Abstract
Mammalian skeletal muscle is a metabolically active tissue that is made up of different types of muscle fibers. These myofibers are made up of important contractile proteins that provide force during contraction of the muscle like actin and myosin. Murine myofibers have been classified into 4 types: Type I, Type IIa, Type IIb and Type IIX. Each muscle fiber has been identified with specific type of MyHC expressed, which in turn gives differential contractility to the muscle.
There have been well-known methodologies to identify different myofibers: histochemical myosin ATPase staining which uses the differential ATPase activity between slow and fast fibers, quantification of metabolic enzymes like malate dehydrogenase and lactate dehydrogenase on specific fragments of muscle fibers. The drawback of these techniques is that they cannot differentiate the subtypes of myofibers, for example, Type IIa and Type IIb. They should be used in conjunction with other known histochemical staining techniques. Here, we devise a direct and robust immunohistochemical staining methodology that utilizes the differential expression of MyHC isoforms in different myofibers types, thus efficiently distinguishing the heterogeneity of the muscle fibers. We use antibodies that specifically recognize Type I, Type IIa and Type IIb fibers on serially cut frozen mouse tibialis anterior sections that can be quantified by ImageJ software.
Keywords: Muscle fibers, Fiber-typing, Immunohistochemistry, Tibialis anterior muscle , Myosin heavy chain isoforms, murine myofiber heterogeneity
Background
Skeletal muscle is a highly organized tissue composed of several distinct compartments of muscle fibers. Each muscle fiber is a multinucleated single cell, enclosed by sarcolemma, and is predominantly made up of myofibrils ( Seeley et al., 2007 ). Muscle fibers that have low contraction speed are called slow twitch or type I fibers. Slow twitch fibers have high mitochondrial density and express myosin isoform Myh7 ( Lompre et al., 1984 ; Talmadge and Roy, 1993). Type II fibers are divided into type IIa and type IIb fibers. Both of these fibers have high contraction speed, thus generating greater force compared to type I fibers. Type IIa fibers have moderately high contraction speed and are anaerobic in nature. Type IIa fibers also have high density of mitochondria with increased oxidative potential. Type IIa fibers predominantly contain myosin isoform Myh2 in mammals. Type IIb fibers have very high contraction speed and are anaerobic in nature. Type IIb fibers have low mitochondrial density and predominantly contain myosin isoform Myh4. Muscle fibers that are neither Type IIa nor Type IIb fibers are called Type IIx or Type IId, and have high expression in the diaphragm and hence is omitted from this study (Schiaffino and Reggiani, 1994; Westerblad et al., 2010 ). Mouse tibialis anterior, a hind limb skeletal muscle made of Type I, Type IIa and Type IIb myofibers serves as a suitable muscle to study the robustness of the immunohistochemical staining procedure.
Other methodologies to distinguish the different muscle fiber types are myosin ATPase histochemical staining of frozen muscle sections pre-incubated in alkaline pH showing light staining for Type I fibers and dark staining for Type II fibers. Muscle sections pre-incubated in acidic pH showed dark ATPase staining for Type I fibers, light staining for Type IIa muscle fibers and intermediate staining for Type IIb fibers (Brooke and Kaiser, 1970, Guth and Samaha 1970). Quantitative estimation of mitochondrial enzymes like glycerophosphate oxidase (GP-OX) and succinate dehydrogenase (SDH) have been used to distinguish Type IIa and Type IIb fibers in mouse and rabbit tibialis anterior muscle but gave variable ratios of enzyme activities in mice and rabbit muscles (Reichmann and Pette, 1984). There have been quantification methodologies of specific metabolic enzymes like lactate dehydrogenase and malate dehydrogenase on frozen serial sections of muscles ( Hintz et al., 1984 ). However, these protocols are extensive and may give non-specific signals. The protocol detailed here uses specific antibodies that recognize different Myosin Heavy chain isoforms on serially cut frozen tibialis anterior mid-belly region sections that is readily detected with a suitable secondary antibody. After image acquisition, the number of each fiber type is quantified using ImageJ software.
Materials and Reagents
High Profile microtome blades 818 (Leica Biosystems, Germany, catalog number: 14035838926)
Microscope glass slides (Globe Scientific, USA, catalog number: GS-1308)
Camel hair brushes
Syringe plunger
1.5 ml microfuge tubes (USA Scientific, catalog number: 1615-5500)
Liquid blocker Pap pen (Sigma Aldrich, catalog number: Z377821)
Freshly dissected mouse hind limb tibialis anterior muscle
Myh fast 2A antibody (Developmental Studies Hybridoma Bank, catalog number: 2F7)
Myh fast 2B antibody (Developmental Studies Hybridoma Bank, Iowa City, IA, catalog number: 10F5)
Myh slow (type1) antibody (Developmental Studies Hybridoma Bank, Iowa City, IA, catalog number: A4.840)
Biotinylated sheep anti-mouse IgG antibody (Abcam, catalog number: ab6807)
Tissue-Tek Optimal cutting temperature (OCT) compound (Sakura Finetek, USA, catalog number: 4583)
Isopentane (Alfa Aesar, catalog number: 19387, CAS number: 78-78-4)
Liquid Nitrogen
Bovine serum albumin (BSA) (Roche, Switzerland, catalog number: 10711454001)
Normal sheep serum (NSS) (Sigma-Aldrich, catalog number: S22)
Tween 20 (RPI Corp., USA, catalog number: P20370, CAS number: 9005-64-5)
4',6-diamidino-2-phenylindole Dihydrochloride (DAPI) (Invitrogen, catalog number: D1306)
ProLong Gold Anti-fade solution (Invitrogen, catalog number: P10144)
Streptavidin Alexa Fluor 488 conjugate (Invitrogen, catalog number: S-11223)
Sodium phosphate, monobasic
Sodium phosphate, dibasic
37% Formaldehyde (Sigma-Aldrich, catalog number: 252549, CAS number: 50-00-0)
Milli-Q water
Blocking mixture (see Recipes)
Diluent (see Recipes)
10% Buffered formalin (see Recipes)
0.2 μg/ml DAPI in 1x PBS (see Recipes)
1x Phosphate buffered saline (PBS), pH 7.4 (see Recipes)
Equipment
Rotary Cryostat Microtome (CM1950, Leica Biosystems, Germany)
Orbital shaker (Boeco, Germany, Ref: BOE 8069200)
Leica CTR 6500 microscope equipped with Leica DFC 310 FX camera
Cryostat
Software
Image J (Fiji, https://imagej.nih.gov/ij/)
Procedure
-
Sample preparation
Dissect hind limb tibialis anterior muscle and remove the surrounding connective tissue.
Holding the tendon side of the muscle, place the tissue on the flat side of the syringe plunger.
Cover the tissue with OCT compound and immediately snap-freeze the tissue by immersing in isopentane cooled with liquid nitrogen for 30-40 s.
Store the frozen tissues at -80 °C for future use.
Cut 10 μm transverse serial sections at the mid-belly region of the muscle in a cryostat maintained at -20 °C.
Support the sections while cryosectioning with a brush and pick the sections by placing a glass slide (kept at room temperature) in very close proximity to the sections.
-
Immunostaining of the muscle sections
Draw a perimeter around the tissue section with a liquid blocker pen. This helps in staining multiple tissue sections on a glass slide and also reduces the volume of diluent required.
Add freshly prepared blocking solution (minimum of 200 μl/section) onto sections to completely cover it and incubate at room temperature for an hour on low speed orbital shaker.
Prepare 1:25 dilutions of Myh fast 2A, Myh fast 2B and Myh slow (type I) primary antibody in separate 1.5 ml microcentrifuge tubes with the diluent and incubate the sections overnight at 4 °C on a low speed orbital shaker.
Wash the sections with 1x PBS 3 times for 5 min each to remove the non-specific antibody bound to the sections.
Fix the primary antibody staining with 10% buffered formalin for 5 min and then wash three times with 1x PBS for five min each. It is recommended (specifically for DSHB MyHC primary antibodies) that the fixation should not be performed before primary antibody incubation, as this would disrupt the efficient binding of primary antibodies to different MyHC in the frozen sections. Hence fixation is performed after primary antibody incubation step.
Incubate the sections with 1:200 dilution of biotinylated sheep anti-mouse IgG secondary antibody made in diluent for one hour at room temperature with shaking.
Wash the sections with 1x PBS 3 times for 5 min each and incubate with 1:400 dilution of streptavidin Alexa Fluor 488 conjugate tertiary antibody in 0.2% BSA and 0.1% PBS-Tween 20 (0.1% Tween 20 made in 1x PBS) for one hour at room temperature in the dark.
Wash the sections with 1x PBS twice for 5 min each.
Stain the myonuclei with DAPI made in 1x PBS diluted at 1:5,000 for 5 min and rinse thoroughly with 1x PBS.
Air-dry the slides co*.xml mpletely to mount with ProLong Gold Anti-fade mountant solution and allow it to dry overnight in the dark.
Visualize using Leica CTR 6500 fluorescent microscope, equipped with the Leica DFC 310 FX camera under the FITC channel with wavelengths of excitation at 488 nm and emission at 536 nm.
Data analysis
To quantify the number of positive myofibers of each antibody used (Myh fast 2A, Myh fast 2B and Myh slow (type I), the images are imported into ImageJ (Fiji) and brightly stained muscle fibers from each antibody stained sections are counted. An example of this can be found in Figure 7 of the original research article Rao et al., 2019 , which is again mentioned in this manuscript as Figure 1. The values were expressed as mean muscle fiber number ± SE of two animals. Level of significance was calculated using two-tailed Student’s t-test in comparison to wild-type muscle (***P < 0.001) ( Rao et al., 2019 ).
Figure 1. Showing the different types of MyHC (type I, type IIa, and type IIb) staining on Akirin1 knockout (Aki1-/-) and wild‐type (WT) tibialis anterior muscle sections .
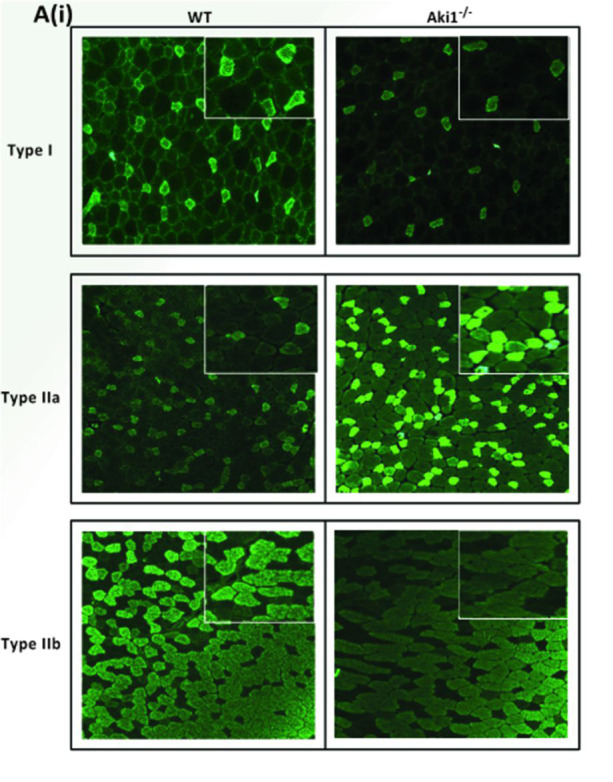
(Adapted from Rao et al., 2019 ).
Recipes
-
Blocking mixture
0.5% Triton X-100
0.2% BSA
10% NSS
1x PBS
-
Diluent
0.2% BSA
5% NSS
0.1% Tween 20 (made in 1x PBS)
-
10% Buffered formalin
Sodium phosphate, monobasic 4 g
Sodium phosphate, dibasic 6.5 g
37% Formaldehyde 100 ml
Milli-Q water 900 ml
-
0.2 μg/ml DAPI in 1x PBS
Make stock concentration of 1 mg/ml DAPI
Take 2 μl of the stock and make up to 10 ml with 1x PBS
-
1x Phosphate buffered saline (PBS), pH 7.4
Distilled water 800 ml
Sodium Chloride 8 g
Potassium Chloride 0.2 g
Sodium phosphate, dibasic 1.44 g
Potassium dihydrogen phosphate 0.24 g
Adjust the pH to 7.4 with HCl
Make up the volume to 1 L
Acknowledgments
This work was supported by the funding agency: BMRC 07/1/21/19/ 521, NMRC/EDG/0026/2008, and Startup grant. The authors acknowledge National University of Singapore for the scholarship provided to Vanitha V. Rao throughout the course of her PhD. The authors acknowledge the original research article Rao et al., 2019 from which this protocol was first discussed.
Competing interests
The authors declare no financial and non-financial competing interests.
Ethics
The animals used in the original research article were housed at the Nanyang Technological University Animal House, Singapore. All experiments were performed as per the approved protocols of Institutional Animal Ethics Committee (Singapore) with approval number ARF‐SBS/NIE-A0270.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Brooke M. H. and Kaiser K. K.(1970). Three"myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18(9): 670-672. [DOI] [PubMed] [Google Scholar]
- 2. Guth L. and Samaha F. J.(1970). Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28(2): 365-367. [PubMed] [Google Scholar]
- 3. Hintz C.S., Coyle E.F., Kaiser K.K., Chi M.M., Lowry O.H.(1984). Comparison of muscle fiber typing byquantitative enzyme assays and by myosin ATPase staining. J Histochem Cytochem 32(6):655-660. [DOI] [PubMed] [Google Scholar]
- 4. Lompre A. M., Nadal-Ginard B. and Mahdavi V.(1984). Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259(10): 6437-6446. [PubMed] [Google Scholar]
- 5. Rao V. V., Sangiah U., Mary K. A., Akira S. and Mohanty A.(2019). Role of Akirin1 in the regulation of skeletal muscle fiber-type switch. J Cell Biochem. doi: 10.1002/jcb.28406. [DOI] [PubMed] [Google Scholar]
- 6. Reichmann H. and Pette D.(1984). Glycerolphosphate oxidase and succinate dehydrogenase activities in IIA and IIB fibres of mouse and rabbit tibialis anterior muscles . Histochemistry 80(5): 429-433. [DOI] [PubMed] [Google Scholar]
- 7. Schiaffino S. and Reggiani C.(1994). Myosin isoforms in mammalian skeletal muscle. J Appl Physiol(2): 493-501. [DOI] [PubMed] [Google Scholar]
- 8. Seeley M., Huang W., Chen Z., Wolff W. O., Lin X. and Xu X.(2007). Depletion of zebrafish titin reduces cardiac contractility by disrupting the assembly of Z-discs and A-bands. Circ Res 100(2): 238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talmadge R. J. and Roy R. R.(1993). Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol(5): 2337-2340. [DOI] [PubMed] [Google Scholar]
- 10. Westerblad H., Bruton J. D. and Katz A.(2010). Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res 316(18): 3093-3099. [DOI] [PubMed] [Google Scholar]


