Abstract
Objective:
To assess the predictors of achieving and maintaining guideline-recommended glycemic control in people with poorly controlled type 2 diabetes.
Methods:
We analyzed data from the Centre for Cardiometabolic Risk Reduction in South Asia (CARRS) Trial (n=1146), to identify groups that achieved guideline-recommended glycemic control (HbA1c<7%) and those that remained persistently poorly controlled (HbA1c>9%) over a median of 28 months of follow-up. We used generalized estimation equations (GEE) analysis for each outcome i.e. achieving guideline-recommended control and persistently poorly controlled and constructed four regression models (demographics, disease-related, self-care, and other risk factors) separately to identify predictors of HbA1c<7% and HbA1c>9% at the end of the trial, adjusting for trial group assignment and site.
Results:
In the final multivariate model, adherence to prescribed medications (RR: 1.46, 95%CI: 1.09, 1.95), adherence to diet plans (RR: 1.79, 95% CI: 1.43, 2.23) and middle-aged: 50-64 years (RR: 1.32; 95% CI: 1.02-1.71) were associated with achieving guideline-recommended control (HbA1c <7%). Presence of microvascular complications (RR: 0.70; 95%CI: 0.53-0.92) reduced the probability of achieving guideline-recommended glycemic control (HbA1c 7%). Further, longer duration of diabetes (>15 years), RR: 1.41; 95% CI: 1.15, 1.72, hyperlipidemia, RR: 1.19; 95% CI: 1.06, 1.34 and younger age group (35-49 years vs. >64 years: RR: 0.61; 95% CI: 0.47-0.79) were associated with persistently poor glycemic control (HbA1c>9%).
Conclusion:
To achieve and maintain guideline-recommended glycemic control, care delivery models must put additional emphasis and effort on patients with longer disease duration, younger people and those having microvascular complications and hyperlipidemia.
Keywords: Type 2 diabetes mellitus, glycemic targets, quality improvement, South Asia
Introduction
Diabetes affects nearly half a billion people globally, yet delivery of quality diabetes care remains persistently sub-optimal resulting in poor outcomes such as macro- and microvascular complications of diabetes (1-4). For example, in South Asia, which is at the epicenter of the diabetes epidemic, only one-third of patients with diabetes have guideline-recommended glycemic control, i.e., HbA1c <7%(5, 6). The situation is only slightly better in the United States and Europe, where only half of patients with diabetes achieved <7% (7, 8).
Quality improvement strategies focused on patients (reminders for clinic visits and laboratory tests), providers (evidence-based treatment prompts), and practice-level improvements have shown to improve adherence to recommended therapy and risk factor control among people with diabetes in high-income countries (9, 10), and the CARRS (Centre for Cardiometabolic Risk Reduction in South Asia) trial also showed this is feasible in low- and middle-income settings. (11, 12). While these quality improvement trials indicate improvement on average, considerable heterogeneity of effect across individuals receiving the intervention remains a challenge. Understanding factors that predict achievement of guideline-recommended glycemic control and persistently poorly controlled glycemia can help improve intervention impact. We conducted a post hoc analysis of data from the CARRS trial to identify groups those achieved guideline-recommended control (HbA1c <7%) and those that remained persistently poorly controlled (HbA1c >9%). Data from both the treatment group (n=1146) were combined and included in this analysis to investigate which factors (patient-level and clinic-level) were associated with glycemic control.
Methods
Study design and setting
The rationale and design of the CARRS trial were reported previously(11). Briefly, the CARRS Trial evaluated the effectiveness of a multicomponent quality improvement (QI) strategy versus usual care among 1146 type 2 diabetes patients with poor cardiometabolic profile (HbA1C level ≥ 8%, and systolic BP ≥ 140mmHg or LDL cholesterol level ≥ 130 mg/dl) in India and Pakistan. The CARRS Trial multicomponent QI intervention featured non-physician care coordinator to improve patient self-care and a decision-support electronic health records (DS-EHR) to enhance physician’s responsiveness to timely treatment modification.
Outcomes
We examined predictors of groups that achieved guideline-recommended control (HbA1C <7.0 %) and those that remained persistently poorly controlled (HbA1c >9%)(12, 13). This analysis included and reported data from all study participants and their baseline, annual, and end of study visit measurements. The end of study visits occurred between 24 and 36 months after randomization.
Exposures and Covariates
Baseline and annual visits data collection included assessing BP, HbA1C, and LDL cholesterol measurements. All blood samples were analyzed by local labs with external quality assurance certification. Blood pressure was measured with an electronic device (Omron T9P). Open ended questions about macro and microvascular complications, adverse events, serious adverse events, or hospitalizations were self-reported by participants. Macrovascular complications were defined as any one of: coronary heart disease (myocardial infarction or unstable angina); chronic stable angina; revascularization (angioplasty [percutaneous coronary intervention]/coronary artery by-pass surgery), stroke, or peripheral vascular disease. Microvascular complications were defined as any of: diabetic retinopathy or neuropathy or nephropathy. Self-reported measure of income categories in Indian rupees (<10,000, 10,000-20,000 and >20,000) was used as a proxy for socioeconomic status. Participant’s age was grouped into 35-49 years (young), 50-64 years (middle-aged) and ≥65 years (elderly). Self-reported education status was categorized as up to primary school, secondary school and graduate and above. Duration of diabetes was categorized as ≤5 years, 6-10 years, 11-15 years and >15 years.
Participants kept a log of their adherence and self-care activities. Adherence to prescribed therapy, diet, and exercise in the past one week was self-reported. Adherence was defined as "Yes" if a participant reported taking medicines and adherence to diet and exercise plan on all seven days in the last week.
Ethical Approval
The trial protocol was approved by ethics review committees of all participating sites in India and Pakistan and the coordinating centers based at the Public Health Foundation of India and Emory University, USA. Written Informed consent was obtained from all study participants after explaining study details. The trial was registered at clinicaltrials.gov: NCT01212328.
Statistical analysis
The baseline characteristics of all enrolled participants were presented as means (SD) or proportions (%). For each study time-point (i.e.12-month, 24 month and end of study (EOS) visit), we used the proportions achieving glycemic control (HbA1c<7.0%) and generalized estimating equations (GEE) to fit log-binomial models to account for correlation of observations within participants over time. The GEE model included baseline risk factors, time (study visit month i.e. 12-month, 24 month and end of study (EOS) visit), treatment group, baseline HbA1c and site. Bivariate and multivariable analysis using GEE models were performed with sequential adjustment for groups of variables. In model 1, we adjusted for socio demographic variables at baseline, including age, gender, socio economic status, and education status. Model 2 was adjusted for model 1 variables, clinical variables at baseline, including duration of diabetes, any macrovascular complication, and any microvascular complication. In model 3, we adjusted for model 1 and 2 variables plus adherence to prescribed medication, diet, and exercise over the study period. In model 4, we further assessed for potential confounding by other clinical factors such as hypertension at baseline, hyperlipidemia at baseline, BMI, and waist circumference over the time period and smoking status at baseline. The final model was adjusted for all of the risk factors included in Models 1-4, and also adjusted for type of hospital type (public vs private). We repeated this same approach for those that remained persistently poorly controlled (HbA1c>9.0 %). We used Poisson regression model where convergence was not achieved with the log-binomial models. We also checked for interaction of time - with each covariate analyzed in models 1-4 (e.g. diabetes duration, medication use, diet and physical activity). Associations are presented as relative risks (RR) with 95% confidence intervals [95%CI]. All the analyses were performed using STATA 15.0 version (College Station, Texas, USA).
Results
Demographic characteristics of the study participants
A total of 1146 participants were included in the analysis (575 in intervention and 571 in control arm) with mean (SD) age of 54.2(9.2) years. Participants were predominantly women (54.0%, n=619), had diabetes for an average of 8.7 years and a mean body mass index (BMI) of 27.4kg/m2. Demographic and clinical characteristics of all CARRS Trial participants at baseline are presented in Table 1.
Table 1:
Baseline characteristics of all the participants in the study (n=1146)
| Characteristics | Mean (SD) or n (%) |
|---|---|
| Age in years, mean (SD) | 54.2(9.2) |
| Gender, n (%) | |
| Men | 527(46.0) |
| Women | 619(54.0) |
| Education, n (%) | |
| Up to Primary School | 337(29.4) |
| Secondary | 499(43.5) |
| College graduate | 303(26.4) |
| Unknown | 7(0.6) |
| Household Income, n (%) | |
| <10,000 | 432(37.7) |
| 10,000-20,000 | 206(18.0) |
| >20,000 | 385(33.6) |
| Unknown | 123(10.7) |
| Duration of diabetes in years, Mean (SD) | 8.7(6.8) |
| Duration of diabetes, years, n (%) | |
| ≤5 | 451(39.4) |
| 6-10 | 305(26.6) |
| 11-15 | 201(17.5) |
| >15 | 175(15.3) |
| Missing | 14(1.2) |
| Smoking status at baseline, n (%) | |
| Yes | 76(6.6) |
| No | 1065(92.9) |
| Missing | 5(0.4) |
| Macrovascular Complications, n (%) | |
| Coronary Heart Disease* | 75(6.5) |
| Myocardial Infarction | 35(3.1) |
| Stroke | 23(2.0) |
| Peripheral Vascular Disease | 66(5.8) |
| Any Macrovascular Complication, n (%) | 135(11.8) |
| Any Microvascular Complication, n (%) | 451(39.4) |
| Microvascular Complications, n (%) | |
| Retinopathy | 100(8.7) |
| Neuropathy | 380(33.2) |
| Nephropathy | 112(9.8) |
| Depression, n (%) | |
| Yes | 25(2.2) |
| No | 1121(97.8) |
| Waist Circumference (cm), mean (SD) | 96.1(11.3) |
| BMI (kg/m2), mean (SD) | 27.4(4.7) |
| BMI (kg/m2), n (%) | |
| <25 | 368(32.1) |
| 25-29.9 | 503(43.9) |
| ≥30 | 275(24.0) |
| HbA1c (%), mean (SD) | 9.9(1.6) |
| Fasting blood glucose level (mg/dl), mean (SD) | 177.6(64.1) |
| LDL Cholesterol level (mg/dl), mean (SD) | 122.4(36.9) |
| Systolic BP (mmHg), mean (SD) | 143.3(19.4) |
| Diastolic BP (mmHg), mean (SD) | 81.7(10.9) |
Coronary Heart Disease (CHD) is defined as Myocardial infarction or Unstable Angina; Angioplasty (PCI)/Open heart surgery (CABG); Chronic Stable Angina; Stroke
Any macrovascular complication is defined as any 1 one these-CHD or peripheral vascular disease
Any microvascular complication is defined as any 1 one these-Retinopathy or neuropathy or nephropathy SD; standard deviation, BMI; body mass index, LDL; low density lipoprotein
Factors associated with achievement of glycemic control (HbA1c < 7%)
Overall, the proportion of participants that achieved guideline-recommended glycemia control were 16.3% (95% CI: 14.6, 18.1). The proportion of subjects achieving glycemic control was higher in males as compared to females but was not statistically significant (adjusted relative risk: 1.17 (95% CI: 0.93, 1.47). In bivariate analyses, higher socioeconomic status, higher education status, longer duration of diabetes, presence microvascular complications, adherence to prescribed medications, adherence to diet plan, adherence to exercise plan, and hyperlipidemia were all found to be significantly associated with HbA1c < 7% (Table 2). Unadjusted and adjusted relative risk ratios showing factors associated with achievement of guideline-recommended glycemic control, i.e., HbA1c <7% are shown in Table 2. In the final multivariate model (adjusted for all of the risk factors included in Models 1-4, refer above statistical analysis section), we found that adherence to prescribed medications and diet plans and middle-aged group (50-64 years) were associated with achievement of guideline-recommended glycemic control HbA1c <7%, RR: 1.46, 95%CI: 1.09-1.95, and RR: 1.79, 95% CI: 1.43-2.23, RR: 1.32; 95%CI: 1.02-1.71, respectively. Presence of microvascular complications reduced the probability of achieving guideline-recommended control (HbA1c <7%): RR: 0.70, 95%CI: 0.53, 0.92. The interaction between time and each of the covariates (medication use, diet and physical activity) was statistically insignificant (p=0.35, 0.09 and 0.35, respectively).
Table 2:
Proportion achieving guideline-recommended glycemic level (HbA1C<7.0%) at 12-month, 24 month and End of study (EOS) visit and factors associated with achievement of guideline-recommended glycemic control
| Factors | Glycaemic level (HbA1c<7.0%) | Crude RR [95% CI] |
Adjusted RR^ [95% CI] |
|||
|---|---|---|---|---|---|---|
| 12m | 24m | EOS | Overall | |||
| Overall | 14.7[12.6,16.9] | 17.5[14.5,20.5] | 17.7[15.4,20] | 16.3[14.6,18.1] | ||
| Age, years | ||||||
| 35-49 | 13.7[10.5,16.9] | 16.2[12.1,20.4] | 16.4[12.7,20.1] | 15.2[12,18.5] | 1.00 | 1.00 |
| 50-64 | 15.5[12.8,18.1] | 18.4[14.8,21.9] | 18.5[15.7,21.4] | 17.3[14.9,19.6] | 1.13[0.88,1.46] | 1.32[1.02,1.71]* |
| ≥65 | 14[10.2,17.9] | 16.6[11.6,21.7] | 16.8[12.1,21.5] | 15.6[11.5,19.8] | 1.03[0.73,1.44] | 1.28[0.89,1.86] |
| Gender | ||||||
| Female | 13.6[11.1,16.1] | 16.1[12.8,19.4] | 16.3[13.5,19] | 15.1[12.8,17.5] | 1.00 | 1.00 |
| Male | 16.1[13.2,18.9] | 19.1[15.3,22.9] | 19.3[16.1,22.5] | 17.9[15.3,20.6] | 1.18[0.96,1.47] | 1.17[0.93,1.47] |
| Socio-economic status | ||||||
| <10,000 | 14.5[10.7,18.3] | 16.9[12.2,21.6] | 17.1[12.9,21.4] | 16[12.2,19.8] | 1.00 | 1.00 |
| 10,000-20,000 | 14.7[10.7,18.7] | 17.1[12.2,22.1] | 17.4[12.6,22.1] | 16.2[12.1,20.4] | 1.01[0.72,1.44] | 0.98[0.7,1.36] |
| >20,000 | 15[11.8,18.2] | 17.6[13.5,21.6] | 17.8[14.2,21.4] | 16.6[13.5,19.7] | 1.04[0.74,1.46] | 0.93[0.68,1.28] |
| Education Status | ||||||
| Up to primary school | 12.7[9.4,16] | 15.2[11.1,19.2] | 15.1[11.2,19] | 14.1[10.7,17.5] | 1.00 | 1.00 |
| Secondary school | 14.8[12,17.6] | 17.7[13.9,21.5] | 17.6[14.5,20.8] | 16.5[13.8,19.2] | 1.17[0.88,1.55] | 1.12[0.83,1.51] |
| Graduate or above | 16.5[12.9,20.1] | 19.7[15,24.5] | 19.7[15.6,23.7] | 18.4[14.8,21.9] | 1.3[0.94,1.81] | 1.23[0.86,1.76] |
| Duration of Diabetes | ||||||
| 5 years or less | 20.6[17,24.2] | 24.5[19.7,29.3] | 24.5[20.5,28.5] | 22.9[19.6,26.3] | 1.00 | 1.00 |
| 6-10 years | 12.2[9.2,15.3] | 14.5[10.7,18.4] | 14.5[11.1,18] | 13.6[10.5,16.7] | 0.59[0.45,0.78]** | 0.63[0.47,0.83]** |
| 11-15 years | 9.9[6.8,13.1] | 11.8[7.8,15.8] | 11.8[8.1,15.5] | 11[7.7,14.4] | 0.48[0.34,0.68]** | 0.56[0.39,0.8]** |
| >15 years | 11.8[7.8,15.8] | 11.8[8.1,15.5] | 10[6.7,13.2] | 11.1[7.6,14.6] | 0.48[0.34,0.68]** | 0.47[0.32,0.69]** |
| Any Macro vascular complication | ||||||
| No | 14.8[12.6,17] | 17.6[14.6,20.7] | 17.8[15.4,20.1] | 16.5[14.7,18.4] | 1.00 | 1.00 |
| Yes | 13.4[6.9,20] | 16[8,23.9] | 16.1[8.4,23.8] | 15 [7.8,22.1] | 0.91[0.55,1.48] | 1.09[0.68,1.76] |
| Any Micro vascular complication | ||||||
| No | 16.8[14.1,19.6] | 20[16.1,23.8] | 20.1[17,23.1] | 18.7[16.2,21.2] | 1.00 | 1.00 |
| Yes | 11.1[8.5,13.7] | 13.2[9.8,16.6] | 13.3[10.2,16.3] | 12.4[9.7,15.1] | 0.66[0.51,0.86]** | 0.70[0.53,0.92]* |
| Adherence to Prescribed Medication Therapy*≠ | ||||||
| Non-Adherent | 9.8[7.1,12.4] | 11.8[8.4,15.1] | 11.9[8.7,15] | 11[8.2,13.8] | 1.00 | 1.00 |
| Adherent | 15.8[13.4,18.1] | 19[15.7,22.4] | 19.2[16.6,21.8] | 17.8[15.8,19.7] | 1.62[1.24,2.11]** | 1.46[1.09,1.95]* |
| Adherence to Diet Plan*≠ | ||||||
| Non-Adherent | 11[9.1,13] | 13.3[10.6,16] | 12.4[10,14.7] | 12[10.1,13.9] | 1.00 | 1.00 |
| Adherent | 21.6[17.7,25.5] | 26[20.9,31.1] | 24.2[20.6,27.7] | 23.5[20.2,26.7] | 1.96[1.59,2.41]** | 1.79[1.43,2.23]** |
| Adherence to Exercise plan*≠ | ||||||
| Non-Adherent | 13.3[11.2,15.4] | 15.9[12.9,18.9] | 15.2[12.7,17.8] | 14.6[12.6,16.6] | 1.00 | 1.00 |
| Adherent | 17.7[14.5,20.9] | 21.1[17.1,25.1] | 20.2[17.3,23.2] | 19.4[16.7,22] | 1.33[1.12,1.57]** | 1.06[0.87,1.3] |
| Hypertension (SBP >140 mmHg) at baseline | ||||||
| ≤ 140 | 15.4[13.1,17.8] | 18.3[15.1,21.6] | 18.1[15.6,20.6] | 17.1[15.1,19] | 1.00 | 1.00 |
| >140 | 12.4[9.1,15.7] | 14.7[10.5,18.9] | 14.6[10.7,18.4] | 13.7[10.3,17.1] | 0.8[0.61,1.05] | 0.86[0.65,1.12] |
| Hyperlipidemia (LDL > 130 mg/dl) at baseline | ||||||
| ≤ 130 | 15.3[13,17.6] | 18[14.8,21.2] | 18.1[15.6,20.5] | 16.9[15,18.8] | 1.00 | 1.00 |
| >130 | 11.6[8.2,15.1] | 13.6[9.4,17.9] | 13.7[9.6,17.8] | 12.8[9.2,16.5] | 0.76[0.56,1.02] | 0.82[0.6,1.14] |
| BMI≠ | ||||||
| <25 | 14.2[11.2,17.2] | 16.8[12.7,20.9] | 17[13.5,20.4] | 15.8[12.8,18.8] | 1.00 | 1.00 |
| 25-29.9 | 16.1[13.2,19] | 19.1[15.3,22.8] | 19.3[16,22.5] | 17.9[15.3,20.6] | 1.14[0.9,1.44] | 1.19[0.93,1.53] |
| ≥ 30 | 13.6[10.5,16.7] | 16.1[12.1,20] | 16.2[12.7,19.8] | 15.1[12,18.2] | 0.96[0.72,1.27] | 1.08[0.79,1.47] |
| Waist Circumference≠ | ||||||
| Males <90 cm, Female <80 cm | 13.4[9.5,17.4] | 16[11.1,20.9] | 16.1[11.5,20.6] | 15[10.9,19.1] | 1.00 | 1.00 |
| Males >90cm, Females >80 cm | 15[12.8,17.3] | 17.9[14.7,21.1] | 18[15.5,20.4] | 16.8[14.8,18.7] | 1.12[0.84,1.5] | 1.33[0.95,1.85] |
| Smoking Status-baseline | ||||||
| No | 14.6[12.4,16.7] | 17.3[14.2,20.4] | 17.5[15.2,19.8] | 16.3[14.5,18] | 1.00 | 1.00 |
| Yes | 18.2[10.3,26.1] | 21.6[12.4,30.8] | 21.8[12.6,31.1] | 20.3[11.8,28.7] | 1.25[0.81,1.91] | 0.93[0.57,1.53] |
| Hospital setting | ||||||
| Public | 1.00 | |||||
| Private | 0.6[0.36,1] | |||||
P value <0.05
P value <0.005
Adjusted RR obtained via Poisson model using generalized estimating equation. Each estimate is adjusted for time, treatment assignment, baseline HbA1C value, site, public private settings and all other factors reported in table
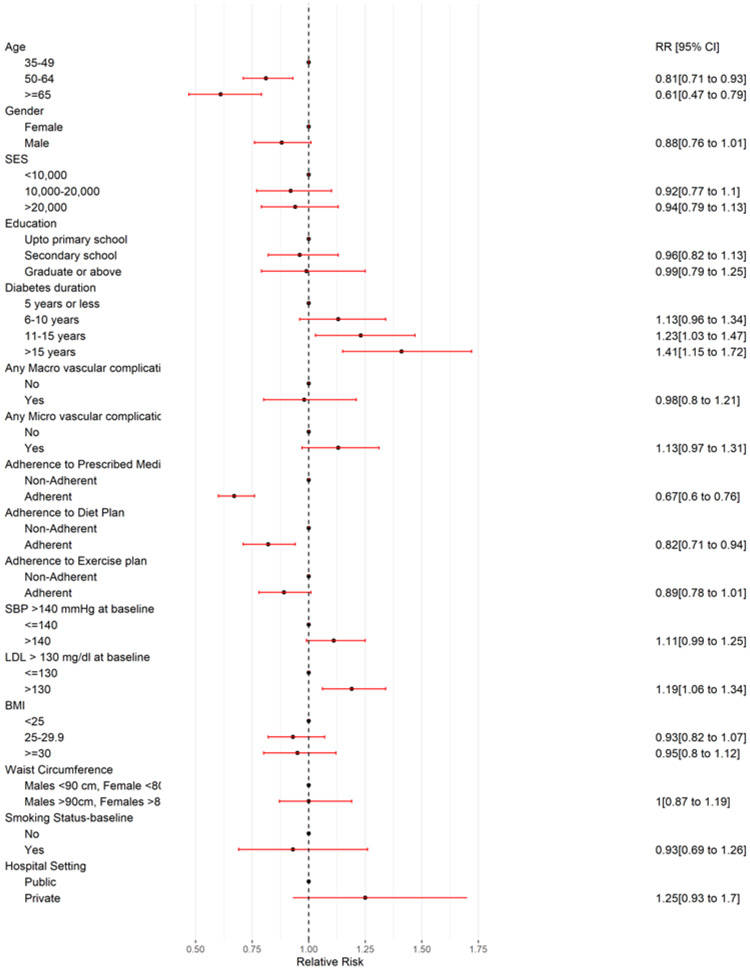
Table 3/Figure 1 demonstrates the factors associated with persistently poorly controlled, i.e., HbA1c > 9%. Longer duration of diabetes (>15 years), RR: 1.41; 95% CI: 1.15, 1.72, hyperlipidemia, RR: 1.19; 95% CI: 1.06, 1.34 and younger age group (35-49 years vs. >64 years old: RR: 0.61; 95% CI: 0.47-0.79) were associated with persistently poorly-controlled (HbA1c>9%).
Table 3:
Factors associated with persistently poorly controlled glycemia (HbA1C> 9.0%) at 12 month, 24 month and End of study (EOS) visit
| Factors | Glycemic level (HbA1c>9.0%) | Crude RR [95% CI] |
Adjusted RR^ [95% CI] |
|||
|---|---|---|---|---|---|---|
| 12m | 24m | EOS | Overall | |||
| Overall | 38.3[35.5,41.1] | 31[27.4,34.5] | 33.4[30.6,36.1] | 34.9[32.7,37] | ||
| Age, years | ||||||
| 35-49 | 42.7[38,47.3] | 34.6[29.6,39.5] | 37.2[32.8,41.5] | 38.9[34.8,42.9] | 1.00 | 1.00 |
| 50-64 | 38.2[34.7,41.7] | 30.9[27,34.8] | 33.3[30,36.6] | 34.8[31.9,37.7] | 0.90[0.78,1.02] | 0.81[0.71,0.93]** |
| ≥65 | 26.5[20.5,32.6] | 21.5[16.3,26.7] | 23.1[17.8,28.4] | 24.2[18.8,29.6] | 0.62[0.49,0.80]** | 0.61[0.47,0.79]** |
| Gender | ||||||
| Female | 41.3[37.7,44.9] | 33.5[29.2,37.8] | 36[32.5,39.5] | 37.6[34.6,40.6] | 1.00 | 1.00 |
| Male | 34.6[30.9,38.3] | 28.1[24.3,31.9] | 30.2[26.8,33.6] | 31.6[28.4,34.7] | 0.84[0.74,0.95]** | 0.88[0.76,1.01] |
| Socio-economic status | ||||||
| <10,000 | 40.7[36.7,44.7] | 31.7[27.2,36.1] | 35.3[31.5,39.2] | 36.8[33.4,40.2] | 1.00 | 1.00 |
| 10,000-20,000 | 36.5[30.7,42.4] | 28.4[23.2,33.6] | 31.7[26.5,36.9] | 33.0[27.9,38.1] | 0.90[0.75,1.07] | 0.92[0.77,1.1] |
| >20,000 | 38.6[33.3,43.9] | 30[25,35.1] | 33.5[28.8,38.2] | 34.9[30.3,39.4] | 0.95[0.8,1.12] | 0.94[0.79,1.13] |
| Education Status | ||||||
| Upto primary school | 41.7[36.8,46.6] | 33.6[28.6,38.6] | 36.5 [32,41] | 38[33.8,42.2] | 1.00 | 1.00 |
| Secondary school | 36.8[33.1,40.5] | 29.6[25.6,33.7] | 32.2[28.7,35.8] | 33.5[30.4,36.7] | 0.88[0.76,1.02] | 0.96[0.82,1.13] |
| Graduate or above | 35.1[29.4,40.8] | 28.3[23.1,33.5] | 30.8[25.7,35.8] | 32[27,37] | 0.84[0.69,1.03] | 0.99[0.79,1.25] |
| Duration of Diabetes | ||||||
| 5 years or less | 33.4[29.2,37.6] | 26.8[22.6,31] | 29.1[25.2,32.9] | 30.4[26.7,34] | 1.00 | 1.00 |
| 6-10 years | 39.8[34.9,44.6] | 31.9[27.1,36.7] | 34.6[30.2,39] | 36.1[32,40.3] | 1.19[1.01,1.4]** | 1.13[0.96,1.34] |
| 11-15 years | 43.4[37.8,49] | 34.8[29.4,40.3] | 37.8[32.7,42.8] | 39.4[34.6,44.3] | 1.30[1.09,1.55]** | 1.23[1.03,1.47]** |
| >15 years | 34.8[29.4,40.3] | 37.8[32.7,42.8] | 41.4[35.3,47.5] | 37.6[32.2,43] | 1.24[1.02,1.5]* | 1.41[1.15,1.72]** |
| Any Macro vascular complication | ||||||
| No | 38[35.2,40.9] | 30.7[27.1,34.3] | 33.1[30.3,36] | 34.6[32.3,36.9] | 1.00 | 1.00 |
| Yes | 41.2[33.2,49.1] | 33.3[26.4,40.1] | 35.9[28.9,42.9] | 37.5[30.5,44.5] | 1.08[0.89,1.32] | 0.98[0.8,1.21] |
| Any Micro vascular complication | ||||||
| No | 34.5[30.8,38.3] | 27.8[23.9,31.7] | 30.1[26.6,33.6] | 31.4[28.2,34.6] | 1.00 | 1.00 |
| Yes | 42.6[38.6,46.6] | 34.3[29.9,38.7] | 37.1[33.4,40.9] | 38.8[35.5,42.1] | 1.24[1.07,1.42]* | 1.13[0.97,1.31] |
| Adherence to Prescribed Medication Therapy*≠ | ||||||
| Non-Adherent | 53.9[48.5,59.4] | 42.1[36.8,47.4] | 45.6[40.9,50.3] | 48.2[43.9,52.5] | 1.00 | 1.00 |
| Adherent | 34.7[31.9,37.5] | 27.1[23.7,30.4] | 29.3[26.6,32] | 31[28.7,33.3] | 0.64[0.58,0.72]** | 0.67[0.6,0.76]** |
| Adherence to Diet Plan*≠ | ||||||
| Non-Adherent | 42[38.6,45.4] | 33.7[29.7,37.7] | 36.3[33.1,39.4] | 38.1[35.4,40.7] | 1.00 | 1.00 |
| Adherent | 31[27.3,34.7] | 24.9[21,28.8] | 26.8[23.2,30.3] | 28.1[24.8,31.4] | 0.74[0.65,0.84]** | 0.82[0.71,0.94]** |
| Adherence to Exercise plan*≠ | ||||||
| Non-Adherent | 41.1[37.9,44.3] | 33.4[29.5,37.4] | 36.2[33,39.4] | 37.6[35,40.2] | 1.00 | 1.00 |
| Adherent | 32.1[28.2,35.9] | 26.1[22.3,29.9] | 28.2[24.8,31.6] | 29.3[26.1,32.6] | 0.78[0.69,0.88]** | 0.89[0.78,1.01] |
| Hypertension (SBP >140 mmHg) at baseline | ||||||
| ≤ 140 | 37.3[34.3,40.3] | 30.1[26.4,33.7] | 32.4[29.6,35.3] | 33.9[31.5,36.3] | 1.00 | 1.00 |
| >140 | 41[36.7,45.3] | 33.1[28.7,37.5] | 35.7[31.6,39.8] | 37.3[33.7,41] | 1.10[0.99,1.23] | 1.11[0.99,1.25] |
| Hyperlipidemia (LDL > 130 mg/dl) at baseline | ||||||
| ≤ 130 | 36.5[33.5,39.4] | 29.7[26.2,33.2] | 31.9[29.1,34.7] | 33.3[30.9,35.6] | 1.00 | 1.00 |
| >130 | 44.5[39.8,49.1] | 36.2[31,41.3] | 38.9[34.5,43.3] | 40.6[36.6,44.6] | 1.22[1.09,1.36]** | 1.19[1.06,1.34]* |
| BMI≠ | ||||||
| <25 | 39[34.9,43] | 31.5[27.2,35.9] | 33.9[30,37.7] | 35.5[32,38.9] | 1.00 | 1.00 |
| 25-29.9 | 37.2[33.3,41] | 30.1[26.1,34.1] | 32.3[28.9,35.7] | 33.8[30.7,37] | 0.95[0.84,1.08] | 0.93[0.82,1.07] |
| ≥ 30 | 39.1[34.1,44] | 31.6[26.5,36.7] | 34[29.3,38.6] | 35.6[31.1,40] | 1.00[0.85,1.18] | 0.95[0.8,1.12] |
| Waist Circumference≠ | ||||||
| Males <90 cm, Female <80 cm | 37.2[31.6,42.7] | 30[24.8,35.2] | 32.2[27,37.3] | 33.8[28.8,38.7] | 1.00 | 1.00 |
| Males >90cm, Females >80 cm | 38.5[35.5,41–5] | 31.1[27.4,34.8] | 33.3[30.5,36.2] | 35[32.6,37.3] | 1.04[0.89,1.21] | 1.00[0.85,1.19] |
| Smoking Status-baseline | ||||||
| No | 38.6[35.8,41.5] | 31.2[27.6,34.8] | 33.7[30.9,36.4] | 35.2[32.9,37.4] | 1.00 | 1.00 |
| Yes | 31.8[22,41.7] | 25.8[17.4,34.1] | 27.8[19,36.5] | 29[20,38] | 0.82[0.6,1.13] | 0.93[0.69,1.26] |
| Hospital setting | ||||||
| Public | 1.00 | |||||
| Private | 1.25[0.93,1.70] | |||||
P value <0.05
P value <0.005
Adjusted RR obtained via Poisson model using generalized estimating equation. Each estimate is adjusted for time, treatment assignment, baseline HbA1C value, site, public private settings and all other factors reported in table.
Figure 1:
Predictors of persistently poorly controlled glycemia i.e. HbA1c >9% (adjusted RR and 95% CI)
Adjusted relative risks for the related variables, which were analyzed in four separate regression models (model 1 consisting of age, gender, SES, education; model 2: diabetes duration, macro- and micro-vascular complications, model 3: adherence to diet, exercise, and medications, and model 4: co-morbid hypertension, hyperlipidemia, BMI and waist circumference) are provided in supplementary table s1. These results were consistent with the above findings.
Discussion
Diabetes is a chronic condition that poses day to day challenges for patients regarding food choices, physical activity, and performing tasks of daily living such as taking medications and self-monitoring blood glucose. Patients need to be consistently motivated and vigilant which can be demanding and lead to patient burnout. This in turn can lead to poor glycemic control which is associated with disabling and sometimes life-threatening diabetes related complications. Our study helps better understand the dynamics involved in promoting or hindering the achievement of guideline-recommended glycemic control. Our focus was to identify the patient related and disease related factors and patient characteristics or associated conditions and type of hospital setting (clinic type) that have an impact on glycemic control.
Overall, we found 16.3% participants achieved good glycemic control (HbA1c<7%), and 34.9% had persistently poor controlled glycemia (HbA1c>9%). Our results are consistent with the findings of a systematic review that evaluated quality of diabetes care in low- and middle- income Asian and Middle Eastern countries involving 115 studies and found that achievement of glycemic targets varied from 17% to 63%. (14)
Age was identified as an important factor and older age was protective in avoiding poor glycemic control. The age group between 35 and 50 years performed worst in terms of achieving good glycemic control, which is supported by the evidence from a community based study in southern Germany where one-fifth of the patients had HbA1c of > 8% and patients aged less than 60 years were at higher risk of poorer glucose control (15-18). This is an important issue as people below 60 years are the most productive age group and that poor glycemic control early on can have a legacy effect in terms of higher probability of diabetes complications in the long-run(19).
Our study results corroborate previous research and showed that the risk of persistently poor glycemic control was significantly higher in patients with 10 or more years of diabetes(20-22), which is also an important predictor of microvascular complications. As disease duration is a non-modifiable factor, focus should be on prevention, early diagnosis, and timely treatment intensification to arrest high blood glucose level. The long-term benefits of intensive treatment have been shown in 10-year United Kingdom Prospective Diabetes Study (UKPDS) follow up study(23). Furthermore, the treatments used often do not prevent a gradual increase of hyperglycemia over time, and more and more medications are needed to achieve control unless patients make sizeable and sustained lifestyle changes.
Adherence to diet plan and prescribed medications stand out as the key factors in patients who achieved glycemic control. These factors are consistently shown to be predictors of guideline-recommended glycemic control in several other studies(24-27). Self-care behaviors provide the foundation in the management of diabetes; and must be integrated in daily lives. A study of behavioral factors alluded that the intention to perform the self-care behavior is a major driver to perform the action(28). Different interventional tools have been tested in improving patient adherence(29). Our study has demonstrated the effectiveness of a multicomponent intervention consisting of a non-physician patient care coordinator and electronic health records with decision support system in achieving diabetes care targets(30).
Presence of any microvascular complications, or hyperlipidemia remain important predictors of poor glycemic control. The direction of these relationships is not clear; for example, microvascular complications influence poor glycemic control, or are influenced by poor glycemic control, or, both directions of influence are likely at play. However, the situation poses a serious challenge. A single clinic-based study in Malaysia has shown that the presence of one or more complications of diabetes as compared to no complications is associated with poor glycemic control(31). Similarly, co-existence of hypercholesterolemia was a predictor of poor glycemic control in a low-income minority of San Diego(16).
This study has important limitations. First, potential confounding factors, such as dietary intake patterns (e.g. quantification of sugar or fat intake) were not assessed. The clinical setting makes it extremely difficult to obtain accurate dietary intake data and affects patient retention in trials. Second, physician characteristics (experience – years practicing and qualification) and health-system level factors (human resources and infrastructure) affecting glycemic control were not analyzed as these data were not fully captured during the trial. Third, the influence of patient-physician-care coordinator interactions and the effect of social and/or family (caregiver) dynamics were not captured during the trial and were not included in the multivariable regression analysis.
This study has several strengths. As previously reported(30), the multicomponent quality improvement strategy used in the CARRS-Trial demonstrated achievement of multiple risk factor control over mean follow-up of 30 months in the randomized controlled trial. The present analysis expands this demonstration by examining the sub-populations, i.e., compared the two “extreme” phenotypes – groups that achieved guideline-recommended control (HbA1c<7%) vs. persistently poorly controlled (hbA1c>9%), adjusting for the randomization. This analysis also identified baseline characteristics of CARRS trial participants that were predictive of maintaining guideline-recommended glycemic control. The sample size was relatively large from a diverse mix of hospital settings (public, private and semi-private) which may improve generalizability of the study findings to a larger population of poorly controlled type 2 diabetes patients. We used standardized methods to measure fasting blood glucose, glycated hemoglobin, lipids and blood pressure.
In conclusion, our study highlighted important factors influencing glycemic control including the non-modifiable factors such as age, duration of diabetes or presence of complications or other comorbid conditions, which re-emphasized the importance of early diagnosis and intensive management early in the course of diabetes. Modifiable factors influencing glycemic control are mainly related to self-care behaviors (adherence to medications, diet and exercise) where patient motivation and empowerment play the key role. To maintain guideline-recommended glycemic control, care delivery models must put an additional emphasis and effort on patients with lower socioeconomic status, longer disease duration and those having microvascular and macrovascular complications.
Supplementary Material
Acknowledgements
The CARRS trial was funded in part by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, under contract number HHSN268200900026C, and by UnitedHealth Group, Minneapolis, Minnesota. Several members of the research team at the Public Health Foundation of India and Emory University were supported by the Fogarty International Clinical Research Scholars and Fellows program through grant number 5R24TW007988 from the National Institutes of Health, Fogarty International Center through Vanderbilt University, Emory Global Health Institute, and D43 NCDs in India Training Program through award number 1D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Fogarty International Center. Dr. Singh is supported by the Fogarty International Center, National Institutes of Health, under award number K43TW 011164. Dr. M K Ali is supported by the National Institute of Mental Health supplemental grant under award number: R01MH100390-04S1.
We also acknowledge the contributions of the software development team: Mr. Prashant Tandon and Mr. Ajeet Kushwaha.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors have any potential conflict of interest to declare.
References
- 1.International Diabetes Federation. http://www.diabetesatlas.org/across-the-globe.html. Accessed on 12 Jan 2018.
- 2.Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390(10111):2437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6(12):e1352–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–96. [DOI] [PubMed] [Google Scholar]
- 5.Deepa M, Grace M, Binukumar B, Pradeepa R, Roopa S, Khan HM, et al. High burden of prediabetes and diabetes in three large cities in South Asia: The Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) Study. Diabetes Res Clin Pract. 2015;110(2):172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, et al. DiabCare Asia--India Study: diabetes care in India--current status. J Assoc Physicians India. 2001;49:717–22. [PubMed] [Google Scholar]
- 7.Stone MA, Charpentier G, Doggen K, Kuss O, Lindblad U, Kellner C, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care. 2013;36(9):2628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613–24. [DOI] [PubMed] [Google Scholar]
- 9.Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–61. [DOI] [PubMed] [Google Scholar]
- 11.Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG, et al. Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia--the CARRS multi-center translation trial. Diabetes Res Clin Pract. 2012;98(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg RB, Stone NJ, Grundy SM. The 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guidelines on the Management of Blood Cholesterol in Diabetes. Diabetes Care. 2020;43(8):1673–8. [DOI] [PubMed] [Google Scholar]
- 13.Katsiki N, Ferrannini E, Mantzoros C. New American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) guidelines for the pharmacotherapy of type 2 diabetes: Placing them into a practicing physician's perspective. Metabolism. 2020; 107:154218. [DOI] [PubMed] [Google Scholar]
- 14.Shivashankar R, Kirk K, Kim WC, Rouse C, Tandon N, Narayan KM, et al. Quality of diabetes care in low- and middle-income Asian and Middle Eastern countries (1993-2012): 20-year systematic review. Diabetes Res Clin Pract. 2015;107(2):203–23. [DOI] [PubMed] [Google Scholar]
- 15.Rothenbacher D, Ruter G, Saam S, Brenner H. Younger patients with type 2 diabetes need better glycaemic control: results of a community-based study describing factors associated with a high HbA1c value. Br J Gen Pract. 2003;53(490):389–91. [PMC free article] [PubMed] [Google Scholar]
- 16.Benoit SR, Fleming R, Philis-Tsimikas A, Ji M. Predictors of glycemic control among patients with Type 2 diabetes: a longitudinal study. BMC Public Health. 2005; 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols GA, Hillier TA, Javor K, Brown JB. Predictors of glycemic control in insulin-using adults with type 2 diabetes. Diabetes Care. 2000;23(3):273–7. [DOI] [PubMed] [Google Scholar]
- 18.Ali MK, Shah MK. Age and Age-old Disparities in Diabetes Care Persist. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care. 2019;42(3):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yigazu DM, Desse TA. Glycemic control and associated factors among type 2 diabetic patients at Shanan Gibe Hospital, Southwest Ethiopia. BMC Res Notes. 2017;10(1):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SL, Chen ZC, Yan L, Chen LH, Cheng H, Ji LN. Determinants for inadequate glycaemic control in Chinese patients with mild-to-moderate type 2 diabetes on oral antidiabetic drugs alone. Chin Med J (Engl). 2011;124(16):2461–8. [PubMed] [Google Scholar]
- 22.Otiniano ME, Al Snih S, Goodwin JS, Ray L, AlGhatrif M, Markides KS. Factors associated with poor glycemic control in older Mexican American diabetics aged 75 years and older. J Diabetes Complications. 2012;26(3):181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leal J, Hayes AJ, Gray AM, Holman RR, Clarke PM. Temporal validation of the UKPDS outcomes model using 10-year posttrial monitoring data. Diabetes Care. 2013;36(6):1541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamuhabwa AR, Charles E. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar es Salaam. Drug Healthc Patient Saf. 2014;6:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demoz GT, Gebremariam A, Yifter H, Alebachew M, Niriayo YL, Gebreslassie G, et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow-up care at a tertiary healthcare setting in Ethiopia. BMC Res Notes. 2019;12(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez LDH, Soto AF, Valenzuela CLC, Ochoa MC, Gonzalez HR and Lopez MCM. Factors Influencing Glycemic Control in Patients with Diabetes Type II in Mexican Patients. J Fam Med. 2016; 3(2): 1051. [Google Scholar]
- 27.Kassahun T, Eshetie T, Gesesew H. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: a cross-sectional survey in Ethiopia. BMC Res Notes. 2016;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatt S, Sammut R. An exploratory study of predictors of self-care behaviour in persons with type 2 diabetes. Int J Nurs Stud. 2008;45(10):1525–33. [DOI] [PubMed] [Google Scholar]
- 29.Costa E, Giardini A, Savin M, Menditto E, Lehane E, Laosa O, et al. Interventional tools to improve medication adherence: review of literature. Patient Prefer Adherence. 2015;9:1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali MK, Singh K, Kondal D, Devarajan R, Patel SA, Shivashankar R, et al. Effectiveness of a Multicomponent Quality Improvement Strategy to Improve Achievement of Diabetes Care Goals: A Randomized, Controlled Trial. Ann Intern Med. 2016;165(6):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almutairi Mansour A., Said Salmiah Md., Zainuddin Huda, Predictors of Poor Glycemic Control among Type two Diabetic Patients, American Journal of Medicine and Medical Sciences, Vol. 3 No. 2, 2013, pp. 17–21. doi: 10.5923/j.ajmms.20130302.01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.