Abstract
Biological invasions by nonindigenous species can have negative effects on economies and ecosystems. To limit this impact, current research on biological invasions uses functional traits to facilitate a mechanistic understanding of theoretical and applied questions. Here we aimed to assess the role of functional traits in the progression of crayfish species through different stages of invasion and determine the traits associated with invasive success. A dataset of thirteen functional traits of 15 species currently occurring or available for sale in the Netherlands was evaluated. Six of these crayfish appeared invasive. Important traits distinguishing successful from unsuccessful invaders were a temperate climate in the native range, a medium to high egg count and producing more than one egg clutch per year. The most successful invaders had different functional trait combinations: Procambarus clarkii has a higher reproductive output, can migrate over longer distances and possesses a higher aggression level; Faxonius limosus is adapted to a colder climate, can reproduce parthenogetically and has broader environmental tolerances. Using a suit of functional traits to analyse invasive potential can help risk management and prevention. For example, based on our data Procambarus virginalis is predicted to become the next successful invasive crayfish in the Netherlands.
Subject terms: Freshwater ecology, Invasive species
Introduction
Over the past decades worldwide trade and traffic have greatly increased, leading to the introduction of many species into ecosystems beyond their native range1. Nonindigenous species that established new populations outside their native range have been widely recognized to have potential negative impacts on local ecosystems and are generally regarded as one of the largest threats to biodiversity besides habitat destruction2,3. Their impacts include competition, grazing, predation and introduction and spread of diseases4,5. In addition to their potential contribution to species extinctions6, nonindigenous species may also have severe economic impacts: between 1992 and 2006 the European Union spent over 130 million euro on projects dealing with nonindigenous species7.
Aquatic ecosystems are considered more vulnerable to species introductions than terrestrial systems, due to active (e.g. fish stocking) and passive (e.g. releasing ballast water) import of nonindigenous species8,9. The United Nations Food and Agriculture Organization’s Database of Invasive Aquatic Species states that on average 63% of aquatic species become established after introduction10. One of the most successful taxa currently invading freshwater systems is decapods, with 46% of the nonindigenous species in Europe being invasive11. In the Netherlands, decapods represent 18% of about 66 recorded nonindigenous macroinvertebrate species12. Within the decapods, crayfish are well known for their invasive potential and are currently displacing native crayfish throughout Europe13,14. Procambarus clarkii, for instance, is considered a highly invasive species throughout Europe with negative impacts on invaded ecosystems15,16. Currently, at least ten nonindigenous crayfish species have established populations in Europe17.
One third of the 100 worst aquatic invasive species18 originates from aquarium releases19. Additionally, worldwide sale of pet crayfish has grown substantially in recent decades: about 130 of roughly 600 different crayfish species were reportedly sold as pets20. In the Netherlands, nine crayfish species are commonly for sale21. In crayfish, functional traits such as bright coloration, smaller size, or single parent reproduction (parthogenesis) seem to contribute to their popularity as pets20. Research by Chucholl & Wendler22 showed that 67% of high risk crayfish sold in Germany possess traits attractive for pet owners. They found such functional traits can be directly related to long-term presence of nonindigenous species in the aquarium trade and concluded that higher risk crayfish are selected for introduction due to popular traits (Table 1). Similarly, Zeng et al.23 found species with large clutch sizes used for non-commercial harvesting (exploitation for food, recreation, and by hobbyists for personal aquariums) and bait to have increased risk of introduction.
Table 1.
Invasion framework by Blackburn et al.24 with functional traits mentioned in literature as relevant to the barriers of each stage.
| Invasion stage | Barrier | Associated traits | References |
|---|---|---|---|
| Transport (A) | Geography | Size of native range | 33,38,62 |
| Introduction (B) | Captivity/cultivation | Preference or tolerance of lentic habitats | 22,23,32,33 |
| Able to reproduce at warm-water aquarium conditions | 22 | ||
| Absence of specialized germination or hatching requirements | 26 | ||
| Bright body coloration | 20,22 | ||
| Small body size (when not brightly colored) | 22 | ||
| Establishment (C) | Survival | Wide environmental and climatic tolerances | 26,33,38,62 |
| Generalist diet | 25,33,38 | ||
| Reproduction | Single parent reproduction | 20,25,26,62 | |
| High reproductive potential | 23,25,26,32,33,38,62 | ||
| Short generation time | 25,26 | ||
| High growth rate | 26 | ||
| Spread (D) | Dispersal | Good dispersal | 25,26,33 |
| Fully invasive (E) | Environmental/competition | Ability to escape or survive natural enemies | 26 |
| Long lived (resist mortality) | 28 | ||
| Large size | 23,32,38 | ||
| High competitive ability | 23,25,26,32,33 |
Blackburn et al.24 suggested a unified framework for biological invasions that combines aspects of both plant and animal ecology and focuses on human-mediated invasions. This framework describes the progressive stages of an invasion from transportation to fully invasive, including specific barriers for each stage (Table 1). A nonindigenous species only progresses through successive stages of invasion when it possesses barrier-specific functional traits11. For example, successful invasive species often have traits such as wide environmental and climatic tolerances, a generalist diet, high reproductive potential and good dispersal ability25,26. By identifying species with traits associated with high invasive potential, invasions with negative impacts can be predicted and prevented more effectively27.
Several efforts were made to assess the functional traits associated with successful aquatic invaders from different taxa, including fish28, gammarids29, plants30 and marine crabs31. For crayfish, Larson & Olden32 classified the invasive potential of 77 species in the United States using a functional trait-based analysis. They identified species with a high latent risk to become invasive outside their native range, taking into account that certain traits can be more beneficial in specific stages of invasion. Their results showed that successful invasive crayfish were grouped together based on the traits large size, high fecundity and lentic, terrestrial and generalist habitat preferences. Zeng et al.23 determined the invasion status of 614 crayfish species according to the framework of Blackburn et al.24 and assessed which functional traits were associated with species progression to the stages of establishment and spread. Their analysis included human-associated traits such as non-commercial harvesting and ornamental trade. They found clutch size to be predictive of the spread stage. An invasiveness screening tool for fish was also adapted into the Freshwater Invertebrate Invasiveness Scoring Kit (FI-ISK) to assess the invasion risk of nonindigenous crayfish species in Italy, based partly on trait related information33.
This paper aims to address the role that functional traits play in the transition of nonindigenous crayfish through the stages of establishment and spread to become fully invasive, and to determine which traits are associated with invasive success. To achieve this, the distribution, relation to their environment and the functional traits of nonindigenous crayfish species currently present in the Netherlands were assessed. The traits of successful and unsuccessful invasive species were compared to determine which specific traits allowed them to pass through the stages of invasion according to the Blackburn framework. Finally, the current invasion status of crayfish in the Netherlands was compared to predictions of invasion risk by other studies. Based on previous research22,23,32 we expected successful invasive species to possess different traits than unsuccessful species, with specific combinations of traits being required to pass all invasion stages leading to invasive success.
Results
Crayfish distribution
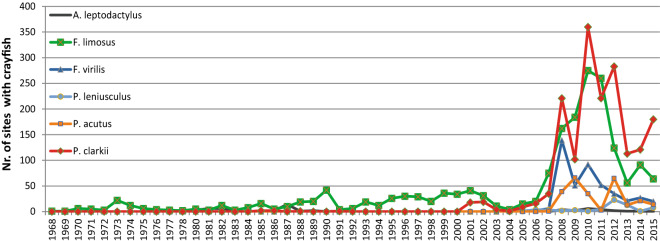
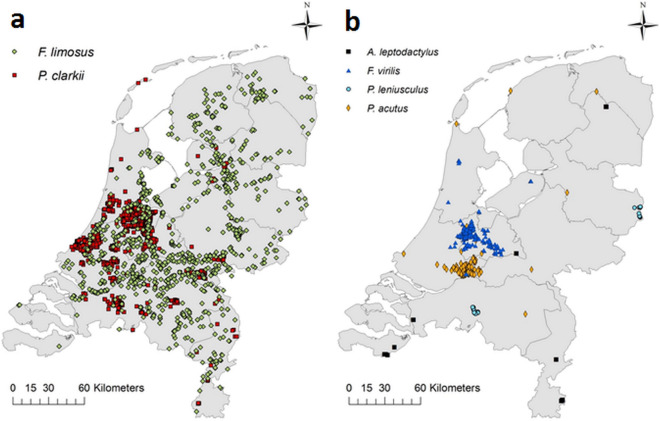
Several nonindigenous crayfish species were first observed in the Netherlands multiple decades ago, such as Faxonius limosus, Astacus leptodactylus, and P. clarkii (1968, 1978, and 1985, respectively) (Fig. 1). More recently introduced species were Faxonius virilis (2004), Pacifastacus leniusculus (2005), and Procambarus acutus (2007). The species found in the highest number of sites were P. clarkii and F. limosus, while A. leptodactylus and P. leniusculus were recorded in the lowest number of sites. Pacifastacus leniusculus was reported in two areas, each with multiple locations nearby. In contrast, A. leptodactylus had been found in multiple locations further apart from each other. Faxonius virilis and P. acutus were sighted at an intermediate number of sites. Although F. virilis and P. acutus hardly co-occurred, there was a similar pattern in sightings (Fig. 2b) as both occur mainly within a limited region with sparse sightings in other regions. Although P. clarkii and F. limosus both occurred at many sites, there is a clear difference between their spatial distributions (Fig. 2a). The former was found mainly in the mid-west of the Netherlands, while the latter was spread more evenly throughout the entire country. Finally, P. acutus showed relatively little overlap with P. clarkii, while F. virilis did occur partially over the same geographical area as P. clarkii.
Figure 1.
Number of sites with crayfish occurrences per year.
Figure 2.
Distributions of (a) F. limosus and P. clarkii and (b) A. leptodactylus, F. virilis, P. leniusculus and P. acutus in the Netherlands. These maps were generated using ArcMap 10.2.2 (https://desktop.arcgis.com/en/arcmap/).
Occurrence in different water types
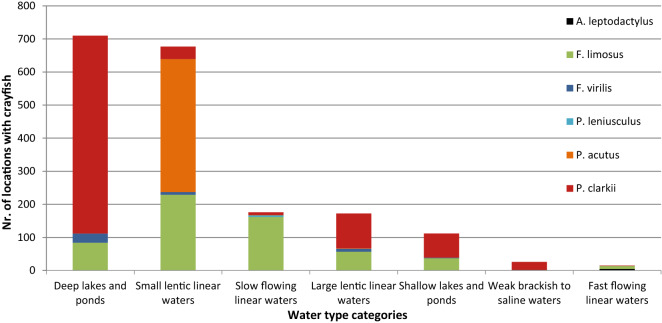
The occurrence of crayfish in different water types is species specific (Fig. 3). Procambarus clarkii occurred in waterbodies of all types but was mostly present in both shallow and deeper lakes and ponds and in larger, linear, lentic waterbodies such as canals. In contrast, P. acutus was found only in small, linear, lentic waters (mainly man-made ditches and canals, all under 3 m deep and 15 m wide). While F. virilis predominantly occurred in deep lakes and ponds, F. limosus was distributed over multiple locations across all water types, except brackish and saline waters. Few crayfish were found in brackish and saline waters and fast flowing waters, only A. leptodactylus was found exclusively in these water types. Pacifastacus leniusculus occurred only in slow flowing waters.
Figure 3.
Number of sites per water type for each of the six crayfish species (data from 2007 to 2015).
Crayfish clustered by traits
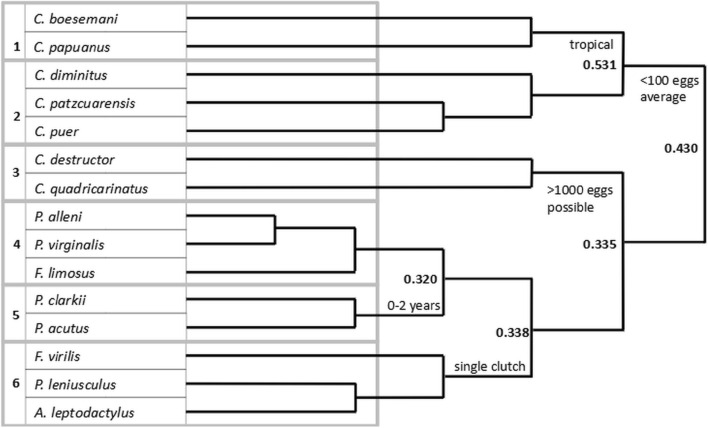
In the first two-way indicator species analysis (TWINSPAN) division all three Cambarellus spp. and both Cherax boesemani and Cherax papuanus were separated based on their low egg production trait (Fig. 4, Table 2). At the second division, the Cambarellus spp. were separated from C. boesemani and C. papuanus due to the tropical native range of both Cherax spp., contrasting to the temperate climate range of Cambarellus spp. Both Cherax destructor and Cherax quadricarinatus were grouped based on their potential to lay over 1000 eggs, where the eight remaining species produce over 100 eggs on average. Of these species, A. leptodactylus, F. virilis and P. leniusculus were grouped based on the production of a single clutch of eggs per year, while the other crayfish produce multiple clutches. Finally, P. acutus and P. clarkii formed their own group due to a shorter lifespan of up to 2 years, while F. limosus, Procambarus alleni and Procambarus virginalis in the last cluster can have a lifespan up to 3 to 4 years. Half of the clusters resulting from the TWINSPAN analysis include invasive species, whereas two groups consisted of exclusively invasive species. The eigenvalues associated with the divisions ranged from 0.320 to 0.531, indicating that the resulting groups were quite similar. While functional traits can have clear phylogenetic patterns, species from both genera Procambarus and Cherax can be found in multiple clusters. This shows that even within a crayfish genus traits can vary substantially. This is further supported by the inclusion of species from three different genera in cluster six.
Figure 4.
TWINSPAN cluster dendrogram with differentiating functional traits and eigenvalues indicated.
Table 2.
Functional traits and invasion category of 15 crayfish species present in the Netherlands in the wild or in aquaria.
| Functional traits | Modalities | Species | Astacus leptodactylus | Cambarellus diminutus | Cambarellus patzcuarensis | Cambarellus puer | Cherax boesemani | Cherax destructor | Cherax papuanus | Cherax quadricarinatus | Faxonius limosus | Faxonius virilis | Pacifastacus leniusculus | Procambarus acutus | Procambarus alleni | Procambarus clarkii | Procambarus virginalis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Invasion category | C | B | B | B | B | B | B | B | E | D | C | D | B | E | B | ||
| Habitat flow preference | Lentic | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Lotic | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Climate tolerance | Tropical | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Arid | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | ||
| Temperate | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Cold | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | ||
| Salinity tolerance | 0–5 g/L | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 5–18 g/L | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | ||
| 18–30 g/L | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Parthenogenetic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Nr. of clutches per year | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 3 + | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Nr. of eggs | < 100 avg | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| > 100 avg | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| > 1000 possible | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Growth rate | Slow | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Medium | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| Fast | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||
| Migration distance | Short | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Medium | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ||
| Long | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Moving over land | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Body size (TLmm) | < 80 mm | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 80–150 mm | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| > 150 mm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| Lifespan | 0–2 y | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | |
| 3–4 y | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| 5–6 y | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 7 + y | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| Aggression | Low | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Medium | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | ||
| High | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | ||
| Crayfish plague resistance | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Uncertainty of trait information
The uncertainty of trait information differs per species as well as per trait (Table 3), with an average uncertainty score across both species and traits of 9%. Cherax boesemani and C. papuanus showed the highest degree of uncertainty (35%). Cambarellus diminitus and Cambarellus patzcuarensis roughly had 20% uncertainty, while Cambarellus puer had about 12%. The functional traits of both aggression and growth rate showed highest uncertainty (about 20%), closely followed by the traits of migration distance and number of clutches per year (about 17%).
Table 3.
Uncertainty calculated in percentage for each functional trait and crayfish species.
| Species | Uncertainty (%) | Traits | Uncertainty (%) |
|---|---|---|---|
| Astacus leptodactylus | 0 | Aggression | 20 |
| Cambarellus diminutus | 23 | Body size | 0 |
| Cambarellus patzcuarensis | 19 | Crayfish plague resistance | 10 |
| Cambarellus puer | 12 | Growth rate | 20 |
| Cherax boesemani | 35 | Habitat flow preference | 0 |
| Cherax destructor | 0 | Lifespan | 7 |
| Cherax papuanus | 35 | Migration distance | 17 |
| Cherax quadricarinatus | 0 | Moving over land | 3 |
| Faxonius limosus | 0 | Native range | 0 |
| Faxonius virilis | 0 | Nr. of clutches per year | 17 |
| Pacifastacus leniusculus | 0 | Nr. of eggs | 10 |
| Procambarus acutus | 8 | Parthenogenetic | 0 |
| Procambarus alleni | 4 | Salinity tolerance | 13 |
| Procambarus clarkii | 0 | Average | 9 |
| Procambarus virginalis | 0 | ||
| Average | 9 |
Discussion
During the stages of invasion, different functional traits can contribute to invasive success of crayfish. Functional traits that separate successful from unsuccessful invaders were a medium egg count and temperate climate adaptation. Crayfish currently in the establishment stage of invasion were separated from fully invasive species by laying only a single clutch per year. The traits parthenogenesis and crayfish plague resistance, contrary to expectation, did not show a clear contribution to invasiveness. Hereafter, the role of functional traits in the invasion stages of establishment, spread, and fully invasive is discussed.
Establishment stage
Environmental tolerances
In the establishment stage local climate becomes an important factor in survival. Cherax boesemani and C. papuanus were separated from the Cambarellus spp. based on their tropical native range (Fig. 4). All currently invasive crayfish are adapted to a temperate climate in their native range, most probably a requirement for invasive success in the Netherlands with a temperate ocean climate (Köppen-Geiger: Cfb). All invasive species, except P. clarkii, also occur in colder climates in their native range, further stressing that adaptation to cold periods can be very useful to survive European Atlantic winters. Larson & Olden34 reported niche shifts in P. clarkii and P. leniusculus with decreased mean maximum temperature and increased minimum temperature variance in comparison to their native range, suggesting that crayfish may survive different climatic condition in their invasive ranges. A separate study confirmed P. clarkii’s cold tolerance through experimental exposure to winter temperatures in a temperate climate35. In contrast, C. quadricarinatus did not survive these experimental conditions, which matches the expectations based on its tropical and arid climate native range. A mismatch between a species’ tolerances and environment means that survival is only potentially possible at locations exhibiting extraordinary environmental circumstances, as was the case where a population of C. quadricarinatus occurred in an oxbow lake in Slovenia with increased temperatures due to occurrence of hot water springs36.
High reproductive potential
Medium to high numbers of eggs contributes to higher invasive success as found in plants37, fish38 and gammarids29. The first TWINSPAN division excluded species with an average egg count lower than 100. Previous research also found high fecundity to be predictive of invasive success32, and clutch size most predictive for transitioning through stages from introduction to establishment to spread23. Yet, despite the high potential of egg numbers, C. destructor and C. quadricarinatus were currently not successful invaders. For C. quadricarinatus its climate tolerance could be the cause, however, C. destructor has been shown capable to survive under temperate climate winter temperatures35. Therefore, a different trait likely determines its lack of invasive success. Besides the number of eggs per clutch, the number of clutches produced also affects invasiveness: the most successful invaders P. clarkii and F. limosus are capable of laying more than a single clutch per year. Larson & Olden32 also reported the trait multiple annual reproductive events to be associated with invasive success, which supports our findings. Most crayfish species currently in the establishment and spread phases produce one clutch of eggs and are moderately successful. Although capable of producing three or more clutches, C. destructor is unsuccessful, suggesting it might lack other important traits.
Spread and dispersal stage
Migration and moving over land
When colonising new freshwater habitats, migration capacity is an important determinant of spread39. However, migration capacity did not determine any division between successful and unsuccessful invaders in the TWINSPAN analysis. Although long migration distances were only found in clusters that included invasive crayfish and five of the nine unsuccessful species have short migration distances, the short migration capacity of F. virilis and P. leniusculus implies that this trait did not clearly contribute to the invasive potential. Moving over land, while less effective than aquatic dispersal, could greatly increase dispersal in crayfish40. While not all currently invasive crayfish can move over land, those that do vary in invasive success. The contribution of dispersal traits to invasiveness appears in our data to be outweighed by other traits. In addition, human mediated propagule pressure, such as repeated introduction through pet releases, could also be important during the spread stage by supporting dispersal20,22.
Fully invasive stage
Large size
A small size can be disadvantageous for crayfish due to increased predation risk and lower competitive capacity41 but did not result in cluster divisions. In contrast, other authors found invasive crayfish species to be characterised by maximum size32. Interestingly, the number of eggs produced appeared an explanatory trait causing grouping and is generally positively related to maximum size42–44. The Cambarellus spp. separated based on low egg count in the first division are also the smallest species considered. Cherax destructor and C. quadricarinatus with the highest potential egg counts are also some of the largest species taken into account. It is possible that potential effects of size on invasive success are masked by the impact of egg count. Zeng et al.23 found clutch size a very important invasive trait when scaled to carapace length, supporting this observation.
Aggression
The level of aggression displayed by current invaders differs from low to high and consequently this trait did not explain invasion success. In contrast, other studies using chelae size as indicator for dominance and competitive ability did find this trait indicative of extraregional invasive success (species that have invaded another continent or crossed major drainage boundaries in their native continent)32 and in the transition to the introduction stage23. While F. limosus is found in lotic waters it prefers to live in ponds and lakes45,46. However, it is less common in the Dutch deep lakes and ponds then its preferences would suggest. This distribution constraint could be the effect of competitive exclusion by the more aggressive P. clarkii, which is mainly found in these waters, forcing F. limosus and other species into less optimal water types16. Although P. clarkii has a high level of aggression and F. limosus a low level of aggression, both are very successful invaders, indicating other traits might offer alternative strategies for high invasiveness.
Crayfish plague
In Europe, the introduction of the crayfish plague, Aphanomyces astaci, from North America has decimated many indigenous crayfish species, which are much more susceptible to this pathogen than North American species47. Although sensitive to this plague, A. leptodactylus is interestingly clustered together with two North American species that are known carriers48. In fact, P. leniusculus was responsible for four recorded mass mortalities of the indigenous Austropotamobius pallipes in France49. This clustering likely results from the shared evolutionary history between A. leptodactylus and P. leniusculus, both belonging to the family Astacidae50. A phylogenetic signal such as this could outweigh the importance of other trait dissimilarities between species during clustering, which might be the case with crayfish plague resistance. In the Netherlands, A. leptodactylus was found almost exclusively in fast flowing and brackish to saline waters, even though it is also adapted to lentic habitats51. Astacus leptodactylus only occurs near North American species at a few locations and is mainly found in water types where those species are absent. It could be excluded from many water types by presence of plague carriers, while its high salinity tolerance allows it to survive where the other species cannot. Similarly, C. destructor, despite possessing several beneficial invasive traits is susceptible to the crayfish plague which might prevent successful establishment48. With the growing presence of crayfish from North America, it seems likely that plague resistance is mandatory to reach the final full invasive stage in the Netherlands.
High invasive success and functional strategies
Crayfish species predicted to become extraregional invaders by Larson & Olden32 were F. virilis, P. acutus and P. clarkii, while the species with the highest FI-ISK scores were P. clarkii, P. leniusculus and F. limosus33. All of these species currently occur in the Netherlands. Faxonius virilis, P. leniusculus, and P. acutus were first reported in 2004, 2005, and 2007 respectively, giving them roughly the same time frame for their invasions. However, P. leniusculus (stage establishment) showed a far smaller geographical distribution compared to both other species (stage spread). This difference between prediction and current invasive status might be caused by the importance given to import for aquaculture in the FI-ISK tool, while this study is focused on the traits of invasion stages that follow after introduction. The other highest risk species, F. limosus and P. clarkii, are fully invasive in the Netherlands, but differ in their traits and invasiveness trajectory. Although more widely distributed, F. limosus is most frequently found in small linear lentic waters and slow flowing waters, which P. clarkii rarely inhabits. This conforms to the difference in aggression between these species; P. clarkii being more aggressive could lead to competitive exclusion of F. limosus. While P. clarkii’s higher migration capacity should bring advantage over F. limosus in occupying new areas, the latter’s widespread occurrence suggests that parthenogenetic reproduction might counteract its lower migration capability. While P. clarkii can theoretically produce more clutches of eggs in a year, it requires individuals from both sexes to do so. In contrast, F. limosus can potentially reproduce with a single individual to establish a new population, increasing its invasiveness. Although facultative parthenogenesis in F. limosus has been experimentally confirmed52, its frequency of occurrence in wild populations is uncertain. And although not reported in the literature, parthenogenesis cannot be excluded in P. clarkii. Furthermore, P. clarkii has been shown to survive Dutch winter temperatures and for F. limosus a cold climate is part of its native range. This might explain the different distributions of these species in the Netherlands, with P. clarkii surviving in deeper waters where it can survive winter conditions and F. limosus being capable of surviving in different water types due to its inherent cold climate tolerance. Ultimately, these two species have a different combination of functional traits leading to alternative strategies that both are successful in becoming fully invasive.
Who is the next invasive species?
FI-ISK invasion risk predictions available for crayfish present in the Dutch aquarium trade range from medium (C. puer, C. quadricarinatus, P. virginalis) to high (C. destructor)33. Cambarellus puer has a small size and produces lower numbers of eggs, resulting in a low risk of invasion for the Netherlands. Due to widespread presence of North American species potentially carrying crayfish plague, the susceptible C. quadricarinatus and C. destructor seem unlikely candidates for widespread invasion in the Netherlands. This notion is supported by the apparent mismatch between their climate tolerance and the Dutch climate. The combination of these traits also excludes the other Cherax spp. in this study. Although not currently found in the wild in the Netherlands, both P. virginalis and P. alleni are clustered with the very successful invader F. limosus, suggesting they also possess those traits necessary to become invasive. However, these Procambarus species and F. limosus differ in their climate tolerance too, with only the latter being adapted to a colder climate. Procambarus virginalis is adapted to a humid subtropical and temperate climate but can survive water temperatures lower than 8 °C and even below 2 °C for several weeks53. Here, climate change could make a difference in the near future. Furthermore, both F. limosus and P. virginalis can reproduce parthonegetically, increasing chances of establishing populations54, but P. alleni has not been reported to be capable of this. Tricarico et al.33 predicted P. virginalis’ invasion risk in Italy to be medium, but caution against its release into the wild. Chucholl55 found that higher human population density and availability of lentic water can increase the likelihood of P. virginalis releases. Procambarus virginalis has established wild populations in Germany, Italy, and Slovakia17,51,56 and has already been recorded once in the Netherlands57. However, no subsequent sightings have been reported. In contrast, no established populations of P. alleni have currently been identified in Europe17 and no sightings have been reported in the Netherlands. Due to availability in the Dutch aquarium trade, its functional traits with high invasive potential and invasion history in Europe, P. virginalis is most likely to become the next successful invader in the Netherlands.
Early detection of P. virginalis introductions is highly important to make informed management decisions, therefore we recommend preventative monitoring. A potential tool for this is environmental DNA monitoring, which has already been successfully applied for targeted detection of P. virginalis in several lakes and rivers in Germany58. In Norway, eDNA detection of invasive crayfish and the spread of the crayfish plague worked so well that it has been adopted in multiple surveillance programmes59. Although not yet suitable for assessing species abundance, presence/absence data can reliably be obtained in Dutch water systems using high throughput sequencing techniques and potentially be gathered through non-targeted monitoring60. An advantage of such approach would possibly provide early detection of P. virginalis or other invasive crayfish during routine eDNA sampling efforts of Dutch water authorities. Additionally, targeted monitoring could be informed by predictions of invasive crayfish distribution through ecological niche models (ENM) and species distribution models (SDM)61. Examples of such predictions are ENMs composed for C. destructor, P. leniusculus and P. clarkii62 and SDMs developed for P. leniusculus and P. carkii63, which show their potential geographic distributions currently and under the impacts of climate change, respectively.
Other conditions affecting invasiveness
Several factors determining the resistance of native communities to non-indigenous species, such as predation and competition, increase with native species richness. Disturbance of aquatic habitats by human activities often reduces local biodiversity, and consequently the resistance of native communities to invasion64. Colautti et al.37 found that increased environmental disturbance and resource availability both significantly contributed to successful invasions. Furthermore, an increase in number of people does increase the chance onto a higher propagule pressure and at the same an increase in environmental pressure providing more opportunity for invasive species. The only crayfish native to the Netherlands, Astacus astacus, has almost gone extinct due to the crayfish plague, opening up a niche space for non-indigenous species. Such niche vacancies can also occur due to anthropogenic environmental influences, e.g., Früh et al.65 reported that German river and stream sites with invasive species were significantly more degraded than sites without non-indigenous species. In the Netherlands, many waters have been thoroughly altered and many are created by humans. Anthropogenic disturbance might have facilitated invasiveness; the distribution of P. clarkii, F. virilis and P. acutus is centred around the largest cities in the Netherlands and nearby densely populated areas, where anthropogenic disturbances are intense.
Evolutionary adaptation is related to time of arrival. Whitney and Gabler26 reported 82 evolutionary changes in 38 species of plants and animals between their native and introduced ranges. Each trait considered was adapted in several species, showing that changes in invasive traits are relatively common. Additionally, some changes occurred within 20 years, a much shorter time-span than F. limosus, A. leptodactylus and P. clarkii are present in the Netherlands. Such rapid evolutionary change could be caused by adaptive phenotypic plasticity, where invasive populations experience directional selection resulting in enhanced fitness66. Niche shifts observed in crayfish regarding climate adaptation34 might be an example of this process and could have contributed to the current invasive success of F. limosus and P. clarkii.
Conclusion
While not all traits studied appeared to contribute to crayfish invasion in the Netherlands, more successful invaders were distinguished by several traits. During establishment, being adapted to a temperate climate is mandatory for survival. Not having a low egg count is required for establishing a population, while producing more than a single clutch per year increases invasive potential. The importance of these traits for crayfish invasiveness is confirmed by literature. Some traits have less straightforward contributions to crayfish invasiveness: besides a temperate climate tolerance, being adapted to a cold climate can improve winter survival. A high level of aggression as seen in P. clarkii might lead to exclusion of competitors and increase invasiveness. Parthenogenetic reproduction potentially contributed to F. limosus passing through stages establishment and spread, and resistance to the crayfish plague has become another important invasion trait due to increased presence of North American crayfish species. Since specific combinations of the traits mentioned above lead to successful invaders, functional trait analysis can be a valuable tool in predicting invasiveness of non-indigenous crayfish species. Using these traits as a descriptive prediction tool, P. virginalis is predicted to become a future successful invader in the Netherlands. This prediction conforms to predictions for P. virginalis from previous risk assessment studies.
Methods
The Dutch food and consumer product safety authority (NVWA) does not officially register crayfish aquaculture companies and records of crayfish trade and import are not digitally available. To determine which nonindigenous crayfish are present in the Netherlands, a web search was performed (20-06-2018) for crayfish being sold. The search terms ‘aquarium store crayfish’ and ‘buy aquarium crayfish’ were used (in Dutch) in two separate searches on www.google.com, resulting in 16 websites offering 19 species of live crayfish in the Netherlands (Supplementary Table S1 online). Species only sold on a single website were excluded due to lower potential for introduction into the wild. Per genus, the three species available on most websites were selected for further research. For C. boesemani several colour morphs were available for sale, which were treated as a single species. Additionally, C. destructor was selected since it is a habitat generalist with a widespread native range51, and it has already established populations in Europe; Italy and Spain67. The search resulted in a list of ten species: C. diminitus, C. patzcuerensis, C. puer, C. quadricarinatus, C. boesemani, C. destructor, C. papuanus, P. alleni, P. clarkii and P. virginalis. Species with verified sightings in the wild in the Netherlands, A. leptodactylus, F. limosus, F. virilis, P. leniusculus, P. acutus and P. clarkii, were obtained from a dataset from Stichting European Invertebrate Survey (EIS)68. This resulted in a total of fifteen crayfish species for further analysis (Table 2). Procambarus virginalis and C. quadricarinatus have been reported only once in the Netherlands21 and were for the purpose of this study not considered to occur in the wild.
Distribution
For the six species in the EIS dataset the number of occurrences (records at 3251 different sites in total) per year was plotted from the first introduction or documented sighting until 2015. Based on this data it appeared that crayfish records increased dramatically around 2007 (Fig. 1). This was due to a strong increase in targeted monitoring by water authorities. Therefore, the more extensive and reliable data from 2007 to 2015 were used for our analyses. Each species was assigned a category of invasive success (Table 2) according to the classification scheme by Blackburn et al.24, simplified by combining subcategories (B1, B2, B3) into main categories (B). This classification was performed based on three factors. First, the presence of juveniles or berried females (carrying eggs) as evidence for self-sustaining populations. Second, the number of sites where they were encountered classified into the categories B = 0, C = > 10, D = > 100 and E = > 1000. Thirdly, the spatial scale of the distribution range of each crayfish species in the Netherlands (Fig. 2) classified as C = local, D = regional and E = national.
Water types
The occurrences of the six successful invasive species were plotted on a map of the Netherlands in ArcMap 10.2.2 (ESRI, Redlands, CA) (Fig. 2a,b). The coordinates of these records were matched to a NLtop10 based aquatic map69, with the closest waterbody within a 200 m radius being assigned to the crayfish sightings. When available within the aquatic map, a Water Framework Directive (WFD) water type was determined as a proxy for environmental conditions, resulting in 1888 records at 615 different sites. Water types were then grouped together in main categories based on their similarity in environmental conditions: deep lakes and ponds, small lentic linear waters, slow flowing linear waters, large lentic linear waters, shallow lakes and ponds, weak brackish to saline waters and fast flowing waters. For each category the number of sites was counted per species.
Functional traits
Functional traits were selected based on the filters described in the invasion framework and commonly used in invasion literature for the stages establishment, spread and fully invasive24–26. The traits included for analysis were: habitat flow preference23,32,33, climate preference33, salinity preference33, parthenogenesis, number of clutches per year23,32,33, number of eggs per clutch23,32,33, growth rate, migration distance33, migration over land33, maximum body size23,32, maximum age, level of aggression23,32,33, and resistance against the crayfish plague (Table 2). The traits generalist diet, short generation time and ability to escape or survive natural enemies from Table 1 were excluded for varying reasons. For example, crayfish have a generalist and flexible diet resulting in high similarity between all species included. For the selected traits, information was obtained through literature research; species were assigned trait modalities based on data from scientific papers (Supplementary Tables S2 and S3 online). For each trait modality a species scored either 1 or 0, with the possibility to score 1 in multiple modalities for some traits. When trait information for a species was unavailable from literature its modalities were assigned through expert judgement, mostly based on confirmed traits of species within the same genus. For the traits growth rate, migration and aggression the information obtained from literature varied in expression, complicating the definition of clear trait modalities. Here, comparative studies between species were used when possible to assign crayfish into generic categories of ‘low’, ‘medium’ and ‘high’. The Köppen-Geiger climate classifications70 of each species’ native range (Supplementary Table S2 online) were used to assign climate preference. Procambarus virginalis is a species that originates from the aquarium trade71 and has no natural home range, thus the native range of its closest relative, Procambarus fallax, was used. No scientific literature was available on the traits growth and aggression for the species C. boesemani and C. papuanus. Therefore, information from aquarium websites was included.
Expert judgement has previously been used to assign trait values to aquatic invertebrates72,73 and plants. In the latter it has been found to correlate strongly with experimentally derived trait information74. However, it does introduce a degree of uncertainty in the species by traits table. To address this issue uncertainty values were calculated (Table 3). Traits taken from literature were scored as 1, traits assigned through expert judgement scored 0.5 and traits based on aquarium websites received a score of 0.1. For each functional trait these scores were added up, divided by the total number of species, multiplied by and subtracted from 100 to obtain percentage-based uncertainty values. The same calculation was performed for each species.
Statistical analysis
Due to the binary nature of our species by traits matrix, the relatively small number of species and expected hierarchy in the data, TWINSPAN hierarchical cluster analysis75 was chosen to determine the relationship between crayfish traits and their invasive success. TWINSPAN analysis has often been applied on plants to identify functional groups based on their traits76,77. For example, TWINSPAN was applied to classify plant communities into multiple stages of succession using functional traits78. The TWINSPAN method constructs hierarchically ordered two-way tables in multiple steps79,80. First, the dataset is split into two subsets roughly in the middle of the primary axis of a correspondence analysis (CA). This is repeated on each subset for further divisions to obtain a divisive hierarchy of the data and convert it into an ordering. Usually this is done on a sites by species matrix with ordering of the data by site, however, we used a species by traits matrix and first ordered the data based on species identity. Secondly, the other side of the data matrix is analysed in a similar manner but includes the constructing of attributes based on the splits in the first divisive hierarchy. In our analysis this step consisted of constructing these attributes for the traits in our matrix and using these to divide the dataset into groups based on functional traits. Finally, a two-way ordered table is created from the previous two steps, showing hierarchical clusters and indicators for each division. In our case the resulting divisions in clusters of crayfish species were indicated by specific functional traits. We performed TWINSPAN analysis in WinTwins (Version 2.3 for Windows79) using a minimum group size of four for division and weighting all traits according to their uncertainty (Table 3). A dendrogram was constructed to visualize the resulting clusters and divisions (Fig. 4). The dissimilarity between clusters was quantified in eigenvalues from low (0) to high (1) at each split.
Supplementary Information
Acknowledgements
This study was part of the DNA Waterscan project funded by the Gieskes-Strijbis Fonds. We thank Bram Koese and NGO EIS for providing verified crayfish data, Maarten van ‘t Zelfde for GIS support and Paula dos Reis Oliveira and anonymous reviewers for constructive manuscript feedback.
Author contributions
T.K. and P.F.M.V. designed the study, B.B.H. directed the project. P.F.M.V., B.B.H. and J.C.B. were involved in planning and supervised the work. T.K. constructed functional traits tables, performed the analysis, drafted the manuscript and designed the figures. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding
This study was funded by the Gieskes-Strijbis Fonds.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82302-4.
References
- 1.Keller RP, Geist J, Jeschke JM, Kühn I. Invasive species in Europe: ecology, status, and policy. Environ. Sci. Eur. 2011;23:1–17. doi: 10.1186/2190-4715-23-23. [DOI] [Google Scholar]
- 2.Parker M, Thompson JN, Weller SG. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001;32:305–332. doi: 10.1146/annurev.ecolsys.32.081501.114037. [DOI] [Google Scholar]
- 3.Allendorf FW, Lundquist LL. Introduction: population biology, evolution, and control of invasive species. Conserv. Biol. 2003;17:24–30. doi: 10.1046/j.1523-1739.2003.02365.x. [DOI] [Google Scholar]
- 4.Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008;6:238–246. doi: 10.1890/070151. [DOI] [Google Scholar]
- 5.van der Veer G, Nentwig W. Environmental and economic impact assessment of alien and invasive fish species in Europe using the generic impact scoring system. Ecol. Freshw. Fish. 2015;24:646–656. doi: 10.1111/eff.12181. [DOI] [Google Scholar]
- 6.Clavero M, García-Berthou E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005;20:110. doi: 10.1016/j.tree.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Scalera R. How much is Europe spending on invasive alien species? Biol. Invasions. 2010;12:173–177. doi: 10.1007/s10530-009-9440-5. [DOI] [Google Scholar]
- 8.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 9.McLellan R, Iyengar L, Jeffries B, Oerlemans N. Living Planet Report 2014: Species and Spaces, People and Places. Gland: WWF International; 2014. [Google Scholar]
- 10.García-Berthou E, et al. Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 2005;62:453–463. doi: 10.1139/f05-017. [DOI] [Google Scholar]
- 11.Karatayev AY, Burlakova LE, Padilla DK, Mastitsky SE, Olenin S. Invaders are not a random selection of species. Biol. Invasions. 2009;11:2009. doi: 10.1007/s10530-009-9498-0. [DOI] [Google Scholar]
- 12.Verdonschot, R. C. M., Vos, J. H., & Verdonschot, P. F. M. Exotische macrofauna en macrofyten in de Nederlandse zoete wateren: voorkomen en beleid in 2012. (WOt-werkdocument 334) (Wettelijke Onderzoekstaken Natuur & Milieu, 2013).
- 13.Holdich DM, Reynolds JD, Souty-Grosset C, Sibley PJ. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009;394–395:11. doi: 10.1051/kmae/2009025. [DOI] [Google Scholar]
- 14.Chucholl C. Invaders for sale: trade and determinants of introduction of ornamental freshwater crayfish. Biol. Invasions. 2013;15:125–141. doi: 10.1007/s10530-012-0273-2. [DOI] [Google Scholar]
- 15.Barbaresi S, Gherardi F. The invasion of the alien crayfish Procambarus clarkii in Europe, with particular reference to Italy. Biol. Invasions. 2000;2:259–264. doi: 10.1023/A:1010009701606. [DOI] [Google Scholar]
- 16.Gherardi F. Crayfish invading Europe: the case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006;39:175–191. doi: 10.1080/10236240600869702. [DOI] [Google Scholar]
- 17.Kouba A, Petrusek A, Kozák P. Continental-wide distribution of crayfish species in Europe: update and maps. Knowl. Manag. Aquat. Ecosyst. 2014;413:5. doi: 10.1051/kmae/2014007. [DOI] [Google Scholar]
- 18.Lowe, S., Browne, M., Boudjelas, S., & De Poorter, M. 100 of the world's worst invasive alien species: a selection from the global invasive species database in Aliens vol. 12 (Invasive Species Specialist Group, 2000).
- 19.Padilla DK, Williams SL. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2004;2:131–138. doi: 10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2. [DOI] [Google Scholar]
- 20.Faulkes Z. The global trade in crayfish as pets. Crustacean Res. 2015;44:75–92. doi: 10.18353/crustacea.44.0_75. [DOI] [Google Scholar]
- 21.Soes, D. M., & Koese, B. Invasive Crayfish in the Netherlands: A Preliminary Risk Analysis. (Bureau Waardenburg bv, Stichting EIS-Nederland, Invasive Alien Species Team, 2010).
- 22.Chucholl C, Wendler F. Positive selection of beautiful invaders: long-term persistence and bio-invasion risk of freshwater crayfish in the pet trade. Biol. Invasions. 2017;19:197–208. doi: 10.1007/s10530-016-1272-5. [DOI] [Google Scholar]
- 23.Zeng Y, Chong KY, Grey EK, Lodge DM, Yeo DC. Disregarding human pre-introduction selection can confound invasive crayfish risk assessments. Biol. Invasions. 2015;17:2373–2385. doi: 10.1007/s10530-015-0881-8. [DOI] [Google Scholar]
- 24.Blackburn TM, et al. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Statzner B, Bonada N, Dolédec S. Biological attributes discriminating invasive from native European stream macroinvertebrates. Biol. Invasions. 2008;10:517–530. doi: 10.1007/s10530-007-9148-3. [DOI] [PubMed] [Google Scholar]
- 26.Whitney KD, Gabler CA. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Divers. Distrib. 2008;14:569–580. doi: 10.1111/j.1472-4642.2008.00473.x. [DOI] [Google Scholar]
- 27.Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 2001;16:199–204. doi: 10.1016/S0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti MP, Moyle PB, Levine R. Invasive species profiling? Exploring the characteristics of non-native fishes across invasion stages in California. Freshw. Biol. 2004;49:646–661. doi: 10.1111/j.1365-2427.2004.01202.x. [DOI] [Google Scholar]
- 29.Grabowski M, Bacela K, Konopacka A. How to be an invasive gammarid (Amphipoda: Gammaroidea)-comparison of life history traits. Hydrobiologia. 2007;590:75–84. doi: 10.1007/s10750-007-0759-6. [DOI] [Google Scholar]
- 30.Thiébaut G. Invasion success of non-indigenous aquatic and semi-aquatic plants in their native and introduced ranges. A comparison between their invasiveness in North America and in France. Biol. Invasions. 2007;9:1–12. doi: 10.1007/s10530-006-9000-1. [DOI] [Google Scholar]
- 31.Swart C, Visser V, Robinson TB. Patterns and traits associated with invasions by predatory marine crabs. NeoBiota. 2018;39:79. doi: 10.3897/neobiota.39.22002. [DOI] [Google Scholar]
- 32.Larson ER, Olden JD. Latent extinction and invasion risk of crayfishes in the southeastern United States. Conserv. Biol. 2010;24:1099–1110. doi: 10.1111/j.1523-1739.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- 33.Tricarico E, Vilizzi L, Gherardi F, Copp GH. Calibration of FI-ISK, an invasiveness screening tool for nonnative freshwater invertebrates. Risk Anal. Int. J. 2010;30:285–292. doi: 10.1111/j.1539-6924.2009.01255.x. [DOI] [PubMed] [Google Scholar]
- 34.Larson ER, Olden JD. Using avatar species to model the potential distribution of emerging invaders. Glob Ecol. Biogeogr. 2012;21:1114–1125. doi: 10.1111/j.1466-8238.2012.00758.x. [DOI] [Google Scholar]
- 35.Veselý L, Buřič M, Kouba A. Hardy exotics species in temperate zone: can “warm water” crayfish invaders establish regardless of low temperatures? Sci. Rep. 2015;5:16340. doi: 10.1038/srep16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaklič M, Vrezec A. The first tropical alien crayfish species in European waters: the redclaw Cherax quadricarinatus (Von Martens, 1868) (Decapoda, Parastacidae) Crustaceana. 2011;84:651–665. doi: 10.1163/001121611X577936. [DOI] [Google Scholar]
- 37.Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: a null model for biological invasions. Biol. Invasions. 2006;8:1023–1037. doi: 10.1007/s10530-005-3735-y. [DOI] [Google Scholar]
- 38.Marchetti MP, Moyle PB, Levine R. Alien fishes in California watersheds: characteristics of successful and failed invaders. Ecol. Appl. 2004;14:587–596. doi: 10.1890/02-5301. [DOI] [Google Scholar]
- 39.Bennett SN, Olson JR, Kershner JL, Corbett P. Propagule pressure and stream characteristics influence introgression: cutthroat and rainbow trout in British Columbia. Ecol. Appl. 2010;20:263–277. doi: 10.1890/08-0441.1. [DOI] [PubMed] [Google Scholar]
- 40.Cruz MJ, Rebelo R. Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii Southwest Iberian Peninsula. Hydrobiologia. 2007;575:191–201. doi: 10.1007/s10750-006-0376-9. [DOI] [Google Scholar]
- 41.Lynas J, Storey AW, Knott B. Aggressive interactions between three species of freshwater crayfish of the genus Cherax (Decapoda: Parastacidae) Mar. Freshw. Behav. Physiol. 2007;40:105–116. doi: 10.1080/10236240701245539. [DOI] [Google Scholar]
- 42.Corey S. Comparative fecundity of four species of crayfish in southwestern Ontario, Canada (Decapoda, Astacidea) Crustaceana. 1987;52(3):276–286. doi: 10.1163/156854087X00510. [DOI] [Google Scholar]
- 43.Somers KM. Characterizing size-specific fecundity in crustaceans. Crustacean Egg Prod. 1991;7:357–378. [Google Scholar]
- 44.Maguire I, Klobučar GIV, Erben R. The relationship between female size and egg size in the freshwater crayfish Austropotamobius torrentium. Bulletin Français de la Pêche et de la Pisciculture. 2005;376–377:777–785. doi: 10.1051/kmae:2005032. [DOI] [Google Scholar]
- 45.Pilotto F, et al. The invasive crayfish Faxonius limosus in Lake Varese: estimating abundance and population size structure in the context of habitat and methodological constraints. J. Crustacean Biol. 2008;28:633–640. doi: 10.1651/07-2967.1. [DOI] [Google Scholar]
- 46.Hobbsła HH., Jr A checklist of the North and Middle American crayfishes (Decapoda: Astacidae and Cambaridae) Smithsonian Contrib. Zool. 1974;166:1–161. [Google Scholar]
- 47.Mrugała A, et al. Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: crayfish plague and white spot syndrome. Biol. Invasions. 2015;17:1313–1326. doi: 10.1007/s10530-014-0795-x. [DOI] [Google Scholar]
- 48.Svoboda J, Mrugała A, Kozubíková-Balcarová E, Petrusek A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: a review. J. Fish Dis. 2017;40:127–140. doi: 10.1111/jfd.12472. [DOI] [PubMed] [Google Scholar]
- 49.Grandjean F, Roques J, Delaunay C, Petrusek A, Becking T, Collas M. Status of Pacifastacus leniusculus and its role in recent crayfish plague outbreaks in France: improving distribution and crayfish plague infection patterns. Aquat. Invasions. 2017;12:541–549. doi: 10.3391/ai.2017.12.4.10. [DOI] [Google Scholar]
- 50.Crandall KA, De Grave S. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J. Crustacean Biol. 2017;37:615–653. doi: 10.1093/jcbiol/rux070. [DOI] [Google Scholar]
- 51.Freshwater Crayfish: A Global Overview. (ed. Kawai, T., Faulkes, Z., & Scholtz, G.) (CRC Press, Boca Raton, 2015).
- 52.Buřič M, Kouba A, Kozak P. Reproductive plasticity in freshwater invader: from long-term sperm storage to parthenogenesis. PLoS ONE. 2013;8:e77597. doi: 10.1371/journal.pone.0077597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaldre, K., Meženin, A., Paaver, T., & Kawai, T. A preliminary study on the tolerance of marble crayfish Procambarus fallax f. virginalis to low temperature in Nordic climate in Freshwater crayfish: global overview, 54–62 (2016).
- 54.Vogt G. Marmorkrebs: natural crayfish clone as emerging model for various biological disciplines. J. Biosci. 2011;36:377–382. doi: 10.1007/s12038-011-9070-9. [DOI] [PubMed] [Google Scholar]
- 55.Chucholl C. Predicting the risk of introduction and establishment of an exotic aquarium animal in Europe: insights from one decade of Marmorkrebs (Crustacea, Astacida, Cambaridae) releases. Biol. Invasions. 2014;5:309–318. doi: 10.3391/mbi.2014.5.4.01. [DOI] [Google Scholar]
- 56.Chucholl C, Morawetz K, Groß H. The clones are coming–strong increase in Marmorkrebs [Procambarus fallax (Hagen, 1870) f. virginalis] records from Europe. Aquat. Invasions. 2012;7:511–519. doi: 10.3391/ai.2012.7.4.008. [DOI] [Google Scholar]
- 57.Soes DM, van Eekelen R. Rivierkreeften, een oprukkend probleem? De Levende Natuur. 2006;107:56–59. [Google Scholar]
- 58.Mauvisseau Q, Tönges S, Andriantsoa R, Lyko F, Sweet M. Early detection of an emerging invasive species: eDNA monitoring of a parthenogenetic crayfish in freshwater systems. Manag. Biol. Invasions. 2019;10:461. doi: 10.3391/mbi.2019.10.3.04. [DOI] [Google Scholar]
- 59.Strand DA, et al. Monitoring a Norwegian freshwater crayfish tragedy: eDNA snapshots of invasion, infection and extinction. J. Appl. Ecol. 2019;56:1661–1673. doi: 10.1111/1365-2664.13404. [DOI] [Google Scholar]
- 60.Beentjes KK, Speksnijder AG, Schilthuizen M, Schaub BE, van der Hoorn BB. The influence of macroinvertebrate abundance on the assessment of freshwater quality in The Netherlands. Metabarcoding Metagenom. 2018;2:e26744. doi: 10.3897/mbmg.2.26744. [DOI] [Google Scholar]
- 61.Melo-Merino SM, Reyes-Bonilla H, Lira-Noriega A. Ecological niche models and species distribution models in marine environments: a literature review and spatial analysis of evidence. Ecol. Model. 2020;415:108837. doi: 10.1016/j.ecolmodel.2019.108837. [DOI] [Google Scholar]
- 62.Zhang Z, et al. Impacts of climate change on the global potential distribution of two notorious invasive crayfishes. Freshw. Biol. 2020;65:353–365. doi: 10.1111/fwb.13429. [DOI] [Google Scholar]
- 63.Capinha C, Leung B, Anastácio P. Predicting worldwide invasiveness for four major problematic decapods: an evaluation of using different calibration sets. Ecography. 2011;34:448–459. doi: 10.1111/j.1600-0587.2010.06369.x. [DOI] [Google Scholar]
- 64.Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB. Aquatic invasive species: challenges for the future. Hydrobiologia. 2015;750:147–170. doi: 10.1007/s10750-014-2166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Früh D, Stoll S, Haase P. Physicochemical and morphological degradation of stream and river habitats increases invasion risk. Biol. Invasions. 2012;14:2243–2253. doi: 10.1007/s10530-012-0226-9. [DOI] [Google Scholar]
- 66.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi: 10.1111/j.1365-2435.2007.01283.x. [DOI] [Google Scholar]
- 67.Scalici M, et al. The new threat to Italian inland waters from the alien crayfish “gang”: the Australian Cherax destructor Clark, 1936. Hydrobiologia. 2009;632:341–345. doi: 10.1007/s10750-009-9839-0. [DOI] [Google Scholar]
- 68.Koese B, Evers CHM. A National Inventory of Invasive Freshwater Crayfish in the Netherlands in 2010. Stichting European Invertebrate Survey Nederland: EIS; 2011. [Google Scholar]
- 69.Clement, J., & van Puijenbroek, P. Basiskaart Aquatisch: de Watertypenkaart Het oppervlaktewater in de TOP10NL geclassificeerd naar watertype (No. 500067004). (Planbureau voor de Leefomgeving 2010).
- 70.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 2007;4:439–473. [Google Scholar]
- 71.Lyko F. The marbled crayfish (Decapoda: Cambaridae) represents an independent new species. Zootaxa. 2017;4363(4):544–552. doi: 10.11646/zootaxa.4363.4.6. [DOI] [PubMed] [Google Scholar]
- 72.Usseglio-Polatera P, Tachet H. Theoretical habitat templets, species traits, and species richness: Plecoptera and Ephemeroptera in the Upper Rhône River and its floodplain. Freshw. Biol. 1994;31:357–375. doi: 10.1111/j.1365-2427.1994.tb01746.x. [DOI] [Google Scholar]
- 73.Poff NL, et al. Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J. North Am. Benthological. Soc. 2006;25:730–755. doi: 10.1899/0887-3593(2006)025[0730:FTNONA]2.0.CO;2. [DOI] [Google Scholar]
- 74.Wyse SV, et al. A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion. Int. J. Wildland Fire. 2016;25:466–477. doi: 10.1071/WF15047. [DOI] [Google Scholar]
- 75.Hill, M. O. TWINSPAN. A FORTRAN program for arranging multivariate data in an ordered two-way table by classification of the individuals and attributes. (Ecology and Systematics, Cornell University, 1979).
- 76.Hu G, et al. Regeneration of different plant functional types in a Masson pine forest following pine wilt disease. PLoS ONE. 2012;7:e36432. doi: 10.1371/journal.pone.0036432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agir SU, Kutbay HG, Surmen B. Plant diversity along coastal dunes of the Black Sea (North of Turkey) Rendiconti Lincei. 2016;27:443–453. doi: 10.1007/s12210-015-0497-z. [DOI] [Google Scholar]
- 78.Andrej P, Andraž Č. Functional response traits and plant community strategy indicate the stage of secondary succession. Hacquetia. 2012;11:209–225. doi: 10.2478/v10028-012-0010-5. [DOI] [Google Scholar]
- 79.Hill, M.O. & Šmilauer, P. TWINSPAN for Windows version 2.3. (Centre for Ecology and Hydrology & University of South Bohemia, Huntingdon & Ceske Budejovice, 2005).
- 80.Roleček J, Tichý L, Zelený D, Chytrý M. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J. Veg. Sci. 2009;20:596–602. doi: 10.1111/j.1654-1103.2009.01062.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.