Abstract
At present, the idea of genome modification has revolutionized the modern therapeutic research era. Genome modification studies have traveled a long way from gene modifications in primary cells to genetic modifications in animals. The targeted genetic modification may result in the modulation (i.e., either upregulation or downregulation) of the predefined gene expression. Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated nuclease 9 (Cas9) is a promising genome-editing tool that has therapeutic potential against incurable genetic disorders by modifying their DNA sequences. In comparison with other genome-editing techniques, CRISPR-Cas9 is simple, efficient, and very specific. This enabled CRISPR-Cas9 genome-editing technology to enter into clinical trials against cancer. Besides therapeutic potential, the CRISPR-Cas9 tool can also be applied to generate genetically inhibited animal models for drug discovery and development. This comprehensive review paper discusses the origin of CRISPR-Cas9 systems and their therapeutic potential against various genetic disorders, including cancer, allergy, immunological disorders, Duchenne muscular dystrophy, cardiovascular disorders, neurological disorders, liver-related disorders, cystic fibrosis, blood-related disorders, eye-related disorders, and viral infection. Finally, we discuss the different challenges, safety concerns, and strategies that can be applied to overcome the obstacles during CRISPR-Cas9-mediated therapeutic approaches.
Keywords: human diseases, CRISPR-Cas9, genome editing, therapeutics, drug development
Graphical Abstract
The CRISPR-Cas9 genome-editing tool has shown promising advancements in clinical trials. However, the results of CRISPR-Cas9-based genome editing are unpredictable and raise safety concerns. Sharma et al. review the preclinical and clinical evidence for CRISPR-Cas9-mediated genome editing and discuss the potential challenges and future strategies to minimize the limitations of CRISPR-Cas9.
Main Text
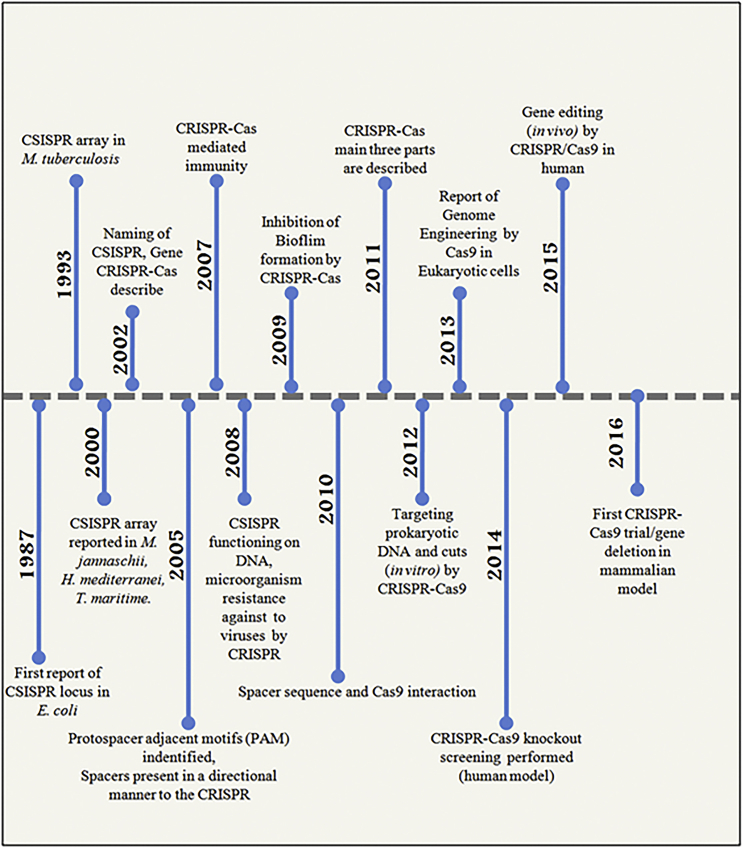
Clustered regulatory interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) is a novel and competent RNA-guided endonuclease-based genome-editing technique1 that is adapted from the naturally occurring bacterial immune system. The CRISPR-Cas9 technique is easily scalable and very cost-effective, and thus it can be applied for a wide range of directed genome editing.2, 3, 4 After the first report of CRISPR in 1987, the technology has evolved step by step (Figure 1). At the present time, CRISPR-Cas9 technology-mediated genetic experiments can be performed on a wide range of models such as plants, yeast, Caenorhabditis elegans, Drosophila, zebrafish, mice, and humans.5, 6, 7, 8, 9, 10, 11 More than 2,000 publications on CRISPR-Cas9 technology have been recorded in PubMed during the last 2 years. This increasing research trend has been portrayed as the “CRISPR craze.”12
Figure 1.
Timeline of the Breakthrough and Progression of CRISPR-Cas9 Systems
CRISPR-Cas9 technology utilizes a single guide RNA (sgRNA) sequence and Cas9 endonuclease. sgRNA is a combination of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA), which identify and attach the sgRNA/Cas9 ribonucleoprotein complex to the target DNA.13 After DNA targeting, Cas9 generates double-stranded breaks (DSBs) at the site of target DNA.14 These DSBs are further repaired by insertions, deletions, additions, or inversions. The DNA repair mechanism can be performed by either the host’s natural repair machinery or by using customized DNA sequences.15
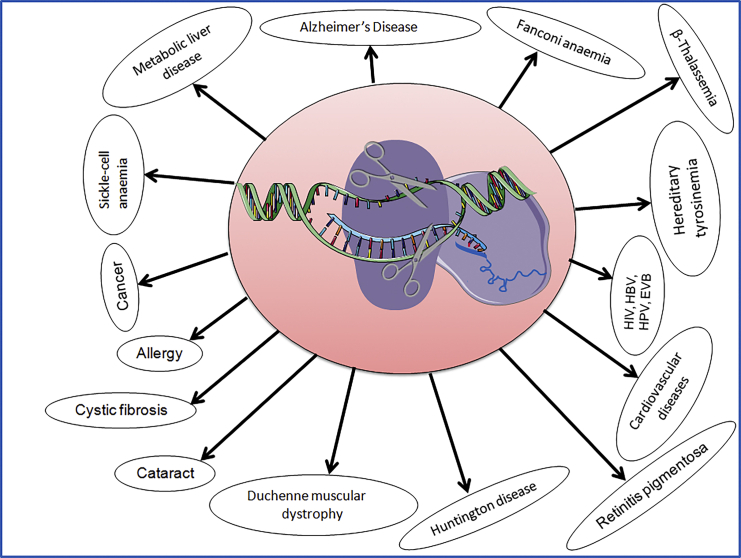
The highlights of CRISPR-Cas9 technology involve its ability to treat various human diseases via genome editing (Figure 2).15 Accumulating evidence suggests successful genome editing in mammalian cells via CRISPR-Cas9 technology.16,17 CRISPR-Cas9 technology has now entered clinical trials in the United States through the National Institutes of Health (NIH) Recombinant DNA Advisory Committee, which has permitted CRISPR technology to assist cancer therapies, relying on the enlistment of a patient’s T cells at the NIH.18 In addition, an analogous type of gene therapy clinical trials received ethical approval in China.19 These clinical trials might establish an efficient therapeutic genome-editing system against hereditary or non-hereditary genetic disorders in humans.
Figure 2.
CRISPR-Cas9 System Dealing for Treatment of Multiple Human Diseases
Although the CRISPR-Cas9 tool has therapeutic potential, unexpected outcomes have challenged the development of more simple and specific gene-editing methodologies.20 In this review, we discuss the preclinical studies on the mechanism of action of CRISPR-Cas9 systems against various diseases, such as cancer, allergies, and immunological disorders. We also discuss the clinical advances, challenges, safety concerns, and strategies to develop efficient CRISPR-Cas9-based gene-editing technology.
Advantages of CRISPR-Cas9 and Other Genome-Editing Tools
Genome-editing technology has gained attention as a therapy against human diseases. However, its success has been previously challenged by unpredictable outcomes. In early gene therapy trials, 5 children, out of 20 participants from different studies, suffering from the severe combined immunodeficiency (SCID) X-1 condition developed T cell leukemia due to non-specific insertion of a correcting gene near to tumor-promoting genes, which resulted in transcriptional activation.21, 22, 23, 24, 25 In another trial, an 18-year-old male with ornithine transcarbamylase (OTC) deficiency developed a lethal immune response induced by viral vector.26 Both of these misfortunes were associated with poor or uncontrolled therapeutic delivery methods.23
Lately, more advanced gene therapy technologies, such as zinc finger nucleases (ZFNs),27 transcription activator-like effector nucleases (TALENs),28 and CRISPR-Cas9,29 were developed, which were capable of site-specific gene editing. ZFNs are a combination of a non-specific FokI cleavage domain and zinc finger proteins (ZFPs). In eukaryotes, ZFPs are associated with protein-protein interaction and regulation of DNA transcription.30 For gene editing, paired ZFNs, one downstream and one upstream of the target site, are used to generate DSBs.31 ZFNs, however, can only recognize nucleotide triplets in the DNA, thus restricting the number of site selections due to limited binding sites for ZFPs.32 Various interventional clinical trials based on ZFN-mediated gene editing are ongoing, e.g., for the treatment of Hunter’s syndrome,33 to cure human immunodeficiency virus (HIV) infection,34 and others. Similar to ZFNs, TALENs include a complex of transcription activator-like effectors (TALEs) and FokI endocuclease.28 TALEs are the amino acid sequences that flank a DNA binding site. TALENs have a benefit over ZFNs, as they can recognize a single nucleotide and are more specific than ZFNs.35 Still, packaging and delivery of TALENs might be a challenge because they have a large size than ZFNs.23
CRISPR-Cas9 is latest gene-editing technique with various advantages over ZFNs and TALENs. First, CRISPR-Cas9 is more cost-effective than other gene-editing techniques.36 Second, the target specificity of CRISPR-Cas9 depends on the ribonucleotide complex, which is probably more specific to protein-based DNA bindings in ZFNs and TALENs. Moreover, it is easy to design sgRNA for a wide range of target DNAs. In addition, various modifications can be performed in the CRISPR-Cas9 cargo system, i.e., plasmid DNA encoding sgRNA and Cas9, the combination of sgRNA and Cas9 mRNA, and the combination of sgRNA and Cas9 protein. Other advantages of CRISPR-Cas9 include the possibility of direct genome modification in the embryo and introduction of more than one mutation at the same time.37 In addition, base edition (i.e., the conversion of cytidine to uracil [C→T] or guanine to adenine [G→A]) that is mediated by the binding of CRISPR-Cas9 with cytidine deaminase is also possible.38 These advancements indicate the possibility of safe and successful clinical application of CRISPR-Cas9 technology in the future.
Preclinical Studies of CRISPR-Cas9
CRISPR-Cas9 research has been applied for the treatment of different human diseases (Table1), which are discussed below.
Table 1.
CRISPR-Cas9 Research and Its Application for the Treatment of Different Human Diseases
| Disease Type | Disease-Related Gene/Protein | Remarks | References |
|---|---|---|---|
| Cancer | additional sex combs like 1 (ASXL1) | CRISPR-Cas9 was used to decrease leukemia cell growth in mouse xenografts | 39 |
| myeloid cell leukemia 1 (MCL-1) | CRISPR-Cas9 was used to delete MCL-1 in human BL cells and induce apoptosis in the BL cells | 40 | |
| cyclin-dependent kinase 11 (CDK11) | CRISPR-Cas9 was used to silence CDK11 in osteosarcoma | 41 | |
| SHC SH2-binding protein 1 (SHCBP1) | SHCBP1 inhibits the proliferation of breast cancer through CRISPR-Cas9 | 42 | |
| Kelch domain containing 4 (KLHDC4) | CRISPR-Cas9 was used to knock out the KLHDC4 gene in a nasopharyngeal carcinoma cell line | 43 | |
| epidermal growth factor receptor (EGFR) | CRISPR-Cas9 was used for possible correction of acquired drug-resistant mutations in EGFR | 44 | |
| Allergy | melanoma cell adhesion molecule (MCAM/MUC18) | CRISPR-Cas9 technology was used to knock out cell surface glycoprotein MUC18 in human primary nasal airway epithelial cells | 45 |
| Janus kinase 3 (JAK3) | CRISPR-Cas9 was used to re-establish the development of normal T cells in JAK3-deficient cells | 46 | |
| Duchenne muscular dystrophy | dystrophin | CRISPR-Cas9 was used to fix DMD gene mutation for the DMD disorder | 47 |
| Cardiovascular diseases | proprotein convertase subtilisin/kexin type 9 (PCSK9) | CRISPR-Cas9 was used to correct the PCSK9 gene in an atherosclerosis mouse model | 48 |
| Huntington disease | huntingtin (HTT) gene | CRISPR-Cas9 was used to suppress the mHTT gene selectively in a mouse model | 49 |
| Alzheimer’s disease | presenilin 1 (PSEN1) and presenilin 2 (PSEN2) genes | CRISPR-Cas9 was used to correct ancestral mutations in AD related to the PSEN gene | 50,51 |
| Metabolic liver disease | Pahenu2 | CRISPR-Cas9 was used to correct the Pahenu2 gene in metabolic liver disease | 52 |
| Fanconi anemia | 17 Fanconi anemia (FA) | CRISPR-Cas9 was used to correct Fanconi anemia | 53 |
| Hereditary tyrosinemia | fumarylacetoacetase (Fah) | CRISPR-Cas9 was used to correct the Fah mutation in mouse models | 54 |
| Sickle cell anemia | β-globin gene | CRISPR-Cas9 was used to treat sickle cell disease patient blood | 55 |
| β-Thalassemia | hemoglobin subunit beta (HBB) gene | CRISPR-Cas9 was used to corrected the HBB gene mutation in human iPSCs from β-thalassemia patients | 56 |
| Cystic fibrosis | cystic fibrosis transmembrane conductance regulator (CFTR) gene | CRISPR-Cas9 was used to correct the CFTR gene in cultured stem cells of cystic fibrosis patients | 57 |
| Retinitis pigmentosa | RP1, RHO, and RPGR genes | CRISPR-Cas9 was used to interrupt the Rho (S334) mutation | 58 |
| Cataract | αA-crystallin gene | CRISPR-Cas9 was used to study the relationship of αA-crystallin mutations and human congenital cataracts | 59 |
| Human immunodeficiency virus (HIV) | long terminal repeats (LTRs) in HIV | CRISPR-Cas9 was used as a tool to mutate LTRs of HIV-1 DNA | 60 |
| Hepatitis B virus (HBV) | covalently closed circular DNAs (cccDNAs) in HBV | CRISPR-Cas9 was used to target cccDNAs of HBV | 61 |
| Human papilloma virus (HPV) | HPVE6 gene | CRISPR-Cas9 was used to target HPVE6 for cancer treatment | 62 |
| Epstein-Barr virus (EBV) | ephrin receptor tyrosine kinase A2 (EphA2) | using the EphA2 extracellular domain, a therapeutic strategy was developed | 63 |
Cancer
Cancer is among the most prevalent lethal diseases that can be illustrated by the accumulation of epigenetic modifications in the genome.64,65 The drawback of conventional chemotherapy is the lack of specific targeting and resistance to chemotherapeutic drugs.66 Therefore, there is a need to identify novel molecular targets that may facilitate cancer treatment. It has been proposed that the mutated oncogenes and tumor suppressor genes in cancer cells might function as smart therapeutic targets, suggesting that the modulation of tumor suppressor genes can induce apoptosis in tumor cells.67,68 Liu et al.69 observed the effective inhibition of bladder cancer cell proliferation, reduced cell motility, and induction of apoptosis via CRISPR-Cas9-mediated regulation of tumor suppressor genes, i.e., hBax, E-cadherin, and p21.
It has been reported that epigenetic regulators are often mutated in myeloid malignancies.70 CRISPR-Cas9 technology corrected the additional sex combs-like 1 (ASXL1) gene and re-established ASXL1 protein expression that significantly decreased leukemia cell growth in mouse xenografts.39 The CRISPR-Cas9 system was also used to delete the myeloid cell leukemia-1 (MCL-1) gene, a member of the emerging B cell lymphoma 2 (BCL2) gene family, in human Burkitt lymphoma (BL) cells for the induction of apoptosis in the BL cells,40 suggesting that the MCL-1 gene can be a novel target for cancer treatment, as it plays a role in cell differentiation, proliferation, and tumorigenesis.71
Cyclin-dependent kinases (CDKs) are critical regulators of the cell cycle. Therefore, dysregulated activation of CDKs may lead to tumorigenesis.72,73 CRISPR-Cas9 technology can silence the CDK11 gene for the treatment of osteosarcoma41 and the CDK7 gene for the treatment of triple-negative breast cancer cells.74 This indicates that CDKs can be novel targets for cancer treatment.
Resistance to chemotherapeutic drugs is a common disadvantage of chemotherapy. The multidrug resistance-1 (MDR1) gene encodes for membrane efflux pump P-glycoprotein. The overexpression of MDR1 facilitates the efflux of anti-cancer drugs from the cells, which results in chemotherapeutic drug resistance. CRISPR-Cas9-mediated knockdown of MDR1 in osteosarcoma cell lines restored the sensitivity toward chemotherapeutic drugs.75 Likewise, the possible management of drug resistance acquired by the secondary mutation in exon 20 at position 790 (T790M) in epidermal growth factor receptor (EGFR) can also be an effective strategy to cure lung cancer.44
SHC SH2-domain binding protein 1 (SHCBP1) is a member of the collagen homolog family that is critical for the regulation of cell proliferation. As overexpression of the SHCBP1 gene is reported in several diseases, especially cancer, it may be a potential therapeutic target as well as a suitable diagnostic biomarker for cancer.42 It has been demonstrated that the CRISPR-Cas9-mediated knockout of the SHCBP1 gene might inhibit cancer cell proliferation and induce apoptosis in breast cancer cells.42
The Kelch-like (KLHL) gene family encodes a group of proteins that are related to several human diseases, along with cancer.76 It was observed that CRISPR-Cas9-mediated knockout of the Kelch domain containing 4 (KLHDC4) gene in a nasopharyngeal carcinoma cell line considerably inhibited cancer cell migration and growth, and it induced apoptosis in both in vitro and in vivo models.43
In addition to the therapeutic domain, CRISPR-Cas9-mediated modification of genes can also be used to develop a mutant cancer model in mice, opening new opportunities to generate mutants in various species and in almost any genetic background to accelerate in vivo studies.77,78 Platt et al.10 developed lung adenocarcinoma mice by knockout of three significant genes (i.e., tumor protein [p53], serine/threonine kinase 11 [STK11] or Lkb1, and Kirsten rat sarcoma 2 viral oncogene homolog [KrasG12D]). In 2015, Chen et al.79 developed in vivo loss-of-function Cas9 screening (genome-wide CRISPR screen) for a tumor metastasis and growth study. CRISPR-Cas9 technology was also used to initiate multiple gene mutations to develop human colonic epithelium in the colorectal cancer model.80 CRISPR-Cas9-mediated knockout of single gene, i.e., patched 1 (Ptch1), or multiple genes, i.e., transformation-related protein 53 (TRP53), neurofibromin 1 (Nf1), and phosphatase and tensin homolog (PTEN), in the brain resulted in the progression of glioblastoma and medulloblastoma in mice.81 CRISPR-Cas9 technology was also used to generate an acute myeloid leukemia (AML) mouse model with a combinatorial genetic lesions system by inducing multiple mutations in the genes of epigenetic modifiers, as well as cytokine signaling and transcription factors in the hematopoietic stem cells of mice.82
Cancer immunotherapy is among the four major line of treatments along with surgery, chemotherapy, and radiotherapy. Clinical trials on gene editing-based immunotherapies have been focusing on chimeric antigen receptor (CAR) T cell therapy83, 84, 85 and genetically modified T cell receptor (TCR) therapy. In recent years, Kymriah from Novartis and Yescarta of KITE Pharma received US Food and Drug Administration (FDA) approval of their CAR T cell therapy products.86,87 CAR, a synthetic receptor, acts as a gene insert that contains a transmembrane domain, a hinge segment, an antibody-derived extracellular-specific target protein binding domain, and a T cell-activating intracellular signaling unit.88 CARs are inserted into the autologous T cells that are collected from the patients, resulting in the expression of CARs on the surface of T cells. When these T cell constructs are introduced again into the patient, they multiply and bind to the target protein and eliminate tumor cells. CAR T cell therapy showed 80%–100% remission in patients with relapsed or refractory B cell acute lymphocytic leukemia (ALL).83,89 However, cytokine release during the therapy may result in various manageable side effects. While CARs recognize surface antigens, TCRs recognize intracellular proteins presented on major histocompatibility complex I (MHC class I). Moreover, TCR therapy is preferred over CAR T cell therapy due to low incidence of cytokine release syndrome.90 The most recent clinical trials are working on CRISPR-Cas9-based immunotherapy against various types of cancer, which are detailed later in this review.
Allergy and Immunological Disorders
The therapeutic role of CRISPR-Cas9 genome editing for allergic and immunological conditions has also been reported.91 It was observed that expression of cell surface glycoprotein MUC18 or CD146 is increased in the alveolar macrophages of bacterial- or viral infection-mediated chronic obstructive pulmonary disease (COPD) or asthma.92 Knockout of the MUC18 gene reduced the level of interleukin (IL-8), a pro-inflammatory chemokine, in human primary nasal airway epithelial cells (AECs) that were stimulated by microbial infection, mimicking Toll-like receptor (TLR) agonists (i.e, TLR2, TLR3, and TLR4).45
It was noted that targeting receptors genes, such as programmed death-1 (PD-1), can stimulate immune responses. PD-1 is a T cell surface protein that is associated with T cell activation. CRISPR-Cas9-mediated disruption of the PD-1 receptor gene in human primary T cells isolated from cancer patients resulted in upregulated interferon (IFN)-γ production and enhanced cytotoxicity,93 suggesting checkpoint inhibitors as novel targets for cancer treatment.
X-linked hyper immunoglobulin M (IgM) syndrome is an immune deficiency disorder identified by defective CD40/CD40L signaling via dysregulated class-switch recombination and somatic hypermutation in B cells.94 It has been observed that the CRISPR-Cas9 gene-editing technique can correct mutations in the CD40 ligand.95 Cheong et al.96 edited the mouse and human Ig genes to obtain class switching of IgH, which might assist in the study of B cell (both normal and lymphoma) biology.
JAK3 (Janus kinase 3) is a protein tyrosine kinase that regulates various pathogenic processes in allergic asthma.46 The deficiency in JAK3 is related to the reduced number of circulating natural killer (NK) cells and T cells, and with normal numbers of inadequately functioning B cells.97 It has been noted that the mutations in the JAK3 gene can cause SCID. CRISPR-Cas9-mediated correction in the human JAK3 gene restored the differentiation potential of T cell progenitors, which are capable of producing T cells/NK cells.97 This suggests that the CRISPR-Cas9 technique can reprogram cells for the prevention of various allergic conditions.
Duchenne Muscular Dystrophy (DMD)
DMD is an X-linked disorder and is characterized by proximal muscle weakness caused by small mutations in the DMD gene that lead to the absence of dystrophin protein. A number of knockout mouse models were produced to imitate the human DMD phenotype by genetic inhibition of dystrophin and/or utrophin/α7β1-integrin genes.98, 99, 100 It was demonstrated that genome editing can be used to restore the DMD gene mutation and correct the DMD disorder.47,101, 102, 103 CRISPR-Cas9 technology has provided a platform for the treatment of DMD.1,104 The CRISPR-Cas9-mediated gene-editing method was used to correct the DNA of an entire region containing CTG/CAG repeats, suggesting a new therapeutic opportunity against DMD.105 Moreover, gold nanoparticles (NPs) were also used as a delivery vehicle for Cas9 ribonucleoprotein and donor DNA to correct the DMD gene in mice with minimal off-target DNA damage.106 Recently, Zhang et al.107 suggested that the self-complementary adeno-associated virus (ScAAV) delivery system can substantially improve the efficiency of CRISPR-Cas9-mediated DMD gene correction.
Cardiovascular Disorders (CVDs)
The proprotein convertase subtilisin/kexin type 9 (PCSK9) gene has an important role in the regulation of cholesterol homeostasis.108 The gain-of-function mutation of the PCSK9 gene can result in hypercholesterolemia and associated artherosclerosis.48 Jiang et al.109 reported CRISPR-Cas9-mediated therapeutic targeting of the PCSK9 gene in mice. In addition, the CRISPR-Cas9 genome-editing tool was also reported to disrupt the low-density lipoprotein receptor (Ldlr) gene and overexpress the PCSK9 gene in adult mice in atherosclerosis research.110 Tessadori et al.111 applied a CRISPR-Cas9-based genome-editing tool in a zebrafish model to correct human genetic cardiovascular disorders. These studies suggest the use of CRISPR-Cas9 as a potential genome-editing tool against cardiovascular disorders, especially conditions associated with hereditary lipid disorders.
Neurological Disorders
Huntington’s disease (HD) is an autosomal inherited neurological disorder that is caused by the extension of CAG repeats in exon 1 of the huntingtin (HTT) gene.112 A CRISPR-Cas9 nucleotide-editing tool was effectively applied to selectively suppress the HTT gene in a mouse model.49 Other studies also supported the use of the CRISPR-Cas9 genome-editing system against HD conditions.113,114
Alzheimer’s disease (AD), a neurodegenerative condition, leads to progressive memory loss. Mutations in the presenilin 1 (PSEN1) and PSEN2 genes are reported in the familial AD condition.115 PSEN1 is a catalytic subunit of γ-secretase, a protease enzyme that cleaves amyloid precursor protein (APP), generating amyloid-β (Aβ). The A79V mutation in the PSEN1 gene increases the Aβ42/Aβ40 ratio by decreasing Aβ40. Studies have reported the correction of A79V and L150P mutations in PSEN1 in an induced pluripotent stem cell (iPSC) line derived from AD patients.50,116 The CRISPR-Cas9 nucleotide-editing system was used to edit the point mutation “T” with wild-type “C” nucleotide in the A79V-hiPSC line. Moreover, mutation in the APP gene increases β-secretase cleavage of APP, resulting in abnormally high Aβ levels in the brain. CRISPR-Cas9 was also used to correct the APP allele, thereby decreasing Aβ pathogenesis.117 CRISPR-Cas9-based genome editing against AD has been reviewed in detail by Rohn et al.51 In summary, CRISPR-Cas9 gene-editing technology can be used as an efficient strategy against genetically-induced neurological disorders.104
Metabolic Disorders
Metabolic liver disease (MLD) is caused by the defect of a transporter protein that results in abnormal metabolism of carbohydrates, protein, and fat. Recently, Villiger et al.52 corrected mutations in the phenylalanine hydroxylase (Pah)enu2 gene to treat phenylketonuria (PKU), an autosomal recessive liver disease, using CRISPR-Cas9 editor systems in mice. In another study, computationally designed hepatocyte-specific CRISPR-Cas9 was used to target the murine factor IX (F9) gene against an MLD-related condition.118 Yang et al.119 worked on CRISPR-Cas9-mediated gene editing-based correction of X-linked deficiency in OTC to treat urea cycle disorder in an infant mice model.
Hereditary tyrosinemia (HT) is an autosomal recessive inherited disease that is associated with a deficiency of the enzyme fumarylacetoacetate hydrolase due to mutations in the Fah gene.120,121 HT type I (HTI) causes severe hepatic disorders, such as cirrhosis, liver failure, and hepatic cancer, due to toxin accumulation. The CRISPR technology-based therapeutic strategy to correct HT was one of the first known studies in mice demonstrating delivery of CRISPR-Cas9 system components to adult mammalian organs.122 In this study, reconstitution of a disease-causing mutation in the Fah gene was done via hydrodynamic injection of Cas9 nuclease, a sgRNA, and a donor oligonucleotide that led to a significant step toward gene therapy.122 VanLith et al.123 also showed hepatocyte-directed Fah gene repair in an HTI mice model using CRISPR-Cas9 against metabolic liver diseases. CRISPR-Cas9-based correction of the Fah gene in the HTI mice model showed weight stability prevention of liver cirrhosis in mice.54
Hunter syndrome, a metabolic disorder, is caused by the mutational dysfunction of an enzyme called iduronate-2-sulfatase (IDS) that leads to damage in lungs, heart, and brain. At the end of 2017, in vivo genetic editing for the treatment of Hunter syndrome via ZFNs was reported.124 This is the first trial reporting the possibility of in vivo gene editing-mediated treatment of genetic diseases. At the end of 2018, Sangamo Therapeutics (Richmond, CA, USA) reported ZFN-based in-body gene editing in people with Hunter syndrome. Although they obtained mixed results, no adverse side effects were observed with this therapy.125,126 This initiative in gene editing-based therapy suggests the possible use of CRISPR-Cas9 for the treatment of metabolic disorders. Nevertheless, CRISPR-Cas9 technology against metabolic disorders needs efforts to enter into clinical trials.
Cystic Fibrosis
Cystic fibrosis (CF), an autosomal recessive monogenic condition, is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene,127 causing damage to the lungs and digestive system. It has been noted that the CRISPR-Cas9 approach may be a suitable method to correct mutations in the CFTR gene.128 Crane et al.129 corrected the mutation of CFTR in iPSCs via the CRISPR-Cas9 approach. In another study, sheep models (CFTR−/− and CFTR+/−) were developed by CRISPR-Cas9-mediated CFTR gene disruption to understand CF pathogenesis.130 The mutated CFTR sheep model is supposed to be a useful resource for the advanced development of new CF therapeutics. In another study, the CRISPR-Cas9 approach was used in the cultured stem cells of CF patients to correct the CFTR gene.57 In primary adult stem cells, CFTR gene alteration was done through homologous recombination.57 Therefore, CRISPR technology might be a potential approach for the treatment of CF in future.
Blood-Related Disorders
Fanconi anemia (FA), an autosomal recessive disorder, is caused by mutations in genes that are responsible for replication-dependent excision of interstrand DNA crosslink.131 CRISPR-Cas9-mediated homology-directed recombination (HDR) in the FA gene implicate the application of genome editing for correcting the defects in the DNA repair pathway.53 CRISPR-Cas9-mediated corrections of disruptive mutation in the FA complementation group F (Fancf) gene132 and correction of the Fanconi anemia I (FANCI) gene133 were reported in iPSCs from primary fibroblasts. The correction of FA mutations is implicated with the therapeutic approach of CRISPR-Cas9 against bone marrow failure.132 Richardson et al.134 found that human Cas9-induced single-strand template repair (SSTR) requires the FA pathway for DNA interstrand crosslink repair in cells.
Sickle cell (SC) anemia, a blood-related disorder, is caused by a mutation in the β-globin gene that results in the formation of abnormal hemoglobin S (HbS) protein.135 As evidenced, CRISPR-Cas9 is a safe and promising gene therapy approach for SC anemia.136, 137, 138 CRISPR-Cas9-based correction of the Hbs gene in hematopoietic stem and progenitor cells (HSPCs) from SC disease patient blood confirmed normal functional reinstitution of hemoglobin.55 In an in vitro study, CRISPR-Cas9 components showed more than 18% gene modifications in CD34+ cells.139 They also reported correction of CD34+ HSPCs derived from the bone marrow of SC anemia patients via CRISPR-Cas9 technology.139 Ye et al.140 created a hereditary persistence of fetal hemoglobin (HPFH) genotype in normal HSPCs using CRISPR-Cas9 and indicated safe autologous transplantation for patients with SC disease and β-thalassemia.
β-Thalassemia, another widespread genetic blood-related disease, is caused by the decreased synthesis of the β-globin chains of the hemoglobin tetramer, which reduces the production of hemoglobin.56,141 Correction of the β-thalassemia splice mutation (IVSII-1G>A) in iPSCs using Cas9 along with the piggyBac transposon-modified donor vector strategy showed repair of the mutated gene with enhanced specificity and accuracy.142 This might be a critical therapeutic approach toward the stem cell-based gene therapy against monogenic disorders in future clinics.
Eye-Related Disorders
Retinitis pigmentosa (RP), an inherited pigmentary retinal dystrophy, may cause loss of vision.143,144 There are several mutations associated with this disease, such as mutations in RP1, rhodopsin (RHO), and RP GTPase regulator (RPGR) genes.145 The CRISPR-Cas9 gene-editing technique can correct the Rho (S334) gene by subretinal injection of guide RNA (gRNA)-Cas9 plasmid, causing improvement of visual function through the discontinuation of retinal degeneration in rats.58 Suzuki et al.146,147 applied CRISPR-Cas9 using homology-independent targeted insertion (HITI), a method that allows the targeted insertion of a gene in non-dividing cells to improve the visual condition in rats. This suggests the possibility of in vivo gene correction using CRISPR-Cas9 technology.
Several mutations at different genetic loci are reported in the cataract condition, i.e., cloudiness of the crystalline lens.148 It has been noted that αA-crystallin gene mutations are associated with an autosomal recessive cataract.149 To understand the role of the αA-crystallin gene in congenital cataracts, Yuan et al.59 used CRISPR-Cas9 to develop mutations in the αA-crystallin gene in an animal model. Recently, it has been noted that missense mutation of GJA8 is also associated with congenital cataracts.150 Yuan et al.151 have developed a GJA8 knockout rabbit model using the CRISPR-Cas9 system to study congenital cataracts in human. Currently, CRISPR-Cas9-mediated in vivo correction of a blindness condition is in a clinical trial that is discussed later in this review.
Viral Infection
HIV
HIV is a pathogen that attacks the human immune system and causes acquired immunodeficiency syndrome (AIDS). Although AIDS is now manageable due to highly active antiretroviral therapy (HAART), the life-long treatment of AIDS is of a great concern. It has been noted that expression of the HIV-1 gene is induced by long terminal repeats (LTRs), which are repeated identical sequences of DNA and assist in the insertion of retroviral DNA into the host chromosome. The genetic variation in the binding sites of LTRs may alter LTR-driven viral transcription.152 It was observed that CRISPR-Cas9 can mutate LTRs in the DNA of HIV-1 provirus, leading to the breakdown of latent HIV-1 provirus.60 In a negative feedback regulation of HIV-1, the Cas9 gene was placed under the control of a minimal HIV-1 promoter to express Cas9 in HIV-1 contagious cells. This might also reduce the complications attributed to the unusual high expression of Cas9 in the cells.153 It has been reported that CRISPR-Cas9-mediated gene editing can inhibit multiple steps of HIV-1 infection.154
Hultquist et al.155 used CRISPR-Cas9 technology for mechanistic examination of HIV host factors in CD4+ T cells. Hartweger et al.156 edited B cells using CRISPR-Cas9 in wild-type mice to reduce the effect of HIV-1 infection. Some factors, such as an apolipoprotein B mRNA-editing enzyme (APOBEC3G) and TRIM5α gene expression, can cause host restriction against HIV infection. CRISPR-Cas9 technology enhanced the expression of these host restriction factors against HIV infection,157,158 suggesting the importance of CRISPR-Cas9 strategies as anti-HIV therapies. However, no curative therapy has been approved at this time.
Hepatitis B Virus (HBV)
HBV causes chronic hepatitis, which is a frequent infectious disease worldwide. It has been observed that covalently closed circular DNAs (cccDNAs) of HBV reside in the contaminated cells, suggesting cccDNA as a potential therapeutic target to treat HBV infection.159, 160, 161 It was found that CRISPR-Cas9 nuclease can mediate interference of episomal cccDNA in reporter cell lines, causing disruption in chromosomally integrated HBV sequences.162 Dong et al.61 demonstrated that CRISPR-Cas9 can reduce HBV cccDNA by inhibiting viral replication. Ramanan et al.163 also showed that CRISPR-Cas9 cleaves viral DNA and suppresses HBV. Wang et al.164 used an RNA interference (RNAi) technique with a sgRNA-microRNA (miRNA)-gRNA cassette along with Cas9 to inhibit the replication of HBV. Thus, it can be suggested that the disruption of the HBV genome through CRISPR-Cas9 technology might be a promising approach as an anti HBV therapy.
Hepatitis C Virus (HCV)
HCV, a single-stranded RNA (ssRNA) virus, is the causative agent of hepatitis C, an inflammatory condition of the liver.165 It has been found that the Cas9 endonuclease enzyme from the Gram-negative bacterium Francisella novicida, known as FnCas9, can target endogenous RNA.166 The CRISPR-FnCas9 system has been used to inhibit HCV within eukaryotic cells.167
Human Papillomavirus (HPV)
HPV, a double-stranded DNA (dsDNA) virus, infects mucosal cells or skin. It causes sexually transmitted disease and accounts for an estimated 11% of the global cancer incidence in women.168,169 The RNA-guided endonuclease offers a therapeutic approach against HPV.170 It was reported that the CRISPR-Cas9-mediated cervical cancer treatment can be done by targeting HPVE6.62 CRISPR-Cas9 technology can also target the conserved regions of HPV6/11 E7 genes, indicating the therapeutic potential of gene editing against genital warts.171
Epstein-Barr Virus (EBV)
EBV, a dsDNA virus, spreads primarily through saliva and causes mononucleosis.172 CRISPR-Cas9 technology has been used for genome editing of EBV in human cells by modifying the BART promoter gene encoding viral miRNAs.173 Ma et al.174 showed that 87 lymphoblastoid cell line (LCL) and 57 BL genes are significant for the survival and growth in LCL and BL cells. Ephrin receptor tyrosine kinase A2 (EphA2) facilitates the entry of EBV in human cells. CRISPR-Cas9-mediated knockout experiments demonstrated that the EphA2 extracellular domain can bind with the EBV- glycoprotein gHgL and provide entry to the cell.63 Thus, EphA2 might be a new potential target for therapeutic development.
Translation of Preclinical Studies into Clinical Use
Although preclinical studies on rodent models show evident efficiency of CRISPR-Cas9-mediated genome editing, variable results may be obtained during clinical translation of preclinical studies due to various reasons.175 Inadequate analysis of the preclinical experimental data is one of the major reasons for variable results.176 In addition, the presence of various backgrounds of the most extensively used C57BL/6 and 129 strains of rodent models might result in improper data analysis.177 Moreover, inbred mice cannot mimic the diversity of humans.178 Another limitation of CRISPR-Cas9-mediated genome editing in rodents is the precise knowledge of the reproductive cycle of rodents, e.g., time of fertilization and time to recover the fertilized eggs. Therefore, it is important to consider these limitations and obtain in-depth knowledge of the animal model used in preclinical studies before entering into clinical use.
Clinical Trials
China performed the first ex vivo clinical trial (ClinicalTrials.gov: NCT02793856) using gene editing with the CRISPR-Cas9 technique in patients with metastatic non-small-cell lung cancer.179 They targeted the PD-1 gene in T cells from peripheral blood of patients using electroporation of sgRNA and Cas9 plasmid, and infused them back into the patients. Very recently, they reported the presence of edited T cells in the peripheral blood of all patients who received infusions.180 They concluded that although this method is feasible and safe, more advanced gene-editing technology is required to enhance the therapeutic efficacy.
Recently, Stadtmauer et al.181 reported the results of a phase 1 in-human CRISPR-Cas9 technology-based clinical trial (ClinicalTrials.gov: NCT03399448), performed in three patients with refractory cancer in advanced stages. They removed TRAC and TRBC genes that encode the chains of endogenous TCR and PDCD1 (encoding PD-1 loci) from the T lymphocytes retrieved from the patients to increase the anti-tumor immunity. They further introduced a transgene (NY-ESO-1) that can recognize tumors. These engineered T lymphocytes were well tolerated by the patients up to 9 months after reintroduction into the patients.181 However, chromosomal translocation was observed, which was reduced after some time.
Another clinical trial (Clincialtrials.gov: NCT03398967) proposed CAR T cell therapy for relapsed or refractory hematological malignancies due to CD19− tumor cells. They performed integration of two CARs (i.e., CD19 and CD20 or CD22) into the TRAC locus of T cells, which were able to recognize CD19− cells.182 In addition, the use of gene-disrupted allogeneic universal CD19-specific CAR T cells (UCART019) using lentivirus (LV) delivery of CARs in patients with relapsed or refractory CD19+ leukemia and lymphoma is also in a clinical trial (ClinicalTrials.gov: NCT03166878). In this study electroporation of CRISPR RNA was used to disrupt endogenous TCR and B2M genes. This system could minimize immunogenicity by avoiding graft-versus-host disease (GVHD). However, the results are still not published. Most recently, the FDA approved a clinical trial (ClinicalTrials.gov: NCT04438083) of CTX130, an allogeneic CRISPR-Cas9-modified T cell line, targeting CD70 against hematologic malignancies and renal cell carcinoma.
In 2019, successful treatment of SC disease and β-thalassemia was reported by Sangamo (ClinicalTrials.gov: NCT03432364) via CRISPR-Cas9-mediated disruption of the BCL11A gene in stem cells that were isolated from the peripheral blood of patients with hemoglobinopathies.183 BCL11A is a transcription regulator that suppress the expression of the β-globin gene. In another partially successful trial (ClinicalTrials.gov: NCT03164135), CCR5-deleted hematopoietic stem cells were transplanted into patients with HIV-1 and acute lymphoblastic leukemia. Although the desired objective was not achieved, no major side effects were observed.184 The study suggested the need for increasing the efficiency of CCR5 disruption in lymphocytes.184
In an in vivo clinical trial (ClinicalTrials.gov: NCT03872479) registered in 2019, the CRISPR-Cas9 gene therapy-based drug AGN-151587 was given directly into the eye, via subretinal injection, to cure a rare blindness condition called Leber’s congenital amaurosis 10 (LCA10) that is caused by mutations in the gene CEP290.185 This trial is the first approach that deploys CRISPR-Cas9 gene-editing therapy directly into the human body. At present, there are approximately 19 registered interventional clinical trials on CRISPR-Cas9-mediated gene-editing technology (Table 2).
Table 2.
Currently Registered Interventional Clinical Trials with CRISPR-Cas9-Based Gene Editing
| Serial No. | NCT No.: ClinicalTrials.gov | Target Gene and Effect | Disease | Intervention | Phase | Study Start Date | Country |
|---|---|---|---|---|---|---|---|
| 1 | NCT04426669 | cytokine-induced SH2 (CISH) protein inhibition | gastrointestinal (GI) cancer | tumor-infiltrating lymphocytes (TILs) inhibited immune checkpoint CISH | I/II | May 15, 2020 | US |
| 2 | NCT04178382 | target adjustment of antibiotics | severe sepsis | detection of alveolar lavage fluid changes the choice of early antibiotics in patients with pneumonia | – | August 1, 2019 | China |
| 3 | NCT04037566 | disruption of HPK1 | refractory B cell malignancies | CD19-CAR-modified T cells with CAR delivered by lentivirus and Cas9 knockout of HPK1 | I | August 2019 | China |
| 4 | NCT03164135 | CCR5 knockout | HIV | modified CD34+ hematopoietic stem cells | – | May 30, 2017 | China |
| 5 | NCT03545815 | programmed cell death protein 1 (PD-1) and TCR knockout | mesothelin-positive solid tumors | CAR T cells to mesothelin with added PD-1 and TCR knockout | I | June 1, 2018 | China |
| 6 | NCT03057912 | E6 and E7 oncogenes of HPV16 and HPV18 deletion | HPV-related malignancy | plasmid in a gel containing a polymer to facilitate delivery | I | January 15, 2018 | China |
| 7 | NCT03342547 | stem cell-derived human intestinal enteroids | gastrointestinal infection | duodenal biopsies, followed by differentiation into mini-guts | – | April 18, 2018 | China |
| 8 | NCT03655678 | disruption of the erythroid enhancer to the BCL11A gene | β-thalassemia | ex vivo-modified hematopoietic stem cells | I/II | September 14, 2018 | UK, Germany, Canada, Italy |
| 9 | NCT03728322 | Correction of the hemoglobin subunit β-globulin gene | β-thalassemia | ex vivo-modified hematopoietic stem cells | I | January 2019 | not specified |
| 10. | NCT04244656 | CTX120 B cell maturation antigen (BCMA)-directed T cell immunotherapy | multiple myeloma | biological safety and efficacy of CTX120 in multiple myeloma | I | January 22, 2020 | US, Spain, Australia |
| 11. | NCT04438083 | CTX130 CD70-directed T cell immunotherapy comprised of allogeneic T cells | renal cell carcinoma | safety and efficacy of CTX130 in relapsed or refractory renal cell carcinoma | I | June 16, 2020 | Australia |
| 12. | NCT03747965 | PD-1 knockout | mesothelin-positive solid tumors | CAR T cells to mesothelin with PD-1 knockout | I | November 2018 | China |
| 13. | NCT03398967 | Cas9-mediated creation of CD19 and CD20 or CD19 and CD22 CAR T cells | B cell leukemia | CAR T cells to CD19 and CD20 or CD19 and CD22 | I/II | January 2, 2018 | China |
| 14. | NCT04035434 | creation of a CD19-directed T cell | refractory B cell malignancies | CD19-directed T cell immunotherapy | I/II | July 22, 2019 | U.S.A., Australia |
| 15. | NCT03745287 | disruption of the erythroid enhancer to the BCL11A gene | sickle cell anemia | ex vivo-modified hematopoietic stem cells | I/II | November 27, 2018 | US |
| 16. | NCT03166878 | βTCRα, TCRβ, β2-microglobulin (B2M) knockout | B cell leukemia | CD19-CAR-modified T cells with CAR delivered by lentivirus and Cas9 knockout B2M and TCR to create universal T cells | I/II | June 2017 | China |
| 17. | NCT03044743 | PD-1 knockout | EBV-positive, advanced stage malignancies | modified T cells selected for those targeting EBV-positive cells | I/II | April 7, 2017 | China |
| 18. | NCT04417764 | PD-1 knockout engineered T cells | hepatocellular carcinoma | TACE combined treatment to block the blood supply of the tumor | I | June 20, 2019 | China |
| 19. | NCT03872479 | removal of alternative splice site in CEP290 | Leber congenital amaurosis 10 | ZFN-mediated removal of intronic alternative splice site in retinal cells | I | September 26, 2019 | U.S.A. |
Search date: July 5, 2020.
Challenges and Future Directions
Although both preclinical work and clinical trials focusing on curative therapies are proceeding globally, the clinical translation of CRISPR-Cas9-mediated gene correction is associated with unpredictable outcomes.186 Factors affecting the success rate of CRISPR-Cas9-mediated gene editing in humans includes off-target effects and cargo delivery methods. It has been observed that off-target effects are principally guided by sgRNAs, and thus rational designs of sgRNAs are necessary to ensure the efficiency of CRISPR-Cas9 gene-editing technology. It was observed that off-target effects were common in human cell culture with persistent Cas9 expression.187,188 while these effects were less common in in vivo models.189 It might be plausible that the occurrence of off-target effects in cell cultures are due to the influence of various factors, such as cell type, expression level, transfection method, cell culture maintenance, consecutive nuclease expression, guide sequence, and repair events.186
Earlier, cuts at off-target sites that generate single-stranded breaks (SSBs) in DNA were considered as a major obstacle and raised concerns about the specificity of CRISPR-Cas9 technology.190 To reduce the possibility of SSBs, paired CRISPR-Cas9 nickase that binds to each forward and reverse DNA sequence on the flanking sides of target DNA was used to generate DSB formation.191,192 Another approach suggested the use of inactive fusion protein consisting of Cas9 and FokI endonuclease enzyme, which becomes functional only after precise binding of sgRNA to both forward and reverse DNA sequences.193 Although these approaches might reduce off-target cutting, there are certain limitations concerning restrictions in the location of protospacer-adjacent motifs (PAMs) near target DNA and designing sgRNAs for PAM alternatives.194 In addition, these approaches might also increase the size of cargo that may generate constraint in the delivery system.
More importantly, repair events or a genomic rearrangement after sgRNA-induced DSBs is also a safety concern in CRISPR-Cas9-based therapeutic interventions. Although CRISPR-Cas9 technology can induce desired changes in the genomic sequences, the poorly understood and less controlled DNA repair mechanism is associated with the undesirable risk of biological dysfunctions. Deletion of a few kilobases in the neighboring CRISPR-Cas9 nickase activity, unexpected insertion (incorrect or partial) of donor DNA sequence to the site of integration, and inversion are also unpredictable consequences of DNA repair mechanisms,195 which might result in unexpected mutations.196 The DSBs are repaired via non-homologous end joining (NHEJ) or HDR. NHEJ is a natural process to join spontaneous breaks in DNA and does not require any DNA template. However, HDR requires either a donor DNA template or DNA that is synthesized by the host’s molecular recombination machinery. NHEJ is an error-prone mechanism that possibly leads to mutations. On the contrary, although HDR repairs DSBs more precisely, the incidence of HDR is very low when compared to NHEJ.197 Various methods are suggested to suppress NHEJ, i.e., chemical suppression of NHEJ,198 cell cycle synchronization,199 silencing of genes,200 and NHEJ-deficient cell lines.201 Therefore, enhancing the efficiency of HDR and decreasing NHEJ is a critical challenge that needs to be fully explored before entering into therapeutic regimes to ensure the desired result of CRISPR-Cas-mediated gene editing.
As the treatment of human diseases needs to be tissue-specific, it is essential to efficiently deliver the CRISPR-Cas9 cargo into target tissue. Therefore, additional consideration should be given to the suitable delivery system that is based on the charge, size, and content of the CRISPR-Cas9 cargo. CRISPR-Cas9 cargo may be of three types, i.e., plasmid DNA encoding sgRNA and Cas9, a combination of sgRNA and Cas9 mRNA, and a combination of sgRNA and Cas9 protein. Various physical, viral, and non-viral systems have been used as vectors for the delivery of CRISPR-Cas9.
AAVs and LVs are the most commonly used viral vectors for gene editing.202 The advantages of AAVs includes a less immunogenic property, serotype specificity, a good safety profile, and the ability to transduce both dividing and non-dividing cells.203 However, mild toxicity is reported at high doses in animal models.204 In addition, LVs also have the advantage of high transducing efficiency and non-immunogenicity. Moreover, LVs have the ability to be pseudotyped, allowing alterations in their cellular tropism. Another important aspect to consider while using viral delivery vectors is their packing limitation. However, it has been observed that the carrying capacity of AAVs can be extended up to 35 kb after modifications. It may be possible that the CRISPR-Cas9 gene-editing complex can activate the host immune response system,205 indicating the need for modifications that can help to avoid an immune response in the host.
In addition to viral delivery systems, the use of non-viral systems or NPs for the delivery of CRISPR-Cas9 is also suggested.205 Lipofectamine is a commercially available source of lipid NPs for sgRNA and Cas9 delivery in various systems.206,207 The cationic property of lipid NPs might allow better packaging of the anionic Cas9/sgRNA complex in NPs. As observed, lipid NPs have low delivery efficiency due to their reduced uptake by cells, low translocation in the nucleus, and the possible entrapment of NPs in the endosomes. However, the use of optimal lipids for the synthesis of lipid NPs, surface modification by a cell targeting agent, avoidance of the immune system, and addition of an endosomal escape system can enhance the delivery efficiency of liposomes.208
Various other non-liposomal delivering techniques are also suggested for the delivery of Cas9 and sgRNA, i.e., FuGENE-6 reagent based on electrostatic interactions,170 calcium phosphate transfection,209, cell-penetrating peptides,210 DNA nanoclew,211, inorganic NPs,212 and polyethylenimine and poly-l-lysine (PLL) polymers. PLL was used to develop a therapeutic delivery system, called multifunctional envelope-type nano devices MENDs, consisting of plasmid DNA and a lipid shell.213 Further modifications in MENDs might allow the targeting of delivery cargo to mitochondria and the nucleus of cells.214 Although MENDs showed a high transfection rate, in vivo validation of this delivery system is still required. Therefore, most of the delivery methods for the CRISPR-Cas9 system have both advantages and disadvantages. This generates a need for extensive work on the development of a suitable delivery system with high specificity, high transfection rate, high capacity, and low immunogenicity. Moreover, long-term safety and low toxicity associated with any delivery system also need consideration before developing CRISPR-Cas9-based gene-editing therapy against human diseases.23
Conclusions
The forthcoming applications of CRISPR-Cas9 are promising for the clinical world. With the progression of genome-editing techniques, the genome-editing research related to therapy for human diseases using CRISPR-Cas9 is developing quickly. Just a few years before, this technology was started, and presently a large number of scientists are working on this technology. Among them, most of the researches are using this genome-editing technology for therapy for human diseases.
However, CRISPR-Cas9 is still a budding technology that is applied to patients with severe conditions at a life-threating stage. Most of the clinical trials are currently in phase I/II. Until now, the clinical trials have been focused on the safety and efficacy of genome editing in humans, to improve molecular processes involved in genome editing. Therapeutic gene-editing technology is now expanding to ethically controversial alteration in the genome of human early-stage embryos to provide protection from HIV infection.215 Such attempts can also suggest that the scientific community establish regulations on gene editing. However, the journey of CRISPR-Cas9 is highly interesting, and it offers a significant hope to researchers for the treatment of human deadly diseases.
Author Contributions
Conceptualization, C.C. and S.-S.L.; Writing – Original Draft, C.C., G.S., and A.R.S; Writing – Review & Editing, A.R.S. and M.B.; Supervision and Funding, S.-S.L. and A.R.S.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by the Hallym University Research Fund and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A2B4012944 and NRF-2020R1C1C1008694).
Contributor Information
Sang-Soo Lee, Email: 123sslee@gmail.com.
Chiranjib Chakraborty, Email: drchiranjib@yahoo.com.
References
- 1.Young C.S., Pyle A.D., Spencer M.J. CRISPR for neuromuscular disorders: gene editing and beyond. Physiology (Bethesda) 2019;34:341–353. doi: 10.1152/physiol.00012.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty C., Teoh S.L., Das S. The smart programmable CRISPR technology: a next generation genome editing tool for investigators. Curr. Drug Targets. 2017;18:1653–1663. doi: 10.2174/1389450117666160527142321. [DOI] [PubMed] [Google Scholar]
- 3.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng R., Lin G., Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283:1218–1231. doi: 10.1111/febs.13586. [DOI] [PubMed] [Google Scholar]
- 5.Bortesi L., Fischer R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015;33:41–52. doi: 10.1016/j.biotechadv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Ryan O.W., Poddar S., Cate J.H.D. 2016. CRISPR-Cas9 genome engineering in Saccharomyces cerevisiae cells. Cold Spring Harb. Protoc. 2016. 10.1101/pdb.prot086827. [DOI] [PubMed] [Google Scholar]
- 7.Friedland A.E., Tzur Y.B., Esvelt K.M., Colaiácovo M.P., Church G.M., Calarco J.A. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratz S.J., Rubinstein C.D., Harrison M.M., Wildonger J., O’Connor-Giles K.M. CRISPR-Cas9 genome editing in Drosophila. Curr. Protoc. Mol. Biol. 2015;111 doi: 10.1002/0471142727.mb3102s111. 31.2.1–31.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Zhou Y., Qi X., Chen J., Chen W., Qiu G., Wu Z., Wu N. CRISPR/Cas9 in zebrafish: an efficient combination for human genetic diseases modeling. Hum. Genet. 2017;136:1–12. doi: 10.1007/s00439-016-1739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt R.J., Chen S., Zhou Y., Yim M.J., Swiech L., Kempton H.R., Dahlman J.E., Parnas O., Eisenhaure T.M., Jovanovic M. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu S., Zhou Y., Wei W. Genome-wide CRISPR/Cas9 screening for high-throughput functional genomics in human cells. Methods Mol. Biol. 2017;1656:175–181. doi: 10.1007/978-1-4939-7237-1_11. [DOI] [PubMed] [Google Scholar]
- 12.Pennisi E. The CRISPR craze. Science. 2013;341:833–836. doi: 10.1126/science.341.6148.833. [DOI] [PubMed] [Google Scholar]
- 13.Chylinski K., Makarova K.S., Charpentier E., Koonin E.V. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42:6091–6105. doi: 10.1093/nar/gku241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouns S.J., Jore M.M., Lundgren M., Westra E.R., Slijkhuis R.J., Snijders A.P., Dickman M.J., Makarova K.S., Koonin E.V., van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porteus M.H. A new class of medicines through DNA editing. N. Engl. J. Med. 2019;380:947–959. doi: 10.1056/NEJMra1800729. [DOI] [PubMed] [Google Scholar]
- 16.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baylis F., McLeod M. First-in-human phase 1 CRISPR gene editing cancer trials: are we ready? Curr. Gene Ther. 2017;17:309–319. doi: 10.2174/1566523217666171121165935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nature. 2016;535:476–477. doi: 10.1038/nature.2016.20302. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer K.A., Wu W.-H., Colgan D.F., Tsang S.H., Bassuk A.G., Mahajan V.B. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat. Methods. 2017;14:547–548. doi: 10.1038/nmeth.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Check E. A tragic setback. Nature. 2002;420:116–118. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003;299:495. doi: 10.1126/science.299.5606.495. [DOI] [PubMed] [Google Scholar]
- 23.Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Adams S., Howe S.J., Al Ghonaium A., Bayford J., Brown L., Davies E.G. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2011;3:97ra79. doi: 10.1126/scitranslmed.3002715. [DOI] [PubMed] [Google Scholar]
- 25.Hacein-Bey-Abina S., Hauer J., Lim A., Picard C., Wang G.P., Berry C.C., Martinache C., Rieux-Laucat F., Latour S., Belohradsky B.H. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 27.Bibikova M., Beumer K., Trautman J.K., Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 28.Christian M., Cermak T., Doyle E.L., Schmidt C., Zhang F., Hummel A., Bogdanove A.J., Voytas D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen R., Embden J.D., Gaastra W., Schouls L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe S.A., Nekludova L., Pabo C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- 31.Doyon Y., Vo T.D., Mendel M.C., Greenberg S.G., Wang J., Xia D.F., Miller J.C., Urnov F.D., Gregory P.D., Holmes M.C. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods. 2011;8:74–79. doi: 10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez C.L., Foley J.E., Wright D.A., Müller-Lerch F., Rahman S.H., Cornu T.I., Winfrey R.J., Sander J.D., Fu F., Townsend J.A. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat. Methods. 2008;5:374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muenzer J., Prada C.E., Burton B., Lau H.A., Ficicioglu C., Foo C.W.P., Vaidya S.A., Whitley C.B., Harmatz P. CHAMPIONS: a phase 1/2 clinical trial with dose escalation of SB-913 ZFN-mediated in vivo human genome editing for treatment of MPS II (Hunter syndrome) Mol. Genet. Metab. 2019;126:S104. [Google Scholar]
- 34.Cannon P., June C. Chemokine receptor 5 knockout strategies. Curr. Opin. HIV AIDS. 2011;6:74–79. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joung J.K., Sander J.D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya D., Marfo C.A., Li D., Lane M., Khokha M.K. CRISPR/Cas9: an inexpensive, efficient loss of function tool to screen human disease genes in Xenopus. Dev. Biol. 2015;408:196–204. doi: 10.1016/j.ydbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H., Wu J.-J., Tang T., Liu K.-D., Dai C. CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci. Rep. 2017;7:7489. doi: 10.1038/s41598-017-07871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valletta S., Dolatshad H., Bartenstein M., Yip B.H., Bello E., Gordon S., Yu Y., Shaw J., Roy S., Scifo L. ASXL1 mutation correction by CRISPR/Cas9 restores gene function in leukemia cells and increases survival in mouse xenografts. Oncotarget. 2015;6:44061–44071. doi: 10.18632/oncotarget.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubrey B.J., Kelly G.L., Kueh A.J., Brennan M.S., O’Connor L., Milla L., Wilcox S., Tai L., Strasser A., Herold M.J. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10:1422–1432. doi: 10.1016/j.celrep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y., Sassi S., Shen J.K., Yang X., Gao Y., Osaka E., Zhang J., Yang S., Yang C., Mankin H.J. Targeting CDK11 in osteosarcoma cells using the CRISPR-Cas9 system. J. Orthop. Res. 2015;33:199–207. doi: 10.1002/jor.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng W., Li H.C., Xu K., Chen Y.F., Pan L.Y., Mei Y., Cai H., Jiang Y.M., Chen T., Feng D.X. SHCBP1 is over-expressed in breast cancer and is important in the proliferation and apoptosis of the human malignant breast cancer cell line. Gene. 2016;587:91–97. doi: 10.1016/j.gene.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 43.Lian Y.-F., Yuan J., Cui Q., Feng Q.-S., Xu M., Bei J.-X., Zeng Y.X., Feng L. Upregulation of KLHDC4 predicts a poor prognosis in human nasopharyngeal carcinoma. PLoS ONE. 2016;11:e0152820. doi: 10.1371/journal.pone.0152820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H., Shrager J.B. CRISPR/Cas-mediated genome editing to treat EGFR-mutant lung cancer: a personalized molecular surgical therapy. EMBO Mol. Med. 2016;8:83–85. doi: 10.15252/emmm.201506006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu H.W., Rios C., Huang C., Wesolowska-Andersen A., Burchard E.G., O’Connor B.P., Fingerlin T.E., Nichols D., Reynolds S.D., Seibold M.A. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015;22:822–829. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaviya R., Laskin D.L., Malaviya R. Janus kinase-3 dependent inflammatory responses in allergic asthma. Int. Immunopharmacol. 2010;10:829–836. doi: 10.1016/j.intimp.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long C., McAnally J.R., Shelton J.M., Mireault A.A., Bassel-Duby R., Olson E.N. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184–1188. doi: 10.1126/science.1254445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seidah N.G. Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors in the treatment of hypercholesterolemia and other pathologies. Curr. Pharm. Des. 2013;19:3161–3172. doi: 10.2174/13816128113199990313. [DOI] [PubMed] [Google Scholar]
- 49.Yang S., Chang R., Yang H., Zhao T., Hong Y., Kong H.E., Sun X., Qin Z., Jin P., Li S., Li X.J. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J. Clin. Invest. 2017;127:2719–2724. doi: 10.1172/JCI92087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pires C., Schmid B., Petræus C., Poon A., Nimsanor N., Nielsen T.T., Waldemar G., Hjermind L.E., Nielsen J.E., Hyttel P., Freude K.K. Generation of a gene-corrected isogenic control cell line from an Alzheimer’s disease patient iPSC line carrying a A79V mutation in PSEN1. Stem Cell Res. (Amst.) 2016;17:285–288. doi: 10.1016/j.scr.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Rohn T.T., Kim N., Isho N.F., Mack J.M. The potential of CRISPR/Cas9 gene editing as a treatment strategy for Alzheimer’s disease. J. Alzheimers Dis. Parkinsonism. 2018;8:8. doi: 10.4172/2161-0460.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villiger L., Grisch-Chan H.M., Lindsay H., Ringnalda F., Pogliano C.B., Allegri G., Fingerhut R., Häberle J., Matos J., Robinson M.D. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 2018;24:1519–1525. doi: 10.1038/s41591-018-0209-1. [DOI] [PubMed] [Google Scholar]
- 53.Osborn M.J., Gabriel R., Webber B.R., DeFeo A.P., McElroy A.N., Jarjour J., Starker C.G., Wagner J.E., Joung J.K., Voytas D.F. Fanconi anemia gene editing by the CRISPR/Cas9 system. Hum. Gene Ther. 2015;26:114–126. doi: 10.1089/hum.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao Y., Wang L., Guo N., Wang S., Yang L., Li Y., Wang M., Yin S., Han H., Zeng L. Cas9-nickase-mediated genome editing corrects hereditary tyrosinemia in rats. J. Biol. Chem. 2018;293:6883–6892. doi: 10.1074/jbc.RA117.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J., Tao W., Hao S., Zu Y. Cellular function reinstitution of offspring red blood cells cloned from the sickle cell disease patient blood post CRISPR genome editing. J. Hematol. Oncol. 2017;10:119. doi: 10.1186/s13045-017-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie F., Ye L., Chang J.C., Beyer A.I., Wang J., Muench M.O., Kan Y.W. Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24:1526–1533. doi: 10.1101/gr.173427.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwank G., Koo B.-K., Sasselli V., Dekkers J.F., Heo I., Demircan T., Sasaki N., Boymans S., Cuppen E., van der Ent C.K. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Bakondi B., Lv W., Lu B., Jones M.K., Tsai Y., Kim K.J., Levy R., Akhtar A.A., Breunig J.J., Svendsen C.N., Wang S. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol. Ther. 2016;24:556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan L., Yao H., Xu Y., Chen M., Deng J., Song Y., Sui T., Wang Y., Huang Y., Li Z., Lai L. CRISPR/Cas9-mediated mutation of αA-crystallin gene induces congenital cataracts in rabbits. Invest. Ophthalmol. Vis. Sci. 2017;58:BIO34–BIO41. doi: 10.1167/iovs.16-21287. [DOI] [PubMed] [Google Scholar]
- 60.Ebina H., Misawa N., Kanemura Y., Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci. Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong C., Qu L., Wang H., Wei L., Dong Y., Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110–117. doi: 10.1016/j.antiviral.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Yoshiba T., Saga Y., Urabe M., Uchibori R., Matsubara S., Fujiwara H., Mizukami H. CRISPR/Cas9-mediated cervical cancer treatment targeting human papillomavirus E6. Oncol. Lett. 2019;17:2197–2206. doi: 10.3892/ol.2018.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J., Sathiyamoorthy K., Zhang X., Schaller S., Perez White B.E., Jardetzky T.S., Longnecker R. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat. Microbiol. 2018;3:172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 65.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 66.Alfarouk K.O., Stock C.-M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., Bashir A.H., Mohammed O.Y., Elhassan G.O., Harguindey S. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Sánchez-Rivera F.J., Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nat. Rev. Cancer. 2015;15:387–395. doi: 10.1038/nrc3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Zeng Y., Liu L., Zhuang C., Fu X., Huang W., Cai Z. Synthesizing AND gate genetic circuits based on CRISPR-Cas9 for identification of bladder cancer cells. Nat. Commun. 2014;5:5393. doi: 10.1038/ncomms6393. [DOI] [PubMed] [Google Scholar]
- 70.Murati A., Brecqueville M., Devillier R., Mozziconacci M.-J., Gelsi-Boyer V., Birnbaum D. Myeloid malignancies: mutations, models and management. BMC Cancer. 2012;12:304. doi: 10.1186/1471-2407-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craig R.W. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 72.Niu Y., Xu J., Sun T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J. Cancer. 2019;10:5504–5517. doi: 10.7150/jca.32628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y., Han C., Li D., Yu Z., Li F., Li F., An Q., Bai H., Zhang X., Duan Z., Kan Q. Cyclin-dependent kinase 11p110 (CDK11p110) is crucial for human breast cancer cell proliferation and growth. Sci. Rep. 2015;5:10433. doi: 10.1038/srep10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Zhang T., Kwiatkowski N., Abraham B.J., Lee T.I., Xie S., Yuzugullu H., Von T., Li H., Lin Z. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu T., Li Z., Zhang Q., De Amorim Bernstein K., Lozano-Calderon S., Choy E., Hornicek F.J., Duan Z. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget. 2016;7:83502–83513. doi: 10.18632/oncotarget.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dhanoa B.S., Cogliati T., Satish A.G., Bruford E.A., Friedman J.S. Update on the Kelch-like (KLHL) gene family. Hum. Genomics. 2013;7:13. doi: 10.1186/1479-7364-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birling M.C., Herault Y., Pavlovic G. Modeling human disease in rodents by CRISPR/Cas9 genome editing. Mamm. Genome. 2017;28:291–301. doi: 10.1007/s00335-017-9703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birling M.C., Schaeffer L., André P., Lindner L., Maréchal D., Ayadi A., Sorg T., Pavlovic G., Hérault Y. Efficient and rapid generation of large genomic variants in rats and mice using CRISMERE. Sci. Rep. 2017;7:43331. doi: 10.1038/srep43331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen S., Sanjana N.E., Zheng K., Shalem O., Lee K., Shi X., Scott D.A., Song J., Pan J.Q., Weissleder R. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–1260. doi: 10.1016/j.cell.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matano M., Date S., Shimokawa M., Takano A., Fujii M., Ohta Y., Watanabe T., Kanai T., Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015;21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 81.Zuckermann M., Hovestadt V., Knobbe-Thomsen C.B., Zapatka M., Northcott P.A., Schramm K., Belic J., Jones D.T., Tschida B., Moriarity B. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat. Commun. 2015;6:7391. doi: 10.1038/ncomms8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heckl D., Kowalczyk M.S., Yudovich D., Belizaire R., Puram R.V., McConkey M.E., Thielke A., Aster J.C., Regev A., Ebert B.L. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 2014;32:941–946. doi: 10.1038/nbt.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., JULIET Investigators Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 86.Vormittag P., Gunn R., Ghorashian S., Veraitch F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 87.Seimetz D., Heller K., Richter J. Approval of first CAR-Ts: have we solved all hurdles for ATMPs? Cell Med. 2019;11 doi: 10.1177/2155179018822781. 2155179018822781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fried L.P., Bush T.L. Morbidity as a focus of preventive health care in the elderly. Epidemiol. Rev. 1988;10:48–64. doi: 10.1093/oxfordjournals.epirev.a036028. [DOI] [PubMed] [Google Scholar]
- 89.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodman M.A., Moradi Manesh D., Malik P., Rothenberg M.E. CRISPR/Cas9 in allergic and immunologic diseases. Expert Rev. Clin. Immunol. 2017;13:5–9. doi: 10.1080/1744666X.2017.1241711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu Q., Case S.R., Minor M.N., Jiang D., Martin R.J., Bowler R.P., Wang J., Hartney J., Karimpour-Fard A., Chu H.W. A novel function of MUC18: amplification of lung inflammation during bacterial infection. Am. J. Pathol. 2013;182:819–827. doi: 10.1016/j.ajpath.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su S., Hu B., Shao J., Shen B., Du J., Du Y., Zhou J., Yu L., Zhang L., Chen F. CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients. Sci. Rep. 2016;6:20070. doi: 10.1038/srep20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arason G.J., Jorgensen G.H., Ludviksson B.R. Primary immunodeficiency and autoimmunity: lessons from human diseases. Scand. J. Immunol. 2010;71:317–328. doi: 10.1111/j.1365-3083.2010.02386.x. [DOI] [PubMed] [Google Scholar]
- 95.Kuo C.Y., Hoban M.D., Joglekar A.V., Kohn D.B. Site specific gene correction of defects in CD40 ligand using the Crispr/Cas9 genome editing platform. J. Allergy Clin. Immunol. 2015;135:AB17. [Google Scholar]
- 96.Cheong T.-C., Compagno M., Chiarle R. Editing of mouse and human immunoglobulin genes by CRISPR-Cas9 system. Nat. Commun. 2016;7:10934. doi: 10.1038/ncomms10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang C.-W., Lai Y.-S., Westin E., Khodadadi-Jamayran A., Pawlik K.M., Lamb L.S., Jr., Goldman F.D., Townes T.M. Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 2015;12:1668–1677. doi: 10.1016/j.celrep.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 98.Min Y.-L., Li H., Rodriguez-Caycedo C., Mireault A.A., Huang J., Shelton J.M., McAnally J.R., Amoasii L., Mammen P.P.A., Bassel-Duby R., Olson E.N. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci. Adv. 2019;5:eaav4324. doi: 10.1126/sciadv.aav4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rooney J.E., Welser J.V., Dechert M.A., Flintoff-Dye N.L., Kaufman S.J., Burkin D.J. Severe muscular dystrophy in mice that lack dystrophin and α7 integrin. J. Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- 100.Guo C., Willem M., Werner A., Raivich G., Emerson M., Neyses L., Mayer U. Absence of α7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum. Mol. Genet. 2006;15:989–998. doi: 10.1093/hmg/ddl018. [DOI] [PubMed] [Google Scholar]
- 101.Tabebordbar M., Zhu K., Cheng J.K.W., Chew W.L., Widrick J.J., Yan W.X., Maesner C., Wu E.Y., Xiao R., Ran F.A. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nelson C.E., Hakim C.H., Ousterout D.G., Thakore P.I., Moreb E.A., Castellanos Rivera R.M., Madhavan S., Pan X., Ran F.A., Yan W.X. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403–407. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long C., Amoasii L., Mireault A.A., McAnally J.R., Li H., Sanchez-Ortiz E., Bhattacharyya S., Shelton J.M., Bassel-Duby R., Olson E.N. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ousterout D.G., Kabadi A.M., Thakore P.I., Majoros W.H., Reddy T.E., Gersbach C.A. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 2015;6:6244. doi: 10.1038/ncomms7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Agtmaal E.L., André L.M., Willemse M., Cumming S.A., van Kessel I.D.G., van den Broek W.J.A.A., Gourdon G., Furling D., Mouly V., Monckton D.G. CRISPR/Cas9-induced (CTG⋅CAG) n repeat instability in the myotonic dystrophy type 1 locus: implications for therapeutic genome editing. Mol. Ther. 2017;25:24–43. doi: 10.1016/j.ymthe.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee K., Conboy M., Park H.M., Jiang F., Kim H.J., Dewitt M.A., Mackley V.A., Chang K., Rao A., Skinner C. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat. Biomed. Eng. 2017;1:889–901. doi: 10.1038/s41551-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y., Li H., Min Y.L., Sanchez-Ortiz E., Huang J., Mireault A.A., Shelton J.M., Kim J., Mammen P.P.A., Bassel-Duby R., Olson E.N. Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci. Adv. 2020;6:eaay6812. doi: 10.1126/sciadv.aay6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergeron N., Phan B.A.P., Ding Y., Fong A., Krauss R.M. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132:1648–1666. doi: 10.1161/CIRCULATIONAHA.115.016080. [DOI] [PubMed] [Google Scholar]
- 109.Jiang C., Mei M., Li B., Zhu X., Zu W., Tian Y., Wang Q., Guo Y., Dong Y., Tan X. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 2017;27:440–443. doi: 10.1038/cr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jarrett K.E., Lee C., De Giorgi M., Hurley A., Gillard B.K., Doerfler A.M., Li A., Pownall H.J., Bao G., Lagor W.R. Somatic editing of Ldlr with adeno-associated viral-CRISPR is an efficient tool for atherosclerosis research. Arterioscler. Thromb. Vasc. Biol. 2018;38:1997–2006. doi: 10.1161/ATVBAHA.118.311221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tessadori F., Roessler H.I., Savelberg S.M.C., Chocron S., Kamel S.M., Duran K.J., van Haelst M.M., van Haaften G., Bakkers J. Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Dis. Model. Mech. 2018;11:11. doi: 10.1242/dmm.035469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saudou F., Humbert S. The biology of huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Shin J.W., Kim K.-H., Chao M.J., Atwal R.S., Gillis T., MacDonald M.E., Gusella J.F., Lee J.M. Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum. Mol. Genet. 2016;25:4566–4576. doi: 10.1093/hmg/ddw286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monteys A.M., Ebanks S.A., Keiser M.S., Davidson B.L. CRISPR/Cas9 editing of the mutant huntingtin allele in vitro and in vivo. Mol. Ther. 2017;25:12–23. doi: 10.1016/j.ymthe.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levy-Lahad E., Wijsman E.M., Nemens E., Anderson L., Goddard K.A., Weber J.L., Bird T.D., Schellenberg G.D. A familial Alzheimer’s disease locus on chromosome 1. Science. 1995;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 116.Poon A., Schmid B., Pires C., Nielsen T.T., Hjermind L.E., Nielsen J.E., Holst B., Hyttel P., Freude K.K. Generation of a gene-corrected isogenic control hiPSC line derived from a familial Alzheimer’s disease patient carrying a L150P mutation in presenilin 1. Stem Cell Res. (Amst.) 2016;17:466–469. doi: 10.1016/j.scr.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 117.György B., Lööv C., Zaborowski M.P., Takeda S., Kleinstiver B.P., Commins C., Kastanenka K., Mu D., Volak A., Giedraitis V. CRISPR/Cas9 mediated disruption of the Swedish APP allele as a therapeutic approach for early-onset Alzheimer’s disease. Mol. Ther. Nucleic Acids. 2018;11:429–440. doi: 10.1016/j.omtn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh K., Evens H., Nair N., Rincón M.Y., Sarcar S., Samara-Kuko E., Chuah M.K., VandenDriessche T. Efficient in vivo liver-directed gene editing using CRISPR/Cas9. Mol. Ther. 2018;26:1241–1254. doi: 10.1016/j.ymthe.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang Y., Wang L., Bell P., McMenamin D., He Z., White J., Yu H., Xu C., Morizono H., Musunuru K. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 2016;34:334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morrow G., Tanguay R.M. Biochemical and clinical aspects of hereditary tyrosinemia type 1. Adv. Exp. Med. Biol. 2017;959:9–21. doi: 10.1007/978-3-319-55780-9_2. [DOI] [PubMed] [Google Scholar]
- 121.Nasrallah F., Hammami M.B., Ben Rhouma H., Fradj S.H., Azzouz H., Omar S., Feki M., Ben Youssef I.T., Messaoud T., Tebib N., Kaabachi N. Clinical and biochemical profile of tyrosinemia type 1 in Tunisia. Clin. Lab. 2015;61:487–492. doi: 10.7754/clin.lab.2014.141009. [DOI] [PubMed] [Google Scholar]
- 122.Yin H., Xue W., Chen S., Bogorad R.L., Benedetti E., Grompe M., Koteliansky V., Sharp P.A., Jacks T., Anderson D.G. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.VanLith C., Guthman R., Nicolas C.T., Allen K., Du Z., Joo D.J., Nyberg S.L., Lillegard J.B., Hickey R.D. Curative ex vivo hepatocyte-directed gene editing in a mouse model of hereditary tyrosinemia type 1. Hum. Gene Ther. 2018;29:1315–1326. doi: 10.1089/hum.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020;5:1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ledford H. First test of in-body gene editing shows promise. Nature. 2018 doi: 10.1038/d41586-018-06195-6. [DOI] [Google Scholar]
- 126.Kaiser J. New gene-editing treatment might help treat a rare disorder, hints first human test. Science Magazine, September 5, 2018. 2018 doi: 10.1126/science.aav3226. [DOI] [Google Scholar]
- 127.Skov M., Hansen C.R., Pressler T. Cystic fibrosis—an example of personalized and precision medicine. APMIS. 2019;127:352–360. doi: 10.1111/apm.12915. [DOI] [PubMed] [Google Scholar]
- 128.Marangi M., Pistritto G. Innovative therapeutic strategies for cystic fibrosis: moving forward to CRISPR technique. Front. Pharmacol. 2018;9:396. doi: 10.3389/fphar.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crane A.M., Kramer P., Bui J.H., Chung W.J., Li X.S., Gonzalez-Garay M.L., Hawkins F., Liao W., Mora D., Choi S. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports. 2015;4:569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan Z., Perisse I.V., Cotton C.U., Regouski M., Meng Q., Domb C., Van Wettere A.J., Wang Z., Harris A., White K.L., Polejaeva I.A. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight. 2018;3 doi: 10.1172/jci.insight.123529. e123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moldovan G.-L., D’Andrea A.D. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skvarova Kramarzova K., Osborn M.J., Webber B.R., DeFeo A.P., McElroy A.N., Kim C.J., Tolar J. CRISPR/Cas9-mediated correction of the FANCD1 gene in primary patient cells. Int. J. Mol. Sci. 2017;18:1269. doi: 10.3390/ijms18061269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osborn M., Lonetree C.L., Webber B.R., Patel D., Dunmire S., McElroy A.N., DeFeo A.P., MacMillan M.L., Wagner J., Balzar B.R., Tolar J. CRISPR/Cas9 targeted gene editing and cellular engineering in Fanconi anemia. Stem Cells Dev. 2016;25:1591–1603. doi: 10.1089/scd.2016.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Richardson C.D., Kazane K.R., Feng S.J., Zelin E., Bray N.L., Schäfer A.J., Floor S.N., Corn J.E. CRISPR-Cas9 genome editing in human cells occurs via the Fanconi anemia pathway. Nat. Genet. 2018;50:1132–1139. doi: 10.1038/s41588-018-0174-0. [DOI] [PubMed] [Google Scholar]
- 135.Williams T.N., Thein S.L. Sickle cell anemia and its phenotypes. Annu. Rev. Genomics Hum. Genet. 2018;19:113–147. doi: 10.1146/annurev-genom-083117-021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Demirci S., Leonard A., Haro-Mora J.J., Uchida N., Tisdale J.F. CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges. Adv. Exp. Med. Biol. 2019;1144:37–52. doi: 10.1007/5584_2018_331. [DOI] [PubMed] [Google Scholar]
- 137.Tasan I., Jain S., Zhao H. Use of genome-editing tools to treat sickle cell disease. Hum. Genet. 2016;135:1011–1028. doi: 10.1007/s00439-016-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sato M., Saitoh I., Inada E. Efficient CRISPR/Cas9-based gene correction in induced pluripotent stem cells established from fibroblasts of patients with sickle cell disease. Stem Cell Investig. 2016;3:78. doi: 10.21037/sci.2016.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoban M.D., Lumaquin D., Kuo C.Y., Romero Z., Long J., Ho M., Young C.S., Mojadidi M., Fitz-Gibbon S., Cooper A.R. CRISPR/Cas9-mediated correction of the sickle mutation in human CD34+ cells. Mol. Ther. 2016;24:1561–1569. doi: 10.1038/mt.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ye L., Wang J., Tan Y., Beyer A.I., Xie F., Muench M.O., Kan Y.W. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl. Acad. Sci. USA. 2016;113:10661–10665. doi: 10.1073/pnas.1612075113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao A., Galanello R. Beta-thalassemia. Genet. Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 142.Alateeq S., Ovchinnikov D., Tracey T., Whitworth D., Al-Rubaish A., Al-Ali A., Wolvetang E. Identification of on-target mutagenesis during correction of a beta-thalassemia splice mutation in iPS cells with optimised CRISPR/Cas9-double nickase reveals potential safety concerns. APL Bioeng. 2018;2:046103. doi: 10.1063/1.5048625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vezinaw C.M., Fishman G.A., McAnany J.J. Visual impairment in retinitis pigmentosa. Retina. 2020;40:1630–1633. doi: 10.1097/IAE.0000000000002649. [DOI] [PubMed] [Google Scholar]
- 144.Shintani K., Shechtman D.L., Gurwood A.S. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80:384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 145.Wang D.Y., Chan W.M., Tam P.O., Baum L., Lam D.S., Chong K.K., Fan B.J., Pang C.P. Gene mutations in retinitis pigmentosa and their clinical implications. Clin. Chim. Acta. 2005;351:5–16. doi: 10.1016/j.cccn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Suzuki K., Izpisua Belmonte J.C. In vivo genome editing via the HITI method as a tool for gene therapy. J. Hum. Genet. 2018;63:157–164. doi: 10.1038/s10038-017-0352-4. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki K., Tsunekawa Y., Hernandez-Benitez R., Wu J., Zhu J., Kim E.J., Hatanaka F., Yamamoto M., Araoka T., Li Z. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shiels A., Hejtmancik J.F. Mutations and mechanisms in congenital and age-related cataracts. Exp. Eye Res. 2017;156:95–102. doi: 10.1016/j.exer.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shiels A., Hejtmancik J.F. Genetic origins of cataract. Arch. Ophthalmol. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- 150.Hadrami M., Bonnet C., Veten F., Zeitz C., Condroyer C., Wang P., Biya M., Sidi Ahmed M.A., Zhang Q., Cheikh S. A novel missense mutation of GJA8 causes congenital cataract in a large Mauritanian family. Eur. J. Ophthalmol. 2019;29:621–628. doi: 10.1177/1120672118804757. [DOI] [PubMed] [Google Scholar]
- 151.Yuan L., Sui T., Chen M., Deng J., Huang Y., Zeng J., Lv Q., Song Y., Li Z., Lai L. CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Sci. Rep. 2016;6:22024. doi: 10.1038/srep22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shah S., Alexaki A., Pirrone V., Dahiya S., Nonnemacher M.R., Wigdahl B. Functional properties of the HIV-1 long terminal repeat containing single-nucleotide polymorphisms in Sp site III and CCAAT/enhancer binding protein site I. Virol. J. 2014;11:92. doi: 10.1186/1743-422X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaminski R., Chen Y., Salkind J., Bella R., Young W.B., Ferrante P., Karn J., Malcolm T., Hu W., Khalili K. Negative feedback regulation of HIV-1 by gene editing strategy. Sci. Rep. 2016;6:31527. doi: 10.1038/srep31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yin L., Hu S., Mei S., Sun H., Xu F., Li J., Zhu W., Liu X., Zhao F., Zhang D. CRISPR/Cas9 inhibits multiple steps of HIV-1 infection. Hum. Gene Ther. 2018;29:1264–1276. doi: 10.1089/hum.2018.018. [DOI] [PubMed] [Google Scholar]
- 155.Hultquist J.F., Hiatt J., Schumann K., McGregor M.J., Roth T.L., Haas P., Doudna J.A., Marson A., Krogan N.J. CRISPR-Cas9 genome engineering of primary CD4+ T cells for the interrogation of HIV-host factor interactions. Nat. Protoc. 2019;14:1–27. doi: 10.1038/s41596-018-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hartweger H., McGuire A.T., Horning M., Taylor J.J., Dosenovic P., Yost D., Gazumyan A., Seaman M.S., Stamatatos L., Jankovic M., Nussenzweig M.C. HIV-specific humoral immune responses by CRISPR/Cas9-edited B cells. J. Exp. Med. 2019;216:1301–1310. doi: 10.1084/jem.20190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bogerd H.P., Kornepati A.V., Marshall J.B., Kennedy E.M., Cullen B.R. Specific induction of endogenous viral restriction factors using CRISPR/Cas-derived transcriptional activators. Proc. Natl. Acad. Sci. USA. 2015;112:E7249–E7256. doi: 10.1073/pnas.1516305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dufour C., Claudel A., Joubarne N., Merindol N., Maisonnet T., Masroori N., Plourde M.B., Berthoux L. Editing of the human TRIM5 gene to introduce mutations with the potential to inhibit HIV-1. PLoS ONE. 2018;13:e0191709. doi: 10.1371/journal.pone.0191709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu A., Liao X., Li S., Zhao H., Chen L., Xu M., Duan X. HBV cccDNA and its potential as a therapeutic target. J. Clin. Transl. Hepatol. 2019;7:258–262. doi: 10.14218/JCTH.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bloom K., Maepa M.B., Ely A., Arbuthnot P. Gene therapy for chronic HBV—can we eliminate cccDNA? Genes (Basel) 2018;9:207. doi: 10.3390/genes9040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee C. CRISPR/Cas9-based antiviral strategy: current status and the potential challenge. Molecules. 2019;24:1349. doi: 10.3390/molecules24071349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karimova M., Beschorner N., Dammermann W., Chemnitz J., Indenbirken D., Bockmann J.H., Grundhoff A., Lüth S., Buchholz F., Schulze zur Wiesch J., Hauber J. CRISPR/Cas9 nickase-mediated disruption of hepatitis B virus open reading frame S and X. Sci. Rep. 2015;5:13734. doi: 10.1038/srep13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramanan V., Shlomai A., Cox D.B., Schwartz R.E., Michailidis E., Bhatta A., Scott D.A., Zhang F., Rice C.M., Bhatia S.N. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci. Rep. 2015;5:10833. doi: 10.1038/srep10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang J., Chen R., Zhang R., Ding S., Zhang T., Yuan Q., Guan G., Chen X., Zhang T., Zhuang H. The gRNA-miRNA-gRNA ternary cassette combining CRISPR/Cas9 with RNAi approach strongly inhibits hepatitis B virus replication. Theranostics. 2017;7:3090–3105. doi: 10.7150/thno.18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li H., Huang M.H., Jiang J.D., Peng Z.G. Hepatitis C: from inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J. Gastroenterol. 2018;24:5297–5311. doi: 10.3748/wjg.v24.i47.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sampson T.R., Saroj S.D., Llewellyn A.C., Tzeng Y.L., Weiss D.S. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497:254–257. doi: 10.1038/nature12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Price A.A., Sampson T.R., Ratner H.K., Grakoui A., Weiss D.S. Cas9-mediated targeting of viral RNA in eukaryotic cells. Proc. Natl. Acad. Sci. USA. 2015;112:6164–6169. doi: 10.1073/pnas.1422340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McMurray H.R., Nguyen D., Westbrook T.F., McAnce D.J. Biology of human papillomaviruses. Int. J. Exp. Pathol. 2001;82:15–33. doi: 10.1046/j.1365-2613.2001.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sanclemente G., Gill D.K. Human papillomavirus molecular biology and pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2002;16:231–240. doi: 10.1046/j.1473-2165.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 170.Kennedy E.M., Kornepati A.V., Goldstein M., Bogerd H.P., Poling B.C., Whisnant A.W., Kastan M.B., Cullen B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014;88:11965–11972. doi: 10.1128/JVI.01879-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y.C., Cai Z.M., Zhang X.J. Reprogrammed CRISPR-Cas9 targeting the conserved regions of HPV6/11 E7 genes inhibits proliferation and induces apoptosis in E7-transformed keratinocytes. Asian J. Androl. 2016;18:475–479. doi: 10.4103/1008-682X.157399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hurt C., Tammaro D. Diagnostic evaluation of mononucleosis-like illnesses. Am. J. Med. 2007;120 doi: 10.1016/j.amjmed.2006.12.011. 911.e1–8. [DOI] [PubMed] [Google Scholar]
- 173.Yuen K.S., Chan C.P., Wong N.M., Ho C.H., Ho T.H., Lei T., Deng W., Tsao S.W., Chen H., Kok K.H., Jin D.Y. CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells. J. Gen. Virol. 2015;96:626–636. doi: 10.1099/jgv.0.000012. [DOI] [PubMed] [Google Scholar]
- 174.Ma Y., Walsh M.J., Bernhardt K., Ashbaugh C.W., Trudeau S.J., Ashbaugh I.Y. CRISPR/Cas9 screens reveal Epstein-Barr virus-transformed B cell host dependency factors. Cell Host Microbe. 2017;10:580–591.e7. doi: 10.1016/j.chom.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Justice M.J., Dhillon P. Using the mouse to model human disease: increasing validity and reproducibility. Dis. Model. Mech. 2016;9:101–103. doi: 10.1242/dmm.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kafkafi N., Golani I., Jaljuli I., Morgan H., Sarig T., Würbel H., Yaacoby S., Benjamini Y. Addressing reproducibility in single-laboratory phenotyping experiments. Nat. Methods. 2017;14:462–464. doi: 10.1038/nmeth.4259. [DOI] [PubMed] [Google Scholar]
- 177.Simpson E.M., Linder C.C., Sargent E.E., Davisson M.T., Mobraaten L.E., Sharp J.J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 178.Bilovocky N.A., Romito-DiGiacomo R.R., Murcia C.L., Maricich S.M., Herrup K. Factors in the genetic background suppress the engrailed-1 cerebellar phenotype. J. Neurosci. 2003;23:5105–5112. doi: 10.1523/JNEUROSCI.23-12-05105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature. 2016;539:479. doi: 10.1038/nature.2016.20988. [DOI] [PubMed] [Google Scholar]
- 180.Lu Y., Xue J., Deng T., Zhou X., Yu K., Deng L., Huang M., Yi X., Liang M., Wang Y. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020;26:732–740. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 181.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., Mangan P.A., Kulikovskaya I., Gupta M., Chen F. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367 doi: 10.1126/science.aba7365. eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fousek K., Watanabe J., Joseph S.K., George A., An X., Byrd T.T., Morris J.S., Luong A., Martínez-Paniagua M.A., Sanber K. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia. 2020 doi: 10.1038/s41375-020-0792-2. Published online March 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.The Lancet Haematology CRISPR-Cas9 gene editing for patients with haemoglobinopathies. Lancet Haematol. 2019;6:e438. doi: 10.1016/S2352-3026(19)30169-3. [DOI] [PubMed] [Google Scholar]
- 184.Xu L., Wang J., Liu Y., Xie L., Su B., Mou D., Wang L., Liu T., Wang X., Zhang B. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N. Engl. J. Med. 2019;381:1240–1247. doi: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
- 185.Ledford H. CRISPR treatment inserted directly into the body for first time. Nature. 2020;579:185. doi: 10.1038/d41586-020-00655-8. [DOI] [PubMed] [Google Scholar]
- 186.Teboul L., Herault Y., Wells S., Qasim W., Pavlovic G. Variability in genome editing outcomes: challenges for research reproducibility and clinical safety. Mol. Ther. 2020;28:1422–1431. doi: 10.1016/j.ymthe.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang X., Wang Y., Wu X., Wang J., Wang Y., Qiu Z., Chang T., Huang H., Lin R.J., Yee J.K. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat. Biotechnol. 2015;33:175–178. doi: 10.1038/nbt.3127. [DOI] [PubMed] [Google Scholar]
- 189.Iyer V., Boroviak K., Thomas M., Doe B., Riva L., Ryder E., Adams D.J. No unexpected CRISPR-Cas9 off-target activity revealed by trio sequencing of gene-edited mice. PLoS Genet. 2018;14:e1007503. doi: 10.1371/journal.pgen.1007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V.V., Thapar V., Wyvekens N., Khayter C., Iafrate A.J., Le L.P. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015;33:187–197. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y., Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., Kim J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsai S.Q., Wyvekens N., Khayter C., Foden J.A., Thapar V., Reyon D., Goodwin M.J., Aryee M.J., Joung J.K. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 2014;32:569–576. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han H.A., Pang J.K.S., Soh B.S. Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing. J. Mol. Med. (Berl.) 2020;98:615–632. doi: 10.1007/s00109-020-01893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kraft K., Geuer S., Will A.J., Chan W.L., Paliou C., Borschiwer M., Harabula I., Wittler L., Franke M., Ibrahim D.M. Deletions, inversions, duplications: engineering of structural variants using CRISPR/Cas in mice. Cell Rep. 2015;10:833–839. doi: 10.1016/j.celrep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 196.Simeonov D.R., Brandt A.J., Chan A.Y., Cortez J.T., Li Z., Woo J.M., Lee Y., Carvalho C.M.B., Indart A.C., Roth T.L. A large CRISPR-induced bystander mutation causes immune dysregulation. Commun. Biol. 2019;2:70. doi: 10.1038/s42003-019-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maruyama T., Dougan S.K., Truttmann M.C., Bilate A.M., Ingram J.R., Ploegh H.L. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vartak S.V., Raghavan S.C. Inhibition of nonhomologous end joining to increase the specificity of CRISPR/Cas9 genome editing. FEBS J. 2015;282:4289–4294. doi: 10.1111/febs.13416. [DOI] [PubMed] [Google Scholar]
- 199.Lin S.R., Yang H.C., Kuo Y.T., Liu C.J., Yang T.Y., Sung K.C., Lin Y.Y., Wang H.Y., Wang C.C., Shen Y.C. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol. Ther. Nucleic Acids. 2014;3:e186. doi: 10.1038/mtna.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chu V.T., Weber T., Wefers B., Wurst W., Sander S., Rajewsky K., Kühn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- 201.Seelos H.J. A new paradigm of medical informatics. Methods Inf. Med. 1992;31:79–81. [PubMed] [Google Scholar]
- 202.Xu C.L., Ruan M.Z.C., Mahajan V.B., Tsang S.H. Viral delivery systems for CRISPR. Viruses. 2019;11:28. doi: 10.3390/v11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yi L., Li J. CRISPR-Cas9 therapeutics in cancer: promising strategies and present challenges. Biochim. Biophys. Acta. 2016;1866:197–207. doi: 10.1016/j.bbcan.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 204.Lau C.H., Suh Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res. 2017;6:2153. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim S., Koo T., Jee H.G., Cho H.Y., Lee G., Lim D.G., Shin H.S., Kim J.S. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018;28:367–373. doi: 10.1101/gr.231936.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu X., Liang X., Xie H., Kumar S., Ravinder N., Potter J., de Mollerat du Jeu X., Chesnut J.D. Improved delivery of Cas9 protein/gRNA complexes using lipofectamine CRISPRMAX. Biotechnol. Lett. 2016;38:919–929. doi: 10.1007/s10529-016-2064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Berardo C., Siciliano V., Di Pasqua L.G., Richelmi P., Vairetti M., Ferrigno A. Comparison between Lipofectamine RNAiMAX and GenMute transfection agents in two cellular models of human hepatoma. Eur. J. Histochem. 2019;63:3048. doi: 10.4081/ejh.2019.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang M., Zuris J.A., Meng F., Rees H., Sun S., Deng P., Han Y., Gao X., Pouli D., Wu Q. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl. Acad. Sci. USA. 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jin W., Lin D., Nguyen A.H., Abdelrasoul G.N., Chen J., Mar A., Qian F., Fang Q., Kovalchuk I., Wang Y., Chen J. Transfection of difficult-to-transfect rat primary cortical neurons with magnetic nanoparticles. J. Biomed. Nanotechnol. 2018;14:1654–1664. doi: 10.1166/jbn.2018.2604. [DOI] [PubMed] [Google Scholar]
- 210.Suresh B., Ramakrishna S., Kim H. Cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA for genome editing. Methods Mol. Biol. 2017;1507:81–94. doi: 10.1007/978-1-4939-6518-2_7. [DOI] [PubMed] [Google Scholar]
- 211.Sun W., Ji W., Hall J.M., Hu Q., Wang C., Beisel C.L., Gu Z. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew. Chem. Int. Ed. Engl. 2015;54:12029–12033. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang P., Zhang L., Zheng W., Cong L., Guo Z., Xie Y., Wang L., Tang R., Feng Q., Hamada Y. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew. Chem. Int. Ed. Engl. 2018;57:1491–1496. doi: 10.1002/anie.201708689. [DOI] [PubMed] [Google Scholar]
- 213.Kogure K., Moriguchi R., Sasaki K., Ueno M., Futaki S., Harashima H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J. Control. Release. 2004;98:317–323. doi: 10.1016/j.jconrel.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 214.Nakamura T., Akita H., Yamada Y., Hatakeyama H., Harashima H. A multifunctional envelope-type nanodevice for use in nanomedicine: concept and applications. Acc. Chem. Res. 2012;45:1113–1121. doi: 10.1021/ar200254s. [DOI] [PubMed] [Google Scholar]
- 215.Rose B.I., Brown S. Genetically modified babies and a first application of clustered regularly interspaced short palindromic repeats (CRISPR-Cas9) Obstet. Gynecol. 2019;134:157–162. doi: 10.1097/AOG.0000000000003327. [DOI] [PubMed] [Google Scholar]