Abstract
Improved thermostability and decreased component costs are desirable features for adjuvanted, recombinant vaccines. We previously showed that a model malaria transmission-blocking vaccine candidate antigen, Pfs25, can be rendered more immunogenic when mixed with liposomes containing cobalt porphyrin–phospholipid (CoPoP) and a synthetic monophosphoryl lipid A (MPLA) variant. CoPoP induces stable particle formation of recombinant antigen based on interaction with the histidine tag. In the present work, different synthetic MPLA variants and concentrations were assessed in CoPoP liposomes. Long-term biophysical stability and immunogenicity were not adversely impacted by a 60 % reduction in MPLA content. When admixed with Pfs25, the adjuvant formulations effectively induced functional antibodies in immunized mice and rabbits. Lyophilized, antigen-bound liposome particles were formed using sucrose and trehalose cryoprotectants, which improved vaccine reconstitution for a variety of model antigens. Compared to liquid storage, the lyophilized Pfs25 and CoPoP liposomes exhibited thermostability with respect to size, biochemical integrity, binding capacity, protein folding and immunogenicity. Following 6 weeks of storage at 60 °C, the most extended storage period assessed, the lyophilized formulation induced functional antibodies in mice with immunization.
Keywords: Vaccines, adjuvants, liposomes, lyophilization, thermostability, malaria
Graphical Abstract
Introduction
Vaccines hold potential for eradicating disease and represent a global health tool that saves millions of lives each year (Ehreth, 2003). They must be provided in a pharmaceutical dosage form that can retain appropriate biological activity during transport and storage prior to use. Thermal instability is a potential cause of vaccine failure (Galazka et al., 1998). The “cold chain” is the approach used to minimize this problem, and refers to the multiple refrigeration systems which are required to maintain the desired temperatures throughout all stages of vaccine transport and storage, usually at 2 – 8 °C (Brandau et al., 2003; Zaffran, 1996).
Malaria is a mosquito-borne infectious disease that causes over 400,000 deaths and over 200 million cases each year. As the female Anopheles mosquito mediates malaria parasite transmission between humans, a unique approach to tackle the disease is a transmission-blocking vaccine (TBV), which allows immunized humans to transfer induced antibodies to mosquitos during their blood meal thus resulting in inhibition of parasite development in the mosquito gut (Huang et al., 2018b). Although TBVs have not yet been tested in large trials, a vaccine reducing parasite transmission is a part of the World Health Organization Malaria Vaccine Roadmap and their introduction could reduce malaria burden (Coelho et al., 2019). Developing a TBV that produces high and sustained levels of transmission-blocking antibodies in humans is the primary goal (Nunes et al., 2014). Pfs25 is a surface protein expressed on Plasmodium falciparum gametes, zygotes and ookinetes which has been studied as a malaria transmission-blocking vaccine antigen (Kaslow et al., 1988). Pfs25 has shown less-than ideal immunogenicity in humans trials (Talaat et al., 2016) that has motivated approaches such as conjugation to toxins (Radtke et al., 2017), gold nanoparticles (Kumar et al., 2015b) and polymer particles (Kumar et al., 2015a), as well as multimerization techniques (Li et al., 2016).
Interest in liposomal vaccine adjuvants has increased over the past years. Composition and preparation technique impact features such as liposome size, charge, size distribution, and antigen complexation efficacy (Schwendener, 2014). Our lab reported a liposomal adjuvant that can sequester recombinant polyhistidine-tagged (his-tagged) Pfs25 on the bilayer surface and increase antigen immunogenicity with simple mixing of antigen and liposome at the time of immunization (Huang et al., 2018a). Via “spontaneous nanoliposome antigen particleization” (SNAP), antigens are stably presented in uniformly orientated display via his-tagged insertion in the bilayer and interaction with CoPoP (Huang et al., 2018a). The liposomes include cobalt porphyrin–phospholipid CoPoP, along with PHAD (Phosphorylated HexaAcyl Disaccharide), a synthetic variant of monophosphoryl lipid A (MPLA). MPLA is a modified and non-pyrogenic derivative of lipopolysaccharide (LPS) and is known for its immunostimulatory effects (Coccia et al., 2017; Sarti et al., 2011).
Although MPLA does not show toxicity in animals or humans (Baldrick et al., 2002; Casella and Mitchell, 2008), its manufacturing can be complex and pure synthetic versions require multi-step organic synthesis. MPLA represents the most expensive component of the SNAP liposome formulation. In this study, we compared 3 structurally-similar synthetic MPLA variants within CoPoP liposomes, in terms of physical stability and the ability to produce functional antibodies. The types of MPLA used were PHAD; PHAD-504, which differs from PHAD in the length of a single fatty acid chain; and PHAD-3D6A a 3-deacyl version of PHAD. The structures of the various synthetic MPLAs are shown in Figure S1. In our initial formulation (Huang et al., 2018a), we used 0.4 μg of PHAD per mouse with 0.1 μg antigen. This is a low dose relative to some studies with AS01, a clinically-used liposomal vaccine adjuvant containing MPLA and QS21, which has been tested pre-clinically with 5 μg of MPLA per mouse (Coccia et al., 2017). Further reduction of the MPLA component would additionally decrease overall costs.
Malaria is endemic mostly in countries with climates with highly elevated temperatures within the Equator, and also in developing African countries according to the United Nations List of Least Developed Countries and the World Health Organization (WHO) list of malaria-infected countries. This may cause problems in maintaining an effective cold chain during mass vaccination and thus there is a need for thermostable vaccines. One of the challenges of a large scale vaccine roll is a narrow range of storage temperature, and WHO guidelines and manufacturer product inserts recommend that most essential vaccines except oral polio vaccine be kept within a 6 degree range (2–8 °C) during distribution (WHO, 2006). Failure of cooling systems either by freezing or elevated temperature can lead to the inactivation of the vaccines. Freeze inactivation is a risk for liposomes as when they are subjected to freeze-thawing, vesicle fusion or leakage of vesicle contents can occur. Disaccharides such as trehalose and sucrose can act as a cryoprotectant to protect vesicle integrity during freezing (Harrigan et al., 1990). Developing a lyophilized formulation could not only afford protection against inadvertent freezing barrier but also exposure to elevated temperatures, as chemicals are generally less thermostable in liquid form compared to solid form following freeze-drying (Flood et al., 2016) or spray-drying (Kunda et al., 2019). This might eliminate the need of refrigeration altogether. In this work, we also develop a lyophilized Pfs25 SNAP vaccine using reduced MPLA that can maintain efficacy when stored at high temperatures (60 °C) for at least 6 weeks.
Materials and Methods
Materials
His-tagged Pfs25 was produced in a baculovirus system, as previously reported (Lee et al., 2016). Pfs230C1, another TBV candidate antigen, was produced in a baculovirus system as previously reported (Lee et al., 2017). Influenza His-tagged recombinant hemagglutinin (H3) was obtained from IRR (Catalog No. FR-1478), Borreliella burgdorferi outer surface protein A (Osp-A) was produced in E. coli as we recently described (Federizon et al., 2020). CoPoP was produced as previously described (Shao et al., 2015). The following lipids were obtained: 1,2-dipalmitoyl-sn-glycero-S-phosphocholine (DPPC, Corden Cat#LP-R4–057, cholesterol (PhytoChol, Wilshire Technologies), synthetic monophosphoryl lipid A (PHAD, Avanti Cat # 699800P), PHAD-504 (Avanti Cat # 699810), and PHAD-3D6A (Avanti Cat # 699855). Sucrose was obtained from VWR (Cat # 97061–428) and trehalose was obtained from TCI (Cat # 6138–23-4).
SNAP liposome preparation
Liposomes were prepared by ethanol injection followed by nitrogen-pressurized lipid extrusion in phosphate buffered saline (PBS) as previously described (Huang et al., 2018a). Briefly, lipids at a mass ratio of DPPC: cholesterol: CoPoP of 4:2:1 were mixed with different ratios and types of MPLA and then were extruded by nitrogen-pressurized lipid extruder at 60 °C followed by dialysis against PBS to remove the ethanol. The final liposome concentration was adjusted to 320 μg/mL of CoPoP equivalent and filtered through a 0.2 μm sterile filter and stored at 4 °C. For sedimentation after long term storage, the liposomes were centrifuged at 1500 × g for 5 min and photographed. Liposome sizes and polydispersity indices were determined by dynamic light scattering (DLS) with a NanoBrook 90 Plus PALS instrument after 200-fold dilution in PBS. To prepare SNAP vaccine, an equal volume of 80 μg/mL antigen was incubated at room temperature with 320 μg/mL CoPoP equivalent liposomes.
Pfs25 binding assay
To test the effect of the type and ratio of MPLA on the binding capacity of the liposomes, Pfs25 was incubated with the liposomes for 3 hours at room temperature and the mixture was then subjected to microcentrifugal filtration at 1500 × g for 1 hour through a 100 kDa filter (PALL Cat # 29300). The protein in the filtrate was assessed with the bicinchoninic acid test (Thermo Cat # 23235) to assess binding, which was calculated using the following equation, where OD is the optical density at 562 nm:
Lyophilized SNAP vaccines
Sucrose and trehalose were tested as cryoprotectants for the liposomes and the SNAP vaccine. Different amounts of the cryoprotectants were added to either the liposomes or the SNAP vaccine. The samples were frozen to −80 °C before lyophilization for at least 6 hours. The resulting powders were sealed in microcentrifuge tubes wrapped with parafilm until reconstitution with deionized water. The integrity of tube closure was not assessed.
Thermostability and binding to his-tagged Pfs25
To test the thermostability of the liposomes, the lipid content of the liquid and the lyophilized liposomes were assessed using liquid chromatography-mass spectrometry (LC-MS) after 2-week incubation at 40 or 60 °C. To test the binding capacity after incubation at different temperatures, Pfs25 was labelled by a fluorescence dye DY-490-NHS-Ester (Dyomics, Cat# 490–01) at a molar ratio of 1:5. 100 μg of antigen was dialyzed into 100 mM sodium bicarbonate buffer (pH= 9) for 4–6 hours at 4 °C twice and later labeled with DY-490 at room temperature for 1 hour, followed by dialysis using slide-a-lyzer (Thermo, Cat # 87729) against PBS three times at 4 °C to remove the free dye. After incubation with the liposomes for 3 hours at room temperature, the mixture was subjected to microcentrifugation through a 100 kDa filter at 1500 ×g for 1 hour (PALL Cat # 29300). The protein in the filtrate was assessed through the fluorophore fluorescence using a microplate reader (TECAN Safire). The binding capacity was calculated using the following equation, where Fl is the fluorescence with excitation at 490 nm and emission at 515 nm
Cryo-electron microscopy
C-flat™ holey carbon grids CFT-2/2–2C washed with chloroform overnight were glow discharged for 15 seconds at 5 mA immediately before the application of the sample. For each grid, a volume of 3.6 μL of the sample was deposited in the grid. All samples were applied to the grids without further dilution and at the concentration indicated in the preparation method described above. Vitrification was performed in a Vitrobot Mark IV (ThermoFisher) by blotting the grids for 3 seconds at a blot force of +2. The blotting chamber in the Vitrobot was set at a relative humidity of 100% and a temperature of 25 °C. Subsequently, grids were plunged into liquid ethane cooled down to near liquid nitrogen temperature. The cryo-TEM images were acquired on a Tecnai F20 electron microscope operated at 200kV using a Gatan 626 single tilt cryo-holder. Images were collected in a Gatan Ultrascan 4000 4k x 4k CCD Camera System Model 895 at a nominal magnification 60,000. Images produced by this camera had a calibrated pixel size of 1.8Å / pixel). The total electron dose per image was ~50 e- /Å2. Images were collected using a defocus range from −2.7 to −3.5 μm. Images were prepared for figures using Adobe Photoshop program.
LC-MS analysis of DPPC and CoPoP
Lipid extraction and experimental protocols for chromatographic separation and MS for liposome samples were performed as previously described with slight modification (Luo et al., 2016; Miranda et al., 2018). The lipid extracts (resuspended in 1:1 chloroform: methanol) were injected to LC-MS for the analysis of CoPoP and DPPC. Mobile phases A and B were 95:5 water:methanol (v/v) and 60:35:5 isopropanol:methanol:water, respectively. Mobile phases were supplemented with 0.1% (v/v) formic acid and 5 mM ammonium formate. For DPPC, the LC gradient started after 3 min at 0 % B and then increased to 100 % B over 10 min followed by 100% B for 7 min. For CoPoP, the LC gradient started after 5 min at 0% B and then increased to 100% B over 40 min followed by 100% B for 7 min.
For targeted analysis of DPPC and CoPoP, the corresponding m/z for each ion (for DPPC m/z=734.5694, [M+H]+; and for CoPoP m/z = 1068.5020 [M]+) was extracted in MassHunter Qualitative Analysis (version B.06.00, Agilent Technologies). Peak areas for each ion in extracted ion chromatograms (EICs) were manually integrated and were presented as ion counts. The identity of observed m/z = 1068.4930 was further confirmed by MS/MS at 15, 35 and 55 V (Luo et al., 2016). Based on m/z= 184.0693, 530.1478, 885.4455, 1009.4237 fragments, m/z=1068.4930 is confirmed as CoPoP.
Immunoprecipitation
Anti-Pfs25 human mAbs 1269 and 1245 which can bind to defined sites “1” and “2”, respectively on Pfs25 as previously described (Scally et al., 2017) were used to test Pfs25 conformational integrity. Each antibody was incubated separately with protein G magnetic beads (New England Biolabs, Cat # S1430S) according to the manufacturer protocol. Then Pfs25 (1.5 μg), either in liquid or reconstituted after lyophilization with 2.5% sucrose, were preincubated at different temperatures for 1 hour with the antibody-bound beads. The beads were then washed 3 times with PBS and were incubated with SDS loading dye for protein analysis with SDS-PAGE.
Mice vaccination
Animal studies were carried out following protocols approved by the University at Buffalo IACUC. For comparison of liposomes containing various MPLA types and concentrations, liposomes were prepared with [DPPC: Cholesterol: MPLA: CoPoP=4:2:(0.2–1):1] mass ratio. Liposomes were admixed with antigen 3 hours prior to immunization unless otherwise noted. 5–6 week old outbred female ICR mice (Envigo) received two intramuscular injections of 10 ng Pfs25 SNAP equivalent on days 0 and 21. Sera were collected on day 42 for ELISA and standard membrane feeding assay (SMFA). For biological activity testing after exposure to elevated temperatures (Figure 5), liquid and 2.5% sucrose lyophilized Pfs25 SNAP vaccine prepared with [DPPC: Chol: PHAD-504: CoPoP=4:2:0.4:1] mass ratio were incubated at different temperatures (4, 40 or 60 °C) for different time points (2, 4 and 6 weeks). 5–6 week old outbred female ICR mice were injected intramuscularly with 100 ng Pfs25 equivalent SNAP formulation per mouse. 21 days after the initial dose, a booster dose was administrated. At the end of the vaccination (42 days from the initial immunization), serum was collected for ELISA and SMFA.
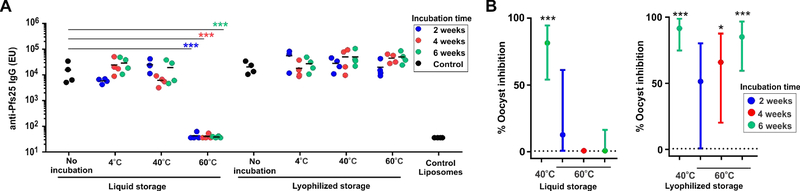
Figure 5. Immunogenicity of lyophilized and liquid Pfs25-bound CoPoP liposomes following storage at elevated temperatures.
Mice were immunized with 100 ng Pfs25 that was first complexed with CoPoP liposomes and stored at the indicated temperatures and durations. A) Anti-Pfs25 IgG ELISA. Statistical analysis based on one-way ANOVA followed by Tukey test, using log transformed data (***P<0.001). Lines represent geometric mean. B) Functional testing in the SMFA. The inhibition activity statistical analysis is based on a zero-inflated negative binomial random effects model and asterisks show significant inhibition compared to the post-immune IgGs from mice immunized with CoPoP liposome without Pfs25. ***P<0.001 and *P < 0.05. Lines show upper and lower 95% confidence intervals. Negative values are assigned as zero for presentation purpose.
New Zealand white rabbit immunization
Rabbit studies were carried out at Pocono Rabbit Farm (Canadensis, PA), following protocols approved by the Pocono Rabbit Farm IACUC. 10–12 weeks old female rabbits received intramuscular injections on day 0 and day 28 of 20 μg of Pfs25 equivalent SNAP formulation. Liposomes were prepared with [DPPC: Cholesterol: PHAD-504: CoPoP=4:2:0.4:1] mass ratio. Serum was collected on day 0, 28 and 56.
ELISA
Anti-Pfs25 IgG titer was measured by ELISA. For Figure 2D and Figure 5, ELISA was carried out at the Laboratory of Malaria and Vector Research (LMVR) at NIAID as previously described (Miura et al., 2008). For other ELISA data, a 96-well plate (Thermo Scientific Cat # Nunc 442404) was coated with 1 μg/mL of Pfs25 for 2 hours at 37 °C, followed by blocking with 2 % bovine serum albumin (BSA) in PBS containing 0.1% Tween20 (PBS-T) for another 2 hours. Later on, mice sera were serially diluted with 1% BSA in PBS-T for 1 hour at 37 °C, followed by incubation with horse radish peroxidase-conjugated goat anti-mouse secondary antibody IgG (Genscript # A00160) for another 30 min. Tetramethylbenzidine was added for color development and after stopping the reaction with HCl, endpoint titers were defined as the reciprocal serum dilution of absorbance (450 nm) with a cutoff value of at least 0.5. For sandwich ELISA to test the thermostability of Pfs25, the plates were coated with mAb 1245 as a capture antibody while mAb 1269 was used as a detection antibody.
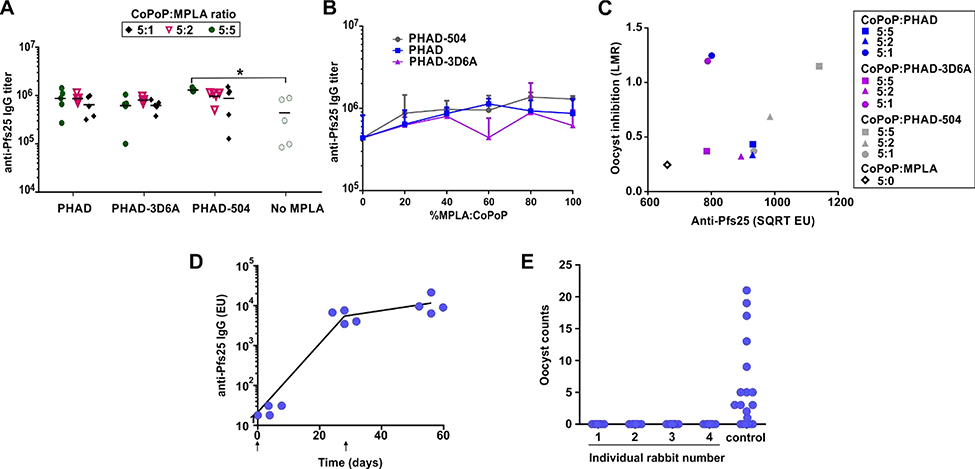
Figure 2. Vaccination using CoPoP liposomes containing varying amounts and types of MPLA.
ICR mice were immunized intramuscularly with 10 ng of Pfs25 and indicated CoPoP liposomes on day 0 and day 21 with serum collection on day 42 (A-C). A) Anti-Pfs25 IgG ELISA data. B) Anti-Pfs25 ELISA as a function of the MPLA ratio of liposomes. C) Functional activity of anti-Pfs25 IgGs judged by SMFA, Pooled IgGs were tested at 750 μg/mL. Anti-Pfs25 in the feeder (on a square root scale, SQRT) and the ratio of mean oocyst (mean oocyst density in control divided by mean in test) is plotted on a log scale (log of mean oocyst ratio, LMR). D) Anti-Pfs25 IgG ELISA from rabbits, injected intramuscularly at day 0 and day 28 (arrows), with serum collected at days 0, 28 and 56 E) SMFA results from IgGs purified from the day 56 rabbit sera. Experiments were performed with n = 20 mosquitos. Figure A was performed with n = 5 and the line shows the geometric mean. * P<0.05 based on one-way ANOVA followed by Tukey test, using log-transformed data.
Standard Membrane-Feeding Assay (SMFA)
Collected mouse and rabbit sera were sent to the LMVR to perform SMFA. Briefly, post-immune sera from all mice from each group were pooled, and the total IgG was purified. For the rabbit sera, total IgGs were purified individually. The mixture of test IgG (0.75 mg/mL for mouse IgGs, and 3.75 mg/mL for rabbit IgGs) and a mature gametocyte culture of P. falciparum (NF54 strain) was fed to Anopheles stephensi (Nijmegen strain) mosquitoes through a membrane feeding apparatus in the absence (for mouse IgGs) or presence (rabbit IgGs) of human complement. After eight days, the midgut of the mosquitoes was dissected to enumerate the oocysts.
Results and discussion
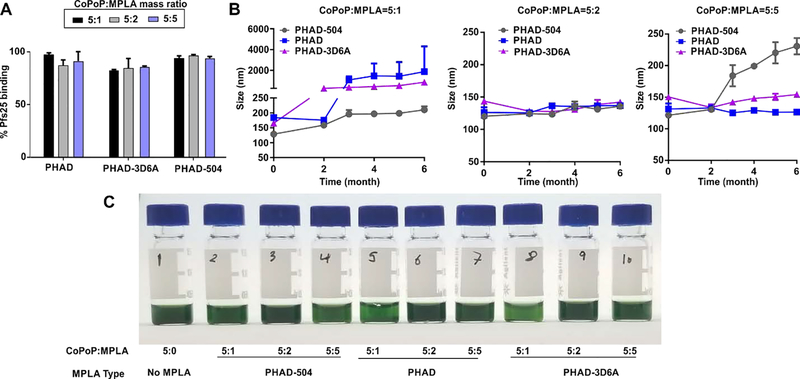
Different batches of liposomes were formed from three different synthetic MPLA variants (PHAD, PHAD-504 and PHAD-3D6A) in varying concentrations relative to the CoPoP component. They were assessed in terms of binding to recombinant Pfs25 and storage stability. The type and ratio of different MPLA amounts did not impact the binding of Pfs25 to the liposomes, as shown in Figure 1A, as all liposomes effectively bound the antigen. The physiochemical characteristics, with respect to hydrodynamic size, varied over 6 months of storage at 4 °C for some formulations. However, all liposomes in the 5:2 mass ratio of CoPoP:MPLA were stable over the 6 months period, as shown in Figure 1B. Formulations with the lower 5:1 mass ratio of CoPoP :MPLA tended to aggregate over storage time prominently. MPLA, which includes a phosphate group and carbohydrate structures, may induce steric stabilization on the liposomes when incorporated in the bilayer in sufficient quantity. Results from additional ratios of MPLA are shown in Figure S2. The formulations which showed aggregation in long term storage at 4 °C, as measured by DLS, also showed a lighter green color after the removal of any precipitation with low-speed centrifugation (Figure 1C) which reflects lower CoPoP concentration in suspension due to liposomal aggregation and precipitation upon centrifugation.
Figure 1. Characterization of CoPoP liposomes containing varying types and amounts of MPLA.
A) Pfs25 binding to the liposomes containing the indicated type of MPLA and ratio of CoPoP:MPLA B) Colloidal stability of the liposomes stored at 4 °C. C) Photograph of different formulations after 6 months of storage and low-speed centrifugation to remove precipitates.
To assess antibody production of the different liposome adjuvants, ICR mice were vaccinated with a low dose of 10 ng of Pfs25, admixed with liposomes before immunization. The CoPoP dose was 40 ng, and the MPLA dose ranged from 0 to 40 ng. Liposomes containing all three MPLAs were able to produce same levels of anti-Pfs25 IgG titer at all MPLA ratios assessed (Figure 2A). However, PHAD-504 at the ratio 5:5 with CoPoP showed the highest titers, which was statistically significant different from the group immunized with liposomes without MPLA. Overall, there was no clear trend to relate the total anti-Pfs25 antibody production to MPLA type or ratios as shown in (Figure 2B), and otherwise there were few significant differences between the antibodies produced by the various groups. All 5:2 formulations showed similar levels of antibody titers but, only PHAD-504 group showed significant improved functionality manifested as oocyst formation inhibition when tested with SMFA as shown in Figure S3.
To compare the quality (i.e., level of antibody which is required to show the same level of inhibition in SMFA) of the produced antibodies, the square root of ELISA units was plotted against the log of mean oocyst ratio (LMR) for the SMFA data. This plot generally produces a linear relationship between oocyst inhibition and antibody concentration, for antibodies of equal quality (Miura et al., 2019). Figure 2C shows a quasi linear relationship for 5:5, 5:2 and 5:0 (without MPLA) liposome formulations, indicating the quality of induced antibodies were similar among them. Raw data are shown in Table S1. Interestingly, the 5:1 liposome formulations with either PHAD or PHAD-3D6A, which suffered from large aggregated size during storage (Figure 1B), appeared to be outliers, with the generated antibodies holding higher functionality in the SMFA. While the mechanisms of this may be interesting to study in further detail, the use of large aggregated material faces challenges in batch to batch reproducibility and also is not possible to sterile filter.
As liposomes formed with CoPoP:PHAD-504 at a ratio of 5:2 showed good activity in the production of functional antibodies and had acceptable stability in storage with respect to size, this formulation was further studied. These liposomes were shown to enhance uptake of Pfs25 into macrophages, as we have previously reported is a key mechanism for CoPoP to enhance the immunogenicity of this antigen (Figure S4) (Huang et al., 2018a). This formulation was next used for a rabbit immunization study, in order to determine the impact of the reduced-MPLA vaccine approach in a larger animal species. where a group of 4 rabbits were vaccinated with 20 μg Pfs25 (and 80 μg CoPoP and 32 μg PHAD-504) per rabbit on day zero and day 28. Production of high anti-Pfs25 IgG was observed 28 days after the initial immunization and was further increased after the booster dose, as shown in Figure 2D. Antibodies from the collected serum at day 56 were used for SMFA testing, where complete inhibition of oocyst formation in mosquito fed these IgGs was observed in all 4 of the rabbits, as shown in Figure 2E.
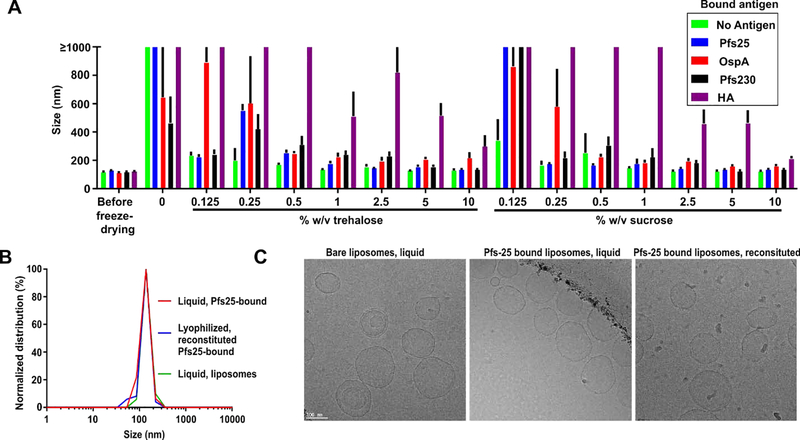
Next, lyophilization was assessed using liposomes with CoPoP:PHAD-504 at a ratio of 5:2. Several antigens that our lab has worked with were bound to the liposomes to assess lyophilization behavior and method versatility. Besides Pfs25 (25 kDa), these included another malaria TBV antigen Pfs230C1 (44 kDa), Lyme Disease antigen OspA (29 kDa), and a hemagglutinin trimeric antigen of the H3 subtype (60 kDa monomer). We assessed these other antigens in order to determine whether a generalized lyophilization approach would be feasible for multiple different proteins. The liposome cryoprotectants sucrose and trehalose were used as these disaccharides are known to prevent fusion or aggregation of liposomes with lyophilization (Kannan et al., 2015). To prepare lyophilized formulations, sucrose or trehalose were added with concentration ranging of 0.125 – 10 % w/v to the liposomes either pre-bound to one of the different proteins or as free liposomes in phosphate buffered saline (PBS). The solution was mixed and then frozen at −80 °C and then lyophilized. The resulting powder was resuspended in deionized water by simple shaking of the tube, and the size was assessed using DLS. As shown in Figure 3A, both sucrose and trehalose were effective at inhibiting antigen-bound liposome aggregation during lyophilization and reconstitution overall, except with HA. For bare liposomes, as little as 0.125 % cryoprotectant inhibited liposome aggregation, whereas, for some protein-bound liposomes, a greater amount was required. In the absence of cryoprotectants, some proteins (Pfs230 and OspA) imparted resistance against lyophilization-induced fusion and aggregation, relative to bare liposomes. This might be due to the binding of his-tagged proteins to the liposomal surface leading to its stabilization where amino acids can stabilize liposomes by hydrogen bonds and electrostatic charges (Mohammed et al., 2007). In general, liposomal aggregation was better inhibited as the molecular weight of the attached protein decreased. Based on the re-dispersibility index (RI), the ratio of size after reconstitution compared the size before lyophilization, liposomes with pre-bound H3 needed 10 % sucrose for a RI of 1.75 while Pfs25 pre-bound liposomes needed only 2.5% sucrose to reach a RI of 1.12 where the size was 134 ± 4 nm with a polydispersity index (PDI) of 0.14 ± 0.02. The reason for this trend is not clear. Bare liposomes were difficult to re-dissolve and showed large visible aggregates when lyophilized with no cryoprotectant, but showed RI of 1.05 when lyophilized with 2.5 % sucrose with a size of 126 ± 3 nm with PDI of 0.09 ± 0.004 which did not differ significantly from before lyophilization (Figure S5). On the other hand, all the formulations acquired negative zeta potential which contributes to the electrostatic repulsion among the particles increasing colloidal stability with no significant effect observed following lyophilization or Pfs25 binding (Figure S5). Similar trends were observed with trehalose, with sucrose appearing slightly more effective for achieving low RI after reconstitution. For Pfs25, the RI showed no further enhancement upon increasing the sucrose concentration further than 2.5 % w/v. The size distribution that was similar to the liposomes themselves, both before and after lyophilization and reconstitution (Figure 3B). Cryo-TEM images showed liposomes having a bilayer with two electron dense bilayer leaflets and a lighter middle layer, with overall spherical morphology (Figure 3C). This appearance appeared largely unaltered for the bare liposomes with or without Pfs25 bound. The Pfs25 antigen would be too small to observe on the TEM. After reconstitution of the lyophilized Pfs25-bound liposomes, they re-adopted their prior morphology for the most part, without obvious fusion or aggregation Some precipitated material was visible as small aggregates following reconstitution, but more research would be required to determine from where this originated and its significance, if any.
Figure 3. Lyophilization of CoPoP:PHAD-504 (5:2) liposomes.
A) Size of the reconstituted lyophilized liposomes using sucrose or trehalose as cryoprotectant at different ratios as measured by DLS. Different proteins were incubated for 3 hours at a weight ratio of 1:4 with the CoPoP liposomal constituent before the addition of the cryoprotectant, lyophilization and re-constitution B) Size distribution of blank liposomes and Pfs25 bound before and after lyophilization using 2.5% sucrose. C) Cryo-TEM images of empty liposomes (left), Pfs25 bound liposomes (middle) and reconstituted lyophilized (with 2.5% sucrose) Pfs25 pre-bound liposomes (right). A 100 nm scale bar is shown. Bar graph in A shows mean ± s.d for n = 3.
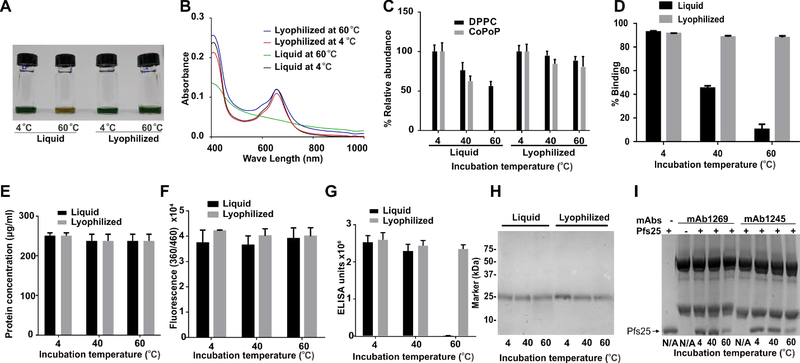
To assess the thermostability of the freeze-dried liposomes, 2.5 % w/v sucrose was used as a cryoprotectant, and the lyophilized formulation, along with the liquid one (before lyophilization), were incubated at 4, 40 or 60°C for two weeks. At the end of the incubation, the lyophilized formulation was rehydrated using deionized water to its original volume and composition with simple shaking. By visual inspection, the liquid formulation at 60 °C lost most of its green color. At the same time, the reconstituted lyophilized formula incubated at the same temperature for two weeks preserved it, as shown in Figure 4A. This is confirmed by the visible absorbance spectrum (Figure 4B), which revealed the loss of the characteristic Q-band peak of CoPoP at 670 nm, which indicates the presence of an intact chlorin macrocycle. On the other hand, the lyophilized formula preserved the intact CoPoP Q-band optical absorption during the incubation period.
Figure 4. Thermostability of liquid or lyophilized Pfs25 SNAP vaccine.
A-D) Accelerated thermal degradation of the liposomes lyophilized and liquid formula stored at 4, 40 and 60 °C for 2 weeks A) Photo of liquid lyophilized formula after reconstitution B) UV-VIS spectrum. C) LC/MS analysis of lipid content D) Pfs25 binding to the liposomes determined by microcentrifugal filtration, E-I) Forced thermal degradation of Pfs25 stored at 4, 40 and 60 °C for 2 weeks E) Total protein content after incubation determined by the BCA assay. F) Free amino groups determined by the OPA assay. G) Sandwich ELISA of the pre-incubated proteins, mAb 1245 was used as a capture antibody while mAb 1269 was used as the detection antibody. H) SDS-PAGE of Pfs25 after storage at various temperatures. I) SDS-PAGE after immune precipitations using anti-Pfs25 monoclonal antibodies using either mAb 1245 or mAb 1269 to capture Pfs25 after incubation at different temperatures for two weeks, the arrow indicates Pfs25 band while the other two bands are the heavy and the light chains of the mAbs. The first lane contains 1μg of Pfs25, second and sixth lane contain only mAbs. Bar graphs in C-G show mean ± s.d. for n = 3.
For more insight into the lipid composition and thermostability, LC-MS was performed following lipid extraction using chloroform. Figure 4C shows the relative abundance of DPPC and CoPoP lipids. The liquid formulation stored at 60 °C lost all intact CoPoP content, while only about 64 % was preserved at 40 °C. On the other hand, at 4 °C, CoPoP remained fully intact. The lyophilized formula was more thermostable and preserved approximately 80% of the CoPoP lipid at 60 °C. As CoPoP is the functional anchor for the his-tagged proteins, the binding capacity of the lyophilized blank liposomes were assessed using fluorescently labeled Pfs25 with a microcentrifugal filtration assay. The liquid formulation stored at 60 °C showed only about 10% binding, whereas the lyophilized and reconstituted one achieved 88% antigen binding (similar to the initial 93 % antigen binding of the liposomes) as shown in Figure 4D.
To check the thermal stability of Pfs25, which is relatively stable owing to its 11 disulfide bonds that would maintain the protein tertiary structure (Kumar et al., 2014; Lee et al., 2016), recombinant Pfs25 was incubated at 4, 40 and 60 °C in both liquid and 2.5% sucrose lyophilized forms. Disaccharides are known to preserve the native conformation of a dried protein (Allison et al., 2000). Thus, Pfs25 was lyophilized in the same conditions of the liposomes (2.5% sucrose) and incubated in both liquid and lyophilized form at 4, 40 and 60 °C. At the end of the 2-week incubation period, the lyophilized Pfs25 was re-dissolved in the same amount of deionized water as before the freeze-drying. The total protein content of all the samples was tested using o-phthaldialdehyde (OPA) and bicinchoninic acid (BCA) tests, as shown in Figure 4E & F, which showed that the concentration of the proteins was not altered. SDS-PAGE showed there was no severe biochemical cleavage and biochemical degradation of the protein in storage, with the exception for liquid storage at 60 °C, which showed a minor smear of smaller molecular weight, which likely represents degradation products (Figure 4H). To test the protein for proper folding, a sandwich ELISA was carried out using two non-competitive conformational anti-Pfs25 antibodies (mAb 1245 recognizes “site 2” in Pfs25 molecule and mAb 1269 for “site 1 a and b”) (Scally et al., 2017). mAb 1245 was used as capture antibody while mAb 1269 was used as detection antibody in the ELISA procedure. While both liquid and lyophilized formulations showed high conformational ELISA reactivity at 40 °C, only the lyophilized protein sustained this with storage at 60 °C as shown in Figure 4G. Despite the resistance of degradation in the primary structure of the protein, at 60 °C the protein lost at in part its conformational stability at site 1 or site 2. This is consistent with another report that showed that Pfs25 loses conformational antibody recognition when incubated at 60 °C for 11 days (Zhu et al., 2017). To elucidate which antibody binding site was impacted, the liquid formulations were subjected to immunoprecipitation using mAbs 1245 and 1269 antibodies, followed by SDS-PAGE. As shown in Figure 4I, both antibodies failed to fully bind to Pfs25 as a result of partial loss of conformational recognition sites, as indicated by the faint bands shown for 60 °C for both antibodies when compared to the protein band at the same concentration. When stored at 4 and 40 °C Pfs25 showed full protein capture in both antibodies.
Next, we assessed the efficacy of a “ready-to-reconstitute-and-inject” lyophilized vaccine. Pfs25 was bound to the liposomes in PBS before lyophilization. Then, to test thermostability, both the lyophilized and the liquid formulations were incubated at 4, 40 or 60 °C for 2, 4 or 6 weeks. At the end of the incubation period, the lyophilized Pfs25 liposomal vaccine was rehydrated with deionized water to its original volume and mice were vaccinated with 100 ng of antigen (400 ng CoPoP and 160 ng PHAD-504). A booster dose was administrated after 21 days of the initial dose injection, and serum was collected on day 42. As shown in Figure 5A, ELISA showed that the liquid formulation failed to elicit any antibody response following storage at 60 °C for any of the incubation times. On the other hand, the lyophilized formulation was able to induce antibodies following at least 6 weeks of incubation at 60 °C, 40 °C and 4 °C.
Antibodies were then assessed for functional activity to inhibit oocyst formation in mosquito midguts in the SMFA. As the SMFA featured a low oocyst intensity in the control IgG, two independent assays were performed (Tables S2, S3). Best estimates of inhibition from the two assays were then calculated using a zero-inflated negative binomial random effects model (Miura et al., 2016), and are shown in Figure 5B and Table S4. The liquid vaccine formulation stored at 60 °C did not induce functional antibodies in the SMFA, with less than 12% inhibition observed when stored for any of the durations tested. On the other hand, the lyophilized formulations significantly preserved the activity of the vaccine over 4 and 6 weeks of storage at 60 °C, and all-time points induced IgG with at least 64% inhibition of oocyst formation. Notably, both lyophilized and liquid Pfs25-CoPoP formulations were able to induce significant functional activity in the SMFA when stored at 40 °C for 6 weeks, which is in agreement with biochemical data showing thermostability in these storage conditions.
Conclusion
In this work, CoPoP liposomes were initially assessed with various types and compositions of synthetic MPLA. The MPLA ratio could be decreased to a CoPoP:MPLA mass ratio of 5:2 without sacrificing physical storage stability or adjuvant immunogenicity. This formulation was amenable to lyophilization and effective reconstitution, when a limited amount of disaccharide was included as a cryoprotectant in the lyophilisate, including 2.5 % sucrose. The liposomes showed the capability of lyophilized storage and reconstitution on their own, or pre-bound to a variety of different his-tagged antigens. The lyophilized formulation of Pfs25 was thermostable and could withstand storage at temperatures of 60 °C for at least 6 weeks while still inducing functional transmission blocking antibodies in mice with 100 ng antigen and 160 ng MPLA. Some limitations of this study include that vial closure integrity was not assessed, nor was the impact of humidity on vaccine stability. Further studies addressing these issues should be conducted, as should testing the stability with longer storage durations. Taken together, this study shows that the CoPoP liposome platform is effective as a vaccine adjuvant with reduced MPLA content and in a thermostable lyophilized format.
Supplementary Material
Acknowledgements
Pfs25, Pfs230C1, 1245 and 1269 were supplied by PATH’s Malaria Vaccine Initiative. This study was supported by PATH’s Malaria Vaccine Initiative, the National Institutes of Health (R21AI122964 and R01CA247771), the intramural program of the National Institute of Allergy and Infectious Diseases/NIH, the Global Health Innovative Technology Fund and a Fulbright scholarship (MM). Recombinant H3 HA with polyhistidine tag, FR-1478, was obtained through the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, Centers for Disease Control and Prevention, Atlanta, GA, USA. We thank Kelly Sears, Mike Strauss, Kaustuv Basu and other staff members of the Facility for Electron Microscopy Research (FEMR) at McGill University for help in electron microscope operation and data collection. FEMR is supported by the Canadian Foundation for Innovation, Quebec government and McGill University.
Footnotes
Declaration of interests
Jonathan Lovell and Wei-Chiao Huang hold equity in POP Biotechnologies. Other authors have no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- UN List of Least Developed Countries (United Nations, December 2018). [Google Scholar]
- WHO World malaria report 2018 (World Health Organization,2018). [Google Scholar]
- Allison SD, Manning MC, Randolph TW, Middleton K, Davis A, Carpenter JF, 2000. Optimization of storage stability of lyophilized actin using combinations of disaccharides and dextran. J. Pharm. Sci 89, 199–214. [DOI] [PubMed] [Google Scholar]
- Baldrick P, Richardson D, Elliott G, Wheeler AW, 2002. Safety evaluation of monophosphoryl lipid A (MPL): an immunostimulatory adjuvant. Regulatory Toxicology and Pharmacology 35, 398–413. [DOI] [PubMed] [Google Scholar]
- Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR, 2003. Thermal stability of vaccines. J. Pharm. Sci 92, 218–231. [DOI] [PubMed] [Google Scholar]
- Casella CR, Mitchell TC, 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cellular and molecular life sciences 65, 3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M, Collignon C, Hervé C, Chalon A, Welsby I, Detienne S, van Helden MJ, Dutta S, Genito CJ, Waters NC, 2017. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ vaccines 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CH, Rappuoli R, Hotez PJ, Duffy PE, 2019. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends Parasitol. 35, 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehreth J, 2003. The global value of vaccination. Vaccine 21, 596–600. [DOI] [PubMed] [Google Scholar]
- Federizon J, Frye A, Huang W-C, Hart TM, He X, Beltran C, Marcinkiewicz AL, Mainprize IL, Wills MKB, Lin Y-P, Lovell JF, 2020. Immunogenicity of the Lyme disease antigen OspA, particleized by cobalt porphyrin-phospholipid liposomes. Vaccine 38, 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood A, Estrada M, McAdams D, Ji Y, Chen D, 2016. Development of a freeze-dried, heat-stable influenza subunit vaccine formulation. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka AM, Milstien JB, Zaffran M, 1998. Thermostabilité de vaccins. Genève: Organisation mondiale de la Santé. [Google Scholar]
- Harrigan P, Madden T, Cullis P, 1990. Protection of liposomes during dehydration or freezing. Chem. Phys. Lipids 52, 139–149. [DOI] [PubMed] [Google Scholar]
- Huang W-C, Deng B, Lin C, Carter KA, Geng J, Razi A, He X, Chitgupi U, Federizon J, Sun B, 2018a. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nature nanotechnology 13, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W-C, Sia ZR, Lovell JF, 2018b. Adjuvant and Antigen Systems for Malaria Transmission-Blocking Vaccines. Advanced Biosystems 2, 1800011. [Google Scholar]
- Kannan V, Balabathula P, Thoma LA, Wood GC, 2015. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. Journal of liposome research 25, 270–278. [DOI] [PubMed] [Google Scholar]
- Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH, 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74. [DOI] [PubMed] [Google Scholar]
- Kumar R, Angov E, Kumar N, 2014. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infection and immunity 82, 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ledet G, Graves R, Datta D, Robinson S, Bansal GP, Mandal T, Kumar N, 2015a. Potent Functional Immunogenicity of Plasmodium falciparum Transmission-Blocking Antigen (Pfs25) Delivered with Nanoemulsion and Porous Polymeric Nanoparticles. Pharm. Res 32, 3827–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ray PC, Datta D, Bansal GP, Angov E, Kumar N, 2015b. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 33, 5064–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda NK, Peabody J, Zhai L, Price DN, Chackerian B, Tumban E, Muttil P, 2019. Evaluation of the thermal stability and the protective efficacy of spray-dried HPV vaccine, Gardasil 9. Hum. Vaccin. Immunother 15, 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-M, Wu C-K, Plieskatt J, McAdams DH, Miura K, Ockenhouse C, King CR, 2016. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malar. J 15, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-M, Wu C-K, Plieskatt JL, Miura K, Hickey JM, King CR, 2017. N-terminal Pfs230 domain produced in baculovirus as a biological active transmission-blocking vaccine candidate. Clin. Vaccine Immunol 24, e00140–00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MDJ, Fyfe AJ, Zakutansky SE, de Cassan S, Long CA, Draper SJ, Hill AVS, Hill F, Biswas S, 2016. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep 6, 18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Li N, Carter KA, Lin C, Geng J, Shao S, Huang WC, Qin Y, Atilla-Gokcumen GE, Lovell JF, 2016. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin–phospholipids. Small 12, 3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda D, Li N, Li C, Stefanovic F, Atilla-Gokcumen GE, Lovell JF, 2018. Detection of Sunlight Exposure with Solar-Sensitive Liposomes that Capture and Release Food Dyes. ACS Applied Nano Materials 1, 2739–2747. [Google Scholar]
- Miura K, Deng B, Wu Y, Zhou L, Pham TP, Diouf A, Wu C-K, Lee S-M, Plieskatt JL, Morin MJ, 2019. ELISA units, IgG subclass ratio and avidity determined functional activity of mouse anti-Pfs230 antibodies judged by a standard membrane-feeding assay with Plasmodium falciparum. Vaccine 37, 2073–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA, 2008. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Stone WJ, Koolen KM, Deng B, Zhou L, van Gemert G-J, Locke E, Morin M, Bousema T, Sauerwein RW, 2016. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar. J 15, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed AR, Coombes AG, Perrie Y, 2007. Amino acids as cryoprotectants for liposomal delivery systems. Eur. J. Pharm. Sci 30, 406–413. [DOI] [PubMed] [Google Scholar]
- Nunes JK, Woods C, Carter T, Raphael T, Morin MJ, Diallo D, Leboulleux D, Jain S, Loucq C, Kaslow DC, 2014. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine 32, 5531–5539. [DOI] [PubMed] [Google Scholar]
- Radtke AJ, Anderson CF, Riteau N, Rausch K, Scaria P, Kelnhofer ER, Howard RF, Sher A, Germain RN, Duffy P, 2017. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci. Rep 7, 40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, Kiparissides C, Bernkop-Schnürch A, 2011. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials 32, 4052–4057. [DOI] [PubMed] [Google Scholar]
- Scally SW, McLeod B, Bosch A, Miura K, Liang Q, Carroll S, Reponen S, Nguyen N, Giladi E, Rämisch S, 2017. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nature communications 8, 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendener RA, 2014. Liposomes as vaccine delivery systems: a review of the recent advances. Therapeutic advances in vaccines 2, 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Geng J, Yi HA, Gogia S, Neelamegham S, Jacobs A, Lovell JF, 2015. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem 7, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, Rausch KM, Zhu D, Muratova O, Herrera R, Anderson C, Jones D, Aebig J, Brockley S, MacDonald NJ, Wang X, Fay MP, Healy SA, Durbin AP, Narum DL, Wu Y, Duffy PE, 2016. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel, a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naïve Adults. PLoS One 11, e0163144–e0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2006. Temperature sensitivity of vaccines. Geneva: World Health Organization. [Google Scholar]
- Zaffran M, 1996. Vaccine transport and storage: environmental challenges. Dev. Biol. Stand 87, 9–17. [PubMed] [Google Scholar]
- Zhu D, Wu Y, McClellan H, Dai W, Rausch K, Jones D, Aebig J, Barnafo E, Butler B, Lambert L, 2017. Accelerated and long term stability study of Pfs25-EPA conjugates adjuvanted with Alhydrogel. Vaccine 35, 3232–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.