Abstract
One of the major advances in our understanding of gene regulation in eukaryotes was the discovery of factors that regulate transcription by controlling chromatin structure. Prominent among these discoveries was the demonstration that Gcn5 is a histone acetyltransferase, establishing a direct connection between transcriptional activation and histone acetylation. This breakthrough was soon followed by the purification of a protein complex that contains Gcn5, the SAGA complex. In this article, we review the early genetic and biochemical experiments that led to the discovery of SAGA and the elucidation of its multiple activities.
Keywords: SAGA, coactivator complex, transcription, histone acetylation, ubiquitination, TBP
1. Introduction
The role of chromatin structure in eukaryotic transcriptional regulation was uncertain for many years. While several studies revealed intriguing correlations between transcription and chromatin structure (for example [8, 9]), the causal relationship between the two was debated. Similarly, other studies suggested that post-translational modifications of histones, specifically histone acetylation, were required for the activation of transcription; however, this was again based upon an association between active transcription and acetylated histones [11].
The chromatin field moved forward dramatically in the 1990’s with important progress coming from biochemical studies in mammalian cell systems and from genetic studies in yeast. Several biochemical studies of mammalian transcriptional activators provided compelling evidence that nucleosomes were inhibitory to transcription in vitro and that the function of activators was to help overcome this repression (for example, [12–14]). Providing a strong complement to these biochemical studies, genetic studies in yeast provided compelling evidence that chromatin structure was dynamic in response to gene activation [15, 16], and furthermore, that altering histone levels caused transcriptional changes in vivo [17–19]. Importantly, a combination of genetic and biochemical studies demonstrated that chromatin structure itself was regulated by the Swi/Snf complex, a conserved chromatin remodeling complex [20–25]. This strengthened the connection between chromatin structure and transcriptional regulation, as Swi and Snf proteins were initially identified as transcriptional regulatory factors in yeast [26–28]; they are now known to be critical tumor suppressors in mammals [29].
A critical breakthrough in establishing the connection between histone acetylation and transcription came in 1996 when Gcn5, a conserved factor previously identified in yeast as a transcriptional regulatory protein, and proposed to function with a group of factors as a bridge between DNA-bound activators and the basal machinery [30–33], was demonstrated to be a histone acetyltransferase [34]. This discovery inspired a number of genetic and biochemical studies that coalesced on the identification of the SAGA (Spt Ada Gcn5 Acetyltransferase) complex [4]. SAGA was one of the first complexes shown to have coactivator activity in vivo, comprised of modular components that contribute diverse transcriptional regulatory functions [1–3, 35–38]. In this chapter, we provide a historical look at the studies that led to the identification of SAGA by our labs and others, and the subsequent studies that elucidated its functions in yeast.
2. The S of SAGA - the identification of SPT genes
SPT genes were identified via a yeast mutant screen whose initial goal was to understand the nature of eukaryotic transposable elements (Ty elements) with respect to their stability and effects on neighboring gene expression. At the time, it was not envisioned that the mutants would identify conserved transcription factors and chromatin proteins.
Ty transposable elements of S. cerevisiae are a family of heterogeneous elements that are structurally and genetically similar to mammalian proviruses [39–44]. Early studies revealed that Ty elements conferred mutant phenotypes when they transposed into the regulatory regions of genes [43, 45–47].
Two Ty insertions were isolated as spontaneous mutations at the HIS4 gene of S. cerevisiae [43]. Both Ty insertion mutations, his4–912 and his4–917, occurred in the 5’ noncoding region of HIS4 and caused histidine auxotrophy, although they differed by position, orientation, and sequence. Selection for His+ revertants of these two insertion mutations revealed many types of cis-acting rearrangements, including recombination between the long terminal repeats (δ sequences) of the Ty element, inversions, translocations, and gene conversions with other Ty elements in the yeast genome [44, 45, 48, 49].
Mutant hunts that focused on finding extragenic suppressors of Ty insertion mutations led to the identification of spt mutants [50, 51]. To avoid the genomic rearrangements isolated as suppressors of complete Ty insertions, these later selections started with single LTR derivatives of his4–912 and his4–917 [43], as well as a Ty-derived allele later isolated at the LYS2 gene [52]. The extragenic suppressors that were identified occurred in several unlinked genes, suggesting that several functions control the behavior of insertion mutations [50, 51]. The genes identified by these mutations were named SPT genes for Suppressor of Ty. Early studies showed that spt mutations suppressed at the level of transcription [51, 53–55]. Additional analysis showed that some, but not all spt mutations impaired transcription of Ty elements themselves [51, 55]. These results as well as additional phenotypic analyses elucidated two major classes of SPT genes, with both classes able to suppress overlapping but distinct classes of insertion mutations, as well as each class conferring distinct mutant phenotypes [56].
Additional studies of spt mutants connected these two classes of SPT genes to known proteins involved in transcription or chromatin structure. One study demonstrated that two SPT genes, initially called SPT11 and SPT12, were the same as two histone genes, HTA1 and HTB1, encoding histones H2A and H2B respectively [17]. These studies showed that either a reduced or increased level of this pair of histones caused suppression of his4–912δ by altering transcription, suggesting that altered histone stoichiometry was responsible. This was consistent with an earlier finding that altered levels of histone pairs impaired chromosome segregation [57]. The mutations of these histone genes caused phenotypes similar to mutations in four other SPT genes, SPT4, SPT5, SPT6, and SPT16, suggesting that all of these function in chromatin-mediated transcription, something now known to be true [16]. Spt4 and Spt5 were later identified in mammalian cells as the elongation factor DSIF (DRB-sensitivity inducing factor; [58, 59]), and Spt16 was later identified in mammalian cells as a component of FACT (Facilitates transcription on a chromatin template; [60]).
Second, the cloning of the SPT15 gene, a member of the second phenotypic class of SPT genes, revealed that it encoded the general transcription factor TATA-binding protein (TBP) [61]. This discovery occurred at the same time as studies from several other labs that used biochemical approaches to identify SPT15 as the gene encoding TBP [62–65]. Although SPT15 is essential for viability, the spt15 mutations isolated in the spt mutant hunt were viable and had genetic and transcriptional phenotypes similar to those caused by four other SPT genes: SPT3, SPT7, SPT8, and SPT20 [61]. The genetic connection of these genes to the gene encoding TBP suggested related roles in transcription initiation. As described below, the Spt3, Spt7, Spt8, and Spt20 proteins are all members of SAGA.
3. The A and G of SAGA – the identification of ADA genes, including the rediscovery of GCN5
ADA genes were identified using a genetic screen for mutations that reduce transcriptional activation. This genetic screen followed the discovery, via biochemical assays, of intermediary factors in transcriptional activation [66–68]. The previous prevailing model for transcriptional activation involved transcriptional activators bound to DNA at enhancer regulatory elements in mammalian cells or their viruses (defined in 1980’s) or at upstream activation sequences (UASs) in yeast, looping or interacting in cis with the basal transcriptional machinery (TFIIA, B, C, D, E, F, H; isolated in 1980’s) bound to DNA near the transcriptional start site [69–71]. However, a major gap in knowledge was the mechanism by which the DNA activators that were bound upstream interacted with the basal machinery: first, which basal factor is the direct target for activation and, second, does contact increase transcription, via basal factor localization, increased concentration, or allosteric change? Moreover, as discussed above, at that time there was a “split” between biochemists and molecular biologists working on transcription, and those working on the function of chromatin and histone modifications, in particular, whether chromatin alterations have a causal role in transcriptional regulation [72].
The in vitro transcription biochemical assay that eventually led to identification of ADA genes utilized a transcriptional activator specifically crippled for DNA binding but retaining an intact activation domain [66]. The concept was based on findings that highly expressed strong transcription factors in vivo can negatively affect transcription due to activation domain titration—or “squelching”—of an unknown critical transcription factor(s) [73]. Thus, in the in vitro assay, using a complete nuclear extract for the assay, which contained all components needed for gene activation, the mutant activator was seen to inhibit transcriptional activation but did not lower basal transcription. This observation suggested that the intact activation domain of the mutant activator bound to – and thus lowered accessibility within the extract – a proposed intermediary bridging factor(s) required specifically for activation. Hence, the direct target appeared to be an accessory factor rather than a basal factor. This factor was named “adaptor”, to reflect a proposed function as a bridge between DNA bound activator and the basal transcriptional machinery [66]. Contemporaneous with these experiments were additional discoveries pointing to accessory factors for transcriptional activation [67, 68], and taken together, these models launched the concept that intermediary factors (co-activators) are crucial for transcriptional activation [74].
Based on these models, the ADA genetic screen, conceptually based on squelching in vivo as described above [73], was set up in yeast to identify genes encoding intermediary factors. The screen selected for mutations that suppressed growth toxicity caused by artificially high expression of a transcriptional activator [33]. The hypothesis was that toxicity resulted from the over-expressed transcriptional activator binding, via its activation domain, to critical adaptors and making them unavailable to genes required for normal growth, and thus was parallel to the in vitro assay described above. Mutations in ADA genes lowered the growth toxicity, presumably by freeing up sufficient adaptor molecules to allow gene activation of the critical growth genes. The ADA mutations also lowered transcriptional activation without lowering basal transcription, again parallel to the initial biochemical identification of intermediary factors discussed above. Genes emerging from this screen were ADA1-ADA5 [1, 32, 33, 75], including two that had been previously isolated. ADA4 was previously isolated as GCN5 and AAS104 in earlier genetic screens [30, 31]. ADA5 was previously isolated as SPT20, as discussed below [75, 76].
The general model that emerged, that of transcriptional activators recruiting intermediary factors to promoters, provided a mechanism for location- and gene-specific chromatin modifications, including histone acetylation, via GCN5. The model informed the Brownell/Allis discovery of GCN5 as the first nuclear histone acetyltransferase (discussed below), providing a mechanistic framework for postulating how acetylation would activate specific genes [34, 77] (Figure 1). Indeed, subsequent studies demonstrated that specific amino acid substitutions in the Gcn5 activation domain that reduced gene transcription in vivo also lowered histone acetylation in vitro [78], and chromatin immunoprecipitation demonstrated histone acetylation at specific genes [79], together confirming that histone acetylation via a specific enzyme at a specific location was required for gene activation.
Figure 1.

A model for SAGA activation as an adaptor, or intermediary complex, to localize histone acetylation. The recruitment of SAGA by gene-specific activators results in gene-specific histone acetylation and transcriptional activation. Figure adapted from [6].
4. Connecting SPT and ADA genes
The idea that SPT and ADA genes might be involved in a common function came from the striking discovery that SPT20 and ADA5, isolated in distinct mutant hunts, were the same gene [75, 76]. Furthermore, these studies showed that ada5 mutants had a broader range of phenotypes compared to the other ada mutants (ada2, ada3, and gcn5), demonstrating two phenotypic classes of ADA genes. Subsequent analysis of the ADA1 gene showed that, like SPT20/ADA5, mutations caused both ada and spt mutant phenotypes [1]. The common phenotypes of mutations in SPT7, SPT20/ADA5, and ADA1 strongly suggested functional overlap. Furthermore, biochemical studies showing physical association of Ada proteins [1, 75, 80, 81] and separate studies showing association of Spt proteins [2], suggested the possibility of a protein complex that might contain all of these proteins.
5. The biochemical identification and characterization of SAGA
The identification of the Tetrahymena thermophila protein p55 as the first nuclear histone acetyltransferase (HAT) linked to a single polypeptide was a remarkable breakthrough [34, 77]. Their development of HAT assays [82] would ultimately allow for the identification of numerous such HAT enzymes. It also provided a direct biochemical link between the process of histone acetylation with transcriptional activation. Strikingly the sequence of p55 clearly identified it as a homolog of yeast Gcn5, a conserved protein that was also isolated in the aforementioned ADA screen as an adaptor required for the full activity of certain transcriptional activators [30, 33, 83].
However, in contrast to the potent in vitro HAT activity of Gcn5 on recombinant or “free” histones, Gcn5 was unable to efficiently acetylate nucleosomal histones [4, 82, 84]. A potential clue that might explain this apparent discrepancy was the fact that genetic screens in yeast, and protein interaction studies, identified Ada proteins as functionally interacting with Gcn5 and that Ada and Spt proteins share certain genetic functions and in some instances are encoded by the same gene (discussed above).
A collaboration between the labs of Workman, Berger, Winston, and Allis was subsequently launched. Using some of the same HAT assays developed by Brownell and Allis, large scale purification of HAT activities from yeast were expected to reveal Gcn5 isolated in its native form, and that either modification of Gcn5 or its physical interaction with other proteins would enable the enzyme to modify nucleosomal histones. This collaboration led to the discovery that native yeast Gcn5 exists in at least two high-molecular-weight complexes—and, indeed, as predicted, multiple subunits of which enable Gcn5 to acetylate nucleosomes [4]. The most notable of these complexes at the time was named SAGA (Spt-Ada-Gcn5 Acetyltransferase), based on the composition and known activity of the complex. It was discovered that SAGA preferentially acetylates multiple lysine residues on the N-terminal tails of histones H3 and H2B [84]. Notably, western blotting revealed that SAGA is composed of certain Ada and Spt proteins, fulfilling the genetic predictions that these proteins functionally interact with Gcn5 and one another. Further analysis of the composition and function of SAGA and its orthologs in larger eukaryotes has revealed that Gcn5 resides in a complex of 18–20 proteins (reviewed by [85, 86]). SAGA has served as a paradigm for our understanding of the regulation of HAT activity and the coupling of histone modifications, in general, with transcriptional co-activation from yeast to humans.
It is interesting that, while the discovery of SAGA was rapidly made after the report of Gcn5 HAT activity, the isolation of SAGA was actually quite fortuitous. It was found that Nickel-NTA agarose resin served to enrich for SAGA and other yeast HAT activities, including the Gcn5-containing ADA complex and the NuA3 and NuA4 HAT complexes ([4]; Figure 2), despite the absence of any known proteins containing stretches of histidines in these complexes. The use of Nickel-NTA agarose resin was likely key in the ability to purify large amounts of SAGA and other complexes from crude extracts, and to enable the eventual mass spectrometric identification of the components of these complexes. Indeed, in follow-up studies using hundreds of liters of yeast whole cell extracts, it was discovered SAGA is composed of at least three protein families known to function in gene expression: the Ada and Spt protein families, and a subset of TATA-binding protein (TBP)-associated factors (TAFs [4, 87]); and also the ATM-related cofactor Tra1 [88] that had been revealed as a coactivator interacting with activation domains [36, 89]. Importantly, the discovery of SAGA clarified years of genetic and biochemical studies on transcriptional regulation by showing that Gcn5 exists in macromolecular complex with the Ada, Spt, and TAF proteins.
Figure 2.
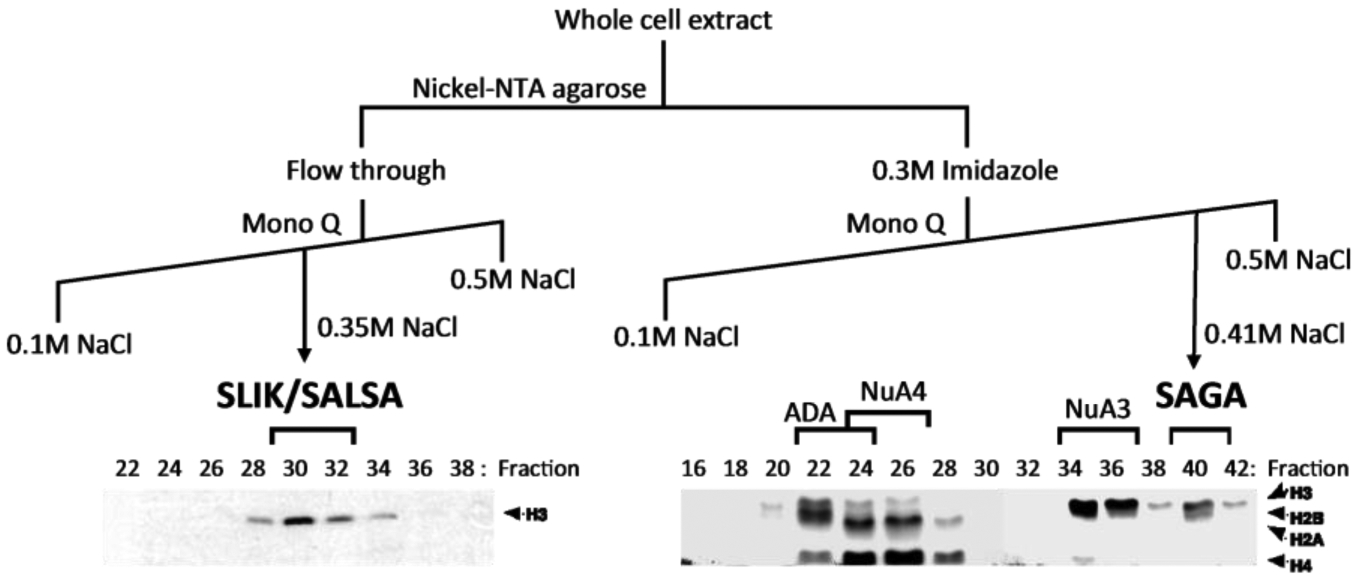
Identification of the SAGA and SLIK/SALSA complexes. Schematic representation of the original isolation of SAGA and SLIK/SALSA from yeast whole cell extracts, adapted from [4, 5]. Lower panel depicts fluorograms of HAT assays performed with nucleosomal histones and Mono Q fractions, which helped isolate the SAGA, SLIK, ADA, NuA3 and NuA4 HAT complexes. SLIK/SALSA was independently purified by Sterner et al, 2002 from synthetic complete media under conditions where it preferentially bound Nickel-nitrilotriacetic acid (NTA) agarose.
6. Genetic and biochemical studies identified distinct functional modules within SAGA
Along with the functional distinctions among SPT and ADA genes, additional genetic studies suggested that SAGA contained multiple activities in addition to Gcn5 that were required for transcriptional activation. One group of SAGA proteins, Spt7, Spt20, and Ada1 appeared to be required for SAGA structure, and hence all function, as these mutants had the most severe growth phenotypes of SAGA mutants tested [1–3, 76]. Mutants lacking any one of these proteins failed to form a SAGA complex [3, 4, 90, 91]. In contrast, Spt3 and Spt8 appeared to form one module of SAGA, likely to be required for TBP recruitment, and Gcn5, Ada2, and Ada3 were thought to form another module, required for histone acetylation [3, 4, 81, 92, 93]. In the latter two classes of mutants, SAGA complexes still formed, showing that these proteins are not required for SAGA structure. Importantly, double mutants that combined null mutations from each module (for example, spt3 and gcn5 null mutations) had more severe phenotypes, similar to spt7, spt20, ada1 mutants, suggesting that the two modules contribute distinct activities [2, 3, 94]. In these double mutants, a SAGA complex still formed, supporting the view that these modules are involved in function, not structure. This led to a model suggesting at least three modules for SAGA, with more likely to be found (Figure 3; [2, 3]). Subsequently a third module with a distinct function, was discovered for SAGA, a histone deubiquitinase activity[7, 91, 95–97].
Figure 3.
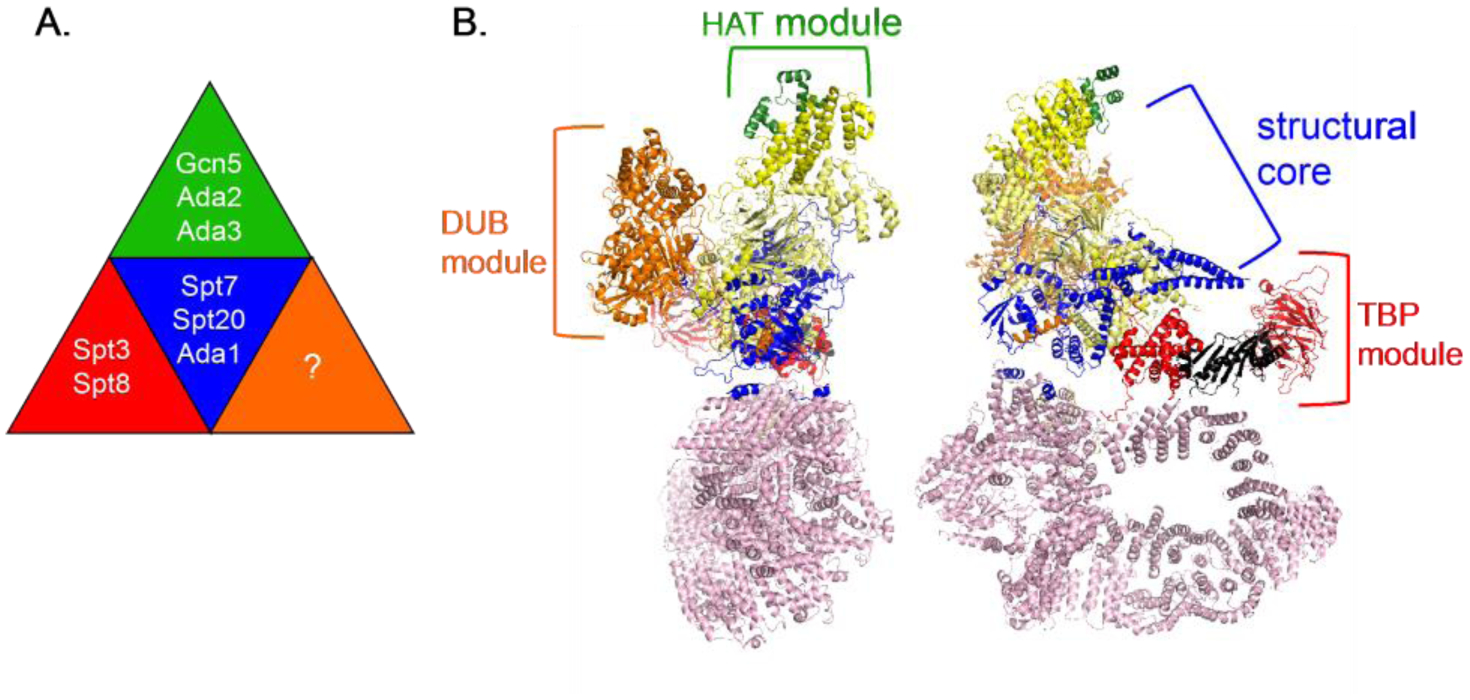
SAGA then and now. A. The proposed SAGA functions based on genetic and biochemical studies [1–3]. The “?” represents a hypothesized activity of SAGA based on genetic studies, later shown to be the histone deubiquitination activity [7]. B. SAGA structure from cryo-EM studies (PDB structure 6TBM; [10]). The modules are colored to correspond to panel A. The TAFs, now known to be part of the structural core, are shown in yellow and Tra1 is shown in pink.
6.1. The Ada2/Ada3/Gcn5 module
Five ADA genes were discovered in the original screen, as described above. Of these five, mutations in ADA1 and ADA5 cause both Ada− and Spt− phenotypes [1, 75, 76], whereas mutations in ADA2, ADA3, and ADA4/GCN5 manifest only Ada− phenotypes [1, 32, 33]. These similar phenotypes led to investigation of whether Ada2, Ada3, and Gcn5 interact in regulating transcription. Multiple genetic and biochemical approaches, including yeast two-hybrid interaction assays, lexA transcription reporter assays, and in vitro transcription/translation assays, demonstrated physical association and interdependent transcriptional activation between the three gene products [80, 93, 98]. Subsequently the three proteins were shown to be co-dependent in effecting histone acetylation [81]. These results suggested that they might assemble into a single module within SAGA, and this was confirmed by in vitro biochemistry using purified proteins; moreover, additional experiments showed that histone and nucleosome acetylation required all three proteins in the module [92]. A fourth subunit of the Gcn5 module, Sgf29, was uncovered through mass spectrometry of protein complexes binding to methylated H3K4 – a transcriptional promoter modification – with interesting implications for cross-talk between histone methylation and histone acetylation/ubiqutination (see below for ubiquitination) in SAGA [99, 100]. Later studies showed that Sgf29 helps to activate transcription and to recruit TBP to promoters [101, 102]. It is important to note that Gcn5 and acetyltransferases in general acetylate numerous substrates well beyond histones [103, 104].
6.2. The connection of Spt3 and Spt8 with TBP
Among the TBP-related set of SPT genes, several genetic results connected SPT15, encoding TBP, with two other members of this group, SPT3 and SPT8. First, starting with a particular allele of SPT15, spt15–21, extragenic suppressors were isolated and all of the suppressor mutations were in SPT3, with the spt3 suppressor mutations found in clusters [105]. In addition, there was allele-specific mutual suppression: spt15–21 and spt3–401 each caused an Spt− phenotype and the double mutant had a wild-type phenotype, suggesting a direct interaction between the two proteins. Additional screens for spt15 suppressors of spt3–401 identified a region encoding one face of TBP as the likely interface with Spt3 [106]. These studies also demonstrated co-immunoprecipitation between these two proteins, suggesting a direction interaction [105, 106]. These genetic studies were supported by subsequent biochemical and structural studies [10, 107, 108], which confirmed and greatly extended our understanding of the Spt3-TBP interaction. In addition to Spt3, other studies provided strong evidence for Spt8-TBP interactions [3, 109, 110]. A less direct role for Spt8 was inferred by the ability of an spt3 mutation to suppress a complete deletion of the SPT8 gene, suggesting that Spt8 served to promote the Spt3-TBP interaction [111]. Interestingly Spt3 and Spt8 were also suggested to have TBP-inhibitory activity at the HIS3 and TRP3 genes [3, 112], consistent with a later study that showed that Spt3 acts negatively in regulation of the HO gene [113]. Thus, while SAGA is generally viewed as a coactivator, it can also repress in some contexts.
With the advent of chromatin immunoprecipitation, later studies of the Spt3/SAGA-TBP connection tested whether SAGA and specific components of SAGA, were required to recruit TBP to promoters. The first study showed that two SAGA components, Spt20 and Spt3, were strongly required to recruit TBP to the GAL1 promoter, while there was only a modest requirement for a third SAGA component, Gcn5 [114]. As SAGA structural studies showed that Spt20 was required for an intact SAGA complex, while Spt3 was not [3, 115, 116], this was consistent with earlier results suggesting a specific role for Spt3 in TBP recruitment. Subsequent studies established a role for SAGA and Spt3 for activation at the GAL1 promoter: Gal4 was required to recruit SAGA and Spt3 of SAGA was required to recruit TBP [35, 37, 90]. These studies, then, provided some of the first in vivo evidence for the hypothesized role for transcriptional co-activators [66–68, 117].
6.3. The histone deubiquitination module within SAGA
Soon after the initial purification of the SAGA complex, a new breakthrough in histone post-translational modification was made with the discovery that histone H2B is ubiquitinated in yeast [118]. The finding of H2B ubiquitination (H2Bub) was closely followed by the identification within SAGA of a putative protein deubiquitinating enzyme, which was named Ubp8 [95, 119, 120]. Deletion of the UBP8 gene revealed elevated H2B ubiquitination in vivo without activity on other core histones; furthermore, Ubp8 within purified SAGA was shown to deubiquitinate H2B in vitro, demonstrating intrinsic activity [7, 91, 121]. These findings ushered in a new paradigm of reversible histone ubiquitination, with SAGA as a key player.
The function of histone ubiquitination/deubiquitination exhibits an interesting twist compared to reversible acetylation, where histone acetylation correlates with transcriptional activation and deacetylation associates with repression of transcription. Instead, H2B ubiquitination and deubiquitination are required in dynamic sequence during the early stages of transcription for full gene activation [7, 122–126]. Mechanistically, Ubp8 within SAGA functions as a gatekeeper during transitions in the transcription process: H2B ubiquitination acts as a barrier to gene association of Ctk1, an RNA polymerase II kinase, and subsequent H2B deubiquitination by Ubp8 triggers Ctk1 recruitment to switch RNA polymerase II into transcription elongation mode [127].
Genetic analysis demonstrated that acetylation by Gcn5 in SAGA and deubiquitination by Ubp8 in SAGA are distinct mechanisms, since loss of one was well-tolerated but loss of both was highly detrimental to gene activation [7]. The genetic separation of histone modifying functions was strengthened by structural investigations uncovering a protein DUB (deubiquitinating) module. Biochemical approaches revealed that Ubp8 associates with the SAGA complex via Sgf11 and Sgf73, with Sus1 as an additional subunit [91, 96, 97, 128–130]. Ubp8 and Sgf11 in the context of SAGA have important roles in physiology of the fly visual system [131] while Sgf73 is the homologue of human Sca7, which in its polyglutamine expanded pathological form, leads to a neurodegenerative disease [132–134]. X-ray crystallography of the DUB module revealed a strikingly interconnected complex, wherein numerous interactions of Sgf73 and Sgf11 allosterically activate the Ubp8 enzyme [135, 136].
The DUB module of SAGA thus emerges as a full functional partner of the Gcn5 acetylation module, and demonstrates the importance of modularity in the coordination of chromatin modification in transcriptional regulation.
7. The discovery of TAFs and Tra1 in SAGA
TAFs 5, 6, 9, 10 and 12, originally discovered as part of TFIID, were also subsequently shown to be integral components of SAGA and SAGA-related complexes in both yeast [87] and mammalian [137] cells. In fact, mammalian SAGA was initially named STAGA because of this finding of TAF subunits [138]. Moreover, and independently identified TBP-free TAF complex (TFTC) in mammalian cells turned out to be SAGA [139, 140]. TAF proteins, along with TBP, make up the general transcription initiation factor, TFIID (reviewed in [141]. TFIID is required for promoter recognition in RNA polymerase II (Pol II)-catalyzed transcription at most genes (reviewed in [142]. The TAF proteins are highly conserved, however metazoans have paralogous proteins for certain TAFs, which are instead incorporated into SAGA, including mammalian TAF5L, TAF6L and TAF9B [86]. The identification of TAFs in SAGA was the first, striking discovery that these proteins function outside the context of TFIID, and physically linked the members of three distinct families of gene products known to be involved in transcriptional activation, namely Ada, Spt, and TAF proteins [87].
The yeast pseudokinase protein Tra1 and the human counterpart TRRAP, which are related to the ataxia telangiectasis mutated (ATM) family of proteins, were also identified as a conserved component of SAGA [88, 89, 143]. Tra1 was subsequently demonstrated to physically interact with transcriptional activators such as VP16, Gal4, Gcn4 and Rap1 [36, 144–147], while TAF12 was found to mediate interactions with Gal4 [147] and Spt3 directly interacts with TBP [10, 107]. The ability of multiple subunits within SAGA to directly interact with the transcriptional machinery allows for the recruitment of SAGA to genes, the facilitation of chromatin acetylation and the assembly of the transcriptional apparatus during gene activation [148–150].
Genome-wide expression analysis indicates that SAGA and TFIID, despite sharing a number of common subunits, are responsible for expression of different subsets of genes. Initially it was suggested that SAGA functions at about 10% of yeast genes and mostly at TATA-containing, highly regulated loci that respond to environmental stresses, such as metabolic starvation, DNA damage and heat, while TFIID plays a more general housekeeping role [151] However, the specific roles of SAGA in transcription is still an active area of investigation [151–157]. More recently SAGA has been found to map to the promoter regions and modify the chromatin of most yeast genes and deletion of key SAGA subunits reduces de novo transcription of nearly all genes [152, 154]. As such it has been suggested that SAGA and TFIID are substantially redundant at most genes. However rapid depletion of TFIID leads to a loss of transcription at 87% of genes analyzed. In contrast, a rapid depletion of Spt3/Spt20 (which does not lead to an immediate disruption of SAGA-dependent histone modifications) leads to a modest decrease in only 13% of genes analyzed [154]. Simultaneous depletion of both TFIID and SAGA leads to a severe transcription defect at these latter locations, termed “coactivator-redundant genes”. This observation is consistent with earlier studies that a combination of SAGA and TFIID mutations can result in greater transcription defects [151, 158]. A model has now emerged of a genome-wide role of SAGA in chromatin modification that regulates global gene transcription, and a gene-specific role for SAGA at a subset of genes, largely overlapping with TFIID, to promote TBP binding [154]. These studies further highlight how the conserved modular nature of SAGA contributes to different aspects of gene regulation and chromatin modification.
8. The alternative complex SLIK/SALSA
Subsequent to the discovery of SAGA, Gcn5 was shown to exist in another chromatographically distinct multiprotein complex, similar in size and composition to SAGA. This complex was independently named SLIK, for SAGA-like or SALSA for SAGA altered, Spt8 absent [5, 159]. Despite containing the vast majority of polypeptides found in SAGA and sharing overlapping functions, SLIK composition is notably distinct in a number of ways. SLIK lacks Spt8 and contains a C-terminally truncated version of Spt7 in a region required for Spt8 interaction [115, 159]. Notably a counterpart of Spt8 has not been identified in metazoan SAGA [86]. At certain gene promoters (HIS3 and TRP3), SAGA was found to have an inhibitory role, mediated at least in part by Spt8, while SLIK/SALSA is associated with activation of these genes [159]. Yeast Pep4 was subsequently identified as a protease required for Spt7 cleavage and formation of SLIK in a study which supported a role for SLIK in response to nutrient starvation [160]. The protein Rtg2, which is a core component of SLIK but not SAGA, is required for the stability of SLIK and links histone acetylation to the retrograde response pathway and yeast longevity [5]. This signaling is responsible for communicating to the nucleus the need to make metabolic adjustments in times of mitochondrial dysfunction and induces expression of genes whose products compensate for defects in the tricarboxylic acid cycle and allows use of acetate as a carbon source (reviewed in [161]. A role for SLIK or SAGA components in anaerobic respiration is also supported by observations that Gcn5 and Ubp8 are required for this process [162]. Despite these observations, it is likely there is much redundancy in the function of SAGA and SLIK/SALSA and the full significance of any different activities of these complexes waits to be determined.
9. SAGA today
Since the discovery of SAGA and the initial genetic and biochemical characterizations discussed in this review, labs around the world have continued to study its functions. Today, SAGA is known to be conserved throughout eukaryotes, with critical functions demonstrated in Drosophila [163, 164], plants [165], and humans [166]. In humans, there are close connections between SAGA and human disease [167, 168].
A major advance in our understanding of SAGA has come from structural studies (reviewed in [169–171]). It has been particularly gratifying that these studies have confirmed the model for distinct functional modules within SAGA, first proposed by the early studies reviewed in this article (Figure 3; [2, 3]). Two of the first structural studies, one using electron microscopy [116] and the other using mass spec/crosslinking studies [145], produced a similar picture of SAGA, with a structure corresponding to the modules defined by the early genetics and biochemistry. Since then, higher resolution studies by crystallography or cryo-EM have provided further insights into the structural organization, as well as elucidating the likely mechanisms by which the deubiquitinating (DUB) module [172], the TBP-recruitment module [10], and the histone acetylation module [108] function.
Highlights.
SAGA was identified by purification of protein complexes that contained the histone acetyltransferase Gcn5.
Characterization of SAGA fulfilled genetic predictions of a protein complex containing groups of factors identified by mutant hunts in yeast, including Spt and Ada proteins.
Biochemical studies identified other SAGA components, including TAF proteins, Ubp8 and Tra1.
Genetic and biochemical studies suggested that SAGA is a coactivator complex with multiple modules for histone acetylation, histone deubiquitylation, and TBP recruitment.
Acknowledgments
We dedicate this review to the memory of Dr. Susan Abmayr, a stellar scientist, friend, and colleague who made many important contributions to understanding gene expression and the characterization of Drosophila SAGA. We thank Jelly Soffers and Jerry Workman for helpful comments on the manuscript.
Funding
This work was supported by grants from the National Institutes of Health: R01GM111911 (to P.G.), R01GM120038 (to F.W), and P01AG031862, R01CA78831, R01AA027202 (to S.L.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Horiuchi J, Silverman N, Pina B, Marcus GA, Guarente L, ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3, Mol Cell Biol 17(6) (1997) 3220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roberts SM, Winston F, Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes, Genetics 147(2) (1997) 451–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sterner DE, Grant PA, Roberts SM, Duggan LJ, Belotserkovskaya R, Pacella LA, Winston F, Workman JL, Berger SL, Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction, Mol Cell Biol 19(1) (1999) 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grant PA, Duggan L, Cote J, Roberts SM, Brownell JE, Candau R, Ohba R, Owen-Hughes T, Allis CD, Winston F, Berger SL, Workman JL, Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex, Genes Dev 11(13) (1997) 1640–50. [DOI] [PubMed] [Google Scholar]
- [5].Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG, Workman JL, Yates JR 3rd, Grant PA, The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway, Mol Cell Biol 22(24) (2002) 8774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Culbertson SJ, Effects of flanking DNA and a transcriptional activator on the histone acetyltransferase activity of the SAGA complex, 2018. [Google Scholar]
- [7].Henry KW, Wyce A, Lo WS, Duggan LJ, Emre NC, Kao CF, Pillus L, Shilatifard A, Osley MA, Berger SL, Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8, Genes Dev 17(21) (2003) 2648–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baer BW, Rhodes D, Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes, Nature 301(5900) (1983) 482–8. [DOI] [PubMed] [Google Scholar]
- [9].Burch JB, Weintraub H, Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene, Cell 33(1) (1983) 65–76. [DOI] [PubMed] [Google Scholar]
- [10].Papai G, Frechard A, Kolesnikova O, Crucifix C, Schultz P, Ben-Shem A, Structure of SAGA and mechanism of TBP deposition on gene promoters, Nature 577(7792) (2020) 711–716. [DOI] [PubMed] [Google Scholar]
- [11].Allfrey VG, Faulkner R, Mirsky AE, Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis, Proc Natl Acad Sci U S A 51 (1964) 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abmayr SM, Workman JL, Roeder RG, The pseudorabies immediate early protein stimulates in vitro transcription by facilitating TFIID: promoter interactions, Genes Dev 2(5) (1988) 542–53. [DOI] [PubMed] [Google Scholar]
- [13].Workman JL, Abmayr SM, Cromlish WA, Roeder RG, Transcriptional regulation by the immediate early protein of pseudorabies virus during in vitro nucleosome assembly, Cell 55(2) (1988) 211–9. [DOI] [PubMed] [Google Scholar]
- [14].Workman JL, Taylor IC, Kingston RE, Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes, Cell 64(3) (1991) 533–44. [DOI] [PubMed] [Google Scholar]
- [15].Almer A, Rudolph H, Hinnen A, Horz W, Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements, EMBO J 5(10) (1986) 2689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rando OJ, Winston F, Chromatin and transcription in yeast, Genetics 190(2) (2012) 351–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clark-Adams CD, Norris D, Osley MA, Fassler JS, Winston F, Changes in histone gene dosage alter transcription in yeast, Genes Dev 2(2) (1988) 150–9. [DOI] [PubMed] [Google Scholar]
- [18].Han M, Grunstein M, Nucleosome loss activates yeast downstream promoters in vivo, Cell 55(6) (1988) 1137–45. [DOI] [PubMed] [Google Scholar]
- [19].Han M, Kim UJ, Kayne P, Grunstein M, Depletion of histone H4 and nucleosomes activates the PHO5 gene in Saccharomyces cerevisiae, EMBO J 7(7) (1988) 2221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cote J, Quinn J, Workman JL, Peterson CL, Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex, Science 265(5168) (1994) 53–60. [DOI] [PubMed] [Google Scholar]
- [21].Imbalzano AN, Kwon H, Green MR, Kingston RE, Facilitated binding of TATA-binding protein to nucleosomal DNA, Nature 370(6489) (1994) 481–5. [DOI] [PubMed] [Google Scholar]
- [22].Kruger W, Peterson CL, Sil A, Coburn C, Arents G, Moudrianakis EN, Herskowitz I, Amino acid substitutions in the structured domains of histones H3 and H4 partially relieve the requirement of the yeast SWI/SNF complex for transcription, Genes Dev 9(22) (1995) 2770–9. [DOI] [PubMed] [Google Scholar]
- [23].Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR, Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex, Nature 370(6489) (1994) 477–81. [DOI] [PubMed] [Google Scholar]
- [24].Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, Workman JL, Crabtree GR, Purification and biochemical heterogeneity of the mammalian SWI-SNF complex, EMBO J 15(19) (1996) 5370–82. [PMC free article] [PubMed] [Google Scholar]
- [25].Hirschhorn JN, Brown SA, Clark CD, Winston F, Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure, Genes Dev 6(12A) (1992) 2288–98. [DOI] [PubMed] [Google Scholar]
- [26].Neigeborn L, Carlson M, Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae, Genetics 108(4) (1984) 845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stern M, Jensen R, Herskowitz I, Five SWI genes are required for expression of the HO gene in yeast, J Mol Biol 178(4) (1984) 853–68. [DOI] [PubMed] [Google Scholar]
- [28].Winston F, Carlson M, Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection, Trends Genet 8(11) (1992) 387–91. [DOI] [PubMed] [Google Scholar]
- [29].Mittal P, Roberts CWM, The SWI/SNF complex in cancer - biology, biomarkers and therapy, Nat Rev Clin Oncol 17(7) (2020) 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Georgakopoulos T, Thireos G, Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription, EMBO J 11(11) (1992) 4145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Penn MD, Galgoci B, Greer H, Identification of AAS genes and their regulatory role in general control of amino acid biosynthesis in yeast, Proc Natl Acad Sci U S A 80(9) (1983) 2704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marcus GA, Silverman N, Berger SL, Horiuchi J, Guarente L, Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors, EMBO J 13(20) (1994) 4807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Berger SL, Pina B, Silverman N, Marcus GA, Agapite J, Regier JL, Triezenberg SJ, Guarente L, Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains, Cell 70(2) (1992) 251–65. [DOI] [PubMed] [Google Scholar]
- [34].Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD, Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation, Cell 84(6) (1996) 843–51. [DOI] [PubMed] [Google Scholar]
- [35].Bhaumik SR, Green MR, SAGA is an essential in vivo target of the yeast acidic activator Gal4p, Genes Dev 15(15) (2001) 1935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bhaumik SR, Raha T, Aiello DP, Green MR, In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer, Genes Dev 18(3) (2004) 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Larschan E, Winston F, The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4, Genes Dev 15(15) (2001) 1946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee KK, Sardiu ME, Swanson SK, Gilmore JM, Torok M, Grant PA, Florens L, Workman JL, Washburn MP, Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes, Mol Syst Biol 7 (2011) 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boeke JD, Garfinkel DJ, Styles CA, Fink GR, Ty elements transpose through an RNA intermediate, Cell 40(3) (1985) 491–500. [DOI] [PubMed] [Google Scholar]
- [40].Cameron JR, Loh EY, Davis RW, Evidence for transposition of dispersed repetitive DNA families in yeast, Cell 16(4) (1979) 739–51. [DOI] [PubMed] [Google Scholar]
- [41].Elder RT, Loh EY, Davis RW, RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA, Proc Natl Acad Sci U S A 80(9) (1983) 2432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garfinkel DJ, Boeke JD, Fink GR, Ty element transposition: reverse transcriptase and virus-like particles, Cell 42(2) (1985) 507–17. [DOI] [PubMed] [Google Scholar]
- [43].Roeder GS, Farabaugh PJ, Chaleff DT, Fink GR, The origins of gene instability in yeast, Science 209(4463) (1980) 1375–80. [DOI] [PubMed] [Google Scholar]
- [44].Farabaugh PJ, Fink GR, Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication, Nature 286(5771) (1980) 352–6. [DOI] [PubMed] [Google Scholar]
- [45].Chaleff DT, Fink GR, Genetic events associated with an insertion mutation in yeast, Cell 21(1) (1980) 227–37. [DOI] [PubMed] [Google Scholar]
- [46].Williamson VM, Young ET, Ciriacy M, Transposable elements associated with constitutive expression of yeast alcohol dehydrogenase II, Cell 23(2) (1981) 605–14. [DOI] [PubMed] [Google Scholar]
- [47].Errede B, Cardillo TS, Sherman F, Dubois E, Deschamps J, Wiame JM, Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes, Cell 22(2 Pt 2) (1980) 427–36. [DOI] [PubMed] [Google Scholar]
- [48].Roeder GS, Fink GR, DNA rearrangements associated with a transposable element in yeast, Cell 21(1) (1980) 239–49. [DOI] [PubMed] [Google Scholar]
- [49].Roeder GS, Fink GR, Movement of yeast transposable elements by gene conversion, Proc Natl Acad Sci U S A 79(18) (1982) 5621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Winston F, Chaleff DT, Valent B, Fink GR, Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae, Genetics 107(2) (1984) 179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Winston F, Dollard C, Malone EA, Clare J, Kapakos JG, Farabaugh P, Minehart PL, Three genes are required for trans-activation of Ty transcription in yeast, Genetics 115(4) (1987) 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Simchen G, Winston F, Styles CA, Fink GR, Ty-mediated gene expression of the LYS2 and HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes, Proc Natl Acad Sci U S A 81(8) (1984) 2431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Clark-Adams CD, Winston F, The SPT6 gene is essential for growth and is required for delta-mediated transcription in Saccharomyces cerevisiae, Mol Cell Biol 7(2) (1987) 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Silverman SJ, Fink GR, Effects of Ty insertions on HIS4 transcription in Saccharomyces cerevisiae, Mol Cell Biol 4(7) (1984) 1246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Winston F, Durbin KJ, Fink GR, The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae, Cell 39(3 Pt 2) (1984) 675–82. [DOI] [PubMed] [Google Scholar]
- [56].Yamaguchi Y, Narita T, Inukai N, Wada T, Handa H, SPT genes: key players in the regulation of transcription, chromatin structure and other cellular processes, J Biochem 129(2) (2001) 185–91. [DOI] [PubMed] [Google Scholar]
- [57].Meeks-Wagner D, Hartwell LH, Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission, Cell 44(1) (1986) 43–52. [DOI] [PubMed] [Google Scholar]
- [58].Hartzog GA, Wada T, Handa H, Winston F, Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae, Genes Dev 12(3) (1998) 357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog GA, Winston F, Buratowski S, Handa H, DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs, Genes Dev 12(3) (1998) 343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D, The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins, Nature 400(6741) (1999) 284–8. [DOI] [PubMed] [Google Scholar]
- [61].Eisenmann DM, Dollard C, Winston F, SPT15, the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo, Cell 58(6) (1989) 1183–91. [DOI] [PubMed] [Google Scholar]
- [62].Cavallini B, Faus I, Matthes H, Chipoulet JM, Winsor B, Egly JM, Chambon P, Cloning of the gene encoding the yeast protein BTF1Y, which can substitute for the human TATA box-binding factor, Proc Natl Acad Sci U S A 86(24) (1989) 9803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hahn S, Buratowski S, Sharp PA, Guarente L, Isolation of the gene encoding the yeast TATA binding protein TFIID: a gene identical to the SPT15 suppressor of Ty element insertions, Cell 58(6) (1989) 1173–81. [DOI] [PubMed] [Google Scholar]
- [64].Horikoshi M, Wang CK, Fujii H, Cromlish JA, Weil PA, Roeder RG, Cloning and structure of a yeast gene encoding a general transcription initiation factor TFIID that binds to the TATA box, Nature 341(6240) (1989) 299–303. [DOI] [PubMed] [Google Scholar]
- [65].Schmidt MC, Kao CC, Pei R, Berk AJ, Yeast TATA-box transcription factor gene, Proc Natl Acad Sci U S A 86(20) (1989) 7785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Berger SL, Cress WD, Cress A, Triezenberg SJ, Guarente L, Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors, Cell 61(7) (1990) 1199–208. [DOI] [PubMed] [Google Scholar]
- [67].Kelleher RJ 3rd, Flanagan PM, Kornberg RD, A novel mediator between activator proteins and the RNA polymerase II transcription apparatus, Cell 61(7) (1990) 1209–15. [DOI] [PubMed] [Google Scholar]
- [68].Pugh BF, Tjian R, Mechanism of transcriptional activation by Sp1: evidence for coactivators, Cell 61(7) (1990) 1187–97. [DOI] [PubMed] [Google Scholar]
- [69].Orphanides G, Lagrange T, Reinberg D, The general transcription factors of RNA polymerase II, Genes Dev 10(21) (1996) 2657–83. [DOI] [PubMed] [Google Scholar]
- [70].Lee TI, Young RA, Transcription of eukaryotic protein-coding genes, Annu Rev Genet 34 (2000) 77–137. [DOI] [PubMed] [Google Scholar]
- [71].Lemon B, Tjian R, Orchestrated response: a symphony of transcription factors for gene control, Genes Dev 14(20) (2000) 2551–69. [DOI] [PubMed] [Google Scholar]
- [72].Cubizolles F, Gasser SM, The nucleosome: from wallflower to Queen of the Ball, Genome Biol 2(10) (2001) REPORTS4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gill G, Ptashne M, Negative effect of the transcriptional activator GAL4, Nature 334(6184) (1988) 721–4. [DOI] [PubMed] [Google Scholar]
- [74].Lewin B, Commitment and activation at pol II promoters: a tail of protein-protein interactions, Cell 61(7) (1990) 1161–4. [DOI] [PubMed] [Google Scholar]
- [75].Marcus GA, Horiuchi J, Silverman N, Guarente L, ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription, Mol Cell Biol 16(6) (1996) 3197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Roberts SM, Winston F, SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae, Mol Cell Biol 16(6) (1996) 3206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brownell JE, Allis CD, HAT discovery: Heading toward an elusive goal with a key biological assist, Biochim Biophys Acta Gene Regul Mech (2020) 194605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang L, Liu L, Berger SL, Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo, Genes Dev 12(5) (1998) 640–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kuo MH, Zhou J, Jambeck P, Churchill ME, Allis CD, Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo, Genes Dev 12(5) (1998) 627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Candau R, Berger SL, Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo, J Biol Chem 271(9) (1996) 5237–45. [DOI] [PubMed] [Google Scholar]
- [81].Candau R, Zhou JX, Allis CD, Berger SL, Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo, EMBO J 16(3) (1997) 555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brownell JE, Allis CD, An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei, Proc Natl Acad Sci U S A 92(14) (1995) 6364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Guarente L, Transcriptional coactivators in yeast and beyond, Trends Biochem Sci 20(12) (1995) 517–21. [DOI] [PubMed] [Google Scholar]
- [84].Grant PA, Eberharter A, John S, Cook RG, Turner BM, Workman JL, Expanded lysine acetylation specificity of Gcn5 in native complexes, J Biol Chem 274(9) (1999) 5895–900. [DOI] [PubMed] [Google Scholar]
- [85].Baker SP, Grant PA, The SAGA continues: expanding the cellular role of a transcriptional co-activator complex, Oncogene 26(37) (2007) 5329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Helmlinger D, Tora L, Sharing the SAGA, Trends Biochem Sci 42(11) (2017) 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Grant PA, Schieltz D, Pray-Grant MG, Steger DJ, Reese JC, Yates JR 3rd, Workman JL, A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation, Cell 94(1) (1998) 45–53. [DOI] [PubMed] [Google Scholar]
- [88].Grant PA, Schieltz D, Pray-Grant MG, Yates JR 3rd, Workman JL, The ATM-related cofactor Tra1 is a component of the purified SAGA complex, Mol Cell 2(6) (1998) 863–7. [DOI] [PubMed] [Google Scholar]
- [89].McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD, The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins, Cell 94(3) (1998) 363–74. [DOI] [PubMed] [Google Scholar]
- [90].Bhaumik SR, Green MR, Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo, Mol Cell Biol 22(21) (2002) 7365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Shukla A, Stanojevic N, Duan Z, Sen P, Bhaumik SR, Ubp8p, a histone deubiquitinase whose association with SAGA is mediated by Sgf11p, differentially regulates lysine 4 methylation of histone H3 in vivo, Mol Cell Biol 26(9) (2006) 3339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S, Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation, J Biol Chem 277(10) (2002) 7989–95. [DOI] [PubMed] [Google Scholar]
- [93].Gamper AM, Kim J, Roeder RG, The STAGA subunit ADA2b is an important regulator of human GCN5 catalysis, Mol Cell Biol 29(1) (2009) 266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Barbaric S, Reinke H, Horz W, Multiple mechanistically distinct functions of SAGA at the PHO5 promoter, Mol Cell Biol 23(10) (2003) 3468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sanders SL, Jennings J, Canutescu A, Link AJ, Weil PA, Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry, Mol Cell Biol 22(13) (2002) 4723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ingvarsdottir K, Krogan NJ, Emre NC, Wyce A, Thompson NJ, Emili A, Hughes TR, Greenblatt JF, Berger SL, H2B ubiquitin protease Ubp8 and Sgf11 constitute a discrete functional module within the Saccharomyces cerevisiae SAGA complex, Mol Cell Biol 25(3) (2005) 1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL, The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex, Mol Cell Biol 25(3) (2005) 1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Horiuchi J, Silverman N, Marcus GA, Guarente L, ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex, Mol Cell Biol 15(3) (1995) 1203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bian C, Xu C, Ruan J, Lee KK, Burke TL, Tempel W, Barsyte D, Li J, Wu M, Zhou BO, Fleharty BE, Paulson A, Allali-Hassani A, Zhou JQ, Mer G, Grant PA, Workman JL, Zang J, Min J, Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation, EMBO J 30(14) (2011) 2829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, Mann M, Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers, Cell 142(6) (2010) 967–80. [DOI] [PubMed] [Google Scholar]
- [101].Kaldis A, Tsementzi D, Tanriverdi O, Vlachonasios KE, Arabidopsis thaliana transcriptional co-activators ADA2b and SGF29a are implicated in salt stress responses, Planta 233(4) (2011) 749–62. [DOI] [PubMed] [Google Scholar]
- [102].Shukla A, Lahudkar S, Durairaj G, Bhaumik SR, Sgf29p facilitates the recruitment of TATA box binding protein but does not alter SAGA’s global structural integrity in vivo, Biochemistry 51(2) (2012) 706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kouzarides T, Acetylation: a regulatory modification to rival phosphorylation?, EMBO J 19(6) (2000) 1176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sterner DE, Berger SL, Acetylation of histones and transcription-related factors, Microbiol Mol Biol Rev 64(2) (2000) 435–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Eisenmann DM, Arndt KM, Ricupero SL, Rooney JW, Winston F, SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae, Genes Dev 6(7) (1992) 1319–31. [DOI] [PubMed] [Google Scholar]
- [106].Laprade L, Rose D, Winston F, Characterization of new Spt3 and TATA-binding protein mutants of Saccharomyces cerevisiae: Spt3 TBP allele-specific interactions and bypass of Spt8, Genetics 177(4) (2007) 2007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mohibullah N, Hahn S, Site-specific cross-linking of TBP in vivo and in vitro reveals a direct functional interaction with the SAGA subunit Spt3, Genes Dev 22(21) (2008) 2994–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang H, Dienemann C, Stutzer A, Urlaub H, Cheung ACM, Cramer P, Structure of the transcription coactivator SAGA, Nature 577(7792) (2020) 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Warfield L, Ranish JA, Hahn S, Positive and negative functions of the SAGA complex mediated through interaction of Spt8 with TBP and the N-terminal domain of TFIIA, Genes Dev 18(9) (2004) 1022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sermwittayawong D, Tan S, SAGA binds TBP via its Spt8 subunit in competition with DNA: implications for TBP recruitment, EMBO J 25(16) (2006) 3791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Eisenmann DM, Chapon C, Roberts SM, Dollard C, Winston F, The Saccharomyces cerevisiae SPT8 gene encodes a very acidic protein that is functionally related to SPT3 and TATA-binding protein, Genetics 137(3) (1994) 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Belotserkovskaya R, Sterner DE, Deng M, Sayre MH, Lieberman PM, Berger SL, Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters, Mol Cell Biol 20(2) (2000) 634–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yu Y, Eriksson P, Bhoite LT, Stillman DJ, Regulation of TATA-binding protein binding by the SAGA complex and the Nhp6 high-mobility group protein, Mol Cell Biol 23(6) (2003) 1910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dudley AM, Rougeulle C, Winston F, The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo, Genes Dev 13(22) (1999) 2940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wu PY, Winston F, Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex, Mol Cell Biol 22(15) (2002) 5367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu PY, Ruhlmann C, Winston F, Schultz P, Molecular architecture of the S. cerevisiae SAGA complex, Mol Cell 15(2) (2004) 199–208. [DOI] [PubMed] [Google Scholar]
- [117].Gill G, Tjian R, Eukaryotic coactivators associated with the TATA box binding protein, Curr Opin Genet Dev 2(2) (1992) 236–42. [DOI] [PubMed] [Google Scholar]
- [118].Robzyk K, Recht J, Osley MA, Rad6-dependent ubiquitination of histone H2B in yeast, Science 287(5452) (2000) 501–4. [DOI] [PubMed] [Google Scholar]
- [119].Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, Schelder M, Brajenovic M, Ruffner H, Merino A, Klein K, Hudak M, Dickson D, Rudi T, Gnau V, Bauch A, Bastuck S, Huhse B, Leutwein C, Heurtier MA, Copley RR, Edelmann A, Querfurth E, Rybin V, Drewes G, Raida M, Bouwmeester T, Bork P, Seraphin B, Kuster B, Neubauer G, Superti-Furga G, Functional organization of the yeast proteome by systematic analysis of protein complexes, Nature 415(6868) (2002) 141–7. [DOI] [PubMed] [Google Scholar]
- [120].Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C, Dewar D, Lin Z, Michalickova K, Willems AR, Sassi H, Nielsen PA, Rasmussen KJ, Andersen JR, Johansen LE, Hansen LH, Jespersen H, Podtelejnikov A, Nielsen E, Crawford J, Poulsen V, Sorensen BD, Matthiesen J, Hendrickson RC, Gleeson F, Pawson T, Moran MF, Durocher D, Mann M, Hogue CW, Figeys D, Tyers M, Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry, Nature 415(6868) (2002) 180–3. [DOI] [PubMed] [Google Scholar]
- [121].Daniel JA, Torok MS, Sun ZW, Schieltz D, Allis CD, Yates JR 3rd, Grant PA, Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription, J Biol Chem 279(3) (2004) 1867–71. [DOI] [PubMed] [Google Scholar]
- [122].Schulze JM, Hentrich T, Nakanishi S, Gupta A, Emberly E, Shilatifard A, Kobor MS, Splitting the task: Ubp8 and Ubp10 deubiquitinate different cellular pools of H2BK123, Genes Dev 25(21) (2011) 2242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA, H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation, Mol Cell 31(1) (2008) 57–66. [DOI] [PubMed] [Google Scholar]
- [124].Sen R, Lahudkar S, Durairaj G, Bhaumik SR, Functional analysis of Bre1p, an E3 ligase for histone H2B ubiquitylation, in regulation of RNA polymerase II association with active genes and transcription in vivo, J Biol Chem 288(14) (2013) 9619–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shukla A, Bhaumik SR, H2B-K123 ubiquitination stimulates RNAPII elongation independent of H3-K4 methylation, Biochem Biophys Res Commun 359(2) (2007) 214–20. [DOI] [PubMed] [Google Scholar]
- [126].Tanny JC, Erdjument-Bromage H, Tempst P, Allis CD, Ubiquitylation of histone H2B controls RNA polymerase II transcription elongation independently of histone H3 methylation, Genes Dev 21(7) (2007) 835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wyce A, Henry KW, Berger SL, H2B ubiquitylation and de-ubiquitylation in gene activation, Novartis Found Symp 259 (2004) 63–73; discussion 73–7, 163–9. [PubMed] [Google Scholar]
- [128].Lee KK, Swanson SK, Florens L, Washburn MP, Workman JL, Yeast Sgf73/Ataxin-7 serves to anchor the deubiquitination module into both SAGA and Slik(SALSA) HAT complexes, Epigenetics Chromatin 2(1) (2009) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Powell DW, Weaver CM, Jennings JL, McAfee KJ, He Y, Weil PA, Link AJ, Cluster analysis of mass spectrometry data reveals a novel component of SAGA, Mol Cell Biol 24(16) (2004) 7249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Durairaj G, Sen R, Uprety B, Shukla A, Bhaumik SR, Sus1p facilitates pre-initiation complex formation at the SAGA-regulated genes independently of histone H2B deubiquitylation, J Mol Biol 426(16) (2014) 2928–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Weake VM, Lee KK, Guelman S, Lin CH, Seidel C, Abmayr SM, Workman JL, SAGA-mediated H2B deubiquitination controls the development of neuronal connectivity in the Drosophila visual system, EMBO J 27(2) (2008) 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Helmlinger D, Hardy S, Abou-Sleymane G, Eberlin A, Bowman AB, Gansmuller A, Picaud S, Zoghbi HY, Trottier Y, Tora L, Devys D, Glutamine-expanded ataxin-7 alters TFTC/STAGA recruitment and chromatin structure leading to photoreceptor dysfunction, PLoS Biol 4(3) (2006) e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].McMahon SJ, Pray-Grant MG, Schieltz D, Yates JR 3rd, Grant PA, Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity, Proc Natl Acad Sci U S A 102(24) (2005) 8478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Palhan VB, Chen S, Peng GH, Tjernberg A, Gamper AM, Fan Y, Chait BT, La Spada AR, Roeder RG, Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration, Proc Natl Acad Sci U S A 102(24) (2005) 8472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kohler A, Zimmerman E, Schneider M, Hurt E, Zheng N, Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module, Cell 141(4) (2010) 606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C, Structural insights into the assembly and function of the SAGA deubiquitinating module, Science 328(5981) (2010) 1025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ogryzko VV, Kotani T, Zhang X, Schiltz RL, Howard T, Yang XJ, Howard BH, Qin J, Nakatani Y, Histone-like TAFs within the PCAF histone acetylase complex, Cell 94(1) (1998) 35–44. [DOI] [PubMed] [Google Scholar]
- [138].Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, Chait BT, Roeder RG, Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo, Mol Cell Biol 21(20) (2001) 6782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Brand M, Yamamoto K, Staub A, Tora L, Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction, J Biol Chem 274(26) (1999) 18285–9. [DOI] [PubMed] [Google Scholar]
- [140].Wieczorek E, Brand M, Jacq X, Tora L, Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II, Nature 393(6681) (1998) 187–91. [DOI] [PubMed] [Google Scholar]
- [141].Green MR, TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes, Trends Biochem Sci 25(2) (2000) 59–63. [DOI] [PubMed] [Google Scholar]
- [142].Burley SK, Roeder RG, Biochemistry and structural biology of transcription factor IID (TFIID), Annu Rev Biochem 65 (1996) 769–99. [DOI] [PubMed] [Google Scholar]
- [143].Saleh A, Schieltz D, Ting N, McMahon SB, Litchfield DW, Yates JR 3rd, Lees-Miller SP, Cole MD, Brandl CJ, Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes, J Biol Chem 273(41) (1998) 26559–65. [DOI] [PubMed] [Google Scholar]
- [144].Brown CE, Howe L, Sousa K, Alley SC, Carrozza MJ, Tan S, Workman JL, Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit, Science 292(5525) (2001) 2333–7. [DOI] [PubMed] [Google Scholar]
- [145].Han Y, Luo J, Ranish J, Hahn S, Architecture of the Saccharomyces cerevisiae SAGA transcription coactivator complex, EMBO J 33(21) (2014) 2534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lin L, Chamberlain L, Zhu LJ, Green MR, Analysis of Gal4-directed transcription activation using Tra1 mutants selectively defective for interaction with Gal4, Proc Natl Acad Sci U S A 109(6) (2012) 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Reeves WM, Hahn S, Targets of the Gal4 transcription activator in functional transcription complexes, Mol Cell Biol 25(20) (2005) 9092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Vignali M, Steger DJ, Neely KE, Workman JL, Distribution of acetylated histones resulting from Gal4-VP16 recruitment of SAGA and NuA4 complexes, EMBO J 19(11) (2000) 2629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Utley RT, Ikeda K, Grant PA, Cote J, Steger DJ, Eberharter A, John S, Workman JL, Transcriptional activators direct histone acetyltransferase complexes to nucleosomes, Nature 394(6692) (1998) 498–502. [DOI] [PubMed] [Google Scholar]
- [150].Ikeda K, Steger DJ, Eberharter A, Workman JL, Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes, Mol Cell Biol 19(1) (1999) 855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Huisinga KL, Pugh BF, A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae, Mol Cell 13(4) (2004) 573–85. [DOI] [PubMed] [Google Scholar]
- [152].Baptista T, Grunberg S, Minoungou N, Koster MJE, Timmers HTM, Hahn S, Devys D, Tora L, SAGA Is a General Cofactor for RNA Polymerase II Transcription, Mol Cell 68(1) (2017) 130–143 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Bonnet J, Wang CY, Baptista T, Vincent SD, Hsiao WC, Stierle M, Kao CF, Tora L, Devys D, The SAGA coactivator complex acts on the whole transcribed genome and is required for RNA polymerase II transcription, Genes Dev 28(18) (2014) 1999–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Donczew R, Warfield L, Pacheco D, Erijman A, Hahn S, Two roles for the yeast transcription coactivator SAGA and a set of genes redundantly regulated by TFIID and SAGA, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Vinayachandran V, Reja R, Rossi MJ, Park B, Rieber L, Mittal C, Mahony S, Pugh BF, Widespread and precise reprogramming of yeast protein-genome interactions in response to heat shock, Genome Res (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ferdoush J, Sen R, Kaja A, Barman P, Bhaumik SR, Two Distinct Regulatory Mechanisms of Transcriptional Initiation in Response to Nutrient Signaling, Genetics 208(1) (2018) 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Uprety B, Sen R, Bhaumik SR, Eaf1p Is Required for Recruitment of NuA4 in Targeting TFIID to the Promoters of the Ribosomal Protein Genes for Transcriptional Initiation In Vivo, Mol Cell Biol 35(17) (2015) 2947–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lee TI, Causton HC, Holstege FC, Shen WC, Hannett N, Jennings EG, Winston F, Green MR, Young RA, Redundant roles for the TFIID and SAGA complexes in global transcription, Nature 405(6787) (2000) 701–4. [DOI] [PubMed] [Google Scholar]
- [159].Sterner DE, Belotserkovskaya R, Berger SL, SALSA, a variant of yeast SAGA, contains truncated Spt7, which correlates with activated transcription, Proc Natl Acad Sci U S A 99(18) (2002) 11622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Spedale G, Mischerikow N, Heck AJ, Timmers HT, Pijnappel WW, Identification of Pep4p as the protease responsible for formation of the SAGA-related SLIK protein complex, J Biol Chem 285(30) (2010) 22793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Butow RA, Avadhani NG, Mitochondrial signaling: the retrograde response, Mol Cell 14(1) (2004) 1–15. [DOI] [PubMed] [Google Scholar]
- [162].Stoppacciaro A, Di Vito S, Filetici P, Epigenetic Factors and Mitochondrial Biology in Yeast: A New Paradigm for the Study of Cancer Metabolism?, Front Pharmacol 9 (2018) 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Li X, Seidel CW, Szerszen LT, Lange JJ, Workman JL, Abmayr SM, Enzymatic modules of the SAGA chromatin-modifying complex play distinct roles in Drosophila gene expression and development, Genes Dev 31(15) (2017) 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Torres-Zelada EF, Weake VM, The Gcn5 complexes in Drosophila as a model for metazoa, Biochim Biophys Acta Gene Regul Mech (2020) 194610. [DOI] [PubMed] [Google Scholar]
- [165].Grasser KD, Rubio V, Barneche F, Multifaceted activities of the plant SAGA complex, Biochim Biophys Acta Gene Regul Mech (2020) 194613. [DOI] [PubMed] [Google Scholar]
- [166].Cheon Y, Kim H, Park K, Kim M, Lee D, Dynamic modules of the coactivator SAGA in eukaryotic transcription, Exp Mol Med (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mustachio LM, Roszik J, Farria A, Dent SYR, Targeting the SAGA and ATAC Transcriptional Coactivator Complexes in MYC-Driven Cancers, Cancer Res 80(10) (2020) 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wang L, Dent SY, Functions of SAGA in development and disease, Epigenomics 6(3) (2014) 329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cheon Y, Kim H, Park K, Kim M, Lee D, Dynamic modules of the coactivator SAGA in eukaryotic transcription, Exp Mol Med 52(7) (2020) 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Helmlinger D, Papai G, Devys D, Tora L, What do the structures of GCN5-containing complexes teach us about their function?, Biochim Biophys Acta Gene Regul Mech (2020) 194614. [DOI] [PubMed] [Google Scholar]
- [171].Soffers JHM, Popova VV, Workman JLW, SAGA Structures Provide Mechanistic Models for Gene Activation, Trends Biochem Sci 45(7) (2020) 547–549. [DOI] [PubMed] [Google Scholar]
- [172].Morgan MT, Haj-Yahya M, Ringel AE, Bandi P, Brik A, Wolberger C, Structural basis for histone H2B deubiquitination by the SAGA DUB module, Science 351(6274) (2016) 725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


