Abstract
Considering the great effect of vaccination and the unpredictability of environmental variations in nature, a stochastic Susceptible-Vaccinated-Infected-Susceptible (SVIS) epidemic model with standard incidence and vaccination strategies is the focus of the present study. By constructing a series of appropriate Lyapunov functions, the sufficient criterion is obtained for the existence and uniqueness of the ergodic stationary distribution of the model. In epidemiology, the existence of a stationary distribution indicates that the disease will be persistent in a long term. By taking the stochasticity into account, a quasi-endemic equilibrium related to the endemic equilibrium of the deterministic system is defined. By means of the method developed in solving the general three-dimensional Fokker-Planck equation, the exact expression of the probability density function of the stochastic model around the quasi-endemic equilibrium is derived, which is the key aim of the present paper. In statistical significance, the explicit density function can reflect all dynamical properties of an epidemic system. Next, a simple result of disease extinction is obtained. In addition, several numerical simulations and parameter analyses are performed to illustrate the theoretical results. Finally, the corresponding results and conclusions are discussed at the end of the paper.
Keywords: Stochastic SVIS epidemic model, Vaccination, Ergodic stationary distribution, Fokker-Planck equation, Probability density function, Extinction
1. Introduction
1.1. Research background
It is well established that many infectious diseases have a critical influence on global social economies and human health. More precisely, the detailed statistics reported by the World Health Organization (WHO) show that approximately one-third of all deaths worldwide are caused by various epidemics. Recently, the global outbreak of COVID-19 with high transmission has also increased awareness of the importance of preventing and controlling infectious diseases. In epidemiology, mathematical models have provided several effective approaches to describe the characteristics and spread of epidemics in the last hundred years. In 1927, by dividing the population into two clusters, which includes people susceptible to the disease and infected individuals, Kermack and McKendrick [1] initially proposed the classical susceptible-infected-susceptible (SIS) epidemic model and established the corresponding threshold theory. Since then, various realistic ordinary differential equations (ODEs) have been extended to analyze and control the transmission of diseases [2], [3], [4], [5], [6], [7], [8]. For instance, Hove-Musekwa and Nyabadza [4] developed a HIV/AIDS model with active screening of disease carriers and obtained the corresponding basic reproduction number. Considering the effect of vertical infection, Tuncer and Martcheva [6] formulated a hepatitis B model with acute infection and carriers.
With the accelerated development of science and technology, vaccination comprises a common precaution that reduces the infection rate and even immunizes against some contagious diseases, such as measles, cholera, and tuberculosis [9]. According to a 2005 WHO report, the eradication of smallpox has been considered the most spectacular success of routine vaccination. Thus, some basic epidemic models with vaccination strategies have been studied in the last several decades [9], [10], [11], [12], [13]. In [9], Liu et al. obtained the global stability of equilibria and analyzed the effect of pulse vaccination. Gao et al. [11] proposed mixed vaccination strategies in the SIRS epidemic model with seasonal variability on infection. However, the vast majority of infection processes are caused by person-to-person contact. As Ma and Wang [2] described, the classical bilinear incidence rate is reasonably assumed by the simple mass-action law. From Anderson and May [14], Hethcote [15], this law is a good approximation for some communicable diseases, such as dengue fever and avian influenza. However, studies (see, e.g., [16], [17]) have shown that the underlying assumption of homogeneous mixing and homogeneous environmental for several sexually transmitted diseases, e.g., HIV/AIDS and syphilis, may be invalid. In addition, owing to the psychological effect, susceptible individuals may tend to reduce the number of contacts with the infected per unit time as the numbers of the infected individuals increase [18], [19]. As a result, the corresponding adequate incidence rate should be modified as a nonlinear form. More importantly, Anderson and May [20], [21] pointed out that various epidemic models with standard incidence are suitable for human beings and some gregarious animals.
Given the above, a SVIS epidemic system with standard incidence and vaccination is the focus of the present study.
1.2. Deterministic SVIS model and dynamical properties
The total population is divided into three compartments, namely susceptible people infected individuals and vaccinees that are in the vaccination process at time . Then, the corresponding deterministic SVIS epidemic model with standard incidence and vaccination strategies takes the form
| (1.1) |
where denotes the recruitment rate of the susceptible, is the effective contact rate, depicts the natural death rate of the population, denotes the additional death rate due to the disease, is the vaccination rate of the susceptible, denotes the immunity loss coefficient of the vaccinated, and reflects the recovery rate of the infected. In epidemiology, these biological parameters are assumed to be positive.
Following similar results described by Ma and Zhou [22], the corresponding basic reproduction number of system (1.1) takes the form
| (1.2) |
By defining a positive invariant set two possible equilibria and their dynamical properties are then given as follows.
Assuming that the disease-free, are then globally asymptotically stable in .
If there is a unique endemic equilibrium where . Moreover, is globally asymptotically stable in but is unstable.
1.3. Stochastic SVIS epidemic model
In fact, Truscott and Gilligan [23] pointed out that the spread of infection, travel of populations, and design of control strategies are critically perturbed by some environmental variations. Therefore, it is more reasonable to construct a corresponding stochastic model to reveal the epidemiological characteristics of infectious diseases by comparison with the deterministic model. Notably, there are various possible approaches to simulate the random effects from biological significance and mathematical perspective [24]. For instance, making use of the fatal properties and multiplex networks, Zhu et al. [25], Jia et al. [26] studied the SIR epidemic spreading process and analyzed individual decision-making behavior. In 2002, the most classical assumption that random changes always fluctuate around some average values due to continuous disturbances in nature, adopted by Mao et al. [27], became a common way of describing environmental variations. Moreover, the above random fluctuations are all assumed to be types of white noise. Therefore, many authors have formulated the relevant stochastic differential equations (SDEs) with linear noises for the transmission of various epidemics [28], [29], [30], [31], [32], [33], [34], [35], [36]. As an example, Qi and Jiang [29] studied the impact of virus carrier screening and actively seeking treatment on the dynamical behavior of a stochastic HIV/AIDS epidemic model with bilinear incidence. In [34], Shi and Zhang focused on the corresponding stochastic avian influenza system and investigated the existence of the unique ergodic stationary distribution. In addition, several dynamical analyses of the stochastic SIS models or epidemic systems with vaccination have been conducted [37], [38], [39], [40]. In [37], Zhao and Jiang creatively proposed a general theory about extinction and persistence in mean based on a stochastic SIS epidemic model with vaccination. Zhang and Jiang [39] obtained sufficient conditions for a stochastic SIS system with saturated incidence and double epidemic diseases. By taking the periodicity effect into account, they still investigated a stochastic SVIR epidemic model with vaccination strategies, and derived the criteria for the existence of non-trivial positive periodic solution [40]. Given the above, in the present study it is assumed that the environmental noises are separately proportional to the compartments and . Then, the corresponding system (1.1) with the stochastic perturbations is described by
| (1.3) |
where and are three independent standard Brownian motions (or Wiener processes), with denoting their intensities.
From the perspective of biomathematics, the existence and ergodicity of stationary distribution indicates that an infectious disease will prevail and persist in long-term development. More importantly, the corresponding probability density function of the stationary distribution can reflect all statistical properties of the individuals and . It can be regarded as a great intersection of epidemiological dynamics and statistics. It should be pointed out that there are relatively few studies devoted to the explicit expression of probability density function due to the difficulty of solving the corresponding Fokker-Planck equation. To the best of our ability, several general methods of solving the corresponding algebraic equations are developed herein that are equivalent to the Fokker-Planck equation, and the exact expression of density function is derived.
The rest of this paper is organized follows. In Section 2, several mathematical notations and important lemmas for the dynamical analyses of system (1.3) are presented. The sufficient conditions for the existence and uniqueness of the ergodic stationary distribution of system (1.3) are obtained in Section 3. By means of the developed approaches in solving the general three-dimensional Fokker-Planck equation, the exact expression of the probability density function for the stationary distribution is derived in Section 4. In Section 5, several simple criteria for the disease extinction of system (1.3) are given. In Section 6, several numerical simulations are performed, together with parameter analyses to validate the theoretical results. Finally, the corresponding result discussions and main conclusions are shown, compared with existing articles, at the end of the paper.
2. Preliminaries and necessary lemmas
Throughout the paper, let be a complete probability space with a filtration with a filtration satisfying the usual conditions (i.e., it is increasing and right continuous, while contains all -null sets. Assuming that is a real matrix, let be the transpose matrix of . If depicts the inverse matrix of . The reader is referred to Mao [41] for detailed descriptions. For convenience, let be the -dimensional Euclidean space, and
Clearly, the values and that satisfy system (1.3) are required to be positive for the corresponding dynamical behavior. To this end, the existence of uniqueness of the global positive solution of system (1.3) is described by the following Lemma 2.1.
Lemma 2.1
For any initial valuethere is then a unique solutionof the system(1.3)onand the solution will remain inwith probability 1 (a.s.).
The detailed proof is almost the same as those in Zhou et al. [28], and thus it is omitted here. Next, let be a homogeneous Markov process defined on that satisfies the following SDE,
where the diffusion matrix and . Then, the corresponding ergodicity theory and the existence of stationary distribution are described by the following Lemma 2.2.
Lemma 2.2
(Has’miniskii[42]) The Markov processhas a unique ergodic stationary distributionif there exists a bounded domainwith a regular boundaryand the following are true.
. In the domainand some neighborhood thereof, the smallest eigenvalue of the diffusion matrixis bounded away from zero.
. There is a non-negative-functionsuch thatis negative for any.
Then, for anyand integral functionwith respect to the measureit follows that
By Zhou et al. [28], two important lemmas of solving the special algebraic equations are given as follows.
Lemma 2.3
([28]) Letbe a symmetric matrix, for the three-dimensional algebraic equationwhere
(2.1) Ifandthenis positive definite, which follows
Lemma 2.4
([28]) Consider the three-dimensional algebraic equationwhereis a symmetric matrix,
(2.2) Assuming thatandthenis positive semi-definite, which takes the form
Finally, combining Lemmas 2.3–2.4 and the Routh-Hurwitz criterion [43], two general theories are developed in solving the similar algebraic equations, i.e., Lemmas 2.5 and 2.6.
Lemma 2.5
For the three-dimensional algebraic equationwhereis a symmetric matrix,
(2.3) Ifthenis a positive semi-definite matrix of the form
Lemma 2.6
For any real matriceswhere. Assume thatis a symmetric matrix, for the three-dimensional algebraic equation
(2.4) By defining the characteristic polynomials ofasifhas all negative real part eigenvalues – that is,– thenis positive definite.
Remark 1
From Zhou et al. [28], and are called standard and matrices, respectively. Similarly, it is assumed that is a standard matrix. In addition, subsection (I) of Appendix A gives the detailed proof of Lemma 2.5. The corresponding proof of Lemma 2.6 and the special form of are shown in subsection (II) of Appendix A.
3. Stationary distribution and ergodicity of system (1.3)
In this section, the focus is on the sufficient conditions for the existence and ergodicity of stationary distribution for system (1.3). Moreover, one must guarantee that the results have no difference from those in the deterministic system (1.1). Define
| (3.1) |
Theorem 3.1
Assuming that then the solution of system (1.3) with any initial value is ergodic and has a unique stationary distribution .
Proof
By Lemma 2.1, for any initial value system (1.3) has a unique global positive solution . Then, the proof of Theorem 3.1 is divided into the following two steps: (i) construct a pair of a -Lyapunov function and bounded domain such that for any and (ii) validate the condition of Lemma 2.2. Step 1. Consider a suitable -function in the form
where are all positive constant and are determined in (3.4), and which indicates
(3.2) Define, for simplicity,
Applying It’s formula to which is shown in subsection (III) of Appendix B, obtains
(3.3) Choosing and such that
(3.4) which means and one can obtain
(3.5) Applying It’s formula to similarly obtains
(3.6)
(3.7) Additionally, note that is a continuous function satisfying
Hence, has a minimum value . Defining a non-negative -function by
and combining (3.2) and (3.5)–(3.7), it can be shown that
(3.8) Next, the corresponding compact subset is contructed by
where is a sufficiently small constant such that the following inequalities hold:
(3.9)
(3.10) For convenience, consider the following four subsets of :
Now, it must be shown that
Case 1. If by (3.8)–(3.9), one can derive
Case 2. For any it follows from (3.8)–(3.9) that
Case 3. Assuming that by (3.8)–(3.9) it can be seen that
Case 4. If from (3.8) and (3.10), one has
Notably, . Hence, one equivalently obtains
Therefore, the condition of Lemma 2.2 holds. The corresponding diffusion matrix is given by
Clearly, is a positive-definite matrix. Then, the assumption of Lemma 2.2 also holds.
Given the above, the global positive solution of system (1.3) follows a unique ergodic stationary distribution . This completes the proof of Theorem 3.1. □
Remark 2
Under the existence of the ergodic stationary distribution for system (1.3) is derived. This reveals that the contagious disease will prevail and persist in a population. Furthermore, from the expressions of and it can be obtained that and the sign holds if and only if . Consequently, not only does this reveal that random fluctuations have a critical effect on the spread of epidemic, but it also indicates that is a unified threshold of the disease persistence of systems (1.1) and (1.3).
4. Probability density function analysis
By Theorem 3.1, one obtains that the global solution of system (1.3) follows a unique ergodic stationary distribution . This section is devoted to deriving the explicit expression of the density function of the distribution while . In fact, the result will present a wide range of possibilities for the further development of epidemiological dynamics. Before this, two necessary transformations of system (1.3) should be first introduced.
4.1. Two important transformations of system (1.3)
(I) (Logarithmic transformation): Let Employing It’s formula, it follows from system (1.3) that
| (4.1) |
By taking the random effect into consideration, another critical value is defined: . Moreover, if then the quasi-stable equilibrium is determined by the following Eq. (4.2):
| (4.2) |
For convenience, let for any . As a result, it can be derived by (4.2) that where .
Notably, it is easily obtained that . This then indicates that if . In addition, if there is no stochastic noise in system (1.3), i.e., model (1.1), then .
(II) (Equilibrium offset transformation): Given the above, let ; thus, the corresponding linearized system of (4.1) takes the form
| (4.3) |
where
4.2. Density function of stationary distribution
Theorem 4.1
For any initial value if then the stationary distribution around follows a unique log-normal probability density function which is given by
(4.4) where is a positive definite matrix, and the special form of is given as follows.
. If and then
. If and then
. If and then
. If and then
. If and then
. If and then
. If and then
. If then
with
and
Proof
For convenience and simplicity, let and
Hence, system (4.3) can be rewritten as . By the theory of Gardiner [44], the unique density function around the quasi-endemic equilibrium satisfies the following Fokker-Plauck equation:
(4.5) Since the diffusion matrix is a constant matrix, Roozen [45] pointed out that can be described by a quasi-Gaussian distribution, i.e., where is determined by the normalized condition and is a symmetric matrix.
Substituting these results into (4.5), one can obtain that obeys the algebraic equation . If is a inverse matrix, by letting an equivalent equation is given by
(4.6) Next, it will be proved that has all negative real-part eigenvalues. The characteristic polynomial of is defined as where
By the expressions of and it can be shown that
Consequently, it follows from (i)–(iv) that
Combining the above (1)-(3) and Lemma 2.6, that of Eq. (4.6) is positive definite can be derived.
However, following the corresponding proof of Lemma 2.6, which is shown in subsection (II) of Appendix A, the exact expression of is given. First, by the finite independent superposition principle, (4.6) can be equivalently transformed into the sum of solution to the following algebraic sub-equations,
where and the symmetric matrices are their respective solutions. Clearly, . Now, the special expression of are derived by the following three steps.
Step 1. For the algebraic equation
(4.7) denote where the elimination matrix and are derived by
where . By the value of the relevant discussion is divided into two subcases:
Case (i). If in view of the method introduced in Zhou et al. [28], it is assumed that where the standardized transformation matrix is
(4.8) By direct calculation, one obtains
where and are the same as above. Furthermore, one can equivalently transform Eq. (4.7) into
Letting where we obtain
Noting that A has all negative real-part eigenvalues, then is a standard matrix. By Lemma 2.3, this means that is positive definite, which takes the form
(4.9) Therefore, .
Case (ii). If i.e., is defined, where another standardized transformation matrix and are obtained by
(4.10) where and is abbreviation. Obviously, is a standard matrix. Additionally, (4.7) can be equivalently transformed into
By letting it can be simplified as
In view of Lemma 2.4, is described by
(4.11) Hence, . Step 2. Consider the algebraic equation
(4.12) For the corresponding elimination matrix is defined, where and are obtained by
where . Similarly, the following two sub-conditions are considered:
Case (1). If let where the relevant standardized transformation matrix
(4.13) In fact, one still derives
which means that is also a standard matrix. By letting where (4.12) is then equivalent to the following equation:
By Lemma 2.3 and the result of A having all negative real-part eigenvalues again, it can be shown that
In other words, .
Case (2). If let where the corresponding standardized transformation matrix and are given by
where and is also shorthand. Similarly, is a standard matrix. By defining (4.12) is then equivalent to
According to Lemma 2.4, takes the form
(4.14) Then, .
Step 3. For the following algebraic equation,
(4.15) and for the following elimination matrix let where and are given by
Hence, (4.15) can be equivalently transformed into
(4.16) where . Similarly, the proof is divided into two subcases by the value of . Case (I). If consider the corresponding standardized transformation matrix
Letting that is still derived. Hence, an equivalent algebraic equation of (4.16) is described as follows:
Denoting where the last equation can be also simplified as
Similarly, one obtains
Consequently, .
Case (II). If then is a standard matrix. Noting that =diag by Lemma 2.5, one can obtain
Based on then is a positive semi-definite matrix, which means that .
In summary, the special form of is divided into eight cases by the different values of and which is shown in Theorem 4.1. Finally, in view of the relation of systems (4.1) and (4.3), the stationary distribution around then has a unique log-normal probability density function
Therefore, this completes the proof. □
Remark 3
If Theorem 4.1 shows that the stationary distribution around has the unique log-normal density function . This reflects the stochastic permanence of system (1.3) from one side. In addition, that if is obtained.
5. Extinction of system (1.3)
As is known, all of the properties of disease persistence of system (1.3) are reflected by Theorems 3.1 and 4.1. For a comprehensive study, a simple extinction result of system (1.3) is described by the following Theorem 5.1.
Theorem 5.1
For any initial valueifthen the solutionof system(1.3)follows:
(5.1) which means that the epidemic of system(1.3)will go to extinction with probability 1 (a.s.).
Proof
Employing It’s formula to one obtains
(5.2) Integrating from 0 to and dividing by on both sides of (5.1), it can be seen that
(5.3) Next, by the strong law of large numbers [1], one derives
(5.4) Taking the superior limit of on both sides of (5.3), the assertion (5.1) can then be obtained by (5.4). Moreover, from the expressions of and one can obtain that .
Consequently, the proof of Theorem 5.1 is confirmed. □
6. Simulations and parameter analyses
In this section, by means of the well-known higher-order method developed by Milstein [46], the corresponding discretization equation of system (1.3) is obtained in the form
| (6.1) |
where the time increment and are three independent Gaussian random variables that follow the distribution for . Furthermore, is the corresponding value of the th iteration of the discretization equation. From AI-Darabsah [13], Zhao and Jiang [37], Liu et al. [38], Zhang and Jiang [39], Arino et al. [47], and the detailed data of the Central Statistical Office of Zimbabwe (CSZ), the corresponding realistic statistics of system (1.3) are shown in Table 1 . Next, several empirical examples are provided to focus on the following five aspects.
Table 1.
List of biological parameters of system (1.3).
| Parameters | Description | Unit | Value | Source |
|---|---|---|---|---|
| Recruitment rate of population | per day | [38], [39] | ||
| Transmission rate of susceptible individuals | per day | [0.390,0.432] | [13] | |
| Natural death rate of population | per day | [47],CSZ data | ||
| Disease mortality of infected people | per day | [13] | ||
| Recovery rate | None | [0.01,0.2] | Estimated | |
| Immune loss rate of vaccinated individuals | None | 0.2 | [37] | |
| Vaccination rate of susceptible individuals | None | [0.371,0.436] | [13] |
(i) The existence of the ergodic stationary distribution of system (1.3) while .
(ii) The exact expression and verification of the unique log-normal density function for the stationary distribution under .
(iii) The influence of random fluctuations on the disease persistence of system (1.3).
(iv) The effects of the main parameters of system (1.3) on the disease dynamics.
(v) The corresponding dynamical behavior of system (1.3) if .
6.1. Dynamical behavior of system (1.3) if
Example 6.1
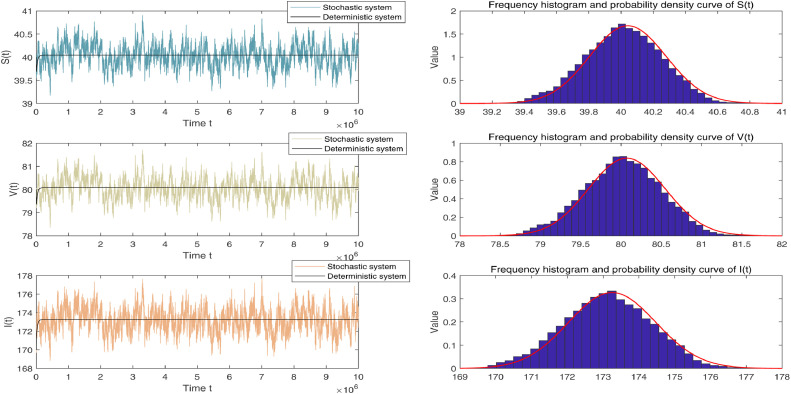
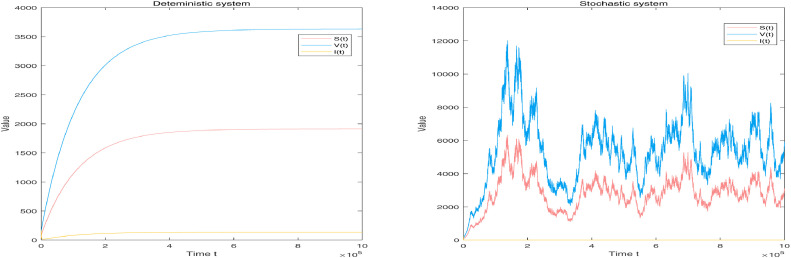
By Table 1, letting the environmental noise intensities and main parameters one then obtains
It follows from Theorem 3.1 that system (1.3) admits a unique ergodic stationary distribution . The left-hand column of Fig. 1 can be seen to validate it. By Theorem 4.1, the stationary distribution around the quasi-endemic equilibrium has a unique log-normal density function . Moreover, it is calculated that
which means
By direct calculation, one can obtain that . Then, the corresponding marginal density functions of and are separately given as follows.
The curves of (i)-(iii) are shown in the right-hand column of Fig. 1. Obviously, this greatly illustrates Theorem 4.1 from the side.
Fig. 1.
Left-hand column shows simulation of compartments and in deterministic system (1.1) and stochastic system (1.3) with noise intensities and main parameters respectively. Right-hand column shows frequency histogram and corresponding marginal density function curves of individuals and .
Combining Remarks 3.1–4.1 and Theorem 5.1, one can derive that all random perturbations and have a critical influence on the dynamical behavior of system (1.3). Therefore, the corresponding parameter analyses of the above three white noises are shown by Example 6.2.
6.2. Impact of random noises on disease extinction and the existence of stationary distribution
Example 6.2
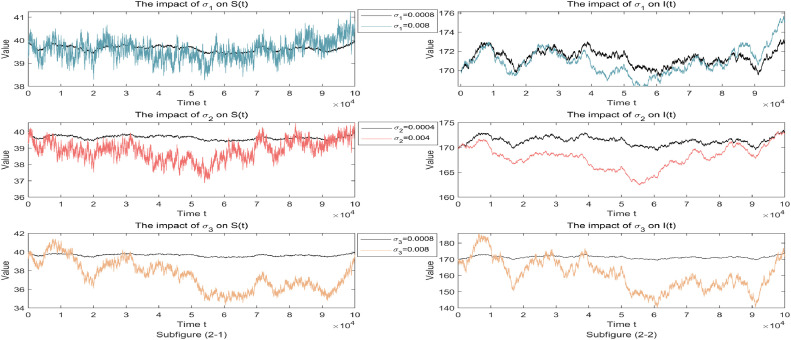
One chooses the epidemiological parameters 0.4) and considers the following four subcases of stochastic perturbations:
First, it should be pointed out that the above four subcases (i)–(iv) all guarantee the existence of a stationary distribution, which has an ergodicity property. For convenience and simplicity, only the population intensities of susceptible and infected individuals are focused on, which are presented in subfigures (2-1) and (2-2) of Fig. 2 , respectively. By only increasing the perturbation intensities of the vaccinated individuals (or infected individuals), i.e., the larger (or ), then the disease infection will be effectively inhibited. In contrast, by only increasing the perturbation intensity of the susceptible individuals, a great destabilizing influence on the population numbers of and manifests.
Fig. 2.
Corresponding simulation of partial compartments and of stochastic system (1.3) under noise intensities and respectively. Other fixed parameters: .
Next, by Zhu et al. [25], Jia et al. [26], the impact of the main parameters of system (1.3) on the individual decision-making behavior is studied. From the expressions of and the disease persistence and extinction of system (1.3) are critically affected by the transmission rate and vaccination rate . Thus, the following Examples 6.3 and 6.4 will reveal the impact. In addition, the corresponding effect of the recruitment rate on the dynamical behavior of system (1.3) is also shown in Example 6.5.
6.3. Impact of transmission rate on dynamics of system (1.3)
Example 6.3
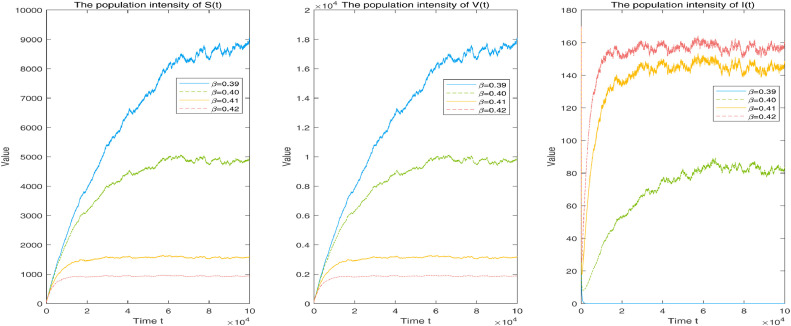
Choosing the epidemiological parameters and random noises and considering the subcases of transmission rate and 0.42, the corresponding numbers of the solution to system (1.3) are described in Fig. 3 . Clearly, a small transmission rate can lead to reduction of disease infection and even elimination, such as per day.
Fig. 3.
Corresponding population numbers of solution to system (1.3) with transmission rates of and 0.42, respectively. Other given parameters: and .
6.4. Impact of vaccination rate on dynamics of system (1.3)
Example 6.4
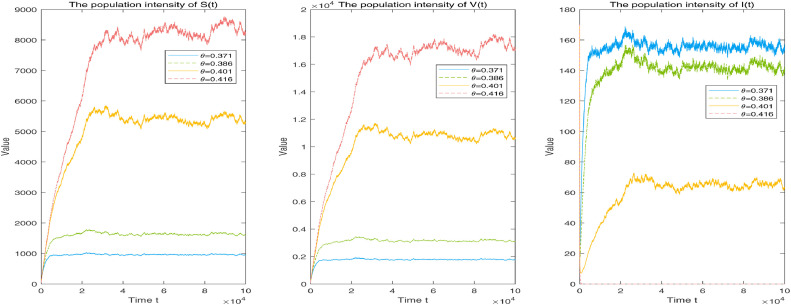
Assuming that the parameters and stochastic perturbations for the corresponding subcases of vaccination rate and 0.416, the corresponding solutions to system (1.3) are shown in Fig. 4 . Similarly, a small vaccination rate can control the disease infection more effectively than a large one.
Fig. 4.
Corresponding simulation of solution to system (1.3) with vaccination rate and 0.416, respectively. Other fixed parameters: and .
6.5. Impact of recruitment rate on dynamics of system (1.3)
Example 6.5
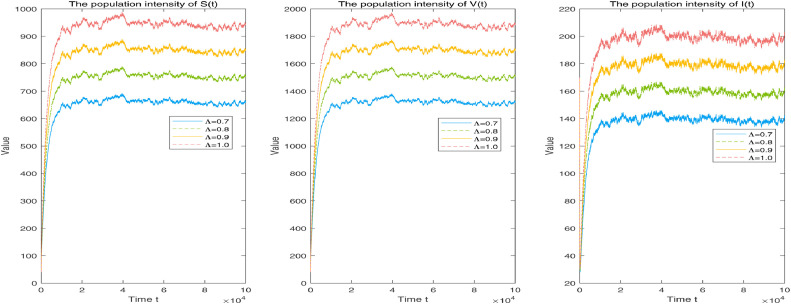
Letting the dynamical parameters be and stochastic fluctuations be and considering the sub-conditions of recruitment rate and 1.0, the corresponding intensities of the compartments and of system (1.3) are reflected in Fig. 5 . Obviously, as the parameter increases to 1 from 0.7, the spread and infection of an epidemic can be effectively controlled by the small recruitment rate.
Fig. 5.
Corresponding population intensities of individuals and of system (1.3) with recruitment rate and 1.0, respectively. Other given parameters: and .
6.6. Dynamical behaviors of system (1.3) under
Example 6.6
Considering the stochastic noises and main parameters one can then obtain
By Theorem 3.1, one cannot derive the existence of the ergodic stationary distribution of system (1.3). In contrast, it follows from Theorem 5.1 that the disease of stochastic system (1.3) will be extinct in a long term. In addition, the deterministic model (1.1) has a globally asymptotically stable endemic equilibrium . On the one hand, these results validate the fact that large white noises lead to disease elimination from the side. On the other hand, the large random fluctuation indicates that it is necessary to isolate and control the infected individuals during the outbreak of an epidemic. These results are verified by Fig. 6 .
Fig. 6.
Corresponding population numbers of solution to system (1.3) with random perturbations and main parameters .
For epidemiological study, combining the above numerical simulations and parameter analyses, several reasonable and effective measures to reduce the threat of infectious diseases to human life, and even eliminate the epidemic, are provided. The special approaches are the following.
(i) Several reasonable policies of joint prevention and control are implemented to reduce the population mobility in differential risk epidemic areas. Then, the small recruitment rate may lead to the elimination of disease (see Fig. 5).
(ii) Controlling the activities of the susceptible individuals in highly pathogenic areas to decrease the contact rate of population. Hence, can be guaranteed, which means and (see Fig. 3).
(iii) Developing several effective vaccines and carrying out other prophylactic measures to improve the immune rate of disease (see Fig. 4).
7. Conclusions and result discussions
7.1. Conclusions
The corresponding theoretical results of this paper are the following.
(i) By Theorem 3.1, system (1.3) admits a unique ergodic stationary distribution under
(ii) By taking the effect of random perturbations into account, a quasi-endemic equilibrium related to is defined while . In view of the expressions of and it is further proved that the stationary distribution around has a log-normal density function in the following form:
where the special form of is shown in Theorem 4.1. (iii) The disease of system (1.3) will go to extinction with probability 1 if . The above results (i) and (ii) reflect the stochastic persistence and ergodicity of the epidemic. Moreover, the corresponding disease extinction of system (1.3) is described by result (iii).
7.2. Result discussions
In this paper, combining the great effect of vaccination and the unpredictability of environmental fluctuations in the real world, a stochastic SVIS infectious disease model with vaccination and standard incidence is the object of concern. Adopting the descriptions in [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], linear perturbation, which is the most intuitive assumption of a random effect, is similarly taken into consideration in this paper. Subsequently, several dynamical properties of stochastic system (1.3) are analyzed, such as the existence and uniqueness of a global positive solution, existence and ergodicity of a stationary distribution, and disease elimination. By comparison with the existing results ([28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]), several highlights developed in the present study are detailed in the following two points.
As is known, the endemic equilibrium and basic reproduction number, two important results of a deterministic epidemic, reflect disease permanence and elimination. Similarly, for the corresponding stochastic model, the existence of stationary distribution indicates the stochastic positive equilibrium state. In this paper, it is first proved that stochastic system (1.3) admits a unique ergodic stationary distribution under the critical value . It should be pointed out that is a unified threshold for the disease persistence of systems (1.1) and (1.3). Moreover, the sufficient condition is obtained for the disease extinction of system (1.3). Both and reveal that the dynamical behavior of system (1.3) is critically affected by the random fluctuations, i.e., and . In view of the method of controlling variables and numerical simulations, this means that a large white noise leads to the disease eradication, while a small one guarantees stochastic permanence. In addition, by the main parameter analyses, several effective measures to stop the spread of an epidemic are provided.
It is generally accepted that the existence of an ergodic stationary distribution incurs difficulty in studying more exact statistical properties. Hence, this paper is devoted to obtaining the corresponding probability density function for further dynamical investigation. The results of Zhou et al. [28] are further perfected and general solving theories of algebraic equations with respect to the three-dimensional Fokker-Planck equation are developed, which are described in Lemmas 2.5 and 2.6. By taking the effect of stochasticity into account again, the quasi-endemic equilibrium corresponding to the endemic equilibrium is defined. For practical application, the exact expression of the log-normal three-dimensional density function of system (1.3) is given. Furthermore, it is worth mentioning that the methods and theories developed herein are still suitable for the case of the diffusion matrix being positive semi-definite, such as delay stochastic differential equations [32], [48].
Several remaining issues are now proposed and analyzed. First, by virtue of the limitation of the present mathematical approaches for epidemiological dynamics, a value gap exists between and and it is unfortunate that difficulty is encountered in obtaining the most precise threshold for disease extinction and persistence. Second, the impact of telegraph noises and periodicity on the dynamics of system (1.3) should also be studied; the reader is referred to [30], [34], [45], [49], [50]. These problems are expected to be studied and solved as planned future work.
CRediT authorship contribution statement
Baoquan Zhou: Validation, Software, Formal analysis, Writing - original draft, Writing - review & editing. Daqing Jiang: Conceptualization, Investigation, Methodology, Writing - review & editing. Yucong Dai: Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Tasawar Hayat: Conceptualization, Writing - review & editing. Ahmed Alsaedi: Investigation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11871473) and Shandong Provincial Natural Science Foundation (Grant Nos. ZR2019MA010 and ZR2019MA006).
Appendix A.
(I) (Proof of Lemma 2.5): Consider the algebraic equation where is a symmetric matrix. Letting by direct calculation one has
| (A.1) |
where . If then it means that is a positive semi-definite matrix. The proof is then completed.
(II) (Proof of Lemma 2.6): Denote
Let be the solutions of the following algebraic equations, respectively:
Obviously, one can obtain
Before proving the positive definiteness of the following two theories of matrix algebra should be described first.
. The positive definiteness of the matrix is not affected by the inverse congruence transformation.
. The similarity transformation does not change the eigenvalues of the matrix.
For convenience and simplicity, an important notation is introduced as follows. For the same dimensional square matrix and define
Given the above, it is easily derived that is also positive definite if is a positive definite matrix.
First, consider the following algebraic equation,
| (A.2) |
after which the relevant proof can be divided into the following two conditions:
Next, one must demonstrate that the elements and have the equivalent status in . Let where and the invertible matrix are given by
respectively. Hence, (A.2) can be equivalently transformed as
| (A.3) |
By defining it can be noticed that
In addition, . Therefore, the validation is completed. Namely, one must only discuss the following two cases, which are equivalent to conditions and respectively:
Case . If by directly solving Eq. (A.2), one obtains
Since has all negative real part eigenvalues, by the similarity invariance of it indicates that
Consequently, has an eigenvalue which has a negative real part. By then . In other words, is positive semi-definite. Moreover,
| (A.4) |
Case . If let .
If which means let where
Thus, (A.2) can be equivalently rewritten as
| (A.5) |
Denoting and one computes
where the parameters can be obtained by (A.5). Because their sign is the only object of concern, they are omitted here. Furthermore, the characteristic polynomial follows from that
By the condition that has all negative real part eigenvalues, it thus means that the equation has two negative real part roots. By the Routh-Hurwitz stability criterion [43], it can be shown that
| (A.6) |
In view of
one hence obtains
| (A.7) |
By means of and it is derived that and are all positive semi-definite. It is then implied that
| (A.8) |
If let where the invertible matrix is given by
Denoting (A.2) can then be equivalently transformed into the following equation:
| (A.9) |
Similarly, by direct calculation, one obtains that and
where are the same as those in Lemma 2.6. Considering
one can therefore derive, by a similar method as that described in (A.7),
Noting that and are all positive semi-definite, one then has
| (A.10) |
Consequently, by (A.4), (A.8) and (A.10), a constant always exists such that
| (A.11) |
In addition, for the following two algebraic equations,
and letting where
Noting that by a method similar to that shown in (A.2), one can see that
where the constants ; that is to say,
| (A.12) |
In summary, it can be derived that
Given the above definitions and discussions, is a positive-definite matrix. This completes the proof.
(III). (SED Preliminaries): For the above complete probability space it is assumed that is an -dimensional standard Brownian motion defined on it. Consider the following -dimensional SDE,
, with the initial value . A common differential operator is given by
Letting the operator act on a function one has
where and . If one has
References
- 1.Kermack W.O., McKendrick A.G.M. A contribution to the mathematical theory of epidemics. Proc R Soc Lond A. 1927;115:700–721. [Google Scholar]
- 2.Ma X., Wang W. A discrete model of avian influenza with seasonal reproduction and transmission. J Biol Dynam. 2010;4:296–314. doi: 10.1080/17513751003793009. [DOI] [PubMed] [Google Scholar]
- 3.Tchuenche J.M., Nwagwo A., Levins R. Global behaviour of an SIR epidemic model with time delay. Math Methods Appl Sci. 2007;30:733–749. [Google Scholar]
- 4.Hove-Musekwa S.D., Nyabadza F. The dynamics of an HIV/AIDS model with screened disease carriers. Comput Math Method M. 2015;10(4):287–305. [Google Scholar]
- 5.Lee H., Lao A., et al. Transmission dynamics and control strategies assessment of avian influenza a (H5N6) in the philippines. Infect Dis Model. 2018;3:35–59. doi: 10.1016/j.idm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuncer N., Martcheva M. Modeling seasonality in avian influenza H5N1. J Biol Syst. 2013;21(4):1–30. [Google Scholar]
- 7.Cai L., Wu J. Analysis of an HIV/AIDS treatment model with a nonlinear incidence. Chaos Solitons Fractals. 2009;41(1):175–182. [Google Scholar]
- 8.Li G., Zhen J. Global stability of an SEI epidemic model with general contact rate. Chaos Solitons Fractals. 2005;23:997–1004. [Google Scholar]
- 9.Liu X., Takeuchi Y., Iwami S. SVIR epidemic models with vaccination strategies. J Theore Bio. 2008;253:1–11. doi: 10.1016/j.jtbi.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Ma Z. Qualitative analyses of SIS epidemic model with vaccination and varying total population size. Math Comput Model. 2002;35:1235–1243. [Google Scholar]
- 11.Gao S., Ouyang H., Nieto J. Mixed vaccination strategy in SIRS epidmeic model with seasonal variability on infection. Int J Biomath. 2011;4:473–491. [Google Scholar]
- 12.Chen F. A susceptible-infected epidemic model with voluntary vaccinations. J Math Biol. 2006;53:253–272. doi: 10.1007/s00285-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 13.AI-Darabsah I. Threshold dynamics of a time-delayed epidemic model for continuous imperfect-vaccine with a generalized nonmonotone incidence rate. Nonlinear Dyn. 2020;101:1281–1300. doi: 10.1007/s11071-020-05825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson R.M., May R.M. Oxford University Press; Oxford: 1991. Infectious diseases in humans: dynamics and control. [Google Scholar]
- 15.Hethcote H.W. The mathematics of infectious diseases. SIAM Rev. 2000;42:599–653. [Google Scholar]
- 16.Yang Q., Jiang D., Shi N., Ji C. The ergodicity and extinction of stochastically perturbed SIR and SEIR epidemic models with saturated incidence. J Math Anal Appl. 2012;388:248–271. [Google Scholar]
- 17.Wang Y., Liu J., Liu L. Viral dynamics of an HIV model with latent infection incorporating antiretroviral therapy. Adv Differ Equ. 2016;225:1–15. [Google Scholar]
- 18.Liu S., Pang L., Ruan S., Zhang X. Global dynamics of avian influenza epidemic models with psychological effect. Comput Math Method Med. 2015;2015:913726. doi: 10.1155/2015/913726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao D., Ruan S. Global analysis of an epidemic model with nonmonotone incidence rate. Math Biosci. 2007;208(2):419–429. doi: 10.1016/j.mbs.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson R., May R. Oxford: Oxford University Press; 1991. Infectious diseases of human: dynamics and control. [Google Scholar]
- 21.Anderson R., May R. Springer-Verlag; Berlin, Heidelberg. New York:: 1982. Population biology of infectious diseases. [Google Scholar]
- 22.Ma Z., Zhou Y., et al. Higher Education Press; Beijing: 2009. Modeling and dynamic of infectious disease. [Google Scholar]; (In Chinese)
- 23.Truscott J.E., Gilligan C.A. Response of a deterministic epidemiological system to a stochastically varying environment. Proc Natl Acad Sci. 2003;100:9067–9072. doi: 10.1073/pnas.1436273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z. Dynamics of positive solutions to SIR and SEIR epidemic models with saturated incidence rates. Nonlin Anal Real World Appl. 2013;14(3):1286–1299. [Google Scholar]
- 25.Zhu P., Wang X., Li S., Guo Y., Wang Z. Investigation of epidemic spreading process on multiplex networks by incorporating fatal properties. Appl Math Comput. 2019;359:512–524. doi: 10.1016/j.amc.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia D., Wang X., Song Z., Rom0ć, Li X., Jusup M., Wang Z. Evolutionary dynamics drives role specialization in a community of players. J R Soc Interface. 2020;17:168. doi: 10.1098/rsif.2020.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]; 20200174
- 27.Mao X., Marion G., Renshaw E. Environmental Brownian noise suppresses explosions in population dynamics. Stoch Pro Appl. 2002;97(1):95–110. [Google Scholar]
- 28.Zhou B., Zhang X., Jiang D. Dynamics and density function analysis of a stochastic SVI epidemic model with half saturated incidence rate. Chaos Soliton Fractals. 2020;137:109865. [Google Scholar]
- 29.Qi K., Jiang D. The impact of virus carrier screening and actively seeking treatment on dynamical behavior of a stochastic HIV/AIDS infection model. Appl Math Model. 2020;85:378–404. [Google Scholar]
- 30.Khan T., Khan A. The extinction and persistence of the stochastic hepatitis b epidemic model. Chaos Soliton Fractals. 2018;108:123–128. [Google Scholar]
- 31.Wang Y., Jiang D. Stationary distribution of an HIV model with general nonlinear incidence rate and stochastic perturbations. J Frank Inst. 2019;356:6610–6637. [Google Scholar]
- 32.Caraballo T., Fatini M.E., Khalifi M.E. Analysis of a stochastic distributed delay epidemic model with relapse and gamma distribution kernel. Chaos Soliton Fractals. 2020;133:109643. [Google Scholar]
- 33.Cai Y., Kang Y. A stochastic epidemic model incorporating media coverage. Commun Math Sci. 2015;14:893–910. [Google Scholar]
- 34.Shi Z., Zhang X., Jiang D. Dynamics of an avian influenza model with half-saturated incidence. Appl Math Comput. 2019;355:399–416. [Google Scholar]
- 35.Yang Q., Jiang D., Shi N., Ji C. The ergodicity and extinction of stochastically perturbed SIR and SEIR epidemic models with saturated incidence. J Math Anal Appl. 2012;388:248–271. [Google Scholar]
- 36.Cai Y., Kang Y. A stochastic epidemic model incorporating media coverage. Commun Math Sci. 2015;14:893–910. [Google Scholar]
- 37.Zhao Y., Jiang D. The threshold of a stochastic SIS epidemic model with vaccination. Appl Math Comput. 2014;243:718–727. [Google Scholar]
- 38.Liu Q., Jiang D., Hayat T., Alsaedi A. Threshold behavior in a stochastic delayed SIS epidemic model with vaccination and double diseases. J Frank Inst. 2019;356:7466–7485. [Google Scholar]
- 39.Zhang X., Jiang D. Dynamics of a stochastic SIS model with double epidemic diseases driven by Lvy jumps. Physica A. 2017;471:767–777. [Google Scholar]
- 40.Zhang X., Jiang D. Dynamical behavior of a stochastic SVIR epidemic model with vaccination. Physica A. 2017;483:94–108. [Google Scholar]
- 41.Mao X. Chichester: Horwood Publishing; 1997. Stochastic differential equations and applications. [Google Scholar]
- 42.Has’miniskii R.Z. Sijthoff Noordhoff, Alphen aan den Rijn; The Netherlands;: 1980. Stochastic stability of differential equations. [Google Scholar]
- 43.Ma Z., Zhou Y., Li C. Science Press; 2015. Qualitative and stability methods for ordinary differential equations. [Google Scholar]
- 44.Gardiner C.W. Chemistry and the natural sciences. Springer Berlin; 1983. Handbook of stochastic methods for physics. [Google Scholar]
- 45.Roozen H. An asymptotic solution to a two-dimensional exit problem arising in population dynamics. SIAM J Appl Math. 1989;49:1793. [Google Scholar]
- 46.Higham D.J. An algorithmic introduction to numerical simulation of stochastic differential equations. SIAM Rev. 2001;43:525–546. [Google Scholar]
- 47.Arino J., Mccluskey C.C., Driessche P. Global results for an epidemic model with vaccination that exhibits backward bifurcation. SIAM J Appl Math. 2003;64(1):260276. [Google Scholar]
- 48.Liu Q., Jiang D., Shi N., Hayat T., Alsaedi A. Asymptotic behavior of stochastic multi-group epidemic models with distributed delays. Physica A. 2017;467:527–541. [Google Scholar]
- 49.Li X., Gray A., Jiang D., Mao X. Sufficient and necessary conditions of stochastic permanence and extinction for stochastic logistic populations under regime switching. J Math Anal Appl. 2011;376:11–28. [Google Scholar]
- 50.Zhang X., Jiang D., Alsaedi A. Stationary distribution of stochastic SIS epidemic model with vaccination under regime switching. Appl Math Lett. 2016;59:87–93. [Google Scholar]