated “stopwatches” in the form of low cost flexible galvanic cells, thereby enabling temporal sweat analysis with passive colorimetric assays (Fig. 2E). Devices that support time-dependent sweat analysis in a battery-free manner are particularly attractive for demanding applications such as athletic performance monitoring. In this context, devices must ensure measurement accuracy by mitigating potential sample loss through evaporation or device deformations. One representative microfluidic device platform[32] employs tortuous microfluidic networks and polymeric materials with low water permeability to significantly reduce evaporation and facilitate use in high-intensity activities in aquatic environments (Fig 2F). A more recent strategy[48] (Fig. 2G) integrates high modulus, impermeable polymers for use as microfluidic structures embedded in low modulus elastomers. Optimized channel geometries enable this configuration to resist mechanical deformation as a result of physical impact while maintaining a conformal, fluid-tight interface with the epidermis. Such application-dependent considerations are essential for fully realizing the promise of these epidermal microfluidic platforms. As the application space for wearable sweat sensors continues to grow, so too will the need for additional fluid-handling techniques.
3.2. Analytical Performance
The intrinsic, time-dynamic nature of sweat necessitates that wearable sensing platforms must operate in rapidly evolving environments where sensor performance and/or changes in biochemistry pose significant challenges in achieving accurate, high-quality measurements. As detailed elsewhere[1,3,4,11,12,18,33,49,50], the rich matrix of electrolytes, metabolites, hormones, and small molecules found in eccrine sweat offer valuable insights into human metabolic and physiological status with many constituents present only in extremely low concentrations. While there is significant interest in the utilization of sweat for tracking disease progression, mental health, and overall physical well-being, the measurement of low-concentration species requires careful consideration of sweat stimulation methodology, correlation with physiological condition, and sample contamination[2,10,11,46,49].
Adequate, facile stimulation and collection of sweat constitute significant challenges for both established sweat-based diagnostics (cystic fibrosis[51]) and novel wearable sweat sensors[46,52]. Typical methods of sweat harvesting require strenuous activity, thermal gradients, or exposure to localized chemical stimulation to generate sweat in sufficient volumes for analysis[3]. Localized sweat stimulation is the primary inducement method for stationary or diagnostic applications to circumvent the need for intense physical activity (Fig. 3A). Here, transcutaneous delivery of sweat-inducing drugs via iontophoresis initiates a localized sweat response for subsequent collection and analysis. While each method can yield adequate sweat volumes for biochemical sensing, the rate of production is method dependent[53]. This, in turn, can result in additional variability in biomarker concentrations. Hence, the establishment of alternative methods that enable repeatable stimulation and consistent flow rates is necessary to support daily health diagnostics. Recent efforts demonstrate potential alternative methods such as capturing sweat generated during showering[30] (Fig. 3B) or employing hydrogel patches[54,55] (Fig. 3C) to wick sweat generated by natural perspiration at a consistent flow rate[56].
Figure 3. Analytical Performance.
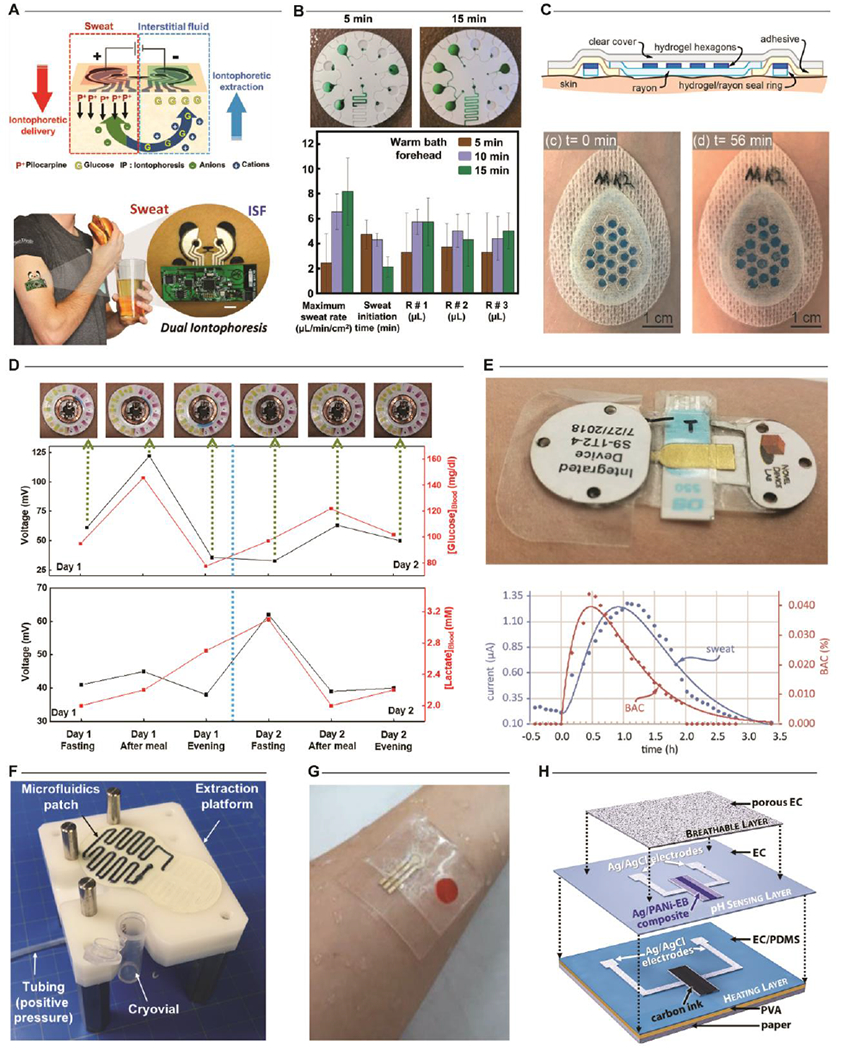
Sweat generation. Typical methods for stimulating a sweat response include stimulation by iontophoresis or exposure to thermal gradients arising from physical exertion. A. A representative example of a device that integrates iontophoretic stimulation and electrochemical analysis. Adapted with permission from Ref. [59]. Copyright 2018 WILEY-VCH Verlad GmbH & Co. B. Exposure to elevated temperatures during bathing (shower or bath) induces sufficient sweat for biochemical analysis. Adapted with permission from Ref. [30]. Copyright 2019 The Royal Society of Chemistry. C. Utilization of absorbent hydrogels enables collection of sweat generated passively during normal daily activities. Adapted with permission from Ref. [55]. Copyright 2020 The Royal Society of Chemistry. Sweat as a blood surrogate. D. One demonstration using an epifluidic sweat sensor correlates blood and sweat glucose concentrations over a multi-day period by periodic sampling. Adapted with permission from Ref. [29]. Copyright 2018 American Association for the Advancement of Science. E. A recent demonstration using a wearable electrochemical sensor with integrated iontophoretic stimulation correlates sweat and blood ethanol concentrations over a 4-hour period. Adapted with permission from Ref. [60]. Copyright 2018 The Royal Society of Chemistry. Contamination. F. Elimination of operator interaction through an optimized device-to-world interface minimizes contamination during sample extraction for external laboratory analysis. Adapted with permission from Ref. [61]. Copyright 2020 Elsevier. G. Optimization of fluid pathways enables strategies for minimizing biofouling of electrochemical sensor surfaces. Here, the embodiment utilizes on-demand sample measurements activated a finger-press to periodically measure sweat cortisol. Adapted with permission from Ref. [62]. Copyright 2020 Elsevier. H. Alternative strategies for mitigating biofouling rely upon multilayer sensor constructs or optimized coatings to maintain consistent sensor performance. Adapted with permission from Ref. [63]. Copyright 2020 Elsevier.
Sweat constituents such as glucose, ammonia, urea, and ethanol serve as surrogates for target analytes in blood for monitoring important metabolic health conditions (i.e. diabetes or kidney health)[50]. Prior to the emergence of skin-interfaced sensing platforms, traditional sampling approaches enabled only limited sweat-based biochemical analysis in the form of aggregated measurements of high-concentration species (electrolytes) at singular sampling instances in time[10]. Initial demonstrations of epidermal microfluidic devices supported the hypothesized relationship between sweat and blood analytes[29,57–59] (Fig. 3D). A more recent embodiment[60] has established the unequivocal correlation through the detection of ethanol via continuous stimulation and sampling of sweat (Fig. 3E). Such clinical validation studies are essential for sweat biomarkers, especially those present in ultra-low concentrations.
To support such validation efforts, minimization of sample contamination during collection and/or extraction is critical. Typical strategies include optimization of the device/epidermal interface and ensuring consistent flow across sensor surfaces to reduce effects of interfering species. One recent approach[61] yields a “clean” sweat sample by eliminating operator interaction during sample extraction by optimization of the device-to-world interface. Custom extraction hardware cleanly evacuates the epidermal sweat collection device into a cryotube for analysis of cytokine concentrations via the application of positive air pressure (Fig. 3F). Other methods circumvent specific contamination issues with electrochemical sensors by implementing novel designs to reduce or eliminate biofouling on the sensor surface[62,63] (Fig. 3G,H).
Establishing operational equivalency for wearable sweat sensors with traditional biochemical methods requires employing strategies that enable stable, predictable sensor operation invariant of external environmental conditions[18]. Wearable systems not only must address the challenges posed by sweat stimulation, physiological relevance, and contamination, but also employ sensor constructs, either electrochemical[62,64–87] or optical[88–91], that can transduce meaningful signals from ultralow concentrations of target analytes[92] under uncontrolled environmental conditions. Such targets could necessitate integration of complementary sensors (e.g. pH, temperature) or complex pretreatment, calibration, or reaction steps for accurate sensing to support operation in a skin-interfaced form factor. Thus, the development of wearable platforms and biochemical sensing systems, that decouple target signals from external sources of noise, while protecting against assay degradation and contamination, represent important directions for continued research.
3.3. Energy Requirements
Power management is an overarching concern for wearable sweat systems with active, continuous-mode sensors due to the requirements for miniaturized form factor, size, and weight needed to support conformal skin-interfacing. Additional considerations result from specific sensor requirements in terms of sampling frequencies, communication bandwidth, operating distances, and device lifecycle. For most wearable platforms, batteries represent the most versatile option for supporting continuous operation[1] with key limitations imposed by the battery footprint and weight. Moreover, wireless communication strategies, compatible with these form factors, have particularly demanding power requirements, especially for short/long range operations, location tracking, and across different bandwidths.
Although advances in energy storage densities and alternative chemistries are important for continued progress, several recent demonstrations that exploit energy harvesting[29,47,93–105] suggest new compelling directions to circumvent these power management issues. Several examples incorporate sweat-activated batteries[96,101,104–106] or supercapacitors[95] to supply power to both sensing and data communication components (Fig. 4A–D). Such platforms utilize a combination of materials and device architectures to achieve power generation performance largely independent of sweat composition. An alternative energy harvesting approach incorporates near-field communication (NFC) capabilities[29,107] to allow for battery-free operation when close proximity to a radio frequency (RF) source is feasible (Fig. 4E,F). One recent demonstration[29] illustrates the use of this approach for nonclinical diagnostics in a device that integrates wireless, skin-interfaced electrochemical sensors for sweat glucose and lactate. Combinations of these energy harvesting approaches may prove attractive for applications in which additional bulk size and footprint would impede operation. Ultimately, power management remains a key area for continued research needed to achieve equivalence between active and passive measurement methods, while preserving simplicity in operation and miniaturized physical designs.
Figure 4. Energy Storage.
Batteries. Two recent demonstrations (A, B) utilize sweat as a biobattery to supply sufficient power to the wearable sensing platform for electrochemical analysis. A is adapted with permission from Ref. [105]. Copyright 2020 American Association for the Advancement of Science. B is adapted with permission from Ref. [96]. Copyright 2020 Springer Nature. C. A similar device utilizes a paper-based battery construct as the measurement method for determining chloride concentrations in sweat. Adapted with permission from Ref. [106]. Copyright 2019 Springer Nature. D. Supercapacitors represent an attractive alternative as demonstrated in a recent example for the measurement of sweat glucose, sodium, and potassium concentrations. Adapted with permission from Ref. [95]. Copyright 2019 Elsevier. Energy Harvesters. Other device platforms (E, F) harvest energy from radio frequency sources (NFC) to support battery-free operation. E is adapted with permission from Ref. [107]. Copyright 2019 Elsevier. F is adapted with permission from Ref. [29]. Copyright 2018 American Association for the Advancement of Science.
4. Opportunities and Outlook
Advances in soft microfluidics, sensing modalities, energy storage technologies, and system integration strategies serve as a powerful foundation for skin-like wearable biochemical systems for sweat analysis. These solutions are capable of non-invasively unlocking clinical grade insights about performance, battlefield hydration, disease screening, stress, and metabolic health tracking in real-world settings. As this class of biosensors continues to mature, additional challenges will rise in prominence with particular consideration in data collection and fluid handling. Broad adoption of platforms capable of long-term continuous monitoring will vastly expand the volume of collected data. This scale up in data will necessitate new algorithms, approaches, and tools to enhance our understanding of large sweat biomarker datasets, in combination with biophysical markers, and their collective physiological relevance. A recent validation study[108] represents one of the first large-scale demonstrations employing advanced rehydration algorithms, developed to optimize athletic performance based on sweat rate and electrolyte loss metrics, with a low-cost, epifluidic sweat sensor. A systematic comparison of sensor performance to gold standard absorbent patches establishes a strong statistical correlation under both controlled laboratory environments and uncontrolled field settings across participants (N=312 athletes). Such large-scale validation studies supported by the standardization of best practices for sensor testing are a critical requirement for the continued broad adoption of such wearable systems.
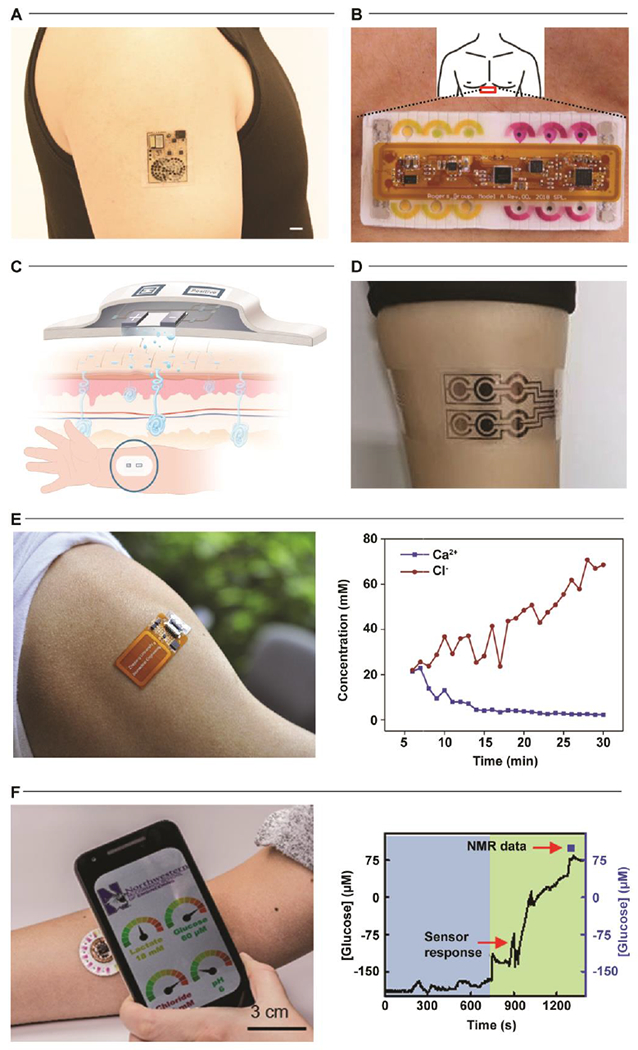
Overall, while the skin-interfaced devices highlighted here represent recent technological milestones in establishing platform utility, many important and interesting challenges remain in translating these prototype systems to commercial products. Such commercialization efforts require regulatory, clinical, and quality strategies to support adoption in conjunction with established manufacturing supply chains to scale production beyond university fabrication laboratories[109] (Fig. 5A). Recent research[110] in manufacturing methodologies demonstrate that electrochemical and optical sensors as well as microfluidic constructs are compatible with low-cost, high-volume process flows. Key representative examples from Epicore Biosystems[111] (the Gx Sweat Patch, commercialized by The Gatorade Company—Fig. 5B; Skin Track pH sweat sensor, commercialized by L’Oreal—Fig. 5C) utilize established roll-to-roll manufacturing methods to establish low-cost wearable microfluidic systems at high volume. Other efforts, such as those by Eccrine Systems[112] (Fig. 5D), seek to leverage fully integrated sweat stimulation and analytical platforms to enable continuous, long-term sweat monitoring for at-home clinical diagnostics. These initial offerings demonstrate the continued convergence of flexible hybrid electronics and established large-scale manufacturing processes to support the commercial deployment of wearable microfluidics and biochemical sweat monitoring. Such wearable sweat sensors represent a class of technology with powerful potential for wide-spread adoption in athletics, military, consumer wellness, and healthcare industries. These solutions are capable of non-invasively unlocking clinical grade insights about performance, battlefield hydration, disease screening, stress, and metabolic health tracking in real-world settings.
Figure 5. Commercialization.
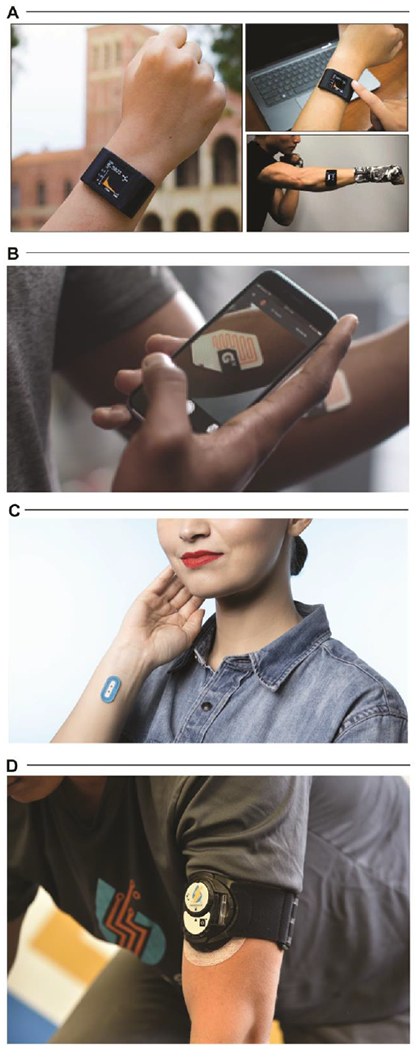
A. Integration of wearable sweat sensors into an existing consumer-adopted watch band. Adapted with permission from Ref. [109]. Copyright 2019 American Association for the Advancement of Science. B. The Gx Sweat Patch developed by Epicore Biosystems. Adapted with permission from Ref. [111]. Copyright 2020 Epicore Biosystems. C. The My Skin Track pH device developed by Epicore Biosystems. Adapted with permission from Ref. [111]. Copyright 2020 Epicore Biosystems. D. The E-AB (electrochemical, aptamer-based) sensing platform developed by Eccrine Systems. Adapted with permission from Ref. [112]. Copyright 2020 Eccrine Systems.
Highlights.
Skin-interfaced wearable sensors offer powerful capabilities for continuous sweat analysis
Wearable sweat sensors enable precise assessments of health status and disease conditions outside of clinical settings
Fundamental challenges in fluid handling, analytical performance, and energy storage restrict widespread deployment
Continued platform maturation via commercialization increases utility for monitoring health, nutrition, and wellness status
Funding:
We would like to acknowledge funding support provided by the Querrey Simpson Institute for Bioelectronics at Northwestern University. This publication was supported by the National Institute on Aging of the National Institutes of Health (R43AG067835). T.R.R. acknowledges additional support as a Junior Investigator from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM113134) and the Hawai‘i Community Foundation (Robert C. Perry Fund).
Author Biographies
Roozbeh Ghaffari obtained his B.S. and M.E. degrees in electrical engineering from the Massachusetts Institute of Technology in 2001 and 2003. He received his Ph.D. degree in biomedical engineering from the Harvard Medical School—MIT Program in Health Sciences and Technology in 2008. He joined the Querrey Simpson Institute for Bioelectronics at Northwestern University in May 2017, where he is currently a research associate professor in the Department of Biomedical Engineering and serves as a Director of Translational Research. His research interests lie at the intersection of soft microfluidics, bioelectronics, biosensors, and precision medicine.
John A. Rogers obtained B.A. and B.S. degrees in chemistry and in physics from the University of Texas Austin, in 1989. From MIT, he received S.M. degrees in physics and in chemistry in 1992 and his Ph.D. degree in physical chemistry in 1995. In 2016, he joined Northwestern University as the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Medicine, with affiliate appointments in Mechanical Engineering, Electrical and Computer Engineering, and Chemistry, where he is also Executive Director of the Querrey Simpson Institute for Bioelectronics.
Tyler R. Ray obtained his B.S. and M.S. degrees in mechanical engineering from the University of South Carolina in 2008 and 2010. He received his Ph.D. in mechanical engineering from the University of California, Santa Barbara in 2015. In 2019, he joined the Department of Mechanical Engineering at the University of Hawai’i at Mānoa as an assistant professor. His research interests include the design and fabrication of multifunctional hierarchical materials, soft microfluidics, and biosensors with a focus on diagnostic applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
T.R.R., and J.A.R. are inventors on patents and patent applications related to epidermal microfluidics, including applications in cystic fibrosis diagnostics. R.G. and J.A.R. are cofounders of Epicore Biosystems, a company that develops epidermal microfluidic devices. T.R.R. has a consulting and advisory relationship with Epicore Biosystems.
References:
- [1].Ray TR, Choi J, Bandodkar AJ, Krishnan S, Gutruf P, Tian L, Ghaffari R, Rogers JA, Bio-Integrated Wearable Systems: A Comprehensive Review, Chem. Rev 119 (2019) 5461–5533. 10.1021/acs.chemrev.8b00573. [DOI] [PubMed] [Google Scholar]
- [2].Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G, Katchman BA, Accessing analytes in biofluids for peripheral biochemical monitoring, Nat Biotechnol. 37 (2019) 407–419. 10.1038/s41587-019-0040-3. [DOI] [PubMed] [Google Scholar]
- [3].Choi J, Ghaffari R, Baker LB, Rogers JA, Skin-interfaced systems for sweat collection and analytics, Sci. Adv. 4 (2018) eaar3921 10.1126/sciadv.aar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Katchman BA, Zhu M, Blain Christen J, Anderson KS, Eccrine Sweat as a Biofluid for Profiling Immune Biomarkers, Prot. Clin. Appl. 12 (2018) 1800010 10.1002/prca.201800010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lin S, Yu W, Wang B, Zhao Y, En K, Zhu J, Cheng X, Zhou C, Lin H, Wang Z, Hojaiji H, Yeung C, Milla C, Davis RW, Emaminejad S, Noninvasive wearable electroactive pharmaceutical monitoring for personalized therapeutics, Proc Natl Acad Sci USA. 117 (2020) 19017–19025. 10.1073/pnas.2009979117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mondal S, Zehra N, Choudhury A, Iyer PK, Wearable Sensing Devices for Point of Care Diagnostics, ACS Appl. Bio Mater. (2020) acsabm.0c00798 10.1021/acsabm.0c00798. [DOI] [PubMed] [Google Scholar]
- [7].Samson C, Koh A, Stress Monitoring and Recent Advancements in Wearable Biosensors, Front. Bioeng. Biotechnol. 8 (2020) 1037 10.3389/fbioe.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang Y, Gao W, Wearable and flexible electronics for continuous molecular monitoring, Chem. Soc. Rev. 48 (2019) 1465–1491. 10.1039/C7CS00730B. [DOI] [PubMed] [Google Scholar]
- [9].Ghaffari R, Choi J, Raj MS, Chen S, Lee SP, Reeder JT, Aranyosi AJ, Leech A, Li W, Schon S, Model JB, Rogers JA, Soft Wearable Systems for Colorimetric and Electrochemical Analysis of Biofluids, Adv. Funct. Mater. (2019) 1907269 10.1002/adfm.201907269. [DOI] [Google Scholar]
- [10].Bandodkar AJ, Jeang WJ, Ghaffari R, Rogers JA, Wearable Sensors for Biochemical Sweat Analysis, Annual Rev. Anal. Chem. 12 (2019) 1–22. 10.1146/annurev-anchem-061318-114910. [DOI] [PubMed] [Google Scholar]
- [11].Brothers MC, DeBrosse M, Grigsby CC, Naik RR, Hussain SM, Heikenfeld J, Kim SS, Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms, Acc. Chem. Res. 52 (2019) 297–306. 10.1021/acs.accounts.8b00555. [DOI] [PubMed] [Google Scholar]
- [12].Zhao J, Guo H, Li J, Bandodkar AJ, Rogers JA, Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids, Trends in Chemistry. 1 (2019) 559–571. 10.1016/j.trechm.2019.07.001. [DOI] [Google Scholar]
- [13].Harris J, Bickford J, Cho P, Coppock M, Farrell ME, Holthoff EL, Ratcliff E, Approaching single molecule sensing: predictive sweat sensor design for ultra-low limits of detection, in: Guicheteau JA, Howie CR (Eds.), Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XX, SPIE, Baltimore, United States, 2019: p. 15 10.1117/12.2518543. [DOI] [Google Scholar]
- [14].Klimuntowski M, Alam MM, Singh G, Howlader MMR, Electrochemical Sensing of Cannabinoids in Biofluids: A Noninvasive Tool for Drug Detection, ACS Sens. 5 (2020) 620–636. 10.1021/acssensors.9b02390. [DOI] [PubMed] [Google Scholar]
- [15].Manjakkal L, Dervin S, Dahiya R, Flexible potentiometric pH sensors for wearable systems, RSC Adv. 10 (2020) 8594–8617. 10.1039/D0RA00016G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Martín Várguez PE, Brunei F, Raimundo J-M, Recent Electrochemical/Electrical Microfabricated Sensor Devices for Ionic and Polyionic Analytes, ACS Omega. 5 (2020) 4733–4742. 10.1021/acsomega.9b04331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lyu Y, Gan S, Bao Y, Zhong L, Xu J, Wang W, Liu Z, Ma Y, Yang G, Niu L, Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors, Membranes. 10 (2020) 128 10.3390/membranes10060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sempionatto JR, Jeerapan I, Krishnan S, Wang J, Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry, Anal. Chem. 92 (2020) 378–396. 10.1021/acs.analchem.9b04668. [DOI] [PubMed] [Google Scholar]
- [19].Teymourian H, Parrilla M, Sempionatto JR, Montiel NF, Barfidokht A, Van Echelpoel R, De Wael K, Wang J, Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs, ACS Sens. 5 (2020) 2679–2700. 10.1021/acssensors.0c01318. [DOI] [PubMed] [Google Scholar]
- [20].Li S, Ma Z, Cao Z, Pan L, Shi Y, Advanced Wearable Microfluidic Sensors for Healthcare Monitoring, Small. 16(2020) 1903822 10.1002/smll.201903822. [DOI] [PubMed] [Google Scholar]
- [21].Shrivastava S, Trung TQ, Lee N-E, Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing, Chem. Soc. Rev. 49 (2020) 1812–1866. 10.1039/C9CS00319C. [DOI] [PubMed] [Google Scholar]
- [22].Ray T, Choi J, Reeder J, Lee SP, Aranyosi AJ, Ghaffari R, Rogers JA, Soft, skin-interfaced wearable systems for sports science and analytics, Current Opinion in Biomedical Engineering. 9 (2019) 47–56. 10.1016/j.cobme.2019.01.003. [DOI] [Google Scholar]
- [23].Ferrari LM, Keller K, Burtscher B, Greco F, Temporary tattoo as unconventional substrate for conformable and transferable electronics on skin and beyond, Multifunct. Mater. 3 (2020) 032003 10.1088/2399-7532/aba6e3. [DOI] [Google Scholar]
- [24].Sreenilayam SP, Ahad IU, Nicolosi V, Acinas Garzon V, Brabazon D, Advanced materials of printed wearables for physiological parameter monitoring, Materials Today. 32 (2020) 147–177. 10.1016/j.mattod.2019.08.005. [DOI] [Google Scholar]
- [25].Lin J, Zhu Z, Cheung CF, Yan F, Li G, Digital manufacturing of functional materials for wearable electronics, J. Mater. Chem. C. 8 (2020) 10587–10603. 10.1039/D0TC01112F. [DOI] [Google Scholar]
- [26].Choi J, Bandodkar AJ, Reeder JT, Ray TR, Turnquist A, Kim SB, Nyberg N, Hourlier-Fargette A, Model JB, Aranyosi AJ, Xu S, Ghaffari R, Rogers JA, Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature, ACS Sens. 4 (2019) 379–388. 10.1021/acssensors.8b01218. [DOI] [PubMed] [Google Scholar]
- [27].Sekine Y, Kim SB, Zhang Y, Bandodkar AJ, Xu S, Choi J, Irie M, Ray TR, Kohli P, Kozai N, Sugita T, Wu Y, Lee K, Lee K-T, Ghaffari R, Rogers JA, A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry, Lab Chip. 18 (2018) 2178–2186. 10.1039/C8LC00530C. [DOI] [PubMed] [Google Scholar]
- [28].Cui Y, Duan W, Jin Y, Wo F, Xi F, Wu J, Ratiometric Fluorescent Nanohybrid for Noninvasive and Visual Monitoring of Sweat Glucose, ACS Sens. 5 (2020) 2096–2105. 10.1021/acssensors.0c00718. [DOI] [PubMed] [Google Scholar]
- [29].Bandodkar AJ, Gutruf P, Choi J, Lee K, Sekine Y, Reeder JT, Jeang WJ, Aranyosi AJ, Lee SP, Model JB, Ghaffari R, Su C-J, Leshock JP, Ray T, Verrillo A, Thomas K, Krishnamurthi V, Han S, Kim J, Krishnan S, Hang T, Rogers JA, Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat, Sci. Adv. 5 (2019) eaav3294 10.1126/sciadv.aav3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Y, Guo H, Kim SB, Wu Y, Ostojich D, Park SH, Wang X, Weng Z, Li R, Bandodkar AJ, Sekine Y, Choi J, Xu S, Quaggin S, Ghaffari R, Rogers JA, Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system, Lab Chip. 19 (2019) 1545–1555. 10.1039/C9LC00103D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim SB, Koo J, Yoon J, Hourlier-Fargette A, Lee B, Chen S, Jo S, Choi J, Oh YS, Lee G, Won SM, Aranyosi AJ, Lee SP, Model JB, Braun PV, Ghaffari R, Park C, Rogers JA, Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat, Lab Chip. 20 (2020) 84–92. 10.1039/C9LC01045A. [DOI] [PubMed] [Google Scholar]
- [32].Reeder JT, Choi J, Xue Y, Gutruf P, Hanson J, Liu M, Ray T, Bandodkar AJ, Avila R, Xia W, Krishnan S, Xu S, Barnes K, Pahnke M, Ghaffari R, Huang Y, Rogers JA, Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings, Sci. Adv. 5 (2019) eaau6356 10.1126/sciadv.aau6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaya T, Liu G, Ho J, Yelamarthi K, Miller K, Edwards J, Stannard A, Wearable Sweat Sensors: Background and Current Trends, Electroanalysis. 31 (2019)411–421. 10.1002/elan.201800677. [DOI] [Google Scholar]
- [34].Francis J, Stamper I, Heikenfeld J, Gomez EF, Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement, Lab Chip. 19 (2019) 178–185. 10.1039/C8LC00968F. [DOI] [PubMed] [Google Scholar]
- [35].Tai L-C, Ahn CH, Nyein HYY, Ji W, Bariya M, Lin Y, Li L, Javey A, Nicotine Monitoring with a Wearable Sweat Band, ACS Sens. 5 (2020) 1831–1837. 10.1021/acssensors.0c00791. [DOI] [PubMed] [Google Scholar]
- [36].Yang Y, Song Y, Bo X, Min J, Pak OS, Zhu L, Wang M, Tu J, Kogan A, Zhang H, Hsiai TK, Li Z, Gao W, A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat, Nat Biotechnol. 38 (2020) 217–224. 10.1038/s41587-019-0321-x. [DOI] [PubMed] [Google Scholar]
- [37].Kim S, Lee B, Reeder JT, Seo SH, Lee S-U, Hourlier-Fargette A, Shin J, Sekine Y, Jeong H, Oh YS, Aranyosi AJ, Lee SP, Model JB, Lee G, Seo M-H, Kwak SS, Jo S, Park G, Han S, Park I, Jung H-I, Ghaffari R, Koo J, Braun PV, Rogers JA, Soft, skin-interfaced microfluidic systems with integrated immunoassays, fluorometric sensors, and impedance measurement capabilities, Proc Natl Acad Sci USA. 117 (2020) 27906–27915. 10.1073/pnas.2012700117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Choi J, Kang D, Han S, Kim SB, Rogers JA, Thin, Soft, Skin-Mounted Microfluidic Networks with Capillary Bursting Valves for Chrono-Sampling of Sweat, Adv. Healthcare Mater. 6 (2017) 1601355 10.1002/adhm.201601355. [DOI] [PubMed] [Google Scholar]
- [39].Zhang Y, Chen Y, Huang J, Liu Y, Peng J, Chen S, Song K, Ouyang X, Cheng H, Wang X, Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis, Lab Chip. 20 (2020) 2635–2645. 10.1039/D0LC00400F. [DOI] [PubMed] [Google Scholar]
- [40].Kim SB, Zhang Y, Won SM, Bandodkar AJ, Sekine Y, Xue Y, Koo J, Harshman SW, Martin JA, Park JM, Ray TR, Crawford KE, Lee K-T, Choi J, Pitsch RL, Grigsby CC, Strang AJ, Chen Y-Y, Xu S, Kim J, Koh A, Ha JS, Huang Y, Kim SW, Rogers JA, Super-Absorbent Polymer Valves and Colorimetric Chemistries for Time-Sequenced Discrete Sampling and Chloride Analysis of Sweat via Skin-Mounted Soft Microfluidics, Small. 14 (2018) 1703334 10.1002/smll.201703334. [DOI] [PubMed] [Google Scholar]
- [41].Clime L, Daoud J, Brassard D, Malic L, Geissler M, Veres T, Active pumping and control of flows in centrifugal microfluidics, Microfluid Nanofluid. 23 (2019) 29 10.1007/s10404-019-2198-x. [DOI] [Google Scholar]
- [42].Lin H, Tan J, Zhu J, Lin S, Zhao Y, Yu W, Hojaiji H, Wang B, Yang S, Cheng X, Wang Z, Tang E, Yeung C, Emaminejad S, A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis, Nat Commun. 11 (2020) 4405 10.1038/s41467-020-18238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nguyen N-T, Wereley ST, Shaegh SAM, Fundamentals and Applications of Microfluidics, Artech House, Boston, 2018. [Google Scholar]
- [44].Lin H, Hojaiji H, Lin S, Yeung C, Zhao Y, Wang B, Malige M, Wang Y, King K, Yu W, Tan J, Wang Z, Cheng X, Emaminejad S, A wearable electrofluidic actuation system, Lab Chip. 19 (2019) 2966–2972. 10.1039/C9LC00454H. [DOI] [PubMed] [Google Scholar]
- [45].Xiao J, Liu Y, Su L, Zhao D, Zhao L, Zhang X, Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose, Anal. Chem 91 (2019) 14803–14807. 10.1021/acs.analchem.9b03110. [DOI] [PubMed] [Google Scholar]
- [46].Liu C, Xu T, Wang D, Zhang X, The role of sampling in wearable sweat sensors, Talanta. 212 (2020) 120801 10.1016/j.talanta.2020.120801. [DOI] [PubMed] [Google Scholar]
- [47].Bandodkar AJ, Choi J, Lee SP, Jeang WJ, Agyare P, Gutruf P, Wang S, Sponenburg RA, Reeder JT, Schon S, Ray TR, Chen S, Mehta S, Ruiz S, Rogers JA, Soft, Skin-Interfaced Microfluidic Systems with Passive Galvanic Stopwatches for Precise Chronometric Sampling of Sweat, Adv. Mater. 31 (2019) 1902109 10.1002/adma.201902109. [DOI] [PubMed] [Google Scholar]
- [48].Choi J, Chen S, Deng Y, Xue Y, Reeder JT, Franklin D, Oh YS, Model JB, Aranyosi AJ, Lee SP, Ghaffari R, Huang Y, Rogers JA, Skin-Interfaced Microfluidic Systems that Combine Hard and Soft Materials for Demanding Applications in Sweat Capture and Analysis, Adv. Healthcare Mater. (2020) 2000722 10.1002/adhm.202000722. [DOI] [PubMed] [Google Scholar]
- [49].McCaul M, Glennon T, Diamond D, Challenges and opportunities in wearable technology for biochemical analysis in sweat, Current Opinion in Electrochemistry. 3 (2017) 46–50. 10.1016/j.coelec.2017.06.001. [DOI] [Google Scholar]
- [50].Qiao L, Benzigar MR, Subramony JA, Lovell NH, Liu G, Advances in Sweat Wearables: Sample Extraction, Real-Time Biosensing, and Flexible Platforms, ACS Appl. Mater. Interfaces. 12 (2020) 34337–34361. 10.1021/acsami.0c07614. [DOI] [PubMed] [Google Scholar]
- [51].LeGrys VA, Moon TC, Laux J, Accurso F, Martiniano SA, A multicenter evaluation of sweat chloride concentration and variation in infants with cystic fibrosis, J Cyst Fibros. 18 (2019) 190–193. 10.1016/j.jcf.2018.12.006. [DOI] [PubMed] [Google Scholar]
- [52].Hussain JN, Mantri N, Cohen MM, Working Up a Good Sweat - The Challenges of Standardising Sweat Collection for Metabolomics Analysis, Clin Biochem Rev. 38 (2017) 13–34. [PMC free article] [PubMed] [Google Scholar]
- [53].Souza SL, Graga G, Oliva A, Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis, Skin Res Technol. 24 (2018) 187–195. 10.1111/srt.12412. [DOI] [PubMed] [Google Scholar]
- [54].Yokus MA, Saha T, Fang J, Dickey MD, Velev OD, Daniele MA, Towards Wearable Electrochemical Lactate Sensing using Osmotic-Capillary Microfluidic Pumping, in: 2019 IEEE SENSORS, IEEE, Montreal, QC, Canada, 2019: pp. 1–4. 10.1109/SENSORS43011.2019.8956651. [DOI] [Google Scholar]
- [55].Zhao FJ, Bonmarin M, Chen ZC, Larson M, Fay D, Runnoe D, Heikenfeld J, Ultra-simple wearable local sweat volume monitoring patch based on swellable hydrogels, Lab Chip. 20 (2020) 168–174. 10.1039/C9LC00911F. [DOI] [PubMed] [Google Scholar]
- [56].Shay T, Saha T, Dickey MD, Velev OD, Principles of long-term fluids handling in paper-based wearables with capillary–evaporative transport, Biomicrofluidics. 14 (2020) 034112 10.1063/5.0010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nyein HYY, Tai L-C, Ngo QP, Chao M, Zhang GB, Gao W, Bariya M, Bullock J, Kim H, Fahad HM, Javey A, A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis, ACS Sens. 3 (2018) 944–952. 10.1021/acssensors.7b00961. [DOI] [PubMed] [Google Scholar]
- [58].Alizadeh A, Burns A, Lenigk R, Gettings R, Ashe J, Porter A, McCaul M, Barrett R, Diamond D, White P, Skeath P, Tomczak M, A wearable patch for continuous monitoring of sweat electrolytes during exertion, Lab Chip. 18 (2018) 2632–2641. 10.1039/C8LC00510A. [DOI] [PubMed] [Google Scholar]
- [59].Kim J, Sempionatto JR, Imani S, Hartel MC, Barfidokht A, Tang G, Campbell AS, Mercier PP, Wang J, Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform, Adv. Sci. 5 (2018) 1800880 10.1002/advs.201800880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hauke A, Simmers P, Ojha YR, Cameron BD, Ballweg R, Zhang T, Twine N, Brothers M, Gomez E, Heikenfeld J, Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation, Lab Chip. 18 (2018) 3750–3759. 10.1039/C8LC01082J. [DOI] [PubMed] [Google Scholar]
- [61].Aranyosi AJ, Model JB, Zhang MZ, Lee SP, Leech A, Li W, Seib MS, Chen S, Reny N, Wallace J, Shin MH, Bandodkar AJ, Choi J, Paller AS, Rogers JA, Xu S, Ghaffari R, Rapid Capture and Extraction of Sweat for Regional Rate and Cytokine Composition Analysis Using a Wearable Soft Microfluidic System, Journal of Investigative Dermatology. (2020) S0022202X20316857. 10.1016/j.jid.2020.05.107. [DOI] [PubMed] [Google Scholar]
- [62].Lee H-B, Meeseepong M, Trung TQ, Kim B-Y, Lee N-E, A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat, Biosensors and Bioelectronics. 156 (2020) 112–133. 10.1016/j.bios.2020.112133. [DOI] [PubMed] [Google Scholar]
- [63].Pal A, Nadiger VG, Goswami D, Martinez RV, Conformal, waterproof electronic decals for wireless monitoring of sweat and vaginal pH at the point-of-care, Biosensors and Bioelectronics. 160 (2020) 112206 10.1016/j.bios.2020.112206. [DOI] [PubMed] [Google Scholar]
- [64].Tai L-C, Gao W, Chao M, Bariya M, Ngo QP, Shahpar Z, Nyein HYY, Park H, Sun J, Jung Y, Wu E, Fahad HM, Lien D-H, Ota H, Cho G, Javey A, Methylxanthine Drug Monitoring with Wearable Sweat Sensors, Adv. Mater 30 (2018) 1707442 10.1002/adma.201707442. [DOI] [PubMed] [Google Scholar]
- [65].Xu H, Lu YF, Xiang JX, Zhang MK, Zhao YJ, Xie ZY, Gu ZZ, A multifunctional wearable sensor based on a graphene/inverse opal cellulose film for simultaneous, in situ monitoring of human motion and sweat, Nanoscale. 10 (2018) 2090–2098. 10.1039/C7NR07225B. [DOI] [PubMed] [Google Scholar]
- [66].An Q, Gan S, Xu J, Bao Y, Wu T, Kong H, Zhong L, Ma Y, Song Z, Niu L, A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring, Electrochemistry Communications. 107 (2019) 106553 10.1016/j.elecom.2019.106553. [DOI] [Google Scholar]
- [67].Bae CW, Toi PT, Kim BY, Lee WI, Lee HB, Hanif A, Lee EH, Lee N-E, Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring, ACS Appl. Mater. Interfaces. 11 (2019) 14567–14575. 10.1021/acsami.9b00848. [DOI] [PubMed] [Google Scholar]
- [68].Lei Y, Zhao W, Zhang Y, Jiang Q, He J, Baeumner AJ, Wolfbeis OS, Wang ZL, Salama KN, Alshareef HN, A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis, Small. 15 (2019) 1901190 10.1002/smll.201901190. [DOI] [PubMed] [Google Scholar]
- [69].Lin Y, Bariya M, Nyein HYY, Kivimäki L, Uusitalo S, Jansson E, Ji W, Yuan Z, Happonen T, Liedert C, Hiltunen J, Fan Z, Javey A, Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring, Adv. Funct. Mater 29 (2019) 1902521 10.1002/adfm.201902521. [DOI] [Google Scholar]
- [70].Parrilla M, Ortiz-Gómez I, Cánovas R, Salinas-Castillo A, Cuartero M, Crespo GA, Wearable Potentiometric Ion Patch for On-Body Electrolyte Monitoring in Sweat: Toward a Validation Strategy to Ensure Physiological Relevance, Anal. Chem 91 (2019) 8644–8651. 10.1021/acs.analchem.9b02126. [DOI] [PubMed] [Google Scholar]
- [71].Sempionatto JR, Martin A, García-Carmona L, Barfidokht A, Kurniawan JF, Moreto JR, Tang G, Shin A, Liu X, Escarpa A, Wang J, Skin-worn Soft Microfluidic Potentiometric Detection System, Electroanalysis. 31 (2019) 239–245. 10.1002/elan.201800414. [DOI] [Google Scholar]
- [72].Toi PT, Trung TQ, Dang TML, Bae CW, Lee N-E, Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-Body Sweat Glucose Detection, ACS Appl. Mater. Interfaces 11 (2019) 10707–10717. 10.1021/acsami.8b20583. [DOI] [PubMed] [Google Scholar]
- [73].Voulgari E, Krummenacher F, Kayal M, A programmable low-power ADC interface for an ISFET sweat sensor used in a wearable multi-sensing system, in: 2019 IEEE 8th International Workshop on Advances in Sensors and Interfaces (IWASI), IEEE, Otranto, Italy, 2019: pp. 210–214. 10.1109/IWASI.2019.8791350. [DOI] [Google Scholar]
- [74].Wang Y, Wang X, Lu W, Yuan Q, Zheng Y, Yao B, A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat, Talanta. 198 (2019) 86–92. 10.1016/j.talanta.2019.01.104. [DOI] [PubMed] [Google Scholar]
- [75].Cheng X, Wang B, Zhao Y, Hojaiji H, Lin S, Shih R, Lin H, Tamayosa S, Ham B, Stout P, Salahi K, Wang Z, Zhao C, Tan J, Emaminejad S, A Mediator-Free Electroenzymatic Sensing Methodology to Mitigate Ionic and Electroactive Interferents’ Effects for Reliable Wearable Metabolite and Nutrient Monitoring, Adv. Funct. Mater 30 (2020) 1908507 10.1002/adfm.201908507. [DOI] [Google Scholar]
- [76].Choi D-H, Kitchen GB, Jennings MT, Cutting GR, Searson PC, Out-of-clinic measurement of sweat chloride using a wearable sensor during low-intensity exercise, Npj Digit. Med 3 (2020) 49 10.1038/s41746-020-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Demuru S, Kunnel BP, Briand D, Real-Time Multi-Ion Detection in the Sweat Concentration Range Enabled by Flexible, Printed, and Microfluidics-lntegrated Organic Transistor Arrays, Adv. Mater. Technol (2020) 2000328. 10.1002/admt.202000328. [DOI] [Google Scholar]
- [78].Lin S, Cheng X, Wang B, Yu W, Ly D, Emaminejad S, A Fouling-Resistant Voltammetric Sensing System for Wearable Electroactive Biomarker Monitoring, J. Microelectromech. Syst 29 (2020) 1059–1063. 10.1109/JMEMS.2020.3006829. [DOI] [Google Scholar]
- [79].Mintah Churcher NK, Upasham S, Rice P, Bhadsavle S, Prasad S, Development of a flexible, sweat-based neuropeptide Y detection platform, RSC Adv. 10 (2020) 23173–23186. 10.1039/D0RA03729J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mondal S, Kim SJ, Choi C-G, Honeycomb-like MoS 2 Nanotube Array-Based Wearable Sensors for Noninvasive Detection of Human Skin Moisture, ACS Appl. Mater. Interfaces 12 (2020) 17029–17038. 10.1021/acsami.9b22915. [DOI] [PubMed] [Google Scholar]
- [81].Pirovano P, Dorrian M, Shinde A, Donohoe A, Brady AJ, Moyna NM, Wallace G, Diamond D, McCaul M, A wearable sensor for the detection of sodium and potassium in human sweat during exercise, Talanta. 219 (2020) 121145 10.1016/j.talanta.2020.121145. [DOI] [PubMed] [Google Scholar]
- [82].Sempionatto JR, Khorshed AA, Ahmed A, De Loyola AN Silva e, Barfidokht A, Yin L, Goud KY, Mohamed MA, Bailey E, May J, Aebischer C, Chatelle C, Wang J, Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition, ACS Sens. 5 (2020) 1804–1813. 10.1021/acssensors.0c00604. [DOI] [PubMed] [Google Scholar]
- [83].Torrente-Rodríguez RM, Tu J, Yang Y, Min J, Wang M, Song Y, Yu Y, Xu C, Ye C, IsHak WW, Gao W, Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System, Matter. 2 (2020) 921–937. 10.1016/j.matt.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wiorek A, Parrilla M, Cuartero M, Crespo GA, Epidermal Patch with Glucose Biosensor: pH and Temperature Correction toward More Accurate Sweat Analysis during Sport Practice, Anal. Chem 92 (2020) 10153–10161. 10.1021/acs.analchem.0c02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xue W, Tan X, Khaing Oo MK, Kulkarni G, Ilgen MA, Fan X, Rapid and sensitive detection of drugs of abuse in sweat by multiplexed capillary based immuno-biosensors, Analyst. 145 (2020) 1346–1354. 10.1039/C9AN02498K. [DOI] [PubMed] [Google Scholar]
- [86].Yoon H, Nah J, Kim H, Ko S, Sharifuzzaman M, Barman SC, Xuan X, Kim J, Park JY, A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection, Sensors and Actuators B: Chemical 311 (2020) 127866 10.1016/j.snb.2020.127866. [DOI] [Google Scholar]
- [87].Zhai Q, Yap LW, Wang R, Gong S, Guo Z, Liu Y, Lyu Q, Wang J, George.p. Simon, Cheng W, Vertically Aligned Gold Nanowires as Stretchable and Wearable Epidermal Ion-Selective Electrode for Noninvasive Multiplexed Sweat Analysis, Anal. Chem 92 (2020) 4647–4655. 10.1021/acs.analchem.0c00274. [DOI] [PubMed] [Google Scholar]
- [88].Dalirirad S, Steckl AJ, Aptamer-based lateral flow assay for point of care cortisol detection in sweat, Sensors and Actuators B: Chemical. 283 (2019) 79–86. 10.1016/j.snb.2018.11.161. [DOI] [Google Scholar]
- [89].Tu E, Pearlmutter P, Tiangco M, Derose G, Begdache L, Koh A, Comparison of Colorimetric Analyses to Determine Cortisol in Human Sweat, ACS Omega. 5 (2020) 8211–8218. 10.1021/acsomega.0c00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].He X, Yang S, Pei Q, Song Y, Liu C, Xu T, Zhang X, Integrated Smart Janus Textile Bands for Self-Pumping Sweat Sampling and Analysis, ACS Sens. 5 (2020) 1548–1554. 10.1021/acssensors.0c00563. [DOI] [PubMed] [Google Scholar]
- [91].Jain V, Ochoa M, Jiang H, Rahimi R, Ziaie B, A mass-customizable dermal patch with discrete colorimetric indicators for personalized sweat rate quantification, Microsyst Nanoeng. 5 (2019) 29 10.1038/s41378-019-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Twine NB, Norton RM, Brothers MC, Hauke A, Gomez EF, Heikenfeld J, Open nanofluidic films with rapid transport and no analyte exchange for ultra-low sample volumes, Lab Chip. 18 (2018) 2816–2825. 10.1039/C8LC00186C. [DOI] [PubMed] [Google Scholar]
- [93].Kim SB, Lee K, Raj MS, Lee B, Reeder JT, Koo J, Hourlier-Fargette A, Bandodkar AJ, Won SM, Sekine Y, Choi J, Zhang Y, Yoon J, Kim BH, Yun Y, Lee S, Shin J, Kim J, Ghaffari R, Rogers JA, Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition, Small. 14 (2018) 1802876 10.1002/smll.201802876. [DOI] [PubMed] [Google Scholar]
- [94].Guan H, Zhong T, He H, Zhao T, Xing L, Zhang Y, Xue X, A self-powered wearable sweat-evaporation-biosensing analyzer for building sports big data, Nano Energy. 59 (2019) 754–761. 10.1016/j.nanoen.2019.03.026. [DOI] [Google Scholar]
- [95].Lu Y, Jiang K, Chen D, Shen G, Wearable sweat monitoring system with integrated micro-supercapacitors, Nano Energy. 58 (2019) 624–632. 10.1016/j.nanoen.2019.01.084. [DOI] [Google Scholar]
- [96].Bandodkar AJ, Lee SP, Huang I, Li W, Wang S, Su C-J, Jeang WJ, Hang T, Mehta S, Nyberg N, Gutruf P, Choi J, Koo J, Reeder JT, Tseng R, Ghaffari R, Rogers JA, Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems, Nat Electron. 3 (2020) 554–562. 10.1038/s41928-020-0443-7. [DOI] [Google Scholar]
- [97].Carr AR, Patel YH, Neff CR, Charkhabi S, Kallmyer NE, Angus HF, Reuel NF, Sweat monitoring beneath garments using passive, wireless resonant sensors interfaced with laser-ablated microfluidics, Npj Digit. Med. 3 (2020) 62 10.1038/s41746-020-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mohammadifar M, Tahernia M, Yang JH, Koh A, Choi S, Biopower-on-Skin: Electricity generation from sweat-eating bacteria for self-powered E-Skins, Nano Energy. 75 (2020) 104994 10.1016/j.nanoen.2020.104994. [DOI] [Google Scholar]
- [99].Mohammadifar M, Tahernia M, Yang J, Koh A, Choi S, A Skin-Mountable Bacteria-Powered Battery System for Self-Powered Medical Devices, in: 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), IEEE, Vancouver, BC, Canada, 2020: pp. 72–75. 10.1109/MEMS46641.2020.9056174. [DOI] [Google Scholar]
- [100].Nagamine K, Nomura A, Ichimura Y, Izawa R, Sasaki S, Furusawa H, Matsui H, Tokito S, Printed Organic Transistor-based Biosensors for Non-invasive Sweat Analysis, Anal. Sci. 36 (2020) 291–302. 10.2116/analsci.19R007. [DOI] [PubMed] [Google Scholar]
- [101].Ortega L, Llorella A, Esquivel JP, Sabaté N, Paper-Based Batteries as Conductivity Sensors for Single-Use Applications, ACS Sens. 5 (2020) 1743–1749. 10.1021/acssensors.0c00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Song Y, Min J, Yu Y, Wang H, Yang Y, Zhang H, Gao W, Wireless battery-free wearable sweat sensor powered by human motion, Sci. Adv. 6 (2020) eaay9842 10.1126/sciadv.aay9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yoshida S, Abe H, Abe Y, Kusama S, Tsukada K, Komatsubara R, Nishizawa M, Totally organic electrical skin patch powered by flexible biobattery, J. Phys. Energy. 2 (2020) 044004 10.1088/2515-7655/abb873. [DOI] [Google Scholar]
- [104].Yoshida S, Mizuno T, Kusama S, Sato K, Raut B, Nishizawa M, Series-Connected Flexible Biobatteries for Higher Voltage Electrical Skin Patches, ACS Appl. Electron. Mater. 2 (2020) 170–176. 10.1021/acsaelm.9b00671. [DOI] [Google Scholar]
- [105].Yu Y, Nassar J, Xu C, Min J, Yang Y, Dai A, Doshi R, Huang A, Song Y, Gehlhar R, Ames AD, Gao W, Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces, Sci. Robot. 5 (2020) eaaz7946 10.1126/scirobotics.aaz7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ortega L, Llorella A, Esquivel JP, Sabaté N, Self-powered smart patch for sweat conductivity monitoring, Microsyst Nanoeng. 5 (2019) 3 10.1038/s41378-018-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Xu G, Cheng C, Yuan W, Liu Z, Zhu L, Li X, Lu Y, Chen Z, Liu J, Cui Z, Liu J, Men H, Liu Q, Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids, Sensors and Actuators B: Chemical. 297 (2019) 126743 10.1016/j.snb.2019.126743. [DOI] [Google Scholar]
- [108].Baker LB, Model JB, Barnes KA, Anderson ML, Lee SP, Lee KA, Brown SD, Reimel AJ, Roberts TJ, Nuccio RP, Bonsignore JL, Ungaro CT, Carter JM, Li W, Seib MS, Reeder JT, Aranyosi AJ, Rogers JA, Ghaffari R, Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications, Sci. Adv. 6 (2020) eabe3929 10.1126/sciadv.abe3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhao Y, Wang B, Hojaiji H, Wang Z, Lin S, Yeung C, Lin H, Nguyen P, Chiu K, Salahi K, Cheng X, Tan J, Cerrillos BA, Emaminejad S, A wearable freestanding electrochemical sensing system, Sci. Adv. 6 (2020) eaaz0007 10.1126/sciadv.aaz0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bariya M, Shahpar Z, Park H, Sun J, Jung Y, Gao W, Nyein HYY, Liaw TS, Tai L-C, Ngo QP, Chao M, Zhao Y, Hettick M, Cho G, Javey A, Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices, ACS Nano. 12 (2018) 6978–6987. 10.1021/acsnano.8b02505. [DOI] [PubMed] [Google Scholar]
- [111].Epicore Biosystems, Epicore Biosystems Homepage, (n.d.). http://www.epicorebiosystems.com/ (accessed October 12, 2020).
- [112].Eccrine Systems, Eccrine Systems Homepage, (n.d.). https://www.eccrinesystems.com/ (accessed October 12, 2020).


