Abstract
Empathy for pain is referred to as an evolutionary behavior of social animals and humans associated with the ability to feel, recognize, understand and share the other's distressing (pain, social rejection and catastrophe) states. Impairment of empathy can definitely lead to deficits in social communication and sociability (attachment, bond, reciprocity, altruism and morality) that may be fundamental to some psychiatric disorders such as autism spectrum disorder (ASD), psychopathy, misconduct, antisocial personality disorder and schizophrenia. So far, the underlying mechanisms of empathy are poorly known due to lack of animal models and scarce understanding of its biological basis. Recently, we have successfully identified and validated the behavioral identities of empathy for pain in rats that can be widely used as a rodent model for studying the underlying biological mechanisms of empathy. Priming dyadic social interaction between a naive cagemate observer (CO) and a cagemate demonstrator (CD), rather than a non-cagemate, in pain for 30 min in a testing box can repeatedly and constantly result in empathic responses of the CO toward the familiar CD's distressing condition, displaying as allo-licking at the injury site, allo-grooming at the body and social transfer of pain. The familiarity-based, distress-specific social consolation and subsequent social transfer of pain can be qualitatively and quantitatively rated as experimental biomarkers for empathy for pain. The rodent model of empathy for pain is state-of-the-art and has more advantages than the existing ones used for social neuroscience since it can reflect sensory, emotional and cognitive processes of the brain in running the prosocial and altruistic behaviors in animals who could not report verbally. Here we would like to provide and share the protocol of the model for wide use.
Keywords: Empathy, Prosocial behavior, Altruism, Pain, Rodent, Model
Background
Empathy for pain is referred to as an evolutionary behavior of social animals and humans associated with the ability to feel, recognize, understand and share the other's distressing (pain, social rejection and catastrophe) states (Chen, 2018). Impairment of empathy can definitely lead to deficits in social communication and sociability (attachment, bond, prosocial reciprocity, altruism and morality) that may be fundamental to some psychiatric disorders such as autism spectrum disorder (ASD), psychopathy, misconduct, antisocial personality disorder and schizophrenia (de Waal and Preston, 2017; Chen, 2018). In humans, empathy for pain has been demonstrated to be processed by a common core neural network mainly involving the anterior cingulate cortex (ACC) and anterior insular cortex which are also associated with direct feeling of emotional pain ( Rainville et al., 1997 ; Singer et al., 2004 ; Lamm et al., 2011 ). Psychologically, empathy for pain can be expressed as changes in emotional state (feeling of pain, anxiety, etc.) and cognitive functions (attentional bias) of a subject who is witnessing distressing condition of others, especially his/her familiars (family members, kin, friends, colleagues, etc.), and this can subsequently motivate empathic concern, consolation and help in behavior (Darwin, 1871; Burkett et al., 2016 ; de Waal and Preston, 2017). Moreover, vicariously felt pain or social transfer of pain from a distressing object to a witnessing subject is frequently evidenced as empathic pain feeling (lowed pain threshold or enhanced pain sensitivity) in humans ( Goubert et al., 2005 ; Williams and Rhudy, 2007; Loggia et al., 2008 ; Villemure and Bushnell, 2009; Godinho et al., 2012 ).
In the past century, the understanding of empathy has been greatly limited to the higher species such as human and non-human primates due to academic rejection of experimental studies in lower animals such as rodents (Panksepp, 1998; Panksepp and Lahvis, 2011; Panksepp and Panksepp, 2013; Chen, 2018). However, more recently, empathy for pain has been reported to consistently exist in both mice ( Langford et al., 2006 ; Martin et al., 2015 ) and rats ( Li et al., 2014 and 2018; Lü et al., 2017 and 2018) following social interactions between dyadic familiar (cagemate) conspecifics but not stranger (non-cagemate) ones for which both or one animal is in pain (for reviews see Martin et al., 2014 ; Mogil, 2015; Chen, 2018). It is of special interest to note that the establishment of familiarity among conspecifics by co-housing them for more than two weeks is essential to induction of empathic responses to other’s pain in both mice and rats ( Martin et al., 2014 ; Mogil, 2015; Chen, 2018). According to the Russian-doll model of the evolution of empathy proposed by de Waal and his colleague, empathy is hierarchical and evolved from the very core (motor mimicry and emotional contagion) to more outer layer (empathic concern and consolation) and to the most outer layer (perspective-taking and targeted-help) (de Waal and Preston, 2017). It is interesting to find that, in our experimental studies, rats have an ability to feel, recognize and share the distressing feeling of another familiar conspecific in pain, resulting in empathic consolation and social pain contagion ( Li et al., 2014 and 2018; Lü et al., 2017 and 2018), highly supporting the Russian-doll model. In comparing to the existing mouse model published previously, the rat model of empathy for pain has several advantages as follows: (1) The CO rat is under naive condition prior to and during priming dyadic social interaction, this can completely exclude the distressing effects of direct physical pain stimulation on itself and make neurobiological, endocrine and other biological assays possible in further tests. However, it is well known that Langford and his colleagues used ‘observer-demonstrator both in pain’ model in their mouse study that could not distinguish between the effects of physical pain and vicariously felt pain (empathy for pain) ( Langford et al., 2006 ). (2) Our rat model of empathy for pain has been validated to have both empathic consolation (allo-grooming and allo-licking) and social pain contagion (empathic pain hypersensitivity) that is consistent with what has been observed in human, non-human primates and some other special species such as socially monogamous, biparental prairie vole ( Burkett et al., 2016 ; de Waal and Preston, 2017). (3) Our rat model of empathy for pain has been approved to be mediated by top-down facilitation from the medial prefrontal cortex (mPFC) and the locus coeruleus (LC)-norepinephrine (NE) system ( Li et al., 2014 ; Lü et al., 2017 ) that are known to be important brain structures involved in empathy for pain in humans ( Singer et al., 2004 ; Terbeck et al., 2016 ). (4) Our unpublished data showed that mice have the same ability as rats in empathy for pain, suggesting no species difference between the two laboratory rodents tested by our experimental setting and designs. (5) Our rodent model of empathy for pain provides a novel bio-psychosocial-brain-behavioral paradigm that can be used in combination with other advanced techniques in neuroscience such as optogenetic, chemogenetic, in-vivo multi-electrode array recordings and other neuroimaging approaches in consciously socially interacting animals. Here we mainly describe a well-established approach used in rat model of empathy for pain in a step-by-step manner.
Materials and Reagents
-
Materials
Microsyringe (50 μl) (Gaoge, China)
-
Animals
-
Sprague-Dawley albino rats (6 weeks old, 150-180 g), purchased from the Laboratory Animal Center of the Fourth Military Medical University (FMMU)
Co-housing 4-6 rats of the same sex per cage in a specific-pathogen free (SPF) animal facility with a humidity of 40-60% and a room temperature (RT) of 22-26 °C for two to three weeks after arrival at the Tangdu hospital of the FMMU.
Keep rats under a 12:12 h light/dark cycle (light off during 20:00-08:00).
Make water and food available at libitum.
-
-
Reagents
Lyophilized whole venom of Apis millifera or bee venom [BV, item number: V3375, Merck Life Science (Shanghai) Co., Ltd, China].
Formalin solution (Sinopharm Chemical Reagent Co., Ltd, China)
0.4% BV solution (see Recipes)
2.5% formalin solution (see Recipes)
Equipment
Video camera recorder (VCR, Sony, FDR-AX40, Japan)
Precise Tactile Sensory Evaluator: von Frey monofilaments (Ugobasile, catalog number: 37450-275, Italy)
-
Mechanical pain sensitivity test device
The mechanical pain sensitivity test setting (customized apparatus) includes a supporting platform and a nontransparent plastic testing box for limiting the range of movement of animals. The supporting platform (160 x 30 x 40 cm) is equipped with metal mesh. The pore size of the mesh (0.5 cm x 0.5 cm) is preferably such that the rat can move freely on the surface without getting caught.
-
Plastic observing cage
Another plastic observing cage covered with sterilized wooden padding at the bottom surface serves as an arena for dyadic social interaction and communication (40 x 30 x 15 cm, customized apparatus).
Software
Statistical test (SPSS 25.0)
Graph and artwork (GraphPad Software Inc. California, USA, GraphPad Prism 7.0)
Procedure
-
Preparation for experiment
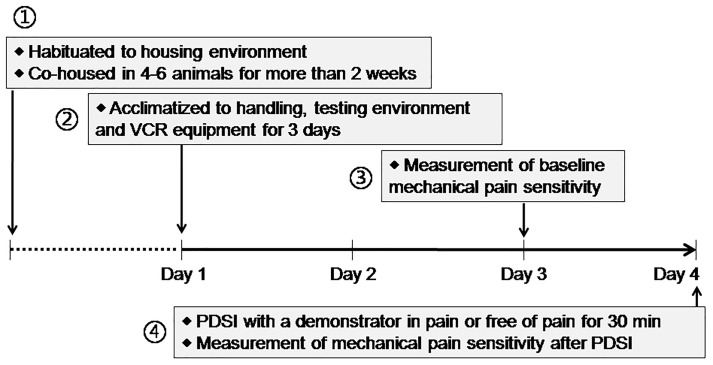
On each day, the habituation tests were performed between 08:00 and 12:00 (for details see Figure 1).
Conduct the experiment in a separate room away from unnecessary interference of scents, noises or movement. No other animals can be seen in this room.
Choose a pair of cagemates randomly serving as observer (CO) or demonstrator (CD) from conspecifics of the same sex co-housed together for more than two weeks immediately before the experiment. During housing, any kind of stressors such as strong odor and noise were avoided.
-
During Days 1-3, allow rats to acclimate to the experimental environment once daily for three days before the experiment (Figure 1).
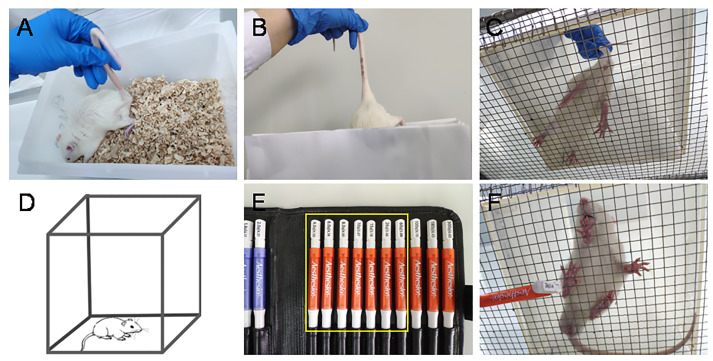
Note: The adaption of experimental environment includes handling, placing, removing from cage to the testing box, VCR and other objects arranged in the observing room. (Figures 2A-2D)
On Day 3, measure the baseline mechanical pain threshold of all animals before the priming dyadic social interaction (PDSI) (Figures 2E-2F, Table 1) (also see Step B2). PDSI was defined as a preemptive condition that allows full body contact and social communication between the dyads of conspecifics so that social transfer or contagion of pain can be achieved (Mogil, 2015; Chen, 2018).
-
On Day 4, pre-positioning the VCR at a top view over the observing cage in which cagemates (or non-cagemates) are randomly paired to serve separately as a demonstrator in pain and a naive observer.
Video 1. Showing experimental protocol for the establishment of empathy for pain model in laboratory animals.
Download video file (76.4MB, mp4) During Days 1-3, environmental and manipulating adaptation to the experimental environment and manual handling by the experimenters. On Day 4, the baseline values of PWMT in both hind paws of a naive CO rat were first measured and determined using a set of von Frey monofilaments, followed by a VCR of 30-min PDSI between a naïve CO and a familiar conspecific (CD) in pain (for detailed behavioral expressions during 30 min PDSI also see Video 2 that was finally succeeded by last measurement of PWMT for determining whether social pain contagion occurred in the naive CO rats.
During Days 1-3, environmental and manipulating adaptation to the experimental environment and manual handling by the experimenters. On Day 4, the baseline values of PWMT in both hind paws of a naive CO rat were first measured and determined using a set of von Frey monofilaments, followed by a VCR of 30-min PDSI between a naïve CO and a familiar conspecific (CD) in pain (for detailed behavioral expressions during 30 min PDSI also see Video 2 that was finally succeeded by last measurement of PWMT for determining whether social pain contagion occurred in the naive CO rats.
-
Experimental design and procedures
-
Priming dyadic social interaction (PDSI)
The test is performed between 08:00 and 14:00 on each experimental day (for details see Figure 1). Place a CO rat in a test cage in advance and allow it to move freely for 30 min. (Figure 3A)
Select pain model for CD rat: according to our previous studies, the BV model ( Li et al., 2014 and 2018), the formalin model ( Li et al., 2018 ) and the acetic acid-induced writhing model ( Lü et al., 2018 ) can stably induce empathic response in the CO rats through 30 min PDSI. Here, we’ll take BV model as an example.
-
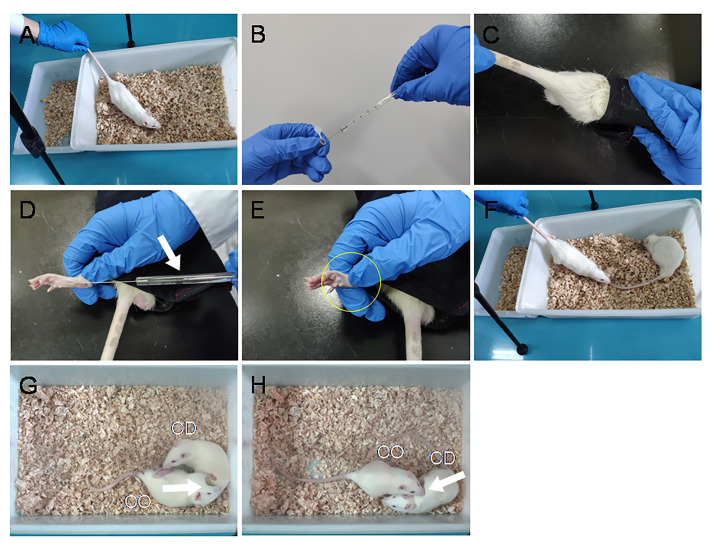
BV model can be produced by subcutaneous (s.c.) injection of BV solution into the left hind-paw pad of the CD rat. (Figures 3B-3E)
Note: The rat with s.c. BV has been shown to express eye-identifiable pain-related behavioral manifestations such as persistent spontaneous paw flinches, paw licking and lifting at the injected paw lasting for at least 1 h. (Chen et al., 1999; Chen and Lariviere, 2010; Li et al., 2014 and 2018; Lü et al., 2017)
Place the CD rat back to the test cage immediately after s.c. BV injection and allow PDSI for 30 min.
Start the VCR to record the behaviors of dyads during 30-min PDSI. (Figures 3F-3H, also see Video 2)
-
Analyze the CO's empathic consolation behaviors (allo-licking and allo-grooming, see Video 2), other social approaching behaviors (allo-mouth sniffing and allo-tail sniffing), and self-grooming through offline VCR. Recording counts and durations of each behavior during the 30-min social interactions in the CO.
Note: The empathic consolation behaviors include allo-grooming and allo-licking (Figures 3G-3H). Judging criteria for empathic consolation behavior: (1) Allo-licking was defined as an observer's sustained licking action to a demonstrator's injury site (left hind-paw for this case). Licking less than 1 s was not counted. Licking directed toward the other part of the body except the injury hind-paw was excluded. (2) Allo-grooming was defined as an observer’s head contact with the head or body of a demonstrator, accompanied by a rhythmic head movement (also see Burkett et al., 2016; Li et al., 2018). Grooming of less than 1 s was excluded. Grooming directed toward the genitals or tail was excluded.
Remove and transport the CO rat to a nontransparent testing box for PWMT measurement so as to evaluate empathic mechanical pain hypersensitivity (EMPH). (For method see Figures 2E-2F, Table 2) (Step B2)
-
Quantitative measurement of PWMT
Place a nontransparent plastic testing box (20 x 20 x 25 cm) in advance on a supporting platform with a metal mesh (1 cm x 1 cm) at the top. (Figure 4A)
Allow the CO rat to acclimate to the test metal mesh platform for 30 min.
Measure the PWMT with a graded series of von Frey monofilament as described above. (Figures 2E-2F, Figure 4B, also see note for Table 1)
Record the number of paw withdrawal reflex in response to stroking by the same vF monofilament (10 times stimuli, 5 s interval) applied to the stimulation site, and increase the stimulation intensity of vF monofilament until the bending force being able to elicit more than 50% paw withdraw reflex. (Table 2, also see note for Table 1, Chen et al., 1999 )
Repeat the measurement by hours until the PWMT restored to the baseline level before the PDSI. (Figure 4C)
-
Figure 1. Timeline of the scheduled experimental design.
Figure 2. Environmental and manipulating adaptation and measurement of baseline pain sensitivity.
A-D. Adaption to the experimental environment and manual handling by the experimenters. E-F. Measurement of paw withdrawal mechanical threshold (PWMT) at bilateral hind-paw pads using a set of von Frey monofilaments (also see Video 1).
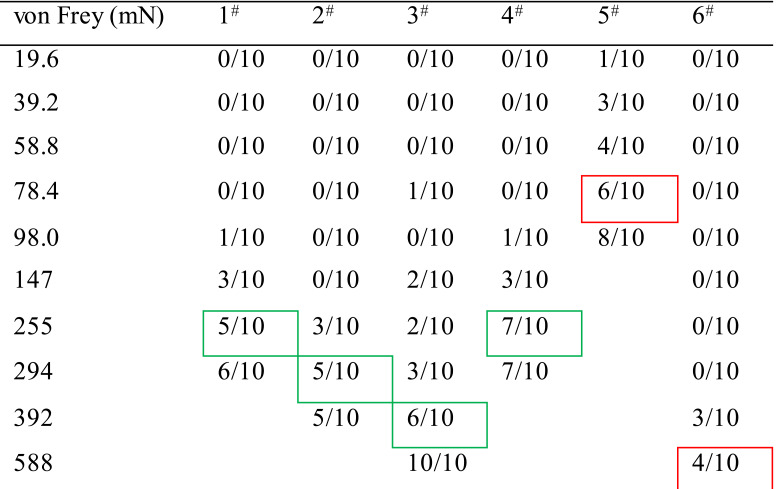
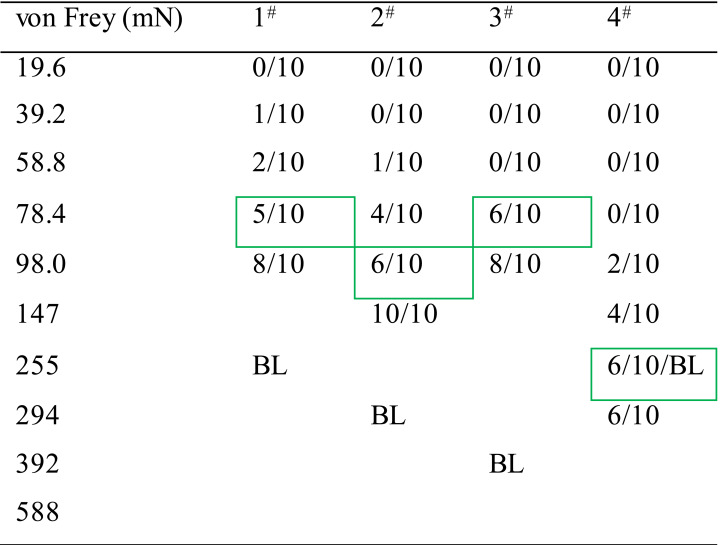
Table 1. Method for determining value of the PWMT.
Note: A 10 graded series of von Frey monofilaments (vF) weighing at different intensities ranging from 19.6 mN to 588 mN were used (see the above Table 1). The measurement of the PWMT was started from the lowest to the higher intensity of the vF, and stopped with the endpoint readout at a given intensity that could induce 50-60% of paw withdrawal reflex to 10 stimuli applied to each paw pad. Table 1 shows an example of results from measurements of baseline values in PWMT (PWMTbaseline, mN) in six animals (#1-6). For details, the animal #1 did not respond to the vF stimuli at intensities of 19.6 mN, 39.2 mN, 58.8 mN and 78.4 mN at all when receiving 10 stroking stimuli (shown as 0/10), while it responded with one paw withdrawal to 10 times of 98.0 mNvF stimuli (1/10), and with three paw withdrawals to those of 147 mN (3/10), and with five and six paw withdrawals to those of 255 mN (5/10) and 294 mN (6/10), thus the PWMTbaseline value could be determined as 255 mN (green rectangled). The green rectangled numbers indicate the readout PWMT for a paw pad of 4 animals (1-4#), while the red rectangled ones indicate extreme bias of either hypersensitive (5#) or hyposensitive (6#) which should be excluded from the experiment.
Figure 3. Procedural display of VCR arrangement, preparation of BV pain model in the CD rat and PDSI between a naive CO and the CD in pain.
A. CO's acclimation to the VCR environment. B-E. BV injection process: draw 50 μl BV solution with a microsyringe (B). Remove and guide the CD rat head into a black canvas bag and expose the left hind-paw (C-D). Then, penetrate the needle of the microsyringe through the skin into the central part of the left hind-paw pad followed by slow injection (D-E, Arrow). Remove the needle immediately after injection and press for several seconds to prevent extravasation of reagents and bleeding (D-E, Circle). F-H. Put the CD rat back to the observing cage and reunify it with the CO rat for 30-min PDSI. (For more details see Video 1)
Video 2. Behavioral expressions of empathy for pain in a naive CO during 30-min PDSI with a conspecific (CD) in pain.

Allo-licking and allo-grooming are shown as consolation behaviors of a naive CO toward a CD in pain.
Table 2. Measurement of CO's PWMT after 30-min PDSI with a CD in pain.
Note: The bending forces (255 mN, 294 mN and 392 mN for CO rat 1#, 2# and 3#) that had been able to evoke 50-60% paw withdrawal reflex (green rectangled number in Table 1) prior to PDSI (BL, baseline) became to be less (78.4 mN, 98.0 mN and 78.4 mN for the same animals, number indicated with green rectangles in Table 2), and this decrease in the value of PWMT was defined as empathic mechanical pain hypersensitivity (EMPH) (see Li et al., 2014 and 2018; Lü et al., 2017). Among the four CO rats, one fourth showed no change in the PWMT after PDSI with a CD in pain (see animal 4#), suggesting no empathy for pain can be seen in someone (see Li et al., 2018).
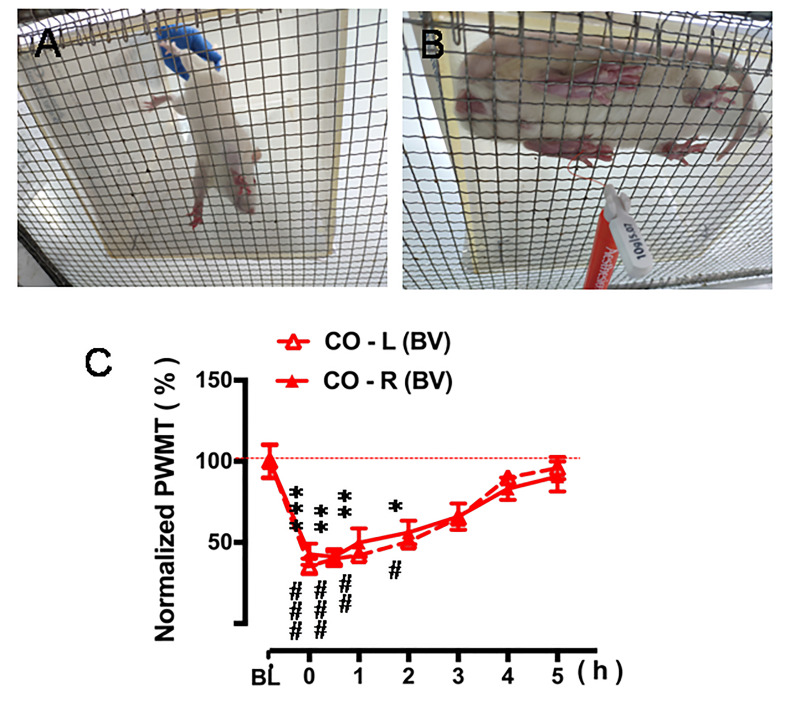
Figure 4. Quantitative measurement of pain sensitivity after PDSI.
A. Place the CO rat on the metal mesh platform lightly and avoid fright. B. Measure the changes in PWMT after 30-min PDSI with a CD in pain every hour interval. C. Five-hour time courses of decrease in bilateral PWMT after 30-min PDSI when compared to the value of baseline (BL) PWMT (Figure 4C from Li et al., 2018 . Validating rat model of empathy for pain: effects of pain expressions in social partners. Front Behav Neurosci, 12:242. With permission according to CC BY license of open access journals).
Data analysis
All statistical analyses are performed using SPSS 25.0. The artwork was created by GraphPad Prism 7.0. All data are shown as mean ± SEM. The data for PWMT are normalized by the following formula:
PWMT = PWMTpost-treatment/PWMTbaseline x 100%
One-way ANOVA with a Turkey’s correction for multiple comparisons was used to determine statistical significance for social or non-social behaviors among different groups. One-way repeated measurement ANOVA was used to determine statistical significance for PWMT.
Notes
Please refer to the original publication for the extended model and examples of the expected results for http://doi:10.3389/fnbeh.2018.00242. Pain models for CD are not limited to BV models. The other pain models in the CD that show eye-identifiable pain manifestations could induce empathic responses in familiar CO conspecifics, such as the formalin model and the acetic acid-induced writhing model. However, pain models without eye-identifiable pain manifestations are likely to fail to induce empathic behavior in familiar observers following 30-min PDSI, such as Complete Freund's adjuvant (CFA) model and the spared nerve injury (SNI) model ( Li et al., 2018 ).
According to our previous experiments, Sprague-Dawley rats or C57 mice, both male and female (unpublished), can use these protocols in the study of empathy for pain.
For a VCR setting, a right top-down vertical view is better than any directions for offline rating of both social and non-social behaviors of the subject animal in an observing cage.
Perform offline VCR analysis and mechanical pain sensitivity test in a double-blind manner.
In this experiment, the occurrence of mechanical hyperalgesia in observer rat is considered to be an important index of pain empathy, thus, the baseline mechanical pain threshold is strictly required. However, some extreme hypersensitive or hyposensitive rats with PWMT less than 147 mN or more than 588 mN should be excluded (see Tables 1 and 2).
Recipes
-
0.4% BV solution
Prepare 0.4% BV solution by dissolving 0.2 mg BV in 50 μl in 0.9% physiological saline (pH 7.4) before use
-
2.5% formalin solution
Prepare 2.5% formalin solution by dissolving 37% formaldehyde stock solution in 0.9% physiological saline (pH 7.4) before use
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to Jun Chen (81571072) and to Chun-Li Li (31600855).
Competing interests
The authors declare that they have no conflicts of interest or competing interests.
Ethics
This study was carried out in accordance with the recommendations of the ARRIVE guidelines ( Kilkenny et al., 2010 ), the U.K. Animals (Scientific Procedures) Act 1986 and associated guidelines, the EU Directive 2010/63/EU for animal experiments, the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978), and the ethical guidelines for investigations of experimental pain in conscious animals of the International Association for the Study of Pain were also critically followed (Zimmermann, 1983).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Burkett J. P., Andari E., Johnson Z. V., Curry D. C., de Waal F. B. and Young L. J.(2016). Oxytocin-dependent consolation behavior in rodents. Science 351(6271): 375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen J.(2018). Empathy for distress in humans and rodents. Neurosci Bull 34(1): 216-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J. and Lariviere W. R.(2010). The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol 92(2): 151-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J., Luo C., Li H. and Chen H.(1999). Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain 83(1): 67-76. [DOI] [PubMed] [Google Scholar]
- 5. Darwin, C.(1871). The descent of man. 2nd edition. London: Penguin Group. [Google Scholar]
- 6. de Waal F. B. M. and Preston S. D.(2017). Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18(8): 498-509. [DOI] [PubMed] [Google Scholar]
- 7. Godinho F., Faillenot I., Perchet C., Frot M., Magnin M., and Garcia-Larrea L.(2012). How the pain of others enhances our pain: searching the cerebral correlates of'compassional hyperalgesia'. Eur J Pain 16: 748-759. [DOI] [PubMed] [Google Scholar]
- 8. Goubert L., Craig K. D., Vervoort T., Morley S., Sullivan M. J., de C. W. A. C., Cano A. and Crombez G.(2005). Facing others in pain: the effects of empathy. Pain 118(3): 285-288. [DOI] [PubMed] [Google Scholar]
- 9. Kilkenny C., Browne W. J., Cuthill I. C., Emerson M. and Altman D. G.(2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1(2): 94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamm C., Decety J. and Singer T.(2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54(3): 2492-2502. [DOI] [PubMed] [Google Scholar]
- 11. Langford D. J., Crager S. E., Shehzad Z., Smith S. B., Sotocinal S. G., Levenstadt J. S., Chanda M. L., Levitin D. J. and Mogil J. S.(2006). Social modulation of pain as evidence for empathy in mice. Science 312(5782): 1967-1970. [DOI] [PubMed] [Google Scholar]
- 12. Li C. L., Yu Y., He T., Wang R. R., Geng K. W., Du R., Luo W. J., Wei N., Wang X. L., Wang Y., Yang Y., Yu Y. Q. and Chen J.(2018). Validating rat model of empathy for pain: effects of pain expressions in social partners. Front Behav Neurosci 12: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z., Lu Y. F., Li C. L., Wang Y., Sun W., He T., Chen X. F., Wang X. L. and Chen J.(2014). Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain 155(7): 1253-1261. [DOI] [PubMed] [Google Scholar]
- 14. Loggia M. L., Mogil J. S. and Bushnell M. C.(2008). Empathy hurts: compassion for another increases both sensory and affective components of pain perception. Pain 136(1-2): 168-176. [DOI] [PubMed] [Google Scholar]
- 15. Lü Y. F., Ren B., Ling B. F., Zhang J., Xu C. and Li Z.(2018). Social interaction with a cagemate in pain increases allogrooming and induces pain hypersensitivity in the observer rats. Neurosci Lett 662: 385-388. [DOI] [PubMed] [Google Scholar]
- 16. Lü Y. F., Yang Y., Li C. L., Wang Y., Li Z. and Chen J.(2017). The locus coeruleus-norepinephrine system mediates empathy for pain through selective up-regulation of P2X3 receptor in dorsal root ganglia in rats. Front Neural Circuits 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin L. J., Hathaway G., Isbester K., Mirali S., Acland E. L., Niederstrasser N., Slepian P. M., Trost Z., Bartz J. A., Sapolsky R. M., Sternberg W. F., Levitin D. J. and Mogil J. S.(2015). Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol 25(3): 326-332. [DOI] [PubMed] [Google Scholar]
- 18. Martin L. J., Tuttle A.H., and Mogil J.S.(2014). The interaction between pain and social behavior in humans and rodents. Curr Top Behav Neurosci 20: 233-250. [DOI] [PubMed] [Google Scholar]
- 19. Mogil J. S.(2015). Social modulation of and by pain in humans and rodents. Pain 156 Suppl 1: S35-41. [DOI] [PubMed] [Google Scholar]
- 20. Panksepp J.(1998). Affective neuroscience: The foundations of human and animal emotions. Oxford Univesity Press. [Google Scholar]
- 21. Panksepp J. and Panksepp J. B.(2013). Toward a cross-species understanding of empathy. Trends Neurosci 36(8): 489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panksepp J. B. and Lahvis G. P.(2011). Rodent empathy and affective neuroscience. Neurosci Biobehav Rev 35(9): 1864-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rainville P., Duncan G. H., Price D. D., Carrier B. and Bushnell M. C.(1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277(5328): 968-971. [DOI] [PubMed] [Google Scholar]
- 24. Singer T., Seymour B., O'Doherty J., Kaube H., Dolan R. J. and Frith C. D.(2004). Empathy for pain involves the affective but not sensory components of pain. Science 303(5661): 1157-1162. [DOI] [PubMed] [Google Scholar]
- 25. Terbeck S., Savulescu J., Chesterman L. P. and Cowen P. J.(2016). Noradrenaline effects on social behaviour, intergroup relations, and moral decisions. Neurosci Biobehav Rev 66: 54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villemure C. and Bushnell M. C.(2009). Mood influences supraspinal pain processing separately from attention. J Neurosci 29(3): 705-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams A.E., and Rhudy J.L.(2007). The influence of conditioned fear on human pain thresholds: does preparedness play a role? J. Pain 8, 598-606. doi: 10.1016/j.jpain.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann M.(1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2): 109-110. [DOI] [PubMed] [Google Scholar]