Abstract
In the nervous system of vertebrates, nerve impulse propagation is accelerated by the ensheathment of neuronal axons with myelin. Myelin sheaths are molecularly specialized, lipid-rich plasma membrane extensions of Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system (CNS). To visualize myelinated nerve fibers and to allow for the morphological analyses of myelin in the brain and the spinal cord, an efficient method for silver impregnation of myelin has originally been developed by Ferenc Gallyas in 1979, referred to as Gallyas silver impregnation. Gallyas’ method is based on the agyrophilic characteristic of myelin to form and bind silver particles, while this process is suppressed in tissues other than myelin. The silver particles are finally enhanced in a developing step (“physical developer”). The main advantage of this method is that it efficiently visualizes both large myelinated fiber tracts and individual myelinated axons. Here we provide our laboratory protocol that is suitable for paraffin embedded sections and the use of light microscopy based on Gallyas’ original protocol and subsequent modifications by Pistorio and colleagues.
Keywords: Myelin, Gallyas, Silver impregnation, Brain, Spinal cord, CNS, Paraffin
Background
Myelin is the multilayered, molecularly specialized plasma membrane of oligodendrocytes in the CNS or Schwann cells in the peripheral nervous system that accelerates nerve conduction by ensheathing axons (for comprehensive reviews: Hildebrand et al., 1993; Kidd et al., 2013; Nave and Werner, 2014; Monk et al., 2015; Simons and Nave, 2015). The myelination of axons provides electrical insulation and thus facilitates saltatory impulse propagation in a highly efficient manner (Tasaki, 1939; Hartline and Colman, 2007). In addition to insulation, myelinating cells may provide trophic support for myelinated axons (Lappe-Siefke et al., 2003; Nave, 2010; Funfschilling et al., 2012; Joseph et al., 2019).
Electron microscopy reveals morphologically distinguishable subcompartments of myelin. The compacted myelin comprises alternating electron-dense and electron-lucent layers while the adaxonal myelin layer and paranodal myelin loops remain non-compacted. At the light microscopic level, large myelinated tracts (i.e., the white matter including the corpus callosum) are clearly distinguishable from the grey matter, in which the majority of axons is non-myelinated. However, even largely unmyelinated CNS regions including the cortex comprise individual myelinated axons that appear as fine fibers if visualized by specific staining.
Ferenc Gallyas described in 1979 a method to specifically visualize myelin in the brain (Gallyas, 1979). A fundamental step is the treatment of the CNS tissue with pyridine and acetic anhydride, which during the subsequent silver impregnation prevents the absorption of silver ions by tissues other than myelin. Another critical step is the physical developer, which contains silver nitrate and tungstosilicic acid, which, once added, reduces silver ions to elemental silver. In 2005, Pistorio and colleagues described a modified version of this method by Gallyas (Pistorio et al., 2006). Here, we provide our lab protocol based on these previous versions. The major difference is the use of a microwave to decrease the staining time as heat causes the molecules to diffuse rapidly across tissues. Other differences include a slight modification in the recipe of the physical developer and the absence of a de-staining step involving potassium ferricyanide. In recent years, silver impregnation of myelin has been mainly used to assess myelinated tracts in mouse mutants (de Monasterio-Schrader et al., 2012; Werner et al., 2013; Patzig et al., 2016; Erwig et al., 2019; Joseph et al., 2019), yet the method can be applied to various species and scientific questions. As we describe the silver impregnation of paraffinated brain sections, we included the paraffin embedding and the de-paraffinization procedure.
Materials and Reagents
Cover slips (Thermo Scientific, Menzel, catalog number: 1011880500)
Microscope glass slides (R. Langenbrinck, catalog number: 03-0003)
Paraffin
50 ml Falcon tube
Mice (postnatal as well as adult brains of any mouse strain are suitable for Gallyas staining)
2-propanol (Roth, catalog number: 6752.4)
Acetic acid (Merck Millipore, catalog number: 1000631000)
Acetic anhydride (Acros organics, catalog number: 149490010)
Ammonium nitrate (Merck Millipore, catalog number: 1011880500)
Ethanol (Roth, catalog number: 9065.4)
Eukitt (O. Kindler, catalog number: 4023.1), store at room temperature
Formaldehyde 37% (Roth, catalog number: P733.1)
KCl
KH2PO4
NaCl
NaH2PO4
Nitric acid (Roth, catalog number: 2616.2)
Paraformaldehyde (Serva, catalog number: 31628.02)
Pyridine (AppliChem, catalog number: A0776.0500)
Silver nitrate (MerckMillipore, catalog number: 1015120025)
Sodium carbonate anhydrous (MerckMillipore, catalog number: 1063920500)
Sodium hydroxide (MerckMillipore, catalog number: 1064981000)
Sodium thiosulfate pentahydrate (J.T. Baker, catalog number: 3946-01)
Sucrose (Sigma-Aldrich, catalog number: S9378-5KG)
Tungstosilicic acid hydrate (MerckMillipore, catalog number: 1006590025)
Xylene (Otto Fischar, catalog number: 27404)
10x PBS stock solution (see Recipes)
4% PFA for tissue (see Recipes)
Incubating solution (see Recipes)
-
Physical developing solution (see Recipes)
Solution A
Solution B
Solution C
Equipment
Filter unit (Corning, catalog number: 431153)
Fume hood (Dueperthal, model: Typ 90)
Glassware (rectangular staining dish with glass cover, removable glass slide rack that, dimensions: 91 mm x 71 mm x 60 mm, sold by e.g., VWR)
Microscope (Zeiss, model: Axiophot)
Microtome (ThermoFisher Scientific, model: HM 430)
Paraffin embedding machine (Microm, model: HMP110)
Procedure
-
Paraffin embedding and microtome sectioning
-
Carefully remove the brain or spinal cord and post-fix it in 4% PFA for tissue for one hour up to overnight at 4 °C in a 50 ml Falcon.
Note: Prior to paraffin embedding of the brain, mice were subjected to transcardial perfusion.
Embed the brain in paraffin by using an automated embedding machine for best results.
-
Use the recommended protocol of your embedding machine. If no particular protocol is recommended, use the following protocol:
Reagent Duration
50% ethanol 1 h
70% ethanol 2x 2 h
96% ethanol 2x 1 h
100% ethanol 2x 1 h
2-propanol 1 h
xylene 2x 2 h
paraffin 2x 2 h
-
Cut 5 µm sections using a microtome and mount sections on microscope glass slides. Air-dry at room temperature (RT). Store at room RT for future use.
Note: A thickness of 5 µm is optimal for subsequent light microscopy. Mounting on slides is most conservative to the integrity of the sections.
-
-
Gallyas silver impregnation (Overview of protocol see Figure 1)
-
De-paraffinize sections by incubating for 10 min each in xylene, again xylene and 2-propanol/xylene (1:1). Afterwards incubate sections in 100%, 90%, 70%, 50% ethanol and distilled water (dH2O) for 5 min each.
Note: Formalin-fixed sections are also suitable for subsequent Gallyas silver staining as described in Pistorio et al., 2005. The conservation of morphology in paraffin sections however is superior to crysections.
To suppress staining of non-myelin tissue, incubate sections in pyridine/acetic anhydride (2:1, e.g., 200 ml of pyridine and 100 ml of acetic anhydride) for 30 min at room temperature (RT). Note: Prepare solution fresh, stored solution will turn yellow and needs to be discarded.
Wash 3 times with dH2O, for 10 min each.
Submerge sections in incubation solution, microwave for 2 min at 440 W. The incubation solution must not come to boil. The temperature should reach approximately 50 °C. Let it cool down for 10 min at RT until lukewarm.
Wash sections 3 times in 0.5% acetic acid for 5 min each.
Develop sections in physical developing solution for 3-15 min. After 1-2 min, start monitoring the reaction under the microscope by placing the sections in 1% acetic acid (= interrupts developing reaction). To determine if staining has reached the desired intensity, compare to the optimal result shown in Figure 2. The color of myelin should be dark brown and should display a good contrast as compared to the background. If too pale, the sections can be exposed once again to the physical developing solution. Repeat until optimal result is reached.
Stop the staining reaction by incubating sections 3 times in 1% acetic acid for 5 min each.
Wash twice in dH2O for 3 min each.
Incubate sections in 2% sodium thiosulfate for 5 min to fix the stain.
Wash twice with dH2O for 5 min each.
Dehydrate section in 50%, 70%, 90%, 100% ethanol for each 5 min, followed by 2-propanol/xylene (1:1), xylene and again xylene for 10 min each.
-
Mount sections with Eukitt and cover slips.
Note: Sections, stored in slides boxes at RT, have a shelf life of many years.
Result: Myelin and erythrocytes are stained black, sometimes nuclei are stained too, background is pale yellow (Figure 2).
-
-
Microscopy
Images can be acquired using a light microscope at different magnifications (Figure 2).
Figure 1. Flowchart depicting individual steps and time specification of deparaffinization, staining, rehydration and mounting procedures.
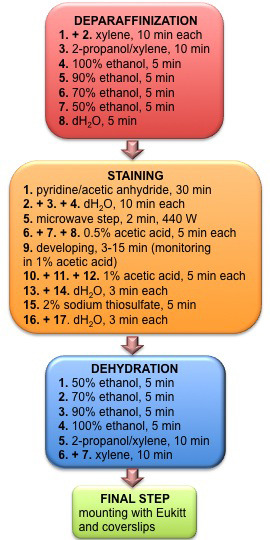
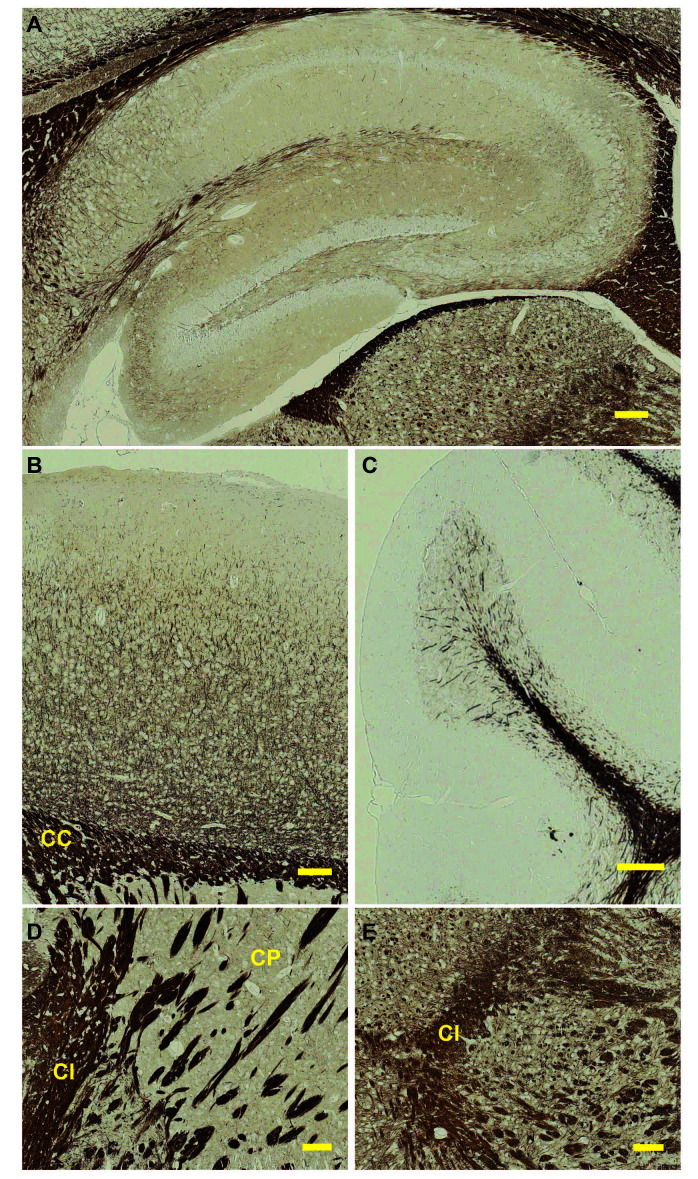
Figure 2. Gallyas silver impregnation of a mouse brain.
Sagittal view of a 3 month-old mouse brain subjected to the Gallyas protocol described here. Depicted are different regions of the brain: A. hippocampus, B. cortex, C. cerebellum, D. striatum, E. midbrain. CC = corpus callosum, CI = capsula interna, CP = caudoputamen. Scale bar = 100 μm.
Data analysis
The images of the silver-impregnated brain sections can be used as representative images or to compare gross morphological differences in myelination between experimental conditions, mouse strains etc.
Notes
Aside from washing steps using water and mounting of sections, carry out the procedure in a chemical fume hood.
Wash glassware to be used for silver staining with 1% nitric acid overnight and rinse with water.
Do not use metal objects such as spatulas, forceps etc.
Do not use metal spatula to weigh silver nitrate.
Discard reagents that contain silver in hazardous waste containers.
Recipes
-
10x PBS stock solution
NaCl 80 g
KCl 2 g
NaH2PO4 14.1 g
KH2PO4 2 g
Dissolve in 1000 ml dH2O and adjust pH to 7.3 with NaOH. Store at RT. To prepare 1x PBS, dilute with dH2O
-
4% PFA for tissue
Paraformaldehyde 4 g
Sucrose 4 g
Dissolve in 100 ml PBS. Can be stored at -80 °C
-
Incubating solution
Ammonium nitrate 1 g
Silver nitrate 1 g
Dissolve in 1000 ml dH2O
Adjust pH to 7.4-7.6 with NaOH
Brown precipitate will disappear after shaking. Can be stored for 8-10 weeks at 4 °C
-
Physical developing solution
Solution A
Sodium carbonate anhydrous 50 g
Dissolve in 1000 ml dH2O. Can be prepared ahead to procedure and stored at 4 °C for several weeks
Solution B
Ammonium nitrate 2 g
Silver nitrate 2 g
Tungstosilicic acid hydrate 10 g
Dissolve in 1000 ml dH2O. Can be prepared ahead to procedure and stored at 4 °C for several weeks.
Solution C
Ammonium nitrate 2 g
Silver nitrate 2 g
Tungstosilicic acid hydrate 10 g
Formaldehyde (37%) 7 ml
Dissolve in 1,000 ml dH2O. Can be prepared ahead to procedure and stored at 4 °C for several weeks
Final physical developing solution
50% solution A (100 ml) + 35% solution B (70 ml) + 15% solution C (30 ml)
Always prepare fresh. Do not store
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG, grant STE1117/11-1).
Competing interests
The authors declare no conflict of interest.
Ethics
The use of animals was approved by LANUV (Nordrhein-Westfalen, 84-02.04.2015.A529).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.de Monasterio-Schrader P., Jahn O., Tenzer S., Wichert S. P., Patzig J. and Werner H. B.(2012). Systematic approaches to central nervous system myelin. Cell Mol Life Sci 69(17): 2879-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erwig M. S., Patzig J., Steyer A. M., Dibaj P., Heilmann M., Heilmann I., Jung R. B., Kusch K., Mobius W., Jahn O., Nave K. A. and Werner H. B.(2019). Anillin facilitates septin assembly to prevent pathological outfoldings of central nervous system myelin. Elife 8: e43888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funfschilling U., Supplie L. M., Mahad D., Boretius S., Saab A. S., Edgar J., Brinkmann B. G., Kassmann C. M., Tzvetanova I. D., Mobius W., Diaz F., Meijer D., Suter U., Hamprecht B., Sereda M. W., Moraes C. T., Frahm J., Goebbels S. and Nave K. A.(2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485(7399): 517-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallyas F.(1979). Silver staining of myelin by means of physical development. Neurol Res 1(2): 203-209. [DOI] [PubMed] [Google Scholar]
- 5.Hartline D. K. and Colman D. R.(2007). Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17(1): R29-35. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand C., Remahl S., Persson H. and Bjartmar C.(1993). Myelinated nerve fibres in the CNS. Prog Neurobiol 40(3): 319-384. [DOI] [PubMed] [Google Scholar]
- 7.Joseph S., Vingill S., Jahn O., Fledrich R., Werner H. B., Katona I., Mobius W., Mitkovski M., Huang Y., Weis J., Sereda M. W., Schulz J. B., Nave K. A. and Stegmuller J.(2019). Myelinating glia-specific deletion of Fbxo7 in mice triggers axonal degeneration in the central nervous System together with peripheral neuropathy. J Neurosci 39(28): 5606-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidd G. J., Ohno N. and Trapp B. D.(2013). Biology of schwann cells. Handb Clin Neurol 115: 55-79. [DOI] [PubMed] [Google Scholar]
- 9.Lappe-Siefke C., Goebbels S., Gravel M., Nicksch E., Lee J., Braun P. E., Griffiths I. R. and Nave K. A.(2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet 33(3): 366-374. [DOI] [PubMed] [Google Scholar]
- 10.Monk K. R., Feltri M. L. and Taveggia C.(2015). New insights on Schwann cell development. Glia 63(8): 1376-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nave K. A.(2010). Myelination and the trophic support of long axons. Nat Rev Neurosci 11(4): 275-283. [DOI] [PubMed] [Google Scholar]
- 12.Nave K. A. and Werner H. B.(2014). Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30: 503-533. [DOI] [PubMed] [Google Scholar]
- 13.Patzig J., Kusch K., Fledrich R., Eichel M. A., Luders K. A., Mobius W., Sereda M. W., Nave K. A., Martini R. and Werner H. B.(2016). Proteolipid protein modulates preservation of peripheral axons and premature death when myelin protein zero is lacking. Glia 64(1): 155-174. [DOI] [PubMed] [Google Scholar]
- 14.Pistorio A. L., Hendry S. H. and Wang X.(2006). A modified technique for high-resolution staining of myelin. J Neurosci Methods 153(1): 135-146. [DOI] [PubMed] [Google Scholar]
- 15.Simons M. and Nave K. A.(2015). Oligodendrocytes: myelination and axonal support. Cold Spring Harb Perspect Biol 8(1): a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasaki I.(1939). The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. Amer J Physiol 127: 211-227. [Google Scholar]
- 17.Werner H. B., Kramer-Albers E. M., Strenzke N., Saher G., Tenzer S., Ohno-Iwashita Y., De Monasterio-Schrader P., Mobius W., Moser T., Griffiths I. R. and Nave K. A.(2013). A critical role for the cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. Glia 61(4): 567-586. [DOI] [PubMed] [Google Scholar]