Abstract
The objective of this study was to examine the effects of obesity on the oxygen (O2) cost of breathing using the eucapnic voluntary hyperpnea (EVH) technique in 10 and 11-year-old children. Seventeen children (8 without and 9 with obesity) underwent EVH trials at two levels of ventilation for assessing the O2 cost of breathing (slope of oxygen uptake, V̇O2 vs. minute ventilation) and a dual energy x-ray absorptiometry scan. Resting and EVH V̇O2 was higher in children with obesity when compared with children without obesity (P=0.0096). The O2 cost of breathing did not statistically differ between children without (2.09 ± 0.46mL/L) and with obesity (2.08 ± 0.64mL/L, P=0.99), but the intercept was significantly greater in children with obesity. Chest mass explained 85% of the variance in resting V̇O2 in children with obesity. Higher resting energy requirements, attributable to increased chest mass, can increase the absolute metabolic costs of exercise and hyperpnea in children with obesity.
Keywords: work of breathing, pediatric obesity, resting oxygen uptake, ventilatory load
Introduction
Childhood obesity is a national public health concern. In children, obesity is associated with reduced functional residual capacity (FRC) at rest (Inselman et al., 1993; Robinson, 2014), increased expiratory flow limitation (EFL) during submaximal exercise (Gibson et al., 2014), and increased metabolic demand (Bandini et al., 1990; Molnar et al., 1985). During exercise, the increases in ventilation (V̇E) might demand a greater work of breathing and constrain breathing range (operational lung volumes and tidal volume, VT, expansion) (Babb, 1999; Mendelson et al., 2012; Parameswaran et al., 2006), which could negatively impact exercise tolerance in children with obesity.
Obesity is associated with a higher oxygen (O2) cost of breathing (Cherniack, 1959; Kaufman et al., 1959; Lorenzo and Babb, 2012) and weight loss is effective in reducing the O2 cost of breathing in adults (Bernhardt et al., 2019; Bhammar et al., 2016). There are no reports on the effects of obesity on the O2 cost of breathing in children. It is important to study this topic because the results could offer important mechanistic information on the effects of obesity on exertional dyspnea, exercise tolerance, and exercise performance in children with obesity. Since growth and development in children affect body composition, lung volumes, respiratory mechanics, airway resistance, and respiratory system compliance (Barlett et al., 1992; Bryan and Wohl, 2011; Lanteri and Sly, 1993; Sharp et al., 1970; Veldhuis et al., 2005; Zapletal et al., 1976), findings from adults should not be generalized to children.
Eucapnic voluntary hyperpnea (EVH) is a technique for measurement of the O2 cost of breathing, where oxygen consumption (V̇O2) is measured at various levels of ventilation with the subject at rest and V̇O2 above rest is attributed to the metabolic cost of respiratory muscle work. During EVH, VT can increase through an increase in end-inspiratory lung volume (EILV) or a decrease in end-expiratory lung volume (EELV), assuming that operational lung volumes are not fixed by the researcher. Increasing EILV, which is consistent with a reduction in inspiratory reserve volume (IRV), is associated with increased work of breathing and dyspnea, particularly when IRV begins to approach total lung capacity [TLC] (O’Donnell et al., 2012; Ora et al., 2009), more so in individuals with obesity who have increased chest wall and abdomen mass. Furthermore, since FRC is already pushed to lower levels in children with obesity, further decreases in EELV during EVH could increase risk of EFL, which poses a mechanical ventilatory constraint (Babb, 2013a). Little is known about the effects of obesity on EELV and EILV during EVH in children. These effects deserve to be studied because they may offer important insights on the regulation of operational lung volumes during voluntary hyperpnea as opposed to spontaneous exercise hyperpnea in children with obesity.
Therefore, the purpose of this study was to test the hypotheses that O2 cost of breathing slope would be higher and operational lung volumes would be lower during EVH in children with obesity when compared with children without obesity. We tested 10 and 11-year-old children in early puberty because the narrow age-range allowed us to reduce the effects of growth and development on respiratory and exercise parameters and because sex differences in pulmonary function are minimal prior to puberty for children of similar height (Polgar and Weng, 1979). The data presented in this report were collected as preliminary data for a larger study investigating the effects of obesity on respiratory function, exercise tolerance, and exertional dyspnea in children (R01 HL136643). However, EVH was not included in the larger study protocol to reduce the time commitment for study participants.
Methods
2.1. Subjects
This study was approved by the UT Southwestern Institutional Review Board (Approval number STU 052012–076). All parents provided written, informed consent and children provided assent. Participants were excluded if they had a history of asthma or if they had a history of cardiovascular, renal, or metabolic disease. All participants completed a self-report Tanner puberty stage questionnaire (Morris and Udry, 1980). Data reported in this paper were collected over two visits with 24 ± 16 days between visits; pulmonary function testing was completed on one day and body composition and EVH assessments were completed on a different day.
2.2. Pulmonary function and body composition
Measurements of height and weight were completed using standard procedures. Pulmonary function tests were completed before and after 360 μg of albuterol. Body composition was assessed using dual energy x-ray absorptiometry (Lunar Prodigy Advance, GE healthcare Lunar, Madison, WI). Whole body fat mass and fat-free mass were estimated using Prodigy enCore software (GE healthcare Lunar, Madison, WI). Custom region of interest analysis was completed for assessing chest mass and abdominal mass. The chest region of interest was selected from the sternal notch to the xiphoid process. The axillae were selected as the lateral boundary of the chest region to exclude the shoulders and arms. The abdomen region of interest was selected from the xiphoid process to the pubic symphysis.
2.3. Eucapnic voluntary hyperpnea (EVH)
Participants completed 6 min of rest and 5 – 6 min trials of EVH at 20 L·min−1 and 40 L·min−1 (girls) or 30 L·min−1 and 50 L·min−1 (boys). Breathing frequency (fB) at each target V̇E (i.e., 20, 30, 40, and 50 L·min−1) was set with a metronome at 20, 30, 30, and 35 bpm, respectively. As such fB and VT were clamped for every target V̇E, but participants were not provided volume feedback to fix their EELV and EILV during EVH (i.e., where they breathed was not fixed). Participants breathed from a 1000 L inspiratory reservoir bag containing 3 or 4% CO2 (21% O2 and balance N2) to maintain eucapnia during the voluntary hyperpnea maneuver (Rundell et al., 2004). Ratings of perceived breathlessness (RPB) were obtained during the last 30 seconds of each hyperpnea trial and physiological data (heart rate, PETCO2, V̇E, V̇O2, etc.) were averaged from 4-min measurements at rest and during the hyperpnea trials.
Expired breath by breath V̇E was monitored in real-time at the mouth with a turbine flow device and a custom computerized gas-exchange system (NEC 486DX), which was calibrated prior to each test, as described previously (Williams and Babb, 1997). V̇E from the breath by breath system was called out to give the participant volume feedback every 3–4 breaths to ensure attainment of the target V̇E (Bhammar et al., 2016). V̇O2, and V̇E were analyzed using the Douglas bag technique and averaged over 4 minutes for each target V̇E. Gas fractions were measured using a mass spectrometer (Marquette Electronics, model 1100, Milwaukee, WI) and expired volume was measured using a 200 L Tissot spirometer as described previously (Bhammar et al., 2017).
Inspiratory flow was measured using a pneumotachograph (Hans Rudolph, Model 4813) and expiratory flow was measured using a heated pneumotachograph (Hans Rudolph, model 3850A). Flow signals were combined into a single bidirectional flow signal (Validyne Buffer Amplifier, model BA112) and digitally integrated to yield volume. Data were collected and processed using the Spike 2 data acquisition and analysis package (Cambridge Electronic Design Limited, Cambridge, England) as described previously (Strozza et al., 2020). Two measurements of inspiratory capacity (IC) were performed at rest and at approximately 5 minutes into each target V̇E by having participants inhale on cue to total lung capacity (TLC). Data processing was completed as described previously (Strozza et al., 2020).
End-expiratory lung volume (EELV) was calculated as TLC – IC and reported as a percentage of TLC. End-inspiratory lung volume (EILV) was calculated as the sum of EELV and VT and was expressed as a percentage of TLC. EFL was defined as the percentage of VT where tidal expiratory flow impinged on maximal expiratory flow using maximal flow-volume loops that were corrected for thoracic gas compression (Babb, 1997).
2.4. Oxygen cost of breathing
O2 cost of breathing was calculated as the slope of the linear regression between V̇O2 (mL·min−1) vs. V̇E (L·min−1) at rest and during the two levels of EVH (Bartlett and Specht, 1957).
2.5. Data analysis
P was set at 0.05, two tailed. Differences between groups were detected with independent t-tests. A two-way analysis of variance was used to detect differences between children with vs. without obesity with repeated measures on one factor (rest vs. lower level vs. higher level of EVH). Post hoc analyses with a Bonferroni correction were completed, where appropriate, for multiple comparisons. Relationships between variables were explored with Pearson correlations and multiple regression analysis. Analyses were completed with SAS 9.3 (Cary, NC) and figures were plotted using Graphpad Prism 8 (San Diego, CA).
Results
3.1. Participant characteristics
Nineteen participants completed the EVH measurements. Data for two children were excluded from all analyses in this paper because of erratic breathing during resting and/or EVH trials, which precluded us from processing the files and correcting for inspiratory/expiratory drift. Therefore, data from 17 children are reported in this paper. Participant characteristics, body composition, fat distribution, and pulmonary function measurements are reported in Table 1. Children with obesity had lower forced expiratory volume in 1 s to forced vital capacity ratios (FEV1/ FVC), peak expiratory flow (% predicted), FRC (%TLC), and expiratory reserve volume when compared with children without obesity (Table 1). Children with obesity carried 4.5kg extra chest mass when compared with children without obesity (Table 1).
Table 1.
Participant characteristics, body composition and fat distribution, pulmonary function, and oxygen cost of breathing data presented as mean ± SD.
| Without obesity | With obesity | p-value | |
|---|---|---|---|
| N | 8 | 9 | |
| Sex (M/F) | 3/5 | 6/3 | 0.229 |
| Tanner stage (1/2/3/4/5) a | 2/4/1/0/1 | 1/3/5/0/0 | 0.251 |
| Age (year) | 10.8 ± 0.6 | 11.2 ± 0.6 | 0.1986 |
| Height (cm) | 143.4 ± 5.8 | 148.6 ± 7.5 | 0.1345 |
| BM (kg) | 36.3 ± 4.2 | 64.1 ± 15.0 | 0.0001 |
| BMI (kg·m−2) | 17.6 ± 1.5 | 28.6 ± 4.2 | <0.0001 |
| BMI Z-score b | 0.11 ± 0.69 | 2.12 ± 0.32 | <0.0001 |
| BMI percentile (CDC) | 54.2 ± 24.7 | 97.9 ± 1.4 | <0.0001 |
| BMI % of the 95th percentile | 75.5 ± 7.1 | 121.1 ± 18.0 | <0.0001 |
| Body surface area (m2) | 1.2 ± 0.1 | 1.6 ± 0.2 | 0.0185 |
| Fat (%) | 27.6 ± 6.1 | 45.9 ± 2.8 | <0.0001 |
| Lean BM (kg) | 26.2 ± 3.7 | 34.6 ± 7.6 | 0.0135 |
| Chest mass (kg) | 5.9 ± 1.4 | 10.4 ± 2.9 | 0.0012 |
| Abdomen mass (kg) | 8.9 ± 1.7 | 18.1 ± 4.6 | 0.0002 |
| Pulmonary function | |||
| FVC (L) | 2.61 ± 0.36 | 2.97 ± 0.49 | 0.1101 |
| FVC (% predicted) | 103.4 ± 10.1 | 107.2 ± 7.9 | 0.3930 |
| FEV1 (L) | 2.32 ± 0.23 | 2.48 ± 0.5 | 0.5056 |
| FEV1 (% predicted) | 104.4 ± 7.2 | 103.6 ± 10 | 0.8509 |
| FEV1/FVC | 89.1 ± 4.8 | 83.7 ± 3.7 | 0.0181 |
| PEF (L/s) | 5.02 ± 0.40 | 4.65 ± 0.45 | 0.0952 |
| PEF (% predicted) | 106.4 ± 7.6 | 91.9 ± 8.0 | 0.0017 |
| MVV (L·min−1) | 85.1 ± 8.4 | 84.4 ± 9.0 | 0.8746 |
| MVV (% predicted) | 102.4 ± 12.2 | 94.2 ± 9.8 | 0.1480 |
| TLC (L) | 3.24 ± 0.42 | 3.55 ± 0.59 | 0.2398 |
| TLC (% predicted) | 95.9 ± 7.8 | 97.4 ± 8.4 | 0.6967 |
| FRC (L) | 1.41 ± 0.27 | 1.18 ± 0.21 | 0.0634 |
| FRC (%TLC) | 43.4 ± 7.2 | 33.5 ± 5.7 | 0.0061 |
| ERV (L) | 0.66 ± 0.13 | 0.49 ± 0.18 | 0.0479 |
| ERV (% predicted) | 69.9 ± 13.5 | 51.9 ± 22.3 | 0.0665 |
| RV (L) | 0.60 ± 0.15 | 0.54 ± 0.16 | 0.4554 |
| RV (% predicted) | 82.5 ± 18.6 | 68.8 ± 18.80 | 0.1514 |
| IC (L) | 1.84 ± 0.33 | 2.37 ± 0.50 | 0.0213 |
| IC (% predicted) | 103.4 ± 15.7 | 126.5 ± 13.1 | 0.0048 |
| Oxygen cost of breathing c | |||
| Slope V̇O2 / V̇E (mL/L) | 2.09 ± 0.46 | 2.08 ± 0.64 | 0.9900 |
| Y intercept (mL) | 183 ± 15 | 229 ± 42 | 0.0124 |
| R2 | 0.97 ± 0.05 | 0.98 ± 0.04 | 0.6116 |
BM: body mass; BMI: body mass index; CDC: Centers for Disease Control and Prevention; FVC: forced vital capacity; FEV1: forced expiratory volume in 1s; PEF: peak expiratory flow; MVV: maximal voluntary ventilation; TLC: total lung capacity; FRC: functional residual capacity; ERV: expiratory reserve volume; RV: residual volume; IC: inspiratory capacity.
Tanner stage for one child was not recorded
Z-score expresses BMI as the number of standard deviations above or below the CDC reference mean
O2 cost of breathing data for two children without obesity were excluded because R2 for relationship between V̇O2 and V̇E was below 0.80.
3.2. Gas exchange and breathing parameters during EVH
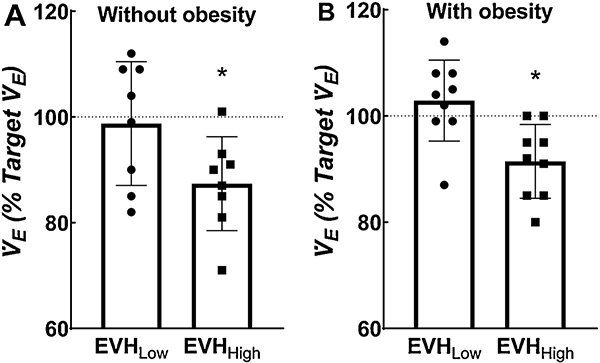
All children had difficulty achieving target V̇E for the higher level of EVH (Figure 1). Since there was unequal distribution of boys and girls in the groups, target V̇E was slightly lower in the children without obesity when compared with children with obesity (data not shown). Breathing pattern (i.e., VT and fB), duty cycle (i.e., Ti/Ttot), inspiratory drive (i.e., VT/Ti), and PETCO2 at rest and during the two levels of EVH did not statistically differ between children with and without obesity and there was no statistical interaction (see Table 2).
Fig. 1.
Individual and mean data for achievement of target ventilation (V̇E) for children without (Panel A) and with (Panel B) obesity. *Indicates significant difference from 100% using a one sample t test.
Table 2.
Gas exchange, breathing parameters, and heart rate measured during rest and eucapnic voluntary hyperpnea trials presented as mean ± S.D.
| Without obesity | With obesity | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | EVHLow | EVHHigh | Rest | EVHLow | EVHHigh | Group | Condition | Interaction | |
| V̇O2 (L·min−1) | 0.20 ± 0.01 | 0.23 ± 0.02 | 0.26 ± 0.03 | 0.25 ± 0.05 | 0.28 ± 0.05 | 0.32 ± 0.06 | 0.0096 | <0.0001§,*,¶ | 0.6840 |
| V̇CO2 (L·min−1) | 0.17 ± 0.02 | 0.17 ± 0.05 | 0.21 ± 0.04 | 0.22 ± 0.05 | 0.21 ± 0.05 | 0.26 ± 0.05 | 0.0255 | <0.0001*,¶ | 0.8659 |
| RER | 0.83 ± 0.07 | 0.70 ± 0.15 | 0.81 ± 0.08 | 0.86 ± 0.08 | 0.73 ± 0.1 | 0.81 ± 0.07 | 0.6716 | <0.0001§,¶ | 0.7194 |
| V̇E (L·min−1) | 8.7 ± 1.1 | 24.9 ± 7.1 | 39.3 ± 6.1 | 10.6 ± 1.5 | 28.5 ± 4.6 | 43.8 ± 5.5 | 0.0910 | <0.0001§,*,¶ | 0.5934 |
| VT (L) | 0.48 ± 0.06 | 0.86 ± 0.16 | 1.16 ± 0.19 | 0.60 ± 0.18 | 1.02 ± 0.16 | 1.31 ± 0.19 | 0.0300 | <0.0001§,*,¶ | 0.8516 |
| fB (b/min) | 18 ± 2 | 29 ± 5 | 34 ± 3 | 19 ± 4 | 28 ± 3 | 34 ± 3 | 0.8165 | <0.0001§,*,¶ | 0.8189 |
| PETCO2 (Torr) | 41 ± 3 | 39 ± 2 | 36 ± 1 | 41 ± 4 | 41 ± 2 | 38 ± 2 | 0.3169 | <0.0001*,¶ | 0.5320 |
| Ti/Ttot (%) | 40 ± 4 | 46 ± 3 | 48 ± 4 | 40 ± 5 | 46 ± 2 | 46 ± 3 | 0.9978 | <0.0001§,* | 0.4941 |
| VT/Ti (L/s) | 0.43 ± 0.07 | 0.97 ± 0.37 | 1.50 ± 0.33 | 0.50 ± 0.14 | 1.05 ± 0.13 | 1.68 ± 0.2 | 0.2387 | <0.0001§,*,¶ | 0.5477 |
| VT/Te (L/s) | 0.29 ± 0.06 | 0.80 ± 0.24 | 1.37 ± 0.26 | 0.33 ± 0.05 | 0.92 ± 0.15 | 1.46 ± 0.2 | 0.2399 | <0.0001§,*,¶ | 0.7365 |
| VT (%FVC) | 18.8 ± 3.8 | 33.0 ± 5.1 | 44.9 ± 7.9 | 20.3 ± 4.6 | 34.9 ± 5.4 | 44.6 ± 5.8 | 0.6031 | <0.0001§,*,¶ | 0.7669 |
| EELV (%TLC) | 49.1 ± 3.2 | 49.1 ± 5.5 | 46.2 ± 4.2 | 37.2 ± 4.6 | 38 ± 4.3 | 37.9 ± 5.0 | <0.0001 | 0.3361 | 0.1622 |
| EILV (%TLC) | 64.6 ± 5.8 | 77.9 ± 8.1 | 85.3 ± 6.1 | 54.4 ± 6.7 | 66.4 ± 4.8 | 76.2 ± 7.3 | 0.0015 | <0.0001§,*,¶ | 0.7397 |
| HR (b/min) | 84 ± 13 | 92 ± 14 | 98 ± 15 | 87 ± 13 | 89 ± 15 | 95 ± 14 | 0.8796 | <0.0001*,¶ | 0.1761 |
| RPB | 0.3 ± 0.5 | 0.9 ± 0.9 | 1.5 ± 1.3 | 0.4 ± 0.5 | 1.1 ± 1.4 | 2.3 ± 1.9 | 0.4407 | <0.0001§,*,¶ | 0.3746 |
V̇O2, Oxygen uptake; V̇CO2, carbon dioxide production; V̇E, minute ventilation; VT, tidal volume; fB, breathing frequency; PETCO2, end tidal CO2; Ti/ Ttot, ratio of inspiratory time to total time; Te, expiratory time; FVC, forced vital capacity; EELV, end-expiratory lung volume; TLC, total lung capacity; EILV, end-inspiratory lung volume; HR, heart rate; RPB, ratings of perceived breathlessness
indicates difference between seated rest and lower level of EVH
indicates significant difference between seated rest and higher level of EVH
indicates significant different from lower level and higher level of EVH
3.3. Operational lung volumes during EVH
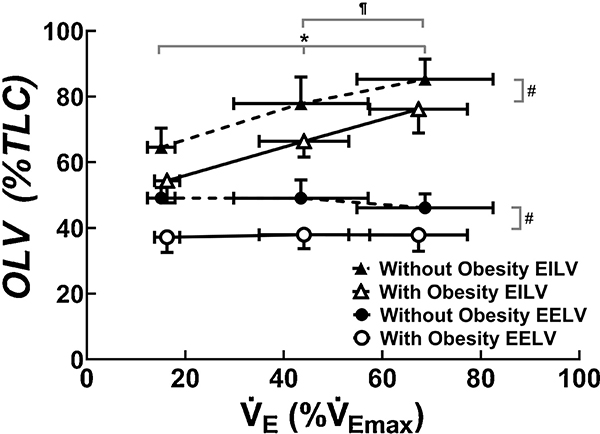
EELV and EILV were significantly lower in children with obesity when compared with children without obesity at rest and during EVH with no statistical interaction (Figure 2 and Table 2). EELV did not significantly decrease from rest to levels of EVH in children with and without obesity. VT therefore increased due to increases in EILV from rest to lower level to higher level of EVH in children with and without obesity. None of the children without obesity experienced EFL during EVH. In children with obesity, two out of nine experienced EFL (27 ± 22%VT) at the lower level of EVH and four out of nine experienced EFL (42 ± 19%VT) at the higher level of EVH.
Fig. 2.
Changes in operational lung volumes (OLV, i.e., end-inspiratory lung volume, EILV, and end-expiratory lung volume, EELV) from rest to two levels of eucapnic voluntary hyperpnea. * indicates significant difference in EILV when compared with rest for all children; ¶ indicates significant difference in EILV when compared with lower level of eucapnic voluntary hyperpnea for all children, # indicates significant group difference between children with and without obesity.
3.4. Oxygen cost of breathing
O2 cost of breathing measurements for two children without obesity were not included because of a poor relationship between the V̇O2 and V̇E slopes (r < 0.80). The O2 cost of breathing did not statistically differ between children with and without obesity and was estimated at 2.09 ± 0.54 mL of V̇O2 for 1 L of V̇E (see Table 1). Anthropometrics, pulmonary variables, and operational lung volumes during EVH did not statistically correlate significantly with the O2 cost slope with all participants combined or within the two individual groups (i.e., with and without obesity).
3.5. Resting oxygen uptake
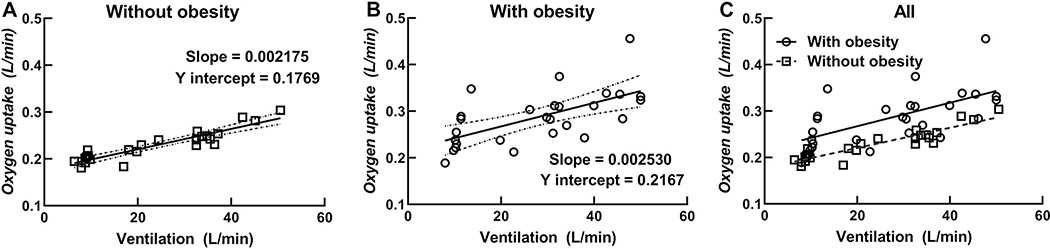
Resting V̇O2 was higher in children with obesity when compared with children without obesity. V̇O2 at the lower and higher levels of EVH were also significantly higher in children with obesity when compared with children without obesity. However, there were no differences in the O2 cost of breathing slope between children with and without obesity. In addition, the Y intercept of the V̇O2 versus V̇E relationship was higher in children with obesity when compared with children without obesity. Figure 3 shows plotted data for all measured V̇O2 and V̇E values in children with and without obesity with Panel C showing higher V̇O2 at each level of V̇E in children with obesity. Figure 3 also shows that the interindividual variability (i.e., the spread of V̇O2 data at each level of V̇E) is much higher for children with obesity when compared with children without obesity.
Fig. 3.
Individual oxygen uptake (V̇O2) and minute ventilation (V̇E) data at rest and two levels of EVH plotted for children without (Panel A) and with (Panel B) obesity. Solid line indicates regression for all data points and dotted lines indicate 95% confidence intervals in Panels A and B. Panel C includes an overlay of data for all children with solid line representing the regression for V̇O2 and V̇E in children with obesity and dashed line representing the regression of V̇O2 and V̇E in children without obesity.
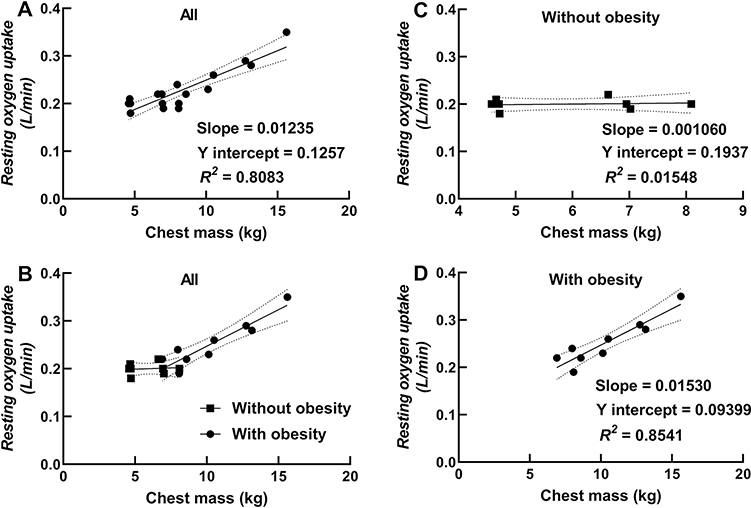
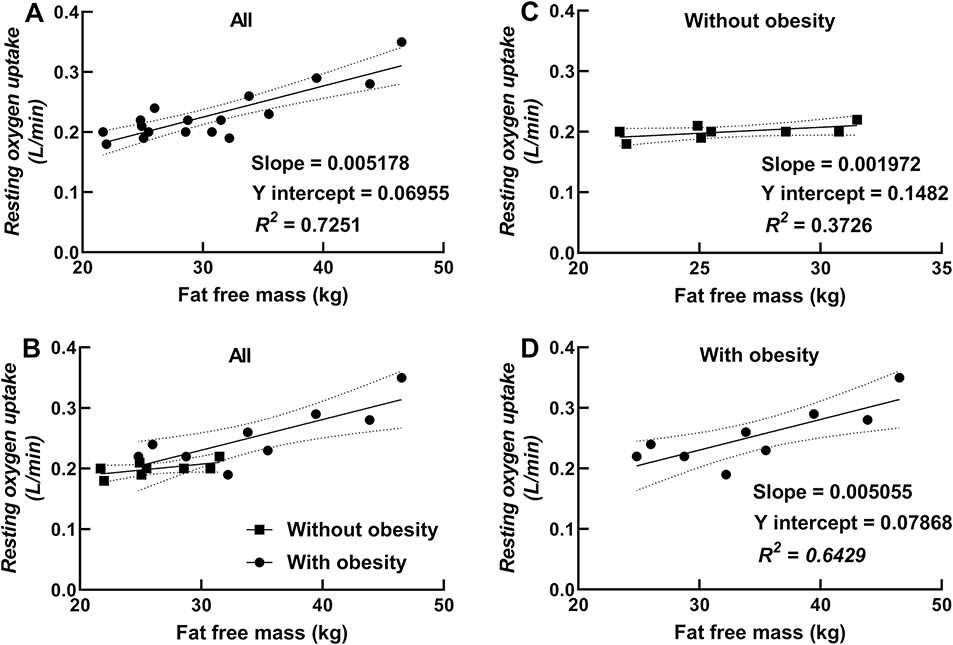
Chest mass was strongly correlated with resting V̇O2 in children with obesity (r = 0.92; P = 0.004) and in all children combined (r = 0.90; P < 0.0001; see Figure 4). Fat free mass was also correlated with resting V̇O2 in children with obesity (r = 0.80; P = 0.0093) and in all children combined (r = 0.85; P < 0.0001; see Figure 5). Stepwise multiple regression analyses showed that chest mass was the strongest predictor of resting V̇O2 (partial R2 = 0.81; β = 0.01346; P < 0.0001) followed by age (partial R2 = 0.03; β = − 0.01409; P = 0.1298) for all children combined (intercept = 0.27216). In children without obesity, percent body fat was the best predictor of resting V̇O2 (R2 = 0.64; β = − 0.00157; P = 0.0170), with an inverse relationship between percent body fat and resting V̇O2 (intercept = 0.24347) (plotted data not shown). In children with obesity, chest mass was the strongest predictor of resting V̇O2 (partial R2 = 0.85; β = − 0.03556; P = 0.0002) followed by fat free mass (partial R2 = 0.11; β = − 0.00801; P = 0.0038).
Fig. 4.
Relationship between resting oxygen uptake and chest mass (Panel A showing a regression for all children, Panel B showing data for all children with different symbols and regression lines for children with and without obesity, and Panels C and D showing regressions for children without and with obesity, respectively).
Fig. 5.
Relationship between resting oxygen uptake and fat free mass (Panel A showing a regression for all children, Panel B showing data for all children with different symbols and regression lines for children with and without obesity, and Panels C and D showing regressions for children without and with obesity, respectively).
Discussion
This is the first study to investigate the O2 cost of breathing and operational lung volumes during EVH in children with obesity. We report that, unlike in adults, obesity in children is not associated with an increase in the O2 cost of breathing slope. We estimated the O2 cost of breathing slope at 2.09 mL/L of V̇E for children in this investigation. Furthermore, we show that the obesity-related increase in resting V̇O2 can be explained by increased chest mass and that chest mass is a stronger predictor of resting V̇O2 as opposed to fat free mass in children with obesity. Unlike exercise hyperpnea, where increases in VT are achieved through reductions in EELV as well as increases in EILV, we show that during voluntary hyperpnea, VT increases are achieved only through increases in EILV in children. Finally, we show that obesity is associated with low lung volume breathing during EVH in children.
4.1. Oxygen cost of breathing
To the best of our knowledge, there are only two published reports on the O2 cost of breathing in healthy children (Davidson and Cayler, 1963; Thomas et al., 1976). Davidson and Cayler (Davidson and Cayler, 1963) reported the O2 cost of breathing as 7.38 ± 1.02 (Range 6.3 – 9.1 mL/L) in six 8 – 9.9-year-old children and 6.43 ± 1.46 (Range 4.5 – 8.7 mL/L) in eight 10 – 14-year-old children. O2 cost of breathing was measured in the reclining position over 1.5 min of hyperventilation. Thomas et al (Thomas et al., 1976) reported O2 cost of breathing as 3.4 ± 1.5 (Range 1.6 – 6.1 mL/L) in 10 healthy 6 – 15-year-old children. O2 cost of breathing was measured in the semi-recumbent position during 1 min of voluntary hyperventilation (Thomas et al., 1976). In the present study, O2 cost of breathing was 2.09 ± 0.54 mL (Range 1.10 – 3.09 mL/L) in 10 and 11-year-old children with and without obesity. Methodological differences such as seated position during testing compared with recumbent or semi-recumbent position, technique of EVH vs. voluntary hyperventilation, longer duration of hyperpnea (i.e., 4 – 6 min) compared with 1 – 1.5 min, and measurement of V̇O2 at two levels of EVH for each participant could explain differences between the present study and previously published work.
There are no published investigations for O2 cost of breathing in children with obesity. O2 cost of breathing was reported by Bernhardt et al (Bernhardt et al., 2013) as 2.1 ± 0.8 (Range 1.1 – 3.9 mL/L) in men with obesity and by Bhammar et al (Bhammar et al., 2016) as 2.5 ± 1.0 (Range 1.2 – 5.7 mL/L) in women with obesity. Kaufman et al reported O2 cost of breathing as 3.4 ± 2.0 (Range 0.8 – 9.7 mL/L) in obese adults (Kaufman et al., 1959). Lower estimates of O2 cost of breathing have been reported in adults without obesity; 1.2 ± 0.4 (Range 0.5 – 1.9 mL/L) by Cherniack (Cherniack, 1959) and 1.2 ± 0.4 (Range 0.6 – 2.1 mL/L) by Lorenzo and Babb (Lorenzo and Babb, 2012). Estimates of O2 cost of breathing for children without obesity in the present study are higher than estimates of O2 cost of breathing in adults without obesity. The higher O2 cost of breathing in children (with and without obesity) in comparison with adults without obesity could be due to higher airway and respiratory system resistance (Lanteri and Sly, 1993) and total respiratory system compliance (Bryan and Wohl, 2011). The present report did not find significant differences in O2 cost of breathing between children with and without obesity. The lack of differences in O2 cost between children with and without obesity could be due to adoption of low lung volume breathing in children with obesity, which allowed EILV to remain below 80% of TLC and reduced the inspiratory work of breathing for majority of children with obesity in the present study.
4.2. Resting metabolic rate
There are numerous reports of higher resting metabolic rate in children with obesity when compared with children without obesity (Bandini et al., 1990; Ekelund et al., 2004; Epstein et al., 1989; Molnar et al., 1985; Rodriguez et al., 2002; Tounian et al., 1993; Treuth et al., 1998; Van Mil et al., 2001). Fat free mass typically stands out as the strongest predictor of resting metabolic rate explaining 59 – 80% of the observed variance in resting metabolic rate (Bandini et al., 1990; Epstein et al., 1989; Goran et al., 1994; Maffeis et al., 1993; Rodriguez et al., 2002; Tounian et al., 1993; Van Mil et al., 2001). After including fat free mass as the strong predictor, the addition of other variables such as fat mass, sex, age, Tanner Stage, and waist circumference can improve the prediction of resting metabolic rate in children with obesity (Bandini et al., 1990; Goran et al., 1994; Maffeis et al., 1993; Rodriguez et al., 2002; Vermorel et al., 2005). The current study showed that chest mass was the strongest predictor of resting V̇O2 in children with obesity, explaining 85.41% of the variance, followed by fat free mass, which explained 11.34% of the variance in resting V̇O2. This is an intriguing finding, albeit in a small number of participants, because it suggests that reduced chest wall compliance and increased work of breathing could play a role in increasing resting oxygen uptake in children with obesity. This elevated V̇O2 at rest could raise the metabolic and ventilatory cost of exercise at every exercise intensity for children with obesity, even though the cost of increasing V̇E per liter (i.e., O2 cost of breathing slope) is not increased when compared with children without obesity.
4.3. Oxygen uptake of the respiratory muscles
When we estimated V̇O2 of the respiratory muscles at rest by extrapolating the oxygen cost of breathing slope back to resting levels of V̇E (Coast et al., 1993), we found that the respiratory muscles utilized 8.9 ± 2.4% of whole body V̇O2 in children with and without obesity, with no differences between groups. Future studies need to partition the contribution of increased chest mass and work of breathing on resting metabolic rate in children with obesity. This is important because although respiratory work may be a relatively small component of whole body V̇O2 at rest in adults without obesity (Bader and Bader, 1955), the increase in respiratory work due to increased chest mass could be an important factor that could influence metabolic and ventilatory cost during exercise and could contribute to exertional dyspnea in children with obesity.
4.4. Operational lung volumes during EVH
Obesity affects respiratory mechanics at rest and during periods of increased ventilatory demand (Babb, 2013b). Consistent with other reports in children (Davidson et al., 2014; Inselman et al., 1993), the current study showed that FRC (%TLC) was 23% lower in children with obesity when compared with children without obesity. Operational lung volumes (i.e., EELV and EILV) were also lower during EVH in children with obesity when compared with children without obesity, which illustrates the impact of excess fat mass on the chest wall and abdomen on dynamic lung volumes (Babb, 1999; Parameswaran et al., 2006). During hyperpnea, increases in VT can be achieved by increasing EILV and/or by reducing EELV. We have previously reported in adults with obesity (Bhammar et al., 2016), that, during EVH, VT increases through increases in EILV with no decreases in EELV. In contrast to adults with obesity, adults without obesity experience decreases in EELV and increases in EILV during EVH, partitioning the increase in VT over both the expiratory and inspiratory reserve volumes (Lorenzo and Babb, 2012). In children with and without obesity, VT during EVH increased through increases in EILV, with no decreases in EELV. This regulation of operational lung volumes (i.e., lack of reduction in EELV) during EVH is different from observations typically made during exercise in children with and without obesity, where EELV reduces, at least during submaximal exercise levels (Mendelson et al., 2012). Reduction in EELV through the recruitment of expiratory muscles is mechanically advantageous during hyperpnea because the energy stored in the abdominal wall due to active expiration can offer passive recoil for the next inspiration and because the diaphragm is able to achieve a more optimal length for tension development (Johnson and Dempsey, 1991). A potential explanation for why EELV did not reduce during EVH as it does during spontaneous exercise hyperpnea could be because exercise can induce bronchodilation (Warren et al., 1984), which allows for reductions in EELV to occur with a reduced risk of airway closure, while EVH may not induce similar bronchodilation. Children also have smaller airways relative to their lung size and are at higher risk for EFL during periods of hyperpnea (Nourry et al., 2005; Nourry et al., 2006; Strozza et al., 2020; Swain et al., 2010), which could consequently limit reductions in EELV during EVH. We did not ask participants to mimic operational lung volumes during EVH to those achieved during exercise because this is standard procedure for EVH studies in our lab, which we have justified previously (Bhammar et al., 2016). Had we asked participants to mimic operational lung volumes during EVH to those achieved during exercise, it is possible that participants could have generated excessive expiratory pressures to achieve a lower EELV, which could have led to an overestimation of the O2 cost of breathing (Klas and Dempsey, 1989).
4.5. Methodological considerations
The EVH trials were completed at 20 L·min−1 and 40 L·min−1 for girls and 30 L·min−1 and 50 L·min−1 for boys. For each level of EVH, we selected the fB such that VT would not exceed 50% of predicted FVC (Younes and Kivinen, 1984) during EVH. The target V̇E levels differed for boys and girls in the present study because, on average, predicted maximum V̇E is lower in girls when compared with boys (Bongers et al., 2014). Our goal was for participants to achieve target V̇E during EVH and we accordingly set a lower target for girls. Since the relationship between V̇O2 and V̇E as well as the mechanical work of breathing and V̇E is relatively linear below ~60 L·min−1 (Bartlett and Specht, 1957; Coast et al., 1993; Margaria et al., 1960). Therefore, we do not expect that the differing levels of target V̇E in girls and boys impacted the O2 cost of breathing results. Future studies could consider two shorter EVH trials especially for the higher target V̇E because it may be difficult for most children to achieve and sustain the higher level of V̇E (i.e., 40 or 50 L·min−1) over 4 – 6 min based on our experience completing these studies in children with and without obesity.
The O2 cost of breathing when measured with the EVH technique is able to detect factors that cannot be captured through the measurement of mechanical work of breathing (i.e., using the esophageal balloon technique) including breathing inertia, chest wall distortion, gas compressibility, antagonistic activity of the respiratory muscles, and the work on the abdominal viscera (Margaria et al., 1960; Milic-Emili and D’Angelo, 1997). There is a strong relationship between the O2 cost of breathing and the mechanical work of breathing (Aaron et al., 1992; Coast et al., 1993; Dominelli et al., 2014), suggesting that the O2 cost of breathing measured using the EVH technique provides a robust assessment of the work of breathing.
4.6. Limitations
This study has a relatively small sample size, in part due to the technical difficulties in obtaining these detailed measurements in younger children and due to the time commitment and costs involved in completing EVH trials in larger samples of children. The small sample size and limited numbers of boys and girls with and without obesity precluded investigations of biological sex differences on the O2 cost of breathing and operational lung volumes during EVH. Furthermore, the generalizability of these results is limited to 10 and 11 year old children. Nevertheless, these results in children ages 10 and 11 years, can provide important insights regarding the potential mechanisms for differences in resting V̇O2 between children with and without obesity, including the effects of chest mass and differences noted in the regulation of operational lung volumes during voluntary hyperpnea.
Conclusions
In conclusion, obesity in children increases resting V̇O2 but does not appear to increase O2 cost of breathing slope. Increase in chest mass is strongly associated with increases in resting V̇O2 in children with obesity. Children with obesity breathe at lower lung volumes at rest and during voluntary hyperpnea when compared with children without obesity. Finally, children with and without obesity were unable to reduce EELV during voluntary hyperpnea, which suggests that regulation of operational lung volumes during spontaneous exercise hyperpnea could be different from voluntary hyperpnea in children. These findings have important clinical implications regarding the evaluation of dyspnea during hyperpnea related to exercise or respiratory illnesses in children with obesity. It is important for clinicians to recognize that the mechanical effects of obesity can lead to low lung volume breathing and to increases in whole body V̇O2 at rest and during hyperpnea, which could explain increases in the work of breathing and dyspnea in children with obesity. Little attention has been paid to the underlying mechanisms for dyspnea in children with obesity despite alarming reports of increased dyspnea in this population (Lang et al., 2015; Sah et al., 2013; Scholtens et al., 2009). Future studies with larger samples are needed to improve our understanding of the physiological mechanisms that contribute to respiratory symptoms in children with obesity.
HIGHLIGHTS.
Obesity in children is not associated with an increase in the O2 cost of breathing slope
Obesity-related increase in resting V̇O2 can be explained by increased chest mass
Regulation of operational lung volumes differs during eucapnic voluntary hyperpnea vs. spontaneous exercise hyperpnea
Acknowledgements
This research was supported by NIH R01 HL136643, Texas Health Presbyterian Hospital Dallas, King Charitable Foundation Trust, and unrestricted funds from Dr. Pepper Snapple. The sponsors had no input in the development of the research or manuscript.
We would like to thank Jonathon Stickford, Vipa Bernhardt, J. Todd Bassett, Rubria Marines-Price, Maria C. Roman, Andreas Kreutzer, Joseph Genovese, Jessica Alcala, and Raksa B. Moran who have helped with data collection.
Footnotes
Conflict of Interest
All authors declare that they have no competing financial interests related to this publication. All authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
No conflicts of interest declared]
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron EA, Johnson BD, Seow CK, Dempsey JA, 1992. Oxygen cost of exercise hyperpnea: measurement. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 72, 1810–1817. [DOI] [PubMed] [Google Scholar]
- Babb TG, 1997. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir. Physiol. 109, 15–28. [DOI] [PubMed] [Google Scholar]
- Babb TG, 1999. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med. Sci. Sports Exerc 31, S12–S22. [DOI] [PubMed] [Google Scholar]
- Babb TG, 2013a. Exercise ventilatory limitation: the role of expiratory flow limitation. Exerc. Sport Sci. Rev. 41, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TG, 2013b. Obesity: Challenges to ventilatory control during exercise-A brief review. Respir. Physiol. Neurobiol. 189, 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader ME, Bader RA, 1955. The work of breathing. Am. J. Med. 18, 851–854. [DOI] [PubMed] [Google Scholar]
- Bandini LG, Schoeller DA, Dietz WH, 1990. Energy expenditure in obese and nonobese adolescents. Pediatr. Res. 27, 198–202. [DOI] [PubMed] [Google Scholar]
- Barlett HL, Kenney WL, Buskirk ER, 1992. Body composition and the expiratory reserve volume of pre-pubertal lean and obese boys and girls. Int.J Obes.Relat Metab Disord. 16, 653–656. [PubMed] [Google Scholar]
- Bartlett RG Jr., Specht H, 1957. Energy cost of breathing determined with a simplified technique. J. Appl. Physiol. 11, 84–86. [DOI] [PubMed] [Google Scholar]
- Bernhardt V, Bhammar DM, Marines-Price R, Babb TG, 2019. Weight loss reduces dyspnea on exertion and unpleasantness of dyspnea in obese men. Respir. Physiol. Neurobiol. 261, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Wood HE, Moran RB, Babb TG, 2013. Dyspnea on exertion in obese men. Respir. Physiol. Neurobiol. 185, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhammar DM, Stickford JL, Bernhardt V, Babb TG, 2016. Effect of weight loss on operational lung volumes and oxygen cost of breathing in obese women. Int. J. Obes. 40, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhammar DM, Stickford JL, Bernhardt V, Babb TG, 2017. Verification of maximal oxygen uptake in obese and nonobese children. Med. Sci. Sports Exerc. 49, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers BC, van Brussel M, Hulzebos E, Takken T, (2014). Pediatric norms for cardiopulmonary exercise testing: in relation to sex and age, Second ed, p. 94. [Google Scholar]
- Bryan AC, Wohl MEB, (2011). Respiratory Mechanics in Children, in: Terjung R (Ed.), Comprehensive Physiology, pp. 179–191. [Google Scholar]
- Cherniack RM, 1959. The oxygen consumption and efficiency of the respiratory muscles in health and emphysema. J. Clin. Invest. 38, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast JR, Rasmussen SA, Krause KM, O’Kroy JA, Loy RA, Rhodes J, 1993. Ventilatory work and oxygen consumption during exercise and hyperventilation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 74, 793–798. [DOI] [PubMed] [Google Scholar]
- Davidson HD, Cayler GG, 1963. Oxygen cost of breathing in children. The Journal of laboratory and clinical medicine 61, 292–301. [PubMed] [Google Scholar]
- Davidson WJ, Mackenzie- Rife KA, Witmans MB, Montgomery MD, Ball GD, Egbogah S, Eves ND, 2014. Obesity negatively impacts lung function in children and adolescents. Pediatr. Pulmonol. 49, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Sheel AW, 2014. Precise mimicking of exercise hyperpnea to investigate the oxygen cost of breathing. Respir. Physiol. Neurobiol. 201, 15–23. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Franks PW, Wareham NJ, Åman J, 2004. Oxygen uptakes adjusted for body composition in normal- weight and obese adolescents. Obes. Res. 12, 513–520. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wing RR, Cluss P, Fernstrom MH, Penner B, Perkins K, Nudelman S, Marks B, Valoski A, 1989. Resting metabolic rate in lean and obese children: relationship to child and parent weight and percent-overweight change. Am. J. Clin. Nutr. 49, 331–336. [DOI] [PubMed] [Google Scholar]
- Gibson N, Johnston K, Bear N, Stick S, Logie K, Hall G, 2014. Expiratory flow limitation and breathing strategies in overweight adolescents during submaximal exercise. Int. J. Obes. 38, 22–26. [DOI] [PubMed] [Google Scholar]
- Goran MI, Kaskoun M, Johnson R, 1994. Determinants of resting energy expenditure in young children. The Journal of pediatrics 125, 362–367. [DOI] [PubMed] [Google Scholar]
- Inselman LS, Milanese A, Deurloo A, 1993. Effect of obesity on pulmonary function in children. Pediatr. Pulmonol. 16, 130–137. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Dempsey JA, 1991. Demand vs. capacity in the aging pulmonary system. Exerc. Sport Sci. Rev. 19, 171–210. [PubMed] [Google Scholar]
- Kaufman BJ, Ferguson GT, Cherniack RM, 1959. Hypoventilation in obesity. J. Clin. Invest. 38, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas JV, Dempsey JA, 1989. Voluntary versus reflex regulation of maximal exercise flow: volume loops. Am. Rev. Respir. Dis. 139, 150–156. [DOI] [PubMed] [Google Scholar]
- Lang JE, Hossain MJ, Lima JJ, 2015. Overweight children report qualitatively distinct asthma symptoms: Analysis of validated symptom measures. J. Allergy Clin. Immunol. 135, 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri CJ, Sly PD, 1993. Changes in respiratory mechanics with age. J. Appl. Physiol. 74, 369–378. [DOI] [PubMed] [Google Scholar]
- Lorenzo S, Babb TG, 2012. Oxygen cost of breathing and breathlessness during exercise in nonobese women and men. Med. Sci. Sports Exerc. 44, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffeis C, Schutz Y, Micciolo R, Zoccante L, Pinelli L, 1993. Resting metabolic rate in six-to ten-year-old obese and nonobese children. J. Pediatr. 122, 556–562. [DOI] [PubMed] [Google Scholar]
- Margaria R, Milic-Emili G, Petit JM, Cavagna G, 1960. Mechanical work of breathing during muscular exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 15, 354–358. [DOI] [PubMed] [Google Scholar]
- Mendelson M, Michallet A-S, Estève F, Perrin C, Levy P, Wuyam B, Flore P, 2012. Ventilatory responses to exercise training in obese adolescents. Respir. Physiol. Neurobiol. 184, 73–79. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J, D’Angelo E, (1997). Work of Breathing, in: Crystal RG, West JB, et al. (Eds.), The Lung: Scientific Foundations. Lippincott-Raven, Philadelphia, pp. 1437–1446. [Google Scholar]
- Molnar D, Varga P, Rubecz I, Hamar A, Mestyan J, 1985. Food-induced thermogenesis in obese children. Eur. J. Pediatr. 144, 27–31. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 9, 271–280. [DOI] [PubMed] [Google Scholar]
- Nourry C, Deruelle F, Fabre C, Baquet G, Bart F, Grosbois JM, Berthoin S, Mucci P, 2005. Exercise flow-volume loops in prepubescent aerobically trained children. J. Appl. Physiol. 99, 1912–1921. [DOI] [PubMed] [Google Scholar]
- Nourry C, Deruelle F, Fabre C, Baquet G, Bart F, Grosbois JM, Berthoin S, Mucci P, 2006. Evidence of ventilatory constraints in healthy exercising prepubescent children. Pediatr. Pulmonol. 41, 133–140. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, O’Donnell CD, Webb KA, Guenette JA, 2012. Respiratory consequences of mild-to-moderate obesity: impact on exercise performance in health and in chronic obstructive pulmonary disease. Pulmonary medicine 2012:818925, doi: 10.1155/2012/818925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE, 2009. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am. J. Respir. Crit. Care Med. 180, 964–971. [DOI] [PubMed] [Google Scholar]
- Parameswaran K, Todd DC, Soth M, 2006. Altered respiratory physiology in obesity. Can. Respir. J. 13, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar G, Weng TR, 1979. The functional development of the respiratory system from the period of gestation to adulthood. Am. Rev. Respir. Dis. 120, 625–695. [DOI] [PubMed] [Google Scholar]
- Robinson PD, 2014. Obesity and its impact on the respiratory system. Paediatr. Respir. Rev. 15, 219–226. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Moreno L, Sarria A, Pineda I, Fleta J, Pérez-González JM, Bueno M, 2002. Determinants of resting energy expenditure in obese and non-obese children and adolescents. J. Physiol. Biochem. 58, 9–15. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Anderson SD, Spiering BA, Judelson DA, 2004. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest 125, 909–915. [DOI] [PubMed] [Google Scholar]
- Sah PK, Teague WG, Demuth KA, Whitlock DR, Brown SD, Fitzpatrick AM, 2013. Poor Asthma Control in Obese Children May Be Overestimated Because of Enhanced Perception of Dyspnea. J. Allergy Clin. Immunol. Pract 1, 39–45. e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens S, Wijga AH, Seidell JC, Brunekreef B, de Jongste JC, Gehring U, Postma DS, Kerkhof M, Smit HA, 2009. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J. Allergy Clin. Immunol. 123, 1312–1318. [DOI] [PubMed] [Google Scholar]
- Sharp J, Druz W, Balagot R, Bandelin V, Danon J, 1970. Total respiratory compliance in infants and children. J. Appl. Physiol. 29, 775–779. [DOI] [PubMed] [Google Scholar]
- Strozza D, Wilhite DP, Babb TG, Bhammar DM, 2020. Pitfalls in Expiratory Flow Limitation Assessment at Peak Exercise in Children: Role of Thoracic Gas Compression. Med. Sci. Sports Exerc 52, 2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain KE, Rosenkranz SK, Beckman B, Harms CA, 2010. Expiratory flow limitation during exercise in prepubescent boys and girls: prevalence and implications. J. Appl. Physiol. 108, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Thomas J, Enecio C, Chehreh M, Young R Jr, 1976. Oxygen cost of breathing III: studies in asthmatic children. J. Natl. Med. Assoc. 68, 374–377. [PMC free article] [PubMed] [Google Scholar]
- Tounian P, Girardet JP, Carlier L, Frelut M, Veinberg F, Fontaine J, 1993. Resting energy expenditure and food-induced thermogenesis in obese children. J. Pediatr. Gastroenterol. Nutr. 16, 451–457. [DOI] [PubMed] [Google Scholar]
- Treuth M, Figueroa-Colon R, Hunter G, Weinsier R, Butte N, Goran M, 1998. Energy expenditure and physical fitness in overweight vs non-overweight prepubertal girls. Int. J. Obes. 22, 440–447. [DOI] [PubMed] [Google Scholar]
- Van Mil E, Westerterp K, Kester A, Saris W, 2001. Energy metabolism in relation to body composition and gender in adolescents. Archives of disease in childhood 85, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY, 2005. Endocrine control of body composition in infancy, childhood, and puberty. Endocrine reviews 26, 114–146. [DOI] [PubMed] [Google Scholar]
- Vermorel M, Lazzer S, Bitar A, Ribeyre J, Montaurier C, Fellmann N, Coudert J, Meyer M, Boirie Y, 2005. Contributing factors and variability of energy expenditure in non-obese, obese, and post-obese adolescents. Reprod. Nutr. Dev. 45, 129–142. [DOI] [PubMed] [Google Scholar]
- Warren JB, Jennings SJ, Clark TJ, 1984. Effect of adrenergic and vagal blockade on the normal human airway response to exercise. Clinical Science 66, 79–85. [DOI] [PubMed] [Google Scholar]
- Williams JS, Babb TG, 1997. Differences between estimates and measured Pa-CO2 during rest and exercise in older subjects. J. Appl. Physiol. 83, 312–316. [DOI] [PubMed] [Google Scholar]
- Younes M, Kivinen G, 1984. Respiratory mechanics and breathing pattern during and following maximal exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 57, 1773–1782. [DOI] [PubMed] [Google Scholar]
- Zapletal A, Paul T, Samanek M, 1976. Pulmonary elasticity in children and adolescents. J. Appl. Physiol. 40, 953–961. [DOI] [PubMed] [Google Scholar]