Abstract
Cannabinoids produce both rewarding and aversive effects in humans and experimental animals. However, the mechanisms underlying these conflicting findings are unclear. Here we examined the potential involvement of CB1 and CB2 receptors in cannabinoid action using transgenic CB1-knockout (CB1-KO) and CB2-knockout (CB2-KO) mice. We found that Δ9-tetrahydrocannabinol (Δ9-THC) induced conditioned place preference at a low dose (1 mg/kg) in WT mice that was attenuated by deletion of the CB1 receptor. At 5 mg/kg, no subjective effects of Δ9-THC were detected in WT mice, but CB1-KO mice exhibited a trend towards place aversion and CB2-KO mice developed significant place preferences. This data suggests that activation of the CB1 receptor is rewarding, while CB2R activation is aversive. We then examined the nucleus accumbens (NAc) dopamine (DA) response to Δ9-THC using in vivo microdialysis. Unexpectedly, Δ9-THC produced a dose-dependent decrease in extracellular DA in WT mice, that was potentiated in CB1-KO mice. However, in CB2-KO mice Δ9-THC produced a dose-dependent increase in extracellular DA, suggesting that activation of the CB2R inhibits DA release in the NAc. In contrast, Δ9-THC, when administered systemically or locally into the NAc, failed to alter extracellular DA in rats. Lastly, we examined the locomotor response to Δ9-THC. Both CB1 and CB2 receptor mechanisms were shown to underlie Δ9-THC-induced hypolocomotion. These findings indicate that Δ9-THC’s variable subjective effects reflect differential activation of cannabinoid receptors. Specifically, the opposing actions of CB1 and CB2 receptors regulate cannabis reward and aversion, with CB2-mediated effects predominant in mice.
Keywords: Δ9-Tetrahydrocannabinol (Δ9-THC), Dopamine, CB1 receptor, CB2 receptor, Reward, Aversion, Place preference
1. INTRODUCTION
The marijuana plant (cannabis sativa) has been cultivated and utilized for recreational, religious, and medicinal purposes for over 4,500 years (Chopra, 1969; Kuddus et al., 2013). The changing political and social landscape in the United States has opened doors to the budding cannabis industry leading to a diverse array of marijuana products with the potential for greater use and abuse. In 2018, an average of 8,400 Americans age 12 or older initiated marijuana use each day (SAMHSA, 2019). Some users describe marijuana’s subjective effects as positive, reporting elation, relaxation, creativity, and friendliness (Ewusi Boisvert et al., 2020; Matheson et al., 2020; Sexton et al., 2019), while others recount a negative experience including anxiety, depression, nausea, and irritability (Ewusi Boisvert et al., 2020; Schlienz et al., 2020; Spindle et al., 2018). Interestingly, a single marijuana user may experience a positive affective state in one smoking session and a negative state in another (Pang et al., 2017). The neurobiological underpinnings that differentiate a positive versus a negative experience with cannabis are not clear.
Pre-clinical data have also demonstrated variation in marijuana’s hedonic profile. Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive component of cannabis, typically produces a place aversion to a location paired with drug delivery, indicative of a negative or aversive subjective effect (Cheer et al., 2000; DeVuono et al., 2017). However, Δ9-THC-induced place preferences have also been observed in this model at similar doses (Braida et al., 2004; Lepore et al., 1995). In intracranial self-stimulation (ICSS) experiments, in which animals are trained to make an operant response to receive electrical stimulation of the medial forebrain bundle, Δ9-THC has been shown to both facilitate (Gardner et al., 1988; Katsidoni et al., 2013; Lepore et al., 1996; Spiller et al., 2019) and attenuate brain-stimulation reward (BSR; Vlachou et al., 2007; Wiebelhaus et al., 2015). Similarly, squirrel monkeys can be trained to respond on a manipulandum for Δ9-THC infusions (Justinova et al., 2003; Tanda et al., 2000), but attempts with other primate species (rhesus, cynomolgus) have failed (Harris et al., 1974; John et al., 2017; Mansbach et al., 1994). Conflicting findings in brain microdialysis studies have also been reported such that Δ9-THC produced an increase (Chen et al., 1991; Tanda et al., 1997) or no change (Castañeda et al., 1991) in extracellular dopamine (DA) levels in the nucleus accumbens (NAc) in rats. The neurobiological mechanisms underlying such paradoxical effects are poorly understood.
Δ9-THC is a partial agonist at the cannabinoid CB1 (Ki =5.05 nM) and CB2 (Ki = 3.13 nM) receptors (Pertwee, 2008). The majority of investigations into the mechanism underlying Δ9-THC’s rewarding vs. aversive effects have focused on the CB1 receptor, given its high expression in brain tissues (Howlett and Abood, 2017; MacKie, 2006). Electrophysiological evidence in brain slices indicates that Δ9-THC may disinhibit DA neurons in the ventral tegmental area (VTA) by reducing presynaptic GABA inputs (Melis et al., 2000). Therefore, it was proposed that cannabinoid reward might be mediated by CB1R activation of VTA GABA neurons (Lupica et al., 2004; Tanda, 2016; Tanda and Goldberg, 2003). However, there is lack of direct behavioral evidence supporting this hypothesis in vivo. This hypothesis also cannot explain how cannabinoids produce aversive effects as described above.
With recent advances in transgenic, RNAscope ISH, and optogenetic techniques, we have attempted to dissect the roles of CB1 and CB2 receptors in cannabis reward vs. aversion. Using conditional CB1-knockout (CB1-KO) mice as controls, we have recently reported that CB1 receptors are expressed not only in VTA GABA neurons, but also in VTA glutamate neurons (Han et al., 2017). Optogenetic activation of VTA glutamatergic neurons was rewarding as assessed by intracranial self-stimulation (ICSS) and inhibited by Δ9-THC, indicating that Δ9-THC is reward-attenuating or aversive. Importantly, selective deletion of CB1Rs on glutamate neurons blocked Δ9-THC action in optical ICSS, implicating CB1Rs on glutamate neurons in cannabinoid aversion (Han et al., 2017). In addition, Δ9-THC or WIN55,212-2 produced biphasic effects on classical ICSS in that low doses enhanced, whereas high doses inhibited electrical BSR (Spiller et al., 2019). Strikingly, these effects were blocked by CB1 or CB2 receptor antagonists, respectively, and mimicked by CB1 or CB2 receptor agonists. Taken together, these findings suggest that both CB1 and CB2 receptor mechanisms mediate cannabinoid reward us. aversion (Spiller et al., 2019).
CB2R expression was previously thought to be limited to the periphery particularly immune cells (Atwood and MacKie, 2010; Howlett and Abood, 2017). However, RNAscope ISH and fluorescent IHC assays enabled the detection of low levels of CB2R expression in the brain (Zhang et al., 2014; Zhang et al., 2017). Indeed, CB2Rs have been identified on dopaminergic neurons in the VTA (Zhang et al., 2014; Zhang et al., 2017; Zhang et al., 2015; Zhang et al., 2019) as well as the NAc (Bystrowska et al., 2018; Foster et al., 2016). Further, activation of CB2Rs inhibits DA neuronal firing in the VTA and decreases DA release in the NAc (Ma et al., 2019; Xi et al., 2011; Zhang et al., 2014; Zhang et al., 2017). Cell type-specific knockout mice with CB2 deleted from DA neurons show enhanced cocaine place preferences (Canseco-Alba et al., 2019). These findings suggest that CB2R activation suppresses BSR and drug reward (Galaj and Xi, 2019; Jordan and Xi, 2019). However, it is unknown whether genetic deletion of CB1 or CB2 receptors alters the affective and neurochemical responses to Δ9-THC.
Therefore, in the present study, we utilized two mutant mouse lines to differentiate the relative contributions of CB1 and CB2 receptors to Δ9-THC reward and aversion as assessed by conditioned place preference (CPP) and brain DA response to Δ9-THC, given the critically important role of DA in drug reward (Galaj et al., 2020; Jordan et al., 2019; Volkow and Morales, 2015). An additional set of microdialysis experiments in rats was included to evaluate possible species differences in NAc DA response to Δ9-THC or SR141716A (a CB1R antagonist or inverse agonist) and compare our findings with previous reports in rats (Chen et al., 1991; Chen et al., 1990; Malone and Taylor, 1999; Tanda et al., 1997).
We also observed the locomotor response to Δ9-THC since Δ9-THC-induced hypoactivity is a classical pharmacological effect of cannabinoids (Ledent et al., 1999; Wang et al., 2020; Zimmer et al., 1999) and locomotor behavior is largely DA-dependent (Beninger, 1983; Li et al., 2009; Xi et al., 2011). We have previously reported that both CB1 and CB2 receptors modulate locomotor activity (Li et al., 2009; Xi et al., 2011; Wang et al., 2020). Specifically, deletion of CB1Rs lowers basal locomotor activity, attenuates cocaine hyperlocomotion, and mediates Δ9-THC-induced catalepsy and immobility (Li et al., 2009; Wang et al., 2020). However, CB2R agonism decreases cocaine’s locomotor activating effects in wild-type, but not CB2-KO mice (Xi et al., 2011). Therefore, we were interested in determining whether deletion of CB1Rs and/or CB2Rs would alter Δ9-THC-induced hypoactivity. We hypothesized that activation of CB1Rs is rewarding and locomotor-stimulating, while activation of CB2Rs is aversive and locomotor-suppressing. Accordingly, genetic deletion of CB1Rs should block Δ9-THC reward, whereas deletion of CB2Rs should block Δ9-THC aversion and lead to an increase in Δ9-THC reward.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Male and female wildtype (WT) and mutant mice (CB1−/− and CB2−/−) were bred at the National Institute on Drug Abuse Intramural Research Program on a C57BL/6J genetic background. Initial CB1+/− breeding pairs (3) were kindly provided by Dr. Andreas Zimmer during his tenure at the National Institute of Mental Health (Bethesda, MD; Zimmer et al., 1999). Dr. George Kunos of the National Institute on Alcohol Abuse and Alcoholism (Rockville, MD) donated 3 CB2+/− breeding pairs (Buckley et al., 2000). Mutant mice from each heterozygous genotype were bred to produce homozygous offspring and genotyped by Charles River Laboratories. In addition, male Long–Evans rats (Charles River Laboratories, Raleigh, NC, USA), weighing 250–300 g, were used for in vivo microdialysis. Animals were maintained on a reverse 12 hr light-dark cycle (lights on at 7am, off at 7pm) with ad lib food and water provided. The vivarium was set to a temperature of 21-23° C with 40-50% humidity. All experimental procedures were subject to the Guide for the Care and Use of Laboratory Animals, 8thedition. The study protocols (#13-BNRB-48 and #13-BNRB-84) were approved by the Institutional Care and Use Committee at the National Institute on Drug Abuse.
2.2. Drugs
Δ9-THC was provisioned by the Pharmacy of the National Institute on Drug Abuse Intramural Research Program (Baltimore, MD, USA). The stock Δ9-THC (50 mg/ml) was dissolved in 100% ethanol. Therefore, we used 0.5% Tween-80 (Sigma-Aldrich, St. Louis, MO, USA) and saline to dilute it by 50× to the final working solution containing 1 mg/ml Δ9-THC, 0.5% Tween-80 and ~2% ethanol. Accordingly, the vehicle for Δ9-THC also contained 0.5% Tween-80 and ~2% ethanol. SR141716A (free base form) was provided by Research Triangle Institute (Research Triangle Park, NC, USA) and dissolved in vehicle containing 0.5% Tween 80 and saline (without ethanol).
2.3. General Procedures
2.3.1. Apparatus
Six CPP boxes (MED-CPP-3013, Med Associates, St. Albans, VT, USA) were utilized for place conditioning testing. Each apparatus contained two large chambers (17.4 × 12.7 × 12.7 cm3) conjoined by a smaller middle compartment (11.7 × 12.7 × 12.7 cm3). Distinct visual and tactile cues were present in each compartment. The left chamber was black with a stainless-steel floor composed of 3.2 mm diameter rods. The right chamber was white with stainless steel mesh flooring. The middle compartment consisted of gray walls and a gray polyvinyl chloride floor. Each chamber housed a clear plexiglass top with an LED light affixed. Photobeams in each apparatus detected movement, which was recorded using Med Associates software (SOF-700RA-4).
2.3.2. CPP procedure
The full CPP procedure is illustrated in Figure 1. During pre-conditioning, naïve mice were given two sessions (1 a day) with full access to the three CPP chambers for 30 minutes. Time spent in each chamber was monitored and no preference was observed for the white or black side (AVG 358.9 s white, AVG 312.3 s black), indicating an unbiased CPP apparatus. Individual subjects with a preference for either compartment (defined as > 600 s) were removed from all analyses. Subjects were randomly assigned to Δ9-THC dose groups such that 48 mice received 1 mg/kg (n = 18 WT, 17 CB1−/−, and 13 CB2−/−) and 48 received 5 mg/kg (n = 20 WT, 12 CB1−/−, and 16 CB2−/−). On conditioning day 1, Δ9-THC was administered i.p. followed by placement in the white or black compartment for 45 minutes. The compartment paired with drug injections was randomly assigned (i.e. a counterbalanced or unbiased procedure). The following day, vehicle (0.5% Tween-80) was injected, and subjects were restricted to the opposite compartment for 45 minutes. This procedure was repeated for a total of 4 drug pairings and 3 vehicle pairings. 24 hours and 48 hours after the final Δ9-THC injection, a test was run under identical parameters to the pre-conditioning sessions. CPP scores were calculated based on the average time spent on the drug paired side - vehicle paired side over the two test days.
Figure 1.
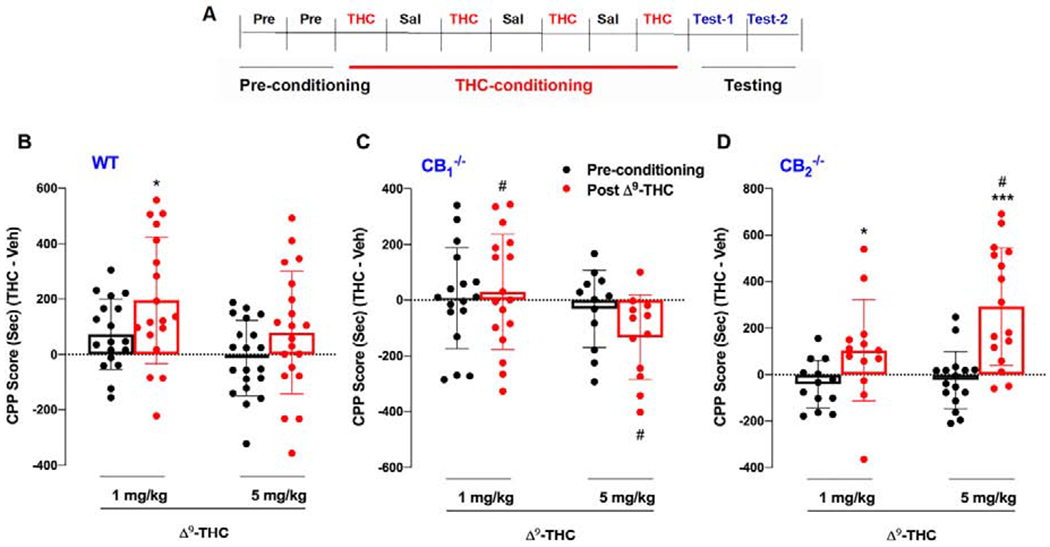
Δ9-THC place conditioning in wild type, CB1−/− and CB2−/−. (A) Experimental timeline of events for each day of CPP conditioning and testing. (B) WT mice displayed a significant increase in CPP score at 1 mg/kg (p<0.05). but not at 5 mg/kg (p>0.05). (C) CB1−/− mutants displayed a dose-dependent trend toward CPA after Δ9-THC administration. (D) Δ9-THC produced a robust dose-dependent place preference in CB2−/− mice. *p<0.05, ***p< 0.001, relative to preconditioning. #p< 0.05 compared to WT mice.
2.3.3. Open-field locomotion
Locomotor activity was next examined in naïve groups of mice to determine the impact of CB1 or CB2 receptor deletion on basal activity levels as well as activity following acute Δ9-THC administration. During the week before locomotor testing, mice were habituated to locomotor detection chambers (Accuscan Instruments, Columbus, OH, USA) during 3 h daily sessions. On the test day, mice were placed in the chamber for 1 h of habituation (baseline), after which the program was paused, and Δ9-THC was injected. Next, mice were placed back in the open-field apparatus and locomotor activity was measured over a 2 h period. Each Δ9-THC dose (0, 1, 3, 10 mg/kg, i.p.) was tested in a balanced manner in 3 genotype groups of mice (n = 8 WT, 8 CB1−/−, and 7 CB2−/−). The time intervals between the drug tests were 2-4 days. The distance traveled before and after injections was collected in 10-min intervals using the VersaMax data analysis system (Accuscan Instruments).
2.3.4. In vivo microdialysis with HPLC assays
Naïve mice were anesthetized using a cocktail of ketamine hydrochloride (80 mg/ml) and xylazine hydrochloride (12 mg/ml) prior to insertion of an intracranial guide cannulae (MAB 4.15.IC, SciPro Inc., Sanborn, NY, USA) into the NAc (stereotaxic coordinates: AP +1.4 mm, ML ±1.5 mm, DV −3.8 mm with an angle of 8° from vertical). Dental acrylic was applied to secure the guide cannulae to the skull. Standard aseptic surgical and stereotaxic procedures were followed. Subjects were given 7 days to recover preceding the experimental procedure. A probe (MAB 4.15.2.PES, SciPro Inc.) was inserted into the NAc 12 hours before sample collection to reduce the occurrence of damage induced neurotransmitter release. A syringe pump (Bioanalytical Systems, West Lafayette, IN, USA) infused dialysate buffer at least 2 hrs prior to sample collection. Baseline microdialysate samples were taken in 20 minute intervals for 1 hr, followed by an i.p. Δ9-THC injection (1 or 3 mg/kg) and further sampling for 3 hrs. Mice were randomly assigned to receive 1 mg/kg (n = 7 WT, 8 CB1−/−, and 6 CB2−/−) or 3 mg/kg (n = 15 WT, 6 CB1−/−, and 11 CB2−/−) Δ9-THC.
The procedures for in vivo brain microdialysis in rats were the same as in mice. The stereotaxic coordinates for the NAc in rats were AP +1.6 mm, ML ±1.8 mm, DV −4.3 mm with an angle of 6° from vertical. Rats (n=20) were randomly divided into 3 dose groups for i.p. injections of Δ9-THC or SR141716A (6-8 per group), and an additional 10 rats were divided into 2 groups for intra-NAc local perfusion of Δ9-THC (n=5) or SR141716A (n=5). Samples were frozen at −80 °C until analysis could be performed using an ESA electrochemical detection system (Chelmsford, MA, USA) for a detailed description see (Li et al., 2009; Xi et al., 2006).
2.5. Statistical Analysis
Data are expressed as means ±SEM. Three-way repeated measures analyses of variance (ANOVA) were utilized to analyze the CPP and locomotor data with the factors of dose, genotype, and test or time. A two-way repeated measures ANOVA assessed differences during baseline microdialysis sampling across genotype and time. Two-way ANOVAs were used to analyze the microdialysis data for each genotype and to directly compare each mutant strain and WT mice. Area under the curve (AUC) was calculated for the normalized locomotor data as the sum of all time points following drug injections. AUC data was analyzed using a two-factor (dose × genotype) ANOVA. Significant interactions were followed by post hoc tests using Fishers Least Significant Difference. Statistical significance was set to p<0.05.
3. RESULTS
3.1. Δ9-THC place conditioning in mutant mice
The general experimental procedures are shown in Figure 1A. Systemic administration of Δ9-THC produced a significant place preference at 1 mg/kg, but not 5 mg/kg, in WT mice (Fig. 1B) , a dose-dependent trend toward conditioned place aversion (CPA) in CB1-KO mice (Fig. 1C), and a dose-dependent THC place preference in CB2-KO mice (Fig. 1D). The three-way repeated measures ANOVA revealed a significant main effect of Genotype (F2,90 =6.256, p<0.01) and Test (F1,90 = 18.52, p<0.001) as well as a significant interaction between Genotype, Test and Dose (F2,90 =3.292, p<0.05). Subsequent post-hoc comparisons indicated that CPP scores were significantly higher during the test than pre-conditioning for WT subjects at 1 mg/kg and CB2-KO mice at 1 and 5 mg/kg. WT mice also showed significantly lower CPP scores at 5 mg/kg relative to CB2-KO mutants and significantly higher scores than CB1-KO mice at both doses.
3.2. Effects of Δ9-THC on extracellular NAc DA in mutant mice
We then compared basal levels of extracellular DA in the NAc between different genotypes of mice. We found a significant decrease in basal levels of extracellular DA in CB1-KO mice (average value across three baseline samples: 0.179 ± 0.046 nM, n=14) and a trend towards a decrease in CB2-KO mice (0.249 ± 0.063 nM, n=17), relative to WT mice (0.433 ± 0.066 nM, n=20). A one-way ANOVA revealed a significant main effect of Genotype (F2,48 =4.651, p<0.05). Post-hoc group comparisons showed a significant reduction in CB1-KO mice (p<0.05), but not CB2-KO mice (p=0.065), compared to WT mice. These findings are consistent with our previous reports (Li et al., 2009; Xi et al., 2011).
Given the differences observed at baseline, Δ9-THC-induced changes in extracellular DA are presented as a percent of baseline to enable data comparisons among the three mouse strains. The results shown in Figure 2 indicate that Δ9-THC produced a dose-dependent reduction in extracellular DA in WT mice (Fig. 2A) and CB1-KO mice (Fig. 2B). In contrast, Δ9-THC elicited a potent and dose-dependent increase in extracellular NAc DA in CB2-KO mice (Fig. 2C). A two-way repeated measures ANOVA on WT mice (Fig. 2A) revealed a significant main effect of Time (F5,86 = 4.704, p<0.01) and Dose (F1,18 = 7.111, p<0.05) as well as a Dose × Time interaction (F5,86 = 3.363, p<0.01). Post-hoc comparisons showed significantly lower DA release relative to the final baseline timepoint beginning 20 minutes post 3 mg/kg Δ9-THC. In CB1−/− mice (Fig. 2B), a main effect of Dose (F1,12 = 8.531, p<0.05), Time (F4,50 = 3.348, p<0.05) and a Dose × Time interaction (F4,50 = 3.346, p<0.05) were uncovered. Post-hoc tests demonstrated decreased DA release relative to baseline 40-60 and 120-180 minutes following Δ9-THC administration (3 mg/kg). The two-way ANOVA on CB2−/− mice (Fig. 2C) revealed a main effect of Time (F1,15 = 5.81, p<0.05), but not Dose (F1,15 = 0.461, p>0.05) and no interaction between Time and Dose (F1,15 = 0.974, p>0.05). Post-hoc comparisons revealed a significant increase in accumbal DA concentrations 40 minutes post Δ9-THC relative to baseline. We also compared Δ9-THC-induced changes in extracellular DA between WT and CB1 - or CB2-KO mice (Fig. 2D). The two-way ANOVA analyzing the effects of 3 mg/kg Δ9-THC across three genotypes of mice revealed a main effect of Genotype (F2,27 = 13.28, p0.001) and a significant Time × Genotype interaction (F22,36 = 1.902, p<0.05). Post-hoc comparisons revealed that DA levels were significantly higher in CB2-KO mice than in WT mice during all timepoints following Δ9-THC administration. An additional two-way ANOVA was run directly comparing CB1-KO and WT mice at 3 mg/kg Δ9-THC (Fig. 2D). There was a main effect of Genotype (F1,19 = 5.109, p<0.05) and Time (F11,209 = 16.853, p<0.001) and a significant interaction between these factors (F11,209 = 3.445, p<0.05). CB1-KO mice exhibited a greater decrease in NAc release 40-80 and 120-160 min post Δ9-THC relative to WT mice (p<0.05). Figure 2E shows a representative image illustrating the location of a microdialysis probe in the mouse brain. Most of the probes (membrane portions) penetrated both the shell and core of the NAc (Fig. 2F).
Figure 2:
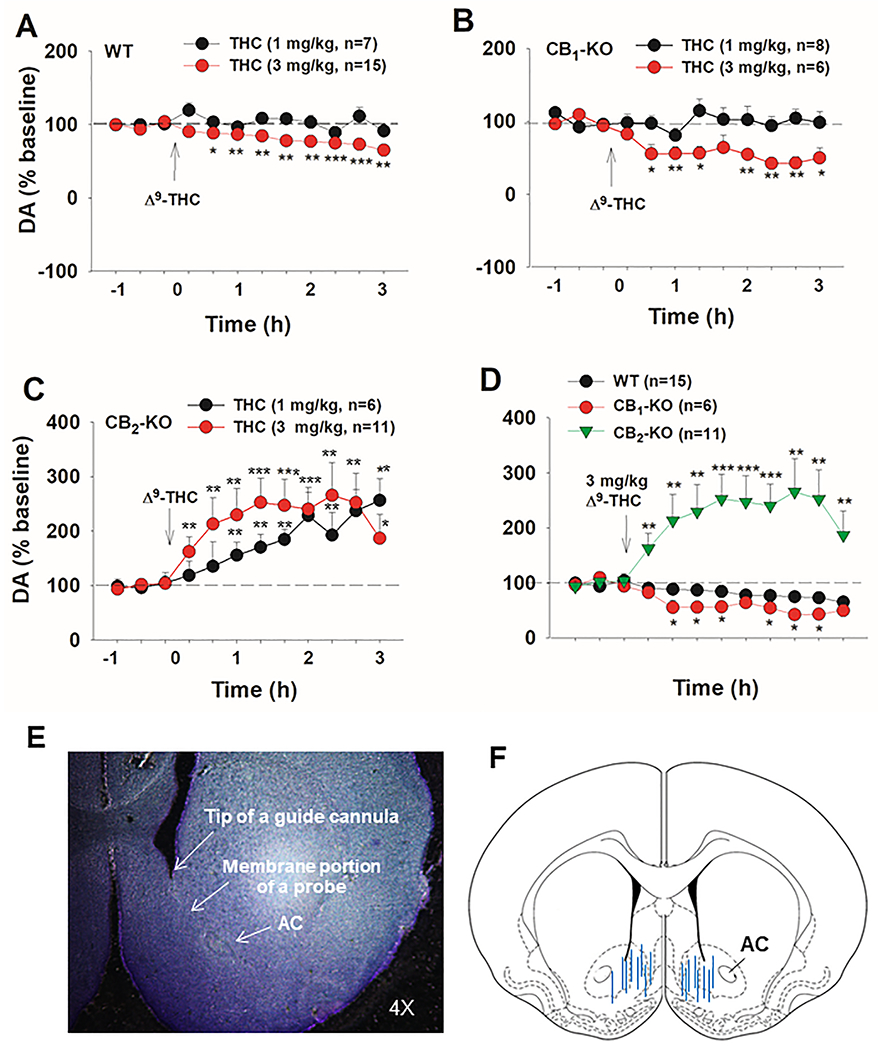
Effects of Δ9-THC on extracellular DA in the NAc. Δ9-THC produced a dose-dependent decrease in extracellular DA in WT (A) and CB1−/− mice (B), while CB2−/− mice demonstrated a dose-dependent increase in extracellular DA relative to baseline (C). (D) Δ9-THC, at 3 mg/kg, reduced extracellular DA in CB1−/− mice and elevated DA in CB2−/− mice compared to WT controls. (E, F) A representative image and histological verification showing that microdialysis probes are located in the shell and core of the NAc. *p<0.05, **p<0.01, ***p<0.001.
3.3. Effects of Δ9-THC and SR141716A on extracellular NAc DA in rats
Given that the above findings in mice contrast with previous findings in rats (Chen et al., 1991; Tanda et al., 1997), the effects of Δ9-THC and SR141716A (a CB1R antagonist or inverse agonist) were evaluated on extracellular NAc DA in rats. Systemic administration of 1, 3 mg/kg Δ9-THC did not produce significant changes in extracellular DA levels compared to vehicle (Fig. 3A). The two-way repeated measures ANOVA did not reveal a significant main effect of Dose (F2,17 = 1.34, p>0.05) or a Dose × Time interaction (F22,187 = 0.91, p>0.05), but revealed a significant Time effect (F11,178 = 4.70, p<0.001). Although extracellular DA displayed a trend to increase at 40 and 60 min compared to baseline, there was no difference observed between vehicle and Δ9-THC groups at any time point. In addition, the effects of systemic administration of SR141716A were evaluated on extracellular DA. We found that 2 and 10 mg/kg SR141617A failed to alter extracellular DA in rats (Fig. 3B). The two-way ANOVA did not reveal a significant main effect of treatment (F2,15 = 0.86, p>0.05), time (F11,165 = 1.02, p>0.05), or a treatment × time interaction (F11,165 = 0.78, p>0.05).
Figure 3:
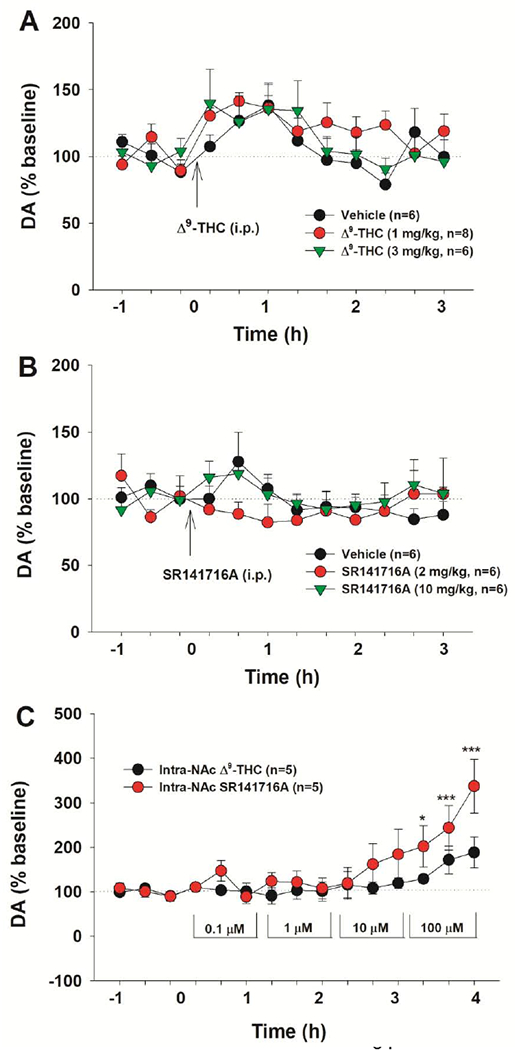
Effects of Δ9-THC and SR141716A on extracellular DA in the NAc in rats. (A) Systemic administration of Δ9-THC or vehicle produced a small decrease in extracellular DA, but no difference was found between the vehicle and THC-treatment groups. (B) Systemic administration of SR141716A failed to alter extracellular DA in the NAc. (C) Local perfusion of Δ9-THC failed to alter extracellular DA, while SR141716A elevated extracellular DA in a concentration-dependent manner. *p<0.05, ***p<0.001, compared to baseline.
To confirm the above finding and also determine whether the 2% alcohol present in the vehicle or Δ9-THC solution may underlie the non-Δ9-THC-specific increase in extracellular DA, we further diluted the Δ9-THC solution from 1 mM to 0.1 μM using 0.5% Tween-80 to minimize ethanol levels in the lower concentrations of Δ9-THC solution, and then locally perfused them into the NAc in a sequence from 0.1 μM to 100 μM. We found that Δ9-THC also failed to alter extracellular DA (Fig. 3C). Unexpectedly, SR141716A produced a dose-dependent increase in extracellular DA in the NAc (Fig. 3C), suggesting that endocannabinoids may tonically inhibit the mesolimbic DA system. Two-way ANOVA for repeated measures over time revealed a marginally significant treatment main effect (F1,8 = 4.91, p=0.057), time main effect (F14,112 = 9.25, p<0.001) and treatment × time interaction (F14,112 = 2.59, p<0.01). Post-hoc individual group comparisons revealed significant increases in DA at 100 μM SR141716A, when compared with baseline (Fig. 3C).
3.4. Δ9-THC-induced changes in open-field locomotion
Lastly, locomotor activity was assessed in WT and mutant mice following Δ9-THC administration. Relative to WT mice, CB1-KO mice displayed a significant reduction, while CB2-KO mice showed a moderate increase in basal levels of locomotion before and/or after vehicle injections (Fig. 4A), suggesting that the endocannabinoids can differentially modulate CB1 and CB2 receptors. A one-way ANOVA for the area under curve (AUC) data revealed a genotype main effect on basal locomotion in the 1 h period before the vehicle injection (Fig. 4B, F2,20=24.72, p<0.001) and for 2 hrs after vehicle injections (Fig. 4C, F2,20=26.12, p<0.001). Post-hoc individual group comparisons revealed a significant reduction in CB1-KO mice before and after vehicle and a significant increase in CB2-KO mice after vehicle compared to WT mice.
Figure 4:
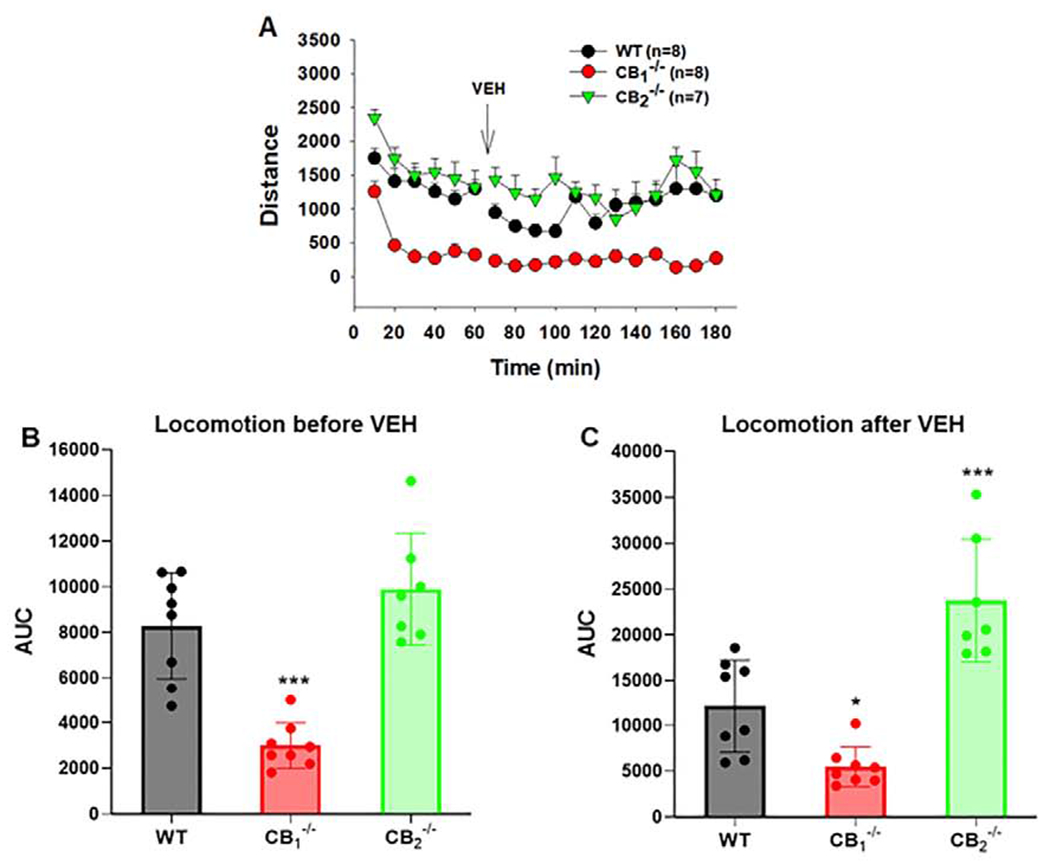
Genetic deletion of CB1 and CB2 receptors differentially alters basal levels of open-field locomotor activity. (A) Time course of locomotion before and after vehicle injection in WT, CB1−/− and CB2−/− mice. (B) Area under curve (AUC) data during the initial 1-h habituation (before vehicle injection) in open-field locomotion chambers. (C) AUC data during 2-h maintenance (after vehicle) in locomotion chambers in three different genotypes of mice. *p<0.05, ***p<0.001, relative to WT mice.
Consistent with the above microdialysis data, systemic administration of Δ9-THC (1,3 mg/kg, i.p.) produced a dose-dependent reduction in open-field locomotor activity in WT mice (Fig. 5A), but not CB2-KO mice (Fig. 5G). The time course in locomotor response to Δ9-THC in WT mice appears to parallel the time course of DA response to Δ9-THC, suggesting a possible correlation between Δ9-THC-induced changes in DA and locomotor activity. CB1-KO mice displayed a robust reduction in basal levels of locomotion (Fig. 4) and the amplitude of locomotor response to Δ9-THC (Fig. 5D). The three-way repeated measures ANOVA showed a significant Genotype × Time × Dose interaction (F102,408 =1.56, p<0.05). Since the basal levels of locomotion differ between WT and mutant mice, Δ9-THC-induced locomotor depression was normalized to percent changes over baseline (Fig. 5–B, E, H). A two-way ANOVA on the ΔAUC data produced a significant Dose Δ Genotype interaction (F6,78 = 2.61, p<0.05). Post-hoc group comparisons revealed that 3 and 10 mg/kg Δ9-THC significantly decreased open-field activity in WT mice, but not CB2-KO mice. CB1-KO mice demonstrated hypolocomotion at 1 and 3 mg/kg Δ9-THC, but not 10 mg/kg.
Figure 5:
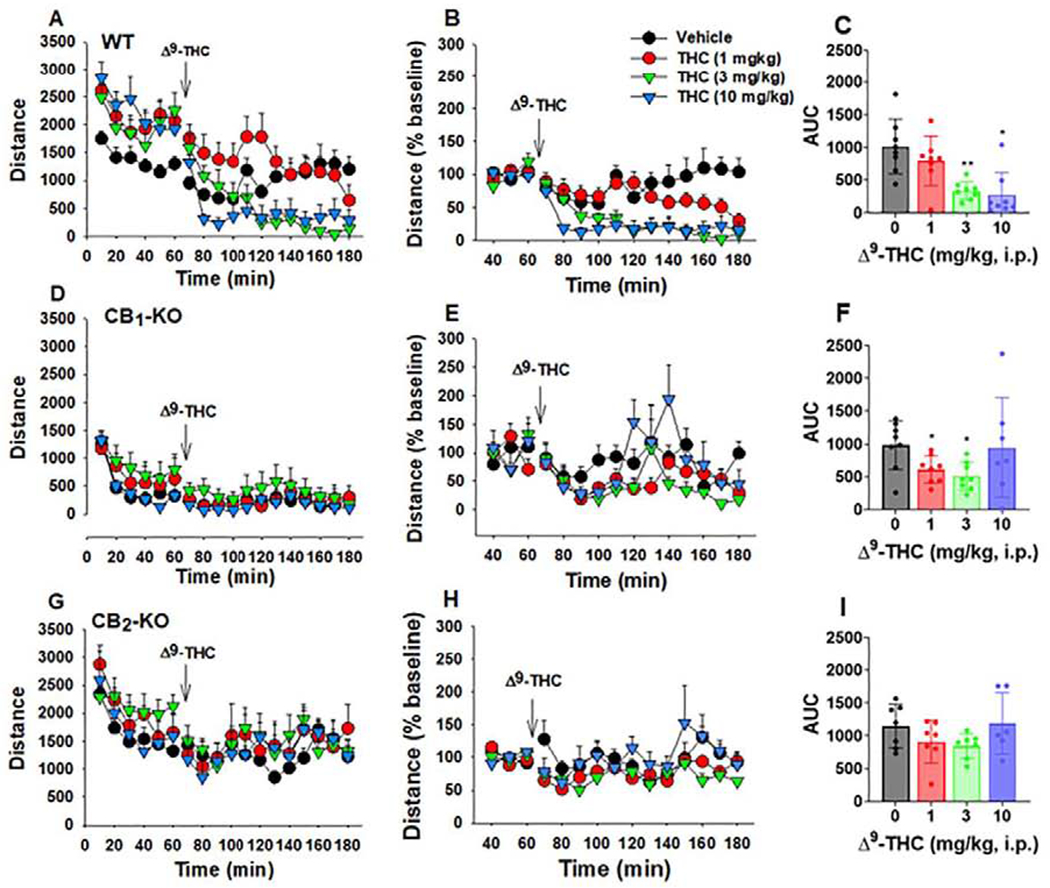
Open field locomotion following Δ9-THC administration in mice. (A, B, C) Original locomotor activity (A), normalized data (% baseline) (B), and ΔAUC (C) in WT mice, illustrating that Δ9-THC dose-dependently decreased locomotor activity (n = 8). (D, E, F) Original locomotor activity (D), normalized data over baseline (% baseline) (E), and ΔAUC (F) in CB1-KO mice, illustrating that CB1-KO mice displayed significantly lower basal level of locomotion and lower locomotor response (amplitude) to Δ9-THC (D) (n = 8). Unexpectedly, the normalized data (E, F) also revealed dose-dependent locomotor depression after Δ9-THC administration in CB1-KO mice. (G, H, I) Original locomotor activity (G), normalized data over baseline (% baseline) (H), and ΔAUC (I) in CB2-KO mice, illustrating that Δ9-THC had no effect locomotor activity (n = 7). *p<0.05, **p<0.01 relative to vehicle.
4. DISCUSSION
In the present experiment we investigated how genetic deletion of the CB1 and CB2 receptors impacted the rewarding versus aversive effects of Δ9-THC as assessed by CPP, NAc DA release, and open-field locomotor activity. In WT mice, Δ9-THC produced a CPP at a low dose (1 mg/kg), which was absent in CB1-KO mice, suggesting a CB1-mediated effect. At 5 mg/kg Δ9-THC, CPP scores were significantly reduced in CB1-KO mice relative to WT. In contrast, CB2-KO mice developed robust dose-dependent CPP, indicating that CB2R activation underlies the aversive effects produced by Δ9-THC. Neurochemical assessments in WT mice revealed a dose-dependent reduction in extracellular DA following Δ9-THC injections. Genetic deletion of CB1Rs potentiated Δ9-THC-induced decreases in extracellular DA, whereas in CB2-KO mice, Δ9-THC produced a significant increase in extracellular DA at both doses. Similarly, deletion of CB1 and CB2 receptors attenuated Δ9-THC-induced locomotor depression. These findings suggest that both CB1 and CB2 receptors likely mediate the hedonic spectrum of Δ9-THC such that CB1R activation underlies cannabis reward and CB2R activation mediates its aversive effects.
The paradoxical effects of Δ9-THC in animal models of reward have been well documented (Panagis et al., 2008; Tanda, 2016). Repeated attempts to train rodents and primates on Δ9-THC self-administration have been unsuccessful (Harris et al., 1974; John et al., 2017; Lefever et al., 2014; Mansbach et al., 1994), although one research group demonstrated that squirrel monkeys can be trained to self-administer cannabinoids (Justinova et al., 2008; Justinova et al., 2003; Tanda et al., 2000). Δ9-THC has been reported to produce biphasic effects in CPP and ICSS experiments, such that low doses are rewarding, while high doses are aversive (Kubilius et al., 2018; Spiller et al., 2019). These effects tend to vary across rat strains, species, and procedures such that rewarding and aversive effects have been described at the same Δ9-THC dose (DeVuono et al., 2017; Valjent and Maldonado, 2000). Discrepancies in the affective properties of cannabinoids have been noted in the literature for decades; however, it is unknown why such variability exists. One theory advanced is that the enjoyment reported by human users is based on negative reinforcement i.e. the removal of an aversive state such as anxiety or pain (Rubino et al., 2007; Rutkowska et al., 2006), which is not measured in typical CPP and ICSS experiments. However, this theory has not been supported by work evaluating the rewarding profile of Δ9-THC following a stressful experience (DeVuono et al., 2017; Fokos and Panagis, 2010).
An alternative explanation is that the affective experience of a cannabinoid is dependent on the underlying neurocircuitry and varying distributions of CB1 and CB2 receptor populations. Prior work from our laboratory has attempted to dissect the role of different cannabinoid receptors in cannabis reward versus aversion (Galaj and Xi, 2019; Han et al., 2017; Jordan and Xi, 2019; Spiller et al., 2019). By using highly sensitive RNAscope ISH assays with CB2-KO mice as controls, we have recently identified functional CB2R expression in VTA DA neurons (Xi et al., 2011; Zhang et al., 2014; Zhang et al., 2017). Given that activation of CB2Rs inhibits firing rates of VTA DA neurons and decreases DA release in the NAc (Foster et al., 2016; Ma et al., 2019; Xi et al., 2011; Zhang et al., 2014; Zhang et al., 2017), we proposed that CB2R activation may in part underlie cannabinoid aversion (Galaj and Xi, 2019; Humburg et al., under review; Jordan and Xi, 2019; Spiller et al., 2019). This hypothesis was supported by work demonstrating that Δ9-THC or WIN55,212-2 attenuates brain-stimulation reward (BSR), maintained by electrical stimulation of the medal forebrain bundle in rats or by optical stimulation of VTA DA neurons in DAT-cre mice, an effect that can be blocked by CB2R antagonism (Humburg et al., under review; Spiller et al., 2019). Furthermore, beta-caryophyllene, a CB2R agonist, inhibits optical BSR and cocaine-enhanced BSR (Galaj et al., in press; He et al., 2020).
As described in the introduction, selective deletion of glutamatergic CB1Rs can block Δ9-THC’s inhibitory action in optical ICSS, suggesting that a CB1R mechanism in glutamate neurons may also contribute to cannabinoid aversion (Han et al., 2017). Thus, stimulation of CB1Rs on glutamate neurons as well as CB2Rs on DA neurons likely mediates cannabinoid aversion and activation of CB1Rs on GABA neurons has been implicated in cannabinoid reward (Figure 6). The final outcome of a given cannabinoid likely depends on the balance of these opposing actions (Galaj and Xi, 2019; Han et al., 2017; Jordan and Xi, 2019; Spiller et al., 2019). This new hypothesis may partially explain why the affective properties of cannabinoids are so inconsistent in the literature, since different species, rodent strains or even individual subjects may have unique cellular distributions of CB1Rs and CB2Rs in the brain.
Figure 6:
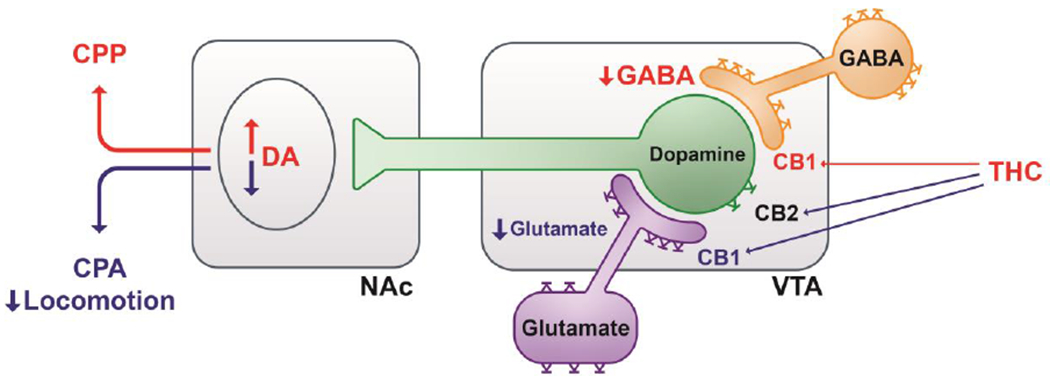
Graphic diagram illustrating how Δ9-THC modulates the mesolimbic DA system and cannabinoid action via CB1 and CB2 receptors. Δ9-THC may produce rewarding effects by binding to CB1 receptors on GABAergic neurons or afferents in the VTA, thereby reducing GABA-mediated inhibition of VTA DA neurons and increasing DA release in the NAc. Conversely, Δ9-THC may produce aversive effects by activating CB1 receptors on glutamatergic neurons or afferents in the VTA as well as CB2 receptors on DA neurons, thereby inhibiting VTA DA release to the NAc. The subjective effects of Δ9-THC may thus depend on the balance of opposing effects produced by activation of CB1 and CB2 receptors in different phenotypes of neurons. VTA: Ventral tegmental area. NAc: Nucleus accumbens, DA: Dopamine.
In the present study, we provide additional evidence supporting this CB1-CB2 hypothesis. Given the wide range of CPP scores often observed with cannabinoids, we used large group sizes (n = 12-20 per group) to reduce variability and found that Δ9-THC was rewarding at a low dose (1 mg/kg) in WT subjects, consistent with prior work in mice (Ghozland et al., 2002; Soria et al., 2004; Valjent and Maldonado, 2000). CB1R deletion blocked Δ9-THC-induced CPP, supporting the conjectured role of these receptors in cannabinoid reward. This is also consistent with earlier studies in which pharmacological antagonism of CB1Rs reduced Δ9-THC-induced CPP, self-administration, and BSR (Braida et al., 2004; Justinova et al., 2008; Spiller et al., 2019). Perhaps the most important finding in this study is that that a high dose of Δ9-THC activates the CB2R, producing an aversive effect that antagonizes CB1-mediated reward. Specifically, 5 mg/kg Δ9-THC produced no effect in WT mice, lowered CPP scores in CB1-KO mice, but induced a robust place preference in CB2-KO mice, implicating a CB2R mechanism in Δ9-THC’s aversive effects. These findings are consistent with our previous report demonstrating that electrical BSR was increased by ACEA, a selective CB1R agonist, but suppressed by JWH133, a selective CB2R agonist (Spiller et al., 2019). Moreover, in prior work JWH133 administration or over-expression of CB2Rs in the brain attenuated cocaine CPP, self-administration and locomotor sensitization (Aracil-Fernandez et al., 2012; Delis et al., 2016; Lopes et al., 2020; Xi et al., 2011), while local infusions of JWH133 into the NAc and VTA inhibited cocaine self-administration in WT and CB1-KO mice, but not CB2-KO mice (Xi et al., 2011; Zhang et al., 2014; Zhang et al., 2017). Further, genetic deletion of CB2Rs from midbrain DA neurons enhanced cocaine-induced hyperactivity, locomotor sensitization, and CPP (Canseco-Alba et al., 2019). Growing evidence indicates that CB2 receptors are expressed in VTA DA neurons and regulate DA neuronal activity (Foster et al., 2016; Xi et al., 2011; Zhang et al., 2014), which may explain how CB2 receptors modulate the mesolimbic DA reward system and cannabinoid aversion (Galaj and Xi, 2019; Jordan and Xi, 2019).
Surprisingly, no increase in extracellular DA was observed following Δ9-THC administration at a dose that induced a place preference in WT mice (1 mg/kg). One explanation for these disparate behavioral and neurochemical results is the technical limitations of microdialysis. Specifically, this approach may not be able to detect subtle changes in extracellular DA produced by Δ9-THC at low doses due to its poor temporal and spatial resolution. Indeed, another report in mice showed that a subthreshold Δ9-THC dose (0.3 mg/kg), increased extracellular DA, but did not induce a place preference (Robledo et al., 2007), suggesting a possible decoupling between the behavioral and neurochemical changes. A second possibility is that Δ9-THC action in DA signaling may display some subregion-specificity within the NAc. Tanda and colleagues demonstrated that Δ9-THC preferentially increases DA in the NAc core over the NAc shell in rats (1997). In the present study, we did not restrict probe placement to the NAc shell or core due to the practical difficulty in separating these subregions in mice.
It is important to note that in previous studies acute Δ9-THC injections increased extracellular DA in the NAc within rats (Chen et al., 1991; Chen et al., 1990; Malone and Taylor, 1999; Tanda et al., 1997). This work conflicts with our own findings in mice. To further address this potential species difference in the DA response to cannabinoids, we performed a microdialysis experiment in rats. Systemic administration of Δ9-THC trended towards an increase in NAc DA. However, vehicle infusions produced an analogous surge in DA, suggesting a non-specific effect. To further confirm this finding and determine whether the ethanol (~2%) present in the vehicle and Δ9-THC solution may underlie the non-specific DA response observed, we diluted the Δ9-THC solution using 0.5% Tween-80 and saline to vary the ethanol level in the Δ9-THC solution. Local perfusion of varying Δ9-THC concentrations (0.1~100 μM) into the NAc also failed to alter extracellular DA. This is consistent with a previous report in rats demonstrating no change in NAc DA following acute Δ9-THC administration (Castañeda et al., 1991). However, a trend towards an increase in DA was observed at 100 μM Δ9-THC, which contained a higher concentration of alcohol (0.625%). These data demonstrate that systemic administration or intra-NAc local perfusion of Δ9-THC has no effect on extracellular DA in rats. The small DA response observed is likely elicited by the presence of ethanol in the vehicle and Δ9-THC solution.
In addition to Δ9-THC, systemic SR141716A (rimonabant) also failed to alter extracellular DA. This is consistent with our previous findings with AM251, another CB1R antagonist or inverse agonist, which failed to alter extracellular NAc DA (Xi et al., 2006). However, intra-NAc local perfusion of SR141716A elevated extracellular DA in a concentration-dependent manner, suggesting that endocannabinoids may tonically inhibit DA release via activation of CB1Rs in the NAc. Although our theory of the affective properties of cannabinoids suggests that activation of CB1Rs is rewarding, this effect is thought to be mediated specifically by GABAergic CB1Rs. These receptors are not limited to GABA neurons but are also expressed on VTA glutamate and a subpopulation of DA neurons (Han et al., 2017; Humburg, 2020). As such, activation of CB1Rs on VTA DA and glutamate neurons may lead to a reduction in DA release. These findings provide additional evidence supporting the presence of a species difference in brain DA response to Δ9-THC between rats and mice (Zhang et al., 2015). Specifically, systemic administration of cannabinoids tends to inhibit NAc DA release in mice but has no significant effect on NAc DA in rats. The CB1-CB2 hypothesis proposed above (Fig. 6) may well explain such species-specific effects in cannabinoid action (Liu et al., 2019; Liu et al., 2009; Zhang et al., 2015).
In relation to the locomotor data, genetic deletion of the CB1 receptor robustly decreased basal locomotor activity, while genetic deletion of the CB2Rs produced a moderate increase, suggesting that both receptors regulate basal locomotor activity. CB1-KO mice also failed to demonstrate Δ9-THC-induced hypolocomotion at 10 mg/kg. This data nicely parallels prior work demonstrating the involvement of CB1Rs in the motor effects of cannabinoids. Our lab has shown that CB1-KO mice display lower basal and cocaine-enhanced locomotion relative to WT mice (Li et al., 2009) and previous reports indicate that CB1R deletion blocks high doses of Δ9-THC-induced catalepsy and immobility (Compton et al., 1996; Grim et al., 2017; Ledent et al., 1999; Varvel et al., 2005; Wang et al., 2020). Surprisingly, when Δ9-THC-induced changes in locomotion were normalized over baseline (% baseline), CB1-KO mice showed no differences from WT at 3 mg/kg, implicating other receptors in the motor effects of cannabinoids. Indeed, genetic deletion of CB2Rs was able to block Δ9-THC-induced locomotor depression at this dose, suggesting that CB2Rs mediate some of Δ9-THC’s locomotor suppressing effects. This finding is somewhat unexpected as CB2-KO mice displayed a robust dose-dependent increase in DA response to Δ9-THC. As such, a corresponding rise in locomotor activity might be predicted. These findings denote a possible dissociation between DA and locomotor activity to cannabinoids in CB2-KO mice. It may be that deletion of CB2RS also modulates neurotransmitter release in the brain, which could counteract DA-induced hyperactivity. Another possibility is that neuroadaptations may occur in CB2-KO mice, which compromise the locomotor activating effects of DA in this context.
In conclusion, the present findings demonstrate that CB1R and CB2R mechanisms differentially underlie cannabinoid reward versus aversion. The final outcome of cannabinoid action likely depends on the relative balance of CB1R and CB2R activation on different phenotypes of neurons. These findings provide insight into the paradoxical hedonic effects described by cannabis users as well as conflicting preclinical reports with Δ9-THC and other cannabinoids.
Acknowledgement
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA) (DA000633-01), National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that there is no any personal financial conflict of interest.
REFERENCES
- Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J, 2012. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology 37, 1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K, 2010. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, 1983. The role of dopamine in locomotor activity and learning. Brain Research Reviews 6, 173–196. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosue S, Pegorini S, Sala M, 2004. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. European Journal of Pharmacology 506, 63–69. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A, 2000. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol 396, 141–149. [DOI] [PubMed] [Google Scholar]
- Bystrowska B, Frankowska M, Smaga I, Pomierny-Chamioło L, Filip M, 2018. Effects of Cocaine Self-Administration and Its Extinction on the Rat Brain Cannabinoid CB1 and CB2 Receptors. Neurotoxicity research 34, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu Q-R, Onaivi ES, 2019. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behavioural Brain Research 360, 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda E, Moss DE, Oddie SD, Whishaw IQ, 1991. THC does not affect striatal dopamine release: Microdialysis in freely moving rats. Pharmacology Biochemistry and Behavior 40, 587–591. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health, S., Quality, 2019. 2018 national survey on drug use and health: Detailed tables. Substance Abuse and Mental Health Services Administration; Rockville,-MD. [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA, 2000. Cannabinoid receptors and reward in the rat: A conditioned place preference study. Psychopharmacology 151, 25–30. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL, 1991. Strain-specific facilitation of dopamine efflux by Δ9-tetrahydrocannabinol in the nucleus accumbens of rat: An in vivo microdialysis study. Neuroscience Letters 129, 136–140. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL, 1990. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 102, 156–162. [DOI] [PubMed] [Google Scholar]
- Chopra GS, 1969. Man and Marijuana. International journal of the addictions 4, 215–247. [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR, 1996. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther 277, 586–594. [PubMed] [Google Scholar]
- Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K, 2016. Attenuation of Cocaine-Induced Conditioned Place Preference and Motor Activity via Cannabinoid CB2 Receptor Agonism and CB1 Receptor Antagonism in Rats. International Journal of Neuropsychopharmacology 20, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono MV, Wills KL, MacPherson DV, Hrelja KM, Parker LA, 2017. Effect of footshock stress on place conditioning produced by Delta(9)-tetrahydrocannabinol and the fatty acid amide hydrolase (FAAH) inhibitor, URB597, in Sprague-Dawley rats. Psychopharmacology (Berl) 234, 3229–3240. [DOI] [PubMed] [Google Scholar]
- Ewusi Boisvert E, Bae D, Pang RD, Davis JP, Kelley-Quon LI, Barrington-Trimis JL, Kirkpatrick MG, Chai SH, Leventhal AM, 2020. Subjective effects of combustible, vaporized, and edible cannabis: Results from a survey of adolescent cannabis users. Drug and Alcohol Dependence 206, 107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokos S, Panagis G, 2010. Effects of Δ9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. Journal of Psychopharmacology 24, 767–777. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2016. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Bi GH, Moore A, Chen K, Gardner EL, Xi ZX, in press Beta-caryophyllene inhibits cocaine taking and seeking by activation of PPRAα and PPARγ receptors: Repurposing a FDA-approved food additive for cocaine use disorder Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Newman AH, Xi Z-X, 2020. Dopamine D3 receptor-based medication development for the treatment of opioid use disorder: Rationale, progress, and challenges. Neuroscience & Biobehavioral Reviews 114, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Xi ZX, 2019. Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs 33, 1001–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D, 1988. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology 96, 142–144. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HWD, Simonin F, Filliol D, Kieffer BL, Maldonado R, 2002. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Thomas BF, Wiley JL, Endres GW, Negus SS, Lichtman AH, 2017. Apparent CB(1) Receptor Rimonabant Affinity Estimates: Combination with THC and Synthetic Cannabinoids in the Mouse In Vivo Triad Model. J Pharmacol Exp Ther 362, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, He Y, Bi GH, Zhang HY, Song R, Liu QR, Egan JM, Gardner EL, Li J, Xi ZX, 2017. CB1 Receptor Activation on VgluT2-Expressing Glutamatergic Neurons Underlies Δ(9)-Tetrahydrocannabinol (Δ(9)-THC)-Induced Aversive Effects in Mice. Sci Rep 7, 12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RT, Waters W, McLendon D, 1974. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia 37, 23–29. [DOI] [PubMed] [Google Scholar]
- He Y, Galaj E, Bi G-H, Wang X-F, Gardner E, Xi Z-X, 2020. β-Caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. British Journal of Pharmacology 177, 2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Abood ME, 2017. CB(1) and CB(2) Receptor Pharmacology. Advances in pharmacology (San Diego, Calif.) 80, 169–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humburg BA, C.J. J, Zhang H-Y, Shen H, Han X, Hempel BJ, Bi G-H, Galaj E, M.H. B, Xi Z-H, under review Evaluation of the abuse potential of cannabinoids using a new animal procedure of optogenetic brain self-stimulation reward in transgenic mice Addiction Biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA, 2017. Behavioral Determinants of Cannabinoid Self-Administration in Old World Monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Cao J, Newman AH, Xi ZX, 2019. Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine. Neuropharmacology 158, 107609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Xi ZX, 2019. Progress in brain cannabinoid CB(2) receptor research: From genes to behavior. Neurosci Biobehav Rev 98, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR, 2008. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33, 2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR, 2003. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology 169, 135–140. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G, 2013. Biphasic effects of Delta9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol 16, 2273–2284. [DOI] [PubMed] [Google Scholar]
- Kubilius RA, Kaplick PM, Wotjak CT, 2018. Highway to hell or magic smoke? The dose-dependence of Delta(9)-THC in place conditioning paradigms. Learn Mem 25, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuddus M, Ginawi IA, Al-Hazimi A, 2013. Cannabis sativa: An ancient wild edible plant of India. Emirates Journal of Food and Agriculture, 736–745. [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M, 1999. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283, 401–404. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL, 2014. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacology, biochemistry, and behavior 118, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL, 1996. Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sciences 58, PF365–PF372. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL, 1995. Conditioned place preference induced by !D–9-tetrahydrocannabinol: Comparison with cocaine, morphine, and food reward. Life Sciences 56, 2073–2080. [DOI] [PubMed] [Google Scholar]
- Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, Xi ZX, 2009. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology (Berl) 204, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-R, Huang NS, Qu H, O’Connell JF, Gonzalez-Mariscal I, Santa-Cruz-Calvo S, Doyle ME, Xi Z-X, Wang Y, Onaivi ES, Egan JM, 2019. Identification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeutics. Acta Pharmacologica Sinica 40, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-R, Pan C-H, Hishimoto A, Li C-Y, Xi Z-X, Llorente-Berzal A, Viveros M-P, Ishiguro H, Arinami T, Onaivi ES, Uhl GR, 2009. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes, Brain and Behavior 8, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JB, Bastos JR, Costa RB, Aguiar DC, Moreira FA, 2020. The roles of cannabinoid CB1 and CB2 receptors in cocaine-induced behavioral sensitization and conditioned place preference in mice. Psychopharmacology 237, 385–394. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF, 2004. Marijuana and cannabinoid regulation of brain reward circuits. British Journal of Pharmacology 143, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Gao F, Larsen B, Gao M, Luo Z, Chen D, Ma X, Qiu S, Zhou Y, Xie J, Xi ZX, Wu J, 2019. Mechanisms of cannabinoid CB(2) receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine 42, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, 2006. Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. International Journal of Obesity 30, S19–S23. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA, 1999. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol 128, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL, 1994. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behavioural pharmacology 5, 219–225. [DOI] [PubMed] [Google Scholar]
- Matheson J, Mann RE, Sproule B, Huestis MA, Wickens CM, Stoduto G, George TP, Rehm J, Le Foll B, Brands B, 2020. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol Biochem Behav, 172937. [DOI] [PubMed] [Google Scholar]
- Melis M, Gessa GL, Diana M, 2000. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Progress in NeuroPsychopharmacology and Biological Psychiatry 24, 993–1006. [DOI] [PubMed] [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG, 2008. Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1, 350–374. [DOI] [PubMed] [Google Scholar]
- Pang RD, Guillot CR, Zvolensky MJ, Bonn-Miller MO, Leventhal AM, 2017. Associations of anxiety sensitivity and emotional symptoms with the subjective effects of alcohol, cigarettes, and cannabis in adolescents. Addictive behaviors 73, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British journal of pharmacology 153, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Trigo JM, Panayi F, de la Torre R, Maldonado R, 2007. Behavioural and neurochemical effects of combined MDMA and THC administration in mice. Psychopharmacology 195, 255–264. [DOI] [PubMed] [Google Scholar]
- Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, Parolaro D, 2007. Cellular Mechanisms Underlying the Anxiolytic Effect of Low Doses of Peripheral [Delta]9-Tetrahydrocannabinol in Rats. Neuropsychopharmacology 32, 2036–2045. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jamontt J, Gliniak H, 2006. Effects of cannabinoids on the anxiety-like response in mice. Pharmacol Rep 58, 200–206. [PubMed] [Google Scholar]
- Schlienz NJ, Spindle TR, Cone EJ, Herrmann ES, Bigelow GE, Mitchell JM, Flegel R, LoDico C, Vandrey R, 2020. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug and Alcohol Dependence 211, 107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, Mischley LK, 2019. A Survey of Cannabis Acute Effects and Withdrawal Symptoms: Differential Responses Across User Types and Age. Journal of alternative and complementary medicine (New York, N.Y.) 25, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castañé A, Berrendero F, Ledent C, Parmentier M, Maldonado R, Valverde O, 2004. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. European Journal of Neuroscience 20, 2203–2213. [DOI] [PubMed] [Google Scholar]
- Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, Xi ZX, 2019. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol 176, 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2018. Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA network open 1, e184841–e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, 2016. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology 233, 1845–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR, 2003. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 169, 115–134. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR, 2000. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature Neuroscience 3, 1073–1074. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Chiara GD, 1997. Cannabinoid and Heroin Activation of Mesolimbic Dopamine Transmission by a Common μ1 Opioid Receptor Mechanism. Science 276, 2048. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R, 2000. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology 147, 436–438. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH, 2005. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther 314, 329–337. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G, 2007. Lack of evidence for appetitive effects of Δ9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behavioural Pharmacology 18, 311–319. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- Wang XF, Galaj E, Bi GH, Zhang C, He Y, Zhan J, Bauman MH, Gardner EL, Xi ZX, 2020. Different receptor mechanisms underlying phytocannabinoid- versus synthetic cannabinoid-induced tetrad effects: Opposite roles of CB(1) /CB(2) versus GPR55 receptors. Br J Pharmacol 177, 1865–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, Negus SS, Lichtman AH, 2015. Δ9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. The Journal of pharmacology and experimental therapeutics 352, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL, 2006. Cannabinoid CB1 Receptor Antagonist AM251 Inhibits Cocaine-Primed Relapse in Rats: Role of Glutamate in the Nucleus Accumbens. The Journal of Neuroscience 26, 8531–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL, 2011. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat Neurosci 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, Gardner EL, Wu J, Xi Z-X, 2014. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proceedings of the National Academy of Sciences 111, E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Shen H, Bi G-H, Yang H-J, Liu Q-R, Wu J, Gardner EL, Bonci A, Xi Z-X, 2017. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biology 22, 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR, 2015. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40, 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Shen H, Jordan CJ, Liu QR, Gardner EL, Bonci A, Xi ZX, 2019. CB(2) receptor antibody signal specificity: correlations with the use of partial CB(2)-knockout mice and anti-rat CB(2) receptor antibodies. Acta Pharmacol Sin 40, 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI, 1999. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96, 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]


