Abstract
Hearing loss is a common sensory deficiency suffered by millions worldwide. It is a heterogeneous condition and genetics plays a critical role in its etiology. Gene variants can fundamentally alter hearing function, or predispose the auditory system towards loss of function resulting from other factors. In mouse studies of hearing loss and gene function, an evoked potential electrophysiological recording, the auditory brainstem response (ABR), is now considered the optimal way to screen large numbers of individuals, either with normal hearing sensitivity or with hearing impairment. Other routinely used methods to assess hearing function (such as acoustic startle responses, or otoacoustic emissions) do not allow assessment of the same broad spectrum of dysfunction nor readily allow the threshold sensitivity of the neural output of the cochlea to be assessed and are less ideal. An optimized recording system to rapidly and reproducibly record high-quality ABRs from mutant mice as part of a high-throughput phenotyping pipeline was developed. Click-evoked ABRs and ABRs evoked by pure-tone frequencies over a range of sound levels from 0 dB to 95 dB, sound pressure levels (SPL) are recorded. This takes approximately 15-20 min per mouse (with 5 tone frequencies), allowing a large number of mutant mice to be screened. This method has been used to measure ABRs on a high-throughput mutant mouse phenotyping pipeline and in laboratory tests to follow-up the hearing loss phenotypes identified on that pipeline.
Keywords: Hearing loss, Phenotyping screen, Electrophysiology, Auditory system, Mutant
Background
Hearing impairment is a widespread sensory deficiency suffered by millions worldwide, of all ages and gender. Effects sizes can range from mild to severe. It can be congenital or develop later in life, often in a frequency-dependent age-related progressive manner. Numerous and varied pathologies can contribute to or be responsible for hearing loss (Davis 1995; Fortnum et al., 2001 ). Environmental conditions, such as noise exposure or drug-toxicity, can also have profound effects on hearing ability. Genetics is known to play many crucial roles in the early development of hearing and in the maintenance of hearing function into old age, as well as in predisposing the auditory system towards pathology or protecting the system against pathological decline. With the development of molecular methods to target the function of specific genes in the mouse and the emergence of phenotyping programs to investigate the systemic roles of these genes, it became necessary to develop a sensitive, rapid, robust and reproducible method to screen hearing function in these mutant animals.
Measurement of the Preyer Reflex (an acoustic startle response) can be used to quickly test very high numbers of animals, but it can detect only severe hearing loss and may be affected by motor system deficiencies in mutant mice. Measurements of otoacoustic emissions can be performed quickly and non-invasively, but they do not detect as many pathological conditions as measures of the output of the cochlea as a whole. The Auditory Brainstem Response (ABR), an electrophysiological response incorporating auditory nerve and auditory brainstem activity, is considered the ideal method to assess hearing function across large numbers of mice.
Methods considered are listed across Table 1: ABR, Auditory Brainstem Response. Startle, Startle Response (such as Preyer Reflex, Pre-Pulse Inhibition, etc.). OAE, Oto-Acoustic Emissions. MEMR, Middle Ear Muscle Reflex. Tymp, Tympanometry. ECoG, Electrocochleography. EP, Endocochlear Potential. The usefulness of a particular method to assess a particular function is indicated as a binary Yes/No choice. Yes, indicates that a test may give results to aid identification/diagnosis of a particular problem. No, indicates the test may not useful for this purpose. Middle Ear Conductive HL, is the test helpful to detect a conductive hearing loss, such as otitis media. IHC dysfunction, to detect Inner Hair Cell dysfunction. OHC dysfunction, to detect Outer Hair Cell dysfunction. SV dysfunction, to detect dysfunction of the Stria Vascularis. Inner Ear developmental problems, to detect early developmental issues affecting the inner ear. Age-Related HL, helpful to detect aspects of progressive hearing loss, if appropriate frequency stimuli are used. Involves other neural systems, can be influenced by dysfunction of other neural systems, such as descending inputs to the cochlea, motor systems, sensori-motor integration, etc. Assessment of cochlear sensitivity, to assess thresholds as an estimate of the sensitivity of cochlear function. Assessment of CAS function, to detect dysfunction within the Central Auditory System. High-Throughput, is the test amenable to high-throughput screening (e.g., simple and quick to set-up, short recording time, able to detect a broad spectrum of auditory dysfunction, etc.). The auditory brainstem response (ABR) is considered the best overall method to detect the widest spectrum of hearing impairment pathologies in a high-throughput screen where very large numbers (1000’s–10,000’s) of individual animals need to be assessed.
Table 1. Comparison of methods of functional assessment of hearing in small mammals.
| Suitability as a test for: | ABR | Startle | OAE | MEMR | Tymp | ECoG | EP |
| Middle Ear Conductive HL | Yes | Yes | Yes | Yes | Yes | Yes | No |
| IHC dysfunction | Yes | No | No | Yes | No | Yes | No |
| OHC dysfunction | Yes | No | Yes | Yes | No | Yes | No |
| SV dysfunction | Yes | No | Yes | Yes | No | Yes | Yes |
| Inner Ear developmental problems | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age-Related HL | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Involves other neural systems | Yes | Yes | No | Yes | Yes | Yes | No |
| Assessment of cochlear sensitivity | Yes | No | Yes | No | No | Yes | No |
| Assessment of CAS function | Yes | Yes | Yes | Yes | No | Yes | No |
| High-Throughput | Yes | Yes | No | No | No | No | No |
The method described here was adopted as the standard protocol used as a screen of hearing function by the International Mouse Phenotyping Consortium (IMPC, https://www.mousephenotype.org) at numerous research centers worldwide (summarized in Bowl et al., 2017 ). Detailed analyses of the data produced using this method at the Wellcome Sanger Institute have been published ( White et al., 2013 ; Ingham et al., 2019 ). Whilst ABRs are commonly used to examine auditory function across a range of species, this method is optimized for rapid measurements in the mouse.
The ABR method, and our customized software, has been used in many secondary phenotyping studies to contribute to more detailed characterization of mutant phenotypes identified in the high-throughput screen ( Ingham et al., 2019 ). The description presented here has been updated to reflect currently available digital signal processing technology. The method can easily be modified to perform evoked potential studies tailored towards more bespoke data collection and analysis requirements of individual studies (e.g., Chen et al., 2014 ; Buniello et al., 2016 ; Ingham et al., 2016 ) and can be used to assess more complex aspects of auditory function. For example, we can measure frequency tuning curves ( Ebrahim et al., 2016 ), forward masking functions ( Kuhn et al., 2011 ), and other measures of auditory temporal processing, based on assessment of ABR wave 1 amplitude (and latency). With training and experience, experimenters can use this system to generate high quality ABR recordings and produce reproducible measurements of threshold sensitivity, waveform shape and peak amplitude and latency, to give mean values with low associated variance helping to increase the statistical power of the method (e.g., Ingham et al., 2017 ).
Materials and Reagents
Mice (Wildtype and Mutant mice, typically generated on a C57BL/6N genetic background, aged 13-14 weeks, were tested on the high-throughput screen [ Ingham et al., 2019 ] although any strain of laboratory mouse aged, 14 days or older can easily be tested)
Antisedan (Atipamezole Hydrochloride 5 mg/ml, Zoetis United States, Parsippany, New Jersery, USA)
Ketaset (Ketamine Hydrochloride 100 mg/ml, Zoetis United States, Parsippany, New Jersery, USA)
Rompun (Xylazine Hydrochloride 20 mg/ml; 2% w/v, Bayer plc, Berkshire, UK)
Viscotears (Liquid Eye Gel 2 mg/g Carbomer, with cetrimide, Bausch & Lomb, Surrey UK)
Ketamine/Xylazine anesthetic mix (made from Materials and Reagents #3 and #4; see Recipes)
Atipamezole mix (made from Materials and Reagents #2; see Recipes)
Equipment
Sound attenuating chamber (MAC1 or MAC2 Industrial Acoustics Company; or equivalent, with Field Noise Reduction of at least 37dB at frequencies of 2,000Hz and above, internal dimensions of approximately 584 x 406 x 356 mm [width x depth x height] or greater, incorporating radio-frequency shielding)
Heating blanket (Homeothermic Blanket System; 50-7221F; Harvard Apparatus, Cambridge UK)
Stimulus Generation and Signal Acquisition digital signal processing (DSP) equipment (RZ6-A-P1 Multi IO Processor, Tucker-Davis Technologies TDT, USA)
Microphone and Signal Conditioner (Model 378C01, Model 480C02; PCB Piezoelectronics, NY, USA)
Sound Transducer (Multifield speaker MF1; TDT, USA)
BNC and other connector cables
Subdermal Needle electrodes (SD51-426-1 NeuroDart, Spes Medica, Italy; 0.4mm diameter, 13mm length, with 1.5mm touchproof connector and 100cm cable length)
Low-Impedance Recording Headstage/Preamplifier (RA4LI + RA4PA, or Medusa4Z; TDT, USA)
Personal Computer (able to house a full-height PCI interface card)
“Optibit” interface card (PO5E, TDT, USA)
Digital Oscilloscope (TBS1000; Tektronix Berkshire UK)
Software
RPvdsEx driver software (TDT, USA; www.tdt.com/support/downloads)
ActiveX control software (TDT, USA; www.tdt.com/support/downloads)
TeeChart AX software (Steema Software; Girona, Spain)
Custom “Averager“ Software, written by Tim Folkard (Medical Research Council Institute of Hearing Research, Nottingham UK) and Neil Ingham, available on request from the author (neil.ingham@kcl.ac.uk)
Custom “Traceview“ software, written by Tim Folkard (Medical Research Council Institute of Hearing Research, Nottingham UK) and Neil Ingham, available on request from the author (neil.ingham@kcl.ac.uk)
ABR Analysis Software (written by Dr. B.N. Buran; https://github.com/bburan/abr) to facilitate peak and latency analyses
Procedure
-
Animal preparation
Auditory Brainstem Response (ABR) recordings are recorded from anesthetized control and mutant mice. Mice are anesthetized using intra-peritoneal Ketamine (100 mg/kg) and Xylazine (10 mg/kg), see Recipe 1.
After injection, mice are kept in a warm sleep cage until deeply sedated (when the righting reflex, corneal-blink reflex and pedal-withdrawal reflex are abolished; which may take from 5-10 minutes following injection) and then placed on a heating blanket located inside the sound attenuating booth where the electrophysiological recordings will take place. To speed up the procedure, the feedback probe is not inserted rectally, but instead is situated inside the blanket cover underneath the mouse. The blanket controller is set to maintain a temperature of 37 °C to ensure the mouse core temperature is not adversely affected during the recording. The induction anaesthetic dose is usually sufficient for the duration of the recording procedure but can be topped-up if necessary, using 30-50% doses.
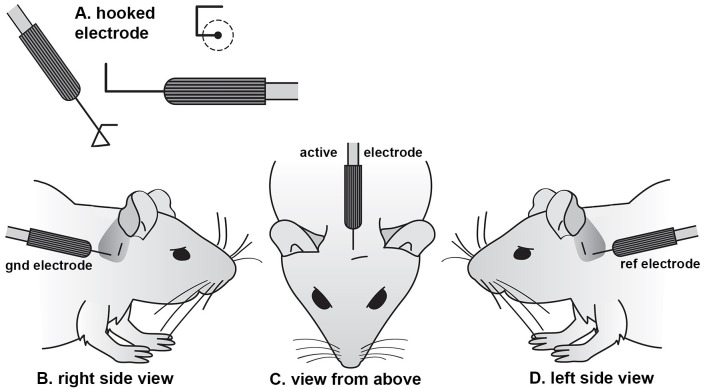
Sub-cutaneous needle electrodes (bent into a hook shape; Figure 1A) are inserted through a pinch of skin on the cranial vertex (connected to the active channel 1 input of the RA4LI or Medusa4Z headstage) and overlying the ventral region of the left bulla (connected to the headstage reference input) and right bulla (connected to the headstage ground input) (see Figures 1B and 1C). This electrode arrangement does not abolish artifact potentials (such as the electrocardiogram (ECG), but does minimize them. The hook shape facilitates an accurate insertion point and ensures that the recorded electroencephalogram (EEG) signal is picked up from a limited anatomical area. No local anesthetic needs to be applied prior to electrode insertion as the mice are already deeply sedated and areflexic.
Stimuli are presented as free-field sounds (not coupled into the ear-canal) from a loudspeaker whose leading edge is positioned 20 cm in front of the mouse’s interaural axis on the midline. This distance gives sufficient space to easily swap mice in and out of the set-up without accidently dislodging the loudspeaker position, whilst maintaining close proximity to ensure 95 dB SPL levels can be achieved at the mouse head position.
-
Stimulus generation
Stimuli are generated under the control the “Averager” software package. A wide range of stimulus parameters are user-definable, or can be easily and quickly loaded from previously saved configurations.
The frequency response of the sound delivery system is equalized using a spectral analysis of the output of the non-equalized output of the system. The PCB microphone is positioned at a 20 cm distance from the leading edge of the loudspeaker (where the mouse head will subsequently be placed), pointing towards the loudspeaker. Under software control, broadband noise bursts (generated from random number sequences) are presented from the loudspeaker. The signal recorded by the microphone is amplified by 40 dB and recorded at approximately 200 kHz sampling rate on the RA6-A-P1 processor. The software calculates a Fast Fourier Transformation of the microphone signal to generate a frequency response representing the maximum output of the loudspeaker across frequency. This frequency response will be subsequently used by the software to calculate the dB attenuation level required to present each stimulus frequency at the desired dB sound pressure level (SPL). This step will need to be performed before the mouse is anesthetized and placed into the experimental setup within the sound attenuating booth.
“Averager” software is used to control the RZ6-A-P1 processor to deliver click (0.01 ms duration) and tone pip (variable frequencies from 3-42 kHz of 5 ms duration, 1 ms rise/fall time) stimuli over a range of intensity levels from 0-95 dB sound pressure level (SPL, re. 5 μPa) in 5 dB steps. Stimuli are generated at a sampling rate of approximately 100 kHz before being attenuated, amplified and delivered to the sound transducer to produce the desired SPL at the mouse head location.
Once triggered to begin, the “Averager” software and the RZ6-A-P1 processor control stimulus presentation and response recording synchronously. The same digital trigger is used to initiate both the presentation of each stimulus and the recording of response snippet evoked by that stimulus.
-
Response recording
Recording electrophysiological responses is controlled by the same “Averager” software used to generate and present stimuli. A wide range of recording parameters are user-definable, or can be loaded from previously saved configurations.
Activity detected by the subcutaneous electrodes is digitized at approximately 25 kHz sampling rate (RA4PA/Medusa4Z) before being passed optically to the bioamplifier input of the RZ6-A-P1 processor.
Under control of the “Averager” software, the digitized electrode signal is captured, bandpass filtered (300 Hz-3 kHz; user definable) and amplified (user definable) before being upsampled to approximately 100 kHz. Snippets of response, 20 ms in duration, are stored in a digital averaging buffer before being retrieved by the software and saved to an output file in comma-separated variable (csv) text format.
Averaged responses are recorded in response to 256 presentations of each stimulus (user definable) presented at 42.6/s (user definable).
-
Stimulus parameter space and response recording protocol
A test ABR trace is recorded first, to ensure the system is functioning correctly, using clicks at 70 dB SPL. This recording can easily be repeated if troubleshooting of the experimental setup is required. In addition to recording no response if the mouse is expected/known to be severely-profoundly impaired, there may be numerous other simple reasons that an evoked response is not seen; for example, the operator should check that a) the loudspeaker is properly connected, b) that the electrodes are properly connected to the mouse and headstage, c) that the electrode headstage and preamplifier are connected and switched on, d) there is no excessive electrical noise on the recording trace and e) that the mouse is still alive.
A series of click-evoked ABRs are then recorded, ranging from 0-95 dB SPL in 5 dB intervals.
Tone-evoked ABRs are recorded for a fixed set of frequencies (typically, 6, 12, 18, 24 and 30 kHz although the data collection can easily be expanded to capture responses to 3, 36 and 42 kHz tone-pips) over sound levels ranging from 0 to 95 dB SPL in 5 dB intervals. Responses are recorded in an array beginning with the lowest stimulus level, in decreasing frequency order before stepping up to the next (5 dB higher) stimulus level.
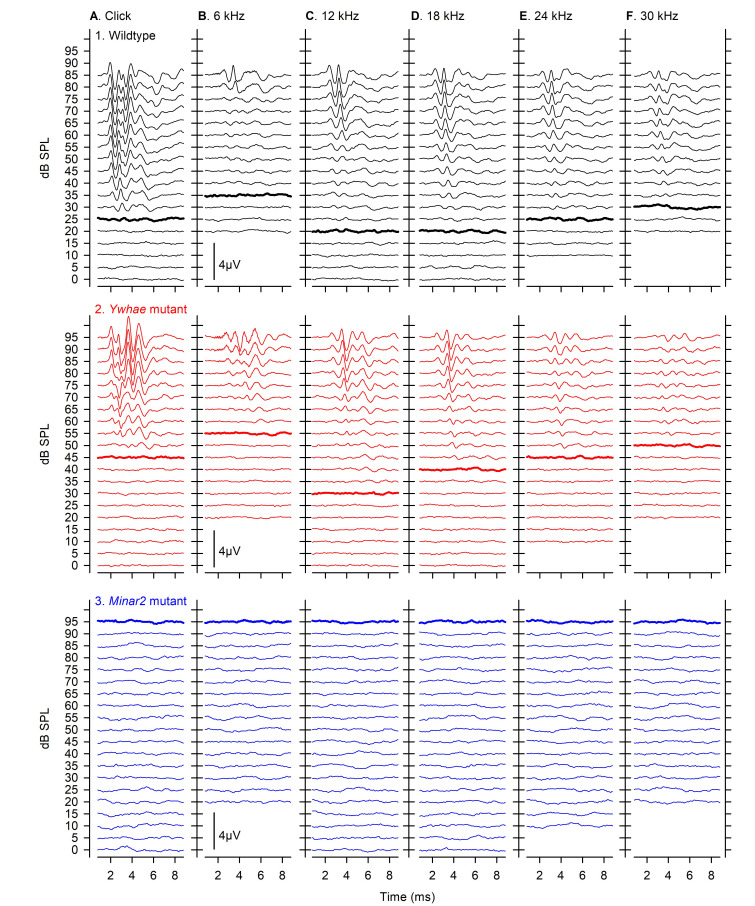
Example ABR traces evoked by click and tone-pip stimuli across 0-95 dB SPL are shown in Figure 2, for a wildtype control mouse (with normal hearing), a mouse carrying a mutant allele of Ywhae (which induces a moderate degree of hearing impairment) and a mouse carrying a mutant allele of Minar2 (which induces a severe hearing impairment, such that no ABRs were evoked even at high stimulus levels).
A final test trace is recorded, again using clicks at 70 dB, to ensure an equivalent ABR to the initial test trace is seen. This can be a helpful measure to determine if a mouse has deteriorated physiologically during the recording procedure.
-
Recovery protocol
Following completion of recording, the electrodes are removed from the skin and a drop of viscotears applied to each eye, to lubricate the cornea during recovery and help the general welfare of the mouse.
Mice are injected with intra-peritoneal Atipamezole (1 mg/kg, see Recipe 2) to promote a faster recovery time from the anesthesia.
Mice should be maintained in warmed cages until they are regaining mobility and judged sufficiently recovered to be returned to their home cage.
Figure 1. Subcutaneous electrode shape and placement.
(A) Individual needle electrodes are bent into a square hook shape using a needle holder, with each side approxiamtely 2 mm long. The hook is inserted through a pinch of skin, such that the contact with the subdermal layers lies on the remaining straight shaft of the electrode. A ground electrode is placed in the skin overlying the bulla behind the right ear (B). The active electrode for the low-impedance bio-amplifier is placed on the midline in a rostro-caudal position lined up with the leading edge of the 2 pinnae (C). The reference electrode for the bio-amplifier is inserted behind the left ear (D), in an equivalent location to the ground electrode (modified from Ingham et al., 2011 ).
Figure 2. Example ABR responses from wildtype and mutant mice.
ABRs are plotted stacked for increasing stimulus dB Sound Pressure Level (SPL), from 0 to 95 dB. Responses in A were evoked by clicks, and those in B-F were evoked by tone pips of 6, 12, 18, 24 and 30 kHz. The threshold sensitivity of each response set, determined by visual inspection of the responses by an experienced experimenter, are indicated by a thickened line on each panel. Typical responses obtained from a wildtype mouse with normal hearing sensitivity are shown in row 1 (black). Responses from a Ywhae homozygous mutant mouse with mild-moderate elevations in hearing sensitivity are shown in row 2 (red). Recordings from a Minar2 homozygous mutant mouse where no visible responses were measured up to 95 dB are shown in row 3 (blue). In such cases, threshold was allocated as the highest dB SPL tested, to allow plotting of data and comparison with other mice. These data have been published previously in Ingham et al. (2019) .
Data analysis
The approach taken to analyze mouse ABR data obtained from a high throughput phenotyping screen of mutant mice has been described in detail in Ingham et al. (2019) . Here, analysis methods appropriate for “secondary phenotyping” laboratory studies are described.
-
ABR threshold and hearing sensitivity
For each stimulus used (click and each tone-pip frequency), ABRs recorded over the range of sound levels tested are plotted as a stack, ordered by increasing dB SPL (Figure 2). Threshold (dB SPL) is estimated by visual inspection of the stacked ABR traces and defined as the lowest sound level where any component of the ABR waveform is recognizable and consistent with responses recorded at higher sound levels, taking into account the characteristic lengthening of peak latency as threshold is approached. Thresholds for each stimulus are plotted to give a profile of the hearing sensitivity of each mouse (Figure 3A). Plots of mean threshold (± standard deviation) in control and mutant groups are used to assess the overall hearing profile of a mutant group. It can also be informative to plot individual mutant and/or control thresholds alongside the groups means (Figure 4).
-
Classification of mouse ABR audiograms
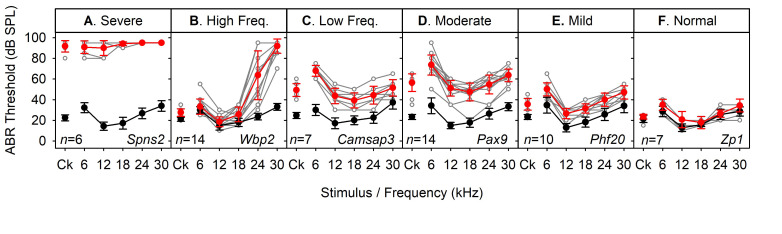
Patterns of raised thresholds for ABRs were classified according to the following criteria: Severe-Profound, if no responses were detected (up to 95 dB SPL) for at least 2 adjacent frequency stimuli, for all mice of that genotype; High-Frequency, if thresholds were elevated at 30 kHz (by > 30 dB) and thresholds were not elevated for at least one of the lower frequency stimuli; Low-Frequency, if thresholds were elevated for 6 and 12 kHz and were normal for at least one of the higher frequency stimuli (with a minimum mean threshold elevation < 15 dB); Moderate, where thresholds were significantly elevated for at least 4 of the 6 stimuli tested (with a minimum mean threshold elevation > 15 dB); Mild, where mean thresholds were elevated by 30 dB or less for up to 3 stimuli tested; Normal Hearing, where no stimuli produced altered thresholds. Figure 4 illustrates one example of each classification of audiogram [see Ingham et al. (2019) for further examples].
-
ABR waveform shape comparisons
Through consistent, reproducible electrode placements and recording with good signal: noise ratio, it is possible to compare, qualitatively and quantitatively, the waveform shapes of stimulus-evoked ABRs. Wave 1 is understood to reflect auditory nerve activity, but as the responses represent a complex summation of responses detected at a single point on the scalp, there is some uncertainty in ascribing specific brain locations to specific features of the ABR waveform. The free-field binaural (i.e., both ear canals open) stimulation conditions we use complicates interpretation further, as there are binaural interactions throughout the auditory brainstem, even as low down as the cochlear nucleus. The ABR represents the summed electrical vectors detected by the electrodes as synchronized action potential volleys (particularly from onset-responding neurons) traverse the central auditory pathways. As these pathways can be both excitatory and inhibitory as well as both ascending and descending, and are distributed in a 3D volume, interpretation is complex.
Waveforms recorded to clicks and tone-pips at fixed dB SPL level, or at fixed levels above threshold (sensation level, dB SL) are plotted for mutant and control mice, along with an average of the ABR amplitude across mice for each genotype. In these responses, 4 waves (positive to negative deflections; Figures 2, 3 and 5) are generally observed. Given that both the latency and amplitude of ABR waves change with changing stimulus level, it is appropriate to plot waveforms (from individual mice and mean waveforms) at a fixed dB SL. This takes account of threshold differences between individuals and gives a mean waveform with reduced variability, facilitating comparison between control and mutant groups.
Additional metrics can be derived from the ABR waveforms, such as the half width of a wave component (for an example of this methodology, see Harris et al., 2018 ). However, such analyses are not discussed further here.
-
Input-Output functions (IOFs)
ABR waves are quantified to determine the amplitude and latency of positive and negative peaks of the waveform at each stimulus level recorded (Figure 3). This can be performed using software routines developed by Dr. B.N. Buran ( Buran et al., 2010 ). From these measures, we calculate the peak-peak amplitude and latencies of waves 1-4 and inter-peak intervals (for example, P1-P2, P1-P3, P1-P4). IOF curves are plotted relative to click threshold for each mouse (i.e., parameter plotted against dB SL). IOFs of individual mice (mutants and littermate controls) are compiled and the mean (± standard deviation) for each parameter is plotted.
-
Statistical analyses
It is rare that populations of the various parameters (for example, thresholds or wave amplitude) derived from ABRs follow a normal distribution. Therefore, the researcher will need to give careful consideration as to the most appropriate statistical comparisons to apply to their datasets. Many of the parameters likely to be tested cannot be considered completely independent (for example, the threshold of adjacent tone-pip stimuli cannot be considered independent due to mechanical coupling along the basilar membrane of the cochlea). Thus, Analysis of Variance (ANOVA) testing is considered more appropriate to compare the range of thresholds tested across different stimuli, rather than multiple individual comparisons of the different stimuli. Non-parametric tests will need to be used for datasets that do not conform to a normal distribution. Such tests are available in many software packages (for example, SigmaPlot, SPSS, etc.)
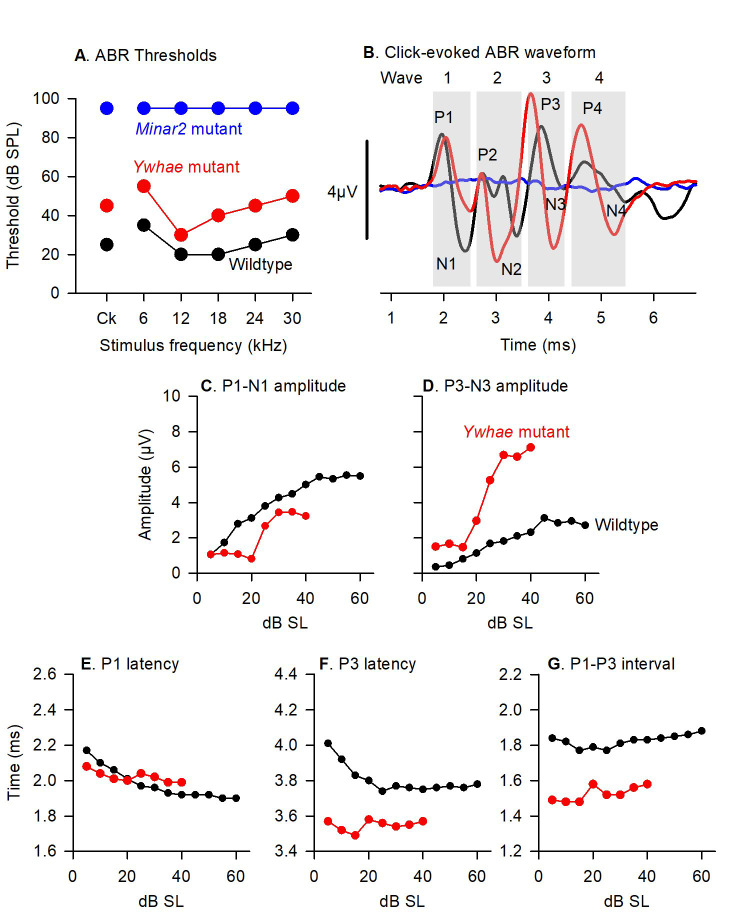
Figure 3. Data representation and analysis methods.
A. ABR thresholds from click and tone stimuli are plotted for the wildtype mouse (black), a Ywhae mutant mouse (red) and a Minar2 mutant mouse (blue) illustrated in Figure 2. B. Click-evoked ABRs recorded from the wildtype mouse (black, 50 dB sensation level, SL), Ywhae mutant (red, 50 dB SL) and Minar2 mutant (blue 95 dB SPL). The grey areas indicate the parts of the waveform corresponding to ABR waves 1-4. Positive and negative peaks of the waveform are labeled P1-P4, and N1-N4, respectively. C-G indicate quantification of the parameters measured from the ABR waveforms, plotted as a function of dB level above threshold (sensation level, dB SL). C. The growth of wave 1, expressed as P1-N1 peak-to-peak ampltiude (P1 positive peak to N1 negative peak) as a function of dB SL is plotted for the wildtype mouse (black) and the Ywhae mutant mouse (red). D. The growth of wave 3, expressed as P3-N3 peak-to-peak ampltiude (P3 positive peak to N3 negative peak) as a function of dB SL is plotted for the wildtype mouse (black) and the Ywhae mutant mouse (red). E. The change in the latency of the peak of wave P1 (the time delay from the onset of the stimulus at 0ms to the time point where P1 reaches it’s maximum amplitude) as a function of dB SL is plotted for the wildtype mouse (black) and the Ywhae mutant mouse (red). F. The change in the latency of the peak of wave P3 as a function of dB SL is plotted for the wildtype mouse (black) and the Ywhae mutant mouse (red). G. The change in the P1-P3 interpeak interval (the time between the occurance of the maximum amplitude of wave P1 and the occurance of the maximum amplitude of the wave P3) as a function of dB SL is plotted for the wildtype mouse (black) and the Ywhae mutant mouse (red). These data have been published previously in Ingham et al. (2019) .
Figure 4. Examples of audiogram classifications of mutant mice.
ABR thresholds from mutant mice classified as having a (A) Severe-Profound, (B) High Frequency, (C) Low Frequency, (D) Moderate, (E) Mild degree of hearing impairmant and (F) Normal hearing sensitivity. Data plotted in grey indicate thresholds from individual mutant animals. Data plotted in red indicate the mean (± standard deviation) thresholds for the mutant animals. Data plotted in black indicate the mean (± standard deviation) thresholds for wildtype control mice recorded in the same weeks as the mutants in the same plot. These data were obtained from a high-throughput phenotyping screen where it was not feasible to record from littermate control mice. These data have been published previously in Chen et al., 2014 ; Maguire et al., 2014 ; Buniello et al., 2016 and Ingham et al., 2019 .
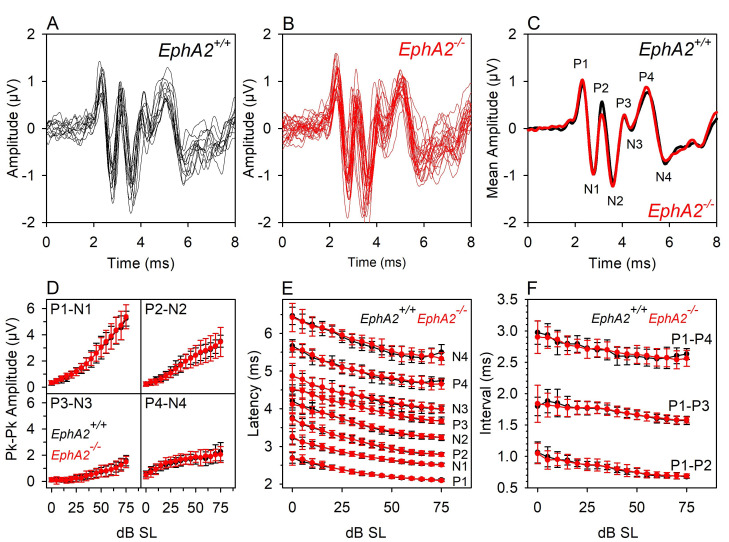
Figure 5. Waveform analyses of tone pip–evoked ABRs.
[These data were described in Ingham et al. (2017) ]. Black lines and symbols indicate data recorded from 12 wildtype littermate control mice ( EphA2+/+). Red lines and symbols indicate data recorded from 23 EphA2 homozygous mutant mice (EphA2-/-). A and B. ABRs recorded from individual mice evoked by 40 dB SL 18 kHz tone pips. C. Mean amplitudes of the wildtype and mutant responses shown in A and B are plotted. Positive-deflection wave components are indicated by P1-P4. Negative-deflection wave components are indicated by N1-N4. D. Input/Output Functions for the peak-peak amplitude of waves 1-4 are plotted. Mean (± standard deviation) amplitude is plotted against sound level above threshold (dB SL, sensation level). E. Input/Output Functions for the peak latency of waves P1-P4 and N-N4 are plotted. Mean (± standard deviation) latency is plotted against sound level above threshold (dB SL). F. Input/Output Functions for 3 inter-peak intervals are plotted. Mean (± standard deviation) interval time is plotted against sound level above threshold (dB SL). These data illustrate that ABR responses between individual animals can be highly reproducible. In this case, there were no significant differences in the waveform response properties recorded from littermate wildtype and EphA2 mutant mice.
Notes
It is crucial to use ABRs from normal hearing littermate control mice to compare responses obtained from mutant mice with abnormal/impaired hearing. This ensures that all mice have the same experiences during their development (such as noise-levels, cage-cleaning disturbance, etc.). This also ensures that control mice used are of the same genetic background.
The background strain can have profound differences on the responses recorded. For example, C57BL/6N mice carry a variant of the Cdh23 gene which predisposes them to early onset high frequency hearing loss, whereas CBA/CaJ mice show very limited age-related elevations in hearing sensitivity. Differences in genetic background may also result in altered modifiers of gene function such that a hearing phenotype present in mutants of a particular strain is lost when the mutation is crossed onto a different background strain.
If researchers are comparing ABRs across different age ranges, either from repeated measurements in the same animals, or from separate cohorts of animals at different ages, it is important to limit the age-variation in each group. When testing 2-3 weeks old mice, it is advisable to perform the ABR tests at an exact age; for example, post-natal day (P) 14 ± 0 days or P21 ± 0 days. As the mice get older, increasing age-variation is more acceptable; for example, 4 weeks old (P28 ± 1 day), 6 weeks old (P42 ± 2 days), 8 weeks old (P56 ± 3 days), 14 weeks (P98 ± 3 days), 6 months old (P182 ± 4 days), 1 year old (P365 ± 7 days). It is vital to age-match control and mutant groups when comparing ABR data in mice. At younger ages, under 6-8 weeks old, the auditory system is still undergoing maturation with improving threshold sensitivity and increasing response amplitude up to 6-8 weeks old. This maturation is particularly rapid after the onset of hearing at around P12, up to around 4 weeks old and therefore it is especially important to exactly age-match younger test groups.
Researchers should give serious consideration to blind-testing their mice, such that the genotype (or other parameters) of individual animals is not known by the experimenter at the time of data collection or data analysis. It is important to remove potential sources of bias during data collection and analysis.
Consideration of gender is important and researchers may need to perform separate analyses on male and female control and mutant mice to fully expose any differences in responses caused by the many different factors present in male vs. female mice. It becoming increasing important to consider sex as a biological variable (SABV) in auditory experimentation.
Researchers should perform a series of pilot experiments to determine the variability of their responses and data and to perform appropriate power calculations to help to estimate the number of experimental animals that might be required to see a given effect size. From experience, the data obtained from these methods are rarely normally distributed (for example, as determined by the Shaprio-Wilk Normality test) and researchers will need to determine whether parametric or non-parametric statistical tests are best suited to their data. In our research, it is often the case that data from a minimum of 6 mice are required to perform statistical comparisons at a sufficient power level, although if responses are more variable, we aim for larger sample sizes.
Use a homeothermic heating blanket system to prevent loss of body temperature in the mice. Anaesthetised mice can become hypothermic very quickly and the accompanying drop in metabolic rate will seriously affect the efficiency of inner ear function and result in artifactually high thresholds across all frequencies (around 20 dB elevations), but especially above 32 kHz (> 30 dB elevations) in the mouse (Henry and Chole, 1984).
Use a sound attenuating booth to reduce background (masking) acoustic noise. The booth should also be grounded (and thus act as a Faraday cage) to ensure low levels of ambient electrical noise which interferes with low-impedance recording systems.
Ensure that the needle electrodes are inserted sub-dermally to give good electrical contact with the animal (lower impedance) and reduced electrical noise on the recorded traces. The hook shape employed here ensures an accurate and reproducible insertion point across many animals and helps to give reproducible ABR waveform shapes. On insertion, the hooked portion of the electrodes is pushed through the pinch of skin, such that the skin sits on the remaining straight shaft of the electrode. Despite the limited area of the needle electrode remaining in the skin, it is still feasible to obtain low impedances of < 5 kΩ (ideally, 1-3 kΩ), tested via the TDT electrode headstage. To achieve better recordings, it is important that the impedance of the active input channel and the reference input channel are in a matching or similar impedance range.
Ensure that the mouse achieves a sufficient depth of anesthesia. With the Ketamine/Xylazine dose suggested here, mice will quickly lose their righting and corneal reflexes. Abolition of pedal withdrawal reflexes may take longer. From a welfare point of view, it is important that the animal does not respond to insertion of the needle electrodes. Furthermore, if the mouse remains only lightly anaesthetized, it will maintain a higher degree of muscle tone. This will introduce excessive myogenic electrical activity onto the recording and should be avoided. Such noise is easily identifiable when viewing the electrode activity on the oscilloscope (rather than the averaged response traces). Recordings can be paused, an anesthesia top-up given if required, and restarted once the myogenic noise has diminished.
It is important to minimize these sources of electrical noise to prevent low amplitude ABR waveforms from being masked. Such masking can produce an artificial elevation of the estimated ABR threshold as the peak and trough features of the waveform used to identify if an ABR is present will be obscured in the noise floor of the recording and only become visible at higher dB SPLs.
The microphone used for calibration of the stimulus system should itself be regularly calibrated (e.g., quarterly/biannually) to ensure that the sound levels presented to mice are consistent over long periods of time. This can be achieved in the users’ own laboratory if appropriate specialist equipment is available, or instead the microphone can be returned to the manufacturer for service & recalibration.
The stimulus and recording parameters described provide a reliable and time-efficient method to estimate ABR thresholds in a high-throughput phenotyping screen. The relatively low number of sweep records (256) contributing to each averaged waveform is made possible by minimizing sources of acoustic and electrical noise in the recording environment. Many other studies need to use many more sweeps (often > 1000) to provide satisfactory waveforms. In this protocol, the stimuli are presented at the relatively fast rate of 42.6/s. This helps to desynchronize any potential noise from the 50 Hz mains electricity supply in the UK. Many other studies use a slower rate of presentation (< 30/s). However, the higher stimulus presentation rate we use has a minimal effect on the recordings and facilitates a shorter time period before the mouse can be recovered from the anesthesia, thus helping to improve the welfare of the mice.
As no artifact rejection is used in the recording system, it is possible that the averaged trace can be significantly influenced by other electrical activity in the body. ECG activity can occasionally approach synchrony with the stimulus repetition rate. Muscle activity from the animals’ breathing rhythm can affect the short snippets of activity recorded, as can prolonged (several seconds) bursts of muscle activity in lightly sedated animals. These can all introduce non-ABR noise/interference on the recorded traces. However, these are a relatively rare occurrence and if necessary, the affected stimulus frequency/level combinations can be repeated manually once the main automated data collection is complete.
This method has been developed for use with laboratory mice. With minor modification, it should be able to be used with most mammalian species; however this has not been tested. If attempting to record ABRs from other species, the experimenter should consider the hearing frequency range of that species and use a loudspeaker with an appropriate matching frequency range. The TDT RZ6-A-P1 Multi-IO processor can drive either magnetic loudspeakers (such as the TDT MF1 speaker) or, if responses to very high frequencies need to be examined, electrostatic speakers (such as TDT EC1 or ES1).
Recipes
-
Ketamine/Xylazine anesthetic mix
1 ml Ketamine HCl (Ketaset 100 mg/ml ketamine HCl, or equivalent)
0.5 ml Xylazine HCl (Rompun, 20 mg/ml xylazine HCl)
8.5 ml H2O
-
Atipamezole mix
0.2 ml antisedan (Atipamezole hydrochloride 5 mg/ml, Pfizer)
9.8 ml H2O
Note: Mixtures are stable at room temperature for long period. Any dilutions stored in a refrigerator should be allowed to warm to room temperature before use. Surplus diluted solutions, or spillages, can be disposed of via incineration (after binding with Clan Uni-Safe chemical spillage reagent; Fisher Scientific). Once opened, stock bottles are stored at room temperature and any unused contents disposed of after 28 days using authorized local protocols.
Acknowledgments
Development of these methods was facilitated by Wellcome and the Medical Research Council funding to Prof. Karen P. Steel (King’s College London & Wellcome Sanger Institute). An early version of this method has been published in Ingham et al. (2011). The complete experimental method and analyses used in high-throughput screening of mutant mice have been published in Ingham et al. (2019). Tim Folkard (Medical Research Council Institute of Hearing Research, Nottingham, UK) performed the early development of the custom “Averager” and “Traceview” software used here. Brad N. Buran developed software to determine waveform peak amplitude and latency (https://github.com/bburan/abr). Prof. Karen P. Steel and Dr. Selina A. Pearson (Wellcome Sanger Institute) gave helpful suggestions during the development of the data collection and analysis methods. Prof. Uwe Drescher (King’s College London) provided the EphA2 mutant mice used here to illustrate the method (see also, Ingham et al., 2017 ). Some data shown here were recorded by the Wellcome Sanger Institute Mouse Genetics Project and has been published in Ingham et al. (2019).
Competing interests
The author declares no competing financial or non-financial interests.
Ethics
Studies were carried out in accordance with UK Home Office regulations and the UK Animals (Scientific Procedures) Act of 1986 (ASPA) under UK Home Office licences, and the study was approved by the King’s College London and Wellcome Sanger Institute Ethical Review Committees. Mice were culled using methods approved under these licences to minimize any possibility of suffering.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Bowl M. R., Simon M. M., Ingham N. J., Greenaway S., Santos L., Cater H., Taylor S., Mason J., Kurbatova N., Pearson S., Bower L. R., Clary D. A., Meziane H., Reilly P., Minowa O., Kelsey L., C. International Mouse Phenotyping, Tocchini-Valentini G. P., Gao X., Bradley A., Skarnes W. C., Moore M., Beaudet A. L., Justice M. J., Seavitt J., Dickinson M. E., Wurst W., de Angelis M. H., Herault Y., Wakana S., Nutter L. M. J., Flenniken A. M., McKerlie C., Murray S. A., Svenson K. L., Braun R. E., West D. B., Lloyd K. C. K., Adams D. J., White J., Karp N., Flicek P., Smedley D., Meehan T. F., Parkinson H. E., Teboul L. M., Wells S., Steel K. P., Mallon A. M. and Brown S. D. M.(2017). A large scale hearing loss screen reveals an extensive unexplored genetic landscape for auditory dysfunction. Nat Commun 8(1): 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buniello A., Ingham N. J., Lewis M. A., Huma A. C., Martinez-Vega R., Varela-Nieto I., Vizcay-Barrena G., Fleck R. A., Houston O., Bardhan T., Johnson S. L., White J. K., Yuan H., Marcotti W. and Steel K. P.(2016). Wbp2 is required for normal glutamatergic synapses in the cochlea and is crucial for hearing. EMBO Mol Med 8(3): 191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buran B. N., Strenzke N., Neef A., Gundelfinger E. D., Moser T. and Liberman M. C.(2010). Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci 30(22): 7587-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J., Ingham N., Kelly J., Jadeja S., Goulding D., Pass J., Mahajan V. B., Tsang S. H., Nijnik A., Jackson I. J., White J. K., Forge A., Jagger D. and Steel K. P.(2014). Spinster homolog 2(spns2) deficiency causes early onset progressive hearing loss. PLoS Genet 10(10): e1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. David A. C.(1995). Hearing in adults: the prevalence and distribution of hearing impairment and reported hearing disability in the MRC institute of hearing research's national study of hearing. London: Whurr. [Google Scholar]
- 6. Ebrahim S., Ingham N. J., Lewis M. A., Rogers M. J. C., Cui R., Kachar B., Pass J. C. and Steel K. P.(2016). Alternative splice forms influence functions of whirlin in mechanosensory hair cell stereocilia. Cell Rep 15(5): 935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortnum H. M., Summerfield A. Q., Marshall D. H., Davis A. C. and Bamford J. M.(2001). Prevalance of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ 323(7312): 536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris K. A., Vaden K. I., McClaskey C.M., Dias J. W. and Dubno J. R.(2018). Complementary metrics of human auditory nerve function derived from compound action potentials. J. Neurophysiol. 119(3): 1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henry K. R. and Chole R. A.(1984). Hypothermia protects the cochlea from noise damage. Hear Res 16: 225-230. [DOI] [PubMed] [Google Scholar]
- 10. Ingham N. J., Carlisle F., Pearson S., Lewis M. A., Buniello A., Chen J., Isaacson R. L., Pass J., White J. K., Dawson S. J. and Steel K. P.(2016). S1PR2 variants associated with auditory function in humans and endocochlear potential decline in mouse. Sci Rep 6: 28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ingham N. J., Pearson S. A., Vancollie V. E., Rook V., Lewis M. A., Chen J., Buniello A., Martelletti E., Preite L., Lam C. C., Weiss F. D., Powis Z., Suwannarat P., Lelliott C. J., Dawson S. J., White J. K. and Steel K. P.(2019). Mouse screen reveals multiple new genes underlying mouse and human hearing loss. PLoS Biol 17(4): e3000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ingham N. J., Pearson S. and Steel K. P.(2011). Using the auditory brainstem response(ABR) to determine sensitivity of hearing in mutant mice. Curr Protoc Mouse Biol 1(2): 279-287. [DOI] [PubMed] [Google Scholar]
- 13. Ingham N. J., Steel K. P. and Drescher U.(2017). On the role of ephrinA2 in auditory function. Hear Res 350: 11-16. [DOI] [PubMed] [Google Scholar]
- 14. Kuhn S., Johnson S. L., Furness D. N., Chen J., Ingham N. J., Hilton J. M., Steffes G., Lewis M. A., Zampini V., Hackney C. M., Masetto S., Holley M. C., Steel K. P. and Marcotti W.(2011). miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells. Proc Natl Acad Sci U S A 108: 2355-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maguire S.,Estabel J. K.,Ingham N.,Pearson S.,Ryder E.,Carragher D. M.,Walker N.,Sanger M. G. P.,Slc25a21 P. T.,Bussell J.,Chan W. I.,Keane T. M.,Adams D. J.,Scudamore C. L.,Lelliott C. J.,Ramirez-Solis R.,Karp N. A.,Steel K. P.,White J. K. and Gerdin A. K.(2014). Targeting of Slc25a21 is associated with orofacial defects and otitis media due to disrupted expression of a neighbouring gene . PLoS One 9(3): e91807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White J. K., Gerdin A. K., Karp N. A., Ryder E., Buljan M., Bussell J. N., Salisbury J., Clare S., Ingham N. J., Podrini C., Houghton R., Estabel J., Bottomley J. R., Melvin D. G., Sunter D., Adams N. C., P. Sanger Institute Mouse Genetics, Tannahill D., Logan D. W., Macarthur D. G., Flint J., Mahajan V. B., Tsang S. H., Smyth I., Watt F. M., Skarnes W. C., Dougan G., Adams D. J., Ramirez-Solis R., Bradley A. and Steel K. P.(2013). Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 154(2): 452-464. [DOI] [PMC free article] [PubMed] [Google Scholar]