Abstract
Prostate cancer (PCa) exhibits highly variable biological behavior ranging from indolent to highly aggressive based on its grade and stage. Conventional imaging consists of computed tomography (CT), magnetic resonance imaging (MRI), and single photon bone scans, but imaging has traditionally played a secondary role in managing prostate cancer patients. Thus, many of the treatment decisions rely on PSA levels and “best guesses” of disease status. The gap between what conventional imaging shows and the suspected status of disease based on clinical factors has encouraged the development of more sensitive molecular imaging probes based on positron emitting radioactive tags. Such Positron Emission Tomography (PET) probes have high sensitivity owing to the nature of PET imaging and several PCa-focused agents have been developed, particularly for detecting sites of recurrence and staging patients with PCa. The expanding list of available PET radiotracers now includes radiolabeled 11C and 18F choline agents, anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (18F-fluciclovine or 18F-FACBC ), 68Ga and 18F bombesin and derivatives, 18F dihydrotestosterone (18F DHT), 68Ga and 18F prostate-specific membrane antigen (PSMA) ligands, and 18F Fluorodeoxyglucose (18F FDG) among others. Of these, radiolabeled PSMA PET agents have emerged as having the best sensitivity and specificity for PCa. However, as use of PSMA PET increases, false negative scans as well as false positives are reported. As a result, it is important to consider the wide range of potential molecular imaging agents that may fill in where PSMA is suboptimal. For instance, PET radiotracers such as bombesin-targeted or antagonist of gastrin releasing-peptide receptor (GRP)-targeted (RM2) and 18F FDG are potential alternatives. Herein, we review the agents currently available or in development for molecular imaging of PCa.
Keywords: Prostate cancer, molecular imaging, prostate specific membrane antigen, biochemical recurrence, staging
Introduction
Prostate cancer (PCa) is a major cause of cancer-related deaths in men and one of the few cancers where imaging, until recently, has not played a major role in the standard diagnostic and risk stratification process (1). Conventional imaging including CT, MRI, and bone scans often fail to detect sites of disease especially at lower PSA levels and may overstate the amount of active disease as PSA rises. Molecular imaging becomes especially important. Detection of intra-prostatic disease at diagnosis is generally handled by multiparametric MRI and fusion biopsy and is covered in detail elsewhere in this special edition. While there may be a role for molecular imaging in early diagnosis, here, we focus on the disease once it has been initially treated and has recurred based on rising PSA or for high risk patients, for optimized staging at the time of initial diagnosis. In the setting of biochemical recurrence (BCR) imaging should be able to distinguish local recurrence in the prostate bed from nodal or distant visceral or osseous metastases. Finally, the same or related molecular entities used in molecular imaging of prostate cancer may also be used to direct targeted radionuclide therapy (TRT), using therapeutic isotopes in place of diagnostic isotopes.
There has been much effort devoted to developing new PET imaging agents for PCa. A distinct problem with many PET agents is that they are often taken up in benign conditions such as benign prostatic hyperplasia or prostatitis, reducing their value for initial diagnosis. Many of the agents lose sensitivity later in the disease when adenocarcinomas undergo neuroendocrine trans-differentiation. The one exception is 18F-FDG PET imaging, which is taken up more avidly in less well differentiated tumors, perhaps a reflection of increased metabolic turnover. As a result 18F-FDG is increasingly used in late castration resistant prostate cancer (CRPC) and increasing uptake is associated with a poor prognosis.
Here, we discuss these molecular probes and compare them using existing literature.
11C/18F Choline
Choline kinase (CK) is overexpressed in PCa, and choline serves as a substrate for this enzyme. CK helps the cells use choline to synthesize phosphatidylcholine, a key component of cell membranes. Therefore, radiolabeled choline is taken up in PCa cells. 11C and 18F radiolabeled choline PET/CT imaging have both been widely used for detecting advanced PCa. Thus, when CK is upregulated radiolabeled choline will be taken up by tumors.
11C-Choline is theoretically an ideal PET tracer because its label, 11C, does not alter the essential chemistry of choline. However, due to the short half-life (t1/2= 20 min) of 11C, it can only be made in institutions with an on-site cyclotron and clinical radiochemistry facility, heavily restricting its use and requiring patients to travel to such institutions simply for a diagnostic test. 18F-labelled choline is a more practical alternative due to its longer half-life (t1/2=110 min.), but this small change in labeling isotope makes the compound chemically and physiologically different from endogenous choline. It has higher urinary excretion into the bladder than 11C-choline, which may obscure visualization of the prostate bed (2,3). Nonetheless, both agents function satisfactorily, and multiple studies have shown similar diagnostic performance for 11C-choline and 18F-choline for malignant lesions in different clinical settings (4). While 11C-choline was approved by the FDA in 2012, 18F-choline is not approved and has no commercial supplier in United States.
In localized disease a central limitation of Choline PET is that its uptake is non-specific. Benign prostatic hyperplasia takes up 11C/18F-choline indistinguishably from cancer. Thus, use is generally restricted to staging/restaging patients with intermediate-high-risk or very-high-risk cancer.
The major role of 11C/18F-choline PET/CT is for detecting recurrence in the setting of BCR, where it has shown better accuracy than conventional imaging (5,6). For instance, among 2686 patients (7) 11C-choline PET/CT had an overall detection rate for recurrent disease of 62% (95%CI: 53%–71%). Such overall numbers, however, are misleading as they are influenced by the distribution of PSA levels in the studied population, therefore, a more meaningful measure is obtained by stratifying patients by their PSA level. Patients with high PSA levels will have their disease easily detected on most imaging methods. However, detecting disease in patients with lower PSA values could be of more use as such patients are more responsive to salvage radiation and therefore, still have a “therapeutic window”. Among 3203 PCa patients with BCR 11C-choline PET/CT (8) achieved a sensitivity of 44.7% in patients with a PSA level between 1 and 2 ng/mL. 11C/18F-choline PET/CT findings in the setting of recurrent disease change clinical decision-making in approximately 50% of cases in which the agent is used (9–11) and it has been incorporated into several professional association guidelines(12). However, this measure of efficacy is limited as a change in management is not necessarily a measure of improved outcome.
The diagnostic performance of choline PET/CT for the detection of bone metastasis in PCa was evaluated in a meta-analysis of 14 studies involving 655 patients(13). On a per-patient basis, the reported sensitivity and specificity ranged from 50% to 100% and from 89% to 100%, respectively. On a per-lesion basis, among 1,619 lesions from 472 patients sensitivity and specificity ranged from 75% to 96% and from 92% to 100, respectively (13). Choline PET/CT imaging was found to exhibit excellent diagnostic performance for the detection of bone lesions, however a negative choline PET did not guarantee that bone metastases were not present (13). Thus, choline PET was among the first molecular imaging agents for PCa that demonstrated improved sensitivity over conventional imaging but is comparatively insensitive when compared to newer agents.
Radiolabeled Amino Acid Analogs
Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, chemically referred to as 18F-FACBC, and recently renamed 18F-fluciclovine, or Axumin™ (Blue Earth Diagnostics, UK), is a synthetic amino acid L-leucine analogue, that binds to amino acid transporters which are heavily upregulated in PCa (14, 15). 18F-fluciclovine exhibits delayed excretion into the urinary tract, thus improving detection of disease near the prostate bed (16,17).
18F-fluciclovine, like choline PET, exhibits non-specific prostate uptake in benign conditions such as BPH. Among 68 patients with primary PCa, 18F-fluciclovine PET/CT was found to have a sensitivity and specificity of 92.5% and 90.1% (18). However, when a more realistic mix of patients and diagnostic criteria are applied the specificity is lower (19−21). In a comparison study (21) of 18F-fluciclovine PET/CT and MRI for the detection of primary PCa in 21 men, MRI had a higher sensitivity and specificity than 18F-fluciclovine (73% vs 67% and 79% vs 66%, respectively). PCa uptake was significantly higher than the normal prostate gland, however, PCa could not be distinguished from benign prostatic hyperplasia (21). This was confirmed in another study in which the high sensitivity of 87% but low specificity of 56% for primary tumor identification was seen with 18F-fluciclovine. Therefore, it is unlikely that 18F-fluciclovine PET will play a role in initial staging of primary PCa.
18F-fluciclovine was granted approval by the US FDA in 2016 for suspected recurrent PCa. In 251 patients with BCR a pooled sensitivity and specificity of 87% (95%CI: 80–92%) and 66% (95%CI: 56–75%), respectively was found with 18F-fluciclovine for recurrent disease (17). Similarly, a high specificity of 96.7% and positive predictive value (PPV) of 95.7% was reported (22) for nodal and boney disease in patients with recurrent PCa (figure 1). This high PPV was confirmed in a trial of 596 patients where the PPV was 92.3% (23). 18F-fluciclovine surpassed the performance of CT in detecting recurrent PCa (24). However, when results were stratified by PSA value, 18F-fluciclovine PET had cancer recurrence detection rates of 72.0%, 83.3%, and 100% at PSA levels of <1 ng/mL, 1–2 ng/mL, and ≥2 ng/mL, respectively (25). When the strata were even more carefully defined, 18F-fluciclovine PET/CT tumor had a detection rate of 31 %, 50%, 66%, and 84% of patients, for PSA 0–0.5ng/mL, >0.5–1.0ng/mL, PSA >1.0–2.0ng/mL and >2.0ng/mL, respectively, on a per patient-basis(26). 18F-fluciclovine results can have a major impact on patient management and 59% of patients in one study had their treatment altered, in some cases significantly, by this PET scan (26). Again, however, altered treatment plans does not necessarily predict better outcomes.
Figure 1:
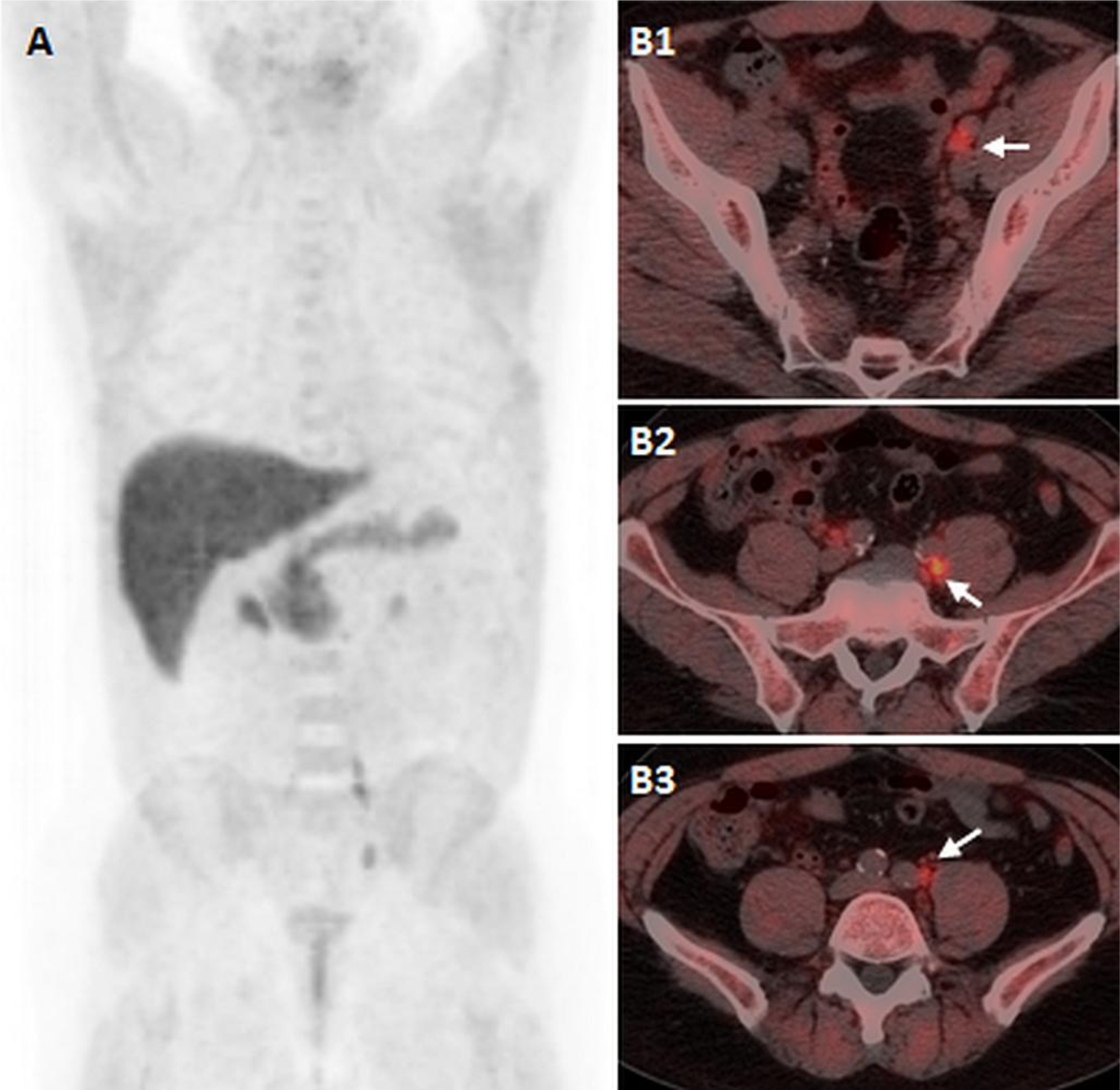
61-years old man with biochemical recurrent prostate cancer, status post HIFU and salvage IMRT 5 ears ago, and recent rising PSA of 2.5ng/ml. 18F-Fluciclovine PET/CT imaging, including maximal intensity projection (A) and axial fused PET/CT images (B1, B2, 3) demonstrates focal increased uptake fusing to sub-centimeter left external iliac (B1), and left common iliac (B2, B3) lymph nodes.
Few studies directly compare 11C/18F-choline and 18F-fluciclovine in the same patient but generally, the performance of 18F-fluciclovine is superior. A prospective study comparing the two scans (27) in 89 BCR patients after radical prostatectomy, revealed sensitivity of 37%, specificity of 67%, PPV of 97%, NPV of 4%, and accuracy of 38% for 18F-fluciclovine, versus 32%, 40%, 90%, 3%, and 32%, respectively for choline PET imaging (27,28). In general, 18F-fluciclovine detects a higher rate of true positive and true negative lesions in the prostate bed, lymph nodes, and bones. Furthermore, 18F-fluciclovine was superior to choline PET at all levels of PSA (28). The longer lived nature of 18F and the commercial availability of an FDA-approved product provide practical advantages for 18F-fluciclovine (29).
18F-fluciclovine, however, has some limitations. It shows only low-to-moderate specificity with a relatively high false positive rate in many patients(30). As a prelude to the next section 68Ga-PSMA-11 PET/CT outperformed 18F-fluciclovine, by detecting findings in half of patients who had a negative 18F-fluciclovine scan, and detecting additional lesions in the 20% of patients who were positive with both scans (31).
Prostate-specific membrane antigen (PSMA) PET/CT imaging
Prostate-specific membrane antigen (PSMA) is a well-known target of PCa but has become the most well known target for PET imaging relatively recently (32). PSMA is overexpressed in most intermediate-high-grade PCa and its expression increases as the disease progresses (32–36). However, in transdifferentiated tumors with neuroendocrine features, PSMA expression may be reduced or absent. In addition to prostate cancer, PSMA is also is expressed in some normal tissues (salivary gland, kidney, small bowel), other neoplasms (especially in the neovasculature) and in some cases of inflammation (37, 36). PSMA is an excellent target for diagnostic imaging and therapy of PCa, due to its high expression in tumors, low background and internalization within cells.
The first clinical attempt to target PSMA for imaging was a radiolabeled monoclonal antibody (mAb), 111Indium-7E11 (111In-capromab; ProstaScint™) that was approved in 1999. Unfortunately, the antibody targeted the intracellular domain of PSMA, which is usually inaccessible to exogenously injected antibodies and thus, lacked sensitivity and specificity (38). Another antibody, hJ591, developed by Neil Bander at Cornell University, binds to an extracellular domain of PSMA and has also been investigated for PET diagnostic imaging in the form of 89Zr-hJ591. While this agent demonstrated higher affinity, and more efficient targeting (39), and was used as a theranostic in the form of 177Lu-hJ591 for therapy in metastatic castrate-resistant PCa patients (mCRPC), it has never advanced commercially. Any of the agents discussed can be labeled with therapeutic isotopes such as the beta particle emitting177Lu or the alpha particle emitting 225Ac. Many other isotopes fall into this therapeutic category and they vary in their emitting energy and half life as well as their “decay” scheme which can release additional therapeutic isotopes.
The more recent development of PSMA radiopharmaceuticals are small-molecule, urea-based inhibitors that bind the active substrate recognition site of the folate hydrolase enzyme which is part of the external domain of the PSMA complex. These ligands bind with high (subnanomolar) affinity and also exhibit rapid plasma clearance producing high tumor-to-background ratios. A variety of PSMA PET agents have been translated into the clinic over recent years, but none has yet been approved by the US FDA. Among the most widely used PSMA compounds in clinical studies, PSMA-HBED-CC is labeled with 68Ga and is known as 68Ga-PSMA-11 (40), which has quickly become the dominant agent worldwide because of the widespread commercial availability of the substrate. However, 68Ga labeling is not ideal in all circumstances due to its short half-life, high energy positron and the need for an onsite generator. An alternative, where labeling can be made off-site with 18F is desirable. To date, the most common labels used for PSMA imaging are 68Ga, 18F, (both PET emitters) and 111In, and 99mTc (both SPECT emitters), such as 18F-DCFBC/18F-DCFPyL, 18F-PSMA-1007, 68Ga/111In-PSMA-617, or 99mTc -MIP-1095 among others.
Unlike other agents discussed, PSMA is highly specific for PCa and is not taken up in benign prostatic hyperplasia. Thus, PSMA PET/CT can be used in localized disease. Some have gone so far as to suggest PSMA PET/CT could guide biopsy and focal therapy of localized prostate cancer (figure 2), (41). Of course, PSMA PET/CT may benefit from being combined with MRI as in PET/MRI since both modalities are useful in PCa diagnosis. Combined 68Ga-PSMA-11 PET/MRI was analyzed in 53 patients with intermediate to high-risk PCa, and PSMA demonstrated superior accuracy with MRI compared to either modality alone, with sensitivities and specificities of 76% and 97% for hybrid 68Ga-PSMA-11 PET/MRI; 58% and 82% for multi-parametric MRI alone, and 64% and 94%, for 68Ga-PSMA-11 PET/CT alone (42). Similarly, other studies. (43) have shown that PSMA PET/MR increased the accuracy of both modalities resulting in a sensitivity of 82% and a specificity of 89%. There is a correspondence between PSMA maximum standard uptake value (SUVmax) and Gleason score at histology, although there is considerable overlap among different Gleason scores. (44). The ability of PSMA PET to discriminate clinically significant high-grade PCa from BPH makes it especially valuable in localized disease (45,46). Even within a specific primary tumor there can be heterogeneity of uptake (47,48) indicating not all parts of a tumor will express PSMA. The significance of non-expression within parts of the tumor is not yet well understood.
Figure 2:
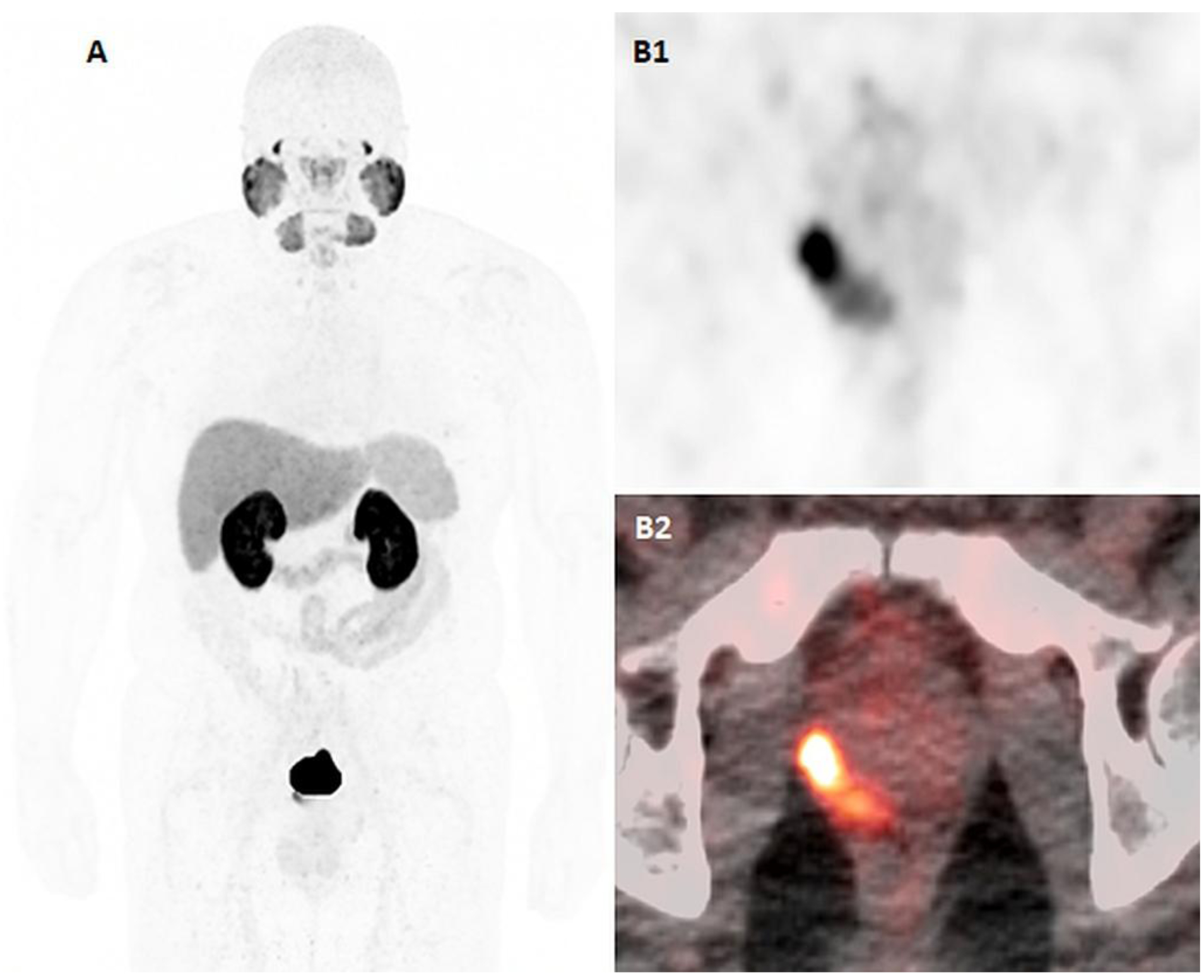
55-years-old man with newly diagnosed high-risk prostate cancer, Gleason 9 (4+5) and PSA of 20.95ng/ml. 18F-DCFPYL PET/CT imaging, including maximal intensity projection (A), axial PET (B1) and axial fused PET/CT (B2) images demonstrate intraprostatic DCFPYL-avid focus at the right mid-base posterolateral peripheral zone of the prostate, consistent with the biopsy-proven primary malignancy. There are no suspicious DCFPYL-avid focus to suggest metastatic disease.
Preoperative staging is important in intermediate and high risk patients as the risk of nodal or bone disease is much higher. Conventional imaging performs poorly for the detection of pelvic lymph node metastases (49), mainly because most nodes measure less than 8 mm and thus, cannot be reported as positive on conventional imaging. When PSMA and CT are compared for lymph node staging (50) PSMA shows its true strength. For instance, the sensitivity, specificity and accuracy for the detection of nodal metastases was 65.9%, 98.9% and 88.5%, respectively for 68Ga-PSMA-11, compared with 43.9%, 85.4% and 72.3% for morphological imaging (50). A recent meta-analysis found a pooled sensitivity, and specificity of 71% and 95% for lymph node staging in patients with newly diagnosed intermediate to high risk PCa, however, again, results must be stratified by PSA values (51, 52,53). In a comparison of 68Ga-PSMA PET/CT with histology in 30 patients (53) it was shown that nodal detection rates were substantially influenced by lymph node size (Fig 3).
Figure 3:
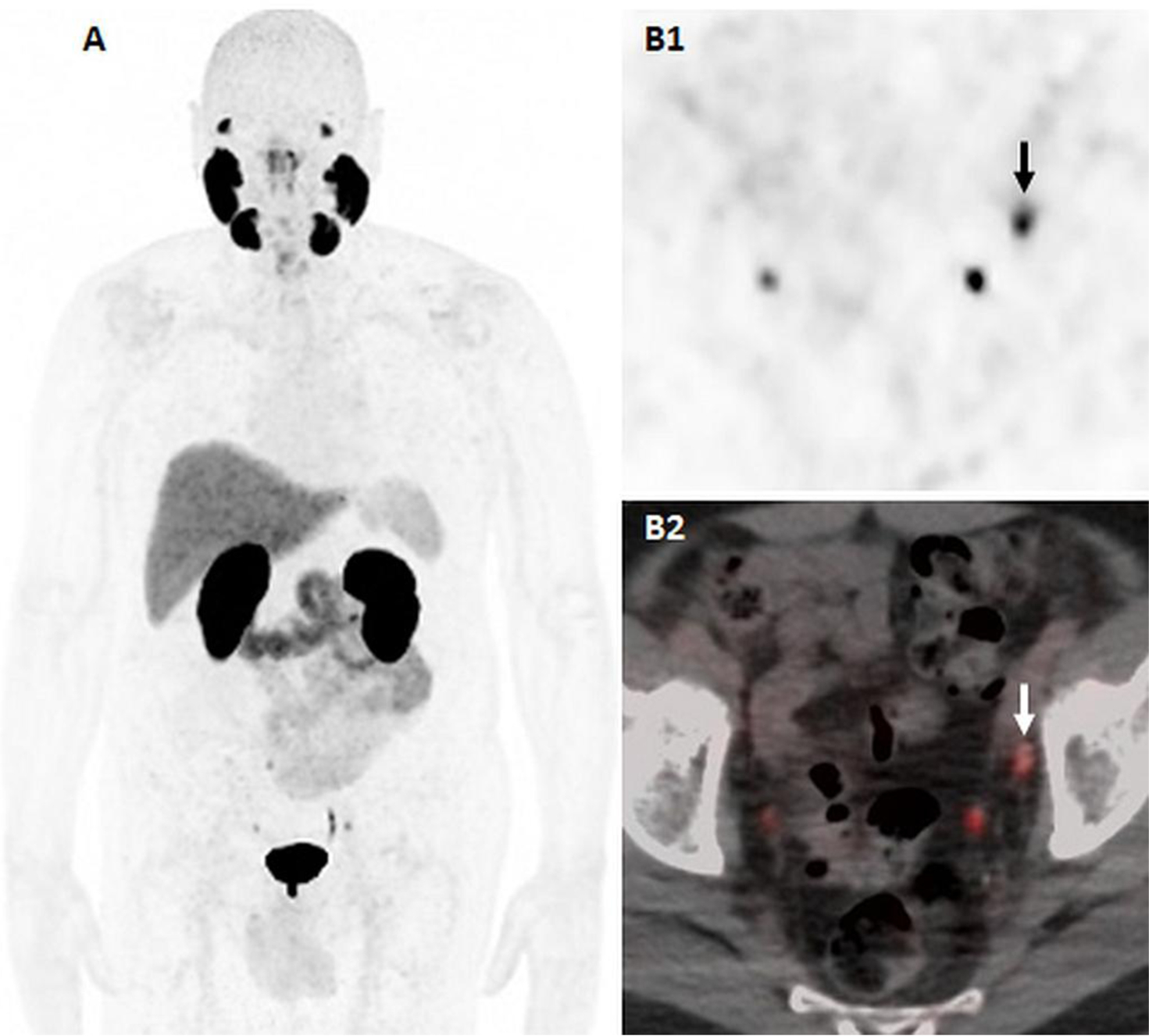
74-years-old man with biochemical recurrence prostate cancer, status post-prostatectomy 5 yers ago, with rising PSA of 2.23ng/ml at the time of the scan. 18F-DCFPYL PET/CT including maximal intensity projection (A), axial PET (B1) and axial fused PET/T (B2) images demonstrate a 0.5cm DCFPYL-avid left obturator lymph node. Physiologic uptake is seen in bilateral ureters.
For initial staging, PSMA PET/CT outperforms conventional bone scan in intermediate-high risk primary patients (54). 68Ga-PSMA uptake showed even higher diagnostic performance for osteolytic lesions than osteoblastic metastases (55).
The most extensive experience with PSMA PET/CT imaging has been in the setting of BCR (PSA ≥0.2 ng/ml after radical prostatectomy, or a 2 ng/ml rise above the PSA nadir post radiation). Early detection of recurrence can guide therapy, and assist in the decision to use aggressive loco-regional salvage therapy or systemic palliative therapy. PSMA-driven changes in management have not yet been shown to positively influence outcomes. This is most important at low serum PSA values, when there are potential curative salvage RT options, which remain most effective at serum PSA values less than 1.0 ng/mL (56).
PSMA PET/CT has shown clear superiority to conventional imaging. (57). PSMA PET/CT was able to detect nodal metastases in 78% of patients with histologically proven metastatic nodes, whereas conventional imaging was positive in only 27% (58). PSMA PET can be used to identify metastatic nodes from the low pelvis to the supraclavicular node chain (figure 4) (figure 5). When PSMA PET/CT data is stratified by PSA a clear relationship between PSA and PSMA PET can be seen. 68Ga-PSMA-11 PET tumor detection rates for the PSA categories of 0–0.2 ng/mL, 0.2–1.0 ng/mL, 1–2 ng/mL, and > 2 ng/mL were 42%, 58%, 76%, and 95%, respectively; and 68Ga-PSMA PET positivity was seen more in patients with shorter PSA doubling time (PSAdt) (59). Using similar strata, a meta-analysis showed a sensitivity of PSMA PET of 50% for PSA of 0.2 to 0.49 and 53% for PSA of 0.50 to 0.99 ng/mL (60). Several large studies with over 1000 patients have been reported (61) and some have found no correlation between PSMA PET/CT uptake and PSAdt or PSA velocity or between Gleason score and PET positivity. However, most studies draw associations between PSA level and PSA doubling time (PSAdt) and a positive 68Ga-PSMA-11 PET/CT (62). A PSAdt of 6.5 months and a PSA of 0.83 ng/ml were optimal cut-off values for predicting 68Ga-PSA PET-positivity, which was observed in 85% of patients with PSA < 2 ng/ml and PSAdt > 6.5 months. Others (63) have confirmed that a shorter PSAdt was significantly associated with the presence of pelvic and extrapelvic LN, bone, and visceral metastases on PSMA PET/CT, and that higher PSA levels and shorter PSAdt were independently associated with scan positivity and extrapelvic metastases(63). The association between positivity and more aggressive disease implies that PSMA positivity has an independent prognostic value in patients with prostate cancer. Likewise, negative PSMA scans carry a favorable prognosis.
Figure 4:
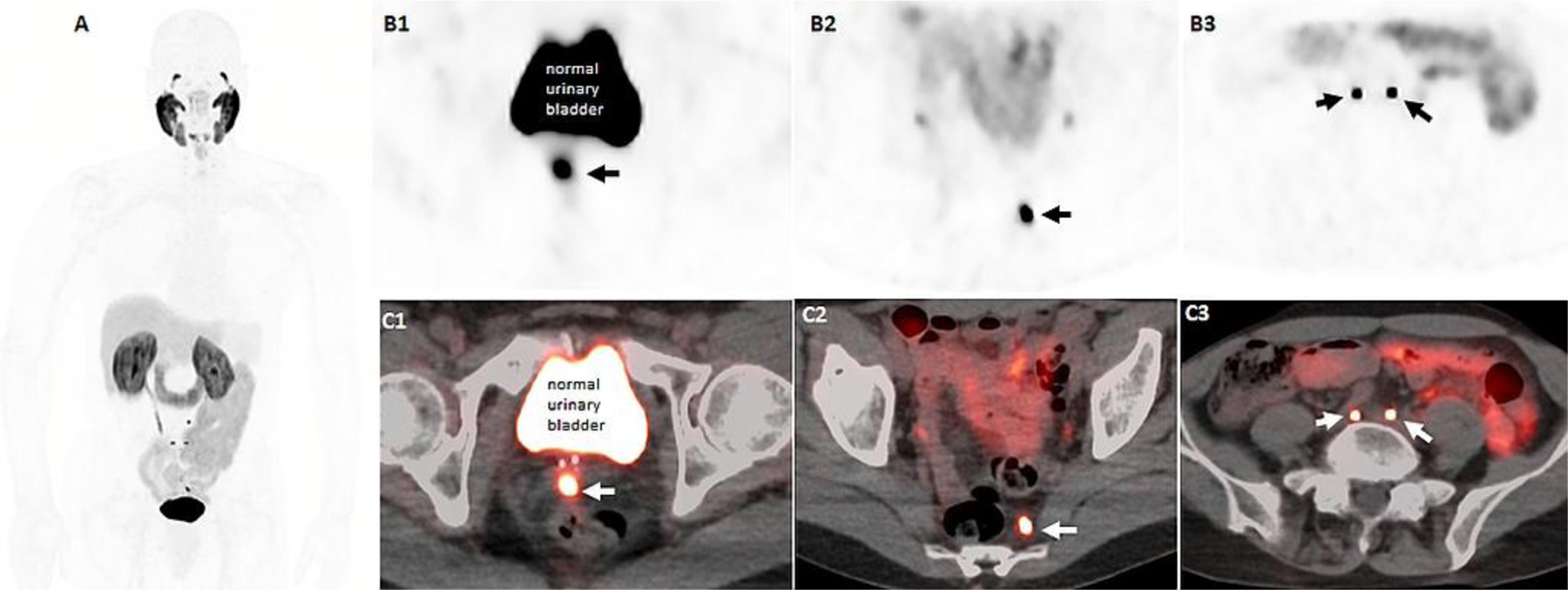
64-years-old man with history of prostate cancer, Gleason 9 (4+5), status post EBRT and 1 year of ADT with PSA nadir of 0.4ng/ml, and recent rising PSA of 2.86ng/ml. 18F-DCFPYL PET/CT imaging, including maximal intensity projection (A), axial PET (B row) and axial fused PET/CT (C row) images demonstrate an intense DCFPYL avid focus at the midline of the seminal sevicles (B1, C1) and several subcentimeter pelvis lymph nodes including left presacral (B2, C2) and bilateral common iliac nodes (B2, C3).
Figure 5:
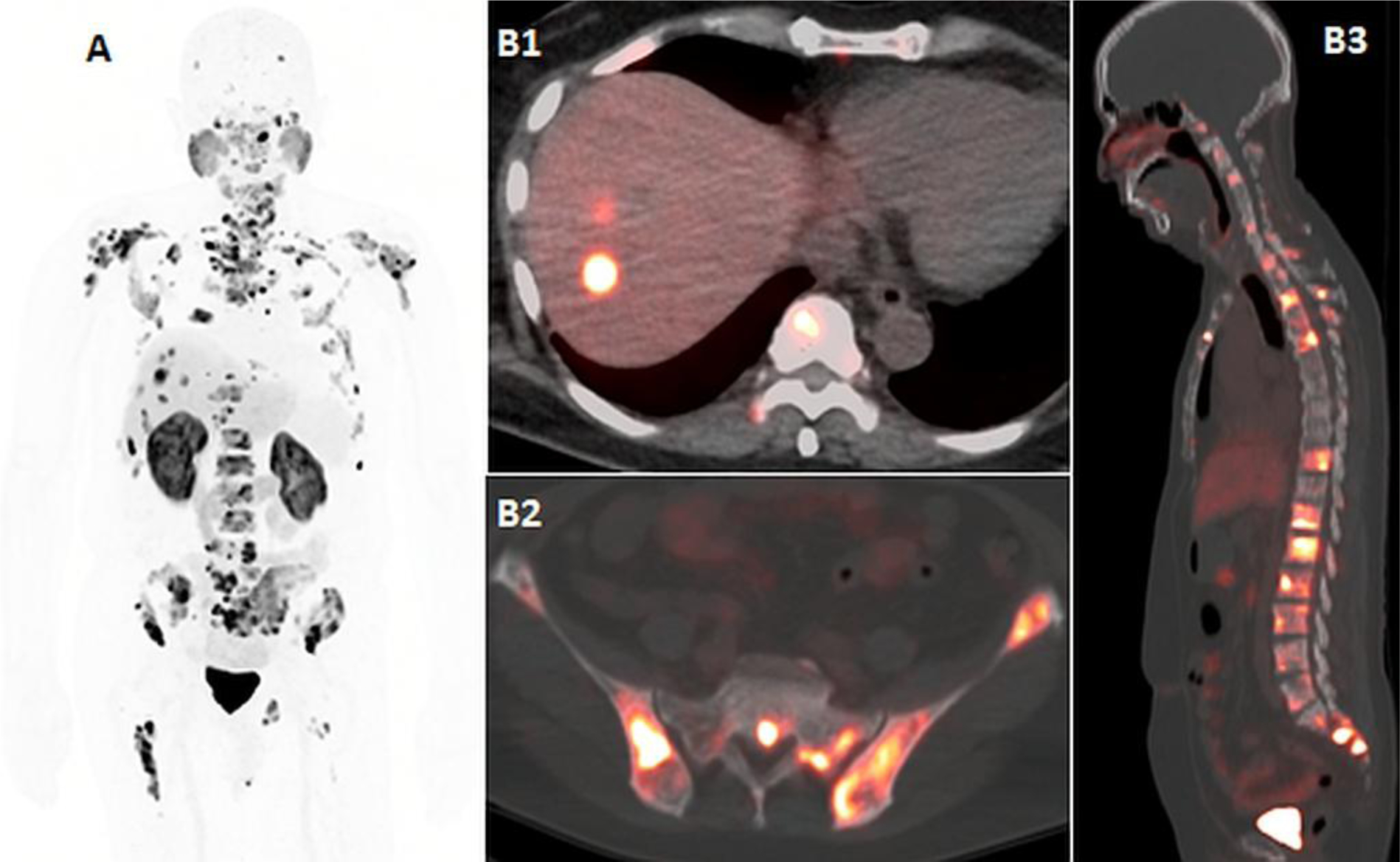
62-year-old man with metastatic castrate resistant prostate cancer, and PSA of 134ng/ml. 18F-DCFPYL imaging, including maximal intensity projection (A), and axial and sagittal fused PET/CT images (B1, B2, B3) demonstrate liver metastases (B1) and wide-spread osseous metastatic disease (A, B2, B3).
In the setting of salvage lymph node resection, PSMA PET/CT can be very useful in guiding surgical assessment (58,64–66). PSMA PET/CT has also been used to guide surgical resection using hand held gamma probes to detect positive nodes. For instance, experimentally, a gamma probe was used in conjunction with 111In-PSMA to facilitate node sampling (67,68). PSMA PET imaging has also shown promise for guiding salvage radiation therapy (RT) (31,69) When a PSMA PET/CT was obtained prior to RT, a change in radiotherapy planning occurred in 20–60% of patients (70,71). PSMA PET obtained after RT can identify sites of residual disease (72), which allows for potential re-irradiation if feasible. In the setting of BCR, a negative PSMA PET scan is associated with favorable RT response compared with patients with a positive scan (73).
PSMA PET imaging has proven more effective than other PET probes heretofore developed. In two studies comparing 68Ga-labeled PSMA-11 ligand to 18F-fluoromethylcholine, the PSMA probe had a higher sensitivity (86% vs 70% and 66% vs 29%) in both and was seen at all PSA strata (74,75). In a similar study of lymphadenectomy specimens (therefore, verified histologically) PSMA PET demonstrated significantly better accuracy and higher negative predictive value than choline-PET (92 vs 83% and 97 vs 89%, respectively) (76). In one study (77) comparing FDG and PSMA PET imaging in patients with advanced metastatic disease (mCRPC) it was shown that discordant studies (FDG positive PSMA negative) were associated with a very poor prognosis.
Some PSMA PET agents are labeled via a chelate with 68Ga while others are directly labeled with 18F. Importantly, in both cases the binding site to PSMA is identical. 68Ga has a shorter half-life (68 min.) and higher positron energies resulting in less sharpness compared to 18F with a 110 min. half-life and lower positron energies. 18F-labeled PSMA agents have advantages of central production and distribution which enables smaller local hospitals without radiopharmacies to offer PSMA PET to their patients. In contrast, 68Ga-labeled PSMA agents require onsite 68Ge/68Ga generators and expertise in radiolabeling thereby favoring hospitals with more resources. One advantage of 68Ga PSMA compounds is that they include a chelate which can also bind therapeutic isotopes such as 177Lu or 225Ac thus creating a theragnostic agent. Only a few studies have been published compare different radiolabeled PSMA ligands in the same patients. In one study 18F-DCFPyL PSMA PET imaging detected 36% more lesions and had a higher sensitivity (88% vs. 66%) for PSA values > 0.5 ng/mL (78) but the study was small and the doses of the agents were not equivalent.
In one prospective, multicenter study 68Ga-PSMA PET/CT scan led to a change in management in 51% of patients with highest impact in the group of patients with biochemical failure vs. those undergoing primary staging (62 vs 21% change) (78) Similar high clinical impact of 68Ga-PSMA/PET was reported in other several retrospective, single-institution studies (70,80, 81), where the rate of management change ranged from 42–76% (70,82,83). However, clinicians should be aware that PSMA PET scans are likely to lead to more complicated workups.
Although PSMA PET/CT agents are found to alter the patients’ clinical management, the impact of enhanced detection on overall survival is yet to be proven. Prospective trials evaluating patient outcome are clearly needed. This is especially important in patients at the very early stages of biochemical recurrence, who would ordinarily be missed with conventional imaging.
Emerging PET radiotracers
18F-Fluorodihydrotestosterone (18F-FDHT)
In addition to the agents previously discussed there are a number of agents that have been developed and used in patients but in small numbers and at limited sites. One of these, 16beta-18F-fluoro-5 alpha-dihydrotestoterone (18F-FDHT) targets the androgen receptor (AR) with radiolabeled dihydrotestosterone. AR plays an important role in growth of PCa since it is a transcription factor that influences many other genes including the genes responsible for PSA. Dihydrotestosterone binds to AR in the cytoplasm mediating its migration to the nucleus of the cell and causing downstream genes to be activated. As the disease progresses the cancer cell may become “androgen independent” especially in the castration resistant (CRPC) phase when the tumor no longer responds to androgen deprivation therapy (ADT). AR still plays a role in CRPC but is often independent of androgen binding by this point. Thus, AR-targeted imaging can play a role in assessing whether androgens can still bind to the AR. 18F-FDHT is a radiolabeled analogue of dihydrotestosterone that has been tested mostly in the context of the development of novel second generation anti-androgen therapies, specifically enzalutamide, which blocks DHT binding to AR (84,85). Decreases in uptake of 18F-FDHT suggest a positive pharmacodynamic effect by enzalutamide and other DHT binding site inhibitors (86). To date, only a few sites around the world produce 18F-FDHT. Larson et al. (87) first developed 18F-FDHT as a method of detecting prostate cancer metastases but it had a lower sensitivity than 18F-FDG (78% vs 97%) in late stage patients. This study actually showed the value of FDG PET in this setting. When 18F-FDHT and 18F-FDG were compared in the same patients (88) with mCRPC, it was initially found that patients whose lesions showed higher 18F-FDHT uptake had significantly shorter overall survival whereas 18F-FDG uptake was not associated with overall survival. However, when a larger group of mCRPC patients were compared (89) it was found that patients with mostly concordant 18F-FDHT and 18F-FDG uptake were found to have the best survival rates, whereas patients whose disease manifested as 18F-FDG >> 18F-FDHT-had the poorest prognosis, reflecting the increased metabolism of late stage prostate cancer (89). 18F-FDHT remains part of the conversation regarding PCa imaging agents as it is the only one that informs about the status of the AR, which plays a central role in PCa but so far has not offered the sensitivity of other agents in detecting recurrent and metastatic disease. It certainly has increased understanding of the dynamics occurring within metastatic PCa cells.
Bombesin and Gastrin Releasing Peptide Receptor Ligands
Another potential target for imaging is the gastrin releasing peptide receptor (GRPR). Bombesin (derived from a rain forest frog) is a natural 14-amino acid peptide, that can be radiolabeled with 68Ga, 64Cu, or 18F, binds to GRPR as a receptor agonist (90,91). GRP receptor antagonists have shown more favorable imaging characteristics with higher tumor to background ratios, without inducing adverse effects (92), and are more sensitive than GRP receptor agonists (93–95). GRPR is reported to be overexpressed in 63–100% of primary PCa lesions, and in 50–85% of nodal and osseous metastases (96,97). By contrast, GRPR is expressed at low levels in benign prostatic hyperplasia and normal prostate tissue (98).
68Ga-RM2 (previously known as BAY 86–7548) is the agent most widely reported upon. It demonstrates a sensitivity, specificity, and accuracy of 89%, 81%, and 83 %, respectively for the detection of primary PCa, and a sensitivity of 70% for nodal metastases using histology as the gold standard; however, it failed to detect skeletal disease in a hormone-refractory patient and revealed 6 false positive foci due to BPH (98). In the setting of BCR, 68Ga-RM2 PET/MRI detected recurrent PCa in 71% of the patients, whereas MRI identified findings compatible with recurrent PCa in only 34% of the patients (99,100). Results were not broken down by PSA strata so direct comparison with other agents is difficult. When comparing 68Ga-RM2 and 68Ga-PSMA-11 PET in a small pilot study of only 7 patients with BCR the two scans had similar results overall, but the patterns of uptake were different (101), possibly reflecting differing aspects of tumor biology worthy of further study. In a comparison of 68Ga-RM2 and 18F-fluoroethylcholine (18F-ECH) PET/CT in 16 patients with BCR 68Ga-RM2 PET/ CT localized PCa recurrence in 62.5% (10/16) of the patients in which the choline scan was negative. One flaw was that the median PSA at the time of 18F-ECH PET/CT was lower than that at the time of 68Ga-RM2 PET/CT (2.4 vs 5.5 ng/ml, respectively) biasing in favor of the 68Ga-RM2 scan. Another GRP receptor-targeting PET radiopharmaceutical, 68Ga-SB3 identified lesions in 5 of 9 patients (55%) with PCa (102). An improved version of this radiopharmaceutical, 68Ga-NeoBOMB1, is being developed (103). While radiolabeled bombesin receptor antagonists are showing encouraging results they clearly must be compared to PSMA PET, the current gold standard. It may be possible that these two agents together cover a larger percentage of patients than either alone but this remains to be proven. If true, it could have implications not only for imaging but also for combination targeted radionuclide therapy of PCa.
Other GRPR antagonists, 68Ga-RM26, and 68Ga-BBN have been developed; 68Ga-RM26 PET/CT was superior to 68Ga-BBN identifying more patients and showing a positive correlation between uptake and GRPR expression(99). Only a small early clinical experience is available for a fluorinated bombesin PET radiotracer, 18F-BAY 86–4367 and there are insufficient results to assess(91). A 64Cu labeled GRP receptor antagonist, 64Cu-CB-TE2A-AR-06, has also shown promising preliminary results, successfully detecting primary tumors in 3 out of 4 patients with newly diagnosed PCa (90).
Urokinase Plasminogen Activator
The serine protease urokinase-type plasminogen activator (uPA) and its receptor (uPAR) have been shown to be up-regulated in a variety of human cancers, including PCa (104), and its presence is associated with advanced disease and poor prognosis (105). Plasma uPAR levels correlate with early progression (105). Radiolabeled uPAR agents with 64Cu, 68Ga, and 18F have been developed and used for PET imaging in human xenograft prostate cancer models (106,107) and only recently, a first-in-human phase I clinical trial was conducted with 64Cu-DOTA-AE105, a radiolabeled chelated small peptide ligand of the uPAR receptor (107,108). These preliminary results are encouraging and support larger scale clinical trials to determine the potential role of uPAR PET in PCa.
Other PET Agents
It is possible that additional experimental tracers will play a role in the future. Among those with preliminary data are 64Cu-TP3805 which targets the VPAC1 receptor, a G-protein coupled receptor that is overexpressed in PCa (109). The cell surface protein 6 transmembrane epithelial antigen of prostate 1 (STEAP1) has also been identified as a target for castration-resistant PCa and antibody imaging against this antigen has been developed using the PET radiotracer, 89Zr-2109A. The STEAP1 antibody was derived from a fully humanized monoclonal antibody targeting STEAP1, and was tested as an imaging agent to measure changes in STEAP1 expression in a preclinical castration-resistant PCa model. 89Zr-2109A was able to localize STEAP1-positive human PCa models, and sensitively measured treatment-induced changes in STEAP1 expression (110). Although these new tracers are promising, much work is needed before they can even be considered for wider clinical use.
Conclusion
During the past two decades, prostate cancer has provided fertile ground for the development of novel PET tracers. Novel imaging agents have allowed for the identification of foci of PCa, particularly in the setting of early biochemical recurrence where the PSA detects small amounts of disease but unfortunately is not able to localize it. To date, PSMA-based PET has shown the best performance among all the candidate probes and has rapidly been adopted across the world. It has also been extended as a targeted radionuclide therapy by adding therapeutic radioisotopes to the PSMA-binding ligand. With this encouraging data, the PSMA-based radiolabelled ligands are likely to become universally available in clinical practice for imaging PCa in the near future. Where they are widely available now they are avidly used by clinicians. In coming years, large and well-defined prospective trials will be needed to understand the impact of PSMA-based PET on the outcomes of patients with PCa.
Table 1.
Comparison of PET tracers in prostate cancer
| PET Agent | Advantages | Disadvantages | Comments |
|---|---|---|---|
| 11C or 18F Choline | Min urinary excretion Availability | Lower sensitivity Non specific uptake | 11C Choi ine approved by US FDA |
| 18F FACBC | Improved sensitivity Local recurrence | Lower sensitivity Non specific uptake | 18FACBC is approved by US FDA (Axumin) |
| 68Ga or 18F PSMA | High sensitivity High specificity | False positive uptake | Soon to be available in US, Current gold standard |
| 18F DHT | Reports AR activity | Difficult synthesis Noisy scans | Not commercially available |
| 68Ga or 18F Bombesin | High sensitivity | False positive uptake | Not commercially available |
| 18F FDG | Prognostic indicator Widely available | Insensitive in early dz. | 18F FDG is approved by US FDA |
Funding
This review received no outside funding support.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests
Dr. Soroush Rais-Bahrami has received research funding from Genomic Health Inc, Blue Earth Diagnostics, and Astellas. Soroush Rais-Bahrami serves as a consultant for Philips/InVivo Corp, Blue Earth Diagnostics, Genomic Health Inc, Intuitive Surgical, and Bayer Healthcare. Other authors have no disclosures.
Research involving human participants and/or animals: N/A
Informed consent: N/A
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 2.Cimitan M, Bortolus R, Morassut S, et al. [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33(12):1387–1398. [DOI] [PubMed] [Google Scholar]
- 3.Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med. 2006;47(2):262–269. [PubMed] [Google Scholar]
- 4.Jadvar H Prostate cancer: PET with 18F-FDG, 18F-or 11C-acetate, and 18F-or 11C-choline. J Nucl Med. 2011;52(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy M, Siew T, Campbell A, et al. (1)(8)F-Fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: prospective comparison with standard imaging. Eur J Nucl Med Mol Imaging. 2011;38(1):14–22. [DOI] [PubMed] [Google Scholar]
- 6.Piccardo A, Paparo F, Piccazzo R, et al. Value of fused 18F-Choline-PET/MRI to evaluate prostate cancer relapse in patients showing biochemical recurrence after EBRT: preliminary results. Biomed Res Int. 2014;2014:103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanti S, Minozzi S, Castellucci P, et al. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43(1):55–69. [DOI] [PubMed] [Google Scholar]
- 8.Graziani T, Ceci F, Castellucci P, et al. (11)C-Choline PET/CT for restaging prostate cancer. Results from 4,426 scans in a single-centre patient series. Eur J Nucl Med Mol Imaging. 2016;43(11):1971–1979. [DOI] [PubMed] [Google Scholar]
- 9.Ceci F, Castellucci P, Mamede M, et al. (11)C-Choline PET/CT in patients with hormone-resistant prostate cancer showing biochemical relapse after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2013;40(2):149–155. [DOI] [PubMed] [Google Scholar]
- 10.Soyka JD, Muster MA, Schmid DT, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39(6):936–943. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein J, Even-Sapir E, Ben-Haim S, et al. Does Choline PET/CT Change the Management of Prostate Cancer Patients With Biochemical Failure? Am J Clin Oncol. 2017; 40:256–259. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Wang L, Hu J, Feng D, Xu L. Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis. PLoS One. 2018;13(9):e0203400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka S, Hattori R, Kurosaki F, et al. A preliminary study of anti-1-amino-3–18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med. 2007;48(1):46–55. [PubMed] [Google Scholar]
- 15.Asano Y, Inoue Y, Ikeda Y, et al. Phase I clinical study of NMK36: a new PET tracer with the synthetic amino acid analogue anti-[18F]FACBC. Ann Nucl Med. 2011;25(6):414–418. [DOI] [PubMed] [Google Scholar]
- 16.Nanni C, Schiavina R, Rubello D, et al. The detection of disease relapse after radical treatment for prostate cancer: is anti-3–18F-FACBC PET/CT a promising option? Nucl Med Commun. 2013;34(9):831–833. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol. 2016;57(4):487–493. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Inoue Y, Fujimoto H, et al. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate cancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol. 2016;46(2):152–162. [DOI] [PubMed] [Google Scholar]
- 19.Schuster DM, Taleghani PA, Nieh PT, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3(1):85–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Jambor I, Kuisma A, Kahkonen E, et al. Prospective evaluation of (18)F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate-to high-risk prostate cancer patients (FLUCIPRO trial). Eur J Nucl Med Mol Imaging. 2018;45:355–364. [DOI] [PubMed] [Google Scholar]
- 21.Turkbey B, Mena E, Shih J, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol. 2017;197(3 Pt 1):676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43(10):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin Nucl Med. 2017;42(1):e22–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andriole GL, Kostakoglu L, Chau A, et al. The Impact of Positron Emission Tomography with (18)F-Fluciclovine on the Management of Patients with Biochemical Recurrence of Prostate Cancer: Results from the LOCATE Trial. J Urol. 2018. [DOI] [PMC free article] [PubMed]
- 27.Nanni C, Zanoni L, Pultrone C, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43(9):1601–1610. [DOI] [PubMed] [Google Scholar]
- 28.Nanni C, Schiavina R, Brunocilla E, et al. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin Nucl Med. 2015;40(8):e386–391. [DOI] [PubMed] [Google Scholar]
- 29.Schuster DM, Nanni C, Fanti S, et al. Anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med. 2014;55(12):1986–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesapane F, Czarniecki M, Suter MB, Turkbey B, Villeirs G. Imaging of distant metastases of prostate cancer. Med Oncol. 2018;35(11):148. [DOI] [PubMed] [Google Scholar]
- 31.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J Nucl Med. 2018;59(5):789–794. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 33.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–2261. [DOI] [PubMed] [Google Scholar]
- 34.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–172. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9(17):6357–6362. [PubMed] [Google Scholar]
- 36.Fendler WP, Eiber M, Beheshti M, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–1024. [DOI] [PubMed] [Google Scholar]
- 37.Malik D, Kumar R, Mittal BR, Singh H, Bhattacharya A, Singh SK. 68Ga-Labeled PSMA Uptake in Nonprostatic Malignancies: Has the Time Come to Remove “PS” From PSMA? Clin Nucl Med. 2018;43(7):529–532. [DOI] [PubMed] [Google Scholar]
- 38.Sodee DB, Malguria N, Faulhaber P, Resnick MI, Albert J, Bakale G. Multicenter ProstaScint imaging findings in 2154 patients with prostate cancer. The ProstaScint Imaging Centers. Urology. 2000;56(6):988–993. [DOI] [PubMed] [Google Scholar]
- 39.Pandit-Taskar N, O’Donoghue JA, Durack JC, et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin Cancer Res. 2015;21(23):5277–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of [(68)Ga]PSMA-HBED-CC in Patients with Prostate Cancer: Characterization of Uptake in Normal Organs and Tumour Lesions. Mol Imaging Biol. 2016;18:428–436. [DOI] [PubMed] [Google Scholar]
- 41.Giesel FL, Sterzing F, Schlemmer HP, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(8):1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol. 2016;70(5):829–836. [DOI] [PubMed] [Google Scholar]
- 43.Zamboglou C, Drendel V, Jilg CA, et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7(1):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uprimny C, Kroiss AS, Decristoforo C, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44(6):941–949. [DOI] [PubMed] [Google Scholar]
- 45.Rowe SP, Gage KL, Faraj SF, et al. (1)(8)F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J Nucl Med. 2015;56(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turkbey B, Mena E, Lindenberg L, et al. 18F-DCFBC Prostate-Specific Membrane Antigen-Targeted PET/CT Imaging in Localized Prostate Cancer: Correlation With Multiparametric MRI and Histopathology. Clin Nucl Med. 2017;42(10):735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fendler WP, Schmidt DF, Wenter V, et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J Nucl Med. 2016;57(11):1720–1725. [DOI] [PubMed] [Google Scholar]
- 48.Zamboglou C, Schiller F, Fechter T, et al. (68)Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics. 2016;6(10):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. [DOI] [PubMed] [Google Scholar]
- 50.Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol. 2016;195(5):1436–1443. [DOI] [PubMed] [Google Scholar]
- 51.Kim SJ, Lee SW, Ha HK. Diagnostic Performance of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging in Newly Diagnosed Intermediate to High-Risk Prostate Cancer Patients: A Systematic Review and Meta-Analysis. Urol Int. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 52.Herlemann A, Wenter V, Kretschmer A, et al. (68)Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur Urol. 2016;70(4):553–557. [DOI] [PubMed] [Google Scholar]
- 53.Budaus L, Leyh-Bannurah SR, Salomon G, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol. 2016;69:393–396. [DOI] [PubMed] [Google Scholar]
- 54.Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ. (68) Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging. 2017. [DOI] [PubMed]
- 55.Janssen JC, Woythal N, Meissner S, et al. [(68)Ga]PSMA-HBED-CC Uptake in Osteolytic, Osteoblastic, and Bone Marrow Metastases of Prostate Cancer Patients. Mol Imaging Biol. 2017;19(6):933–943. [DOI] [PubMed] [Google Scholar]
- 56.King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012;84(1):104–111. [DOI] [PubMed] [Google Scholar]
- 57.Giesel FL, Fiedler H, Stefanova M, et al. PSMA PET/CT with Glu-urea-Lys-(Ahx)-[(6)(8)Ga(HBED-CC)] versus 3D CT volumetric lymph node assessment in recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(12):1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J Nucl Med. 2016;57(11):1713–1719. [DOI] [PubMed] [Google Scholar]
- 59.Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70(6):926–937. [DOI] [PubMed] [Google Scholar]
- 60.von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. (68)Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2016. [DOI] [PubMed]
- 61.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ceci F, Castellucci P, Graziani T, et al. (68)Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med Mol Imaging. 2018. [DOI] [PubMed]
- 63.Verburg FA, Pfister D, Heidenreich A, et al. Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging. 2016;43:397–403. [DOI] [PubMed] [Google Scholar]
- 64.Jilg CA, Drendel V, Rischke HC, et al. Diagnostic Accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics. 2017;7(6):1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarebrough E, Duncan C, Christidis D, Lavoipierre A, Lawrentschuk N. PSMA-PET guided hook-wire localization of nodal metastases in prostate cancer: a targeted approach. World J Urol. 2018. [DOI] [PubMed]
- 66.Porres D, Pfister D, Thissen A, et al. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis. 2017;20(1):85–92. [DOI] [PubMed] [Google Scholar]
- 67.Schottelius M, Wirtz M, Eiber M, Maurer T, Wester HJ. [(111)In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siriwardana A, Thompson J, van Leeuwen PJ, et al. Initial multicentre experience of (68) gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int. 2017;120(5):673–681. [DOI] [PubMed] [Google Scholar]
- 69.Guler OC, Engels B, Onal C, et al. The feasibility of prostate-specific membrane antigen positron emission tomography(PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin Transl Oncol. 2018;20(4):484–490. [DOI] [PubMed] [Google Scholar]
- 70.Bluemel C, Linke F, Herrmann K, et al. Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016;6(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calais J, Czernin J, Cao M, et al. (68)Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients with a PSA Level of Less Than 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J Nucl Med. 2018;59(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hruby G, Eade T, Kneebone A, et al. Delineating biochemical failure with (68)Ga-PSMA-PET following definitive external beam radiation treatment for prostate cancer. Radiother Oncol. 2017;122(1):99–102. [DOI] [PubMed] [Google Scholar]
- 73.Emmett L, van Leeuwen PJ, Nandurkar R, et al. Treatment Outcomes from (68)Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J Nucl Med. 2017;58(12):1972–1976. [DOI] [PubMed] [Google Scholar]
- 74.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J Nucl Med. 2015;56(8):1185–1190. [DOI] [PubMed] [Google Scholar]
- 76.Pfister D, Porres D, Heidenreich A, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(8):1410–1417. [DOI] [PubMed] [Google Scholar]
- 77.Hofman MS, Violet J, Hicks RJ, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–833. [DOI] [PubMed] [Google Scholar]
- 78.Dietlein F, Kobe C, Neubauer S, et al. PSA-Stratified Performance of (18)F-and (68)Ga-PSMA PET in Patients with Biochemical Recurrence of Prostate Cancer. J Nucl Med. 2017;58(6):947–952. [DOI] [PubMed] [Google Scholar]
- 79.Roach PJ, Francis R, Emmett L, et al. The Impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J Nucl Med. 2018;59(1):82–88. [DOI] [PubMed] [Google Scholar]
- 80.Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat Oncol. 2015;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dewes S, Schiller K, Sauter K, et al. Integration of (68)Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiat Oncol. 2016;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sterzing F, Kratochwil C, Fiedler H, et al. (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albisinni S, Artigas C, Aoun F, et al. Clinical impact of (68) Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120(2):197–203. [DOI] [PubMed] [Google Scholar]
- 84.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51(2):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31(28):3525–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344–350. [DOI] [PubMed] [Google Scholar]
- 87.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 88.Vargas HA, Wassberg C, Fox JJ, et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271(1):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox JJ, Gavane SC, Blanc-Autran E, et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgen Receptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2018;4(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wieser G, Mansi R, Grosu AL, et al. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist--from mice to men. Theranostics. 2014;4(4):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sah BR, Burger IA, Schibli R, et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J Nucl Med. 2015;56:372–378. [DOI] [PubMed] [Google Scholar]
- 92.Roivainen A, Kahkonen E, Luoto P, et al. Plasma pharmacokinetics, whole-body distribution, metabolism, and radiation dosimetry of 68Ga bombesin antagonist BAY 86–7548 in healthy men. J Nucl Med. 2013;54(6):867–872. [DOI] [PubMed] [Google Scholar]
- 93.Cescato R, Maina T, Nock B, et al. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49(2):318–326. [DOI] [PubMed] [Google Scholar]
- 94.Baratto L, Jadvar H, Iagaru A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol Imaging Biol. 2018;20(4):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansi R, Wang X, Forrer F, et al. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin Cancer Res. 2009;15(16):5240–5249. [DOI] [PubMed] [Google Scholar]
- 96.Mansi R, Fleischmann A, Macke HR, Reubi JC. Targeting GRPR in urological cancers--from basic research to clinical application. Nat Rev Urol. 2013;10(4):235–244. [DOI] [PubMed] [Google Scholar]
- 97.Chatalic KL, Franssen GM, van Weerden WM, et al. Preclinical comparison of Al18F-and 68Ga-labeled gastrin-releasing peptide receptor antagonists for PET imaging of prostate cancer. J Nucl Med. 2014;55(12):2050–2056. [DOI] [PubMed] [Google Scholar]
- 98.Kahkonen E, Jambor I, Kemppainen J, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86–7548. Clin Cancer Res. 2013;19(19):5434–5443. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Niu G, Fan X, et al. PET using a GRPR Antagonist 68Ga-RM26 in Healthy Volunteers and Prostate Cancer Patients. J Nucl Med 218; 59(6):922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Minamimoto R, Sonni I, Hancock S, et al. Prospective Evaluation of (68)Ga-RM2 PET/MRI in Patients with Biochemical Recurrence of Prostate Cancer and Negative Findings on Conventional Imaging. J Nucl Med. 2018;59(5):803–808. [DOI] [PubMed] [Google Scholar]
- 101.Minamimoto R, Hancock S, Schneider B, et al. Pilot Comparison of (6)(8)Ga-RM2 PET and (6)(8)Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J Nucl Med. 2016;57(4):557–562. [DOI] [PubMed] [Google Scholar]
- 102.Maina T, Bergsma H, Kulkarni HR, et al. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [(6)(8)Ga]SB3 and PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(5):964–973. [DOI] [PubMed] [Google Scholar]
- 103.Nock BA, Kaloudi A, Lymperis E, et al. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J Nucl Med. 2017;58(1):75–80. [DOI] [PubMed] [Google Scholar]
- 104.Rasch MG, Lund IK, Almasi CE, Hoyer-Hansen G. Intact and cleaved uPAR forms: diagnostic and prognostic value in cancer. Front Biosci. 2008;13:6752–6762. [DOI] [PubMed] [Google Scholar]
- 105.Shariat SF, Roehrborn CG, McConnell JD, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25(4):349–355. [DOI] [PubMed] [Google Scholar]
- 106.Persson M, Liu H, Madsen J, Cheng Z, Kjaer A. First (18)F-labeled ligand for PET imaging of uPAR: in vivo studies in human prostate cancer xenografts. Nucl Med Biol. 2013;40(5):618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Persson M, Skovgaard D, Brandt-Larsen M, et al. First-in-human uPAR PET: Imaging of Cancer Aggressiveness. Theranostics. 2015;5(12):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skovgaard D, Persson M, Brandt-Larsen M, et al. Safety, Dosimetry, and Tumor Detection Ability of (68)Ga-NOTA-AE105: First-in-Human Study of a Novel Radioligand for uPAR PET Imaging. J Nucl Med. 2017;58:379–386. [DOI] [PubMed] [Google Scholar]
- 109.Tripathi S, Trabulsi EJ, Gomella L, et al. VPAC1 Targeted (64)Cu-TP3805 Positron Emission Tomography Imaging of Prostate Cancer: Preliminary Evaluation in Man. Urology. 2016;88:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doran MG, Watson PA, Cheal SM, et al. Annotating STEAP1 regulation in prostate cancer with 89Zr immuno-PET. J Nucl Med. 2014;55(12):2045–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]


