Abstract
Tremendous innovation is underway among a rapidly expanding repertoire of promising personalized immune-based treatments. Therapeutic cancer vaccines (TCVs) are attractive systemic immunotherapies that activate and expand antigen-specific CD8+ and CD4+ T cells to enhance anti-tumor immunity. Our review highlights key issues impacting TCVs in clinical practice and reports on progress in development. We review the mechanism of action, immune-monitoring, dosing strategies, combinations, obstacles, and regulation of cancer vaccines. Most trials of personalized TCVs are ongoing and represent diverse platforms with predominantly early investigations of mRNA, DNA, or peptide-based targeting strategies against neoantigens in solid tumors, with many in combination immunotherapies. Multiple delivery systems, routes of administration, and dosing strategies are used. Intravenous or intramuscular administration is common, including delivery by lipid nanoparticles. Absorption and biodistribution impact antigen uptake, expression, and presentation, affecting the strength, speed, and duration of immune response. The emerging trials illustrate the complexity of developing this class of innovative immunotherapies. Methodical testing of the multiple potential factors influencing immune responses, as well as refined quantitative methodologies to facilitate optimal dosing strategies, could help resolve uncertainty of therapeutic approaches. To increase the likelihood of success in bringing these medicines to patients, several unique development challenges must be overcome.
Keywords: personalized therapeutic cancer vaccine, cancer immunotherapy, drug development, clinical trials, tumor neoantigen, adjuvant, clinical pharmacology
Graphical Abstract
Shemesh et al. present a detailed review of the tremendous innovation underway among a rapidly expanding repertoire of personalized cancer vaccines (PCVs) in phase 1 or 2 development. The findings reveal PCVs as substantially complex immunotherapies with unique challenges requiring forward-thinking approaches to influence their translational success.
Main Text
Therapeutic cancer vaccines (TCVs) have been heavily investigated in clinical trials for the past 50 years as investigational immunotherapies that aim to elicit new, or strengthen existing, CD8+ cytotoxic T cell lymphocyte (CTL) tumor antigen-specific responses.1,2 As TCVs target antigens predominantly associated with tumor cells, this approach can be safer than other therapies by avoiding off-target effects. TCVs have evolved as a promising class of drugs in the immuno-oncology space, and they comprise a diverse set of antigens, adjuvants, delivery vectors, and administration methods.3 Historically, hundreds of TCV clinical trials including dozens of pivotal investigations were largely unsuccessful in demonstrating a clear clinical benefit.4,5 This is likely due to a combination of factors not limited to (1) suboptimal antigens, (2) lack of effective adjuvants, (3) poorly immunogenic platforms, and (4) an insufficient number of CTLs entering the tumor due to immunosuppression related to high disease burden, poor immune fitness, or an immunosuppressive tumor microenvironment.6,7
Renewed investment and innovation are now underway, among a rapidly expanding repertoire of advanced TCV platforms. Among recent advances are personalized neoantigen-based TCVs with selective individualized antigens and new combination approaches to enhance immune activities compared to conventional TCVs against shared antigens.8, 9, 10 Given that neoantigen load has been correlated with response to existing immunotherapies,11,12 these represent compelling targets for personalizing TCVs to enhance activity. More than 799 TCVs are in the global drug development pipeline as of 2019, with more than 400 active clinical trials.13 Of these, at least 23 are personalized vaccination approaches, which are well suited to investigate therapeutically as custom-tailored medicines in patients. Our review aims to provide a detailed account of the key components and common mechanisms of action of TCVs, while focusing on personalized TCVs, including (1) assessing the current clinical trial landscapes, (2) summarizing vaccination strategies, combination immunotherapies, common obstacles in development, and the regulatory framework of personalized TCVs, and, lastly, (3) by providing insight into additional development aspects important for the clinical development of personalized TCVs.
Mechanism of Action and Key Elements
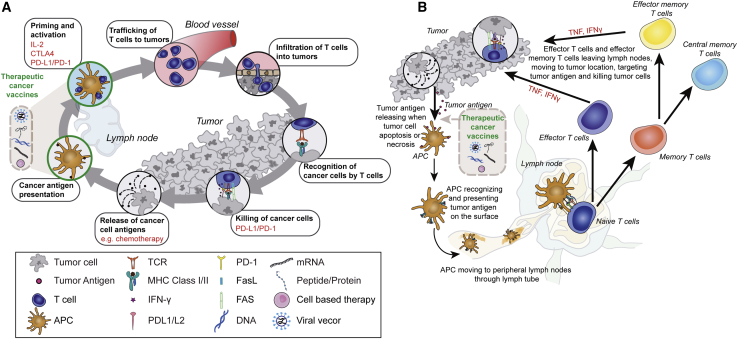
Many of the mechanisms of action and biology relevant for TCVs also apply to personalized TCVs. To successfully induce anti-tumor T cell responses in the human body, TCVs act on and exploit multiple aspects of cancer immunity, including cancer antigen presentation, T cell priming and activation, recognition of cancer cells by T cells, and several effector mechanisms to eliminate tumor cells.14 TCVs engage both innate and adaptive immunity with the use of an adjuvant and antigen to trigger an innate and adaptive response, respectively. Nonspecific innate immune responses are activated via pattern recognition receptors, such as Toll-like receptors, that recognize and respond to pathogen- or damage-associated molecular patterns. Engagement of these receptors activates transcription factor nuclear factor κB (NF-κB), stimulates cytokine and chemokine production, and recruits and activates lymphocytes.15 To induce adaptive CTL-mediated anti-tumor responses, TCVs must assist in (1) presentation and recognition of immunogenic tumor antigens by antigen-presenting cells (APCs); (2) recruitment, antigen processing, and maturation of APCs; (3) induced expression of T cell costimulatory signals and cytokines by APCs; (4) interaction of APCs with the adaptive immune system to prime and activate CD8+ T cells; and, lastly, (5) localization of these elements to the tumor.16, 17, 18 A schematic of common TCV immune mechanisms to induce human anti-tumor T cell responses is provided in Figure 1. Through these processes TCVs can generate long-lasting immunological memory capable of controlling tumor growth and inhibiting relapse and metastasis. In preclinical studies, it has been shown that long-lived memory T cell responses regenerate effector T cells to eliminate tumor cells.19 Unfortunately, TCVs have rarely met the criteria among the number of biological processes that must be engaged for a TCV to be efficacious; however, new approaches hold promise for improved performance.20
Figure 1.
Immune Mechanisms That Underlie Tumor Immunity to Successfully Induce Anti-tumor T Cell Responses in the Human Body
Therapeutic cancer vaccines (TCVs) aim to generate potent immune responses by presentation of antigens to dendritic cells that traffic through the lymphatics and present cancer antigens to naive T cells. Activated cytotoxic T lymphocytes proliferate, multiply, and traffic throughout the body, and they can provide long-lasting immunologic memory. (A) TCV action and combination immunotherapy impacts on specific components of the cancer immunity cycle. (B) T cell activation, effector function, and immunological memory specific to TCV therapy. Adapted with permission from presentation by Chen and Mellman14 and Song et al.16
To strengthen anti-tumor immunity, TCVs must activate tumor-associated antigen-specific CTLs, and thus targeting neoantigens expressed on a tumor cell surface via the use of a personalized TCV may be an effective strategy. The choice of target antigen is a major determinant of immunogenicity and takes advantage of distinctions between tumor and normal cells. Numerous approaches are used to identify one or multiple antigens for a TCV. These include selection of overexpressed or dysregulated tumor-associated proteins, such as the melanoma antigen gene (MAGE), New York esophageal squamous cell carcinoma 1 (NY-ESO01), or human epidermal growth factor receptor 2 (HER2) proteins, or by personalized approaches targeting cancer-associated neoantigens that arise by mutations found in a specific tumor.16,21 Most TCVs to date have been directed against the first type, i.e., antigens overexpressed in malignant cells and expressed at lower levels in healthy tissues.1
CD4+ T cells play a complex and pivotal helper role orchestrating cancer immunity by regulating and priming antigen-specific CD8+ T cells (boosting function, magnitude, quality, persistence, and memory); additionally, these cells provide protective immunity through effector function, cytokine secretion, and activation of tumoricidal macrophages.22, 23, 24 Interferon (IFN)-γ release by CD4+ T cells is required for elimination of tumor cells25,26, and immune attack against tumors depend on both CD4+ and CD8+ T cells, with depletion of either subset limiting tumor inhibition.27 Mechanistic insights into the crosstalk between T lymphocyte subsets, as well as future work to optimize and modulate CD4+ T cells against specific tumor antigens, are likely crucial determinants for improved clinical response of personalized TCVs.27, 28, 29, 30, 31
TCVs currently in development rely on various methods of antigen delivery, including cell-based, protein/peptide-based, RNA- or DNA-based, and viral/bacterial-based approaches. Each of these has unique considerations related to manufacturing, delivery, antigen selection, immunogenicity, and tolerability.1 A wide spectrum of adjuvants may also be used to enhance immune responses.32 The optimal adjuvant must contain attributes to produce more immunity than the antigen alone and succeed at increasing cell-mediated immunity to an optimal amplitude, specificity, and effector profile, some of which can be optimized preclinically.32,33 Most adjuvants activate damage- or pathogen-associated pattern recognition receptors, initiating a cascade of innate immune response aiding presentation of antigens on APCs. The multitude of diverse approaches for selection, engineering, packaging, and delivery of antigens, in conjunction with the complex biology, give rise to great complexity in comparing TCV platforms systematically.
Personalized TCVs
Neoantigen-based vaccines are individualized tumor-specific therapies,34 typically targeting multiple tumor antigens unique to each patient. To identify and confirm expression of non-synonymous somatic mutations expressed in the tumor for inclusion in a personalized TCV, a biopsy of tumor tissue is taken for whole-exome and RNA sequencing. Mutations are analyzed using major histocompatibility complex (MHC) class I epitope prediction algorithms and prioritized. Ranked lists of candidate antigens are further refined based on in vitro binding assay results in which synthetic peptides are tested for binding to the appropriate class I human leukocyte antigen allele of interest.9 Selected neoantigens are tumor-specific and, hence, unlike tumor-associated antigens, neoantigen-specific T cells are less likely to have been eliminated during development of immune self-tolerance. This enhances their immunogenicity and ability to stimulate robust T cell responses and increases the breadth and diversity of the response.8 Various types of variant mutations can be targeted by neoantigen-based vaccines. These include the common use of single nucleotide variants resulting from a single nucleotide change from one base to another, or indels as an insertion or deletion of a sequence of nucleotides from the genome resulting in a frameshift mutation that may alter protein function. Neoantigens selected may be of clonal origin present in all tumor cells or subclonal, which are present only in a subset, both of which influence immunoreactivity.35 Mutations can also be classified by their role in tumor growth, and either passenger mutations lacking intrinsic growth advantages, or driver mutations that provide growth advantages selected during tumor evolution, can be incorporated into personalized TCVs.36
Patients harbor extensive variability in tumor neoantigen expression and clonality that gives rise to evasion of immune effectors and formation of resistance mechanisms, which are key challenges to reducing variability and increasing efficacy for immunotherapies such as TCVs.37,38 Tumors with high neoantigen intratumoral heterogeneity have a higher degree of branched mutations that give rise to an increased amount of subclones expressed with specific neoantigens, resulting in weaker neoantigen-specific T cell responses.39 Provided that T cell infiltration and anti-tumor effect are related to selected antigens and the percentage of tumor cells expressing selected antigens, high fractions of subclonal neoantigens have had a negative impact on the response to immunotherapy.35 Innovative multi-epitope approaches targeting more neoantigens by multiplexed personalized TCVs in addition to multi-regional tumor sampling that account for temporal changes following longitudinal liquid biopsy at follow-up may be key to combating tumor antigen heterogeneity,39,40 allowing for diverse targeting of both dominant subclones and low-abundance neoantigens to increase T cell reactivity.
Following vaccination, augmentation of initial CTL responses through epitope spread leads to distinct immune responses to additional untargeted cryptic tumor antigens not present in TCVs, which have been reported in mouse and human cancers.31,41, 42, 43, 44, 45 These become additional targets of ongoing immune response important in control and protection against heterogeneous tumors in a robust, durable, and adaptive process that broadens and expands over time.46 Correlations between epitope spread and tumor regression show significant value in immune response diversification by TCV therapies.47
Foundational pre-clinical work of tumor neoantigens led to the first proof-of-concept study in mice, which revealed that mice treated with a peptide-based neoantigen vaccine against mLama4 and mAlg8 using poly(IC) as an adjuvant conferred strong anti-tumor immunity and high response rates in treated animals.48 Further supportive preclinical efforts in other tumor models using other neoantigen-based vaccines have since confirmed anti-tumor immunity through de novo CD8+ T cell responses that are capable of inducing tumor rejection of aggressive tumors in mice, conferring a survival benefit.49 The anti-tumor efficacy of neoantigen-based cancer vaccines has been established with the first neoantigen vaccine clinical trials in patients with melanoma, and later in patients with glioblastoma, validating the potential benefit of neoantigen-based cancer vaccines as effective and even curative cancer therapies.50, 51, 52, 53
Now more than a dozen companies and academic institutions have partnered to explore personalized TCVs and have many studies ongoing or poised to begin enrolling patients. Advances in DNA/RNA sequencing, epitope prediction algorithms, and artificial intelligence are helping to identify more potent neoantigens. Additionally, optimization and expansion of manufacturing capacity is underway with the goal of supporting more widespread use of personalized TCVs.54,55 Many personalized TCVs have entered this space with accelerated development plans, despite considerable investment risk and uncertainty regarding the best platform given the many unproven methodologies among diverse algorithms for neoantigen prediction. Additional questions remain around optimal delivery, dosing, and identifying the best therapeutic setting.56, 57, 58 More human data are needed to substantiate the evolving field of diverse immunizing platforms.
Clinical Trial Landscape of Personalized TCVs
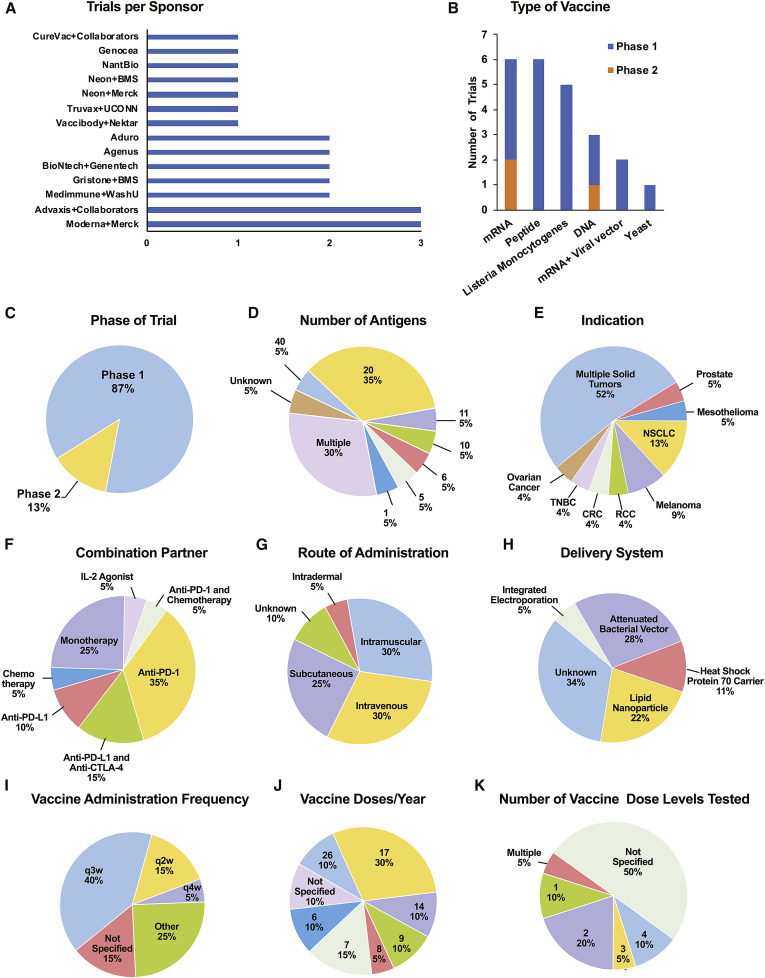
An analysis of personalized TCVs in clinical trials conducted by 13 major companies and partnerships was performed to investigate the various platforms, antigens, algorithms, delivery systems, clinical settings, endpoints, and preliminary clinical findings. Parameters for trial selection were limited to a search of personalized TCVs acting through neoantigens, which included both completed and ongoing trials in 2017 through March 2020. Sources included ClinicalTrials.gov, company websites and press releases, conference presentations, and abstracts within the last 5 years at the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the Society for Immunotherapy of Cancer (SITC), and the American Association for Cancer Research (AACR), in addition to PubMed search results for relevant publications. Keywords included personalized cancer vaccines, personalized neoantigens/neoepitopes, personalized immunotherapy, personalized vaccines, and relevant national clinical trial (NCT) numbers. Our survey of innovative approaches to personalized TCVs revealed at least 23 distinct phase 1 and 2 trials, most which are ongoing (Table 1). Among these trials, 20 (87%) are phase 1 investigations, with up to three trials per sponsor, with most (17; ~75%) using mRNA-, DNA-, or peptide-based antigen delivery platforms (Figure 2). The bulk of trials are enrolling patients with multiple solid tumor types; trials in non-small-cell lung cancer (NSCLC) and melanoma are the next most common personalized TCV trial indications (Figure 2E). We identified 15 unique antigen-selection algorithms based on proprietary artificial intelligence, machine learning, advanced data analytics, and deep analysis bioinformatics that are being used to predict, rank, and validate high-quality and high-frequency neoepitopes for optimal MHC binding and T cell recognition for personalized TCV therapy (Table 1). The studies show a broad range in the number of target antigens selected, with more than 75% of personalized TCVs currently in phase 1–2, targeting a total of ≥10 neoantigens (Figure 2D). As it stands, the best delivery method and route of administration are not yet defined. Lipid nanoparticles are the most commonly used delivery vehicle, and the route of administration is primarily intravenous (30%), intramuscular (30%), or subcutaneous (25%; Figure 2G). A dosing frequency of every 3 weeks (q3w) is used in 40% of trials (Figure 2I). The annual number of vaccinations varies widely from 6 to 26 (Figure 2J). At least 40% of these trials will evaluate multiple dose levels (Figure 2K). Many TCVs are being tested in combination with other treatments, including chemotherapy, interleukin (IL)-2, and anti-programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1), given as single agents or in combination with anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (Figure 2F). Taken together, these diverse efforts recall earlier TCV trials, where a similar lack of consensus on the best approach to the systematic testing of antigen and vaccine efficacy was evident.59 As shown in Figure 2, immunogenic platforms that (1) benefit broader patient populations, (2) are comprised of fewer antigens, (3) offer a less frequent immunization schedule, (4) require a less invasive delivery route, and (5) are more amendable to combination approaches hold practical advantages for late-stage development.
Table 1.
Clinical Trials of Personalized Therapeutic Cancer Vaccines
| NCT No., Patients Expected (Trial Status): ClinicalTrials.gov | Sponsors | Antigen Platform | Antigen Selection Algorithm | Delivery | Dose and Schedule | Indication | Combination Therapy | Biomarkers | Endpoints | References |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT03289962, n = 770 (phase 1 recruiting, 2020 completion) | BioNTech and Genentech | neoantigen mRNA | MHC class I & II prediction, mutations ranked by HLA allele-specific antigen binding affinity | i.v. infusion RNA-lipoplex, size and charge optimized | 25–100 μg qw prime + boosters | multiple solid tumors | atezolizumab | IFN-γ ELISPOT, flow cytometry, TCR analysis, T cell killing | AEs, imAEs, DLTs, ORR, DOR, PFS, OS | 60 |
| NCT03815058, n = 132 (phase 2 recruiting, 2022 completion) | BioNTech and Genentech | neoantigen mRNA | ˆ | ˆ | not specified; qw prime + boosters | melanoma | pembrolizumab | ˆ | AEs, ORR DOR, PFS, OS | 61 |
| NCT03313778, n = 90 (phase 1 recruiting, 2021 completion) | Moderna and Merck | neoantigen mRNA | based on HLA type; analyzes IEDB data for immunogenicity prediction | intramuscular via lipid nanoparticles | 40–1, 000 μg nine q3w cycles | multiple solid tumors | pembrolizumab | neoantigen-specific T cell response | AEs, DLTs, RFS, DOR, PFS, OS | 62 |
| NCT03897881, n = 150 (phase 2 recruiting, 2023 completion) | Moderna and Merck | neoantigen mRNA | ˆ | ˆ | 1,000 μg nine q3w cycles | melanoma | pembrolizumab | ˆ | AEs, RFS, DMFS, OS | 63 |
| NCT03948763, n = 100 (phase 1 recruiting, 2024 completion) | Moderna and Merck | TAAs mRNA | targets four most prevalent KRAS mutations | ˆ | not specified | CRC, NSCLC, pancreatic | pembrolizumab | T cell receptor clonality and diversity | AEs, DLTs, discontinuation, ORR | 64,65 |
| NCT03380871, n = 15 (phase 1 active, not recruiting, 2021 completion) | Neon Therapeutics and Merck | neoantigen peptides | Recon bioinformatics engine quantifies epitope quality from mass spectrometry binding to predict peptide presentation | subcutaneous (up to four sites) with poly(ICLC) | 5 priming doses then qw + boosters | NSCLC | pembrolizumab, pemetrexed, carboplatin | IFN-γ ELISPOT, T cell phenotyping | AEs, SAEs, ORR, DOR, RCR, CBR, PFS, OS | 66 |
| NCT02897765, n = 55 (phase 1 active not recruiting, 2020 completion) | Neon Therapeutics and BMS | neoantigen peptides | ˆ | ˆ | not specified | multiple solid tumors | nivolumab | IFN-γ ELISPOT, cytokines, flow cytometry | AEs, SAEs, ORR, DOR, RCR, CBR, PFS, OS | 67 and P.A. Ott et al., 2019, Soc. Immunother. Cancer, conference |
| NCT03639714, n = 214 (phase 1/2 recruiting, 2022 completion) | Gritstone Oncology and BMS | neoantigen adenovirus vector + self-amplifying mRNA | Edge, novel AI model for antigen prediction for optimal immunogenicity/T cell recognition | intramuscular via viral vector | ChAdV prime 1 × 1012 vp SAM boosts 30–300 μg | NSCLC, MSS-CRC, EC, BC | nivolumab ipilimumab | ex vivo ELISPOT | AEs, immune response, ORR, DOR, CBR, PFS, OS | 68 |
| NCT03953235, n = 144 (phase 1/2 recruiting, 2023 completion) | Gritstone Oncology and BMS | neoantigen tumor-specific shared neoantigen peptides | ˆ | ˆ | not specified | NSCLC, CRC, pancreatic, other solid tumors | ˆ | ex vivo ELISPOT | ˆ | 69,70 |
| NCT02992977, n = 5 (phase 1 terminated in 2019) | Agenus | neoantigen peptides linked to heat shock protein | AIM algorithm for ID and immunogenicity prediction in vitro mass spectrometry validation | subcutaneous peptides are linked to recombinant HSP70 protein carrier + QS-21 Stimulon adjuvant | 30–240 μg of AutoSynVax + 50 μg of QS adjuvant q2w up to 1 year | melanoma, NSCLC, bladder, TNBC, RCC, HNC, CRC, solid tumors | none | ELISPOT, cytokines | AEs, T cell response, ORR, PFS, OS | 71 |
| NCT03673020, n = 3 (phase 1 recruiting, 2020 completion) | Agenus | neoantigen peptides linked to heat shock protein | ˆ | ˆ | 50 μg + 240 μg of Hsc70 + 50 μg of QS q2w | multiple solid tumors | none | ˆ | AEs. time of recurrence | 71 |
| NCT03633110, n = 99 (phase 1/2 recruiting, 2022 completion) | Genocea | neoantigen peptides | Atlas proprietary system, ex vivo assay for ID, epitope prediction based on predicted binding to HLA | subcutaneous with Hiltonol poly(ICLC) adjuvant | not specified; days 1, 22, and 43 with boosters at weeks 12 and 24 | melanoma, NSCLC, RCC, HNC, UC | nivolumab or pembrolizumab | IFN-γ ELISPOT, cytokines, immuno-phenotyping | anti-tumor activity | 72 |
| NCT03265080, n = 5 (phase 1 active not recruiting, 2020 completion) | Advaxis Immunotherapies and Amgen | neoantigen Listeria monocytogenes- secreting TAAs | MINE system, machine learning to predict and weigh rankings, allele frequency, tumor drivers, MHC binding | i.v. infusion −15 min attenuated bacterial vector + adjuvant fusion protein tLLO-NEO | 1 × 108–1 x 109 CFU, q3w for 2 years | NSCLC, MSS-CRC, HNC, UC | pembrolizumab | ELISPOT, cytokines | AEs, ORR, DOR, DCR, PFS, OS | 73 |
| NCT03847519, n = 74 (phase 1/2 recruiting, 2023 completion) | Advaxis Immunotherapies and Personalis | TAAs (public or shared hotspots) Listeria monocytogenes secreting TAA | ˆ + ImmunoID NeXT deep analysis platform | ˆ | ˆ | NSCLC | pembrolizumab | ELISPOT, cytokines, gene expression, immuno-sequencing of T cell repertoire | AEs, anti-tumor activity, PFS, OS | 74 |
| NCT02325557, n = 51 (phase 1/2 completed 2018) | Advaxis Immunotherapies and Merck | TAAs Listeria monocytogenes-secreting TAAs | MINE system with machine learning to predict and weigh rankings, allele frequency, tumor drivers, MHC binding | ˆ | 1 x 109–1 × 1010 CFU q3w for 2 years | prostate | pembrolizumab | ELISPOT, gene expression, flow cytometry, TCR sequencing | AEs, immune responses, PSA, anti-tumor activity, PFS, PROs | 75 |
| NCT03189030, n = 28 (phase 1 active, not recruiting, 2020 completion) | Aduro Biotech | TAAs Listeria monocytogenes- secreting TAAs | ZoomX workflow for neoantigen ID and selection | i.v. infusion −1 h with live attenuated Listeria monocytogenes | 1 × 108–1 × 109 CFU q3w 17 injections per year | MSS-CRC | none | IFN-γ ELISPOT | AEs | 76 |
| NCT01675765, n = 60 (phase 1completed 2019) | Aduro Biotech | TAAs Listeria monocytogenes- secreting TAAs | ˆ | ˆ | 1 × 109 CFU two infusions q2w apart followed by two boosts q3w | malignant pleural mesothelioma | pemetrexed, cisplatin | IFN-γ ELISPOT, flow cytometry | AEs, immune responses, serum mesothelin, ORR, TTP, OS | 77 |
| NCT03548467, n = 65 (phase 1/2 recruiting, 2023 completion) | Vaccibody and Nektar- Therapeutic | neoantigen DNA plasmid | NeoSELECT neoepitope selection for high-frequency, high-quality neoepitopes | intramuscular needle-free jet injection DNA plasmid pUMVC4a vector | 3 mg of multiple dose induction q4w until week 50, 14 injections | melanoma NSCLC, RCC, UC, SCCHN | bempegaldesleukin | IFN-γ ELISPOT, flow cytometry | AEs, immune responses, ORR, DOR, PFS | J. Krauss et al., 2019, Soc. Immunother. Cancer, conference |
| NCT03552718, n = 16 (phase 1 recruiting, 2020 completion) | NantBioscience | neoantigen yeast based | not specified | injectable suspension recombinant yeast-based vector | not specified | CRC, BC, HNC, NSCLC, pancreatic, liver | none | not specified | AEs, RR, DFS, PFS, OS | 78,79 |
| NCT03164772, n = 56 (phase 1/2 recruiting, 2024 completion) | CureVac, Boehringer Ingelheim, and MedImmune | neoantigen mRNA | MutSig algorithm and sparse partial correlation estimation algorithm | intradermal injection needle-free lipid nanoparticles | 2 × 200 μL as six components, total of 14 doses in 12 cycles | NSCLC | durvalumab, tremelumumab | ELISPOT, flow cytometry, cytokines, CD8+ T cell response | AEs, ORR, DOR, PFS, OS | 80 |
| NCT03199040, n = 24 (phase 1 recruiting, 2022 completion) | MedImmune and Washington University St. Louis | neoantigen DNA | public tools, including NetMHC, NetMHCpan, and NetChop algorithms to predict binding and epitope processing | intramuscular electroporation administration delivery system with two injections at separate sites | not specified; D1, D29, D57, D85, D113, D141 q3w between injections | TNBC | durvalumab | ELISPOT, flow cytometry | AEs, immune responses | 81 |
| NCT03598816, n = 48 (phase 2 not yet recruiting 2022 completion) | MedImmune and Washington University St. Louis | neoantigen DNA | ˆ | intramuscular electroporation administration delivery system one injection into each deltoid or lateralis, two injections at each vaccination | not specified; C1D1, C1D15, C2D1, C3D1, C4D1, and C5D1 total of six doses with two injections per dose | RCC | durvalumab, tremelumumab | ELISPOT, tetramer staining | AEs, ORR, PFS, OS | 81 |
| NCT02933073, n = 15 (phase 1 recruiting, 2026 completion) | Truvax and University of Connecticut | neoantigen peptides | Epi-Seq pipeline and differential agretopic index to rank epitopes by MHC score differences | not specified | not specified; six injections monthly for 6 months | ovarian | none | immune responses, CD8+ T cell proliferation and phenotyping | AEs, immune responses | 82,83 |
Information was obtained from public documents (scientific literature, posters, patents, and/or corporate presentations); ˆ indicates the same finding as the prior row. AE, adverse event; AI, artificial intelligence; BC, breast cancer; CBR, clinical benefit rate; CFU, colony-forming units; ChAdV chimpanzee adenoviral vector; CRC, colorectal cancer; DCR, disease control rate; DFS, disease-free survival; DLT, dose-limiting toxicity; DMFS, distant metastasis-free survival; DOR, duration of response; EC, endometrial cancer; ELISPOT, enzyme-linked immunospot; HLA, human leukocyte antigen; HNC, head and neck cancer; IEDB, Immune Epitope Database and Analysis Resource; IFN-γ, interferon γ; imAE, immune-mediated adverse event; i.v., intravenous; MHC, major histocompatibility complex; mRNA, messenger RNA; MSS, microsatellite stable; NSCLC, non-small-cell lung cancer; ORR, objective response rate; OS, overall survival; poly(ICLC), carboxymethylcellulose polyinosinic-polycytidylic acid, and poly-l-lysine double-stranded RNA; PFS, progression-free survival; PSA, prostate-specific antigen; PRO, patient reported outcomes; qw, once weekly; q2w, every 2 weeks; q3w, every three weeks; RCC, renal cell carcinoma; RCR, radiologic complete response; RFS relapse-free survival; RP2D, recommended phase 2 dose; RR, relapse rate; SAE, serious adverse event; SAM self-amplifying; SCCHN, squamous cell carcinoma of the head and neck; TAA, tumor-associated antigen; TCR, T cell receptor; TNBC, triple-negative breast cancer; TTP, time to progression; UC, urothelial cancer; VP, viral particles.
Figure 2.
Clinical Trial Landscape for Personalized TCVs
(A–K) Twenty-three personalized TCVs currently in phase 1 or 2 from 13 major sponsors: (A) trials per sponsor; (B) type of vaccine; (C) phase of trial; (D) number of antigens; (E) indication; (F) combination partner; (G) route of administration; (H) delivery system; (I) vaccine administration frequency; (J) vaccine doses per year; and (K) number of vaccine dose levels tested.
Personalized TCV Platform Influence on ADME Processes
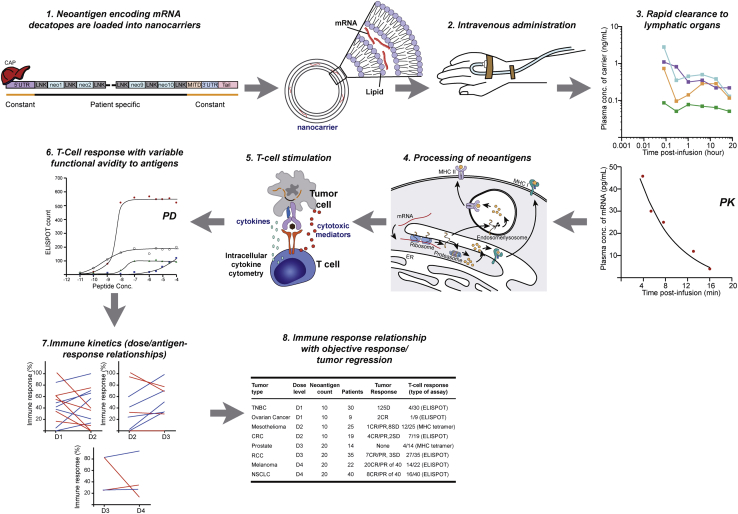
Limited absorption, distribution, metabolism, and excretion (ADME) characterization due to poorly conducted dose-finding trials jeopardize the chance of approval of some personalized TCVs that might have otherwise provided clinical benefit to patients.84, 85, 86 Figure 3 illustrates the complexity of the components impacting dosing strategy and dose response for a personalized TCV. Absorption and biodistribution of personalized TCVs are influenced by a myriad of factors, including delivery method, lymphatic uptake by mononuclear phagocytes, formulation, critical quality attributes, payload/carrier ratio, and in vivo “leakage” (premature release) of antigen-encoding material from carriers.87 Absorption is influenced by the route of administration, and biodistribution is dependent on the carrier, which impacts antigen uptake, expression, and presentation. After processing of neoantigens, immune responses can be detected and evaluated. However, due to heterogeneity in dose-response relationships across multiple antigens or epitopes in a personalized TCV, it can be difficult to determine specific associations between various personalized TCV components and any resulting clinical response. Hence, favorable absorption and distribution of antigen-encoder/antigen to lymphoid organs are precursors to successful immune activation, anti-tumor CTL activity, and effective tumor killing.88,89
Figure 3.
Complexity of Dosing Strategy and Dose-Response for a Personalized Neoantigen-Based TCV
Favorable absorption and distribution of antigen-encoder/antigen to lymphoid organs are precursors to enable successful immune activation, anti-tumor CTL activity, and effective tumor killing. After neoantigen encoding mRNA is packaged into nanocarriers and infused (steps 1 and 2), mRNA and carrier component concentrations are measured in systemic circulation, which may relate to uptake by lymphoid organs for processing of neoantigens (steps 3 and 4). After processing of neoantigens, immune monitoring of antigen-specific T cell responses is evaluated (step 5); however, due to heterogeneity in dose-response relationships across multiple antigens or epitopes in a personalized TCV (steps 6 and 7), it can be difficult to determine specific associations between various personalized TCV components and any resulting clinical response (step 8). Illustrated findings are hypothetical and do not represent actual clinical trial data. CR, complete response; D, dose level; ELISPOT, enzyme-linked immunospot assay; LNK, linker; MHC, major histocompatibility complex; Neo, neoantigen; PD, pharmacodynamics; PK, pharmacokinetics; PR, partial response; SD, stable disease; UTR, untranslated region.
The presence of antigen, or amount of antigen surrogate (such as a lipid carrier), in systemic circulation after personalized TCV administration may or may not reflect distributed amounts of antigen to lymphoid organs. Understanding how novel adjuvants, excipients, and carrier systems, such as nanoparticles, bacterial ghosts, heat shock proteins, or other vesicles (see Table 1), influence the ADME of personalized TCVs may provide insights to improve efficacy and/or safety.87,90,91 Additional aspects related to interactions and use in special populations should be considered in development of these novel modalities.92, 93, 94
Immune Cell Responses as Surrogate Biomarkers for Efficacy
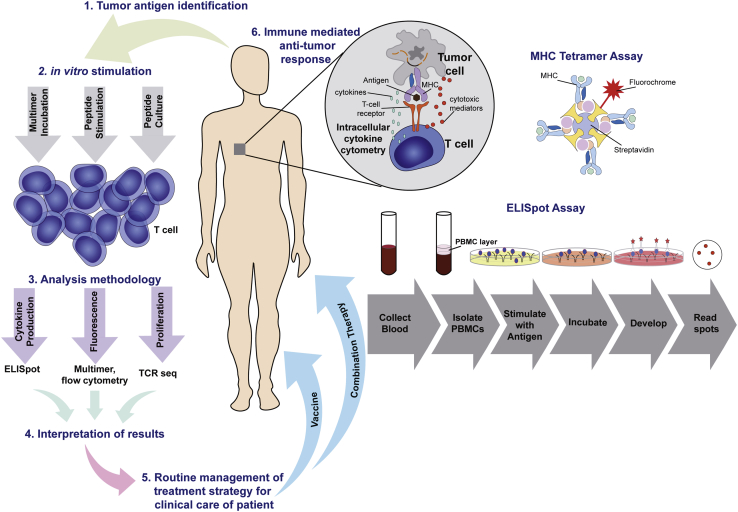
Certain human immune cell functions allow for quantitative measurement of responses to personalized TCV therapy. This includes tumor antigen-specific T cell responses that potentially lead to tumor rejection, and hence these techniques are an essential component of optimal dose selection in cancer vaccine trials. Immune-monitoring techniques include (1) analysis of cell populations by flow cytometry, including phenotypes, functionalities, and activation status; (2) enzyme-linked immunospot (ELISPOT) assays to measure cytokine release after antigen-specific immune responses, and peripheral cytokine profiling using and enzyme linked immunosorbent assay (ELISA) to evaluate innate immune responses; (3) tetramer analysis using MHC multimers loaded with antigen peptides to measure antigen-specific CD8+ T cells; and (4) T cell receptor analysis by sequencing and polymerase chain reaction to elucidate the immune repertoire, including genetic arrangement, and specificity.95 Together, these assays are applied to immune cells in peripheral blood mononuclear cells (PBMCs) acquired from patients to detect and describe T cell responses (Figure 4). ELISPOT is one of the most common techniques to identify CD8+ T cell responses to a given antigen.96,97 Quantitative ELISPOT output is correlated with the strength of the antigen-specific T cell response, although it cannot be used to determine the absolute number of antigen-specific T cells. An alternative approach to CTL detection, tetramer analysis, uses MHC multimers loaded with antigen peptides to detect and quantitatively measure the number of T cells specific to a particular peptide-MHC complex in response to a personalized TCV. Together, these tools offer high specificity; however, they are considered exploratory, and obstacles remain to their robust clinical use.98 Immune monitoring requires careful evaluation of stability and assay conditions with the use of proper controls to accurately evaluate induced cellular immune responses. Efforts must be taken to reduce potential sources of variability and improve reliability, as immune-monitoring relationships in response to personalized TCVs are routinely used to inform clinical decisions. More recently, the National Cancer Institute has supported an initiative to fund four Cancer Immune Monitoring and Analysis Centers in support of researchers conducting state-of-the-art analyses such as immune profiling characterization of patient responses in selected immunotherapy trials.
Figure 4.
Immune Cells in PBMCs Acquired from Patients Detect and Inform on Antigen-Specific T Lymphocyte Response
ELISPOT identifies CD8+ T cell responses to a given antigen after PBMCs are treated with an antigen of interest and stimulated ex vivo, leading to CD8+ T cell activation in response to a tumor-specific antigen and secretion of IFN-γ captured on an immobilized surface as insoluble spots that are enumerated. Tetramer analysis using MHC multimers loaded with antigen peptides detects T cells specific to a particular peptide-MHC complex in response to a TCV. Immune-monitoring relationships in response to TCVs may be used to help inform clinical decisions. Adapted from presentation by Caushi and Smith.95
Vaccination Strategy: Dosing and Administration
A first-in-human trial evaluates safety, dose, frequency, immune monitoring, and clinical activity under a suitable schedule of vaccinations to identify the optimal dose, number, and interval of doses required to achieve a peak immune response and anti-tumor activity.84,88,99 Lower doses of antigen may not be effective, while higher doses may have practical limitations and safety issues, such as strong activation of the innate immune response and cytokine release.97 To the extent that tolerability allows, the vaccination dose should be increased until the cellular immune response plateaus.85 Only high-avidity T cells are presumed capable of being stimulated by extremely low concentrations of antigen, whereas higher concentrations have the potential to stimulate lower avidity T cells.100 Mouse models are commonly used to inform fundamental understanding around the most effective route of administration, adjuvant, carrier, dose, and schedule, providing insight into the kinetics of antigenic stimulation to be further refined in clinical trials.101 These studies may administer multiple vaccine doses that are 2 weeks apart to evaluate the kinetics of immune responses. Additional studies acquire knowledge on timing of administrations, total number of doses, and induction (priming) and booster (maintenance) intervals to establish a rationale for an initial clinical schedule.84,102 A lack of widely accepted allometric scaling approaches to calculate an equivalent dose across species limits translatability, providing only a rough estimate to support clinical trial designs.103, 104, 105 The optimal dose in a personalized TCV will likely vary across antigens, and the total antigen dose must ensure delivery of adequate amounts of the less immunogenic antigens.
To address this gap and optimize clinical vaccination strategies, quantitative modeling methodologies, such as immunostimulation/immunodynamic (IS/ID) modeling, have used novel statistical and mechanistic approaches parameterized using relevant preclinical and clinical observations.105, 106, 107 Implementing quantitative modeling methodologies may overcome some of the challenges in establishing an optimal dosing strategy in humans (Table 2). Models using IFN-γ dose-response relationships in mice and humans to identify ideal regimens have been credited with avoiding suboptimal dosing in late-stage development of certain vaccines.108,109 Other recent modeling efforts focus on optimizing cancer vaccine dosage and delivery rates to maximize recruitment of high-avidity CTLs to the tumor.110
Table 2.
Obstacles to Determining an Optimal Dose and Regimen for a Personalized Therapeutic Cancer Vaccine
| Key Issues |
| • Preclinical models used to inform on the best route of administration, adjuvant, carrier, dose, and schedule do not scale allometrically and may not translate across species103,105 |
| • Limited understanding of vaccine ADME to allow optimization of biopharmaceutical properties90 |
| • Lack of systematic approaches for testing various platforms to induce immune responses, e.g., approaches are scattered across various competing commercial entities5,59 |
| • Poor methods for selecting highly immunogenic antigens and lack of adjuvants able to overcome substantial immunosuppression111 |
| • Antigens with relatively low avidity for MHC must be given at higher doses to achieve adequate lymphatic exposure, which may not be feasible97 |
| • Platform-specific toxicity issues requiring use of short-acting prophylaxis may limit dose escalation4 |
| • Lack of knowledge of the optimal interval between immunizations for induction and maintenance phases84,88 |
| • Selecting a non-ideal study population, e.g., metastatic population with rapidly progressive disease, non-immunogenic cancers, presence of immune suppression, and/or insufficient time for immune response99,112 |
| • Lack of clear dose-response relationships, due to small trial populations and disease heterogeneity, makes it difficult to use surrogate markers of immunity to identify clinical responders, and induced T cell responses in true clinical responders may be too low for statistical assessment4,113 |
| • Clinical anti-tumor efficacy not observed despite evidence of immunological activity4,113 |
| • Inadequate characterization of the shape of the immune response curve due to insufficient dose levels/patients tested105 |
| • Lack of implementation of quantitative modeling approaches to provide insight on optimal dose-response relationships106,107 |
| • Speed of development and commercial pressure leading to trial design with inadequate testing of dosing strategies and, during late-stage development dose selection, bias to favor safety over efficacy, jeopardizing future licensure84, 85, 86 |
Combination Immunotherapy
Appreciation of the minimal toxicity observed with many personalized TCV platforms has encouraged investigators to combine these agents with immune checkpoint inhibitors (ICIs), chemotherapies, radiation, targeted therapies, hormone therapies, or other immunomodulators that have overlapping effects on immune cells.1,114 Co-administered therapies that may offer unique synergistic immunopotentiation with personalized TCVs include (1) cytokines, such as IL-2, IFN, and transforming growth factor (TGF)-β, which may promote differentiation of immature T cells into effector T cells; (2) radiotherapy to release tumor neoantigens, increase inflammation and secretion of immunomodulatory cytokines, and sensitize tumor cells to immune-mediated killing; (3) ICIs, such as anti-CTLA-4 and/or anti-PD-1/PD-L1, to activate different T cell populations; (4) small molecules, such as tyrosine kinase or histone deacetylase inhibitors, to promote immune cell function by decreasing regulatory T cells and myeloid-derived suppressor cells; (5) endocrine therapy to increase production of naive T cells and decrease regulatory T cells; and, lastly, (6) chemotherapy to increase immune-supportive M1 macrophages and induce tumor immunosurveillance by natural killer (NK) cells.114, 115, 116 Table 1 lists agents currently being tested in combination with personalized TCVs. The biological rationale for combination, and individual contribution of each individual agent, should be established.117 Active combinations will be influenced by the patient population, and dose/schedule, sequencing, and safety evaluation of combination therapies in relationship to a personalized TCV should be considered.118 One such example highlighting the importance of proper sequencing for combinations was recently revealed in anti-PD-1-resistant models, which indicated that simultaneous anti-PD-1 and vaccine therapy reversed resistance, while PD-1 blockade before antigen priming abolished therapeutic outcomes.119
Common Obstacles in Development
Cell-mediated immunity directing T cells toward tumor-specific antigens has proven difficult. Many completed TCV trials have yielded disappointing results. Provenge (sipuleucel-T) has demonstrated statistically significant associations of antigen-specific CD8+ T cell responses with overall survival in patients with metastatic castration-resistant prostate cancer and is the only US Food and Drug Administration (FDA)-approved TCV.120,121 A quantitative analysis across 451 clinical trials of TCVs from 1999 to 2014 indicated a lack of consistency in TCV approaches with no clear benefit of any particular adjuvant or platform to induce immune or objective responses.5 Despite evidence of immunological activity, many TCVs tested failed to reveal correlations between vaccine-induced immunity and clinical benefit. Interpatient heterogeneity affects the immunogenicity of TCVs, and true clinical responders in trials may be too few to allow for robust statistical assessment of TCV-induced T cell responses.113
More potent and versatile TCV platforms are needed, and testing of new approaches such as the ongoing trials of personalized TCVs to enhance efficacy are underway.122 There is also a recent shift to testing TCVs in patients with early stage diseases in tumor types presumed more responsive to immunotherapy.99,112 The tailor-made approaches to vaccination described herein will advance the field; however, there is little clarity as to which antigen selection algorithm, adjuvant, or delivery approach is ideal to elicit potent tumor-specific T cell responses. More systematic approaches to testing could help improve the likelihood of better outcomes. Recently, efforts to launch an international, multidisciplinary human vaccines consortium to create a roadmap for systematic testing to assist with reducing the complexity of personalized TCV development have been discussed. Suggested collaborations include a large multi-center pilot clinical study, or iterative studies, to compare delivery platforms, materials, antigens, doses, adjuvants, prime/boost schedules, frequency, and route of administration via selected cohorts of approximately 10 individuals to provide clues as to the potency of various approaches.19
Regulatory Framework
Stringent regulations aim to minimize risks and protect patients. To fulfill approval standards a personalized TCV must indicate that the therapy is safe, of sufficient quality, and clinically effective. Understanding the regulatory framework, including classification and implementation of suitable and specific guidance documents to support investigation and marketing of personalized TCVs, is essential. The neoantigen-based personalized TCV approaches reviewed herein are regulated as gene therapies. In the US, these therapies are regulated by the Center for Biologics Evaluation and Research (CBER) within the FDA, while in EU the Committee for Advanced Therapies (CAT) of the European Medicines Agency (EMA) is responsible for reviewing individualized TCV regulatory submissions, and their decisions are ratified by the Committee for Medicinal Products for Human Use (CHMP).123 CBER’s Office of Cellular, Tissue, and Gene Therapies and other global agencies have published guidance documents to inform development of TCVs. These documents cover critical quality attributes, manufacturing process controls, potency, nonclinical safety, pharmacology, and clinical development. However, these are not all-inclusive and do not account for the broad range of potential issues impacting the varied personalized and state-of-the-art TCV modalities in development. In the long-term, advances in scaling out manufacturing of these agents are needed to (1) enhance production lines and equipment utilization, (2) reduce the cost of goods and increase purchasing volume for raw materials, and (3) increase automation, leading to more efficient quality control. Additionally, earlier optimization of formulation and manufacturing processes will lessen the need for comparability and extensive bridging studies to the proposed commercial product.
Input from regulatory bodies should be sought and obtained as soon as a clear plan and rationale are established for the development strategy.124 As the science progresses along with advancement in technologies to produce personalized TCVs, developers and regulators need to collaborate to evolve the regulatory landscape for these modalities. Additionally, fostering and implementing policies that support the application of model-informed drug development (MIDD) to personalized TCVs may facilitate dose optimization and clinical trial design.125, 126, 127 Overall, given the current lack of established clinical pharmacology programs to assist with the clinical development of this novel class of medicines, new approaches, solutions, and adaptive practices to address important technical, clinical, and regulatory questions should be considered to bring these innovative therapies to patients faster.
Conclusions
With the success of ICI-based immunotherapy, there has been an explosion of renewed interest in cancer vaccines and a rapidly expanding repertoire of tailored approaches. However, to date TCVs have delivered only modest clinical benefit and have not yet matured as a major pillar of cancer treatment. Many early clinical trials and ombination studies of personalized TCVs are now underway. The field is crowded, with a flurry of recent collaborations indicating excitement about the potential of personalized TCVs, yet considerable uncertainty remains as to which platform will perform best. The next decade is expected to bring significant advances in high-throughput sequencing, antigen prediction algorithms, modeling and simulation efforts, manufacturing, and regulatory guidance. As part of this effort, implementing traditional quantitative clinical pharmacology techniques may have a role to play in more rational personalized TCV study designs, and dose/schedule selection. Methodical testing of complex platforms and quantitative modeling are forward-thinking approaches emerging to assist clinical development. Overcoming key limitations unique to vaccine-based treatment compared to other immunotherapies remains a significant hurdle. In addition to challenges detailed in this review, production and associated development costs leading to affordability and patient access issues must be addressed. Nonetheless, personalized TCVs are certainly worth pursuing to further explore their potential to combat cancer.
Author Contributions
C.S.S. wrote the manuscript. J.C.H., I.H., B.-Q.S., A.R., P.T., S.G., and B.W. provided strategic input and helped review and revise the article. All authors read and approved the final manuscript.
Conflict of Interest
C.S.S., J.C.H., I.H., B.-Q.S., P.T., S.G., and B.W. are employees and stockholders of Genentech, Inc. and F. Hoffmann-La Roche Ltd. A.R. declares no competing interests.
Acknowledgments
This article was sponsored by Genentech and F. Hoffmann-La Roche. The sponsor was involved in the design of the review; the collection, analysis, and interpretation of the data; and in the writing the manuscript. We thank Anshin BioSolutions Corporation for assistance with medical writing support and figure creation, which was provided under the direction of the authors. We thank Chris Petry of Genentech, Inc. for helpful manufacturing insights.
References
- 1.Finn O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2018;18:183–194. doi: 10.1038/nri.2017.140. [DOI] [PubMed] [Google Scholar]
- 2.Falzone L., Salomone S., Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes A., Vandermeulen G., Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019;38:146. doi: 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahma O.E., Gammoh E., Simon R.M., Khleif S.N. Is the “3+3” dose-escalation phase I clinical trial design suitable for therapeutic cancer vaccine development? A recommendation for alternative design. Clin. Cancer Res. 2014;20:4758–4767. doi: 10.1158/1078-0432.CCR-13-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan A.C.L., Goubier A., Kohrt H.E. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J. Immunother. Cancer. 2015;3:48. doi: 10.1186/s40425-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Burg S.H. Correlates of immune and clinical activity of novel cancer vaccines. Semin. Immunol. 2018;39:119–136. doi: 10.1016/j.smim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Tran T., Blanc C., Granier C., Saldmann A., Tanchot C., Tartour E. Therapeutic cancer vaccine: building the future from lessons of the past. Semin. Immunopathol. 2019;41:69–85. doi: 10.1007/s00281-018-0691-z. [DOI] [PubMed] [Google Scholar]
- 8.Wirth T.C., Kühnel F. Neoantigen Targeting—dawn of a new era in cancer immunotherapy? Front. Immunol. 2017;8:1848. doi: 10.3389/fimmu.2017.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Sharma P.K., Peter Goedegebuure S., Gillanders W.E. Personalized cancer vaccines: targeting the cancer mutanome. Vaccine. 2017;35:1094–1100. doi: 10.1016/j.vaccine.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 11.Desrichard A., Snyder A., Chan T.A. Cancer neoantigens and applications for immunotherapy. Clin. Cancer Res. 2016;22:807–812. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- 12.Łuksza M., Riaz N., Makarov V., Balachandran V.P., Hellmann M.D., Solovyov A., Rizvi N.A., Merghoub T., Levine A.J., Chan T.A. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin Yu J., Hubbard-Lucey V.M., Tang J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019;18:899–900. doi: 10.1038/d41573-019-00167-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Rezaei N., Keshavarz-Fathi M. Academic Press; 2018. Vaccines for Cancer Immunotherapy: An Evidence-Based Review on Current Status and Future Perspectives. [Google Scholar]
- 16.Song Q., Zhang C.D., Wu X.H. Therapeutic cancer vaccines: from initial findings to prospects. Immunol. Lett. 2018;196:11–21. doi: 10.1016/j.imlet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Coventry B.J. Therapeutic vaccination immunomodulation: forming the basis of all cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2019;7 doi: 10.1177/2515135519862234. 2515135519862234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., Shi Z., Xu X., Yu Z., Mi J. The influence of microenvironment on tumor immunotherapy. FEBS J. 2019;286:4160–4175. doi: 10.1111/febs.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero P., Banchereau J., Bhardwaj N., Cockett M., Disis M.L., Dranoff G., Gilboa E., Hammond S.A., Hershberg R., Korman A.J. The human vaccines project: a roadmap for cancer vaccine development. Sci. Transl. Med. 2016;8:334ps9. doi: 10.1126/scitranslmed.aaf0685. [DOI] [PubMed] [Google Scholar]
- 20.Wong K.K., Li W.A., Mooney D.J., Dranoff G. Advances in therapeutic cancer vaccines. Adv. Immunol. 2016;130:191–249. doi: 10.1016/bs.ai.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Jäger D., Jäger E., Knuth A. Immune responses to tumour antigens: implications for antigen specific immunotherapy of cancer. J. Clin. Pathol. 2001;54:669–674. doi: 10.1136/jcp.54.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardoll D.M., Topalian S.L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 23.Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 24.Tay R.E., Richardson E.K., Toh H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-0183-x. Published online May 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D., Schreiber H. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl. Acad. Sci. USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alspach E., Lussier D.M., Schreiber R.D. Interferon γ and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb. Perspect. Biol. 2019;11:a028480. doi: 10.1101/cshperspect.a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alspach E., Lussier D.M., Miceli A.P., Kizhvatov I., DuPage M., Luoma A.M., Meng W., Lichti C.F., Esaulova E., Vomund A.N. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sillito F., Holler A., Stauss H.J. Engineering CD4+ T cells to enhance cancer immunity. Cells. 2020;9:1721. doi: 10.3390/cells9071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson K.L., Disis M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostroumov D., Fekete-Drimusz N., Saborowski M., Kühnel F., Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018;75:689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreiter S., Vormehr M., van de Roemer N., Diken M., Löwer M., Diekmann J., Boegel S., Schrörs B., Vascotto F., Castle J.C. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temizoz B., Kuroda E., Ishii K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016;28:329–338. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermaelen K. Vaccine strategies to improve anti-cancer cellular immune responses. Front. Immunol. 2019;10:8. doi: 10.3389/fimmu.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacher T.N., Scheper W., Kvistborg P. Cancer neoantigens. Annu. Rev. Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 35.McGranahan N., Furness A.J.S., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., Jamal-Hanjani M., Wilson G.A., Birkbak N.J., Hiley C.T. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aurisicchio L., Pallocca M., Ciliberto G., Palombo F. The perfect personalized cancer therapy: cancer vaccines against neoantigens. J. Exp. Clin. Cancer Res. 2018;37:86. doi: 10.1186/s13046-018-0751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris L.G., Riaz N., Desrichard A., Şenbabaoğlu Y., Hakimi A.A., Makarov V., Reis-Filho J.S., Chan T.A. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7:10051–10063. doi: 10.18632/oncotarget.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shembrey C., Huntington N.D., Hollande F. Impact of tumor and immunological heterogeneity on the anti-cancer immune response. Cancers (Basel) 2019;11:1217. doi: 10.3390/cancers11091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fennemann F.L., de Vries I.J.M., Figdor C.G., Verdoes M. Attacking tumors from all sides: personalized multiplex vaccines to tackle intratumor heterogeneity. Front. Immunol. 2019;10:824. doi: 10.3389/fimmu.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanta G., Jahn S.W., Bonin S., Hoefler G. Tumour heterogeneity: principles and practical consequences. Virchows Arch. 2016;469:371–384. doi: 10.1007/s00428-016-1987-9. [DOI] [PubMed] [Google Scholar]
- 41.GuhaThakurta D., Sheikh N.A., Fan L.Q., Kandadi H., Meagher T.C., Hall S.J., Kantoff P.W., Higano C.S., Small E.J., Gardner T.A. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-t and its association with improved clinical outcome. Clin. Cancer Res. 2015;21:3619–3630. doi: 10.1158/1078-0432.CCR-14-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbière V., Chapiro J., Stroobant V., Ma W., Lurquin C., Lethé B., van Baren N., Van den Eynde B.J., Boon T., Coulie P.G. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 43.Disis M.L., Gooley T.A., Rinn K., Davis D., Piepkorn M., Cheever M.A., Knutson K.L., Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J. Clin. Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield L.H., Ribas A., Dissette V.B., Amarnani S.N., Vu H.T., Oseguera D., Wang H.J., Elashoff R.M., McBride W.H., Mukherji B. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 45.Wierecky J., Müller M.R., Wirths S., Halder-Oehler E., Dörfel D., Schmidt S.M., Häntschel M., Brugger W., Schröder S., Horger M.S. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- 46.Gulley J.L., Madan R.A., Pachynski R., Mulders P., Sheikh N.A., Trager J., Drake C.G. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J. Natl. Cancer Inst. 2017;109:djw261. doi: 10.1093/jnci/djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribas A., Timmerman J.M., Butterfield L.H., Economou J.S. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24:58–61. doi: 10.1016/s1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 48.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duperret E.K., Perales-Puchalt A., Stoltz R., G H H., Mandloi N., Barlow J., Chaudhuri A., Sardesai N.Y., Weiner D.B. A synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 2019;7:174–182. doi: 10.1158/2326-6066.CIR-18-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., Ly A., Lie W.R., Hildebrand W.H., Mardis E.R., Linette G.P. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 53.Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., Oliveira G., Giobbie-Hurder A., Felt K., Gjini E. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hundal J., Miller C.A., Griffith M., Griffith O.L., Walker J., Kiwala S., Graubert A., McMichael J., Coffman A., Mardis E.R. Cancer immunogenomics: computational neoantigen identification and vaccine design. Cold Spring Harb. Symp. Quant. Biol. 2016;81:105–111. doi: 10.1101/sqb.2016.81.030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bräunlein E., Krackhardt A.M. Identification and characterization of neoantigens as well as respective immune responses in cancer patients. Front. Immunol. 2017;8:1702. doi: 10.3389/fimmu.2017.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Y., Lei K., Tang L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front. Immunol. 2018;9:1499. doi: 10.3389/fimmu.2018.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terbuch A., Lopez J. Next generation cancer vaccines—make it personal! Vaccines (Basel) 2018;6:3. doi: 10.3390/vaccines6030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Türeci Ö., Löwer M., Schrörs B., Lang M., Tadmor A., Sahin U. Challenges towards the realization of individualized cancer vaccines. Nat. Biomed. Eng. 2018;2:566–569. doi: 10.1038/s41551-018-0266-2. [DOI] [PubMed] [Google Scholar]
- 59.Lu L., Yan H., Shyam-Sundar V., Janowitz T. Cross-sectional and longitudinal analysis of cancer vaccination trials registered on the US Clinical Trials Database demonstrates paucity of immunological trial endpoints and decline in registration since 2008. Drug Des. Devel. Ther. 2014;8:1539–1553. doi: 10.2147/DDDT.S65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genentech (2020). Pipeline. https://www.gene.com/medical-professionals/pipeline.
- 61.BioNTech (2020). Pipeline. https://biontech.de/science/pipeline.
- 62.Burris H.A., Patel M.R., Cho D.C., Clarke J.M., Gutierrez M., Zaks T.Z., Frederick J., Hopson K., Mody K., Binanti-Berube A. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019;37(15 Suppl):2523. [Google Scholar]
- 63.Moderna (2020). Moderna’s mRNA clinical trials. CMV, MMA, Zika, several types of cancer and other diseases. https://investors.modernatx.com/static-files/70785838-06c4-4e35-bb7f-502866bf6b00.
- 64.Moderna (2020). Moderna’s pipeline. https://www.modernatx.com/pipeline.
- 65.Merck (2020). Trials. https://www.merck.com/clinical-trials/search.html?kw=V941.
- 66.Govindan R., Awad M.M., Cleary L.D., Moles M.A., Gaynor R., Goldstein M.J., Spigel D.R. An open-label, phase 1B study of NEO-PV-01 with pembrolizumab plus chemotherapy in patients with advanced or metastatic nonsquamous non-small cell lung cancer. J. Clin. Oncol. 2018;36(15 Suppl) TPS3134. [Google Scholar]
- 67.Bushway M.E., Ting Y.S., Besada R.H., Sciuto T.E., Prabhakara J., Scherer J., Balogh K.N., Lamb A., Kaplan J.A., Cleary L.D. Comprehensive gene expression analysis of the tumor microenvironment in patients with advanced cancer treated with a personalized neoantigen vaccine, NEO-PV-01, in combination with anti-PD1. Cancer Res. 2019;79(13 Suppl):5006. https://seekingalpha.com/article/4317592-gritstone-oncology-grts-presents-38th-annual-j-p-morgan-healthcare-conference-slideshow. [Google Scholar]
- 68.Gritstone, J.P. Morgan SFO Conference Jan 16. https://jpmorgan.metameetings.net/events/hc20/sessions/29771-gritstone-oncology/webcast.
- 69.Gritstone Oncology (2020). Building a pipeline of immunotherapies. https://gritstoneoncology.com/our-pipeline/.
- 70.Gritstone Oncology (2019). Gritstone oncology reports promising early immunogenicity activity and safety data from its phase 1 studies evaluating its neoantigen-based immunotherapies, GRANITE and SLATE. http://ir.gritstoneoncology.com/news-releases/news-release-details/gritstone-oncology-reports-promising-early-immunogenicity.
- 71.Wesolowski R., Wilkey B.A., O’Neill A., Chi S., Gonzalez A.M., Drouin E.E., Dow E., Uduman M., Tanne A.J., Agarwal M. A phase 1 study of safety and tolerability of AutoSynVax vaccine in patients with advanced cancer. J. Immunother. Cancer. 2018;6(Suppl 1) P189. [Google Scholar]
- 72.Cohen R.B., Johnson M.L., Twardowski P., Stein M.N., Vaishampayan U.N., Dobson J.R., Foti J., Agnihotri P., Dowal L., Broom W. A phase 1/2a study of GEN-009, a neoantigen vaccine based on autologous peptide immune responses. J. Clin. Oncol. 2019;37(15 Suppl):2611. [Google Scholar]
- 73.Hecht J.R., Goldman J.W., Hayes S., Balli D., Princiotta M.F., Dennie J.G., Heyburn J., Sands T., Sheeri S., Petit R. Safety and immunogenicity of a personalized neoantigen-Listeria vaccine in cancer patients. Cancer Res. 2019;79(13 Suppl) https://seekingalpha.com/article/4372484-advaxis-adxs-investor-presentation-slideshow CT007. [Google Scholar]
- 74.Advaxis Corporate Presentation. https://www.advaxis.com/static-files/83029cdc-3ac2-4924-b109-a6178d21c7c8.
- 75.Stein M.N., Fong L., Mega A.E., Lam E.T., Heyburn J.W., Gutierrez A.A., Parsi M., Vangala S., Haas N.B. Effects of ADXS-PSA in combination with pembrolizumab on survival in metastatic, castration-resistant prostate cancer patients with or without prior exposure to docetaxel. J. Clin. Oncol. 2020;38(6 Suppl):126. [Google Scholar]
- 76.Deng W., Hudson T.E., Lemmens E.E., Hanson B., Rae C.S., Burrill J., Skoble J., Katibah G., Murphy A.L., deVries M. Development of personalized, live, attenuated double-deleted Listeria monocytogenes (pLADD) immunotherapy targeting tumor-specific neoantigens to treat cancer. J. Immunother. Cancer. 2016;4(Suppl 1):107–221. abstract P348. [Google Scholar]
- 77.Hassan R., Alley E., Kindler H., Antonia S., Jahan T., Honarmand S., Nair N., Whiting C.C., Enstrom A., Lemmens E. Clinical response of live-attenuated, Listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin. Cancer Res. 2019;25:5787–5798. doi: 10.1158/1078-0432.CCR-19-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.NANT (2018). NANT received FDA authorization of new clinical trials with personalized, next-generation neoepitope cancer vaccine. https://ichgcp.net/clinical-trials-registry/NCT03552718.
- 79.Good Clinical Practice Network (2020). Clinical trials sponsored by NantBioScience+Inc. https://ichgcp.net/clinical-trials-registry/research/find?spons=NantBioScience%2BInc.
- 80.Papachristofilou A., Hipp M.M., Klinkhardt U., Früh M., Sebastian M., Weiss C., Pless M., Cathomas R., Hilbe W., Pall G. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer. 2019;7:38. doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Washington University in St. Louis (2020). Clinical drug experience knowledgebase. http://cdek.wustl.edu/query/.
- 82.Truvax Pipeline. https://truvax.com/.
- 83.Srivasta, P.K., Mandoiu, I., and Duan, F. (2015). Identification of tumor-protective epitopes for the treatment of cancers. US patent 20150252427, filed September 27, 2013, granted September 10, 2015.
- 84.Mackiewicz J., Mackiewicz A. Design of clinical trials for therapeutic cancer vaccines development. Eur. J. Pharmacol. 2009;625:84–89. doi: 10.1016/j.ejphar.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 85.Wages N.A., Slingluff C.L., Jr., Bullock T.N., Petroni G.R. Tailoring early-phase clinical trial design to address multiple research objectives. Cancer Immunol. Immunother. 2020;69:95–102. doi: 10.1007/s00262-019-02442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conaway M.R., Petroni G.R. The impact of early-phase trial design in the drug development process. Clin. Cancer Res. 2019;25:819–827. doi: 10.1158/1078-0432.CCR-18-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong E., Dobrovolskaia M.A. Addressing barriers to effective cancer immunotherapy with nanotechnology: achievements, challenges, and roadmap to the next generation of nanoimmunotherapeutics. Adv. Drug Deliv. Rev. 2019;141:3–22. doi: 10.1016/j.addr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Wang W., Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines. 2015;14:1509–1523. doi: 10.1586/14760584.2015.1081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J. Clin. Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez-Mantilla J.D., Tronconiz I.F., Garrido M.J. John Wiley & Sons; 2015. ADME Processes in Vaccines and PK/PD Approaches for Vaccination Optimization: ADME and Translational Pharmacokinetics/Pharmacodynamics of Therapeutic Proteins. Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development and Manufacturing. [DOI] [Google Scholar]
- 91.Goldberg M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 92.Pellegrino P., Clementi E., Capuano A., Radice S. Can vaccines interact with drug metabolism? Pharmacol. Res. 2015;92:13–17. doi: 10.1016/j.phrs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Lau S.W.J., Cheung L.K., Chow D.S. Application of pharmacokinetics to specific populations: geriatric, obese, and pediatric patients. In: Shargel L., Yu A.B.C., editors. Applied Biopharmaceutics and Pharmacokinetics. Seventh Edition. McGraw-Hill; 2016. pp. 735–774. [Google Scholar]
- 94.Fulop T., Witkowski J.M., Hirokawa K., Larbi A., Pawelec G. Immunosenescence and cancer immunotherapy at old age: basics. In: Extermann M., editor. Geriatric Oncology. Springer; 2020. [DOI] [Google Scholar]
- 95.Caushi J.X., Smith K.N. Quantifying the anti-tumor immune response in patients receiving immunotherapy. Discov. Med. 2017;24:59–68. [PubMed] [Google Scholar]
- 96.Slota M., Lim J.B., Dang Y., Disis M.L. ELISpot for measuring human immune responses to vaccines. Expert Rev. Vaccines. 2011;10:299–306. doi: 10.1586/erv.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lehmann P.V., Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol. Biol. 2012;792:3–23. doi: 10.1007/978-1-61779-325-7_1. [DOI] [PubMed] [Google Scholar]
- 98.van der Burg S.H., Kalos M., Gouttefangeas C., Janetzki S., Ottensmeier C., Welters M.J., Romero P., Britten C.M., Hoos A. Harmonization of immune biomarker assays for clinical studies. Sci. Transl. Med. 2011;3:108ps44. doi: 10.1126/scitranslmed.3002785. [DOI] [PubMed] [Google Scholar]
- 99.Kudrin A. Cancer vaccines: what do we need to measure in clinical trials? Hum. Vaccin. Immunother. 2014;10:3236–3240. doi: 10.4161/hv.27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Billeskov R., Beikzadeh B., Berzofsky J.A. The effect of antigen dose on T cell-targeting vaccine outcome. Hum. Vaccin. Immunother. 2019;15:407–411. doi: 10.1080/21645515.2018.1527496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johansen P., Storni T., Rettig L., Qiu Z., Der-Sarkissian A., Smith K.A., Manolova V., Lang K.S., Senti G., Müllhaupt B. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. USA. 2008;105:5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sellers R.S., Nelson K., Bennet B., Wolf J., Tripathi N., Chamanza R., Perron Lepage M.F., Adkins K., Laurent S., Troth S.P. Scientific and regulatory policy committee points to consider: approaches to the conduct and interpretation of vaccine safety studies for clinical and anatomic pathologists. Toxicol. Pathol. 2020;48:257–276. doi: 10.1177/0192623319875085. [DOI] [PubMed] [Google Scholar]
- 103.Mestas J., Hughes C.C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 104.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Afrough S., Rhodes S., Evans T., White R., Benest J. Immunologic dose-response to adenovirus-vectored vaccines in animals and humans: a systematic review of dose-response studies of replication incompetent adenoviral vaccine vectors when given via an intramuscular or subcutaneous route. Vaccines (Basel) 2020;8:131. doi: 10.3390/vaccines8010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rhodes S.J., Guedj J., Fletcher H.A., Lindenstrom T., Scriba T.J., Evans T.G., Knight G.M., White R.G. Using vaccine immunostimulation/immunodynamic modelling methods to inform vaccine dose decision-making. NPJ Vaccines. 2018;3:36. doi: 10.1038/s41541-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rhodes S.J., Knight G.M., Kirschner D.E., White R.G., Evans T.G. Dose finding for new vaccines: the role for immunostimulation/immunodynamic modelling. J. Theor. Biol. 2019;465:51–55. doi: 10.1016/j.jtbi.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rhodes S.J., Zelmer A., Knight G.M., Prabowo S.A., Stockdale L., Evans T.G., Lindenstrøm T., White R.G., Fletcher H. The TB vaccine H56+IC31 dose-response curve is peaked not saturating: data generation for new mathematical modelling methods to inform vaccine dose decisions. Vaccine. 2016;34:6285–6291. doi: 10.1016/j.vaccine.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 109.Rhodes S.J., Sarfas C., Knight G.M., White A., Pathan A.A., McShane H., Evans T.G., Fletcher H., Sharpe S., White R.G. Using data from macaques to predict gamma interferon responses after Mycobacterium bovis BCG vaccination in humans: a proof-of-concept study of immunostimulation/immunodynamic modeling methods. Clin. Vaccine Immunol. 2017;24:3. doi: 10.1128/CVI.00525-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumbhari A., Kim P.S., Lee P.P. Optimisation of anti-cancer peptide vaccines to preferentially elicit high-avidity T cells. J. Theor. Biol. 2020;486:110067. doi: 10.1016/j.jtbi.2019.110067. [DOI] [PubMed] [Google Scholar]
- 111.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baxevanis C.N., Papamichail M., Perez S.A. Therapeutic cancer vaccines: a long and winding road to success. Expert Rev. Vaccines. 2014;13:131–144. doi: 10.1586/14760584.2014.852961. [DOI] [PubMed] [Google Scholar]
- 113.Keilholz U., Martus P., Scheibenbogen C. Immune monitoring of T-cell responses in cancer vaccine development. Clin. Cancer Res. 2006;12:2346s–2352s. doi: 10.1158/1078-0432.CCR-05-2540. [DOI] [PubMed] [Google Scholar]
- 114.Gatti-Mays M.E., Redman J.M., Collins J.M., Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum. Vaccin. Immunother. 2017;13:2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schlom J., Gulley J.L. Vaccines as an integral component of cancer immunotherapy. JAMA. 2018;320:2195–2196. doi: 10.1001/jama.2018.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 117.US Food and Drug Administration (2013). Codevelopment of two or more new investigational drugs for use in combination. https://www.fda.gov/media/80100/download.
- 118.Ciccolini J., Barbolosi D., André N., Benzekry S., Barlesi F. Combinatorial immunotherapy strategies: most gods throw dice, but fate plays chess. Ann. Oncol. 2019;30:1690–1691. doi: 10.1093/annonc/mdz297. [DOI] [PubMed] [Google Scholar]
- 119.Verma V., Shrimali R.K., Ahmad S., Dai W., Wang H., Lu S., Nandre R., Gaur P., Lopez J., Sade-Feldman M. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 2019;20:1231–1243. doi: 10.1038/s41590-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McNeel D.G. Therapeutic cancer vaccines: how much closer are we? BioDrugs. 2018;32:1–7. doi: 10.1007/s40259-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Antonarakis E.S., Small E.J., Petrylak D.P., Quinn D.I., Kibel A.S., Chang N.N., Dearstyne E., Harmon M., Campogan D., Haynes H. Antigen-specific CD8 lytic phenotype induced by sipuleucel-T in hormone-sensitive or castration-resistant prostate cancer and association with overall survival. Clin. Cancer Res. 2018;24:4662–4671. doi: 10.1158/1078-0432.CCR-18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van Nuffel A.M.T., Boudousquié C., Tuyaerts S. Editorial: approaches to advance cancer vaccines to clinical utility. Front. Immunol. 2019;10:2032. doi: 10.3389/fimmu.2019.02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Halioua-Haubold C.L., Peyer J.G., Smith J.A., Arshad Z., Scholz M., Brindley D.A., MacLaren R.E. Regulatory considerations for gene therapy products in the US, EU, and Japan. Yale J. Biol. Med. 2017;90:683–693. [PMC free article] [PubMed] [Google Scholar]
- 124.Heelan B.T. Regulatory considerations for clinical development of cancer vaccines. Hum. Vaccin. Immunother. 2014;10:3409–3414. doi: 10.4161/21645515.2014.982999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y., Zhu H., Madabushi R., Liu Q., Huang S.M., Zineh I. Model-informed drug development: current us regulatory practice and future considerations. Clin. Pharmacol. Ther. 2019;105:899–911. doi: 10.1002/cpt.1363. [DOI] [PubMed] [Google Scholar]
- 126.Madabushi R., Benjamin J.M., Grewal R., Pacanowski M.A., Strauss D.G., Wang Y., Zhu H., Zineh I. The US Food and Drug Administration’s model-informed drug development paired meeting pilot program: early experience and impact. Clin. Pharmacol. Ther. 2019;106:74–78. doi: 10.1002/cpt.1457. [DOI] [PubMed] [Google Scholar]
- 127.Marshall S., Madabushi R., Manolis E., Krudys K., Staab A., Dykstra K., Visser S.A.G. Model-informed drug discovery and development: current industry good practice and regulatory expectations and future perspectives. CPT Pharmacometrics Syst. Pharmacol. 2019;8:87–96. doi: 10.1002/psp4.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]