Abstract
Objective
Low levels of thyroid hormones have been associated with poor clinical outcomes. This metabolic situation, designated euthyroid sick syndrome, has been interpreted as a state of adaptation to different pathological processes, characterized by the decrease in plasma triiodothyronine. The present study seeks to determine the incidence of this disorder in patients with septic shock and its relationship with other severity indices and clinical outcomes.
Methods
This prospective analytical study evaluated patients admitted to the intensive care unit with septic shock between April 2018 and July 2019. Variables associated with septic shock and thyroid profile were recorded at the time of the septic shock diagnosis and 7, 14, and 21 days later.
Results
A total of 27 patients who met the inclusion criteria were analyzed. The incidence of an altered thyroid axis was 96.3%, with a mortality at 28 days of 36.7%. Patients without hormonal alteration did not present negative outcomes. Among those with low triiodothyronine, 42.3% recovered their thyroid function within 28 days, in whom mortality was 0%; 57.7% did not recover their thyroid function, in whom mortality was 66.7%. Those whose thyroid axis was altered and who did not normalize its function required more doses of vasoactives and had deteriorated lactate clearance.
Conclusion
Patients with septic shock have a high incidence of alteration of the thyroid axis, and this dysfunction is associated with higher mortality.
Keywords: Sepsis, Infection, Hemodynamics, Metabolism, Prognosis
Abstract
Objetivo
Se ha visto asociación entre los bajos niveles de hormonas tiroideas y malos resultados clínicos. Esta situación metabólica designada bajo el término de enfermedad eutiroidea, ha sido interpretada como un estado de adaptación a diferentes procesos patológicos, caracterizada por la disminución plasmática de triiodotironina T3. El presente estudio busca determinar la incidencia de este trastorno en los pacientes con shock séptico y su relación con otros índices de gravedad, y resultados clínicos.
Métodos
Estudio de corte prospectivo analítico, evaluó a los pacientes que ingresaron con shock séptico a la unidad de terapia intensiva, durante el periodo abril 2018 - julio 2019. Se registraron variables asociadas al shock séptico, y el perfil tiroideo al momento del diagnóstico de shock séptico, a los 7, 14 y 21 días.
Resultados
Se analizaron 27 pacientes que cumplieron con los criterios de inclusión. La incidencia de alteración del eje tiroideo fue del 96,3%s, con una mortalidad a los 28 días de 36,7%. Los pacientes sin alteración hormonal no presentaron desenlaces negativos. Entre los que presentaron baja triiodotironina, 42,3% recupero la función tiroidea dentro de los 28 días, con mortalidad del 0%. No recuperaron función tiroidea (57,7%), con una mortalidad del 66,7%. Comparativamente se observó que aquellos que presentaron alteración del eje y no normalizaron la función, requirieron más dosis de vasoactivos, y deterioro del clearence de lactato.
Conclusión
Los pacientes con shock séptico presentan una alta incidencia de alteración del eje tiroideo y esta disfunción se asoció a mayor mortalidad.
Keywords: Sepsis, Infecciones, Hemodinámica, Metabolismo, Prognóstico
INTRODUCTION
In critically ill patients, it is common to observe low concentrations of thyroid hormones. This hormonal imbalance is known as euthyroid sick syndrome.(1,2) It is characterized by low serum triiodothyronine (T3) and normal or low levels of thyroid-stimulating hormone (TSH), increases in serum reverse T3, and decreased thyroxine (T4). This syndrome is described as an adaptive mechanism to stress that seeks to reduce energy requirements. Some publications question this hypothesis, stating that this alteration has a role in the pathophysiology of critical illness and especially in septic states.(3)
Septic shock is a disease state characterized by an inflammatory response to infection that triggers a state of tissue hypoperfusion, and it is associated with high mortality. Its pathophysiology involves a profound hemodynamic deterioration, characterized by vasoplegia that can be associated with deteriorated myocardial function, together with different degrees of alterations at the microcirculatory level, with cytopathic lesions up to failure of mitochondrial respiration (mitochondrial and microcirculatory distress syndrome). In this context, it could be interpreted that the actions of T3 are part of the pathophysiological mechanism of septic shock.
The cardiovascular system and oxygen metabolism at the mitochondrial level are some of the most important targets of thyroid hormones. Triiodothyronine is the metabolically active hormone that is produced by peripheral conversion of 80% of the T4 secreted by the thyroid gland. Triiodothyronine plays a fundamental role in modulating cardiovascular performance and mitochondrial activity.(4-6) The actions of this hormone are produced by genomic mechanisms (actin and myosin synthesis, adrenergic receptors, etc.) as well as nongenomic mechanisms (Ca+ modulation) on the cardiovascular system, improving cardiac output and increasing blood flow to tissues. Additionally, by means of mitochondrial T3 receptors, a direct effect on these organelles is produced, increasing the production and use of adenosine triphosphate and increasing metabolism and oxygen consumption.
The main pathophysiological mechanism involved in low-T3 syndrome (LT3S) is low activity of the 5′ monodeionidase enzyme that converts T4 to T3 in peripheral tissues.(7-9) In septic states, pro-inflammatory cytokines (interleukin - IL - 1, IL-6, tumor necrosis factor alpha - TNF-α) perturb thyroid hormone levels by decreasing the activity of type I deiodinase, thereby decreasing the peripheral conversion of T4 to T3.(10-15)
The primary objective of this study was to measure the incidence of LT3S in our population of patients with septic shock. The secondary objectives were to analyze its impact on prognosis and its relationship with other severity indices associated with septic shock.
METHODS
This analytical prospective cohort study was designed to evaluate the incidence of impaired thyroid function in patients with septic shock during admission to the intensive care unit (ICU). The period of enrollment and follow-up of patients was April 1, 2018 to June 30, 2019. The patients admitted to the study were followed up until their institutional discharge.
Patients older than 18 years who were admitted consecutively to the ICU and who met the inclusion criteria were included. Septic shock was diagnosed according to the Third Consensus Conference SEPSIS-3.(16) In this conference, sepsis was defined as infection associated with organ failure, which in turn was defined as a rise in the Sequential Organ Failure Assessment (SOFA) score greater by ≥ 2 points from baseline. In addition, septic shock was defined as sepsis with persistent hypotension that requires vasoactive to achieve mean arterial pressure ≥ 65mmHg and serum lactate ≥ 2mmol/L after adequate volume resuscitation. Patients with a history of thyroid disease (hypo/hyper/tumor) or with a previous diagnosis of endocrine abnormality (thyroid or adrenal axis) were excluded, and patients under treatment with amiodarone or any other alteration that could perturb the thyroid hormone profile of the patient were excluded.
The general demographic data of sex, age, date of admission to the ICU, reason for admission, diagnosis of sepsis, and suspected/probable focus were collected. The Charlson comorbidity score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and SOFA score. Thyroid hormone profile with dosages every 7 days until day 21 of diagnosis (T0, T7, T14, T21) was as described in table 1. Likewise, serum lactate, hemodynamic parameters, and the requirement of vasoactive drugs and their doses were recorded. Length of ICU stay and mortality at 28 days were recorded.
Table 1.
Laboratory method and thyroid profile values
| Thyroid profile | Measurement | Laboratory ranges | Method |
|---|---|---|---|
| TSH | At SS diagnosis, days 7, 14, and 21 | 0.27 - 4.200µU/mL | Roche© Electrochemiluminescence |
| T4 free | At SS diagnosis, days 7, 14, and 21 | 0.93 - 1.70ng/dL | COBAS e411 analyzer |
| T4 total | At SS diagnosis, days 7, 14, and 21 | 5.10 - 14.10µg/dL | |
| T3 | At SS diagnosis, days 7, 14, and 21 | 80 - 200ng/dL |
TSH - thyroid-stimulating hormone; SS - septic shock; T4 - thyroxine; T3 - triiodothyronine.
Patients were first divided into two groups according to the presence or absence of LT3S, considered in this study as T3 below 80ng/dL. Then, patients who presented LT3S were subdivided into those whose low T3 persisted throughout the study period and those who normalized their T3 in the same period.
This information was recorded in the AVICENNA program and was then reduced to an Excel Microsoft® workbook to be incorporated into the statistical analysis program.
Statistical analysis
The data collected were analyzed statistically using IBM Statistical Package for Social Science (SPSS®) 25 software. The data were condensed to location (mean), dispersion (standard deviation), and aggregation (percentages), respectively. The existence of differences between the means, proportions, and results was evaluated using comparison tests of proportions based on the Z distribution. A cutoff of 5% was chosen for significance. Survival was analyzed using the Kaplan-Meier nonparametric test.
RESULTS
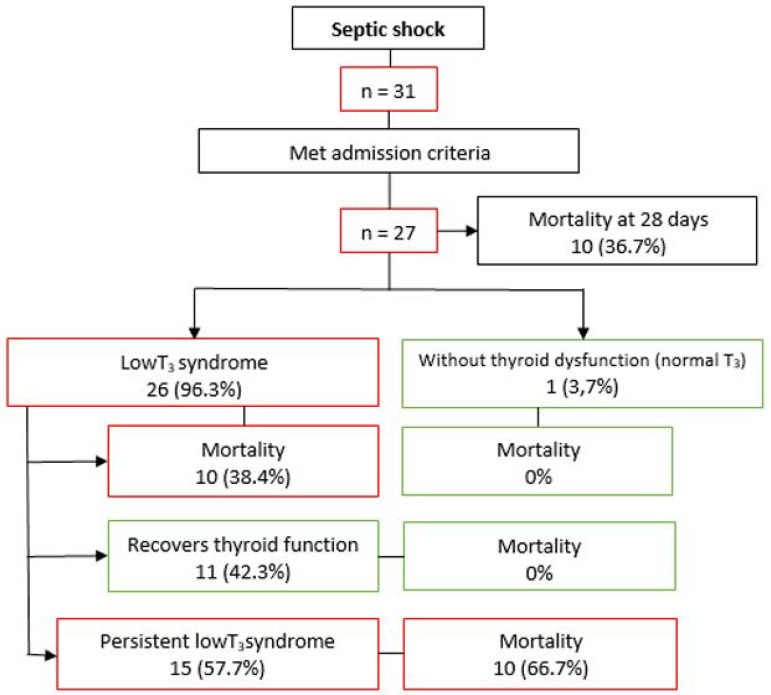
During the study period, 31 patients meeting the diagnostic criteria for septic shock were recruited; in 27 of these, all the procedures designed for the study were completed (Figure 1). Table 2 shows the baseline data of these patients. The mean concentrations of thyroid hormones are given in table 3. Of the patients included in the study, 96.3% (n = 26) had LT3S. Of these, 42.3% (n = 11) recovered thyroid axis function within 28 days of follow-up (Table 4).
Figure 1.
Patient flowchart. The mortality expressed in the figure is at 28 days. T3 - triiodothyronine.
Table 2.
Clinicodemographic data of the whole population, survivors, and nonsurvivors
| Sample total n = 27 |
Survivors n = 17 |
Nonsurvivors n = 10 |
p value | |
|---|---|---|---|---|
| Age (years) | 55.9 ± 16 | 56 ± 19 | 55..9 ± 11 | 0.98 |
| Sex, female | 51.9 | 60 | 36.4 | 0.24 |
| APACHE II | 16.2 ± 6.6 | 14.9 ± 6.22 | 18..5 ± 6.9 | 0.17 |
| SOFA | 9.7 ± 3.6 | 8.9 ± 2.96 | 11.2 ± 4.3 | 0.11 |
| Kidney failure | 15 (55.6) | 7 (41.2) | 8 (80.0) | 0.054 |
| AKI I | 4 (26.6) | 3 (42.8) | 1 (12.5) | 0.2 |
| AKI II | 3 (20) | 2 (28.6) | 1 (12.5) | 0.45 |
| AKI III | 8 (53.4) | 2 (28.6) | 6 (75.0) | 0.082 |
| Focus | ||||
| Abdominal | 55.6 | 64.7 | 40.0 | 0.32 |
| Respiratory | 18.5 | 11.8 | 30.0 | 0.248 |
| Urinary | 14.8 | 11.8 | 20.0 | 0.57 |
| Endovascular | 3.7 | 5.9 | NC | |
| Other | 7.4 | 5.9 | 10.0 | 0.7 |
| Charlson index | 3.6 ± 2.7 | 3 ± 2.85 | 4.7 ± 2.31 | 0.12 |
| Days of hospitalization | 19.5 ± 7.6 | 24 ± 22 | 11.9 ± 8.19 | 0.11 |
| Mortality 28 days | 10 (36.7) | NC | NC | NC |
| Thyroid dysfunction | 26 (96.3) | 16 (94.1) | 10 (100) | 0.44 |
| Recovered function | 44.4 | 70.6 | 0 | 0.0005 |
APACHE II - Acute Physiology and Chronic Health Evaluation II; SOFA - Sequential Organ Failure Assessment; AKI - acute kidney injury; NA - not applicable; 95%CI - 95% confidence interval. Results expressed as mean ± standard deviation, %, or n (%).
Table 3.
Thyroid profile of survivors versus nonsurvivors
| Survivors (n = 17) |
Nonsurvivors (n = 10) |
p value | |
|---|---|---|---|
| TSH at diagnosis | 1.02 ± 0.98 | 3.05 ± 4.83 | 0.102 |
| TSH day 7 | 4.98 ± 3.06 | 3.77 ± 5.73 | 0.47 |
| TSH day 14 | 3.84 ± 2.05 | 8.64 ± 14.80 | 0.18 |
| TSH day 21 | 3.19 ± 2.11 | 3.63 | NC |
| T3 at diagnosis | 52.06 ± 17.83 | 48.62 ± 17.40 | 0.53 |
| T3 day 7 | 69.81 ± 21.09 | 52.09 ± 17.19 | 0.033 |
| T3 day 14 | 96.07 ± 30.12 | 41.89 ± 10.68 | 0.0009 |
| T3 day 21 | 72.33 ± 16.99 | 55.60 | NC |
| T4 at diagnosis | 5.01 ± 1.76 | 4.15 ± 1.33 | 0.19 |
| T4 day 7 | 5.72 ± 1.31 | 3.54 ± 2.18 | 0.0032 |
| T4 day 14 | 7.24 ± 1.33 | 3.41 ± 1.97 | 0.0001 |
| T4 day 21 | 6.20 ± 1.30 | 3.34 | NC |
TSH - thyroid-stimulating hormone; T3 - triiodothyronine; T4 - thyroxine; NA - not applicable. Results expressed as mean ± standard deviation or absolute value.
Table 4.
Comparison of recovered thyroid function versus persistent low T3 syndrome
| Recovery of T3 plasma level n = 11 |
Persistent low T3 syndrome n = 15 |
p value | |
|---|---|---|---|
| Age (years) | 56.8 ± 20.6 | 55.4 ± 16 | 0.84 |
| Sex, female | 50.0 | 53.3 | 0.87 |
| APACHE II | 13.6 ± 5.22 | 18.3 ± 7 | 0.07 |
| SOFA | 8.8 ± 3 | 10.5 ± 3.9 | 0.23 |
| Focus | |||
| Abdominal | 75 | 40 | 0.07 |
| Respiratory | 8.3 | 26.7 | 0.23 |
| Urinary | 8.3 | 20 | 0.41 |
| Endovascular | 6.7 | NC | |
| Other | 8.3 | 6.7 | 0.87 |
| Charlson index | 2.8 ± 2.6 | 4.2 ± 2.7 | 0.19 |
| Days of hospitalization | 15.6 ± 5.7 | 13.8 ± 13.5 | 0.68 |
| Mortality 28 days | 0 | 66.7% | 0.013 |
| T3 at the diagnosis of shock (ng/dL) | 53.15 ± 13.7 | 48.9 ± 20 | 0.54 |
| T3 day 7 | 73.9 ± 20 | 54.4 ± 17.9 | 0.015 |
| T3 day 14 | 99.8 ± 28 | 44.6 ± 11.7 | 0.0001 |
| T3 day 21 | 73.2 ± 20 | 62.5 ± 9.9 | 0.084 |
| Lactate 0 hour (mmol/L) | 5.4 ± 4.1 | 3.04 ± 3.4 | 0.12 |
| Lactate 24 hours (mmol/L) | 3.1 ± 2.7 | 3.5 ± 4.4 | 0.79 |
| Clearance lactate 24 hours | 42 | -15 | 0.0046 |
| Lactate 48 hours (mmol/L) | 2.4 ± 1.6 | 2.3 ± 1.1 | 0.85 |
| Lactate 72 hours (µ/kg/min) | 1.7 ± 0.9 | 2.22 ± 1.58 | 0.33 |
| Clearance lactate 72 hours | 68.7% | 36.5% | 0.11 |
| NDL day 1 (µ/kg/min) | 0.31 ± 0.26 | 0.79 ± 0.58 | 0.017 |
| NDL day 2 (µ/kg/min) | 0.46 ± 0.38 | 0.63 ± 0.6 | 0.41 |
| NDL day 3 (µ/kg/min) | 0.14 ± 0.05 | 0.55 ± 0.68 | 0.058 |
| NDL day 4 (µ/kg/min) | 0.05 ± 0.05 | 0.68 ± 0.7 | 0.0068 |
T3 - triiodothyronine; APACHE II - Acute Physiology and Chronic Health Evaluation II; SOFA - Sequential Organ Failure Assessment; NA - not applicable; OR - odds ratio; 95%CI - 95% confidence interval NDL - noradrenaline. Results expressed as mean ± standard deviation or %.
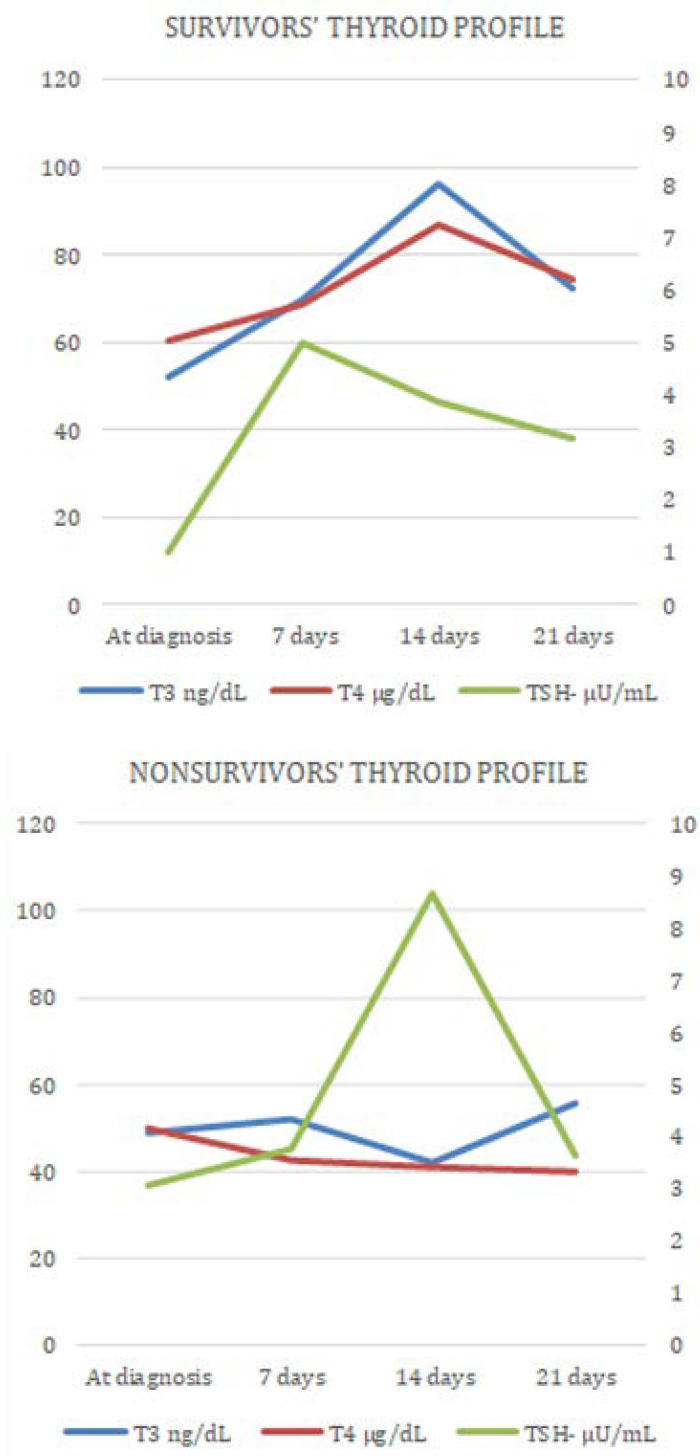
Triiodothyronine was lower in those who died than those who survived (admission 53.15ng/dL ± 13.7 versus 48.9ng/dL ± 20, p = 0.54; 7th day 73.9ng/dL ± 20 versus 54.4ng/dL ± 17.9, p = 0.015; 14th day 99.8ng/dL ± 28 versus 44.6ng/dL ± 11.7, p = 0.0001; 21st day 73.2ng/dL ± 20 versus 62.5 ng/dL ± 9.9, p = 0.084), associated with the changes to the rest of the thyroid axis (T4, TSH). See figure 2, where the dynamic behavior of thyroid hormone values (TSH, T3, T4) during the follow-up is compared between survivors and nonsurvivors.
Figure 2.
Thyroid profile behavior in survivors versus nonsurvivors. T3 - triiodothyronine; TSH - thyroid-stimulating hormone; T4 - thyroxine.
Patients who normalized their thyroid function within the study period and those who persisted with LT3S did not show statistically significant differences in age, sex, or APACHE II, SOFA, or Charlson comorbidity index score (Table 2). A difference was observed in the infectious focus: those who normalized their thyroid function had a higher incidence of abdominal sepsis, but without being statistically significant or suggesting any inferences we could make.
The T4 values in the patients who survived remained within normal values throughout the study time, while nonsurvivors had low, progressively decreasing T4 (admission 5.01µg/dL ± 1.76 versus 4.15µg/dL ± 1.33, p = 0.19; 7th day 5.72µg/dL ± 1.3 versus 3.54µg/dL ± 2.18, p = 0.0032; 14th day 7.24µg/dL ± 1.33 versus 3.41µg/dL ± 1.97, n = 5; 21st day 6.20µg/dL ± 1.30 versus 3.34, n = 1). Similarly, TSH was normal in the patients who survived, but in patients who died, a peak TSH stimulation was observed at day 14. The thyroid profile in its entirety could not be adequately analyzed at day 21 because the patients who died did so before this date, so there were hormonal values in only one case.
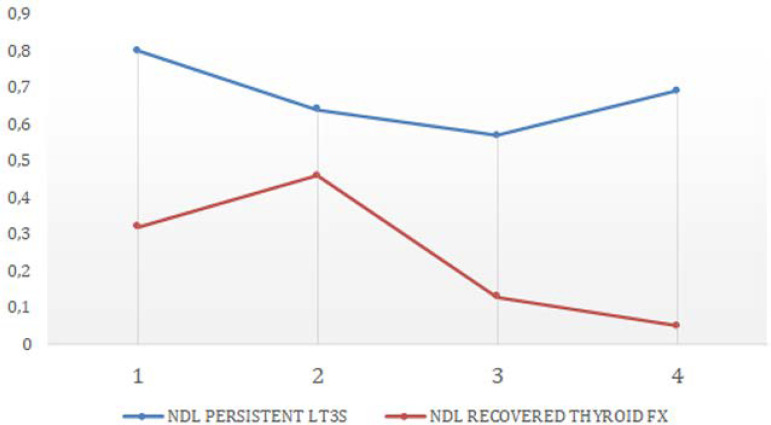
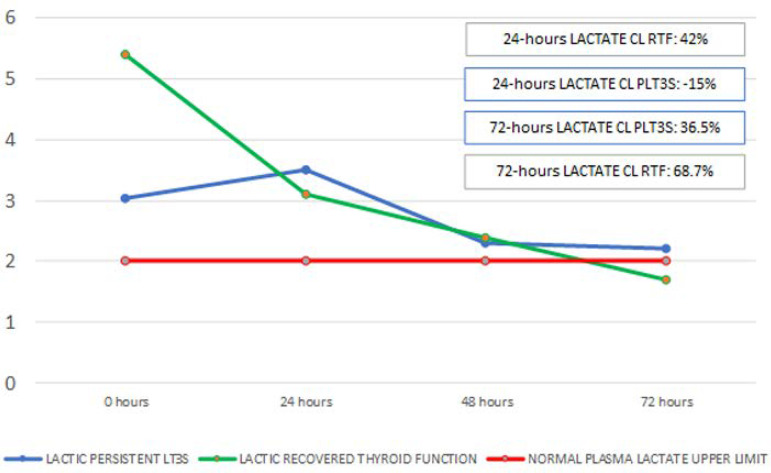
When hemodynamic parameters were compared between both groups, only vasoactive drug requirements at admission were higher in patients who died than those who survived (0.79 ± 0.58 versus 0.31 ± 0.26; p = 0.017); see figure 3A. In both groups, after initial resuscitation, lactate levels were modified. In the persistent LT3S group, lactate stayed above 2mmol/L, whereas the group that normalized thyroid function had lactate that decreased to normal at 72 hours after admission (Figure 3B).
Figure 3A.
Differences in noradrenaline requirements between patients with persistent low T3 syndrome and those who recovered their T3 level. NDL - noradrenaline; LT3S - low T3 syndrome; FX - function.
Figure 3B.
Differences in the behavior of plasma lactate between patients with persistent low T3 syndrome and those who recovered their T3 level. PLT3S - persistent low T3 syndrome; FX - function; CL - clearance; RTF - recovers thyroid function.
When the incidence of kidney failure related to thyroid disorder was analyzed, there were no statistically significant differences between the two groups, although there was a tendency to see more kidney failure in the group of nonsurvivors (Table 2).
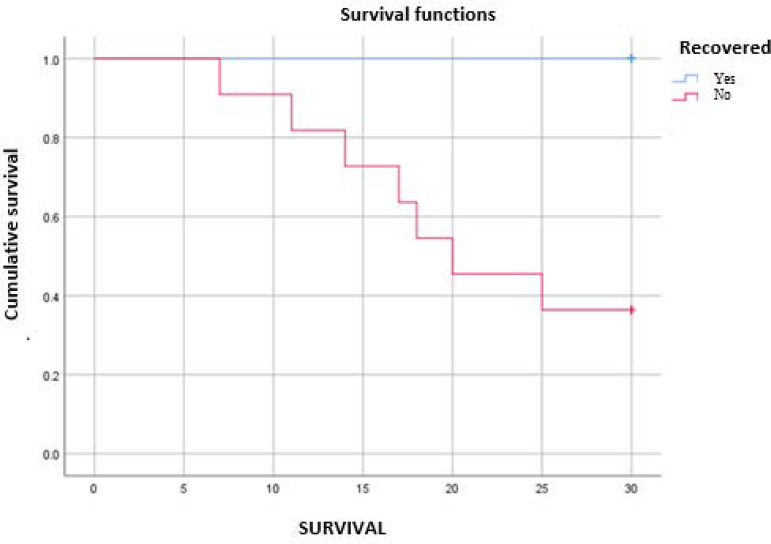
The total mortality of the sample at 28 days was 36.7%. When mortality was analyzed according to the presence of thyroid dysfunction, those who normalized their thyroid function had a mortality of 0%, compared with 66.7% among the patients who did not normalize their thyroid function within 28 days (Figure 1). Figure 4 shows the Kaplan-Meier survival curve, which shows a difference in mortality at 28 days.
Figure 4.
Persistent low-T3 syndrome survival curve versus recovery of thyroid function.
DISCUSSION
The present study evaluated the relationship between low T3 and septic shock and the probable relationship of low T3 with disease severity.
Thyroid hormones play an important role in the hemodynamic and metabolic functions of the body, among others. In the course of different disease states, the levels of T3, T4, and TSH decrease, a phenomenon called euthyroid sick syndrome or nonthyroidal illness syndrome.(17) These changes in circulating hormone levels are proportionally associated with disease severity and survival.
Neuroendocrine dysfunction is common in critically ill patients, including septic states,(18-20) and it has been described as an independent predictor of mortality in this group of critically ill patients, as well as in other pathologies of similar severity.(21-24) Likewise, studies carried out on brain-dead patients during body maintenance show that more organs can be harvested for transplantation from those who undergo hormonal resuscitation therapy, with supplementation of thyroid hormones, corticosteroids, and insulin, with the objective of generating greater hemodynamic stability.(25-28)
The levels of thyroid hormones are lower in septic patients than nonseptic patients with equal severity of disease, and low T3 can contribute to associated organ dysfunction; these alterations are associated with worse evolution.(19)
The present study shows the high incidence of impaired thyroid function in this group of patients and shows a relationship between the severity of septic patients and thyroid axis dysfunction. Patients with persistent deterioration of hormone levels (T3, T4) during the study period had higher mortality than those who normalized the function of the thyroid axis.
There was also evidence of greater hemodynamic deterioration as evaluated by SOFA (vasoactive drug requirement) (Figure 3A) and lower lactate clearance associated with thyroid deterioration (Figure 3B). This might have been associated with the deterioration of the physiological effects of thyroid hormones on cardiovascular performance (hemodynamic effects), as well as their metabolic actions on oxygen metabolism at the mitochondrial level.
On this basis, it could be hypothesized that LT3S in patients with septic shock is part of the pathophysiology of this disease and/or an associated organ (endocrine-metabolic) failure and not just an adaptive phenomenon. Therefore, substitution treatment with synthetic thyroid hormones could modify the hemodynamic symptoms of septic patients, contributing in part to the decrease in their morbidity and mortality. There are few publications, either experimental and clinical, on this topic, particularly in this group of patients, and they have had small samples and some contradictory results.(17)
As noted above, the results of this study, despite coming from a small sample, reflect a probable association between LT3S and septic shock severity and mortality at 28 days. The role of hormone replacement therapy in septic patients with LT3S, and therefore its positive or negative effect on the clinical course of the disease, remains to be clarified.
CONCLUSION
Patients with septic shock have a high incidence of altered thyroid axis, characterized by low T3. In this study, hormonal alterations were more severe and persistent in patients who died, suggesting an association between low T3 and mortality. T4 behaved similarly. Persistently low T3 was associated with higher vasoactive requirements and deterioration of lactate clearance.
In summary, low-T3 syndrome had a high incidence in our series of patients with septic shock, and this dysfunction was a marker of severity in this specific group of patients. The limitations of our study are that it was done in a single center in a small sample, precluding a multivariate analysis to assess thyroid alteration as an independent predictor of mortality.
Footnotes
Conflicts of interest: None.
Responsible editor: Gilberto Friedman
REFERENCES
- 1.Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993;39(5):499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 2.McIver B, Gorman CA. Euthyroid sick syndrome: an overview. Thyroid. 1997;7(1):125–132. doi: 10.1089/thy.1997.7.125. [DOI] [PubMed] [Google Scholar]
- 3.Peeters RP. Non thyroidal illness: to treat or not to treat? Ann Endocrinol (Paris) 2007;68(4):224–228. doi: 10.1016/j.ando.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE. Guyton and Hall textbook of medical physiology. 13th ed. PA: Elsevier; 2016. [Google Scholar]
- 5.Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 6.Danzi S, Klein I. Thyroid hormone and the cardiovascular system. Med Clin North Am. 2012;96(2):257–268. doi: 10.1016/j.mcna.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Perez A, Palos-Paz F, Kaptein E, Visser TJ, Dominguez-Gerpe L, Alvarez-Escudero J, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle an adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) 2008;68(5):821–827. doi: 10.1111/j.1365-2265.2007.03102.x. [DOI] [PubMed] [Google Scholar]
- 8.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van der Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88(7):3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 9.Debaveye Y, Ellger B, Mebis L, Darras VM, Van der Berghe G Regulation of tissue iodothyronine deiodinase activity in a model of prolonged critical illness. Thyroid. 2008;18(5):551–560. doi: 10.1089/thy.2007.0287. [DOI] [PubMed] [Google Scholar]
- 10.Boelen A, Platvoet-ter Schiphorst MC, Bakker O, Wiersinga WM. The role of cytokines in the lipopolysaccharide-induced sick euthyroid syndrome in mice. J Endocrinol. 1995;146(3):475–483. doi: 10.1677/joe.0.1460475. [DOI] [PubMed] [Google Scholar]
- 11.Kimura T, Kanda T, Kotajima N, Kuwabara A, Fukumura Y, Kobayashi I. Involvement of circulating interleukin-6 and its receptor in the development of euthyroid sick syndrome in patients with acute myocardial infarction. Eur J Endocrinol. 2000;143(2):179–184. doi: 10.1530/eje.0.1430179. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya T, Fujieda M, Otsuka G, Yang JP, Okamoto T, Seo H. A potential role of activated NF-kappa B in the pathogenesis of euthyroid sick syndrome. J Clin Invest. 2000;106(3):393–402. doi: 10.1172/JCI7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Poll T, Romijn JA, Wiersinga WM, Sauerwein HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. 1990;71(6):1567–1572. doi: 10.1210/jcem-71-6-1567. [DOI] [PubMed] [Google Scholar]
- 14.Michalaki M, Vagenakis AG, Makri M, Kalfarentzos F, Kyriazopoulou V. Dissociation of the early decline in serum T(3) concentration and serum IL-6 and TNFalpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab. 2001;86(9):4198–4205. doi: 10.1210/jcem.86.9.7795. [DOI] [PubMed] [Google Scholar]
- 15.Boelen A, Schiphorst MC, Wiersinga WM. Relationship between serum 3,5,3'-triiodothyronine and serum interleukin-8, interleukin-10 or interferon gamma in patients with nonthyroidal illness. J Endocrinol Invest. 1996;19(7):480–483. doi: 10.1007/BF03349894. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopra IJ. Nonthyroidal illness syndrome or euthyroid sick syndrome. Endocr Pract. 1996;2(1):45–52. doi: 10.4158/EP.2.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Avalos Méndez MA, Cruz Martínez E, Espinosa Larrañaga F, Graef Sanchéz A, Molinar Ramos F. Correlación entre las hormonas tiroideas circulantes y la mortalidad en pacientes con sepsis asociada a síndrome de disfunción orgánica múltiple. Rev Asoc Mex Med Crit Ter Intensiva. 1996;10(4):147–153. [Google Scholar]
- 19.Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. 2011;164(2):147–155. doi: 10.1530/EJE-10-0695. [DOI] [PubMed] [Google Scholar]
- 20.Economidou F, Douke E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critically illness. Hormones (Athens) 2011;10(2):117–124. [Google Scholar]
- 21.Slag MF, Morley JE, Elson MK, Crowson TW, Nuttall FQ, Shafer RB. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245(1):43–45. [PubMed] [Google Scholar]
- 22.Friberg L, Drvota V, Bjelak AH, Eggertsen G, Ahnve S. Association between increased levels of reverse triiodothyronine and mortality after acute myocardial infarction. Am J Med. 2001;111(9):699–703. doi: 10.1016/s0002-9343(01)00980-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Pan W, Wang H, Wang S, Pan S, Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Crit Care. 2012;16(1):R11–R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelnour T, Rieke S. Relationship of hormonal resuscitation therapy and central venous pressure on increasing organs of transplant. J Heart Lung Transplant. 2009;28(5):480–485. doi: 10.1016/j.healun.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Novitzky D, Cooper DK, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006;82(11):1396–1401. doi: 10.1097/01.tp.0000237195.12342.f1. [DOI] [PubMed] [Google Scholar]
- 26.Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff LG, Garrity ER, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75(8):1336–1341. doi: 10.1097/01.TP.0000062839.58826.6D. [DOI] [PubMed] [Google Scholar]
- 27.Novitzky D, Cooper DK, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43(6):852–854. [PubMed] [Google Scholar]
- 28.Maldonado LS, Murata GH, Hershman JM, Braunstein GD. Do thyroid function tests independently predict survival in the critically ill. Thyroid. 1992;2(2):119–123. doi: 10.1089/thy.1992.2.119. [DOI] [PubMed] [Google Scholar]