Abstract
Leishmaniasis is a parasitic disease caused by the obligatory intracellular protozoa Leishmania spp. Current therapeutic options are limited and thus, drug discovery against leishmaniasis is very important. Nevertheless, there is a great difficulty to develop therapeutic strategies against the disease because the parasite deploys various mechanisms to evade the immune system and multiply inside the host. Among the main factors of the immunity that are recruited to confront the Leishmania infection are the macrophages (MΦs) that produce effector molecules such as Nitric Oxide (NO) and Reactive Oxygen Species (ROS). Therefore, efficient drug agents should combine the antileishmanial effect of these gaseous transmitters along with the enhancement of the host’s adaptive immunity. In the quest of therapeutic alternatives, natural products have been extensively studied and are considered as candidate antileishmanial agents since they exhibit specific properties in modulating the host’s immune response towards an effective anti-leishmanial cell-mediated immunity capable to eliminate parasitic dissemination. In the current protocol, Leishmania-infected MΦs (J774A.1 cell line) that have been treated with various increasing concentrations of a natural compound, are tested for the production of the aforementioned molecules. In order to detect NO production, we employ the Griess colorimetric nitrite assay and quantification relies on the construction of an accurate standard curve using appropriate standards of known concentration. ROS detection and quantification is achieved by flow cytometry and relies on the use of carboxy-H2DCFDA, an indicator that converts to a fluorescent form when interacts with ROS molecules.
Keywords: Nitric Oxide, Reactive Oxygen Species, Innate immunity, Immune mechanisms, Natural products, Leishmania spp. , Macrophages
Background
Macrophages (MΦs) are important innate immune effector cells that participate in host defense, providing enhanced antimicrobial activity. They are characterized by a remarkable plasticity and unique abilities to polarize toward different phenotypes. On one hand, classically activated macrophages are known to have major roles in host defense against various microbial pathogens, while alternatively activated macrophages are instrumental in immune regulation and wound healing (Leopold Wager and Wormley, 2014). The term “classically” activated is used to designate the effector macrophages that are produced during cell-mediated immune responses upon the dual signal of interferon-γ (IFN-γ) and tumor-necrosis factor (TNF), characterized by enhanced microbicidal or tumoricidal capacity and secretion of high levels of pro-inflammatory cytokines and immune mediators. More specifically, IFN-γ is produced by innate (NK cells) or adaptive immune cells (T helper 1 cells) and primes macrophages to secrete pro-inflammatory cytokines and to produce increased amounts of superoxide anions, oxygen and nitrogen radicals that increase their killing ability ( Dale et al., 2008 ).
The obligatory intracellular parasites of the genus Leishmania have deployed sophisticated mechanisms to evade and modulate the host’s immune system for their benefit and consequently, they are able to survive and persist within MΦs. Consequently, in the case of Leishmania infection, MΦs are having a dual role either as effector cells with leishmanicidal activity or as host cells. The fate of Leishmania spp. parasites is determined by the activation status of MΦs (Tomiotto- Pellissier et al., 2018 ). “Classically” activated MΦs are capable of killing parasites effectively via the production of Nitric Oxide (NO) upon activation of inducible nitric oxide synthase (iNOS) and other leishmanicidal molecules, such as Reactive Oxygen Species (ROS). NO and ROS are two key players in the macrophage defense system against intracellular parasites ( Horta et al., 2012 ; Roy et al., 2017 ). Nevertheless, Leishmania spp. parasites have been adapted to survive and replicate in this hostile environment by deploying antioxidant systems or by suppressing ROS and NO production ( Kumar et al., 2001 ; Denkers and Butcher, 2005; Gupta et al., 2013 ).
In recent years, there is an increasing interest in the study of natural products against leishmaniasis because several plant extracts or isolated compounds exhibit a promising antileishmanial activity which may not only be due to their direct action on parasite but also to a concomitant effect on the host’s immune response ( Rodrigues et al., 2015 ). Several natural products have been tested for their ability to increase the production of ROS and/or NO in in vitro and in vivo experimental models of leishmaniasis (Torres- Santos et al., 1999 ; do Socorro et al., 2003 ; Georgopoulou et al., 2007 ; Patricio et al., 2008 ).
In this protocol, we aim to quantify the NO and ROS production by Leishmania spp.-infected J774A.1 macrophages as a response to treatment with various increasing concentrations of Total Phenolic Fraction (TPF) derived from Extra Virgin Olive Oil ( Angelis et al., 2017 ). The quantification of NO is achieved with the use of Griess assay which relies on the accumulation of nitrites in cell culture supernatants. Respectively, the relative quantification of TPF-mediated ROS generation is determined with the use of cell-permeable carboxy-H2DCFDA fluorescent probe which is deacetylated by cellular esterases to form 2',7'-dichlorodihydrofluorescein (H2DCF). In the presence of ROS, predominantly H2O2, H2DCF is rapidly oxidized to 2',7'-dichlorofluorescein (DCF), which is highly fluorescent, with excitation and emission wavelengths of 498 and 522 nm, respectively ( Bae et al., 2000 ). These protocols can be used to gain a first in vitro evidence, about whether or not, these immune mechanisms are activated by an antimicrobial agent.
Materials and Reagents
Cell Culture Flasks with filter cap, 25 cm2 (Thermo Fisher Scientific, catalog number: 156367)
Cell culture Flasks, plug seal cap, 25 cm2 (Greiner Bio-One, catalog number: 690160)
Cell scrapers (Sarstedt, catalog number: 83.1830)
Cover glasses square (VWR, catalog number: 6311570)
Falcon® Round-Bottom Tubes, 5 ml (Corning, catalog number: 352008)
96-well flat bottom tissue culture plates (Sarstedt, catalog number: 83.3924.005)
24-well flat bottom tissue culture plates (Sarstedt, catalog number: 83.3922.005)
Pipettes tips: 0.5-10 μl, 10-200 μl, 200-1,000 μl (Greiner Bio-One, catalog numbers: 771291, 739290, 740290)
Pleated filter paper (Sigma-Aldrich, catalog number: WHA1201150)
Reaction tubes, 1.5 ml (Greiner Bio-One, catalog number: 616201)
Serological pipettes 2 ml, 5 ml, 10 ml (Sarstedt, catalog numbers: 86.1252.001, 86.1253.001, 86.1254.001)
Sterile syringe filter 0.22 μm (Millipore, catalog number: SLGVV255F)
Syringes 5 ml (Terumo, catalog number: SS+05S21381)
Cell lifter (Corning, catalog number: 3008)
Leishmania infantum promastigotes (zymodeme GH8, strain MHOM/GR/2001/GH8)
Leishmania major promastigotes (zymodeme LV39, strain MRHO/SU/59/P)
Immortalized macrophage cell line J774A.1 (ATCC; Rockville, USA/ ATCC No: TIB-67)
Total Phenolic Fraction (derived from Extra Virgin Olive Oil from agricultural cooperative in Zaros region, Crete, Greece)
RPMI-1640 (w/o) L-glutamine (Biowest, catalog number: L0501)
Fetal Bovine Serum (Biowest, catalog number: S181B)
L-glutamine (Biosera, catalog number: LM-R1641)
HEPES buffer 1 M (Biowest, catalog number: L0180)
Penicillin-Streptomycin solution 10,000 U/ml (Biowest, catalog number: L0022)
Dimethyl sulfoxide (DMSO) Cell culture grade (PanReac Applichem, catalog number: A3672,0050)
Ethanol absolute (Sigma-Aldrich, catalog number: 32205-M)
Formalin (Sigma-Aldrich, catalog number: R04586-82)
Trypan blue dye for vital staining (BDH, catalog number: 34078)
Milteforan® 20 mg/ml (Hexadecylphosphocholine, [HePC], Virbac S.A.)
Hydrochloric acid (HCl) solution, 1 M (Sigma-Aldrich, catalog number: 150696)
Sodium chloride 99.9% (NaCl) (Applichem, catalog number: 381659)
Potassium chloride (KCl), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 746336)
Sodium phosphate dibasic (Na2HPO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795410)
Potassium phosphate monobasic (KH2PO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795488)
Sodium hydroxide (NaOH) solution, 1 M (Sigma-Aldrich, catalog number: 79724)
Carboxy-H2DCFDA (Thermo Fisher Scientific, catalog number: C400)
Hydrogen Peroxide solution 30% (w/w) (Sigma-Aldrich, catalog number: H1009)
ortho-Phosphoric acid 85% (AppliChem, catalog number: A0989)
Sulfanilamide (Sigma-Aldrich, catalog number: S9251-100G)
N-(1-Naphthyl) ethylenediamine dihydrochloride (Sigma-Aldrich, catalog number: 222488)
Sodium nitrite (NaNO2) (Sigma-Aldrich, catalog number: 237213)
Complete RPMI-1640 medium (see Recipes)
Fetal Bovine Serum solution (FBS) (see Recipes)
Phosphate Buffer Saline (PBS), 10x, pH 7.2-7.4 (see Recipes)
0.4% (w/v) Trypan blue exclusion dye (see Recipes)
Sodium nitrite (NaNO2) solution (see Recipes)
Griess reagent (see Recipes)
H2O2 solution (see Recipes)
Equipment
New BrunswickTM Galaxy® 170 S CO2 Incubator (Eppendorf, catalog number: Galaxy 170 S)
Refrigerated Incubator 26 °C (Sanyo, catalog number: MIR-253)
BD FACSCalibur Flow Cytometer: 3-Color (BD FACSCaliburTM, catalog number: 342973)
ELISA Microplate reader (Dynatech Laboratories, catalog number: MRX)
Optical microscope (Olympus, catalog number: BHB)
Sterile biosafety cabinet (Telstar, catalog number: Bio-II-A)
Gilson pipettes (Gilson, catalog numbers: PIPETMAN Classic P-10, P-20, P-200, P-1000)
Malassez counting chamber (Paul Marienfeld GmbH & Co., catalog number: 0640610)
Multichannel pipette (Brand, catalog number: 703710)
pH Meter (Thermo Fisher Scientific, catalog number: 13-644-928)
Pipette controller (Brand, catalog number: accu-jet® pro 26300)
Water distiller (Sartorius, catalog number: H2O-I-1-UV-T)
Software
Microsoft® Office Excel 2010 (Microsoft)
FlowJo, version 10 (BD)
Procedure
-
In vitro quantification of nitric oxide production by Leishmania-infected J774A.1 macrophage treated with increasing concentrations of TPF
-
Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Note: The recovery of TPF from extra virgin olive oil was carried out by Centrifugal Partition Extraction (CPE) technique, which is an innovative solid support free separation technique derived from Centrifugal Partition Chromatography (CPC). Briefly, liquid-liquid chromatography was performed using a laboratory scale centrifugal partition extractor FCPE300®, which was equipped with a rotor composed of 7 stacked partition disks engraved with a total of 231 partition cells, while the total volume of the column was 300ml (Angelis et al., 2017; Koutsoni et al., 2018).
Cultivate J774A.1 macrophage in 25 cm2 cell culture flasks with filter cap containing 10 ml of complete RPMI-1640 medium (Recipe 1) at 37 °C under 5% CO2 humidified air.
-
Allow macrophages to reach a high density population of about 70% confluence.
Note: J774A.1 macrophages cultured in complete RPMI-1640 medium at 37 °C under 5% CO2 humidified air, usually reach 70% confluence within 3 to 4 days.
Then, remove the majority of culture medium with a sterile disposable transfer pipette and leave about 2 ml medium in the flask and detach macrophage monolayer by scrapping cells gently and slowly with a cell scraper at 45° angle.
-
Determine the number of macrophages per ml by differential counting of dead and live cells using the Trypan blue exclusion dye (Recipe 4) in a Malassez counting chamber under an optical microscope.
Note: The final dilution of macrophages in Trypan blue dye depends on the density of the culture. Usually, a 1:20 final dilution of macrophages is suitable at the 3rd or 4th day of the in vitro culture.
Seed 5 x 104 J774A.1 macrophages per well in a final volume of 100 μl of complete RPMI-1640 medium in a 96-well flat bottom tissue culture plate under a sterile biosafety cabinet.
Incubate the plate for 18 h at 37 °C under 5% CO2 humidified air in order to achieve cell adhesion.
-
Determine the number of Leishmania spp. promastigotes per ml (promastigotes must be in their stationary phase of growth) by differential counting of dead and live parasites using the Trypan blue exclusion dye in a Malassez counting chamber under an optical microscope.
Notes:
Leishmania spp. promastigotes usually enter the stationary-growth phase after 3 to 5 days, depending on the Leishmania strain. For example, L. major, a commonly used strain, approximately enters the stationary growth phase at Day 4 when reaches the number of 3.5 x 107 parasites/ml (Nasiri et al., 2013).
The final dilution of promastigotes in Trypan blue dye depends on the density of the in vitro parasite culture. Usually, a 1:20 final dilution of promastigotes is suitable at the 3rd or 4th day of the in vitro culture. Additionally, promastigotes have to be fixed with 2% v/v formalin before counting.
Seed 7.5 x 105 Leishmania spp. early stationary phase promastigotes in each well (i.e., ratio of 15:1 parasites/macrophage) in a total final volume of 200 μl of complete RPMI-1640 medium.
Incubate the plate for 48 h at 37 °C under 5% CO2 humidified air.
Then, remove the non-internalized promastigotes by washing thrice with RPMI-1640 medium pre-warmed at 37 °C.
-
Add various increasing concentrations of TPF (5-400 μg/ml) in triplicates and fill in the wells with complete RPMI-1640 medium until the final volume of 200 μl per well.
Note: Concentrations of plant extracts may vary depending on the natural product.
-
Add one reference drug (i.e., one drug currently subscribed for the chemotherapy of leishmaniasis) in triplicate at the appropriate 50% inhibitory concentration (IC50). Hexadecylphosphocholine (HePC) is used at the concentrations of 0.6 μg/ml and 3.2 μg/ml for L. infantum and L. major respectively, as previously described ( Koutsoni et al., 2018 ).
Note: Control triplicates of the equivalent volumes of TPF solvents must also be included.
Triplicate of a negative control group must also be included. More specifically, a triplicate of Leishmania-infected J774A.1 macrophages cultured only in the presence of complete RPMI-1640 medium without any drug influence, serves as a negative control group.
Incubate the plate for 48 h at 37 °C under 5% CO2 humidified air.
-
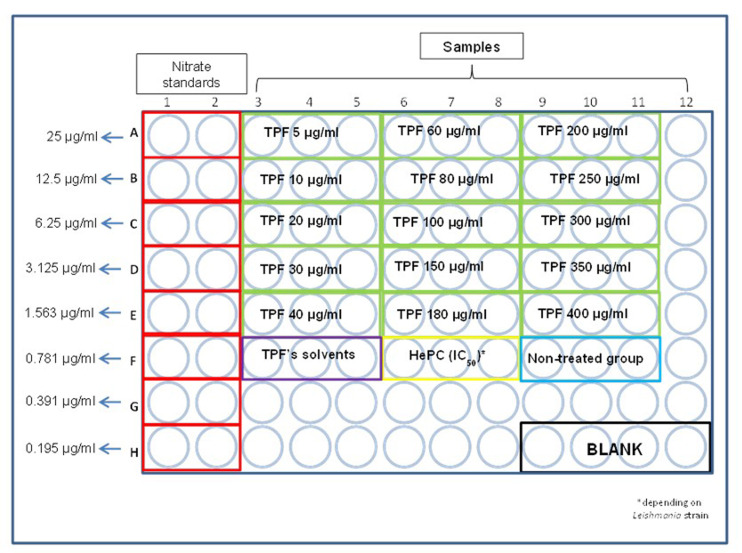
Collect culture supernatants and transfer 50 μl of each sample into a new 96-well flat bottom tissue culture plate, as designated in Figure 1. It is also recommended to use again triplicates for each sample.
Note: Culture supernatants contain the secreted reactive nitrogen intermediates.
A sodium nitrite standard curve is required. Place 100 μl of NaNO2 solution (Recipe 5) at concentration of 75 μg/ml at A1 and A2 wells, as designated in Figure 1.
-
Place 50 μl of distilled H2O in B1, B2 etc. until H1 and H2 wells and perform serial dilutions by transferring 50 μl of NaNO2 solution from A1 and A2 to B1 and B2 respectively until H1 and H2, with the use of a multichannel pipette.
Note: Discard the excessive 50 μl obtained from H1 and H2 wells.
-
Place 100 μl of Griess reagent (Recipe 6) in both standards and samples subsequently.
Notes:
Griess reagent must be freshly prepared. When preparing Griess reagent, avoid contact with the bottleneck because this usually contains Griess-positive material that gives an increase in background absorbance.
The final standard NaNO2 concentrations range from 0.195 μg/ml to 25 μg/ml.
A quadruplicate that contains only 50 μl of distilled water that serves as blank is also included.
-
Read the plate by using an absorbance microplate reader with excitation at 570 nm.
Note: Color change from transparent to pink is immediately noticed. The intensity of the color is greater as nitrite concentration gets higher.
-
-
In vitro quantification of reactive oxygen species production by Leishmania-infected J774A.1 macrophages treated with increasing concentrations of TPF
-
Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Note: The recovery of TPF from extra virgin olive oil was carried out by Centrifugal Partition Extraction (CPE) technique, which is an innovative solid support free separation technique derived from Centrifugal Partition Chromatography (CPC), as mentioned in Procedure section, Step A1.
Cultivate J774A.1 macrophages in 25 cm2 cell culture flasks with filter cap, containing 10 ml of complete RPMI-1640 medium, as previously described in Step A2.
Allow macrophages to reach a high density population of about 70% confluence, as mentioned in Step A3.
Then, determine the number of macrophages per ml by differential counting of dead and live cells using the Trypan blue exclusion dye in a Malassez counting chamber under an optical microscope, as already described in Steps A4 and A5.
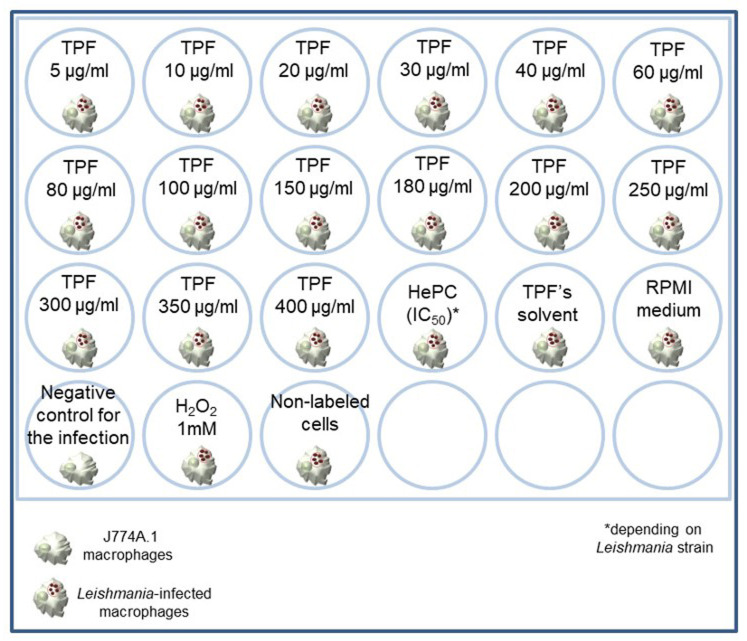
Seed 5 x 105 J774A.1 macrophages per well in a final volume of 500 μl of complete RPMI-1640 medium in a 24-well flat bottom tissue culture plate under a sterile biosafety cabinet (Figure 2).
Incubate the plate for 18 h at 37 °C under 5% CO2 humidified air in order to achieve cell adhesion.
-
Determine the number of Leishmania spp. promastigotes per ml (promastigotes must be in their stationary phase of growth) by differential counting of dead and live parasites using the Trypan blue exclusion dye in a Malassez counting chamber under an optical microscope.
Notes:
Leishmania spp. promastigotes usually enter the stationary-growth phase after 3 to 5 days, depending on the Leishmania strain. For example, L. major, approximately enters the stationary growth phase at Day 4 when reaches the number of 3.5 x 107 parasites/ml (Nasiri et al., 2013).
The final dilution of promastigotes in Trypan blue dye depends on the density of the in vitro parasite culture. Usually, a 1:20 final dilution of promastigotes is suitable at the 3rd or 4th day of the in vitro culture. Additionally, promastigotes have to be fixed with 2% v/v formalin before counting.
-
Seed 7.5 x 106 Leishmania spp. early stationary phase promastigotes in each well (i.e., ratio of 15:1 parasites/macrophage) in a total final volume of 1,000 μl of complete RPMI-1640 medium.
Note: Infection negative control group is included and is consisted of promastigote-free macrophages.
Incubate the plate for 48 h at 37 °C under 5% CO2 humidified air.
Then, remove the non-internalized promastigotes by washing thrice with RPMI-1640 medium pre-heated at 37 °C.
-
Add various increasing concentrations of TPF (5-400 μg/ml) and fill in the wells with complete RPMI-1640 medium until the final volume of 1,000 μl per well.
Note: Concentrations of plant extracts may vary depending on the natural product.
-
Add one reference drug (i.e., one drug currently subscribed for the chemotherapy of leishmaniasis) in triplicate at the appropriate 50% inhibitory concentration (IC50). Hexadecylphosphocholine (HePC) is used at the concentrations of 0.6 μg/ml and 3.2 μg/ml for L. infantum and L. major respectively, as previously described ( Koutsoni et al., 2018 ).
Notes:
Control of the equivalent volumes of TPF solvents must also be included.
Three different control groups must be included i) Leishmania spp. infected-macrophages that will serve as treatment negative control, ii) Leishmania spp. infected-macrophages that will serve later as positive control for ROS production and iii) Leishmania spp. infected-macrophages that will serve later in the flow cytometry as the non-labeled sample.
Incubate the plate for 48 h at 37 °C under 5% CO2 humidified air.
-
Remove culture supernatants and add 500 μl of PBS 1x solution (Recipe 3) in each well except from the positive control for ROS production where you add 500 μl of 1 mM H2O2 solution (Recipe 7).
Note: H2O2 is an inducer of ROS production by the macrophages and therefore is useful as positive control for the ROS production (Ogawa et al., 2004).
Incubate the plate for 15 min at 37 °C under 5% CO2 humidified air.
Remove the culture supernatant from the positive control group and replace with 500 μl of PBS 1x solution.
-
Add 5 μM of the carboxy-H2DCFDA in each well.
Note: In the non-labeled group, avoid adding the carboxy-H2DCFDA.
Incubate the plate for 30 min at 37 °C under 5% CO2 humidified air.
Remove the culture supernatants and add in each well 500 μl of PBS 1x solution.
Detach cells with the use of a cell lifter.
-
Collect one by one cell suspensions and transfer them into a 5 ml round bottom tube each.
Note: Tubes containing cell suspensions should be placed on ice during the procedure of the flow cytometric data collection.
Analyze the samples in the FACSCalibur.
-
Figure 1. Representative illustration of the 96-well flat bottom plate that Griess assay takes place.
Figure 2. Representation of the 24-well tissue culture plate.
Data analysis
-
In vitro quantification of NO production by Leishmania-infected J774A.1 macrophages treated with increasing concentrations of TPF
Griess colorimetric nitrite assay relies on the conversion of the nitric oxide in a sample to a stable azo compound that absorbs at around 540 nm ( Sun et al., 2003 ). Put the plate in the microplate reader in order to obtain the Optical Density (OD) value of each well.
Calculate the mean Optical Density (OD) value of the blank quadruplicate and subtract it from every other OD value.
Calculate the mean of the OD values for each of the standard nitrite concentrations.
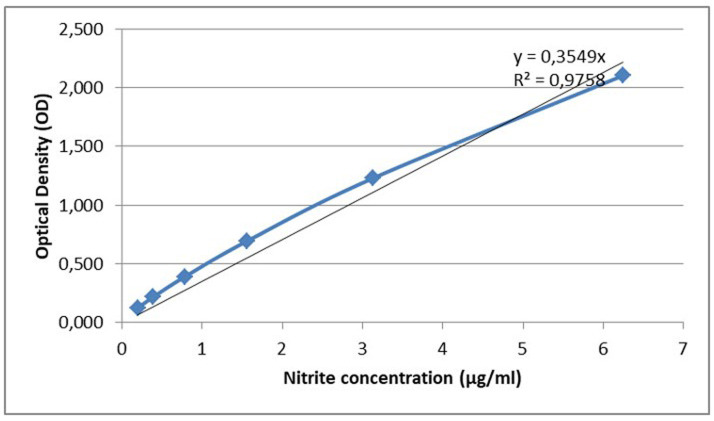
Select all the values that correspond to the standard nitrite concentrations and insert a scatter plot that depicts the nitrite concentration in the x-axis and the mean of the corresponding OD values in the y-axis.
Extract the linear region of the curve.
Select “add trendline” and pick “linear” trendline options.
-
Add the trendline in the chart and extract the equation and R-squared value of the trendline (Figure 3.).
Note: A trendline is most reliable when its R-squared value is at or near 1.
Calculate the mean of the OD values that corresponds to each sample.
Based on the equation, find the nitrite concentration for each sample.
-
In vitro quantification of ROS production by Leishmania-infected J774A.1 macrophages treated with increasing concentrations of TPF
For the data analysis, FlowJo software is used.
-
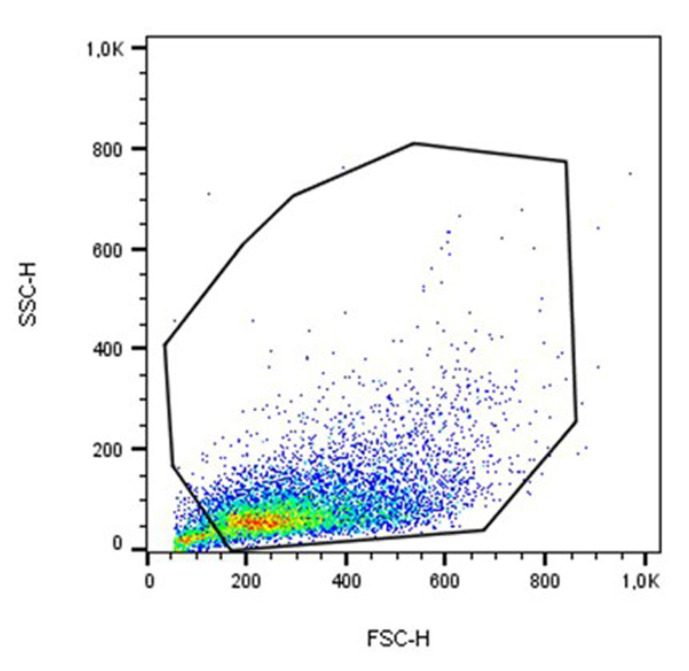
Analyze the samples according to their size (forward-scattered light/ FSC) and cell granularity (side-scattered light/ SSC) on a dual parameter dot plot and set a gating region to exclude cellular debris (Figure 4).
Note: As a general guide, cellular debris is FSC-low.
Analyze the gated cell population in a single-parameter histogram (FL1-H channel).
-
Extract the Geo-mean for each sample (Figure 5).
Note: Geo-mean is representative of the fluorescence intensity of the sample. The greater the Geo-mean, the more is the fluorescence intensity and consequently the more is the presence of ROS in the cells (Eruslanov and Kusmartsev, 2010).
-
Figure 3. Linear region of the standard curve with trendline.
Figure 4. An example of gating J774A.1 macrophages analyzed by FSC and SSC dot plot.
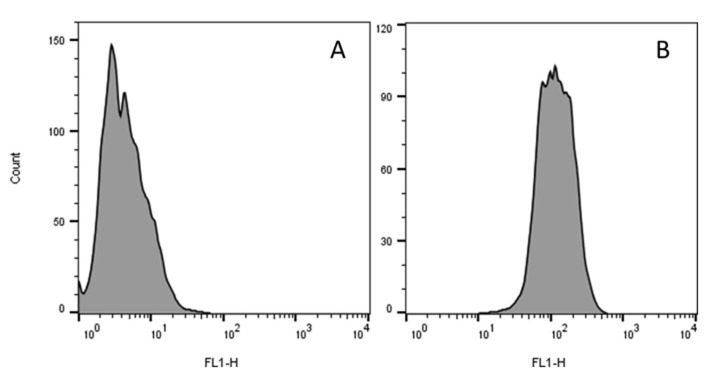
Figure 5. Representative single-parameter histograms of flow-cytometric analysis for the quantification of ROS production.
FL1-histogram of the fluorescence intensity (Geo Mean) in gated population of J774A.1 cells loaded with H2DCFDA upon stimulation with (A) RPMI-1640 medium and (B) TPF at 400 µg/ml.
Recipes
-
Complete RPMI-1640 medium
Add 5 ml of L-glutamine (stock solution 200 mM, stored at -20 °C), 5 ml of Penicillin-Streptomycin (stock solution 10,000 U/ml, stored at -20 °C) and 5 ml of HEPES (stock solution 1 M) to 500 ml RPMI-1640 medium
Store at 4 °C
Take the appropriate volume and add FBS to 10% (v/v) final concentration
-
Fetal Bovine Serum solution (FBS)
Thaw FBS and heat-inactivate it at 56 °C for 30 min in a water-bath under constant agitation
Store at -20 °C until use
-
Phosphate Buffer Saline (PBS), 10x, pH 7.2-7.4
Place 800 ml of distilled water in a suitable container and dissolve 80 g NaCl, 2 g KCl, 11.5 g of Na2HPO4, and 2 g of KH2PO4. Agitate until the complete salt dilution and adjust solution to the desired pH (typically 7.2-7.4) with NaOH (1 M) or HCl (1 M) solution, if needed
Add distilled water until the final volume is 1,000 ml and sterilize through a 0.22 μm pore size syringe filter unit
Prepare the 1x working solution by diluting 50 ml of 10x stock solution in 450 ml of sterile and distilled water. Store at 4 °C
-
0.4% (w/v) Trypan blue exclusion dye
Dissolve 0.4 g of Trypan blue in 100 ml of PBS 1x. Agitate well
Filter through a pleated filter paper and sterilize through a 0.22 μm pore size syringe filter unit. Store at room temperature
-
Sodium nitrite (NaNO2) solution
Prepare a stock solution of 75 µg/ml
Dissolve 750 µg in 10 ml of de-ionized water
-
Griess reagent
Griess reagent consists of two separate solutions (A and B) that they are mixed together (ratio 1:1) at the moment of the experiment operation
Griess reagent A
First, prepare a 3% (v/v) solution of phosphoric acid. Then, dissolve the appropriate amount of sulfanilamide at a final concentration of 1% (w/v)
Griess reagent B
Dissolve the appropriate amount of N-(1-Naphthyl) ethylenediamine dihydrochloride in distilled water in order to obtain a final concentration of 0.1 % (w/v)
-
H2O2 solution
Prepare 100 ml working solution of 1 mM
Dissolve 10 μl of stock solution (30% w/w) in 100 ml of de-ionized water
Acknowledgments
Part of this research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under the HFRI Ph.D. Fellowship grant (GA. no. 6ΝΔΘ46ΨΖ2Ν-ΣΣΟ). Also, this work was supported by KRHPIS II (MIS 5002486) and EATRIS (MIS 5028091).
The protocols were adapted and modified by Koutsoni et al., 2018 and Kyriazis et al., 2016 .
Competing interests
Authors declare that they have no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Angelis A., Hamzaoui M., Aligiannis N., Nikou T., Michailidis D., Gerolimatos P., Termentzi A., Hubert J., Halabalaki M., Renault J. H. and Skaltsounis A. L.(2017). An integrated process for the recovery of high added-value compounds from olive oil using solid support free liquid-liquid extraction and chromatography techniques. J Chromatogr A 1491: 126-136. [DOI] [PubMed] [Google Scholar]
- 2. Bae Y. S., Sung J. Y., Kim O. S., Kim Y. J., Hur K. C., Kazlauskas A. and Rhee S. G.(2000). Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase . J Biol Chem 275(14): 10527-10531. [DOI] [PubMed] [Google Scholar]
- 3. Dale D. C., Boxer L. and Liles W. C.(2008). The phagocytes: neutrophils and monocytes. Blood 112(4): 935-945. [DOI] [PubMed] [Google Scholar]
- 4. Denkers E. Y. and Butcher B. A.(2005). Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol 21(1): 35-41. [DOI] [PubMed] [Google Scholar]
- 5. do Socorro S. R. M. S., Mendonca-Filho R. R., Bizzo H. R., de Almeida Rodrigues I., Soares R. M., Souto-Padron T., Alviano C. S. and Lopes A. H.(2003). Antileishmanial activity of a linalool-rich essential oil from Croton cajucara . Antimicrob Agents Chemother 47(6): 1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eruslanov E. and Kusmartsev S.(2010). Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594: 57-72. [DOI] [PubMed] [Google Scholar]
- 7. Georgopoulou K., Smirlis D., Bisti S., Xingi E., Skaltsounis L. and Soteriadou K.(2007). In vitro activity of 10-deacetylbaccatin III against Leishmania donovani promastigotes and intracellular amastigotes . Planta Med 73(10): 1081-1088. [DOI] [PubMed] [Google Scholar]
- 8. Gupta G., Oghumu S. and Satoskar A. R.(2013). Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol 82: 155-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horta M. F., Mendes B. P., Roma E. H., Noronha F. S., Macedo J. P., Oliveira L. S., Duarte M. M. and Vieira L. Q.(2012). Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res 2012: 203818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koutsoni O. S., Karampetsou K., Kyriazis I. D., Stathopoulos P., Aligiannis N., Halabalaki M., Skaltsounis L. A. and Dotsika E.(2018). Evaluation of total phenolic fraction derived from extra virgin olive oil for its antileishmanial activity. Phytomedicine 47: 143-150. [DOI] [PubMed] [Google Scholar]
- 11. Kumar R., Pai K. and Sundar S.(2001). Reactive oxygen intermediates, nitrite and IFN-gamma in Indian visceral leishmaniasis. Clin Exp Immunol 124(2): 262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyriazis I. D., Koutsoni O. S., Aligiannis N., Karampetsou K., Skaltsounis A. L. and Dotsika E.(2016). The leishmanicidal activity of oleuropein is selectively regulated through inflammation- and oxidative stress-related genes. Parasit Vectors 9: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leopold Wager C. M. and Wormley F. L., Jr (2014). Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol 7(5): 1023-1035. [DOI] [PubMed] [Google Scholar]
- 14. Nasiri V., Karimi G., Dalimi A., Paykari H. and Ghaffarifar F.(2013). Effects of sheep and mouse urine on the growth pattern of Leishmania major promastigotes. Biomed Res Int 2013: 748592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogawa Y., Kobayashi T., Nishioka A., Kariya S., Ohnishi T., Hamasato S., Seguchi H. and Yoshida S.(2004). Reactive oxygen species-producing site in hydrogen peroxide-induced apoptosis of human peripheral T cells: involvement of lysosomal membrane destabilization. Int J Mol Med 13(3): 383-388. [PubMed] [Google Scholar]
- 16. Patricio F. J., Costa G. C., Pereira P. V., Aragao-Filho W. C., Sousa S. M., Frazao J. B., Pereira W. S., Maciel M. C., Silva L. A., Amaral F. M., Rebelo J. M., Guerra R. N., Ribeiro M. N. and Nascimento F. R.(2008). Efficacy of the intralesional treatment with Chenopodium ambrosioides in the murine infection by Leishmania amazonensis . J Ethnopharmacol 115(2): 313-319. [DOI] [PubMed] [Google Scholar]
- 17. Rodrigues I. A., Mazotto A. M., Cardoso V., Alves R. L., Amaral A. C., Silva J. R., Pinheiro A. S. and Vermelho A. B.(2015). Natural products: insights into leishmaniasis inflammatory response. Mediators Inflamm 2015: 835910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy S., Dutta D., Satyavarapu E. M., Yadav P. K., Mandal C., Kar S. and Mandal C.(2017). Mahanine exerts in vitro and in vivo antileishmanial activity by modulation of redox homeostasis . Sci Rep 7(1): 4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun J., Zhang X. J., Broderick M. and Fein H.(2003). Measurement of nitric oxide production in biological systems by using Griess Reaction assay. Sensors 3(8): 276-284. [Google Scholar]
- 20. Tomiotto-Pellissier F., Bortoleti B., Assolini J. P., Goncalves M. D., Carloto A. C. M., Miranda-Sapla M. M., Conchon-Costa I., Bordignon J. and Pavanelli W. R.(2018). Macrophage polarization in leishmaniasis: broadening horizons. Front Immunol 9: 2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Torres-Santos E. C., Moreira D. L., Kaplan M. A., Meirelles M. N. and Rossi-Bergmann B.(1999). Selective effect of 2',6'-dihydroxy-4'-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis . Antimicrob Agents Chemother 43(5): 1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]