Abstract
Introduction
Multilineage myelosuppression is an acute toxicity of cytotoxic chemotherapy, resulting in serious complications and dose modifications. Current therapies are lineage specific and administered after chemotherapy damage has occurred. Trilaciclib is a cyclin-dependent kinase 4/6 inhibitor that is administered prior to chemotherapy to preserve hematopoietic stem and progenitor cells and immune system function during chemotherapy (myelopreservation).
Methods
In this randomized, double-blind, placebo-controlled phase II trial, patients with previously treated extensive-stage small cell lung cancer (ES-SCLC) were randomized to receive intravenous trilaciclib 240 mg/m2 or placebo before topotecan 1.5 mg/m2 on days 1–5 of each 21-day cycle. Primary endpoints were duration of severe neutropenia (DSN) in cycle 1 and occurrence of severe neutropenia (SN). Additional endpoints were prespecified to further assess the effect of trilaciclib on myelopreservation, safety, patient-reported outcomes (PROs), and antitumor efficacy.
Results
Thirty-two patients received trilaciclib, and 29 patients received placebo. Compared with placebo, administration of trilaciclib prior to topotecan resulted in statistically significant and clinically meaningful decreases in DSN in cycle 1 (mean [standard deviation] 2 [3.9] versus 7 [6.2] days; adjusted one-sided P < 0.0001) and occurrence of SN (40.6% versus 75.9%; adjusted one-sided P = 0.016), with numerical improvements in additional neutrophil, red blood cell, and platelet measures. Patients receiving trilaciclib had fewer grade ≥ 3 hematologic adverse events than patients receiving placebo, particularly neutropenia (75.0% versus 85.7%) and anemia (28.1% versus 60.7%). Myelopreservation benefits extended to improvements in PROs, specifically in those related to fatigue. Antitumor efficacy was comparable between treatment arms.
Conclusions
Compared with placebo, the addition of trilaciclib prior to topotecan for the treatment of patients with previously treated ES-SCLC improves the patient experience of receiving chemotherapy, as demonstrated by a reduction in chemotherapy-induced myelosuppression, improved safety profile, improved quality of life and no detrimental effects on antitumor efficacy.
Trial Registration
ClinicalTrials.gov: NCT02514447
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01538-0) contains supplementary material, which is available to authorized users.
Keywords: Anemia, Chemotherapy, Myelopreservation, Myelosuppression, Neutropenia, Patient-reported outcomes, Small cell lung cancer, Topotecan, Trilaciclib
Key Summary Points
| Why carry out this study? |
| Topotecan is an intravenous (IV) topoisomerase I inhibitor indicated for the treatment of small cell lung cancer (SCLC) in patients with platinum-sensitive disease after failure of first-line chemotherapy. |
| Although topotecan remains an important treatment option for patients with relapsed SCLC, it is commonly associated with chemotherapy-induced myelosuppression (CIM), which results in complications such as increased risk of infection, fatigue, and bleeding and the associated need for dose reductions and delays. |
| Trilaciclib is an IV cyclin-dependent kinase 4/6 inhibitor that transiently arrests hematopoietic stem and progenitor cells in the G1 phase of the cell cycle during chemotherapy exposure, thereby preserving them from chemotherapy-induced damage (myelopreservation). |
| In this randomized, placebo-controlled phase II study, the myelopreservation effects of trilaciclib administered prior to topotecan for the treatment of patients with previously treated extensive-stage SCLC (ES-SCLC) were evaluated. |
| What was learned from the study? |
| Compared with placebo, administering trilaciclib prior to topotecan reduced CIM and the need for supportive care interventions, improved the safety profile of topotecan, and improved the quality of life of patients, particularly with regard to endpoints associated with fatigue. |
| The data extend the evidence for the clinical benefits of trilaciclib as a first-in-class myelopreservation agent for patients with ES-SCLC treated with chemotherapy and demonstrate that trilaciclib can reduce the risk of CIM that might otherwise result in a substantial risk of additional intervention, hospitalization, and even death. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digitalfeatures for this article go to https://doi.org/10.6084/m9.figshare.13078844.
Introduction
Small cell lung cancer (SCLC) is highly sensitive to chemotherapeutic agents given in the first-line setting. However, despite high response rates to initial chemotherapy with etoposide plus cisplatin or carboplatin, irinotecan, or combination therapy with cyclophosphamide, vincristine, and an anthracycline, most patients relapse [1]. For > 15 years, the topoisomerase I inhibitor, topotecan, has been the only United States Food and Drug Administration-approved standard of care for patients with relapsed SCLC after failure of front-line chemotherapy, and it continues to be an important treatment option in this setting, both in the US and globally [2, 3]. However, topotecan is associated with significant chemotherapy-induced myelosuppression (CIM), which has long been a major concern to clinicians using this agent. The standard 5-day schedule of intravenous (IV) topotecan 1.5 mg/m2 results in high rates of grade 3 and 4 neutropenia, anemia, and thrombocytopenia [4–6], which increase the risk of infection, fatigue and bleeding among patients with SCLC and reduce patient quality of life. Furthermore, clinical concerns raised by CIM commonly lead to chemotherapy dose reductions and/or delays, which limit therapeutic dose intensity and, potentially, its intended antitumor efficacy [6–8]. CIM is currently managed with supportive care interventions such as hematopoietic growth factors and transfusions [9–12]. However, these are often administered reactively when signs or symptoms appear, are specific to individual hematopoietic lineages and impart their own set of risks for adverse reactions, highlighting the need for alternative approaches that can proactively prevent CIM.
Trilaciclib is a selective, reversible cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor that is administered intravenously prior to chemotherapy to preserve hematopoietic stem and progenitor cells (HSPCs) and lymphocytes during chemotherapy (myelopreservation). Because HSPCs and lymphocytes are dependent on CDK4/6 activity for proliferation, they are arrested in the G1 phase of the cell cycle upon exposure to trilaciclib. This transient, drug-induced cell cycle arrest prevents HSPCs and lymphocytes from proliferating in the presence of cytotoxic chemotherapy, thereby protecting them from chemotherapy-induced damage [13–15]. The myelopreservation effects of trilaciclib are in contrast to the myelosuppressive effects of oral CDK4/6 inhibitors currently approved for the treatment of hormone receptor-positive breast cancer. Oral CDK4/6 inhibitors are dosed chronically to inhibit CDK4/6-dependent tumor proliferation, with the continued blockade of HSPC proliferation in the bone marrow resulting in myelosuppression (most commonly neutropenia) [16]. By contrast, trilaciclib is administered intravenously and intermittently (i.e., only prior to the administration of chemotherapy) to prevent damage to HSPCs. This allows for more precise control over the period of HSPC cycle arrest and the avoidance of lingering myelosuppressive effects [13].
Clinically, the myelopreservation benefits of trilaciclib have primarily been studied in patients with extensive stage (ES)-SCLC. SCLC tumor cells replicate independently of CDK4/6 through the obligate loss of the retinoblastoma protein [17], thereby allowing assessment of trilaciclib’s effects on the host without any potential direct effects on the tumor. In a randomized, double-blind, placebo-controlled phase II trial in patients with newly diagnosed ES-SCLC, administration of trilaciclib prior to etoposide plus carboplatin (E/P) improved myelosuppression endpoints across multiple hematopoietic lineages, without impairing chemotherapy efficacy [18]. Compared with the placebo arm, fewer supportive care interventions and dose reductions were required in the trilaciclib arm. Furthermore, safety was improved, with fewer grade ≥ 3 adverse events (AEs) reported with trilaciclib, primarily because of less high-grade hematologic toxicity [18].
Here, we report the myelopreservation, safety, health-related quality of life (HRQoL) and antitumor efficacy results from a randomized, double-blind, placebo-controlled phase II trial of trilaciclib administered prior to topotecan in patients with previously treated ES-SCLC. The current study was performed to assess the myelopreservation effects of trilaciclib in the setting of a chemotherapy regimen that is associated with significant hematologic toxicity and to evaluate the effects of trilaciclib when administered to patients with HSPCs that have already been damaged by prior lines of chemotherapy.
Methods
Study Design and Participants
This was a global, multicenter, phase Ib/IIa study (NCT02514447) of trilaciclib administered prior to topotecan for patients with ES-SCLC being treated in a second-/third-line setting. Data from the phase II portion of the study are presented.
Eligible patients were aged ≥ 18 years, with a confirmed diagnosis of ES-SCLC. Patients must have had disease progression during or after first- or second-line chemotherapy and been eligible to receive topotecan. Additional inclusion criteria (Supplementary Methods) included ≥ 1 measurable target lesion per Response Evaluable Criteria in Solid Tumors Version 1.1 (RECIST v1.1), adequate organ function, and Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 2. Patients were excluded if they had a history of topotecan treatment for SCLC or brain metastases requiring immediate treatment.
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. The protocol and all study-related materials were approved by the institutional review board or independent ethics committee of each participating center. All patients provided written informed consent.
Patients were randomized to receive trilaciclib or placebo by an interactive web response system according to a randomization schedule generated by an unblinded statistician (Supplementary Methods). Randomization was stratified based on ECOG PS (0/1 versus 2) and sensitivity to first-line treatment, as defined by the investigator (sensitive versus resistant, whereby sensitivity was defined as having a complete response, partial response or stable disease with first-line treatment, and a progression-free interval ≥ 90 days after completion of first-line treatment; resistance was defined as a best response of progressive disease or a progression-free interval < 90 days). The sponsor, patients, investigators, and other staff were blinded to the treatment arm.
All patients received trilaciclib 240 mg/m2 or placebo administered as a 30-min IV infusion ≤ 4 h prior to topotecan 1.5 mg/m2 on each day that chemotherapy was administered. Treatment was administered on days 1–5 of each 21-day cycle. Patients were treated until progression, unacceptable toxicity, withdrawal of consent, or discontinuation by the patient or investigator. No dose modifications of trilaciclib were allowed. Topotecan dose reductions were only allowed twice for any patient and were permanent. To ensure an unconfounded assessment of trilaciclib’s ability to prevent CIM, administration of erythropoiesis-stimulating agents (ESAs) and primary prophylaxis with granulocyte colony-stimulating factors (G-CSFs) was prohibited in cycle 1, although therapeutic G-CSF was allowed in all cycles. As the risk of febrile neutropenia (FN) is predicted to be > 20% with topotecan, primary prophylaxis with G-CSF during cycle 1 would be indicated per standard guidelines. However, the safety monitoring committee agreed that for this study, prohibiting primary prophylaxis with G-CSF was permissible as long as the risk to patients receiving placebo was minimized by implementing a 2:1 (trilaciclib: placebo) randomization ratio, allowing the therapeutic use of G-CSF in cycle 1 and allowing investigators to only enroll those patients whose safety (as assessed by the treating physician) was not substantially compromised by this approach. Following completion of cycle 1, supportive care measures, including ESAs and G-CSF, were permitted per American Society of Clinical Oncology guidelines [19] and current prescribing information. Red blood cell (RBC) and platelet transfusions were allowed per investigator discretion throughout the entire treatment period.
Objectives, Endpoints, and Assessments
The primary objective was to assess the safety and tolerability of trilaciclib administered prior to topotecan. Unless otherwise specified, myelosuppression endpoints were measured using hematologic laboratory parameters (e.g., complete blood counts) and their derivatives rather than AEs. Primary endpoints were the duration of severe neutropenia (DSN) in cycle 1 and occurrence (percent of patients) of severe neutropenia (SN), whereby SN was defined as absolute neutrophil count < 0.5 × 109 cells/l. Key secondary endpoints were the occurrence of RBC transfusions on/after week 5, G-CSF administration, platelet transfusions, and number of all-cause dose reductions. Supportive secondary endpoints were the occurrence of FN AEs, ESA administration, IV antibiotic use, and infection serious AEs (SAEs) as well as overall response rate (ORR), progression-free survival (PFS), and overall survival (OS).
Additional endpoints included patient-reported outcomes (PRO; exploratory), the occurrence and incidence (per 100 cycles) of hospitalization (all cause and due to CIM [neutropenia, anemia, thrombocytopenia] or sepsis), AEs, and additional safety endpoints. AEs were monitored throughout the study and were graded according to National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. Hematologic AEs were defined as neutropenia, neutrophil count decreased, anemia, anemia macrocytic, RBC count decreased, hemoglobin decreased, thrombocytopenia, and platelet count decreased. Exploratory assessment of trilaciclib’s effects on HRQoL was based on validated PRO instruments (e.g., Functional Assessment of Cancer Therapy [FACT]-Anemia [An], and FACT-Lung [FACT-L]), using literature-based thresholds of meaningful within-patient change [20–23]. Antitumor efficacy evaluation was based on responses derived from investigator measurements, as per RECIST v1.1.
Statistical Analysis
The sample size was determined based on having ≥ 90% power to detect treatment effects between trilaciclib versus placebo with respect to each of the primary endpoints, at a significance level of two-sided 0.20 (Supplementary Methods).
The intent-to-treat (ITT) analysis set, used for myelopreservation, PRO and PFS/OS endpoints, included all randomized patients, with data analyzed by randomly assigned treatment. Safety analyses included all patients who received ≥ 1 dose of any study drug, with data analyzed by actual received treatment. Analyses of tumor response were performed in patients who had measurable disease at the baseline tumor assessment, and had ≥ 1 post-baseline tumor assessment, clinical progression as noted by the investigator before their first post-baseline tumor scan, or died because of disease progression before their first post-baseline tumor scan.
Continuous variables were summarized by descriptive statistics, and categorical variables summarized in frequency tables. DSN in cycle 1 was assessed using a nonparametric analysis of covariance, and occurrence of SN was evaluated using a modified Poisson model. Both models included the stratification factors of ECOG PS (0/1 versus 2), sensitivity to first-line treatment (sensitive or resistant), and treatment as fixed effects, with baseline absolute neutrophil count as a covariate. For counting variables, treatment effects were evaluated using a negative binomial model with the same fixed terms, using corresponding baseline laboratory values as covariates.
For the two primary endpoints and key secondary endpoints, a Hochberg-based gatekeeping procedure was used to control the family-wise error rate across the multiple null hypotheses at the one-sided level of 0.1.
For PFS and OS, median time to event was estimated using the Kaplan-Meier method. Treatment group differences were tested using a stratified log-rank test, and a Cox regression model was used to estimate the hazard ratio (HR) and 80% confidence interval (CI) for trilaciclib versus placebo, with stratification factors as covariates.
Analyses were implemented using SAS® version 9.4. Final myelopreservation and PRO analyses were conducted after all patients had had the opportunity to receive ≥ 12 weeks of treatment or had discontinued from study treatment prior to week 12 (database lock 1; data cutoff September 28, 2018). Safety, hospitalization, and antitumor endpoint analyses were conducted when ≥ 70% of patients had died (database lock 2; data cut-off May 31, 2019).
Results
Patients and Treatment
Patients were enrolled at 17 sites in the US, 5 in Serbia, and 1 each in Belgium, Croatia, and Republic of Macedonia. Sixty-one patients were randomized (ITT population; 32 to trilaciclib and 29 to placebo), and 60 were treated per protocol (Fig. S1). Of the 60 treated patients, 59 (98.3%) discontinued study treatment, usually because of disease progression (37 patients; 61.7%). Of the ITT population, 60 patients (98.4%) discontinued the study, including 53 patients (86.9%) who discontinued because of death, most commonly attributed to lung cancer.
Baseline demographics and disease characteristics were generally comparable between the trilaciclib and placebo arms, except that there were more male patients (68.8% versus 41.4%), more ex-US patients (56.3% versus 37.9%), more current smokers (40.6% versus 24.1%), and more patients with brain metastases (25.0% versus 17.2%) enrolled in the trilaciclib arm (Table 1).
Table 1.
Baseline demographics and disease characteristics
| Category | Trilaciclib prior to topotecan 1.5 mg/m2 (n = 32) | Placebo prior to topotecan 1.5 mg/m2 (n = 29) |
|---|---|---|
| Age, median, (min, max) years | 62 (47, 77) | 64 (47, 82) |
| Age group, n (%) | ||
| 18– < 65 years | 20 (62.5) | 18 (62.1) |
| ≥ 65 years | 12 (37.5) | 11 (37.9) |
| Gender, n (%) | ||
| Male | 22 (68.8) | 12 (41.4) |
| Female | 10 (31.3) | 17 (58.6) |
| Region, n (%) | ||
| US | 14 (43.8) | 18 (62.1) |
| Ex-US | 18 (56.3) | 11 (37.9) |
| ECOG PS, n (%) | ||
| 0/1 | 29 (90.6) | 27 (93.1) |
| 2 | 3 (9.4) | 2 (6.9) |
| Smoking history, n (%) | ||
| Never | 3 (9.4) | 2 (6.9) |
| Former | 16 (50.0) | 20 (69.0) |
| Current | 13 (40.6) | 7 (24.1) |
| Treatment line, n (%) | ||
| Second | 26 (81.2) | 24 (82.8) |
| Third | 6 (18.8) | 5 (17.2) |
| Sensitivity to first-line treatment, n (%) | ||
| Sensitive | 14 (43.8) | 13 (44.8) |
| Resistant | 18 (56.3) | 16 (55.2) |
| Brain metastases at baseline, n (%) | 8 (25.0) | 5 (17.2) |
| Baseline LDH, n (%) | ||
| ≤ ULN | 15 (46.9) | 15 (51.7) |
| > ULN | 16 (50.0) | 13 (44.8) |
| Missing | 1 (3.1) | 1 (3.4) |
| Weight loss ≥ 6 months prior to randomization, n (%) | ||
| No | 22 (68.8) | 21 (72.4) |
| Yes | 10 (31.3) | 8 (27.6) |
| Weight loss > 5% | 9 (90.0) | 6 (75.0) |
| Weight loss ≤ 5% | 1 (10.0) | 2 (25.0) |
ECOG PS Eastern Cooperative Oncology Group performance status, LDH lactate dehydrogenase, max maximum, min minimum, SCLC small cell lung cancer, ULN upper limit of normal, US United States
Myelopreservation
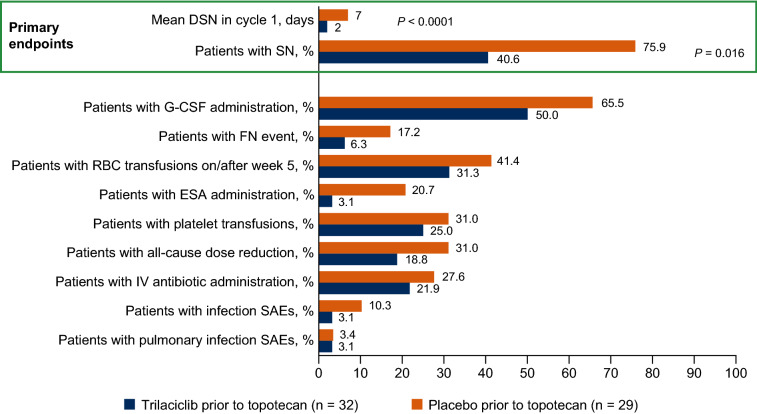
The addition of trilaciclib decreased CIM relative to placebo when administered prior to topotecan, as measured by statistically significant improvements in the primary endpoints of DSN in cycle 1 (P < 0.0001) and occurrence of SN (P = 0.016; Fig. 1 and Table S1). Fewer patients receiving trilaciclib required RBC and platelet transfusions, the use of G-CSFs and ESAs, and all-cause chemotherapy dose reductions compared with placebo (Fig. 1; Table S1).
Fig. 1.
Myelopreservation outcomes. DSN duration of severe neutropenia, ESA erythropoiesis-stimulating agent, FN febrile neutropenia, G-CSF granulocyte colony-stimulating factor, IV intravenous, RBC red blood cell, SAE serious adverse event, SN severe neutropenia
Patient Experience
PRO completion rates were high (> 80% in both arms) throughout the study. At baseline, mean PRO scores were higher in the placebo arm (indicating better HRQoL) than in the trilaciclib arm. Patients receiving trilaciclib consistently showed improvement or remained stable from baseline to the end of cycle 4 in all domains except emotional wellbeing, whereas patients receiving placebo showed deterioration (Fig. S2A). From baseline to the end of each of the first four cycles, a larger proportion of patients receiving trilaciclib had improvement and a smaller proportion had deterioration in fatigue subscale scores (symptoms and functional limitations) than in the placebo arm (Fig. S2B). Benefits with trilaciclib were seen for each measure of patient functioning and symptoms, in particular for fatigue, anemia symptoms, and functional limitations. Median time to deterioration for patients receiving trilaciclib was longer than for patients receiving placebo (HR range: 0.25–0.75; Fig. 2). The time to deterioration among patients receiving trilaciclib was approximately 5.5 months longer than placebo for functional wellbeing, 3 months longer for fatigue, and 2 months longer for Anemia-Trial Outcome Index.
Fig. 2.
Median time to confirmed deterioration in patient-reported outcomes. CI confidence interval, EWB emotional wellbeing, FACT-An Functional Assessment of Cancer Therapy-Anemia [An], FACT-G Functional Assessment of Cancer Therapy-General, FACT-L Functional Assessment of Cancer Therapy-Lung, FWB functional wellbeing, LCS lung cancer symptoms, NYR not yet reached, PWB physical wellbeing, SWB social wellbeing, TOI trial outcome index, TTD time to confirmed deterioration, Worsening decrease from baseline by a clinically meaningful threshold for two consecutive visits: ≤ 3 points for PWB, SWB, EWB, FWB, LCS, and fatigue; ≤ 6 points for FACT-L, lung TOI, and anemia TOI points; ≤ 7 points for FACT-G and FACT-An total scores
Safety
On average, patients in the trilaciclib and placebo arms completed five and four cycles of topotecan, respectively. Fewer patients receiving trilaciclib (18.8%) had per-protocol chemotherapy dose reductions compared with those receiving placebo (32.1%; Table S2). The incidence of dose delays was similar across the trilaciclib and placebo arms (65.6% versus 60.7%).
Almost all patients experienced ≥ 1 AE (Table 2). Compared with placebo, patients receiving trilaciclib had fewer high-grade (grade 3 and 4) AEs overall, including fewer high-grade hematologic AEs (Table 2). Hematologic AEs were the most commonly reported high-grade events (Table 2). Grade 3 or 4 FN AEs were reported in 6.3% of patients receiving trilaciclib compared with 17.9% of patients receiving placebo (Fig. 1). Among patients receiving trilaciclib, three (9.4%) were hospitalized for CIM or sepsis versus six patients (21.4%) receiving placebo (P = 0.1879). The incidence of hospitalization due to CIM or sepsis was 1.97/100 cycles with trilaciclib versus 9.73/100 cycles with placebo (Table S1).
Table 2.
Overall safety summary and most common adverse events (≥ 5 patients with any grade adverse event in either treatment arm)
| Trilaciclib prior to topotecan 1.5 mg/m2 (n = 32) |
Placebo prior to topotecan 1.5 mg/m2 (n = 28)* |
|
|---|---|---|
| Patients with any AE, n (%) | 32 (100) | 27 (96.4) |
| Patients with AE related to any study drug, n (%) | 30 (93.8) | 27 (96.4) |
| Trilaciclib-/placebo-related | 8 (25.0) | 12 (42.9) |
| Topotecan-related | 30 (93.8) | 27 (96.4) |
| Patients with AE leading to discontinuation, n (%) | 1 (3.1) | 7 (25.0) |
| Patients with any grade ≥ 3 AE | 28 (87.5) | 27 (96.4) |
| Patients with any grade ≥ 4 AE | 18 (56.3) | 21 (75.0) |
| Patients with grade ≥ 3 AE related to any study drug, n (%) | 25 (78.1) | 27 (96.4) |
| Trilaciclib-/placebo-related | 7 (21.9) | 6 (21.4) |
| Topotecan-related | 24 (75.0) | 27 (96.4) |
| Patients with grade ≥ 3 hematologic AE, n (%) | 26 (81.3) | 26 (92.9) |
| Patients with grade ≥ 4 hematologic AE, n (%) | 16 (50.0) | 21 (75.0) |
| Patients with any serious AE, n (%) | 12 (37.5) | 7 (25.0) |
| Patients with any serious AE related to any study drug, n (%) | 5 (15.6) | 6 (21.4) |
| Trilaciclib-/placebo-related | 1 (3.1) | 0 |
| Topotecan-related | 5 (15.6) | 6 (21.4) |
| Patients with AE leading to death, n (%) | 3 (9.4)† | 1 (3.6) |
| Most common AEs | Trilaciclib prior to topotecan 1.5 mg/m2 (n = 32) |
Placebo prior to topotecan 1.5 mg/m2 (n = 28)* |
||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Neutropenia | 24 (75.0) | 15 (46.9) | 7 (21.9) | 24 (85.7) | 4 (14.3) | 20 (71.4) |
| Thrombocytopenia | 20 (62.5) | 8 (25.0) | 9 (28.1) | 19 (67.9) | 5 (17.9) | 11 (39.3) |
| Anemia | 17 (53.1) | 9 (28.1) | 0 (0) | 24 (85.7) | 17 (60.7) | 0 (0) |
| Fatigue | 13 (40.6) | 2 (6.3) | 0 (0) | 10 (35.7) | 2 (7.1) | 0 (0) |
| Nausea | 9 (28.1) | 0 (0) | 0 (0) | 14 (50.0) | 1 (3.6) | 0 (0) |
| Pyrexia | 8 (25.0) | 0 (0) | 0 (0) | 5 (17.9) | 0 (0) | 0 (0) |
| Hypokalemia | 7 (21.9) | 0 (0) | 1 (3.1) | 5 (17.9) | 2 (7.1) | 0 (0) |
| Decreased appetite | 6 (18.8) | 1 (3.1) | 0 (0) | 5 (17.9) | 0 (0) | 0 (0) |
| Diarrhea | 5 (15.6) | 0 (0) | 0 (0) | 8 (28.6) | 0 (0) | 1 (3.6) |
| Leukopenia | 4 (12.5) | 1 (3.1) | 1 (3.1) | 9 (32.1) | 4 (14.3) | 3 (10.7) |
| Dyspnea | 4 (12.5) | 1 (3.1) | 0 (0) | 5 (17.9) | 2 (7.1) | 0 (0) |
| Cough | 3 (9.4) | 0 (0) | 0 (0) | 6 (21.4) | 0 (0) | 0 (0) |
| Vomiting | 2 (6.3) | 0 (0) | 0 (0) | 9 (32.1) | 1 (3.6) | 0 (0) |
| Dehydration | 2 (6.3) | 0 (0) | 0 (0) | 7 (25.0) | 1 (3.6) | 0 (0) |
| Febrile neutropenia | 2 (6.3) | 0 (0) | 2 (6.3) | 5 (17.9) | 2 (7.1) | 3 (10.7) |
| Dizziness | 2 (6.3) | 0 (0) | 0 (0) | 5 (17.9) | 0 (0) | 0 (0) |
AE adverse event
*One patient randomized to the placebo arm was not treated
†One AE that led to death was considered related to topotecan; none were considered related to trilaciclib
One patient in the trilaciclib arm had an AE leading to treatment discontinuation versus seven patients receiving placebo. Fatal AEs were reported in three patients in the trilaciclib arm (respiratory failure, acute respiratory failure, and cerebrovascular accident); none were considered related to trilaciclib. One patient in the placebo arm had a fatal AE (sepsis). One trilaciclib-related SAE was reported (infusion-related grade 3 thrombophlebitis), which was also considered by the investigator to be related to topotecan. Infusion-related reactions/injection site reactions or phlebitis AEs were reported in four patients in the trilaciclib arm and no patients in the placebo arm; all were grade 1 or 2 in severity.
Antitumor Efficacy
The ORR was comparable between the trilaciclib and placebo arms (16.7% [5/30 patients] versus 23.1% [6/26 patients]; P = 0.5494; Table S3). Median duration of response was numerically longer with trilaciclib (6.8 months) than with placebo (4.9 months), with overlapping CIs. Investigator-assessed PFS and OS were comparable between the trilaciclib and placebo arms; median PFS was 4.2 versus 4.2 months (HR [80% CI] 0.88 [0.61, 1.27]; P = 0.5886]), and median OS was 6.2 versus 6.5 months (HR 1.38 [0.95, 2.01]; P = 0.3377), respectively (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of the probability of progression-free survival and overall survival. a PFS in the ITT population. b OS in the ITT population. CI confidence interval, HR hazard ratio, ITT intent-to-treat, OS overall survival, PFS progression-free survival
Discussion
Data from this study indicate that trilaciclib demonstrates myelopreservation efficacy in previously treated patients with ES-SCLC, whose bone marrow was damaged by first- or second-line chemotherapy. Myelopreservation benefits manifested as statistically significant, clinically meaningful improvements in the primary endpoints of DSN in cycle 1 and occurrence of SN, consistent with the results of the previous phase II trial of trilaciclib in patients who received E/P chemotherapy for newly diagnosed ES-SCLC [18]. These measures are clinically relevant since the severity and duration of SN are associated with an increased risk of FN, infection, IV antibiotic use, and hospitalizations [24–26]. Indeed, consistent with the significant reduction in DSN in cycle 1, and occurrence of SN, there was an approximately threefold decrease in the occurrence of FN AEs among patients receiving trilaciclib compared with placebo, although the total number of events was small. Patients receiving trilaciclib also experienced less chemotherapy-induced anemia, consistent with the observation that fewer patients receiving trilaciclib needed RBC transfusions and ESA administrations. This finding is also clinically meaningful; not only does anemia negatively impact patients’ HRQoL, but it is also associated with decreased survival, decreased tumor response, delays in therapy, and reduced patient compliance and therefore contributes to considerable morbidity and mortality among patients with cancer [7, 27]. Additional myelopreservation endpoints also consistently favored trilaciclib over placebo, with improvements observed in the use of other supportive care measures, namely G-CSF and ESA administration, and platelet transfusions. This finding is particularly pertinent, as current supportive care interventions for myelosuppression are associated with additional risks, such as bone pain with G-CSF, thromboembolic events with ESAs, and hemolytic reactions with platelet transfusions [11, 12, 28]. Also important is the finding that trilaciclib reduced the occurrence of chemotherapy dose reductions compared with placebo, allowing the standard dose of topotecan to be maintained. Of note, > 50% of patients diagnosed with SCLC are aged > 65 years [29]. Older patients often present with additional comorbidities, meaning they are particularly vulnerable to CIM and more likely to experience clinically significant side effects leading to clinical intervention and delayed chemotherapy treatment and/or dose reductions. The proactive management of CIM in elderly patients with SCLC is therefore essential to ensure delivery of standard-of-care chemotherapy regimens while improving the patient experience [30].
Patients with ES-SCLC who received trilaciclib prior to topotecan had a better experience receiving chemotherapy than patients receiving placebo. PRO assessments, using validated instruments, demonstrated that trilaciclib administered prior to topotecan resulted in meaningful delays in deterioration and even showed signs of improvement in fatigue, as well as other symptoms and functional limitations associated with cancer and CIM. Given the poor prognosis associated with relapsed SCLC, some patients may consider improved HRQoL a more important therapeutic goal than traditional efficacy outcomes [31]. The benefit of trilaciclib was particularly apparent in endpoints associated with anemia and fatigue, providing further evidence that trilaciclib may reduce the burden of CIM and its associated symptoms among patients with ES-SCLC. The fact that these data were collected in a randomized, placebo-controlled, double-blind study, with limited missing data, supports the robustness of these findings.
In line with previous findings [18], an improved overall safety profile for topotecan was evidenced by a reduction in high-grade hematologic AEs (neutropenia and anemia), which are commonly associated with CIM, providing further evidence for the myelopreservation effects of trilaciclib. Rates of thrombocytopenia, particularly high-grade events, were reported at a similarly low frequency in the trilaciclib and placebo arms. Among patients receiving trilaciclib, there was a 56% decrease in the number of patients hospitalized for CIM or sepsis compared with placebo (9.4% versus 21.4%). No discontinuations or deaths due to AEs were considered to be related to trilaciclib treatment. AEs of special interest with trilaciclib were primarily low grade and included injection-site reactions and phlebitis/thrombophlebitis.
Differences in measures of antitumor efficacy (ORR, PFS, and OS) between trilaciclib and placebo were nonsignificant; however, for OS, there was a trend for the HRs to favor placebo for both the ITT population and most subgroups. The trend appears to reflect an imbalance between prognostic factors for antitumor efficacy between the treatment arms (Supplementary Methods; Table S4). Compared with the placebo arm, for example, more patients in the trilaciclib arm were male and were current smokers, both of which are poor prognostic factors for survival [32, 33]. Survival outcomes in this study were similar to those seen in other studies of topotecan, where median survival times rarely exceed 6 months [2, 3]. The data reiterate the dismal prognosis of patients with relapsed or refractory ES-SCLC and highlight the urgent need for more effective treatment options in this setting. In June 2020, lurbinectedin was approved for the treatment of adult patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy. Approval was based on efficacy data from a single-arm, phase II basket trial of 105 patients treated with IV lurbinectedin every 21 days [34]. In this study, the ORR (primary endpoint) was 35.2%, with a median response duration of 5.1 months. The most common grade 3/4 AEs were hematologic abnormalities. Eleven patients had SAEs, including five patients with neutropenia and five patients with FN [34]. Considering the efficacy and safety profile of lurbinectedin, it would be interesting to investigate whether trilaciclib might be advantageously combined with lurbinectedin, and/or other emerging agents, to improve patient outcomes. Importantly, however, comparable antitumor efficacy outcomes between the two treatment arms corroborate previous findings that trilaciclib does not negatively impact the antitumor efficacy of chemotherapy [18].
A limitation of this study is that, due to the small sample size, only large differences in OS would be detected. Therefore, although trilaciclib reduced the occurrence of chemotherapy dose reductions compared with placebo, detecting the impact of any potential differences in topotecan dose intensity on survival outcomes would be limited. Studies in patients with SCLC have shown, however, that increasing the relative dose intensity of chemotherapy beyond the standard of care rarely translates into significant improvements in response rates or survival [35]. The small sample size may have also reduced the ability to observe statistically significant differences in secondary myelopreservation measures, such as the occurrence of FN AEs, infection SAEs, and IV antibiotic use. However, large treatment effects were not expected for these endpoints given that patients in both arms could receive supportive care interventions, with the exception of prophylactic G-CSF in cycle 1. Further investigation of the effects of trilaciclib on CIM and antitumor efficacy in larger studies is needed, including in tumor types or settings that may be more responsive to dose intensification. Studies to further delineate the effects of trilaciclib compared with other supportive care interventions, such as prophylactic G-CSF, would also be of interest to establish the real-world impact of trilaciclib on CIM.
Conclusions
Overall, these data extend the evidence for the clinical benefits of trilaciclib as a first-in-class myelopreservation agent for patients with SCLC treated with myelotoxic chemotherapy. The study demonstrates that trilaciclib reduces the risk of CIM in patients with HSPCs that have been damaged by prior lines of cytotoxic chemotherapy who are being treated with a chemotherapy regimen associated with significant hematologic toxicity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank and acknowledge all the patients, their families, and study personnel for participating in the study.
Funding
This study and medical writing support, along with the Rapid Service and Open Access fees, were funded by G1 Therapeutics, Inc. (Research Triangle Park, NC).
Medical Writing Assistance
Medical writing assistance was provided by Fiona Scott, contracted by Alligent Europe (Envision Pharma Group), funded by G1 Therapeutics, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
The submitted work was sponsored and funded by G1 Therapeutics, Inc., Research Triangle Park, NC, USA. Jie Xiao and Joyce M. Antal are employees of, and have stock ownership in, G1 Therapeutics, Inc. Shannon R. Morris was a paid employee of, and had stock ownership in, G1 Therapeutics, Inc., at the time of study completion and manuscript preparation and submission, and is currently a paid consultant to G1 Therapeutics, Inc., via SRM Consulting LLC. Outside of the submitted work, Renata Ferrarotto reports personal fees from Regeneron-Sanofi, Ayala Pharma, Klus Pharma, Medscape, Cellestia Biotech, Carevive and Prelude, and grants from AstraZeneca, Merck, Genentech, Pfizer, Oropharynx Program Stiefel clinical trials, ASCO Career Development Award, and the MD Anderson Khalifa Award. Maen A. Hussein is on the speaker bureau for Bristol-Myers Squibb, Incyte, and Pfizer. Lowell L. Hart reports personal consulting fees from Genentech, Novartis, Lilly, Nanostring, Astra Zeneca and Daiichi Sankyo outside of the scope of the current work. Zoran G. Andric, J. Thaddeus Beck, Janakiraman Subramanian, Davorin Z. Radosavljevic, Bojan Zaric, Wahid T. Hanna, Raid Aljumaily, Taofeek K. Owonikoko, and Didier Verhoeven have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. The protocol and all study-related materials were approved by the institutional review board or independent ethics committee of each participating center. All patients provided written informed consent.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract. 2018;14(6):359–366. doi: 10.1200/JOP.18.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quoix E. Topotecan in the treatment of relapsed small cell lung cancer. Oncol Targets Ther. 2008;1:79–86. doi: 10.2147/OTT.S3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin A, Kalemkerian GP. Treatment options for relapsed small-cell lung cancer: What progress have we made? J Oncol Pract. 2018;14(6):369–370. doi: 10.1200/JOP.18.00278. [DOI] [PubMed] [Google Scholar]
- 4.von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 6.Smith RE. Trends in recommendations for myelosuppressive chemotherapy for the treatment of solid tumors. J Natl Compr Canc Netw. 2006;4(7):649–658. doi: 10.6004/jnccn.2006.0056. [DOI] [PubMed] [Google Scholar]
- 7.Lyman GH. Chemotherapy dose intensity and quality cancer care. Oncology (Williston Park, NY) 2006;20(14 Suppl 9):16–25. [PubMed] [Google Scholar]
- 8.Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93(3):203–210. doi: 10.1016/j.critrevonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Taylor SJ, Duyvestyn JM, Dagger SA et al. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci Transl Med. 2017;9(402). [DOI] [PubMed]
- 10.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg N, Heal JM, Phillips GL. Platelet transfusions: trigger, dose, benefits, and risks. F1000 Med Rep. 2010;2:5. [DOI] [PMC free article] [PubMed]
- 12.Bohlius J, Bohlke K, Lazo-Langner A. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. J Oncol Pract. 2019;15(7):399–402. doi: 10.1200/JOP.19.00111. [DOI] [PubMed] [Google Scholar]
- 13.He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387):eaal3986. doi: 10.1126/scitranslmed.aal3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy-induced myelosuppression. Mol Cancer Ther. 2016;15(5):783–793. doi: 10.1158/1535-7163.MCT-15-0775. [DOI] [PubMed] [Google Scholar]
- 15.Lai A, Sorrentino JA, Dragnev K, et al. CDK4/6 inhibition enhances anti-tumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J Immunother. 2020;8(2):e000847. doi: 10.1136/jitc-2020-000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10:1758835918793326. doi: 10.1177/1758835918793326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–1621. doi: 10.1093/annonc/mdz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 20.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 21.Butt Z, Webster K, Eisenstein AR, et al. Quality of life in lung cancer: the validity and cross-cultural applicability of the Functional Assessment of Cancer Therapy-Lung scale. Hematol Oncol Clin N Am. 2005;19(2):389–420. doi: 10.1016/j.hoc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the functional assessment of cancer therapy-lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55(3):285–295. doi: 10.1016/S0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manag. 2002;24(6):547–561. doi: 10.1016/S0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A. Management of chemotherapy induced neutropenia – an unmet clinical need. Am J Biomed Sci Res. 2019;4:313–318. doi: 10.34297/AJBSR.2019.04.000823. [DOI] [Google Scholar]
- 25.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Klippel Z, Shih X, Reiner M, Wang H, Page JH. Relationship between severity and duration of chemotherapy-induced neutropenia and risk of infection among patients with nonmyeloid malignancies. Support Care Cancer. 2016;24(10):4377–4383. doi: 10.1007/s00520-016-3277-0. [DOI] [PubMed] [Google Scholar]
- 27.Jamil K, Kalyani P, Perimi R, Kameshwari SV. Assessment of severity of anemia and its effect on the quality of life (QOL) of patients suffering with various types of neoplasia. Biol Med (Aligarh) 2009;1(3):63–72. [Google Scholar]
- 28.Xu H, Gong Q, Vogl FD, Reiner M, Page JH. Risk factors for bone pain among patients with cancer receiving myelosuppressive chemotherapy and pegfilgrastim. Support Care Cancer. 2016;24(2):723–730. doi: 10.1007/s00520-015-2834-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7(1):1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balducci L. Myelosuppression and its consequences in elderly patients with cancer. Oncology (Williston Park, NY) 2003;17(11 Suppl 11):27–32. [PubMed] [Google Scholar]
- 31.Eckardt JR. Second-line treatment of small-cell lung cancer. The case for systemic chemotherapy. Oncology (Williston Park, NY). 2003;17(2):181–8, 191; discussion 91–2, passim. [PubMed]
- 32.Liu X, Jiang T, Li W, et al. Characterization of never-smoking and its association with clinical outcomes in Chinese patients with small-cell lung cancer. Lung Cancer. 2018;115:109–115. doi: 10.1016/j.lungcan.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–953. doi: 10.2147/CMAR.S187317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645–654. doi: 10.1016/S1470-2045(20)30068-1. [DOI] [PubMed] [Google Scholar]
- 35.Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.