Abstract
Background and Objectives
Data regarding the safety of atrial fibrillation (AF) ablation using high-power (50 W) radiofrequency (RF) energy in Asian populations are limited. This study was conducted to evaluate the incidence and pattern of esophageal injury after high-power AF ablation in an Asian cohort.
Methods
We searched the prospective AF ablation registry to identify patients who underwent AF ablation with 50 W RF energy using the smart touch surround flow catheter (Biosense Webster, Diamond Bar, CA, USA). Visitag™ (Biosense Webster) was used for lesion annotation with predefined settings of catheter stability (3 mm for 5 seconds) and minimum contact force (50% of time >5 g). All patients underwent upper gastrointestinal endoscopy at 1 or 3 days after the ablation.
Results
A total of 159 patients (mean age: 63±9 years, male: 69%, paroxysmal AF: 45.3%, persistent AF: 27.7%, long-standing persistent AF: 27.0%) were analyzed. Initially, 26 patients underwent pulmonary vein isolation with 50 W for 5 seconds at each point. The remaining 133 patients underwent prolonged RF duration (anterior 10 seconds and posterior 6 seconds). The incidence rates of esophageal erythema/erosion and superficial ulceration were 1.3% for each type of the lesion. Food stasis, a suggestive finding of gastroparesis, was observed in 25 (15.7%) patients. There were no cases of cardiac tamponade, stroke, or death.
Conclusions
In Asian patients, AF ablations using 50 W resulted in very low rates of mild esophageal complications.
Keywords: Atrial fibrillation, Complications, Endoscopy, Esophagus, Radiofrequency catheter ablation
INTRODUCTION
Catheter ablation is a standard treatment in patients with symptomatic atrial fibrillation (AF) refractory to or intolerant to antiarrhythmic drugs (AADs), but it is associated with significant complications and even mortality. Atrioesophageal fistula (AEF), one of the most serious complications following AF catheter ablation,1) is associated with lesions along the left inferior pulmonary vein (PV) in patients with older age, low body weight, and higher CHA2DS2VASc score.2) Several strategies are used in ablation procedures along the posterior wall especially along the course of the esophagus, including avoidance of delivery of radiofrequency (RF) energy along the course of the esophagus, lower power delivery,3) esophageal temperature monitoring,4) temperature-controlled RF delivery,5) and high-power short-duration (HPSD) ablation.
HPSD ablation is distinguished from conventional ablation in terms of power (≥40 W vs. 25–30 W) and duration (2–5 seconds vs. 20–30 seconds). HPSD ablation can reduce convective heating as well as subsequent collateral damage,6) also, HPSD leads to the formation of wider and shallow lesions,7) which makes it suitable for use in thin atrial walls, especially in the posterior wall. HPSD ablation also has advantages over conventional low power, long-duration ablation strategy in terms of shorter procedure time, less radiation exposure, and shorter RF application time.8),9),10) Most studies on the application of HPSD ablation in AF ablation were conducted in Western countries, and data on the safety of HPSD in Asian populations are limited. We thus evaluated the incidence and pattern of esophageal injury in Asian patients undergoing AF ablation with high-power (50 W), short-duration RF energy.
METHODS
Patient population
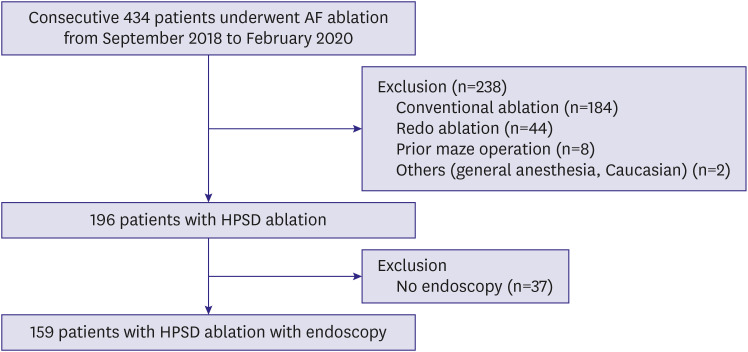
This single-center, prospective observational study involved consecutive patients with drug-refractory, symptomatic atrial fibrillation who received their first RF catheter ablation (RFCA) at Asan Medical Center (Seoul, Republic of Korea) between September 2018 and February 2020. Patients with previous surgical- or catheter-based AF ablation and those who did not undergo endoscopic evaluations after the index RF ablation for AF were excluded (Figure 1). Written informed consent for data collection was obtained from all patients. This study was approved by the Institutional Review Board of Asan Medical Center (2020-0464).
Figure 1. Study flow diagram for inclusion and exclusion.
AF = atrial fibrillation; HPSD = high-power, short-duration.
Atrial fibrillation radiofrequency catheter ablation
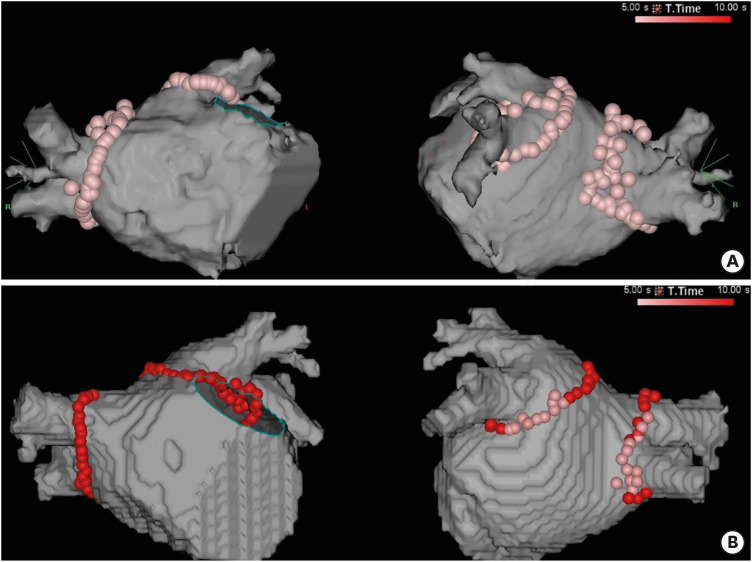
AADs were discontinued for at least 5 half-lives before the procedure. Transesophageal echocardiography (TEE) was performed to exclude left atrium (LA) thrombus in 88 patients. Majority of patients underwent contrast-enhanced magnetic resonance (MR) imaging (n=114) or computed tomography (CT) (n=26, due to claustrophobia, presence of pacemaker/defibrillator, etc.) for evaluation of the anatomy of the LA and PVs and exclusion of the LA appendage thrombus before the procedure. Remifentanil with/without midazolam or dexmedetomidine was used for sedation/analgesia. General anesthesia was used in none of patients. Uninterrupted oral anticoagulation was administered in all patients. Of the 71 patients who did not undergo TEE, 27 patients have received oral anticoagulation for more than 1 year. Mean duration of anti-coagulation of the remaining 44 patients were 166 days and all patients underwent uninterrupted anticoagulation during minimum 38 days. Supplementary unfractionated heparin was administered to maintain the activated clotting time (ACT) at above 300 seconds. Venous access and catheterization were performed as previously described.11) Transseptal catheterization was performed under the guidance of hemodynamic monitoring, fluoroscopy, and intracardiac echocardiography (SOUNDSTAR catheter; Biosense Webster, Diamond Bar, CA, USA). Two 8 Fr SL1 sheaths (St. Jude Medical, St. Paul, MN, USA) were advanced into LA to handle a mapping catheter and an ablation catheter, respectively. Three-dimensional electroanatomic geometry of the LA was created using a multipolar mapping catheter (PentaRay; Biosense Webster). Voltage and activation mappings of LA were performed during constant pacing from the right atrium or distal coronary sinus (CS) at a pacing cycle length of 500–600 ms. All patients underwent PV isolation (PVI) using a 3.5 mm irrigated tip contact-force sensing RF ablation catheter (Thermocool smarttouch SF bidirectional navigation catheter; Biosense Webster). The point-by-point RF applications were delivered using the power-controlled mode, a temperature limited to 42°C, and normal saline irrigation (15 mL/min). For each point, RF energy was delivered with a power output of 50 W for 5 seconds initially. Due to high recurrence rate within blanking period, we prolonged the RF time to 10 seconds at the anterior wall and 6 seconds at the posterior wall. Automated lesion tagging (VisiTag™; Biosense Webster) was used to mark the location of each lesion. The VisiTag setting was as follows: catheter stability range of motion 3 mm during 3 seconds and minimum contact force >5 g over time of 50%. A tag size of 2 was used and further ablation was delivered to close the visual gap (Figure 2). The contact force in the posterior wall was maintained between 5–10 g. Entrance block into the PVs was demonstrated by the absence of PV potentials distal to the ablation line during constant pacing from the right atrium or distal CS. And the entrance block was also visualized by performing post-PVI voltage mapping of LA and PVs with inferior cut-off voltage of 0.05 mV. Additional anatomical ablations (LA roof/posterior wall, mitral isthmus, cavotricuspid isthmus, and/or superior vena cava) were performed at the discretion of the operator. The endpoint of the linear lesions was bidirectional block as confirmed by conventional pacing maneuvers.12),13) Isoproterenol infusion (5–10 μg/min) with burst atrial pacing was carried out after the ablations to induce non-PV triggers or atrial tachyarrhythmia.
Figure 2. Representative examples of HPSD ablation. (A) shows an example of a patient who underwent PVI using the initial 5-second protocol. Automated lesion tagging with VisiTag (size of 3) was used. (B) shows an example of a patient who underwent PVI using the 6–10 seconds protocol. RF energy was delivered with 50 W for 10 seconds in the anterior and superior wall and 6 seconds in the posterior wall. A tag size of 2 was used, and further ablation was delivered to close the visual gap.
HPSD = high-power, short-duration; PVI = pulmonary vein isolation; RF = radiofrequency.
Endoscopic evaluation of the esophagus
Endoscopic examination of the upper gastrointestinal tract was performed under conscious sedation by an experienced gastroenterologist on the day after the AF ablation procedure. Esophageal lesion was classified based on the following endoscopic appearance: 1) erythema/erosion (without disruption of mucosa), 2) superficial ulcer involving only the mucosa, or 3) deep ulcer involving up to the muscularis externa.14) All patients received proton pump inhibitor (PPI) for 4 weeks after the ablation. In the case of esophageal injury, the management plans and determining whether and when to perform follow-up endoscopy were made in consultation with gastroenterologists. The primary outcome of this study is the occurrence of thermal injury of the esophagus detected by endoscopic evaluation after AF ablation using HPSD RF energy. Before discharge, the patients were informed on the possible symptoms associated with AEF (e.g., fever, chest pain, any neurologic symptom, hematemesis) and possible actions that should be taken (e.g., visiting a doctor's office or emergency room, taking chest CT, avoiding endoscopy).
Post-ablation follow-up
During the index hospitalization after RFCA, 12-lead electrocardiogram (ECG) and telemetry monitoring were performed daily and the recurrence of atrial arrhythmia was screened. The follow-up visits to the outpatient clinics were scheduled at 1, 3, 6, and 12 months after the RFCA and then every 6 months thereafter. At each visit, 12-lead ECG was performed; 24-hours Holter monitoring was carried out at discharge and at 3, 6 and 12 months post-RFCA. Recurrence was defined by an episode of AF or atrial tachycardia (AT) lasting at least 30 seconds after the 3-month blanking period. If the arrhythmic recurrence was suspected, further Holter or event recording test was performed. The use of AADs after index ablation was determined at the physician's discretion.
Statistical analysis
All continuous variables were compared by Student's t-test and are reported as mean±standard deviation. Categorical variables were compared with the χ2 test or Fisher's exact test as appropriate and are expressed as percentages. The cumulative probability and survival curves were constructed from Kaplan-Meier estimates and compared by the log-rank test. The p values <0.05 were considered statistically significant. All statistical analysis was performed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
During the study period, 196 patients with atrial fibrillation underwent their first RFCA using 50 W of RF energy at our hospital. After excluding 37 patients who did not undergo upper GI endoscopy evaluation after the AF ablation, 159 patients were included in the study (Figure 1). The baseline clinical characteristics of the entire population are shown in Table 1. The mean age was 63±9 years, and 68.6% were male. Paroxysmal, persistent, and long-standing persistent AF were observed in 72 (45.3%), 44 (27.7%) and 43 (27.0%) patients, respectively. The mean LA anteroposterior diameter measured by echocardiography was 44.4±6.7 mm.
Table 1. Baseline characteristics.
| Characteristics | Total (n=159) |
|---|---|
| Age (years) | 63±9 |
| Body weight (kg) | 71.2±11.4 |
| Body mass index (kg/m2) | 25.9±3.4 |
| Male | 109 (68.6) |
| Paroxysmal AF | 72 (45.3) |
| Persistent AF | 44 (27.7) |
| Long-standing persistent AF | 43 (27.0) |
| CHA2DS2-VASc score | 1.9±1.4 |
| Hypertension | 74 (46.5) |
| Diabetes mellitus | 29 (18.2) |
| Previous stroke | 14 (8.8) |
| Chronic renal insufficiency* | 34 (21.4) |
| Heart failure | 37 (23.3) |
| Valvular heart disease | 6 (3.8) |
| Coronary artery disease | 15 (9.4) |
| Anticoagulation with NOAC | 146 (91.8) |
| Anticoagulation with warfarin | 13 (8.2) |
| B-type natriuretic peptide (pg/mL) | 198±393 |
| LA diameter (mm) | 44±7 |
| LV ejection fraction (%) | 56±9 |
Data are shown as mean±standard deviation or number (%).
AF = atrial fibrillation; LA = left atrium; LV = left ventricle; NOAC = non-vitamin K antagonist oral anticoagulant.
*Estimated glomerular filtration rate <60 mL/min/1.73 m2.
Atrial fibrillation ablation procedure
PVI was successfully achieved by anatomical PV antral ablation in all patients. Additional RF ablations beyond PVI were performed in 120 (75.5%) patients (Table 2). Linear ablations of the LA roof, left lateral mitral isthmus, cavotricuspid isthmus, and/or LA posterior wall isolation was performed in 62 (39.0%), 49 (30.8%), 61 (38.4%) and/or 40 patients (25.2%), respectively. Of the 49 patients who underwent ablation for the left lateral mitral isthmus, epicardial ablation inside the CS was required in 34 (69.4%) patients to achieve bidirectional block of the mitral isthmus. Three patients underwent slow pathway ablation due to the induction of typical atrioventricular nodal tachycardia during the index procedure.
Table 2. Procedural characteristics.
| Characteristics | Total (n=159) | |
|---|---|---|
| Procedure time (minutes) | 155.5±43.4 | |
| Ablation time (minutes) | 34.3±14.4 | |
| Fluoroscopy time (minutes) | 8.6±4.8 | |
| DAP during procedure (mcGym2) | 268.8±337.6 | |
| Initial RF protocol (5 seconds) | 26 (16.4) | |
| Prolonged RF time (6–10 seconds) | 133 (83.6) | |
| Ablation lesions | ||
| Pulmonary vein isolation | 159 (100.0) | |
| LA roof line | 62 (39.0) | |
| Posterior wall isolation | 40 (25.2) | |
| Cavotricuspid isthmus | 61 (38.4) | |
| Mitral isthmus | 49 (30.8) | |
| Superior vena cava | 46 (28.9) | |
Data are shown as mean±standard deviation or number (%).
DAP = dose area product; LA = left atrium; RF = radiofrequency.
Acute pulmonary vein reconnection
Acute PV reconnection during a 20-minute waiting period occurred in 50/159 (31.4%) patients with the initially successful PVI. Acute reconnection of left, right PVs and bilateral PVs were observed in 33, 10, and 7 patients, respectively. Eleven patients (11/50, 22.0%) showed PV reconnections at multiple PVs. Patients underwent PVI with 5 seconds ablation protocol showed significantly higher rate of acute reconnection than the patients with 6–10 seconds ablation protocol (14/26 [53.8%] vs 36/133 [27.1%], p=0.007). Isoproterenol infusion test was done in 109 patients of all patients and PV reconnection was observed in 11 patients (10.1%). Higher rate of reconnection after isoproterenol infusion was also observed in patients with 5 seconds ablation protocol (7/26 [28.0%] vs. 4/84 [4.8%], p=0.003). All reconnected PVs were re-isolated finally.
Endoscopic findings
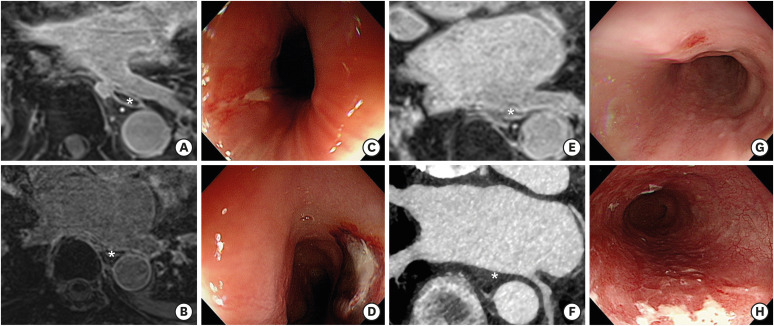
Out of the 159 patients, 4 (2.5%) had esophageal thermal injury and their characteristics are summarized in Table 3. Two (1.3%) patients had superficial esophageal ulcer and another 2 (1.3%) patients had erythematous erosion of the esophagus. None of these patients had gastrointestinal symptoms including indigestion, dysphagia, and chest pain. One of the patients with esophageal ulcer had a longitudinal ulcer of 1 cm in length at 27 cm from the upper incisor (Figure 3). Chest CT did not show mural thickening, defect along the esophagus, or evidence of pneumomediastinum. Follow-up endoscopy at 1 month after the initial endoscopy showed healed state of the esophageal ulcer. Patients with esophageal injury were older (75±6 vs. 63±9, p=0.015) and had higher CHA2DS2-VASc scores (3.8±2.1 vs. 1.9±1.3, p=0.007) than those without thermal lesions of the esophagus. Other baseline or procedural characteristics were not significantly different according to the presence of thermal injury. No significant difference of RF delivery time was observed between patients with and without esophageal injury (esophageal injury [+]: 36.9±13.4 minutes vs. esophageal injury [−]: 34.2±14.4 minutes, p=0.712). And other procedural characteristics, including procedural time, ablation lesion set and applied RF protocol (5 seconds vs. 6–10 seconds), were not different between groups. Among the 4 patients with esophageal injury, 1 patient (patient #1) underwent PV isolation using initial RF protocol (5 seconds) and the other 3 patients (patient #2–4) underwent PVI using prolonged RF protocol (6–10 seconds).
Table 3. Summary of patients with esophageal complications.
| Patient | Sex/age | AF type | CHA2DS2-VASc | Comorbidity | Preprocedural TEE | RF lesion | RF time, min | Esophageal injury | PPI treatment |
|---|---|---|---|---|---|---|---|---|---|
| #1 | M/80 | PeAF | 5 | HTN, prior stroke | Yes | PVI + SVC isolation | 24.6 | Superficial ulcer | Lansoprazole 60 mg, 1 month |
| #2 | F/79 | LsPeAF | 6 | HF, HTN, DM, CKD | No | PVI + SVC isolation | 27.9 | Erosion | Esomeprazole 40 mg, 1 month |
| #3 | M/73 | PeAF | 2 | Prior HF, CKD, COPD | No | PVI + LA roof | 41.3 | Erosion | Lansoprazole 30 mg, 1 month |
| #4 | M/66 | LsPeAF | 2 | HF, HCMP | No | PVI + LA roof + MI | 53.8 | Superficial ulcer | Esomeprazole 40 mg, 1 month |
AF = atrial fibrillation; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; F = female; HCMP = hypertrophic cardiomyopathy; HF = heart failure; HTN = hypertension; LA = left atrium; LsPeAF = long-standing persistent atrial fibrillation; M = male; MI = mitral isthmus; PeAF = persistent atrial fibrillation; PPI = proton pump inhibitor; PVI = pulmonary vein isolation; RF = radiofrequency; SVC = superior vena cava; TEE = transesophageal echocardiography.
Figure 3. MR images, computed tomographic images, and endoscopic findings of patients with esophageal complications. (A, B) show the MR images of LA, LIPV, and adjacent esophagus (marked with an asterisk) of patients with an esophageal ulcer. (C, D) show the endoscopic findings of superficial esophageal ulcers located at 27 cm and 34 cm from the upper incisor, respectively. (E, F) show the CT images of the esophagus (marked with an asterisk) adjacent to LIPV and posterior wall of LA in patients with esophageal erosion. (G, H) are endoscopic findings of esophageal erosions.
CT = computed tomography; LA = left atrium; LIPV = left inferior pulmonary vein; MR = magnetic resonance.
Food stasis, a suggestive finding of gastroparesis, was observed in 25 (15.7%) patients; among them, 3 patients had clinical symptoms of indigestion, one of whom required conservative treatment with a prokinetic agent. All 3 patients with indigestion showed symptom improvement within a month after the AF ablation procedure. There was no statistically significant predictor for the occurrence of food stasis in this study population.
Other complications
During the periprocedural period, there were no major procedure-related complications such as cardiac tamponade, phrenic nerve palsy, stroke, or death. All patients completed the outpatient clinic visits as scheduled with a median follow-up duration of 6.2 months (interquartile range [IQR], 3.4–8.7). No patients reported symptoms suggestive of AEF beyond 3 months after the index procedure. None of the patients who did not undergo endoscopy had symptoms or signs suggestive of AEF.
Recurrence of atrial tachyarrhythmia
Among the total patient cohort, 133 patients (paroxysmal AF, n=57 [42.9%]; persistent AF, n=39 [29.3%]; long-standing persistent AF, n=37 [27.8%]) were followed-up over the blanking period of 3 months after the index procedure. During a median follow-up of 193 days (IQR, 117–283), 35 (26.3%) patients showed recurrence of AF (n=23 [65.7%]) or AT (n=12 [34.3%]) after a single RF ablation.
DISCUSSION
In this study on 159 consecutive Asian patients who underwent HPSD ablation for AF, we found that the incidence of esophageal erosion and ulcer were both 1.3%. Patients who developed esophageal injury were older and had a higher CHA2DS2-VASC score than those who did not. There were no other major complications such as cardiac tamponade, esophageal perforation, AEF, stroke, or death during the periprocedural and follow-up periods. Thus, in our cohort, AF ablation using the high-power (50 W), short-duration strategy was not significantly associated with major procedural risks.
Of the possible complications of RF ablation for AF occurring in the form of esophageal injury, AEF is the most severe type with a high fatality rate. AEF is estimated to occur in 0.02% to 0.15% of patients undergoing conventional RF ablation for AF.2),15) The HPSD strategy during AF ablation emerged from the biophysical knowledge that the occurrence of collateral damage could be prevented by reducing the conductive heating of deep tissues.16) After Bunch and Day6) reported that no esophageal injury occurred in more than 1,000 AF ablation procedures using a 50 W RF energy with very short duration, several others have consistently demonstrated the safety of HPSD ablation.9),17) Particularly, in a multicenter study on 11,436 AF ablations, there was only one (0.0087%) case of AEF.9) Compared with the incidence of AEF in conventional RF ablation,2) the risk of AEF seems to be lower in HPSD.6),9)
Data in the East Asian population regarding the incidence of AEF are limited, but three recent studies reported the incidence of AEF from 0.06% to 0.15%.2),18),19) Kim et al.2) suggested that East Asian patients could be more vulnerable to AEF formation due to the lower body weight compared with Western patients. This hypothesis led to the clinical reasoning that the HPSD strategy for AF ablation might be more useful in the East Asian population.
Our findings on the low incidence and mild severity of esophageal injury are in line with the results reported by other studies on HPSD ablation.20),21),22) Previous studies regarding the esophageal injury in conventional AF ablation reported the incidence of esophageal ulcer from 2.9% to 14.6%.3),23),24) The incidence of esophageal ulcer in the HPSD method also appears to be lower than that in the conventional method, although the incidence varied between studies.
Food stasis was observed in 25 (15.7%) patients, of whom only 3 had clinical symptoms of indigestion that were recovered within a month with or without the aid of prokinetic agents. Similar to the incidence of food stasis in our findings, a study on endoscopic examination after AF ablation in 425 patients reported that the incidence of gastric food retention was 17%.25) One study reported that the injury of periesophageal vagal plexus was a predictor of favorable outcomes26); however, we did not find a significant association between the occurrence of food stasis and arrhythmic recurrence.
There are several limitations to our study. First, this is a single-center, single-arm, observational study with a relatively small number of patients. All procedures were performed by a single operator (JK) with an experience of >1,000 cases of AF ablation. Considering low incidence of esophageal thermal injury, a randomized clinical trial comparing HPSD vs conventional ablation in terms of safety superiority requires 3,000–10,000 patients, which is practically hard to conduct. Second, we did not perform esophageal temperature monitoring during the ablation procedure because multi-sensor esophageal temperature probes were not available during the study period. Thermal latency is a well-known phenomenon after RF energy delivery, and to prevent the possible overheating of tissues, we waited more than 10 seconds between contiguous lesions, especially for those along the posterior wall.27) Also, there is controversy on whether real-time monitoring of the esophageal luminal temperature plays a significant role in preventing esophageal injury during AF ablation.28),29) Safety of HPSD ablation without esophageal temperature monitoring in our study in line with other study.30) Third, we did not extend the ablation duration to meet a certain value of the novel index such as the ablation index. Ablation index-guided ablation using 50 W requires a longer duration of RF energy than that used in our study, which may change the study outcomes. And we stopped performing an endoscopy routinely after interim analysis due to low incidence rate of mild damage of esophagus. Before the time of interim analysis, 11 patients did not undergo endoscopy due to refusal and they had no symptoms or signs suggestive of AEF during the regular follow-up visits. Lastly, we did not measured the distance between esophagus and LA on the MR/CT imaging. Further evaluation of the association between the minimal distance to esophagus and esophageal thermal injury could be helpful for understanding and prevention of this esophageal complication. In conclusion, AF catheter ablation using high-power (50 W), short-duration RF applications could be safely performed and resulted in very low rates of esophageal complications, all of which were mild in nature in Asian population. Further studies evaluating the long-term clinical outcomes of the novel ablation technique are needed to confirm its efficacy.
Footnotes
Funding: JK received honoraria from Medtronic, Biosense-Webster, and BostonScientific and research funding from Boston Scientific.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Do U, Kim J.
- Data curation: Do U, Kim J, Kim M, Cho MS, Nam GB, Choi KJ, Kim YH.
- Formal analysis: Do U, Kim J.
- Investigation: Do U, Kim J.
- Methodology: Do U, Kim J.
- Project administration: Kim J.
- Software: Do U.
- Supervision: Kim J, Nam GB, Choi KJ, Kim YH.
- Writing - original draft: Do U, Kim J.
- Writing - review & editing: Do U, Kim J.
References
- 1.Han HC, Ha FJ, Sanders P, et al. Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10:e005579. doi: 10.1161/CIRCEP.117.005579. [DOI] [PubMed] [Google Scholar]
- 2.Kim YG, Shim J, Kim DH, et al. Characteristics of atrial fibrillation patients suffering atrioesophageal fistula after radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2018;29:1343–1351. doi: 10.1111/jce.13671. [DOI] [PubMed] [Google Scholar]
- 3.Martinek M, Bencsik G, Aichinger J, et al. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol. 2009;20:726–733. doi: 10.1111/j.1540-8167.2008.01426.x. [DOI] [PubMed] [Google Scholar]
- 4.Leite LR, Santos SN, Maia H, et al. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011;4:149–156. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Romero J, Stevenson WG, et al. Impact of lowering irrigation flow rate on atrial lesion formation in thin atrial tissue: preliminary observations from experimental and clinical studies. JACC Clin Electrophysiol. 2017;3:1114–1125. doi: 10.1016/j.jacep.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Bunch TJ, Day JD. Novel ablative approach for atrial fibrillation to decrease risk of esophageal injury. Heart Rhythm. 2008;5:624–627. doi: 10.1016/j.hrthm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskaran A, Chik W, Pouliopoulos J, et al. Five seconds of 50–60 W radio frequency atrial ablations were transmural and safe: an in vitro mechanistic assessment and force-controlled in vivo validation. Europace. 2017;19:874–880. doi: 10.1093/europace/euw077. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson B, Chen X, Pehrson S, Svendsen JH. The effectiveness of a high output/short duration radiofrequency current application technique in segmental pulmonary vein isolation for atrial fibrillation. Europace. 2006;8:962–965. doi: 10.1093/europace/eul100. [DOI] [PubMed] [Google Scholar]
- 9.Winkle RA, Mohanty S, Patrawala RA, et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019;16:165–169. doi: 10.1016/j.hrthm.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Bunch TJ, May HT, Bair TL, et al. Long-term outcomes after low power, slower movement versus high power, faster movement irrigated-tip catheter ablation for atrial fibrillation. Heart Rhythm. 2020;17:184–189. doi: 10.1016/j.hrthm.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Nam GB, Go TH, et al. Alternative strategies to improve success rate of mitral isthmus block. Medicine (Baltimore) 2018;97:e13060. doi: 10.1097/MD.0000000000013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 13.Hocini M, Jaïs P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- 14.Yarlagadda B, Deneke T, Turagam M, et al. Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm. 2019;16:204–212. doi: 10.1016/j.hrthm.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leshem E, Zilberman I, Tschabrunn CM, et al. High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol. 2018;4:467–479. doi: 10.1016/j.jacep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Winkle RA, Mead RH, Engel G, Patrawala RA. Atrial fibrillation ablation: “perpetual motion” of open irrigated tip catheters at 50 W is safe and improves outcomes. Pacing Clin Electrophysiol. 2011;34:531–539. doi: 10.1111/j.1540-8159.2010.02990.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Zhang YY, Ye Q, et al. Characteristics of atrial fibrillation patients suffering esophageal injury caused by ablation for atrial fibrillation. Sci Rep. 2020;10:2751. doi: 10.1038/s41598-020-59539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaji T, Shizuta S, Morimoto T, et al. Very long-term clinical outcomes after radiofrequency catheter ablation for atrial fibrillation: a large single-center experience. Int J Cardiol. 2017;249:204–213. doi: 10.1016/j.ijcard.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Marrouche NF, Guenther J, Segerson NM, et al. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrum isolation: procedural parameters, outcomes, and the effect on esophageal injury. J Cardiovasc Electrophysiol. 2007;18:583–588. doi: 10.1111/j.1540-8167.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Schmidt B, Bordignon S, et al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electrophysiol. 2019;30:2724–2731. doi: 10.1111/jce.14219. [DOI] [PubMed] [Google Scholar]
- 22.Castrejón-Castrejón S, Martínez Cossiani M, Ortega Molina M, et al. Feasibility and safety of pulmonary vein isolation by high-power short-duration radiofrequency application: short-term results of the POWER-FAST PILOT study. J Interv Card Electrophysiol. 2020;57:57–65. doi: 10.1007/s10840-019-00645-5. [DOI] [PubMed] [Google Scholar]
- 23.Halm U, Gaspar T, Zachäus M, et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105:551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 24.Halbfass P, Berkovitz A, Pavlov B, et al. Incidence of acute thermal esophageal injury after atrial fibrillation ablation guided by prespecified ablation index. J Cardiovasc Electrophysiol. 2019;30:2256–2261. doi: 10.1111/jce.14193. [DOI] [PubMed] [Google Scholar]
- 25.Knopp H, Halm U, Lamberts R, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11:574–578. doi: 10.1016/j.hrthm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Jhuo SJ, Lo LW, Chang SL, et al. Periesophageal vagal plexus injury is a favorable outcome predictor after catheter ablation of atrial fibrillation. Heart Rhythm. 2016;13:1786–1793. doi: 10.1016/j.hrthm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Irastorza RM, d'Avila A, Berjano E. Thermal latency adds to lesion depth after application of high-power short-duration radiofrequency energy: results of a computer-modeling study. J Cardiovasc Electrophysiol. 2018;29:322–327. doi: 10.1111/jce.13363. [DOI] [PubMed] [Google Scholar]
- 28.Black-Maier E, Pokorney SD, Barnett AS, et al. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14:1328–1333. doi: 10.1016/j.hrthm.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Schoene K, Arya A, Grashoff F, et al. Oesophageal probe evaluation in radiofrequency ablation of atrial fibrillation (OPERA): results from a prospective randomized trial. Europace. 2020;22:1487–1494. doi: 10.1093/europace/euaa209. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Schmidt B, Seegeer A, et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation: with or without esophageal temperature probe? Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]