Abstract
Aim
To evaluate whether left bundle branch block with residual conduction (rLBBB) is associated with worse outcomes after cardiac resynchronisation therapy (CRT).
Methods
All consecutive CRT implants at our institution between 2006 and 2013 were identified from our local device registry. Pre- and post-implant patient specific data were extracted from clinical records.
Results
A total of 690 CRT implants were identified during the study period. Prior to CRT, 52.2% of patients had true left bundle branch block (LBBB), 19.1% a pacing-induced LBBB (pLBBB), 11.2% a rLBBB, 0.8% a right bundle branch block (RBBB), and 16.5% had a nonspecific intraventricular conduction delay (IVCD) electrocardiogram pattern. Mean age at implant was 67.5 years (standard deviation [SD] = 10.6), mean left ventricular ejection fraction (LV EF) was 25.7% (SD = 7.9%), and mean QRS duration was 158.4 ms (SD = 32 ms). After CRT, QRS duration was significantly reduced in the LBBB (p < 0.001), pLBBB (p < 0.001), rLBBB (p < 0.001), RBBB (p = 0.04), and IVCD groups (p = 0.03). LV EF significantly improved in the LBBB (p < 0.001), rLBBB (p = 0.002), and pLBBB (p < 0.001) groups, but the RBBB and IVCD groups showed no improvement. There was no significant difference in mortality between the LBBB and rLBBB groups. LV EF post-CRT, chronic kidney disease, hyperkalaemia, hypernatremia, and age at implant were significant predictors of mortality.
Conclusion
CRT in patients with rLBBB results in improved LV EF and similar mortality rates to CRT patients with complete LBBB. Predictors of mortality post-CRT include post-CRT LV EF, presence of CKD, hyperkalaemia, hypernatremia, and older age at implant.
Keywords: Cardiac resynchronisation therapy, Heart failure, Left bundle branch block, Left bundle branch block with residual conduction
This retrospective study examined CRT response and mortality of patients with rLBBB, in comparison to patients with true LBBB. The groups did not exhibit any significant difference in post-CRT LV EF or mortality. Predictors of mortality post-CRT include post-CRT LV EF, presence of CKD, and older age at implant.
What’s new?
-
•
The retrospective study analysed cardiac resynchronisation therapy (CRT) response in the largest patient population with left bundle branch block and residual conduction (rLBBB) described in the literature to date.
-
•
In contrast to previously reported findings, this study shows that there is no difference in CRT response or mortality between patients with rLBBB and those with true LBBB.
-
•
The analysis demonstrated that hypernatremia and hyperkalaemia, renal insufficiency, a lack of increase in LV function, and minimal shortening of QRS duration were associated with an increased risk of mortality. (see Table 1, Table 2, Table 3, Fig. 1, Fig. 2, Fig. 3)
Table 1.
Baseline patient characteristics.
| Variable |
LBBB |
rLBBB |
IVCD >120 ms |
p-value (LBBB vs rLBBB |
p-value (cLBBB vs IVCD |
|||
|---|---|---|---|---|---|---|---|---|
| SD | SD | SD | ||||||
| Age | 66.4 | 10.1 | 65.8 | 10.7 | 71.3 | 9.1 | 0.68 | <0.01 |
| QRS duration | 159.5 | 24.6 | 158.1 | 28.4 | 155.7 | 26.9 | 0.71 | 0.41 |
| LVEF | 25.6 | 7.6 | 23.9 | 7.4 | 27.5 | 7.6 | 0.10 | 0.166 |
| DM | 98 | 31.3 | 20 | 29.9 | 17 | 44.7% | 0.82 | 0.08 |
| ICMP | 158 | 50.2% | 37 | 55.2% | 25 | 67.6% | 0.45 | 0.04 |
| Male sex | 247 | 77.9% | 56 | 82.4% | 33 | 84.6% | 0.42 | 0.29 |
| Renal failure | 103 | 32.8% | 20 | 30.3% | 15 | 40.5% | 0.69 | 0.08 |
Abbreviations: LBBB = complete left bundle branch block; rLBBB = left bundle branch block with residual conduction, IVCD = intraventricular conduction delay, SD = standard deviation, DM = diabetes mellitus, ICMP = ischaemic cardiomyopathy.
Table 2.
Comparison between LBBB and rLBBB and between LBBB and IVCD >120 ms I.
| Variable | QRS pre-CRT (ms) | SD (ms) | QRS post- CRT (ms) | SD (ms) | LVEF pre | SD | LVEF post | SD | p value (QRS duration pre vs post) | p value (LV-F pre vs post) |
|---|---|---|---|---|---|---|---|---|---|---|
| LBBB (n = 287) | 160 | 24.8 | 137.3 | 22.8 | 25.6% | 8.1% | 31.7% | 9.7% | <0.0 | <0.001 |
| rLBBB (n = 65) | 158.8 | 28.8 | 137.1 | 22.4 | 24.1% | 8.0% | 29.6% | 10.5% | <0.001 | 0.001 |
| IVCD > 120 ms | 154.2 | 27.8 | 142.6 | 24.5 | 28.8% | 7.6% | 29% | 6.8% | 0.309 | 0.05 |
Abbreviations: LBBB = complete left bundle branch block; rLBBB = left bundle branch block with residual conduction, IVCD = intraventricular conduction delay, SD = standard deviation, LVEF = left ventricular ejection fraction.
Table 3.
Comparison between LBBB and rLBBB and between LBBB and IVCD >120 ms II.
| Variable | QRS post-CRT (ms) | LV-F post-CRT (ms) | P value |
|---|---|---|---|
| LBBB vs rLBBB | 137.5 vs 137.1 | 31.7 vs 29.6 | 0.27 |
| LBBB vs IVCD > 120 ms | 137.5 vs 144.3 | 31.7 vs 29.1 | 0.10 |
Abbreviations: LBBB = complete left bundle branch block; rLBBB = left bundle branch block with residual conduction, IVCD = intraventricular conduction delay, SD = standard deviation.
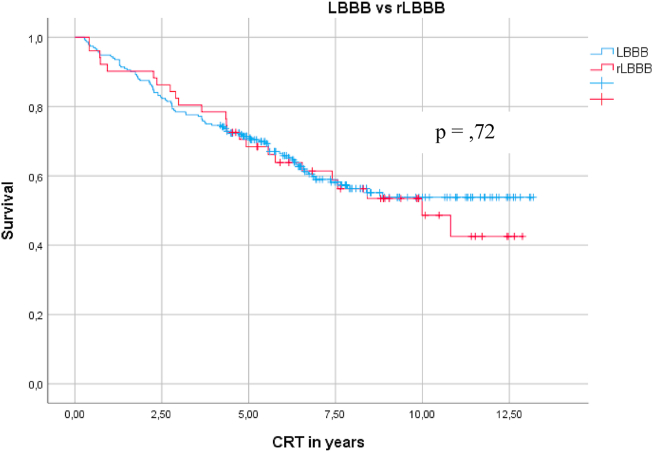
Fig. 1.
Kaplan-Meier curve comparing mortality between LBBB and rLBBB.
Fig. 2.
Kaplan-Meier curve comparing mortality between LBBB and pLBBB.
Fig. 3.
Kaplan-Meier curve comparing mortality between LBBB and IVCD >120 ms.
1. Introduction
Cardiac resynchronisation therapy (CRT) is an effective and established guideline recommended therapy for patients with symptomatic heart failure and reduced ejection fraction [[1], [2], [3], [4]]. In addition to the improvement in left ventricular ejection fraction (LV EF) and the reduction of mortality and hospitalisation rates, studies have shown significant symptomatic improvement associated with CRT use [2,[5], [6], [7]]. However, only 70%–80% of the patients are CRT ‘responders’; thus, it is essential to screen patients to ensure a favourable risk-benefit ratio for implant [[6], [7], [8]]. A subgroup analysis of the PREDICT trial suggested that a left bundle branch block (LBBB) morphology with residual conduction (rLBBB) was not associated with the same benefits of CRT as complete LBBB [7,9].
Electrocardiographically rLBBB is similar to complete LBBB, but with an r wave in V1 (>1 mm) and/or q wave in aVL >1 mm. The rLBBB pattern does not have complete block of the left bundle branch, but instead septal depolarisation is present from left to right [[9], [10], [11]]. Given that previous study results suggested a worse response to CRT in patients with rLBBB, these patients may not be offered CRT. However, this is based on a sub-group analysis; thus, the data are less robust than other CRT response indicators.
The present study aimed to investigate whether patients with the electrocardiographic criteria of a rLBBB will benefit less from a CRT than those with a true LBBB morphology.
2. Methods
We performed a retrospective analysis of all consecutive patients undergoing CRT at the German Heart Centre Munich between January 1, 2006 and December 31, 2013. Data including patient demographics, CRT indication, co-morbidities, and echocardiographic indices as well as electrocardiograms (ECGs) before and after CRT are collected prospectively at the time of implant and recorded in our internal device registry. Follow-up data including serial echocardiograms, ECGs, and mortality data were extracted from electronic clinical records consisting of regular follow-up visits, on both outpatient and inpatient bases. Patients who did not attend follow-up in our outpatient clinic were contacted for a telephone interview. If the survival data were unclear, the patients or their attending physicians were contacted.
Electrocardiographic QRS morphologies were quantified both manually and digitally (1SEMA version: 2.70.1.1, Schiller®, Austria). If both analogue and digital ECGs were available, the digital measurements were used. The presence of LBBB, RBBB, pLBBB, and intraventricular conduction delay (IVCD) was defined according to the published guidelines.8. Patients with LBBB were subdivided into those with complete LBBB and those with rLBBB defined as an r wave in V1 ≥1 mm (r-V1) and/or a q wave in aVL ≥1 mm (q-aVL). Measurements were taken from the beginning of the QRS complex (earliest deflection) to the end. Furthermore, device programming as well as pre- and post-procedural echocardiographic scans (1 and 12 months) were analysed and compared.
The study was reviewed and approved by the local ethics board.
2.1. Implantation and programming of devices
Our centre implants a variety of devices from Medtronic, Biotronik, St. Jude Medical/Abott, Sorin ELA/Livanova, and Boston Scientific.
Target vessels for the left ventricular lead were lateral midventricular or basal branches of the coronary sinus. Vessels with phrenic stimulation were only accepted as the final placement in the absence of alternatives due to anatomical constraints. The leads were implanted both transvenously as first line, or epicardially if the transvenous access was contra-indicated or ineffective. The right atrial lead was preferentially placed in the right atrial appendage (RAA), whereas the right ventricular lead was placed in the right ventricular apex (RVA) or inferior septum. Lead position was confirmed radiologically in several projections (AP, LAO, RAO) at the time of implant. After the implantation, optimisation of the AV delay and RV/LV timing by surface ECG was performed [[11], [12], [13]].
Statistical analysis was performed using SPSS Statistics 22 (IBM®). A p value ≤ 0.05 was considered significant.
Data are displayed as mean and standard deviation (SD) where appropriate. Student’s t-test was used for independent samples, but the Mann-Whitney U test was used in cases of non-normally distributed data. The chi-square test was used to assess significance in discrete data. Kaplan-Meier’s survival curves were used to calculate the probability of death at a certain point in time with log-rank testing to compare groups.
3. Results
A total of 690 patients were analysed. The majority (79.3%) were male and had an ischemic cardiomyopathy as the underlying pathology (55.3%). The patients were divided into the following 6 groups: LBBB (52.2%), rLBBB (11.2%), pLBBB (19.1%), RBBB (0.8%), IVCD >120 ms (6.5%), and IVCD <120 ms (10.2%). At the end of 2017, 35.7% (n = 246) were still alive, 39.6% (n = 273) had died, and survival data were not available in 24.8% (n = 171) of the patients.
There was no difference in the final LV EF (31.7% vs 29.6%; p = 0.25) and final QRS duration (137.4 vs 137 ms; p = 0.833) after CRT between the LBBB and rLBBB groups. There was no difference in mortality between the LBBB and rLBBB groups (8.9 SD, 35 confidence interval (CI) 8.2–9.6 vs 8.5 SD, 67 CI 7.2–9.1; p = 0.72). The same result was found between patients with LBBB and pLBBB in terms of mortality (p = 0.48).
The QRS complex duration was shortened (181 ± 32 ms to 142.1 ± 25.8 ms; p < 0.001) and the LV EF improved (27.8% ± 8.1%–34.2% ± 10.8%, p < 0.001) after CRT in patients with pLBBB. Patients with IVCD >120 ms showed a significant reduction in QRS duration (154.2 ± 27.8 ms to 142.6 ± 24.5 ms; p = 0.05), but no improvement in LV EF (28.8% ± 7.6%–29% ± 6.8%; p = 0.31). Patients with baseline LBBB had improved survival compared to those with IVCD >120 ms (p = 0.02).
4. Discussion
This retrospective study revealed that there is no difference in the mortality rate between patients with electrocardiographically confirmed LBBB and rLBBB morphology after CRT. Owing to the design of our study, the extent to which the patients clinically benefited from CRT cannot be determined, but both groups showed a significant increase in LV EF and decrease in QRS duration, which were previously shown to be predictors of clinical CRT response [2,[14], [15], [16]]. Furthermore, the patients in our cohort who had an improvement in their LV EF and/or a reduction in their QRS duration had decreased mortality risk.
Our data were consistent with previous literature suggesting the greater benefit from CRT in patients with LBBB than in those with RBBB or IVCD >120 ms [1,[17], [18], [19], [20]]. In our cohort, these patients had a higher mortality rate, no improvement in LV EF, and no reduction in QRS duration. Electrolyte disorders, such as hyperkalaemia (>5 mmol/l) and hypernatremia (>145 mmol/l) were also associated with increased mortality; however, this may be related to the concurrent diagnosis of chronic renal failure.
Our study is retrospective in design and is subject to the usual limitations of such a design. In particular, in our study, we were not able to establish the clinical response to CRT therapy. Lead position was not established in each case; however, it is our policy that no LV leads are to be positioned apically (which is known to be associated with poor CRT outcomes); thus, we do not believe that this have affected the results. Moreover, echocardiographical evaluation was performed in an un-blinded fashion by various operators, which may have introduced bias into the results.
Our data represent real-world results of CRT in a sub-group that is less thoroughly investigated (rLBBB) and provide evidence to counter the previous sub-group analysis suggesting that rLBBB is associated with poorer outcomes. We believe that patients with rLBBB should be offered CRT therapy similar to those with complete LBBB.
5. Conclusion
CRT in patients with rLBBB resulted in improvement in LV EF, shortened QRS duration, and reduced mortality risk similar to those with LBBB. In the rLBBB group (as in others), CKD, older age at implant, failure to improve LV FE, and failure to narrow QRS were all predictors of mortality.
Funding
None.
Consent
Informed consent was not required.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Zareba W., Klein H., Cygankiewicz I., Hall W.J., McNitt S., Brown M. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 2.Hsu J.C., Solomon S.D., Bourgoun M., McNitt S., Goldenberg I., Klein H. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study. J Am Coll Cardiol. 2012;59:2366–2373. doi: 10.1016/j.jacc.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 3.Moss A.J., Hall W.J., Cannom D.S., Klein H., Brown M.W., Daubert J.P. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Fung J.W., Auricchio A., Chan J.Y., Kum L.C., Wu L.W. Differential change in left ventricular mass and regional wall thickness after cardiac resynchronization therapy for heart failure. Eur Heart J. 2006;27:1423–1430. doi: 10.1093/eurheartj/ehi885. [DOI] [PubMed] [Google Scholar]
- 6.Tang A.S., Wells G.A., Talajic M., Arnold M.O., Sheldon R., Connolly S. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W., Klein H., Cygankiewicz I., Hall W.J., McNitt S., Brown M. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator. Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 8.European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA), Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P. ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eurospace 2013. 2013;15:1070–1118. doi: 10.1093/europace/eut206. [DOI] [PubMed] [Google Scholar]
- 9.Perrin M.J., Green M.S., Redpath C.J., Nery P.B., Keren A., Beanlands R.S. Greater response to cardiac resynchronization therapy in patients with true complete left bundle branch block: a PREDICT substudy. Europace. 2012;14:690–695. doi: 10.1093/europace/eur381. [DOI] [PubMed] [Google Scholar]
- 10.Lev M., Unger P.N., Rosen K.M., Bharati S. The anatomic substrate of complete left bundle branch block. Circulation. 1974;50:479–486. doi: 10.1161/01.cir.50.3.479. [DOI] [PubMed] [Google Scholar]
- 11.Padanilam B.J., Morris K.E., Olson J.A., Rippy J.S., Walsh M.N., Subramanian N. The surface electrocardiogram predicts risk of heart block during right heart catheterization in patients with preexisting left bundle branch block: implications for the definition of complete left bundle branch block. J Cardiovasc Electrophysiol. 2010;21:781–785. doi: 10.1111/j.1540-8167.2009.01714.x. [DOI] [PubMed] [Google Scholar]
- 12.Boriani G., Biffi M., Müller C.P., Seidl K.H., Grove R., Vogt J. A prospective randomized evaluation of VV delay optimization in CRT-D recipients: echocardiographic observations from the RHYTHM II ICD study. Pacing Clin Electrophysiol. 2009;32:S120–S125. doi: 10.1111/j.1540-8159.2008.02267.x. [DOI] [PubMed] [Google Scholar]
- 13.Gras D., Gupta M.S., Boulogne E., Guzzo L., Abraham W.T. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol. 2009;32:S236–S239. doi: 10.1111/j.1540-8159.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 14.Shanks M., Delgado V., Ng A.C., Auger D., Mooyaart E.A., Bertini M. Clinical and echocardiographic predictors of nonresponse to cardiac resynchronization therapy. Am Heart J. 2011;161:552–557. doi: 10.1016/j.ahj.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Bax J.J., Bleeker G.B., Marwick T.H., Molhoek S.G., Boersma E., Steendijk P. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Ypenburg C., Schalij M.J., Bleeker G.B., Steendijk P., Boersma E., Dibbets-Schneider P. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33–41. doi: 10.1093/eurheartj/ehl379. [DOI] [PubMed] [Google Scholar]
- 17.Chung E.S., Leon A.R., Tavazzi L., Sun J.P., Nihoyannopoulos P., Merlino J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 18.Kashani A., Barold S.S. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 19.Birnie D.H., Tang A.S. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006;21:20–26. doi: 10.1097/01.hco.0000198983.93755.99. [DOI] [PubMed] [Google Scholar]
- 20.Auricchio A., Stellbrink C., Butter C., Sack S., Vogt J., Misier A.R. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109–2116. doi: 10.1016/j.jacc.2003.04.003. [DOI] [PubMed] [Google Scholar]