Abstract
Introduction
Few studies have evaluated whether the pharmacokinetics of N-acetyl-cysteine (NAC) are different in Chinese and Caucasian individuals.
Methods
This single- and multiple-dose, single-centre, open-label, phase I clinical study was conducted in healthy adult volunteers. All participants received oral NAC 600-mg uncoated tablets, which were administered first as a single dose and, following a 48-h wash-out period, twice daily for 3 days. Blood and urine were collected after single- and multiple-dose NAC administration. Adverse event (AE) data were collected throughout the study.
Results
Fifteen Chinese and 15 Caucasian (mostly Italian) individuals (males 66.7%, mean age 36.8 years) participated in the study. Pharmacokinetic characteristics of NAC were similar in the two cohorts. Following both single- and multiple-dose administration, plasma concentration of NAC increased rapidly, reaching a peak at approximately 1.0 h. Maximum plasma concentration and extent of exposure were higher after multiple doses than after a single dose. The accumulation ratio was relatively consistent in both Chinese (mean ± standard deviation 1.5 ± 0.4) and Caucasian (1.4 ± 0.2) participants. The half-life was 15.4 h in Chinese and 18.7 h in Caucasian participants, and the fraction of NAC excreted in urine in the 36 h following administration was 3.7% in Chinese and 3.8% in Caucasian participants. Two Caucasian participants had a total of 3 AEs (headache, presyncope and dysmenorrhoea). No AEs occurred in Chinese participants.
Conclusions
The pharmacokinetic characteristics of NAC are similar in healthy Chinese and Caucasian individuals after single and repeated administration. NAC has a favourable tolerability profile.
Keywords: Caucasian, Chinese, Healthy volunteer, N-acetylcysteine, Pharmacokinetics, Phase I
Key Summary Points
| The pharmacokinetic characteristics of N-acetyl-cysteine are similar in healthy Chinese and Caucasian volunteers after the administration of both single and multiple doses. |
| N-acetyl-cysteine was well tolerated in both groups of participants. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.13102892.
Introduction
For several decades, N-acetylcysteine (NAC) has been used in clinical practice worldwide in a variety of indications: as a mucolytic, in acetaminophen intoxication, doxorubicin-induced cardiotoxicity and in other cardiovascular, respiratory and psychiatric disorders [1]. NAC appears to exert its biological activity by acting as a precursor of glutathione, a ubiquitous compound with anti-oxidant properties that is involved in the metabolism of xenobiotics, signal transduction and gene expression, regulation of the cell cycle and apoptosis, and prostaglandin and leukotriene synthesis, among other processes [1, 2]. NAC can be administered intravenously as well as orally. Oral dosage forms include capsules, granulate and effervescent, fast-dissolving and slow-release tablets. In the present study, NAC uncoated tablets were investigated.
The pharmacokinetics of NAC have been extensively studied in Caucasian patients and healthy volunteers [3–9]. Following oral administration, NAC is rapidly absorbed in the gastrointestinal tract, and the maximum plasma concentration (Cmax) is reached after 1–2 h [6]. The terminal half-life (t½) of NAC is approximately 6 h [6].
However, few studies of NAC have been conducted in Chinese individuals and, to our knowledge, only one in healthy volunteers [10]. Furthermore, no study has compared the pharmacokinetics of NAC in Chinese and Caucasian individuals. Genetic differences between populations can affect both metabolism and responses to drugs [11]. Therefore, this study was designed to evaluate the plasma concentrations, pharmacokinetic parameters and safety/tolerability of single and multiple doses of NAC (Fluimucil® 600-mg tablets, Zambon, Italy) in healthy Chinese and Caucasian individuals.
Methods
Study Design and Participants
This was a single- and multiple-dose, single-centre, open-label, phase I clinical study. The study was performed at the Phase I Unit of CROSS Research Arzo, Switzerland.
Healthy Chinese and Caucasian men and women aged 18–50 years with a bodyweight of ≥ 45 kg and a body mass index (BMI) of 18–29 kg/m2 who provided written informed consent for participating in the study were included. Additional inclusion criteria were: systolic blood pressure (BP) 100–139 mmHg, diastolic BP 50–89 mmHg, heart rate (HR) 50–90 bpm, good comprehension and use of the written and spoken Italian language. In addition, women of childbearing potential were required to use an effective method of contraception, and all women had to have a negative pregnancy test result at screening and Day − 1.
Individuals were excluded if they had clinically significant abnormalities on electrocardiogram, physical examination or laboratory analyses; a history of significant disease; positive test for HIV, hepatitis B, hepatitis C or Treponema pallidum; hypersensitivity to any ingredient of the investigational drug or a history of allergic reactions; surgery (except diagnostic surgery) within 2 months before screening; used any medications, including over-the-counter, traditional Chinese and herbal remedies, within 2 weeks before the start of the study (hormonal contraceptives for women were allowed); had vaccination within 4 weeks before screening; participated in a clinical trial within 3 months of the study; donated blood within 3 months before the study; had a history of drug, alcohol (> 1 drink/day for women and > 2 drinks/day for men), caffeine (> 5 cups coffee/tea/day) or tobacco (≥ 10 cigarettes/day) abuse, had a positive result of urine drug assay either at screening or upon admission or tested positive at the breath alcohol assay upon entrance to the clinic; consumed < 1600 or > 3500 kcal/day or changed their diet substantially in the 4 weeks before the study; or were vegetarian.
Study Drug and Procedures
Participants were required to remain at the study site from the evening of Day − 1 until the morning of Day 7.
All participants received NAC 600-mg uncoated tablets (Fluimucil®; batch number 20039498, expiry December 2021). NAC was administered orally with 150 mL of still mineral water. On Day 1, a single tablet was administered at 08:00 ± 1 h under fasting conditions. After a wash-out period of 48 h, NAC was administered twice daily (at 08:00 ± 1 h before breakfast and at 20:00 ± 1 h before dinner) on Days 3, 4 and 5. On Day 6, a single tablet was administered at 08:00 ± 1 h under fasting conditions. Steady state of NAC was expected to be attained after seven consecutive doses administered up to the morning of Day 6 with a τ of 12 h on the basis of mean half-life values of up to 14 h found in previous studies of the same dosage form.
On Day 1 and Day 5, a standardised light dinner was served. On Day 1 and Day 6, participants were not allowed any food or drinks (except water) for at least 10 h before, and for 5 h after, NAC administration. Water was allowed ad libitum, except for 1 h before and 1 h after NAC administration. During the 4 h following NAC administration, when not involved in study procedures, participants remained seated and were not allowed to lie down.
On Day 1 and Day 6, standardised lunch and dinner were served at approximately 5 h and 12 h after NAC administration, respectively. On Days 2, 3, 4 and 5, standardised breakfast, lunch and dinner were served at approximately 09:00, 13:00 and 20:00, respectively. One cup of tea or coffee was allowed after each meal only. Any other food or drinks containing xanthines (e.g. chocolate) were forbidden for the duration of stay at the study site. Alcohol and grapefruit were forbidden from 24 h before NAC administration on Day 1 until the end of the study. A single cigarette after each meal was allowed during the stay at the study site. Routine daily activities were encouraged, but hazardous, strenuous or athletic activities were not permitted.
Assessments
In order to measure pharmacokinetic parameters, 6 mL of blood was collected from a vein in the forearm using an indwelling catheter with a switch valve. Blood for pharmacokinetic analyses was collected at 20:00 ± 1 h on Day 1; before NAC administration, and 5, 15, 30 and 45 min and 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 16, 24 and 36 h after NAC administration on Day 1 and Day 2; and before NAC administration, and 5, 15, 30 and 45 min and 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h after NAC administration on Day 6 and Day 7. Urine was collected before the end of each of the following intervals: 0–4 h, 4–8 h, 8–12 h, 12–24 h and 24–36 h after NAC administration on Day 1 and Day 2, and 0–4 h, 4–8 h, 8–12 h and 12–24 h on Day 6 and Day 7. The concentrations of NAC in plasma and urine were determined at Ardena Bioanalytical Laboratory, The Netherlands.
Samples were incubated with dithiotreitol to reduce intermolecular disulphide bonds. NAC and its isotope-labelled internal standard (d3-N-acetylcysteine) were extracted from human Li-heparin plasma by protein precipitation using acetonitrile, and from human urine using liquid–liquid extraction. Extracted samples were injected into the chromatographic system using isocratic elution on an Alltima HP hydrophilic interaction chromatography (HILIC) column for plasma (W.R. Grace, Columbia, MD, USA), and on an XBridge ethylene bridged hybrid HILIC column (Waters, Milford, MA, USA) for urine. The mass spectrometer was equipped with a Turbo Ion Spray interface and operated in the negative ion mode.
The bioanalytical methods used for the quantification of NAC in human Li-heparin plasma and urine were validated for samples stored at ≤ − 70 °C. A full validation of the methods was performed according to the current guidelines. The long-term stability of NAC was tested and the results showed that NAC stored frozen at ≤ − 18 °C or ≤ − 70 °C is stable for up to 65 d ays in plasma and 71 days in urine. Both liquid chromatography with tandem mass spectrometry (LC–MS/MS) methods produced accurate and precise results. Validation studies showed that the absolute biases for the quality control (QC) samples at the low, medium and high levels were − 0.6%, − 5.0% and − 5.6% in plasma, and 4.6%, − 9.0% and − 7.0% in urine, respectively. The precision results [expressed as total coefficients of variation (CV)] were as follows: 5.3%, 2.1% and 1.9% in plasma, and 4.5%, 1.6% and 3.1% in urine, for the low, medium and high QC samples, respectively. Intra-run CVs (repeatability) were 3.0%, 1.0% and 1.0% in plasma, and 4.5%, 2.1% and 2.3% in urine, for the low, medium and high QC samples, respectively. The calibration range covered 20.0–5000 ng/mL in human plasma and 0.100–20.0 µg/mL in human urine.
The pharmacokinetic parameters assessed after single dose administration on Day 1 were: maximum concentration (Cmax), time to Cmax (tmax), terminal elimination rate constant (λz, calculated using log-linear regression from at least three points), area under the concentration–time curve (AUC) until time t (AUC0–t, calculated using the trapezoidal method), AUC extrapolated to infinity (AUC0–∞, calculated as AUC0–t + Ct/λz, where Ct is the last measurable drug concentration), proportion of residual AUC extrapolated to infinity (AUCextra, calculated as 100 × [Ct/λz]/AUC0–∞), AUC from administration to 12 h on Day 1 (AUC0–12, calculated using the trapezoidal method), t½ (calculated as ln2/λz), volume of distribution [Vz, calculated as dose/(AUC0–∞ × λz)] and total body clearance (CLt, calculated as dose/AUC0–∞) of NAC in plasma, and total amount excreted until time t (Ae0–t), fraction excreted until time t (Fe0–t) and renal clearance (CLr, Ae0–t/AUC0–∞) of NAC in urine. The pharmacokinetic parameters assessed after multiple doses on Day 6 and Day 7 were: Cmax at steady state (Cmax,ss), tmax at steady state (tmax,ss), minimum concentration at steady state (Cmin,ss), AUC0–t at steady state (AUC0–t,ss), AUC during the interval τ at steady state (AUCτ,ss, calculated using the trapezoidal method), average concentration at steady state (Cave,ss, calculated as AUCt,ss/t), accumulation ratio (ARAUC, calculated as AUCτ,ss/AUC0–12) and peak–trough fluctuation [PTF, calculated as (Cmax,ss − Cmin,ss)/Cave,ss × 100)] of NAC in plasma, and Ae0–t at steady state (Ae0–t,ss) of NAC in urine.
The plasma pharmacokinetic parameters were calculated using baseline-corrected concentrations because quantifiable levels of endogenous NAC were detected at both baseline measurements (evening of Day 1 and pre-dose on Day 1). For each participant, an individual mean baseline value was calculated as the arithmetic mean of the two measured baseline concentrations, and this mean value was subtracted from each concentration value measured on Days 1–2 and 6–7. The quality of log-linear regression and, consequently, the reliability of the extrapolated pharmacokinetic parameters (namely λz, t½, AUC0–∞, AUCextra, Vz, CLr and CLt) was demonstrated by a determination coefficient, R2 ≥ 0.8. Individual extrapolated parameters, when considered unreliable, were reported as not calculated. In the pharmacokinetic analysis, the scheduled sampling times were used for calculation, as the deviations observed were considered not relevant for the analysis purposes, except for one sampling time for one participant. As per the study protocol, deviations from the scheduled sampling times were recommended not to exceed predetermined ranges.
Safety assessments included treatment-emergent adverse events (AEs), vital signs (BP and HR), bodyweight, physical examinations and laboratory parameters.
Statistical Methods
No formal sample size calculation was performed. Instead, we estimated the number of participants that would be sufficient for the descriptive purposes of the study.
Results are presented using descriptive statistics, including the number of observations, geometric mean (for pharmacokinetic parameters only), arithmetic mean, standard deviation (SD), coefficient of variation, minimum, median and maximum for quantitative variables, and frequency for qualitative variables. The pharmacokinetic analysis was performed using Phoenix WinNonlin® v.6.3 (Pharsight) and SAS® v.9.3 TS1M1 for Windows®.
Ethics
The study protocol was approved by an independent ethics committee (Comitato Etico Cantonale, Canton Ticino, Switzerland). The study was conducted in accordance with the protocol and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines, Declaration of Helsinki and applicable Swiss and European Union regulations.
Results
A total of 30 participants, 15 Asian (Chinese) and 15 Caucasian (mainly Italian), were enrolled. All participants received the planned study treatment and completed the study per protocol.
The majority of Chinese participants were male (80.0%), while the proportions of male and female Caucasian participants were roughly equal (53.3% and 46.7%, respectively) (Table 1). On average, Chinese participants were older than their Caucasian counterparts (mean age 39.8 years vs. 33.8 years, respectively).
Table 1.
Baseline characteristics
| Asian (n = 15) |
Caucasian (n = 15) |
Overall (n = 30) |
|
|---|---|---|---|
| Sex, n (%) | |||
| Male | 12 (80.0) | 8 (53.3) | 20 (66.7) |
| Female | 3 (20.0) | 7 (46.7) | 10 (33.3) |
| Age, years | |||
| Mean ± SD | 39.8 ± 9.7 | 33.8 ± 9.6 | 36.8 ± 10.0 |
| Median (range) | 44.0 (19–50) | 36.0 (18–46) | 39.0 (18–50) |
| Height, cm | |||
| Mean ± SD | 170.9 ± 7.7 | 169.9 ± 7.7 | 170.4 ± 7.6 |
| Median (range) | 171.0 (158–189) | 171.0 (154–182) | 171.0 (154–189) |
| Weight, kg | |||
| Mean ± SD | 64.7 ± 6.8 | 66.3 ± 12.3 | 65.5 ± 9.8 |
| Median (range) | 64.7 (50.4–75.5) | 64.9 (47.7–88.6) | 64.8 (47.7–88.6) |
| BMI, kg/m2 | |||
| Mean ± SD | 22.1 ± 2.0 | 22.8 ± 3.1 | 22.5 ± 2.6 |
| Median (range) | 21.4 (19.2–25.8) | 21.8 (19.3–27.9) | 21.6 (19.2–27.9) |
BMI body mass index, SD standard deviation
Concentration of NAC in Plasma
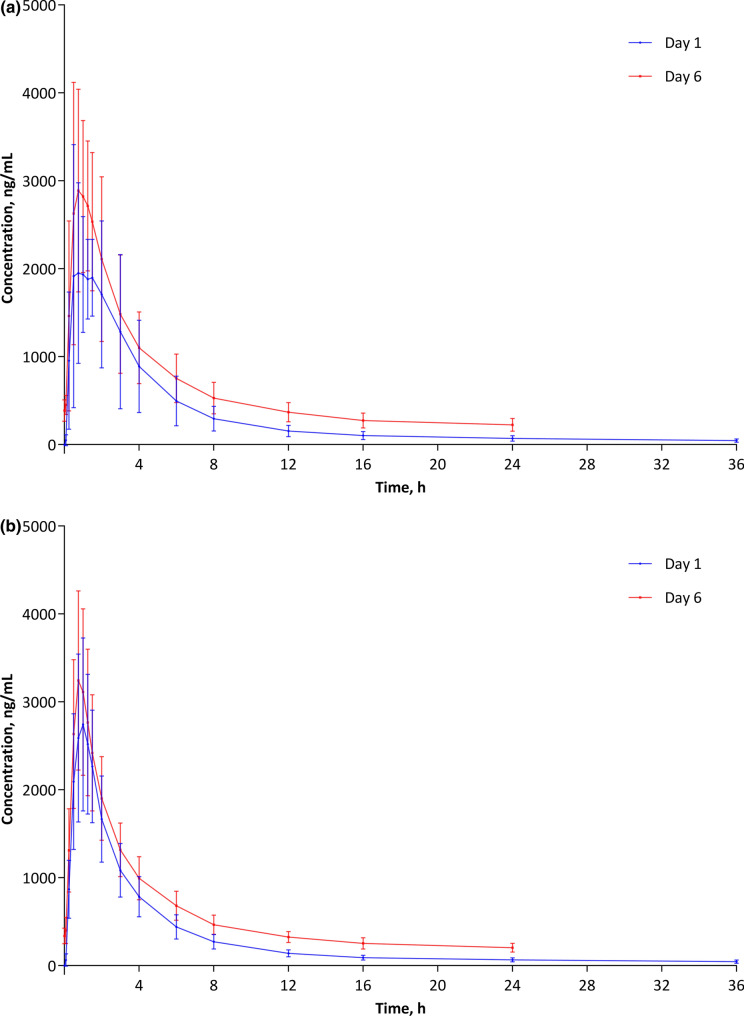
Mean baseline-corrected plasma concentrations of NAC on Day 1 and Day 6 are shown in Fig. 1a (Chinese participants) and Fig. 1b (Caucasian participants). After a single dose (Day 1), the plasma concentration of NAC increased rapidly in both groups. In Chinese participants, the mean ± SD maximum observed plasma concentration of NAC was 1950.9 ± 1026.1 ng/mL at 45 min after NAC administration, while, in Caucasian participants, the maximum observed plasma concentration was 2743.9 ± 985.2 ng/mL at 1.0 h. After that, plasma concentrations of NAC declined rapidly in both groups. At steady state (Day 6), pre-dose baseline-corrected NAC plasma concentrations were approximately ten times higher than on Day 1 (mean ± SD 36.4 ± 11.5 ng/mL vs. 4.22.5 ± 130.5 ng/mL in Chinese and 39.7 ± 13.9 ng/mL vs. 377.7 ± 92.7 ng/mL in Caucasian participants). Plasma concentration–time curves at steady state were otherwise similar to those after a single dose.
Fig. 1.
Baseline-corrected plasma concentration of N-acetyl-cysteine: a Chinese participants; b Caucasian participants
Pharmacokinetic Parameters
The elimination phase after the single NAC administration could not be defined for two Caucasian participants according to the established condition (quality of extrapolated pharmacokinetic parameters as demonstrated by a determination coefficient, R2 < 0.8), and therefore λz, t½, AUC0–∞, AUCextra, Vz and CLt for these individuals were not calculated.
Mean calculated Cmax was similar in Chinese and Caucasian participants both after a single dose of NAC and at steady state (Table 2). In Chinese participants, mean calculated Cmax was approximately 1.3 times higher at steady state than after a single dose, while, for Caucasians, it was approximately 1.2 times higher. After a single dose, median tmax was 1.25 h in Chinese participants and 1.00 h in Caucasian participants. At steady state, median tmax was 1.00 h in both groups. Mean AUC0–t and AUCτ,ss were also similar in Chinese and Caucasian participants. As expected, the extent of exposure increased at steady state relative to single dose administration. However, mean ARAUC was quite low in both groups (1.5 in Chinese and 1.4 in Caucasians). Mean ± SD t½ was 15.4 ± 3.5 h in Chinese and 18.7 ± 7.2 h in Caucasian participants. Mean Vz was higher than 1000 L in both groups (1250.0 L in Chinese and 1400.8 L in Caucasian participants).
Table 2.
Baseline-corrected pharmacokinetic parameters of N-acetylcysteine after single- (Days 1–2) and multiple-dose (Days 6–7) administration
| Days 1–2 | Days 6–7 | ||||
|---|---|---|---|---|---|
| Asian (n = 15) |
Caucasian (n = 15) |
Asian (n = 15) |
Caucasian (n = 15) |
||
| Cmax, ng/mL | 2711.7 ± 1270.3 | 2895.3 ± 842.4 | Cmax,ss, ng/mL | 3513.1 ± 1142.6 | 3419.3 ± 920.0 |
| tmax, h | 1.3 (0.5–3.0) | 1.0 (0.8–1.5) | tmax,ss, h | 1.0 (0.5–2.0) | 1.0 (0.5–1.5) |
| AUC0-t, ng h/mL | 10,657.5 ± 4108.7 | 10,614.0 ± 3114.5 | AUC0-t,ss, ng h/mL | 15,804.4 ± 4770.8 | 14,702.0 ± 3409.8 |
| AUC0-12, ng h/mL | 8792.1 ± 3353.0 | 8895.5 ± 2578.8 | AUCτ,ss, ng h/mL | 12,543.2 ± 3824.2 | 11,737.6 ± 2782.6 |
| AUC0-∞, ng h/mL | 11,691.8 ± 4623.6 | 12,031.1 ± 4119.0a | Cave,ss, ng/mL | 1045.3 ± 318.7 | 978.1 ± 231.9 |
| t½, h | 15.4 ± 3.5 | 18.7 ± 7.2a | Cmin,ss, ng/mL | 354.9 ± 109.6 | 306.9 ± 74.7 |
| Vz, L | 1250.0 ± 474.9 | 1400.8 ± 508.5a | PTF, % | 303.6 ± 58.6 | 318.0 ± 38.8 |
| CLt, L/h | 56.9 ± 16.2 | 56.0 ± 20.1a | ARAUC | 1.5 ± 0.4 | 1.4 ± 0.2 |
All data are presented as mean ± SD, except for tmax, which is presented as median (range)
AUC area under the curve, ARAUC accumulation ratio based on AUC, CL clearance, PTF peak-trough fluctuation, SD standard deviation
an = 13
The mean Ae0–t was small (approximately 2% of the dose) and relatively constant after single and multiple doses of NAC (Table 3). Differences between Chinese and Caucasian participants were minor. The mean Fe0–t was also relatively low and similar in Chinese and Caucasian participants (Table 3).
Table 3.
Total amount of N-acetylcysteine excreted in urine after single- (Days 1–2) and multiple-dose (6–7) administration, and fraction of N-acetylcysteine excreted in urine and renal clearance after single- (Days 1–2) and multiple-dose (6–7) administration
| Days 1–2 | Days 6–7 | ||||
|---|---|---|---|---|---|
| Asian (n = 15) |
Caucasian (n = 15) |
Asian (n = 15) |
Caucasians (n = 15) |
||
| Ae0–t, µg | 21,963.2 ± 7331.4 | 22,775.4 ± 6191.0 | Ae0–t,ss, µg | 21,599.4 ± 7846.0 | 20,523.8 ± 5592.7 |
| Fe0–t, % | 3.7 ± 1.2 | 3.8 ± 1.0 | NA | – | – |
| CLr, L/h | 2.0 ± 0.4 | 2.0 ± 0.6a | NA | – | – |
All data are presented as mean ± SD
CL clearance, NA not applicable, SD standard deviation
an = 13
Safety
During the study, a total of three AEs occurred in two Caucasian participants (6.7%, n = 2/30). No AEs occurred in Chinese participants. These AEs were headache, presyncope and dysmenorrhoea, were mild or moderate in intensity, and none were considered to be related to study treatment. All AEs resolved and none led to study discontinuation. No serious AEs occurred during the study.
Study treatment had no relevant effects on BP, HR, bodyweight or laboratory parameters.
Discussion
The results of the present study show that the single-dose and steady-state pharmacokinetics of NAC are similar in healthy Chinese and Caucasian individuals. Differences in the rate and extent of exposure observed between the two groups were not considered to be clinically significant.
Cmax was reached approximately 1.0 h after single and multiple doses of NAC. Predictably, Cmax and extent of exposure were higher at steady state than after a single dose. However, the accumulation ratio was relatively low. Mean Vz calculated in the present study was relatively high (> 1000 L), indicating that the drug was able to reach deep tissue compartments. Mean CLt was also relatively high (> 50 L/h), explaining the rapid decline in plasma concentrations in the first 6–8 h after administration. On the other hand, the relatively long t½ (15.36 h for Chinese and 18.69 h for Caucasian participants) is consistent with the endogenous nature of NAC and likely explains the slow terminal elimination. This is supported by the findings of a study conducted in patients with respiratory disorders which showed that, after an oral administration of 100 mg of 35S-labelled NAC, plasma radioactivity concentrations remained high after 24 h, and that approximately 22% of radioactivity (range 13–38%) was excreted in urine after 24 h [3]. The fraction of NAC excreted in urine in the 36 h following administration (3.66% in Chinese and 3.80% in Caucasian participants) was consistent with the fact that NAC is known to undergo extensive metabolism and transformation, and is excreted as sulfate and sulfur [12].
The results of the present study are generally consistent with those of previous studies conducted in Caucasian individuals. In the study conducted by Borgström and colleagues in 10 healthy volunteers, the mean Cmax of orally administered NAC 600 mg was 2758.00 ± 1223.96 ng/mL and mean tmax was 0.75 ± 0.21 h [4]. However, that study also found that approximately 30% of oral dose of NAC was excreted through the kidneys [4], whereas, in the present study, only 3.7–3.8% of the oral dose of NAC was found to be excreted in urine. A study conducted in six healthy volunteers reported that, after a single dose of oral NAC 400 mg, the tmax was 0.5 h in five participants and 1.0 h in one participant [5]. However, the median t½ was shorter than in the present study (6.25 h, range 4.59–10.6 h) [5]. On the other hand, similar t½ (mean ± SD 18.1 ± 3.96 h) and Vz (1720 ± 731 L) were reported after the administration of a single NAC 500-mg effervescent tablet in a study of 29 healthy volunteers, while the Cmax (26,500 ± 7580 ng/mL) and tmax (2.12 ± 0.677 h) reported in that study were quite different [9].
At the same time, the findings of the present study parallel those reported in a study of a single oral dose of NAC 600 mg in 24 healthy Chinese volunteers [10]. In that study, Cmax was 2397.81 ± 709.54 ng/mL, tmax was 1.01 ± 0.73 h and AUC0–∞ was 8287.27 ± 2418.68 ng h/mL. Nevertheless, the t½ was also shorter than in the present study (6.07 ± 2.41 h) [10]. The analytical technique in that study (liquid chromatography–isotopic dilution mass spectrometry) more closely resembles the LC–MS/MS assay we used than does the HPLC technique used by Borgström and colleagues in their 1986 study [4].
Another potential reason for the differences between the findings of the present study and those by Borgström et al. could be the fact that, in the latter study, proteins in the plasma samples were precipitated before disulfide bonds were reduced, which could have affected their findings [4, 13]. Furthermore, in the studies by Borgström et al. and Olsson et al., samples were collected for 12 h after NAC administration, while, in the study by Liu et al., samples were collected for 24 h [4, 5, 10]. In contrast, samples were collected over a period of 36 h after NAC administration in the present study. These differences together with the above-mentioned differences in bioanalytical methods also the pharmacokinetic calculation methods (e.g., DAS instead of WinNonLin in the work by Liu et al.) and the paucity of the sample size in the studies by Borgström et al. and Olsson et al. could have affected NAC t½ calculations.
NAC had a favourable safety profile, with only three non-treatment-related AEs occurring in two participants (6.7%). These findings are in line with the above-mentioned study in healthy Chinese volunteers, in which two participants (8.3%) had AEs [10].
The main limitation of the present study is the fact that the demographic characteristics of Chinese and Caucasian participants were different, including a higher proportion of males and higher mean age in the Chinese cohort.
To our knowledge, this is the first study to compare the pharmacokinetics of NAC in healthy Chinese and Caucasian individuals. The present study shows that the pharmacokinetic characteristics of NAC after oral administration of tablets are similar in healthy Chinese and Caucasian individuals after single and repeated administration. It also shows that NAC has a favourable safety profile, without major differences between Chinese and Caucasians.
Acknowledgements
We would like to thank the participants of the study. The pharmacokinetic analysis was performed using Phoenix WinNonlin® v.6.3 (Pharsight) and SAS® v.9.3 TS1M1 for Windows®.
Funding
This study was funded by Zambon S.p.A., Italy. The sponsor also paid the journal’s Rapid Service and Open Access Fees.
Medical Writing Assistance
We would like to thank Georgii Filatov of Springer Healthcare Communications who wrote the first draft of this manuscript. This medical writing assistance was funded by Zambon.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Milko Radicioni reviewed and approved the design of the study, was responsible for the clinical activities and collected the data. Andrea Francesco Daniele Di Stefano took part in designing the study and wrote the clinical study protocol and report. Alberto Papi reviewed the design of the study and reviewed drafts of the manuscript. All authors read and approved the manuscript.
Disclosures
Andrea Francesco Daniele Di Stefano and Milko Radicioni: The relationship between the Sponsor, Zambon S.p.A., and CROSS Research S.A., Switzerland, are governed by financial agreements. Alberto Papi: has received grants and personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici and TEVA; personal fees, non-financial support and other from Mundipharma, Zambon, Novartis, Menarini and Sanofi/Regeneron; personal fees from Roche and Edomond Pharma; and grants from Fondazione Maugeri and Fondazione Chiesi. None were related to the present study.
Compliance with Ethics Guidelines
The study protocol was approved by an independent ethics committee (Comitato Etico Cantonale, Canton Ticino, Switzerland). The study was conducted in accordance with the protocol and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) guidelines, Declaration of Helsinki and applicable Swiss and European Union regulations.
Data Availability
The datasets generated and analysed during the current study are not publicly available. These data are protected by a confidentiality agreement with the study sponsor, Zambon S.p.A., Italy, due to their ethically and commercially sensitive nature. Further information about the data and conditions for access are available at www.zambon.com.
References
- 1.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27(9–10):916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 3.Rodenstein D, DeCoster A, Gazzaniga A. Pharmacokinetics of oral acetylcysteine: absorption, binding and metabolism in patients with respiratory disorders. Clin Pharmacokinet. 1978;3(3):247–254. doi: 10.2165/00003088-197803030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–222. doi: 10.1007/bf00606662. [DOI] [PubMed] [Google Scholar]
- 5.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77–82. doi: 10.1007/bf01061422. [DOI] [PubMed] [Google Scholar]
- 6.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20(2):123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 7.Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/bf00609183. [DOI] [PubMed] [Google Scholar]
- 8.De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989;39(3):382–386. [PubMed] [Google Scholar]
- 9.Greene SC, Noonan PK, Sanabria C, Peacock WF. Effervescent N-acetylcysteine tablets versus oral solution N-acetylcysteine in fasting healthy adults: an open-label, randomized, single-dose, crossover, relative bioavailability study. Curr Ther Res Clin Exp. 2016;83:1–7. doi: 10.1016/j.curtheres.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YM, Liu Y, Lu C, Jia JY, Liu GY, Weng LP, et al. Relative bioavailability of generic and branded acetylcysteine effervescent tablets: A single-dose, open-label, randomized-sequence, two-period crossover study in fasting healthy Chinese male volunteers. Clin Ther. 2010;32(12):2097–2105. doi: 10.1016/j.clinthera.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Huang T, Shu Y, Cai YD. Genetic differences among ethnic groups. BMC Genomics. 2015;16:1093. doi: 10.1186/s12864-015-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffner AL, Medler EM, Bailey KR, Gallo DG, Mueller AJ, Sarett HP. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- 13.Kagedal B, Kallberg M, Martensson J. Determination of non-protein-bound N-acetylcysteine in plasma by high-performance liquid chromatography. J Chromatogr. 1984;311(1):170–175. doi: 10.1016/s0378-4347(00)84705-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available. These data are protected by a confidentiality agreement with the study sponsor, Zambon S.p.A., Italy, due to their ethically and commercially sensitive nature. Further information about the data and conditions for access are available at www.zambon.com.