Abstract
Mild traumatic brain injury (mTBI), or concussion, accounts for 85% of all TBIs. Yet survivors anticipate full cognitive recovery within several months of injury, if not sooner, dependent upon the specific outcome/measure. Recovery is variable and deficits in executive function, e.g., working memory (WM) can persist years post-mTBI. We tested whether cognitive deficits persist in otherwise healthy undergraduates, as a conservative indicator for mTBI survivors at large. We collected WM performance (change detection, n-back tasks) using various stimuli (shapes, locations, letters; aurally presented numbers and letters), and wide-ranging cognitive assessments (e.g., RBANS). We replicated the observation of a general visual WM deficit, with preserved auditory WM. Surprisingly, visual WM deficits were equivalent in participants with a history of mTBI (mean 4.3 years post-injury) and in undergraduates with recent sports-related mTBI (mean 17 days post-injury). In seeking the underlying mechanism of these behavioral deficits, we collected resting state fMRI (rsfMRI) and EEG (rsEEG). RsfMRI revealed significantly reduced connectivity within WM-relevant networks (default mode, central executive, dorsal attention, salience), whereas rsEEG identified no differences (modularity, global efficiency, local efficiency). In summary, otherwise healthy current undergraduates with a history of mTBI present behavioral deficits with evidence of persistent disconnection long after full recovery is expected.
Subject terms: Psychology, Human behaviour, Neuroscience, Learning and memory, Working memory
Introduction
Mild traumatic brain injury (mTBI), or concussion accounts for nearly 235,000 hospitalizations each year1–4, with 85% of cases categorized as mTBI5,6 in the USA alone. Many mTBI cases go untreated because people do not seek medical care7. Treatment involves initial rest followed by a gradual return to normal activities. Full recovery of cognitive function is expected within months without further rehabilitation8. But does cognition fully recover after mTBI?
Certainly, in moderate and severe TBI cognitive impairment persists9–11. In acute mTBI (0–3 days post injury) impairments include deficits in processing speed12,13, attention14–16, and episodic memory17. However, the data on cognitive outcomes long after mTBI (> 3 months) are mixed. For example, recent reviews reported no lasting cognitive deficits18–20, whereas athletes and veterans at > 1-year post-injury have lasting executive dysfunction21–40, and impaired working memory (WM)41,42. In these populations, effects may be heightened by blast injury43, and/or repeated head injury44. Reports in civilian mTBIs do not note cognitive deficits but do identify impaired peripheral vision, tandem gait, and psychosocial ability45,46. As many reconsider the consequences of brain trauma, we recently reported that healthy undergraduates self-reporting a history of mTBI (hmTBI, > 4 years post-injury) performed significantly worse than their peers on visual WM tasks (e.g., 3-color patches × 900 ms delay)47,48. We replicated this observation in six experiments testing 135 hmTBI participants while manipulating encoding, maintenance durations, retrieval demands, and presence of feedback47,48. The undergraduates reported mTBIs from typical childhood experiences (e.g., bike accidents, falling off the monkey bars), as well as sports. Meta-analysis of our work from the six experiments suggests that number of mTBIs, time since injury, and etiology did not predict visual WM performance, however, loss of consciousness did. Surprisingly, loss of consciousness predicted less WM impairment, we speculate that perhaps those who reported loss of consciousness sought medical treatment48. Nonetheless, there was a consistent, significant visual WM deficit in a young, healthy population who is not seeking or receiving treatment. Visual WM is an important executive function that is needed to integrate visual experience across eye movements, meaning deficits might interfere with the seamless representation of the external world.
Given that roughly 50% of mTBIs are undiagnosed or are not managed appropriately makes post injury care difficult to manage49. This is vitally important as initial presentation to a concussion specialty clinic within a week of injury tends to result in faster recovery compared to athletes evaluated 2–3 weeks post-injury50,51. If no injury assessment occurs, specific managements strategies to aid in recovery may not be implemented, which could lead to longer recovery time. Protracted recovery may lead to reorganization of functional networks, for example due to chronic exposure to pain52.
Why is visual WM impaired so long after mTBI? Lingering tissue damage can arise from shearing forces53–58 that disrupt neural connections59,60 in several WM relevant networks including the default mode network (DMN)61,62. Connectivity measurements identify altered default mode network (DMN) activity 10 years post mTBI63. DMN connectivity is also associated with processing speed64, and visuospatial task performance65,66. Several other networks demonstrate altered activity post-mTBI67–69, including the central executive network (CEN)70,71, dorsal attentional network (DAN)72–74, and salience network (SN)75,76.
It could be that visual WM is the only area where hmTBI participants are impaired, but this seems improbable. Here, we had several goals. First, to test whether our observation of a visual WM deficit extended to other visual WM tasks, and other visual stimuli, and whether group-level impairment would extend to auditory WM. We also wanted to test cognition more broadly to see if there were other areas of deficit in this undergraduate population. Second, to compare lasting WM deficit with initial effects, we included a group of undergraduates who had recently experienced an mTBI. Third, we tested whether the cause of performance deficits was due to altered network-level activity. In short, our goal was to understand the breadth of cognitive deficits in the hmTBI group, whether WM performance significantly improves post-mTBI, and to identify neural mechanisms underlying observed deficits. Importantly, if hmTBI alters cognitive outcomes in undergraduates—it can serve as a bellwether for the impact of mTBI in the general population. Experiments 1A-B tested the breadth of WM deficits in undergraduates with a hmTBI by including two tasks (3-back, change detection) and three kinds of stimuli (locations, shapes, letters) Experiment 2 compared cognitive performance in undergraduates with a hmTBI (no persistent symptoms, > 3 months post-mTBI) to those with a recent sports related-mTBI (SR-mTBI: 4 days–3 months post-injury); see Table 1. Experiment 3 probed connectivity (rsEEG, rsfMRI) to link behavior with underlying neural mechanisms. We hypothesized a pattern of generally impaired WM performance in the hmTBI group. We predicted a specific deficit in WM rather than a general cognitive deficit across all cognitive domains. We anticipated significantly worse performance in the SR-mTBI group consistent with the typical timeline of recovery over time. Additionally, we predicted the hmTBI group would demonstrate reduced connectivity following mTBI, in the networks selected for their involvement in WM (Table 3).
Table 1.
Demographics for all experiments.
| Experiment | Group | Age (SD) | # (# F) | # TBI | Range # | Time (SD) | Range time |
|---|---|---|---|---|---|---|---|
| Experiment 1A | hmTBI | 20.9 (3.2) | 25 (16) | 1.9 (1.5) | 1–7 | 3.9 y (6) | 6 mo–25 y |
| Control | 22.2 (2.9) | 25 (17) | |||||
| Experiment 1B | hmTBI | 20.5 (2.53) | 30 (22) | 2.7 (2.1) | 1–9 | 3.4 y (3.2) | 7 mo–sss11.6 y |
| Control | 20.9 (2.33) | 33 (23) | |||||
| Experiment 2 | hmTBI | 22.2 (3.3) | 25 (11) | 3.6 (2.6) | 1–12 | 4.3 y (3.7) | 5 m–12.2 y |
| SR-mTBI | 20.3 (2.0) | 21 (15) | 2.2 (1.5) | 1–6 | 17 days (19.3) | 3–80 days | |
| Control | 25.1 (4.8) | 25 (15) | |||||
| Experiment 3 | hmTBI | 22.3 (3.4) | 23 (10) | 3.6 (2.7) | 1–12 | 4.2 y (3.6) | 5 mo–12.2 y |
| Control | 24 (5.5) | 23 (11) |
Separate cohorts were tested in each experiment. The mean number of mTBIs and the Time (in years) since the most recent mTBI, averaged across participants.
# number of mTBIs, F female, hmTBI history of mTBI group, mo months, Range # range of number of mTBIs, Range Time range of time since last mTBI, recent SR-mTBI recent sport related mTBI, SD standard deviation in years, y years.
Table 3.
ROI seed locations.
| Network | Seed locations | MNI (X, Y, Z) |
|---|---|---|
| DMN | rPCC, vmPFC | (5, − 49, 40), (− 4, 54, 0) |
| CEN | rDLPFC, rPPC | (44, 36, 20), (42, − 56, 50) |
| DAN | rIPS, FEF | (39, − 42, 51), (− 26, 10, 51) |
| SN | rAI, BA 47 | (42, 0, 2), (38, 24, − 12) |
All seed locations were selected using prior rsfMRI research that used WM as a behavioral correlate of connectivity.
Results
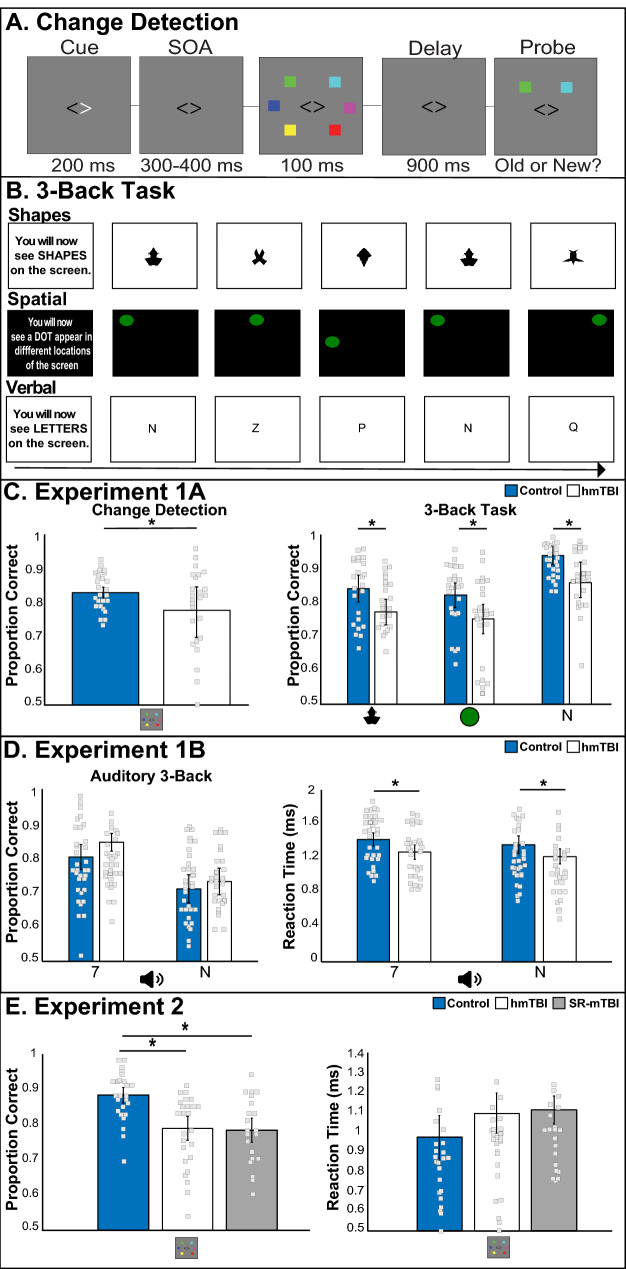
The first two behavioral experiments tested WM in undergraduates with a hmTBI more broadly by including two WM tasks (change detection, 3-back) and three tasks (shapes, spatial, letters); Experiment 1A. We included two sensory modalities (vision, audition) to see if WM deficits were constrained to one modality or were supramodal; Fig. 1B. We first replicated the pattern of impaired change detection accuracy in those with a hmTBI. An independent sample t-test indicated that accuracy measures in the change detection task revealed that there significantly less information maintained in visual WM in the hmTBI (t(48) = 2.25, p = 0.03, d = 0.58; Fig. 1C); without effecting reaction times (t(48) = 0.27, p = 0.78, observed β = 0.92, n.s.). Performance on the 3-back task was examined with a repeated measures ANOVA across the two groups (control, hmTBI) and the three task types (shapes, spatial, letters). The analysis revealed a significant main effect of group (F1,48 = 9.62, p = 0.004, η2p = 0.16; Fig. 1C; reaction time: p = 0.30, η2p = 0.003, n.s.). Accuracy was superior for the letter stimuli (F1,48 = 44.4, p < 0.0001, η2p = 0.39). Reaction times were faster responding to the shapes (F1,48 = 6.8, p = 0.002, η2p = 0.12). There was no group × stimulus interaction (p = 0.73, observed β = 0.1). Experiment 1A confirmed a general visual WM deficit in undergraduates with a hmTBI. This raises the question of whether WM deficits are restricted to the visual domain or whether they extend to other sensory domains, such as auditory WM.
Figure 1.
WM tasks and behavioral results. (A) Change detection and (B) 3-back task showing the shapes, spatial locations, and verbal stimuli used in Experiment 1A. Behavioral Results for Experiment 1A visual change detection and 3-back tasks (C). Those with a hmTBI performed significantly less accurately than controls across tasks and stimuli. (D). Experiment 1B used aurally presented digits and letters and observed no accuracy difference between groups but did reveal a significant speeding in the hmTBI group. (E) Behavioral results showing accuracy, but no reaction time deficit in both hmTBI and SR-mTBI compared to controls, in Experiment 2. *p < 0.05 and error bars represent 95% confidence intervals. hmTBI history of mTBI, SR-mTBI sports related mTBI.
We then tested whether WM deficits were process-general by probing auditory WM in new cohorts (Exp. 1B). Participants completed an auditory 3-back task with blocks of digits and letters. Performance on the 3-back task was examined with a repeated measures ANOVA across the two groups (control, hmTBI) and the two auditory stimulus types (numbers, letters). Importantly, there was no main effect of group and no interaction on accuracy (ps > 0.4, observed β = 0.05). Accuracy was significantly higher during the digit trials (F1,61 = 64.6, p < 0.00001, η2p = 0.51). Unexpectedly, reaction times in the hmTBI group were significantly faster than controls (F1,61 = 5.7, p = 0.02, η2p = 0.08; Fig. 1D), and significantly faster for letter trials (F1,61 = 4.72, p = 0.03, η2p = 0.07). No other comparisons approached significance (all ps > 0.15). These findings suggest that auditory WM was intact, and even more rapid, in those with h mTBI.
To compare results between experiment 1A and 1B we conducted repeated measures ANOVA with group (control, hmTBI) and experiment (1A, 1B) as factors for both the accuracy and reaction times of the letters task. There was a significant main effect of experiment (F(1,109) = 80, p < 0.00001, η2p = 0.4), where participants had higher accuracy scores in the visual WM task. Additionally, there was a significant interaction of group × experiment (F(1,103) = 4.2, p = 0.04, η2p = 0.04) indicating that control participants performance was higher across experiment. No other comparisons reached significance (all ps > 0.4, observed β = 0.14). The reaction times indicated a main effect of experiment (F(1,109) = 77.9, p < 0.00001, η2p = 0.42) where hmTBI participants had faster reaction times across experiments and a borderline effect of group (F(1,109) = 2.9, p = 0.08, η2p = 0.02) where hmTBI had faster reaction times. No other comparisons reached significance (all ps > 0.25, observed β = 0.2).
To test cognitive performance across domains we replicated the change detection WM test and conducted neuropsychological assessments of attention, episodic memory and learning in new participants in Experiment 2. We recruited a third group, athletes with a recent SR-mTBI to clarify the effect of recovery time on post-mTBI WM performance. This third group was necessary to begin to capture the amount of continued recovery to visual WM that could be anticipated over time. Finally, we collected resting state EEG connectivity in search of a biomarker sensitive to the visual WM impairment.
Change detection accuracy showed that compared to controls, both mTBI groups were impaired (F(2,68) = 13.65, p < 0.00001, η2p = 0.28; see Fig. 1E), but not different from each other (p = 0.82, observed β = 0.7). There were no significant differences in reaction times (F(2,68) = 1.1, p = 0.3, η2p = 0.03; see Fig. 1E). The neuropsychological tests revealed that both mTBI groups showed low index scores for immediate and delayed memory, consistent with the impaired learning and delayed recall observed on a test of verbal learning (CVLT; see Table 2). A measure of executive function identified a significant group difference (TMT-B: one-way ANOVA: F(2,68) = 8.17, p = 0.001, η2p = 0.19) with significantly slower performance in the hmTBI group (mean: 59.3 s, SD: 18.5, p = 0.0004; control mean: 41.7 s, SD: 10.3), but only a trend in the recent SR-mTBI group (mean: 50.2 s, SD: 16.3, p = 0.15). No group differences emerged in the sustained attention task (PVT: F(2,68) = 1.3, p = 0.27, η2p = 0.04). These data show preserved attention, visuospatial and recognition memory ability in the mTBI groups.
Table 2.
Neuropsychological assessment data.
| hmTBI | SR-mTBI | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| RBANS indexes | ||
| Immediate memory | 88.7 (15.2) | 88.7 (11.4) |
| Visuospatial/constructional | 100 (16.4) | 100.2 (13.9) |
| Language | 96 (16.4) | 94.6 (15.8) |
| Attention | 98.3 (16) | 95.1 (20.9) |
| Delayed memory | 91 (14.4) | 84 (18.3) |
| Sum of index scores | 472.6 (51.6) | 459 (45.5) |
| Total scale | 92.4 (12.8) | 88.6 (11.2) |
| CVLT-short | ||
| Trial 1: Free recall correct | − 0.92 (0.69) | − 1.2 (0.6) |
| Trial 2: Free recall correct | − 1.21 (0.69) | − 1.62 (0.5) |
| Trial 3: Free recall correct | − 2 (0.5) | − 2.02 (0.5) |
| Trial 4: Free recall correct | − 2.24 (0.63) | − 2.4 (0.7) |
| Trials 1–4 free recall total correct (T Score) | 42 (9.4) | 37 (7.5) |
| Short-delay free recall correct | − 1.72 (0.6) | − 1.8 (0.6) |
| Long-delay free recall correct | − 2.38 (0.74) | − 2.3 (0.8) |
| Long-delay cued recall correct | − 2.64 (0.8) | − 2.6 (0.7) |
| Free recall intrusions | 0.06 (0.7) | 0.2 (0.8) |
| Cued recall intrusions | − 0.3 (0.3) | − 0.3 (0.5) |
| Total intrusions | − 0.24 (0.7) | 0.02 (0.7) |
| Total repetitions | − 0.92 (0.5) | − 0.8 (0.6) |
| Long-delay yes/no recognition hits | − 4.92 (0.2) | − 4.95 (0.1) |
| Long-delay yes/no recognition false positives | − 0.26 (0.56) | 0.07 (0.6) |
| Long-delay forced-choice recognition accuracy | 0.98 (0.04) | 0.97 (0.05) |
All RBANS index score values were between 90 and 109 and normal. CVLT-Short standardized scores > 1.96 are considered impaired (bold italicized).
hmTBI history of mTBI, SR-mTBI sports related mTBI.
We were interested in testing whether rsEEG connectivity measures would be sensitive to the visual WM deficit in the hmTBI group. Identifying a reliable biomarker would benefit recovery assessment and could serve as a potential target for continued rehabilitation. RsEEG data in the theta band were evaluated using measures of modularity, global efficiency and local efficiency. The theta band is linked to WM performance77,78. Modularity reflects the integration (global communication) or segregation (local processing) of within-network connections. Global efficiency measures information transfers across node-pairs to clarify integration. Local efficiency measures segregation of information transfer between neighboring electrodes. Data were compared using a mixed model ANOVA with the between-subject factor of group (control, hmTBI, SR-mTBI), and the within-subject factor of network cost (10%, 15%, 20%, 25%, 30%) to ensure results were not due to specific threshold values. There were no significant main effects of group across any measure (modularity: p = 1, global efficiency: p > 0.4, local efficiency: p > 0.5). For each measure, there was the expected main effect of network cost (all ps < 0.00001), as increasing network cost increases the threshold of the values. There were no interactions of group and network cost (all ps > 0.4). The rsEEG data found no evidence of altered connectivity in the hmTBI group at any network cost model.
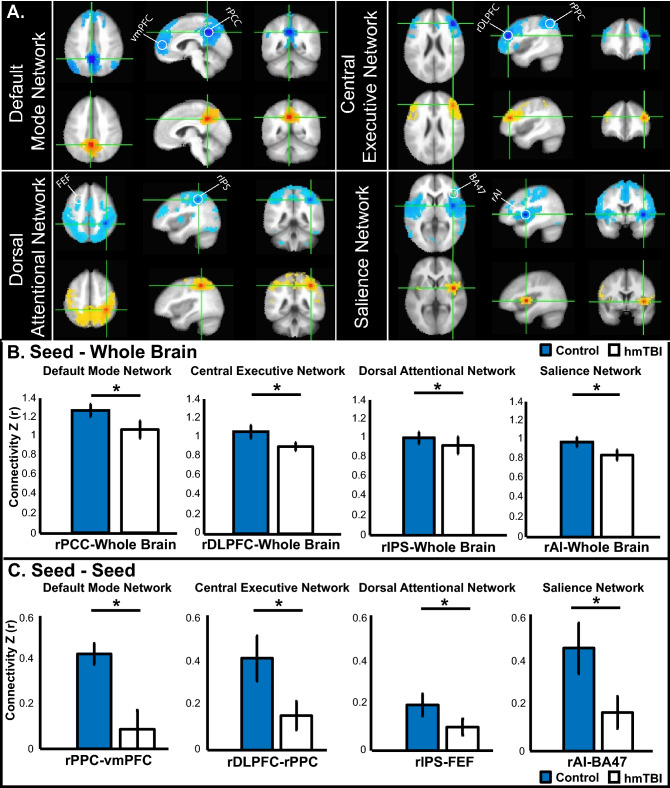
Next, we examined rsfMRI data from the hmTBI group who provided rsEEG (Exp. 2) to test for weakened connectivity. Using one seed region per network, we evaluated functional connectivity between our primary seed location and the whole brain (rPCC-Whole Brain, rDLPFC-Whole Brain, rIPS-Whole Brain, rAI-Whole Brain). Next, we selected a second seed location within each network and calculated connectivity from seed to seed (rPCC-vmPFC, rDLPFC-rPPC, rIPS-FEF, rAI-BA47). The first approach sampled whole brain connectivity, whereas the second approach probed within network connectivity. As shown by our independent sample t-test the hmTBI group had reduced seed—whole brain connectivity across all seeds (DMN: rPCC-Whole Brain: t (44) = − 3.4, p = 0.001 (hmTBI mean: 1.05, SD: 0.22; control mean: 1.24, SD: 0.15), rPCC-vmPFC: t (44) = − 6, p < 0.00001 (hmTBI mean: 0.12, SD: 0.2; control mean: 0.43, SD: 0.12); CEN: rDLPFC-Whole Brain: t (44) = − 2.1, p = 0.04 (hmTBI mean: 0.9, SD: 0.2; control mean: 1.05, SD: 0.17), rDLPFC-rPPC: t (44) = − 4.2, p < 0.00013 (hmTBI mean: 0.16, SD: 0.16; control mean: 0.43, SD: 0.26); DAN: rIPS-Whole Brain: t (44) = − 2.4, p = 0.02 (hmTBI mean: 0.93, SD: 0.17; control mean: 1.01, SD: 0.23), rIPS-FEF: t (44) = −3.4, p ≤ 0.003 (hmTBI mean: 0.1, SD: 0.08; control mean: 0.2, SD: 0.1). SN: rAI-Whole Brain t (44) = 3.9, p = 0.0003 (hmTBI mean: 1.05, SD: 0.22; control mean: 0.94, SD: 0.12), rAI-BA 47 t (44) = − 2.9, p < 0.006 (hmTBI mean: 0.05, SD: 0.07; control mean: 0.11, SD: 0.09); see Fig. 2A–C. The results indicate there is weaker network connectivity in the hmTBI between our seed locations and the whole brain and even in our more conservative approach of seed-seed connectivity.
Figure 2.
RsfMRI reveals weaker connectivity in participants with hmTBI. (A) DMN, CEN, DAN, and SN functional whole brain connectivity results show weaker connectivity in those with a hmTBI (yellow) compared to controls (blue). White circles identify seed locations. (B) Bar plots indicate z-score seed analysis based on seed-whole brain, indicating greater seed-whole brain connectivity in the control group. (C) Bar plots indicating z-score seed analysis showing connectivity between our seed-seed locations indicating greater connectivity in the control group. Error bars represent 95% confidence intervals. hmTBI history of mTBI, SR-mTBI sports related mTBI.
Finally, to understand whether hmTBI alters the relationship between visual WM accuracy and connectivity, we conducted a Pearson correlation. There was a borderline effect in DMN (rPCC-Whole Brain: r = 0.37, p = 0.08), but no other evidence of significant correlations (DMN: rPPC-vmPFC: r = 0.07, p = 0.74); CEN: rDLPFC-Whole Brain: r = − 0.14, p = 0.5, rDLPFC-rPPC: r = − 0.05, p = 0.8; DAN: rIPS-Whole Brain: r = − 0.28, p = 0.2, rIPS-FEF: r = − 0.15, p = 0.5; SN: rAI-Whole Brain: r = − 0.28, p = 0.2, rAI-BA 47: r = 0.22, p = 0.3.
Discussion
The consequences of mTBI can persist for years. To date, relatively few studies examine the long-term consequences of mTBI in civilian populations. Research typically tests military veterans with a history of blast injury or elite athletes, both at higher risk for repeated head injury. We adopted a different approach by testing otherwise healthy undergraduates with a hmTBI who were past the typical recovery stage. Findings in this population likely underestimate effects in the general population. These data support the existing literature that suggests mTBI can have long-term cognitive effects. Our data suggest that undergraduates with a history of show the traces of an mTBI ~ 3.8 years post-mTBI and that these effects are not different from student athletes who are only ~ 17 days post-mTBI.
We replicated our previous finding showing visual WM deficits in those with a hmTBI, who were well over 4 years post-injury. Unexpectedly, this visual WM deficit was equivalent to performance in those with a recent SR-mTBI. In other words—time may not heal this deficit. Surprisingly, auditory WM was well preserved as our findings suggest no differences in accuracy between the groups (control, hmTBI) and even faster reaction times in the hmTBI group. This is important because neuropsychological tests, such as the RBANS and the CVLT rely on auditory testing. Consequently, modality specific deficits may be obscured with auditory testing. Indeed, the cognitive battery revealed generally high function, as would be expected from an undergraduate sample. The exception was poor performance in immediate and delayed memory tasks in the hmTBI group. Connectivity analyses identify reductions across WM-relevant networks in the hmTBI group; see Fig. 2A–C. Even in otherwise healthy undergraduates there are lingering traces of an mTBI. An alternative hypothesis is that low WM capacity individuals experience mTBI more often than high WM capacity individuals, a hypothesis requiring prospective testing and long-term follow-up.
We speculated that longer white matter tracts, such as those associated with visual WM, would be more susceptible to heterogeneous trauma. This view is challenged by the overlap between network activity associated with auditory and visual WM79, and the observation of superior auditory WM in the hmTBI group. Future work including visual and auditory WM performance paired with connectivity measures is needed to understand how mTBI alters brain and behavior.
MTBI in the general population falls under the radar as large investigations examine mTBI in specific populations, such as American football players and veterans33,80,81. Yet, the current investigation may be targeting the 14% of mTBI patients seen privately and the estimated 25% who receive no medical attention82. MTBI can be consequential with detectible effects years after injury. To our knowledge, there are no ongoing therapeutic interventions designed to rehabilitate cognitive deficits in the hmTBI population. The functional connectivity data suggest that reduced connectivity persists after a mTBI and should be a target for future interventions.
This work has several important limitations. The unexpected observation that auditory WM was superior in the hmTBI group deserves additional research including both visual and auditory WM tasks. Secondly, the observation of weaker connectivity in the hmTBI population would benefit from a much larger sample so that the individual differences could be teased apart. However, we did attempt to identify relationships between accuracy and connectivity, but our sample size is too small to begin to draw strong conclusions. Thirdly, the participants tested in this project were heterogeneous and we accepted all self-reports of hmTBI. We imposed no restrictions based on time since injury, number of mTBIs, nature of medical treatment received, or etiology. We argue that this ‘open-door’ policy would avoid cherry-picking and would add noise thereby making it more difficult to detect an effect of mTBI. Because we did not require medical verification of participants’ mTBI history it is possible someone would deliberately lie. This seems unlikely as there was the concurrent opportunity to serve as a control participant. This is different from the SR-mTBI participants who were recruited from UNR Athletics after mTBI diagnosis. The similar performance between the two mTBI groups was unexpected. It may be that their peak physical condition allows them to perform better than the typical subacute mTBI participant and that these data present an inflated level of post-mTBI WM ability. Regardless, these findings raise concerns that all mTBIs should be taken seriously. Furthermore, we acknowledge that better powered studies are needed to look at more subtle effects. Overall, these data highlight the need for continued rehabilitation approaches as they confirm the presence of lasting WM deficits long after an mTBI.
Methods
Participants
Table 1 provides demographic information for each experiment. Based on our empirically derived large effect size of group (Cohen’s d = 0.92) from our first paper. To preserve power (0.90), we needed 25 participants per group (G-Power)47,83. Participants self-reported their hmTBI by keypress response indicating if they had a hmTBI (‘Y’ or ‘N’). Participation did not depend on their mTBI history. Follow-up questions included reporting the number of mTBIs incurred, when the most recent took place, and the etiology of their mTBI (19 hx mTBI were SR-mTBI). Recent SR-mTBI participants were recruited from the athletic department and had medically diagnosed mTBI and were under active treatment. The Institutional Review Board at the University of Nevada, Reno approved all protocols. All participants provided written informed consent consent and were reimbursed $15/h or course bonus credit (their choice). All methods were carried out in accordance to the guidelines and regulations set by the University of Nevada, Reno ethics committee.
Stimulus and procedure
Exp. 1A, Exp. 2. Change detection task
The task was presented on a 16″ MGC CRT monitor (75 Hz refresh rate, 1024 × 768) in MATLAB (The MathWorks, Natick, MA) using Psychophysics Toolbox 3.0 extension, using a Mac mini-1.4-GHz dual-core Intel Core i5. Participants were seated 57 cm from the display and instructed to maintain fixation throughout. Stimuli were presented in two rectangular areas subtending 7.1° × 12.2° of visual angle centered 4.6° from the fixation cross on a uniform medium gray background. Each trial, six colored squares (0.7° × 0.7°) were drawn from a set (cyan, white, red, blue, yellow, green, magenta). Stimuli were briefly presented symmetrically in each visual hemifield with a probe item appearing after a delay (Fig. 1A). Participants indicated whether the encoded stimulus and probe item matched. If no response was registered, the trial was considered incorrect. Trials were self-paced. Participants completed 24 practice and 120 experimental trials.
Exp. 1A. 3-Back task
The task was presented on a 24″ LCD monitor (Dell 1707 FPc,) using an Intel Core i7 CPU 2.93 GHz running E-Prime v2.0 (Psychology Software Tools, PA, USA; https://pstnet.com/e-prime-publications/). Participants completed three 3-back WM blocks using different task type (shapes, spatial, verbal; Fig. 1B) and randomized across participants. During the spatial 3-back task, participants maintained the location of green circles (3° visual angle, 500 ms) appearing sequentially in one of nine locations (followed by an inter-stimulus interval, 3000 ms). The shapes block using symmetrical novel polygons84, the verbal block used 20 consonants (Palatino size 30). The button presses, trial count, and timing of the task matched the spatial task. Participants pressed ‘J’ when the stimulus matched the item presented three items earlier; they pressed ‘F’ if they did not match. Participants completed 45 practice and 120 experimental trials (66% non-target, 7 min).
Exp. 1B. Auditory WM 3-back
Participants completed a 3-back WM task while hearing letters (consonants) or numbers (digits 1–9) spoken in separate blocks. Block order and response inputs were counterbalanced. The task was presented on a 16-in. MGC CRT monitor (75 Hz refresh rate, 1024 × 768) in MATLAB (The MathWorks, Natick, MA) with Psychophysics Toolbox 3.0 extension, using a Mac mini-1.4-GHz dual-core Intel Core i5.
Exp. 2. Methods
We collected behavioral, neuropsychological, and rsEEG data from participants who self-reported a hmTBI. We also collected data from SR-mTBI participants were recently (< 3 months) injured college-aged students. The inclusion of the subacute sample serves to clarify the relative impairment experienced by the hmTBI group. It also provided a comparison group in examining the time course of connectivity changes after an mTBI. We were interested in seeing if connectivity measures could be sensitive to detecting the cognitive and neural deficits in subacute and those with a hmTBI. We hypothesized that there would be more severely impaired performance and worse connectivity in the subacute mTBI group compared to those with a hmTBI; but that both would be worse than the control group. We predicted more severely abnormal rsEEG measures in the subacute mTBI participants compared to the group with a hmTBI.
Cognitive testing
Participants completed several tests to measure cognitive performance, verbal learning, and attention. Tests included: Repeated Battery for the Assessment of Neuropsychological Status (RBANS)85, Trail Making Test Part B (TMT-B)86, California Verbal Learning Test-II (CVLT-II)87-Short, and the Psychomotor Vigilance Test (PVT)88.
rsEEG
EEG recordings were collected over 6 intervals of 3 min each, alternating between eyes open and closed (not analyzed)89. Participants remained still and maintained neutral thoughts. During eyes-open blocks, participants-maintained fixation. Blocks ended with a 1000 Hz tone. EEG data was recorded (1000 Hz, 256 high-impedance electrodes in a HydroCel Geodesic Sensor Net, vertex reference, Net Amps 300 amplifier, Net Station 4.5.5 EGI, Eugene, OR), running on a 2.7 GHz dual-core Apple Power Mac G5.
EEG processing and analysis
Offline preprocessing (EEGLAB v14.1.290 and ERPLAB v8.0191) included band-pass filtering (1–100 Hz), segmentation from block onset—180 s, and downsampled (10–10 international electrode system, 70 electrode channels) to reduce highly correlated signals. Independent components analysis (ICA, SOBI) removed artifacts without removing trials92,93. For consistency, we automated the process using Multiple Artifact Rejection Algorithm94,95. Subsequent analyses were done in Fieldtrip96 separately in the delta (1–5 Hz), theta (5–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and low gamma (30–80 Hz) bands97–99.
rsEEG analyses
We used the following measures: weighted phase lag index100,101, modularity102, local and global efficiency102–104, and network cost105. RsEEG data was analyzed adopting prior methods deemed successful in clinical populations106. These values measure synchronization, within network connections, neighboring and node pair interactions, and node-pair interactions, respectively.
Weighted Phase Lag Index (wPLI)
WPLI is a measure of synchronization that address issues of volume conduction in the assessment of connectivity measures in EEG100,101. WPLI allows for the measurement of coherence that does not rely on correlation or partial correlations. WPLI weights the cross spectrum based on magnitude of the imaginary component. This allows for limits in the influence of cross spectrum elements around the real axes. Overall, wPLI measures asymmetry in the distribution of the phase differences from instantaneous phases of the two-time series. The wPLI contribution of the observed phase leads and lags is weighted by the magnitude of the imaginary component of the cross-spectrum. WPLI is defined as follows107,108:
The resulting WPLI is an absolute value between 0 and 1, such that 0 is the random phase difference with minimal strength of connectivity and 1 is the constant phase difference with maximum strength of connectivity109. denotes the imaginary and real component of the cross-spectrum. The wPLI was calculated using FieldTrip and was calculated for each electrode pair for every participant which created a wPLI matrix (70 electrodes × 70 electrodes) with wPLI values for each cell.
Graph theoretical metrics
In EEG connectivity, graph theoretical methods depict vertices and edges represent electrodes and connectivity strengths. We used the Brain Connectivity Toolbox (BCT)104 for Matlab.
Modularity
Modularity (Q) is the number of within-network module connections to all within-network module connections102,104 reflecting the balance of local versus broader interactions110,111. Modularity is measured from 0 (integration) to 1 (segregation). Integration across modules allows for global communication whereas segregation benefits local processing103. Modularity (Q) is defined by this equation104:
Each network is subdivided into a set of non-overlapping modules M, and is the proportion of all links that connect nodes in module with nodes in module 104,112.
Global efficiency
Global efficiency measures information transfer among node-pairs to clarify integration within a neural network104,113. Global efficiency is calculated as follows104,113:
here, is the efficiency of node 104,113 and dij is the shortest path between nodes and .
Local efficiency
Local efficiency quantifies network segregation which measures information transfer among neighboring nodes102–104,113. It is calculated as follows104:
where the is the local efficiency node , and is the length of the shortest path between and , that contains neighbors of 104.
Network cost
The analyses were conducted at network costs ranging from 10 to 30%, step size 5%, to ensure that results were not due to specific threshold values105. The range of thresholds was based on those values that have previously produced graphs with small world characteristics105.
Exp. 3. RsfMRI
In Experiment 3 we collected rsfMRI data from the hmTBI group tested in Experiment 2. We were interested in identifying reduced connectivity measured by rsfMRI in the hmTBI group. All but two of the hmTBI cohort from Experiment 2 (see Table 1) provided rsfMRI data. The control group was taken from a prior rsfMRI study in participants reporting no history of head-injury, psychiatric or neurological conditions114. Participants received $50/h or course bonus credit.
fMRI methods and parameters
Participants completed 3 rsfMRI runs (5.3 min each). Participants closed their eyes, relaxed and maintained neutral thoughts. Functional images were acquired on a 3 T Philips (Andover, MA) MRI with an eight channel SENSE parallel head coil. A set of 155 T2*-weighted volumes were obtained (TR = 2000 ms, TE = 30 ms, 32 slices per volumes, slice thickness = 3 mm, FOV = 240 mm, matrix size 128 × 128). Data were aligned to a high-resolution 3D structural dataset using an echo-planar 3D T1-weighted image.
fMRI preprocessing
Preprocessing used AFNI115 (http://afni.nimh.nih.gov/afni/), SUMA116; http://afni.nimh.nih.gov/afni/suma/), and FreeSurfer117,118 (http://surfer.nmr.mgh.harvard.edu/) [afni_proc.py (http://afni.nimh.nih.gov/pub/dist/doc/program_help/afni_proc.py.html)]. The first two TRs were removed, data were despiked, slice-time and motion corrected, and spatially normalized to the MNI template. The data were bandpass filtered (0.01–0.2 Hz). Censoring relied on motion parameters and signal outliers119,120. Six motion parameter estimates, ventricular and white matter signals, and baseline, linear, quadratic, and cubic trends were removed by linear regression121.
Regions of interest and seeds
Neurosynth (neurosynth.org) was used to identify commonly used seeds for the networks of interest based on prior research indicating interactions between functional connectivity and WM performance; see Table 361,62,67–69,122–126. Primary seed regions of interest (5 mm spheres, 19 voxels; rPCC, rDLPFC, rIPS, rAI) and secondary brain regions within the network (4 ROIs; 5 mm spheres, 19 voxels; vmPFC, rPPC, FEF, BA 47) were generated manually using AFNI.
Resting state analysis
To examine functional connectivity, we first evaluated connectivity between the time series data of the seed region and the rest of the brain (e.g., rPCC-Whole Brain). Additionally, we selected a second brain region within the selected network and correlated the time series between the two seed regions as a measure of network connectivity. AFNI’s 3dUndump created the ROI from the specified coordinates. 3dmaskave generated the time course in the seed region. 3dfim+ correlated time courses within the seed regions and the whole brain and the primary seed region-second brain region to generate connectivity maps of Pearson’s r values. To normalize the r values, we converted them to z-scores using Fisher’s r-to-z transformation and the expression ‘log((1 + a)/(1 − a))/2’. We then compared the mean z-scores for each group.
Acknowledgements
This material is based upon work supported in part by several funding sources including NSF OIA 1632849 (MB), NSF OIA 1632738 (MB), NIGMS P20GM103650 (MB), the Tahoe Institute for Rural Health Research (MB), NINDS F99 NS113419 (HA), and NINDS K00 NS113419-02 (HA). The research from the presented projects were submitted in fulfillment of the requirements for the degree of Doctor of Philosophy in Neuroscience at the University of Nevada, Reno. The content is solely the responsibility of the authors and does not represent the official views of any funding agency.
Author contributions
H.A. and M.B. wrote the manuscript text, H.A. and A.K.G. collected and analyzed the data. J.S. helped code the experiments and analyze the data. A.C. collected control MRI data. S.F. helped with EEG analysis. N.M. helped recruit participants and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cameron KL, Marshall SW, Sturdivant RX, Lincoln AE. Trends in the incidence of physician-diagnosed mild traumatic brain injury among active duty US military personnel between 1997 and 2007. J. Neurotrauma. 2012;29:1313–1321. doi: 10.1089/neu.2011.2168. [DOI] [PubMed] [Google Scholar]
- 2.Mann B. Forensic neuropsychology – a scientific approach. J Forensic Psychiatry Psychol. 2012;23(5–6):744–745. doi: 10.1080/14789949.2012.720838. [DOI] [Google Scholar]
- 3.Corrigan JD, Selassie AW, Orman JAL. The epidemiology of traumatic brain injury. J. Head Trauma Rehabilit. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 4.Taylor CA, Greenspan AI, Xu L, Kresnow M-J. Comparability of national estimates for traumatic brain injury-related medical encounters. J. Head Trauma Rehabilit. 2015;30:150–159. doi: 10.1097/HTR.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazarian JJ, et al. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005;19:85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- 6.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 7.Setnik L, Bazarian JJ. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj. 2007;21:1–9. doi: 10.1080/02699050601111419. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DB, et al. Treatment of persistent post-concussive symptoms after mild traumatic brain injury: A systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging Behav. 2015;9:403–420. doi: 10.1007/s11682-015-9440-2. [DOI] [PubMed] [Google Scholar]
- 9.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatry. 2003;15:341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 10.Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- 11.Millis SR, et al. Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabilit. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Shumskaya E, Andriessen TMJC, Norris DG, Vos PE. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology. 2012;79:175–182. doi: 10.1212/WNL.0b013e31825f04fb. [DOI] [PubMed] [Google Scholar]
- 13.De Monte VE, Geffen GM. Effects of mild traumatic brain injury: Comparison of direct and indirect injury groups. Brain Impairment. 2005;6:109–116. doi: 10.1375/brim.2005.6.2.109. [DOI] [Google Scholar]
- 14.Mayer AR, et al. A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging Behav. 2012;6:343–354. doi: 10.1007/s11682-012-9178-z. [DOI] [PubMed] [Google Scholar]
- 15.Catale C, Marique P, Closset A, Meulemans T. Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J. Clin. Exp. Neuropsychol. 2008;31:331–338. doi: 10.1080/13803390802134616. [DOI] [PubMed] [Google Scholar]
- 16.Konrad C, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011;41:1197–1211. doi: 10.1017/S0033291710001728. [DOI] [PubMed] [Google Scholar]
- 17.Wammes JD, Good TJ, Fernandes MA. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017;111:112–126. doi: 10.1016/j.bandc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Iverson GL, Karr JE, Gardner AJ, Silverberg ND, Terry DP. Results of scoping review do not support mild traumatic brain injury being associated with a high incidence of chronic cognitive impairment: Commentary on McInnes et al. 2017. PLoS ONE. 2019;14:e0218997. doi: 10.1371/journal.pone.0218997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCrea M, et al. An integrated review of recovery after mild traumatic brain injury (MTBI): Implications for clinical management. Clin. Neuropsychol. 2009;23:1368–1390. doi: 10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- 20.Christensen BK, et al. Recovery of cognitive function after traumatic brain injury: A multilevel modeling analysis of Canadian outcomes. Arch. Phys. Med. Rehabil. 2008;89:S3–S15. doi: 10.1016/j.apmr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Alosco ML, et al. Interactive effects of racial identity and repetitive head impacts on cognitive function, structural MRI-derived volumetric measures, and cerebrospinal fluid tau and Aβ. Front. Hum. Neurosci. 2019 doi: 10.3389/fnhum.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 2016;27:529–535. doi: 10.1016/j.nec.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with overall cognitive status in blast-related mTBI. Brain Imaging Behav. 2017;11:1129–1138. doi: 10.1007/s11682-016-9593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 2016;37:220–229. doi: 10.1002/hbm.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobeissy, F. H. (ed) Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects (CRC Press/Taylor & Francis, 2015). [PubMed]
- 26.Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. NeuroImage. Clin. 2015;8:148–156. doi: 10.1016/j.nicl.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mez J, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: Rationale and methods for the UNITE study. Alzheimers Res. Ther. 2015;7:62. doi: 10.1186/s13195-015-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peskind ER, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage. 2011;54(Suppl 1):S76–82. doi: 10.1016/j.neuroimage.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sponheim SR, et al. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage. 2011;54(Suppl 1):S21–29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Samuelson KW, et al. Longitudinal effects of PTSD on memory functioning. J. Int. Neuropsychol. Soc. 2009;15:853–861. doi: 10.1017/S1355617709990282. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JM, Scott KC, Dubinsky L. Battlefield brain: Unexplained symptoms and blast-related mild traumatic brain injury. Can. Fam. Phys. 2008;54:1549–1551. [PMC free article] [PubMed] [Google Scholar]
- 32.Mouzon B, et al. Chronic white matter degeneration, but No Tau pathology at one-year post-repetitive mild traumatic brain injury in a Tau Transgenic Model. J. Neurotrauma. 2019;36:576–588. doi: 10.1089/neu.2018.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montenigro PH, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma. 2017;34:328–340. doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manley G, et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017;51:969–977. doi: 10.1136/bjsports-2017-097791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamm JM, et al. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma. 2015;32:1768–1776. doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole VN, et al. MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev. Neuropsychol. 2014;39:459–473. doi: 10.1080/87565641.2014.940619. [DOI] [PubMed] [Google Scholar]
- 37.Gysland SM, et al. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann. Biomed. Eng. 2012;40:14–22. doi: 10.1007/s10439-011-0421-3. [DOI] [PubMed] [Google Scholar]
- 38.Guskiewicz KM, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 39.Shenton ME, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broadway JM, et al. Executive function predictors of delayed memory deficits after mild traumatic brain injury. Cortex J. Devoted Study Nervous Syst. Behav. 2019;120:240–248. doi: 10.1016/j.cortex.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosselin N, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg. Focus. 2012;33(E7):1–7. doi: 10.3171/2012.10.FOCUS12253. [DOI] [PubMed] [Google Scholar]
- 42.Hudac CM, Cortesa CS, Ledwidge PS, Molfese DL. History of concussion impacts electrophysiological correlates of working memory. Int. J. Psychophysiol. 2017 doi: 10.1016/j.ijpsycho.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld JV, et al. Blast-related traumatic brain injury. Lancet Neurol. 2013;12:882–893. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 44.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Vanderploeg RD, Curtiss G, Luis CA, Salazar AM. Long-term morbidities following self-reported mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 2007;29:585–598. doi: 10.1080/13803390600826587. [DOI] [PubMed] [Google Scholar]
- 46.Murray N, et al. Baseline postural control and lower extremity injury incidence among those with a history of concussion. J. Athl. Train. 2020;55:109–115. doi: 10.4085/1062-6050-187-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arciniega H, et al. Visual working memory deficits in undergraduates with a history of mild traumatic brain injury. Atten. Percept Psychophys. 2019;81:2597–2603. doi: 10.3758/s13414-019-01774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arciniega H, Kilgore-Gomez A, McNerney WM, Lane S, Berryhill ME. Loss of consciousness, but not etiology, predicts better working memory performance years after concussion. J. Clin Transl. Res. 2020;5(4):169–177. doi: 10.18053/jctres.05.202004.003. [DOI] [Google Scholar]
- 49.McCrory P, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 50.Eagle SR, et al. Association of time to initial clinic visit with prolonged recovery in pediatric patients with concussion. J. Neurosurg. Pediatr. 2020 doi: 10.3171/2020.2.peds2025. [DOI] [PubMed] [Google Scholar]
- 51.Terwilliger VK, Pratson L, Vaughan CG, Gioia GA. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J. Neurotrauma. 2016;33:761–765. doi: 10.1089/neu.2015.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, et al. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157:1970–1978. doi: 10.1097/j.pain.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hume AJ, et al. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 54.van Eijck MM, Schoonman GG, van der Naalt J, de Vries J, Roks G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: A systematic review and meta-analysis. Brain Inj. 2018;32:395–402. doi: 10.1080/02699052.2018.1429018. [DOI] [PubMed] [Google Scholar]
- 55.Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Geschwind N. Disconnexion syndromes in animals and man: Part I. Neuropsychol. Rev. 2010;20:128–157. doi: 10.1007/s11065-010-9131-0. [DOI] [PubMed] [Google Scholar]
- 57.Geschwind N. Disconnexion syndrome in animals and man. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 58.Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain J. Neurol. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 59.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peerless SJ, Rewcastle NB. Shear injuries of the brain. Can. Med. Assoc. J. 1967;96:577–582. [PMC free article] [PubMed] [Google Scholar]
- 61.Yakushev I, et al. Metabolic and structural connectivity within the default mode network relates to working memory performance in young healthy adults. NeuroImage. 2013;79:184–190. doi: 10.1016/j.neuroimage.2013.04.069. [DOI] [PubMed] [Google Scholar]
- 62.Vatansever D, Manktelow AE, Sahakian BJ, Menon DK, Stamatakis EA. Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 2017;38:41–52. doi: 10.1002/hbm.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajesh A, et al. Differences in brain architecture in remote mild traumatic brain injury. J. Neurotrauma. 2017;34:3280–3287. doi: 10.1089/neu.2017.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin F, et al. Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: A pilot study. J. Am. Geriatr. Soc. 2016;64:1293–1298. doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chien HY, Gau SSF, Tseng WYI. Deficient visuospatial working memory functions and neural correlates of the default-mode network in adolescents with autism spectrum disorder. Autism Res. 2016;9:1058–1072. doi: 10.1002/aur.1607. [DOI] [PubMed] [Google Scholar]
- 66.Santangelo V, Bordier C. Large-scale brain networks underlying successful and unsuccessful encoding, maintenance, and retrieval of everyday scenes in visuospatial working memory. Front. Psychol. 2019;10:233. doi: 10.3389/fpsyg.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li F, et al. Disrupted functional network connectivity predicts cognitive impairment after acute mild traumatic brain injury. CNS Neurosci. Ther. 2020 doi: 10.1111/cns.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Horn HJ, et al. Brain network dysregulation, emotion, and complaints after mild traumatic brain injury. Hum. Brain Mapp. 2016;37:1645–1654. doi: 10.1002/hbm.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson B, Dodd A, Mayer AR, Hallett M, Slobounov S. Are there any differential responses to concussive injury in civilian versus athletic populations: A neuroimaging study. Brain Imaging Behav. 2020;14:110–117. doi: 10.1007/s11682-018-9982-1. [DOI] [PubMed] [Google Scholar]
- 70.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson DE, et al. Neurophysiological evidence of impaired attention and working memory in untreated hematologic cancer patients. Clin. Neurophysiol. 2019;130:1243–1252. doi: 10.1016/j.clinph.2019.04.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn. Affect Behav. Neurosci. 2005;5:144–155. doi: 10.3758/CABN.5.2.144. [DOI] [PubMed] [Google Scholar]
- 73.Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 2005;16:965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- 74.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 75.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen AC, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. PNAS Proc. Natl. Acad. Sci. USA. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 78.Maurer U, et al. Frontal midline theta reflects individual task performance in a working memory task. Brain Topogr. 2015;28:127–134. doi: 10.1007/s10548-014-0361-y. [DOI] [PubMed] [Google Scholar]
- 79.Mencarelli L, et al. Stimuli, presentation modality, and load-specific brain activity patterns during n-back task. Hum. Brain Mapp. 2019;40:3810–3831. doi: 10.1002/hbm.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warden DL, French L. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;353:633–634. doi: 10.1056/NEJM200508113530621. [DOI] [PubMed] [Google Scholar]
- 81.Okie S. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;352(20):2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- 82.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 83.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 84.Jiang Y, Olson IR, Chun MM. Organization of visual short-term memory. J. Exp. Psychol. Learn. Memory Cognit. 2000;26:683–702. doi: 10.1037/0278-7393.26.3.683. [DOI] [PubMed] [Google Scholar]
- 85.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 86.Lezak MD. Neuropsychological Assessment. 3. Oxford: Oxford University Press; 1995. [Google Scholar]
- 87.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test–second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol. 2006;21(5):413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J. Neurosci. Methods. 2014;222:250–259. doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barry RJ, De Blasio FM. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol. Psychol. 2017;129:293–304. doi: 10.1016/j.biopsycho.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 90.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014 doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belouchrani A, Abed-Meraim K, Cardoso J-F, Moulines E. A blind source separation technique using second-order statistics. IEEE Trans. Signal Process. 1997;45:434–444. doi: 10.1109/78.554307. [DOI] [Google Scholar]
- 93.Tang AC, Sutherland MT, McKinney CJ. Validation of SOBI components from high-density EEG. NeuroImage. 2005;25:539–553. doi: 10.1016/j.neuroimage.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 94.Winkler I, et al. Robust artifactual independent component classification for BCI practitioners. J. Neural Eng. 2014;11:035013. doi: 10.1088/1741-2560/11/3/035013. [DOI] [PubMed] [Google Scholar]
- 95.Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-Components for artifact removal in EEG signals. Behav. Brain Funct. 2011 doi: 10.1186/1744-9081-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gasser T, Rousson V, Gasser US. EEG power and coherence in children with educational problems. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2003;20:273–282. doi: 10.1097/00004691-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51:1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Munck JC, Goncalves SI, Mammoliti R, Heethaar RM, da Silva FHL. Interactions between different EEG frequency bands and their effect on alpha-fMRI correlations. Neuroimage. 2009;47:69–76. doi: 10.1016/j.neuroimage.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 100.Ortiz E, et al. Weighted phase lag index and graph analysis: Preliminary investigation of functional connectivity during resting state in children. Comput. Math. Methods Med. 2012 doi: 10.1155/2012/186353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage. 2011;55:1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 102.Cohen JR, D'Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sporns O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013;23:162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Bullmore ET, Bassett DS. Brain graphs: Graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 106.Furlong S, et al. Resting-state EEG connectivity in young children with ADHD. J. Clin. Child Adolesc. Psychol. 2020 doi: 10.1080/15374416.2020.1796680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stam CJ, Nolte G, Daffertshofer A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toth SL, Cicchetti D. The historical origins and developmental pathways of the discipline of developmental psychopathology. Isr. J. Psychiatry Relat. Sci. 2010;47:95–104. [PubMed] [Google Scholar]
- 109.Tóth B, et al. Large-scale network organization of EEG functional connectivity in newborn infants. Hum. Brain Mapp. 2017;38:4019–4033. doi: 10.1002/hbm.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghaderi AH, Nazari MA, Darooneh AH. Functional brain segregation changes during demanding mathematical task. Int. J. Neurosci. 2019;129:904–915. doi: 10.1080/00207454.2019.1586688. [DOI] [PubMed] [Google Scholar]
- 111.Fox PT, Friston KJ. Distributed processing; distributed functions? NeuroImage. 2012;61:407–426. doi: 10.1016/j.neuroimage.2011.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 113.de Pasquale F, Della Penna S, Sporns O, Romani GL, Corbetta M. A dynamic core network and global efficiency in the resting human brain. Cereb. Cortex. 2016;26:4015–4033. doi: 10.1093/cercor/bhv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cerreta AGB, Mruczek REB, Berryhill ME. Predicting working memory training benefits from transcranial direct current stimulation using resting-state fMRI. Front. Psychol. 2020;11:570030. doi: 10.3389/fpsyg.2020.570030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 116.Saad Z, Reynolds R, Cox RJ, Argall B, Japee S. SUMA: An interface for surface-based intra- and inter-subject analysis. PROC ISBI. 2004;2:1510–1511. [Google Scholar]
- 117.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 118.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 119.Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fox CJ, Iaria G, Barton JJ. Defining the face processing network: Optimization of the functional localizer in fMRI. Hum. Brain Mapp. 2009;30:1637–1651. doi: 10.1002/hbm.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ward AM, et al. Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiol. Aging. 2015;36:265–272. doi: 10.1016/j.neurobiolaging.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Disbrow EA, et al. Resting state functional connectivity is associated with cognitive dysfunction in non-demented people with Parkinson's disease. J. Parkinson's Disease. 2014;4:453–465. doi: 10.3233/JPD-130341. [DOI] [PubMed] [Google Scholar]
- 124.Tessitore A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79:2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- 125.Zhang, Q., Zhang, G., Yao, L. & Zhao, X. Impact of real-time fMRI working memory feedback training on the interactions between three core brain networks. Front. Behav. Neurosci.9 (2015). [DOI] [PMC free article] [PubMed]
- 126.Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Hum. Brain Mapp. 2014;35(3):1004–1017. doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]