Supplemental Digital Content is Available in the Text.
Key Words: ischemic stroke, vinpocetine, NLRP3 inflammasome
Abstract:
Ischemic stroke is the leading cause of globe death and permanent disability, but its therapeutic strategies are limited. Over the past decades, multiprotein complexes called inflammasomes have been shown as promising targets in ischemic stroke. Here, we examined vinpocetine (Vinp), a synthetic drug, playing a neuroprotective role against ischemic stroke in mice through regulating NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation. Middle cerebral artery occlusion/reperfusion (MCAO/R) was applied to mimic ischemic stroke in vivo. Vinp was administrated by intraperitoneal injection with different dose (5 or 10 mg/kg) 1 hour after reperfusion. Then, neurological assessment and infarct size were performed, and interleukin-1β (IL-1β) and IL-18 levels were evaluated using ELISA. The levels of NLRP3 inflammasome components and its upstream nuclear factor-κB (NF-κB) were determined using real-time PCR or Western blot. The experimental results indicated that posttreatment with Vinp decreased cerebral infarct size, improved behavior recover, reduced NLRP3 inflammasome expression, and suppressed the transfer of NF-κB to nucleus and proinflammatory cytokine release in middle cerebral artery occlusion/reperfusion mice. In conclusion, this study demonstrates that Vinp alleviates ischemic stroke by regulating levels of NLRP3 inflammasome, NF-κB, and proinflammatory cytokines in vivo, offering an alternative medication for ischemic stroke associated with inflammation.
INTRODUCTION
Ischemic stroke is one of the most common cerebral vascular diseases with high morbidity, high mortality, and high disability worldwide. The molecular mechanisms responsible for the cerebral ischemia injury progression affected by stroke are complex, involving acidosis, excitotoxicity, oxidative stress, and inflammation.1–3 Among them, poststroke inflammation activated by innate local immune responses is thought to play a critical role in the pathophysiology of ischemic stroke.4 Recent findings have evidenced that inflammasome emerges as a newly discovered component involved in cerebral ischemia.5,6 Currently, the best characterized inflammasome, the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, is thought to participate in the pathophysiology of ischemic stroke.7
NLRP3 inflammasome contains a receptor protein NLRP3, a caspase activation recruitment domain (ASC) and precursor caspase-1.8,9 Caspase-1 cleaves into active caspase-1 and triggers the maturation of proinflammatory cytokines such as interleukin-1β (IL-1β) and IL-18 to engage in innate and acquired immune defenses.10 NLRP3 inflammasome has been found to express in microglia of the central nervous system.11 Numerous research studies have demonstrated that NLRP3 inflammasome mediates inflammation in neurological diseases.12,13 Previously, researchers used different models of ischemia and found the level of NLRP3 inflammasome proteins, IL-1β, and IL-18 were increased after ischemia injury.14,15 Meanwhile, either the deficiency or inhibition of NLRP3 inflammasome could ameliorate cerebral injury induced by ischemic stroke in different degrees.15 Overall, the expression of NLRP3 inflammasome is associated closely with the pathogenesis of ischemic stroke.
Vinpocetine (Vinp) is a synthetic derivative of vincamine, which is extracted from the periwinkle plant.16 Vinp has long been used in patients with ischemic stroke due to its ability to block Na+ channels and inhibit Ca2+ influx.17,18 Recent meta-analysis research have indicated that Vinp also works as a potent anti-inflammatory agent in monocytes/macrophages19 and brain microglial cells.20 The similar effect of Vinp has also been observed in the rat cerebral ischemia/reperfusion injury model21 and patients with anterior cerebral circulation occlusion and onset of stroke.22 Vinp has been supposed to influence many steps in ischemic stroke via inhibiting the release of numerous inflammatory mediators and regulating adaptive immune response.23 However, little is currently known about the specific role of NLRP3 inflammasome in the Vinp treatment of ischemic stroke.
In this study, we examine the effect of Vinp on NLRP3 inflammasome activation, NF-κB transfer, and pro-inflammatory cytokine expressions in middle cerebral artery occlusion/reperfusion (MCAO/R) of mice. We aim to explore the effect of Vinp on NLRP3 inflammasome-mediated inflammatory response, and provide an avenue for a profound understanding of Vinp in the clinical application of ischemic stroke.
MATERIALS AND METHODS
Animals and Treatment
All experimental procedures were performed in conformity with institutional guidelines of Shengjing Hospital of China Medical University (Shenyang, China). Thirty-six adult male C57BL/6 mice (22–25 g) were provided from Qinglongshan Animal Breeding Centre (Nanjing, China). Mice were fed freely in a temperature-controlled room (22 ± 2°C), with a 12-hour light–dark cycle. After surgery, mice were randomly allocated to 4 groups: sham, MCAO/R, Vinp high dose (10 mg/kg, Vinp-H), and Vinp low dose (5 mg/kg, Vinp-L) group (n = 9). For evaluation of infarct size, neurological deficiency, and ELISA analysis, n = 6 were assigned for each group. For real-time PCR and Western blot, n = 3 were assigned for each group.
Drug Administration
Vinp were supplied by Gedeon Richter Plc (A65132A, 2 mL: 10 mg). The mice were received different doses of Vinp (10 and 5 mg/kg) by intraperitoneal (i.p) injection once at 1 hour after reperfusion, with a volume of 0.25 mL/10 g. The sham and model mice were administrated with same volume of vehicle (normal saline).
Focal Cerebral Ischemia Establishment
MCAO/R injury was served as ischemic stroke model in our experiment as described previously.24,25 Mice were anesthetized using 2% chloral hydrate (400 mg/kg, i.p) and fixed in the supine position. The cerebral blood flow was monitored using Laser Speckle Contrast Imager (Moor Instruments, Essex, United Kingdom). After surgical exposure of the right carotid artery bifurcation, a silicon-coated monofilament nylon suture (diameter about 0.22 ± 0.01 mm, Beijing Cinontech Co, Ltd, Beijing, China) was advanced 11 mm from the common carotid artery bifurcation to obstruct the MCA. The filament was left for 1 hour for obstruction and then withdrawn for reperfusion. The sham group contains all surgical procedures, except occlusion of the MCA. Other groups were all performed MCAO/R surgery.
Neurological Scoring
All mice received behavior tests at 24 hours after reperfusion. The degrees of neurological deficit were assessed based on the method of Bederson.24 The scoring system was graded on a scale of 0–4 (minimal score, 0; maximal score, 4), where 0: no neurological deficit; 1: consistent forelimb flexion to the injured hemisphere when lifted by tail; 2: reduced resistance to lateral push on the shoulder; 3: circling to the paretic side; and 4: no spontaneous movements.
Assessment of Infarct Size
Mice were sacrificed and removed the whole brain at 24 hours after reperfusion. Each brain was dissected and sliced into 2-mm thick coronal sections. Brain slices were incubated in 1% 2, 3, 5-triphenyltetrazolium chloride (TTC, Sangon Biotech Co, Ltd, Shanghai, China) at 37°C for 10 minutes and flipped every 5 minutes.26 Using the ImageJ software (version, 1.50; National Institutes of Health, Bethesda, MD), the area of infarction was analyzed and integrated across 5 slices. The infarct size in each slice was calculated according to the following formula:
Infarct size (%) = (contralateral area − ipsilateral noninfarct area)/contralateral area × 100%.
Measurement of IL-1β and IL-18 in Brain Tissue and Serum
Blood samples were collected from venous plexus of fundus at 1 hour after last administration. Tissue samples for ELISA analysis were collected from ischemic core (located at lateral striatum and overlying cortex,27 Fig. 2A) after execution and reperfusion with normal saline. Samples were collected by centrifuging the tissue homogenate and blood sample for 20 minutes at 2500 rpm. IL-1β and IL-18 levels were determined using ELISA kits (Shanghai Enzyme-linked Biotechnology Co, Ltd, Shanghai, China).
FIGURE 2.
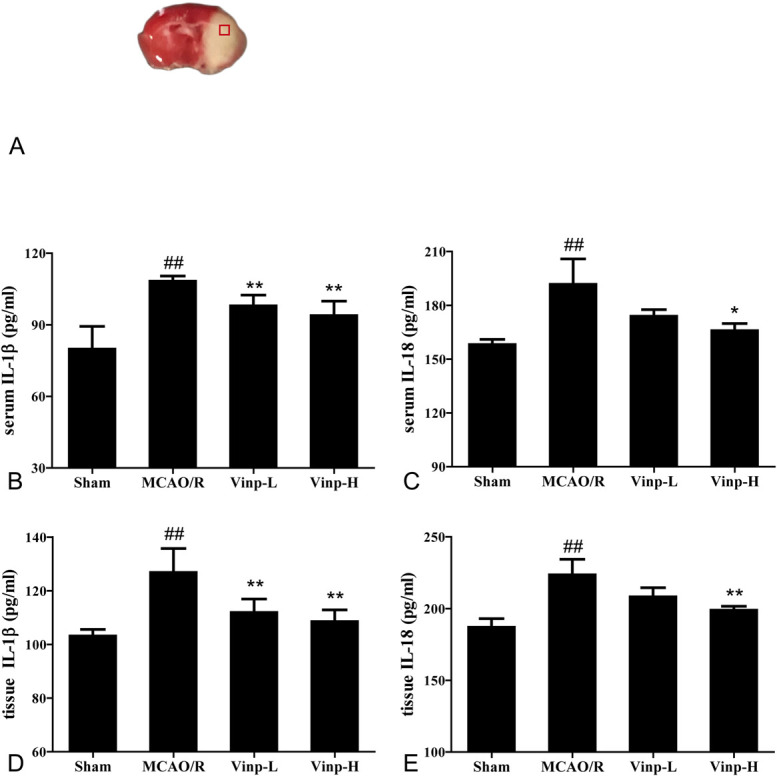
Effect of Vinp on the expressions of IL-1β and IL-18 in serum and ischemic brain at 24 hours after MCAO/R. A, Coronal brain diagrams showing locations of regions for molecular analysis in infarct cortex. The level of serum IL-1β (B), IL-18 (C), cerebral homogenate IL-1β (D), and IL-18 (E) at 24 hours after MCAO/R (n = 6). Values are shown as mean ± SD. #P < 0.05, ##P < 0.01, versus sham group; *P < 0.05, **P < 0.01, versus MCAO/R group.
Real-Time PCR
To detect the changes of NLRP3, ASC, and caspase-1 mRNA levels in brain tissue, real-time PCR was performed as described previously.28 The brain tissue was collected from ischemic core (Fig. 2A) and homogenized with Trizol reagent (Vazyme, Nanjing, China). The suspension was centrifuged at 4°C 12,000g for 5 minutes. Then, the 1/5 Trizol reagent volume of chloroform (Lingfeng Chemical Reagent Co, Ltd, Shanghai, China) was added. After being shaken vigorously, the emulsion was centrifuged at 12000g, 4°C for 10 minutes. Next, the supernatant was centrifuged at 12000g, 4°C for 10 minutes with an equal volume of isopropanol (Sinopharm Chemical Reagent Co, Ltd, Shanghai, China). The precipitate was added to 1 mL 75% ethanol (Titan Scientific Co, Ltd, Shanghai, China) and centrifuged at 7500g, 4°C for 5 minutes. Finally, DEPC water was added to dissolve the precipitate. The cDNA synthesis was reverse-transcribed from the isolated RNA with a HiScript II Q RT SuperMix (Vazyme, Nanjing, China) according to manufacturer's instructions. Real-time PCR was performed using ChamQ SYBR qPCR Master Mix kit (Vazyme, Nanjing, China) in a Mastercycler (Eppendorf, Hamburg, BRD). The mRNA expressions were determined by using comparative threshold cycle method. The results were normalized to β-actin and reported as fold changes versus sham group. The primers (Sangon Biotech Co, Ltd, Shanghai, China) used for the amplification are shown as below:
NLRP3: F: ATGCTGCTTCGACATCTCCT, R: AACCAATGCGAGATCCTGAC;
ASC: F: GAAGCTGCTGACAGTGCAAC, R: GCCACAGCTCCAGACTCTTC;
Caspase-1: F: AGATGGCACATTTCCAGGAC,
R: GATCCTCCAGCAGCAACTTC;
β-actin: F: AGAGGGAAATCGTGCGTGAC, R: CAATAGTGATGACCTGGCCGT.
Western Blot
The protein extracts of brain tissue were prepared as described previously. To determine NLRP3, ASC, and cleaved caspase-1 protein expressions in the ischemic core of the brain (Fig. 2A), the protein concentrations were measured using BCA assay. Protein samples were separated by SDS-PAGE and were transferred onto polyvinylidene fluoride membranes. The membrane was blocked with 5% nonfat milk in TBST (0.1% Tween 20 in TBS) for 2 hours at room temperature and incubated with primary antibodies of anti-NLRP3 (1:1000, A12694; ABclonal Technology, Wuhan, China), anti-ASC (1:1000, A16672; ABclonal Technology, Wuhan, China), anti-cleaved caspase-1 (1:1000, WL03325; Wanleibio, Shenyang, China), and anti-NF-κB (1:500, WL01980; Wanleibio, Shenyang, China) at 4°C overnight. After being washed 3 times, the membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (H + L) secondary antibodies (1:5000, AB0101; Abways Technology, Shanghai, China) for 2 hours. Then, the blots were visualized by enhanced chemiluminescence detection system (Chemidoc XRS+, Bio-Rad; Berkeley, CA). The results were normalized to anti-β-actin (1:1000, AY0573; Abways Technology, Shanghai, China) or anti-H3 (1:500, WL09804a; Wanleibio, Shenyang, China) and the intensity of bands was quantified by ImageJ.
Statistical Analysis
The data were presented as mean ± SD. Statistical analysis was performed using IBM SPSS Statistics v19.0 software. One-way analysis of variance and least significant difference test were used to compare statistical differences between groups. The neurological scores and real-time PCR were compared with other groups by using Mann–Whitney U test. Tests were considered statistically significant at P < 0.05. Statistical figures were performed with Photoshop CS6, Image J, and GraphPad Prism Version 5.0.
RESULTS
Vinp Attenuated Ischemic Outcome in Mice After MCAO/R
All mice that underwent the MCAO surgery were excluded for successful occlusion (cerebral blood flow fell less than 75% of baseline, Supplemental Digital Content 1, http://links.lww.com/JCVP/A544). The infarct size in the mice brain was detected by TTC staining. Conversely, no infarct area was detected in the sham group, suggesting that the occlusion initiated infarct volume (Fig. 1B). The infarct size of MCAO/R group increased significantly in comparison with sham group (P < 0.01), whereas Vinp (5 and 10 mg/kg) treatment groups significantly reduced (P < 0.01).
FIGURE 1.
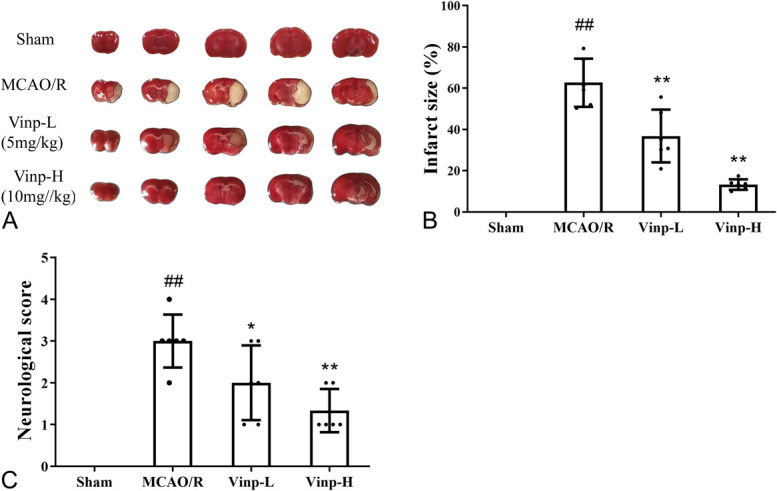
Effect of Vinp on brain infarct size and neurological behavior at 24 hours after MCAO/R. A, Representative images of TTC-stained brain sections. B, Brain infarct size. C, Neurological score. Data presented as mean ± SD (n = 6). #P < 0.05, ##P < 0.01, versus sham group; *P < 0.05, **P < 0.01, versus MCAO/R group.
The neurological severity score was conducted at 24 hours after MCAO/R to examine the effect of Vinp treatment on the behavior dysfunction. Compared with MCAO/R group, the scores of Vinp group greatly decreased. Vinp-H group displayed more significant difference with the MCAO/R group (P < 0.01, Fig. 1C) than the Vinp-L group.
Vinp Inhibited Inflammatory Cytokine Releasing Both in Ischemic Brain and Serum After MCAO/R
As secretion of IL-1β and IL-18 dynamically participating in the pathological progression of ischemia stroke, we used ELISA to observe whether Vinp was able to regulate their secretion. As shown in Figure 2, the levels of both IL-1β and IL-18 increased in mice of MCAO/R group compared with sham group at 24 hours after MCAO/R (P < 0.01). In comparison with MCAO/R group, the dramatic increase of IL-1β in brain tissue and serum was inhibited by treatment with Vinp (P < 0.01, Figs. 2A, C). Similarly, Vinp also downregulated the expression of IL-18 at high dose (Figs. 2B, D).
Vinp Downregulated NLRP3, ASC, and Caspase-1 mRNA Expressions in Mice After MCAO/R
First, we investigated the influence of Vinp on inflammasome-related cytokines in vivo (Fig. 2). The releasing of cytokines IL-1β and IL-18 was induced by active caspase-1, a component in NLRP3 inflammasome.29 To investigate whether NLRP3 inflammasome was involved in ischemic stroke and Vinp treatment, we determined the mRNA expressions of NLRP3, ASC, and caspase-1 in MCAO/R mice (Fig. 3). The mRNA levels of NLRP3, ASC, and caspase-1 were low in sham mice and enhanced greatly after cerebral ischemia (P < 0.01). Compared with MCAO/R group, the mRNA expressions of NLRP3, ASC, and caspase-1 in brain tissue were reduced by treatment with Vinp (P < 0.05).
FIGURE 3.
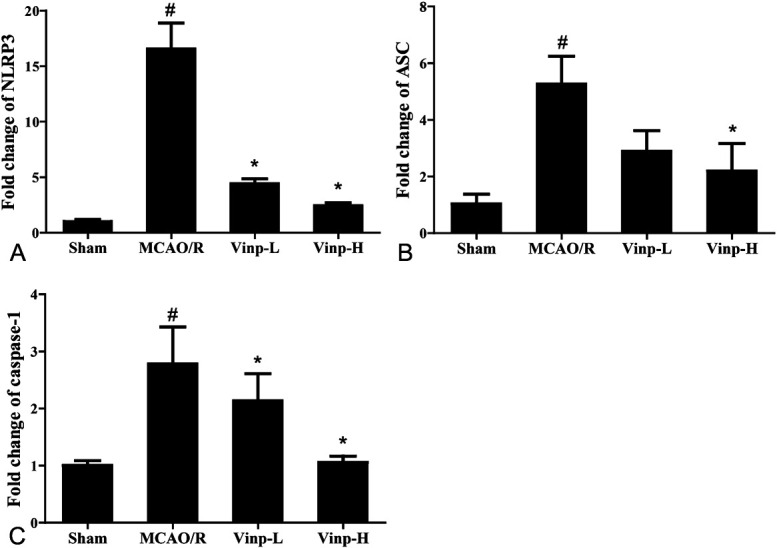
Effect of Vinp on the mRNA expressions of NLRP3, ASC, and caspase-1 in ischemic brain at 24 hours after MCAO/R. Real-time PCR for mRNA expressions of NLRP3 (A), ASC (B), and caspase-1 (C) at 24 hours after MCAO/R (n = 3). Values were shown as mean ± SD. #P < 0.05, ##P < 0.01, versus sham group; *P < 0.05, **P < 0.01, versus MCAO/R group.
Vinp Downregulated NLRP3, ASC, and Caspase-1 Protein Expressions in Mice After MCAO/R
We further analyzed the protein level of NLRP3 inflammasome using Western blot. Consistently, the protein levels of NLRP3, ASC, and caspase-1 elevated in the MCAO/R group (Fig. 4, P < 0.01). As Figure 4A showed, the protein expression of NLRP3 in brain tissue significantly reduced after Vinp treatment, consistent with the results of real-time PCR. Similar trends were also observed in the expressions of ASC, caspase-1, and cleaved caspase-1 (Figs. 4C–E). Moreover, the Vinp-H group (P < 0.01, Figs. 4B, C) exhibited more significant difference than Vinp-L group (P < 0.05) when compared with MCAO/R group.
FIGURE 4.
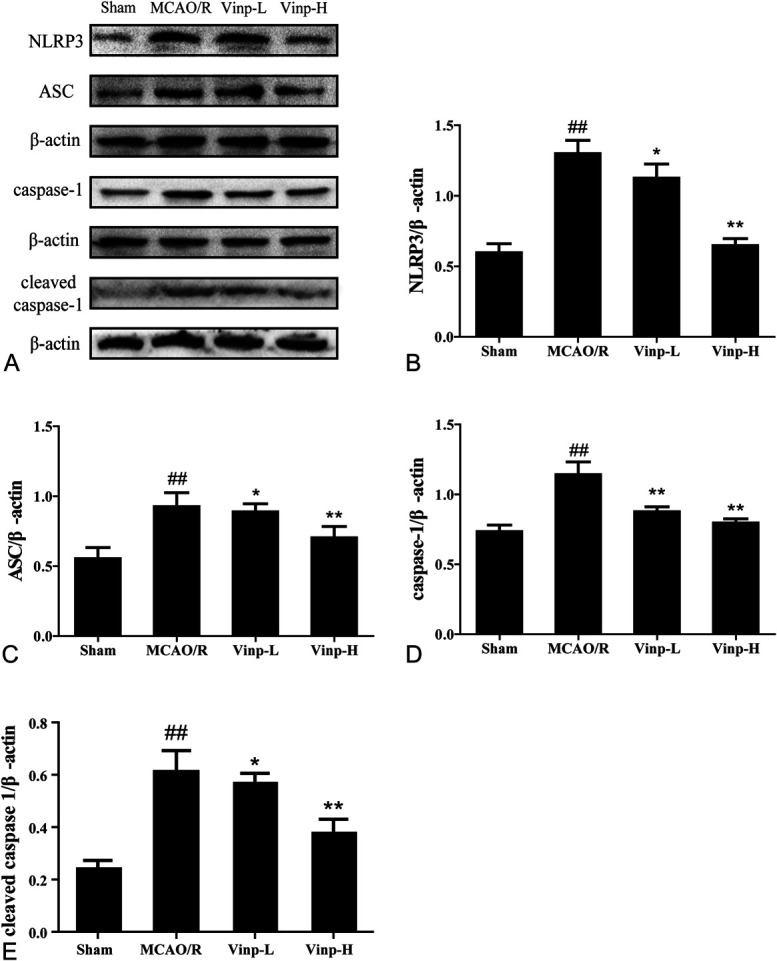
Effect of Vinp on protein expressions of NLRP3, ASC, caspase-1, and its cleaved form in ischemic brain at 24 hours after MCAO/R. A, Representative immunoblots of inflammasome proteins. Western blot for expressions of NLRP3 (B), ASC (C), caspase-1 (D), and cleaved caspase-1 (E) in ischemic brain. Data were shown as mean ± SD (n = 3). #P < 0.05, ##P < 0.01, versus sham group; *P < 0.05, **P < 0.01, versus MCAO/R group.
Vinp Suppressed the Transfer of NF-κB in Mice After MCAO/R
As shown in Figure 5A, the protein levels of NF-κB enhanced in the MCAO/R group. Vinp treatment significantly reduced the total expression of NF-κB in brain tissue. The cytoplasm NF-κB expression of the MCAO/R group significantly decreased (Fig. 5B), whereas nucleus NF-κB expression increased (Fig. 5C) when compared with sham group. After Vinp treatment, the expression of cytoplasm NF-κB elevated, whereas that of nucleus NF-κB reduced.
FIGURE 5.
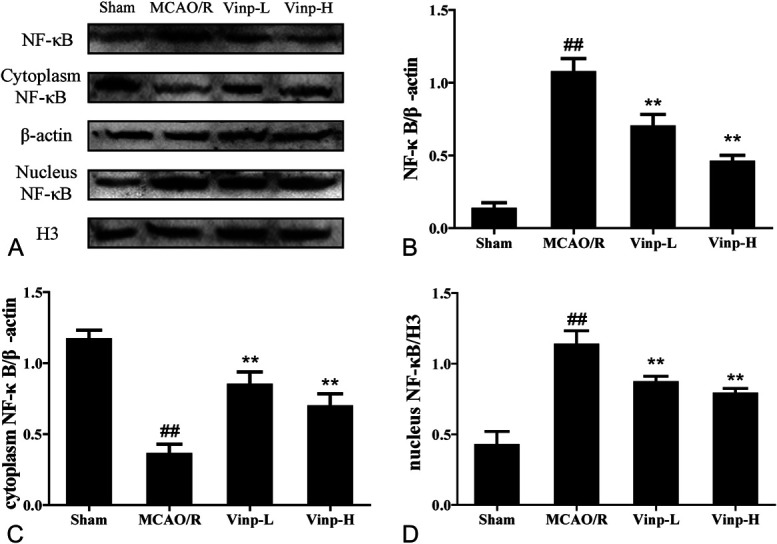
Effect of Vinp on protein expressions of nucleus and cytoplasm NF-κB in ischemic brain at 24 hours after MCAO/R. A, Representative immunoblots of inflammasome proteins. Western blot for expressions of total (B), cytoplasm (C), and nucleus (D) NF-κB in ischemic brain. Data were shown as mean ± SD (n = 3). #P < 0.05, ##P < 0.01, versus sham group; *P < 0.05, **P < 0.01, versus MCAO/R group.
DISCUSSION
Ischemic stroke is the leading cause of globe death and a major cause of permanent disability. At present, the only way approved for treating ischemic stroke is tissue plasminogen activator.30 However, due to its narrow therapeutic window, contraindications, and complications, researchers have been taking effort to explore new treatment with efficiency and safety in recent years.31 In this study, we performed experiments in vivo and found that Vinp mitigated the outcomes of ischemic stroke. In MCAO/R mice, the infarct size decreased and the neurological behavior improved after Vinp treatment. The results show that Vinp has neuroprotective effects against cerebral ischemic injury, which is associated with inhibiting NLRP3 inflammasome expression and reducing proinflammatory cytokine production.
Vinp exerts neuroprotective effect by its action on improving brain metabolism and cerebral circulation. It increases both oxygen and glucose utilization to enhance cerebral function.32 Recently, the protocol of meta-analysis for treating stroke-related disease has been established to evaluate the efficacy and safety of Vinp.33 Researchers assumed that it could also be protective in conditions of hypoxia and ischemia. The assumption has been evidenced by multiple clinical studies on patients suffering ischemic stroke.34,35 Previous article has revealed that Vinp could reduce oxidative stress, inflammation, and apoptosis in brain tissues to attenuate cerebral I/R injury.36 As a PDE-1 inhibitor, Vinp is believed to selectively inhibit purinergic receptor-stimulated mitochondrial Ca2+ efflux and subsequent H2O2 production to alleviate neuronal damage.37,38 Furthermore, inflammation comprises a large proportion in ischemic disease progression, and researchers also found that Vinp could inhibit this process to prevent ischemic stroke.36,39 The neuroprotective effect has been achieved by reducing the expression and activation of NLRP3 and proinflammatory cytokines in retinal pigment epithelial cells.40 The anti-inflammatory effect of Vinp has been displayed to correlate with its inhibition of the NF-κB pathway and subsequent cytokines release.21,41 The results in our study demonstrates that Vinp can exert a protective effect against ischemia injury by reducing expression of the NF-κB/NLRP3 pathway.
As the neuroinflammatory response exists from early arterial occlusion to late brain injury in ischemic stroke, inhibition of inflammatory reaction is a potential therapeutic target in patients with stroke.42,43 New evidence is emerging to support the role of specific inflammasome involved in the development of ischemic stroke. The NLRP3 inflammasome elicits a cascade of inflammatory responses to mediate postischemic inflammation.44 Previously, researchers found that the NLRP3 inflammasome was involved in mice and patients with stroke under ischemic conditions.14,45 Either pharmaceutical inhibitor or genetical knockout of NLRP3 inflammasome could protect neuronal cells in experimental stroke models.14,15 Consistently, our research on realtime PCR and Western blot provides experimental proof that NLRP3 inflammasome plays a crucial role during ischemic stroke and a potential pharmaceutical inhibitor of NLRP3 inflammasome.
Recent evidence suggests that the activation of NLRP3 inflammasome is induced by NF-κB and MAPK signaling pathways, which participate in acute inflammatory response and chronic inflammation.46 The blockade of NF‐κB pathway could reduce NLRP3 inflammasome component expression under the inflammatory condition. Researchers have proved that activation of NF-κB provokes the transcription of NLRP3 gene.47 It is supposed that NLRP3 inflammasome may be involved in the pathogenesis of ischemic stroke as a downstream substrate of NF-κB signaling pathway. Numerous articles reported that NF-κB/NLRP3 signaling pathway was activated after cerebral ischemia.48 Intriguingly, drugs and adequate exercise that regulate the expression of these proteins perform protective function against ischemic stroke.48–50 Congruous with published literature, our results also display the activation of NF-κB/NLRP3 signaling pathway induced by cerebral ischemia. The upregulation associates closely with exacerbated brain injury. After Vinp treatment, the expression of NF‐κB and NLRP3 inflammasome obviously reduced, suggesting a possible mechanism that Vinp involves.
In addition to block NF‐κB and NLRP3 inflammasome expression, Vinp also effectually suppressed cytokine production in our study. As the decrease of NLRP3 protein leads to less recruitment of caspase-1, pro-caspase-1 cannot be cleaved without incorporating into the NLRP3 complex. Consequently, there is less IL-1β and IL-18 being secreted in brain tissue and serum. IL-1β is believed to induce adhesion molecules and promote the infiltration of circulating immune cells in cerebral ischemia. Besides, IL-1β contributes to amplify the inflammatory response by stimulating the release or synthesis of other proinflammatory mediators.51 IL-18 is a proinflammatory cytokine primarily secreted by macrophage cells, blood mononuclear cells, and neurons. It is likely to be the first-line immune defense for the brain.52 Recently, increased IL-18 level has been found to associate with increased expressions of many types of inflammatory cytokines (including IL-6 and C-reactive protein), which can lead to changes in atherosclerosis plaques, hypertension, and hyperlipidemia, ultimately resulting in the development of stroke.53 Taken together, these affected cytokines may act on different aspects of the inflammatory pathway in the process of ischemic stroke.
Although this study demonstrates that Vinp alleviates ischemic stroke through reducing NLRP3 inflammasome expression, additional questions remain. How Vinp inhibits NLRP3 inflammasome still needs profound investigation.
In conclusion, Vinp negatively regulated NF‐κB, NLRP3 inflammasome, and subsequent IL-1β and IL-18 activation in MCAO/R mice. The data demonstrate that Vinp treatment inhibits NF-κB/NLRP3 signaling pathway to exert its effect. The results suggest that NLRP3 inflammasome could be a potential target for Vinp to treat cerebral ischemia.
ACKNOWLEDGMENTS
This study was sponsored by Key projects of Liaoning Natural Science Foundation (No. 20170541053).
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
Contributor Information
Dong Han, Email: handonghawk@163.com.
Jue Wang, Email: wjw_999@126.com.
Miao Sun, Email: Wenluluwilla@126.com.
Hang Liu, Email: liuhangkitty@sina.com.
REFERENCES
- 1.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. [DOI] [PubMed] [Google Scholar]
- 2.Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem. 2007;14:857–874. [DOI] [PubMed] [Google Scholar]
- 3.Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. [DOI] [PubMed] [Google Scholar]
- 4.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. [DOI] [PubMed] [Google Scholar]
- 6.Barrington J, Lemarchand E, Allan SM. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2017;27:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Lan P, Hou X, et al. Hbv inhibits lps-induced nlrp3 inflammasome activation and il-1β production via suppressing the nf-κb pathway and ros production. J Hepatol. 2017;66:693–702. [DOI] [PubMed] [Google Scholar]
- 8.Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2011;469:221–225. [DOI] [PubMed] [Google Scholar]
- 9.Tschopp J, Schroder K. Nlrp3 inflammasome activation: the convergence of multiple signalling pathways on ros production? Nat Rev Immunol. 2010;10:210–215. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. [DOI] [PubMed] [Google Scholar]
- 11.Gustin A, Kirchmeyer M, Koncina E, et al. Nlrp3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One. 2015;10:e0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SM, Ka SM, Wu HL, et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-nf-κb/nlrp3 inflammasome-mediated inflammation, enhancement of nrf2 antioxidant activity and inhibition of apoptosis. Diabetologia. 2014;57:424–434. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Xu X, Li Y, et al. Quercetin, luteolin and epigallocatechin gallate alleviate txnip and nlrp3-mediated inflammation and apoptosis with regulation of ampk in endothelial cells. Eur J Pharmacol. 2014;745:59–68. [DOI] [PubMed] [Google Scholar]
- 14.Fann DY, Lee S, Manzanero S, et al. Intravenous immunoglobulin suppresses nlrp1 and nlrp3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Wang Z, Wei X, et al. Nlrp3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cerebr Blood F Met. 2014;34:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurolo. 2006;59:467–477. [DOI] [PubMed] [Google Scholar]
- 17.Bönöczk P, Gulyás B, Adam-Vizi V, et al. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Res Bull. 2000;53:245–254. [DOI] [PubMed] [Google Scholar]
- 18.Sitges M, Chiu LM, Reed RC. Effects of levetiracetam, carbamazepine, phenytoin, valproate, lamotrigine, oxcarbazepine, topiramate, vinpocetine and sertraline on presynaptic hippocampal na(+) and ca(2+) channels permeability. Neurochem Res. 2016;41:758–769. [DOI] [PubMed] [Google Scholar]
- 19.Jeon KI, Xu X, Aizawa T, et al. Vinpocetine inhibits nf-kappab-dependent inflammation via an ikk-dependent but pde-independent mechanism. Proc Natl Acad Sci U S A. 2010;107:9795–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YY, Yu JZ, Li QY, et al. Tspo-specific ligand vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation. Neuron Glia Biol. 2011;7:187–197. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Zhang K, Zhao L, et al. Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa b and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury. Neurosci Lett. 2014;566:247–251. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Yan C, Wei C, et al. Vinpocetine inhibits nf-κb-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res. 2018;9:174–184. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature. Molecules. 2015;20:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. [DOI] [PubMed] [Google Scholar]
- 25.Moisse K, Welch I, Hill T, et al. Transient middle cerebral artery occlusion induces microglial priming in the lumbar spinal cord: a novel model of neuroinflammation. J Neuroinflammation. 2008;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raza S, Khan M, Ahmad A, et al. Neuroprotective effect of naringenin is mediated through suppression of nf-κb signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. [DOI] [PubMed] [Google Scholar]
- 27.Ashwal S, Tone B, Tian HR, et al. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29:1037–1046; discussion 1047. [PubMed] [Google Scholar]
- 28.Wang L, Zhang L, Chen ZB, et al. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing sirt1. Eur J Pharmacol. 2009;609:40–44. [DOI] [PubMed] [Google Scholar]
- 29.Boucher D, Monteleone M, Coll RC, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215:827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. The Lancet. 2011;377:1693–1702. [DOI] [PubMed] [Google Scholar]
- 31.dela Peña I, Borlongan C, Shen G, et al. Strategies to extend thrombolytic time window for ischemic stroke treatment: an unmet clinical need. J Stroke. 2017;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farooq MU, Min J, Goshgarian C, et al. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31:759–776. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Yin Y, Lu QL, et al. Vinpocetine in the treatment of poststroke cognitive dysfunction: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Huang Y, Li Y, et al. Efficacy and safety of vinpocetine as part of treatment for acute cerebral infarction: a randomized, open-label, controlled, multicenter cavin (Chinese assessment for vinpocetine in neurology) trial. Clin Drug Invest. 2016;36:697–704. [DOI] [PubMed] [Google Scholar]
- 35.Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;2008:CD000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M, Hou S, Feng L, et al. Vinpocetine protects against cerebral ischemia-reperfusion injury by targeting astrocytic connexin43 via the pi3k/akt signaling pathway. Front Neurosci. 2020;14:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nivison-Smith L, Acosta ML, Misra S, et al. Vinpocetine regulates cation channel permeability of inner retinal neurons in the ischaemic retina. Neurochem Int. 2014;66:1–14. [DOI] [PubMed] [Google Scholar]
- 38.Svab G, Doczi J, Gerencser AA, et al. The mitochondrial targets of neuroprotective drug vinpocetine on primary neuron cultures, brain capillary endothelial cells, synaptosomes, and brain mitochondria. Neurochem Res. 2019;44:2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konsman JP, Drukarch B, Van Dam AM. (peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clinical Sci. 2007;112:1–25. [DOI] [PubMed] [Google Scholar]
- 40.Liu RT, Wang A, To E, et al. Vinpocetine inhibits amyloid-beta induced activation of nf-κb, nlrp3 inflammasome and cytokine production in retinal pigment epithelial cells. Exp Eye Res. 2014;127:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC, et al. Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and nf-κb. Chem Biol Interact. 2015;237:9–17. [DOI] [PubMed] [Google Scholar]
- 42.Alawieh A, Langley EF, Tomlinson S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci Transl Med. 2018;10:eaao6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayaraj RL, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, Dong Q, Song Z, et al. Nlrp3 inflammasome: a promising target in ischemic stroke. Inflamm Res. 2017;66:17–24. [DOI] [PubMed] [Google Scholar]
- 45.Hong P, Li FX, Gu RN, et al. Inhibition of nlrp3 inflammasome ameliorates cerebral ischemia-reperfusion injury in diabetic mice. Neural Plasticity. 2018;2018:9163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fann DY, Lim YA, Cheng YL, et al. Evidence that nf-κb and mapk signaling promotes nlrp inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55:1082–1096. [DOI] [PubMed] [Google Scholar]
- 47.Shao A, Wu H, Hong Y, et al. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of nf-κb pathway and nlrp3 inflammasome. Mol Neurobiol. 2016;53:3462–3476. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Liang W, Guo C, et al. Renshen shouwu extract enhances neurogenesis and angiogenesis via inhibition of tlr4/nf-κb/nlrp3 signaling pathway following ischemic stroke in rats. J Ethnopharmacol. 2020;253:112616. [DOI] [PubMed] [Google Scholar]
- 49.Yang B, Sun Y, Lv C, et al. Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting tlr4-nlrp3 inflammasome signal pathway. Psychopharmacology. 2020;237:3283–3293. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Xu X, Wang Z, et al. Exercise ameliorates post-stroke depression by inhibiting pten elevation-mediated upregulation of tlr4/nf-κb/nlrp3 signaling in mice. Brain Res. 2020;1736:146777. [DOI] [PubMed] [Google Scholar]
- 51.Touzani O, Boutin H, Chuquet J, et al. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura K, Kassem S, Cleynen A, et al. Dysregulated il-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell. 2018;33:634–648.e635. [DOI] [PubMed] [Google Scholar]
- 53.Hao Y, Ding J, Hong R, et al. Increased interleukin-18 level contributes to the development and severity of ischemic stroke. Aging. 2019;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]


