Abstract
Objective or Purpose:
Report factors associated with failure and complication in a cohort of children who have undergone glaucoma drainage device (GDD) implantation.
Design:
Retrospective case series.
Subjects, Participants, and/or Controls:
Consecutive pediatric GDD eyes that met criteria between May 1997 and July 2019.
Methods, Intervention, or Testing:
Entries were included for analysis if age < 18 years at time of GDD implantation. Failure was defined as an intraocular pressure (IOP) greater than 21mmHg or IOP reduction < 20% or IOP < 5mmHg at 2 consecutive follow-up visits > 3 months after implantation, reoperation for glaucoma, or loss of light perception vision. Complications were defined as postoperative events attributed to the GDD that required additional examination under anesthesia or additional surgery.
Main Outcome Measures:
Survival analyses of failures and complications following surgery.
Results:
Over a mean follow-up period of 5.4 years, 58 (38%) of 150 first-time GDD implants failed. Glaucoma associated with acquired conditions had decreased risk for failure when compared to glaucoma secondary to non-acquired systemic diseases (HR=12, p=0.0063), non-acquired ocular anomalies (HR=12, p=0.0054), and primary congenital glaucoma (HR=5.4, p=0.041). There was also an increased risk of failure for first-time tubes in younger patients with a 23% reduction of failure with each 3-year increase in age (HR=0.77, p=0.034). 38 (25.3%) of the first-time GDD had a complication. Higher preoperative intraocular pressure (5 mmHg increase, HR=1.2, p=0.038) and younger age at time of implantation (< 3 years, HR=2.1, p=0.024; < 2 years, HR=1.9, p=0.046) increased the risk of complication. There were 22 second-time GDD implants in the study, of which 11 failed (50%), and increased risk for failure was associated with younger age at time of implantation (< 1 year, HR=27, p=0.0053); in subgroup analysis when compared to eyes with no concurrent surgery, additional glaucoma-related procedures with or without anterior segment surgery at the time of implantation (HR=13, p=0.0085).
Conclusion:
Although GDD implantation in children is relatively safe and effective, this data should be interpreted in the context of children’s relative longevity. These findings offer an outcome metric to which future novel glaucoma procedures in children can be compared.
Precis:
Pediatric tube failures and complications are influenced by age at time of surgery as well as other factors such as pre-operative intra-ocular pressure, etiology of glaucoma, and concurrent procedures at the time of implantation.
Introduction:
Glaucoma drainage devices (GDD) were first introduced in adult patients by Molteno in 1968.1 Shortly thereafter, they were adopted for pediatric patients and have since become part of the treatment for patients with disease refractory to angle surgery.2,3 Despite its long history, outcomes data in pediatric GDD are scarce. In the American Academy of Ophthalmology’s 2014 Ophthalmic Technology Assessment, they found no level I evidence in the field and only 10 studies including 393 eyes providing level II and level III evidence.4
This paucity of evidence results in highly variable treatment algorithms based heavily on anecdotes. To optimize surgical outcomes, delineating the factors which predict GDD failure and complication for both first-time GDD and second-time GDD eyes are key objectives. In this study, we report a large cohort of pediatric patients who underwent GDD implantation and evaluate the risk factors for complication and failure.
Methods:
Institutional Review Board/Ethics Committee approval was obtained for this study. In addition, this study protocol adhered to the tenets of the Declaration of Helsinki and complied with the Health Information Portability and Accountability Act. Potential patients were identified using Current Procedural Terminology codes for glaucoma drainage device implantation at the Bascom Palmer Eye Institute. Records from May 1997 to July 2019 were reviewed. Entries were included for analysis if aged <18 years at time of GDD implantation. Age, sex, race/ethnicity, eye laterality, preoperative IOP, concurrent procedures at time of GDD implantation, preoperative number of glaucoma medications, type of implant, tube location, type of glaucoma, and lens status were collected. Preoperative IOP was defined as the median of the three IOP measurements immediately prior to the procedure. The type of glaucoma was based on the Childhood Glaucoma Research Network classification system.5
“Failure” was defined, over two consecutive follow-up visits at three or more months after the procedure, as having intraocular pressure (IOP) greater than 21 mmHg, or an IOP reduction < 20% from baseline, or IOP < 5 mmHg; or any reoperation for glaucoma; or loss of light perception vision in accordance to the 2008 World Glaucoma Association consensus on defining surgical success.6 “Complications” were defined as postoperative events attributed to the GDD that required additional, unplanned examination under anesthesia and/or surgery.
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means and standard deviations. Time to failure was assessed with Cox proportional hazards regression, and Kaplan-Meier survival analysis was used to create survival curves. Association between outcomes of right and left eyes, for children who received bilateral first or second tubes was assessed with chi-square and Fisher Exact tests. All analyses were done using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). A p-value < 0.05 was considered statistically significant.
Results:
Our search algorithm resulted in 175 GDD implantations in 152 eyes of 119 patients: 150 eyes with first-time GDD implantation, 22 eyes with second time GDD implantation, one eye with third-time GDD implantation, and one eye that had two tubes placed at the same time. The data from the eye that had two tubes placed at the same time were excluded from the analyses, as were data from the third-time GDD implantation. The baseline characteristics (just prior to tube implantation) are summarized in Supplementary Table 1.
In all GDD implants that failed by IOP criteria with at least one medication at baseline, 15.0% had fewer medications at the time of failure. In those that failed due to NLP (four in first-time GDD and four in second-time GDD), four eyes lost vision due to inoperative retinal detachment (three with retinopathy prematurity that had undergone multiple vitreoretinal surgeries, one with Peters anomaly and required keratoprosthesis after failing multiple grafts). One eye became NLP due to trauma; one eye was enucleated due to intractable pain (with light perception vision at the visit prior to enucleation); and two eyes with Peters anomaly became phthisical after multiple failed corneal surgeries.
First-time GDD failure and complication survival analyses
In the 150 first-time GDD eyes, the gender distribution was nearly equal and patients predominantly self-identified as “Caucasian, Hispanic” (62, 41.3%; Supplementary Table 1). The most commonly placed GDD was a Baerveldt (Johnson and Johnson Vision, Santa Ana, CA; 112, 75.2%), followed by Ahmed (New World Medical, Rancho Cucamonga, CA; 37, 24.8%), and then Molteno (Molteno Ophthalmic Limited, Dunedin, New Zealand; 1, 0.7%). The most common location for tube placement was the anterior chamber (96, 64%) followed by pars plana (42, 28%) and sulcus (12, 8%). Primary congenital glaucoma (40, 26.7%) was the most common form of glaucoma represented in the sub-population of first-time GDD eyes, followed by glaucoma associated with acquired conditions (GAAC; 38, 25.3%), glaucoma following cataract surgery (31, 20.7%), and glaucoma associated with non-acquired ocular anomalies (22, 14.7%; Supplementary Table 1).
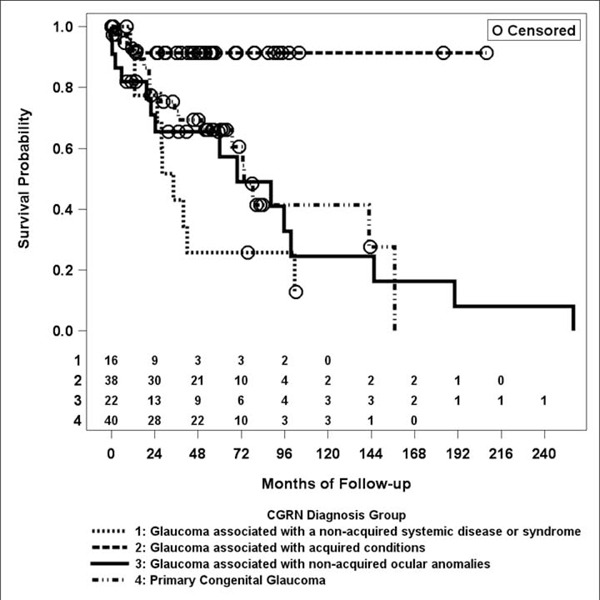
Of the 150 first-time GDD implants (150 eyes of 117 patients), 58 failed during the study period (38%). The mean follow-up duration was 5.4 +/− 4.1 years. The mean time from implantation to failure was 47 +/− 51 months (Supplementary Table 1). GAAC such as juvenile idiopathic arthritis and trauma had significantly decreased risk for failure when compared to glaucoma associated with non-acquired systemic diseases (HR = 12, 95% confidence interval (CI): 2.0, 74; p = 0.0063), non-acquired ocular anomalies (HR = 12, 95% CI: 2.0, 68; p = 0.0054), and primary congenital glaucoma (HR = 5.4, 95% CI: 1.1, 27; p = 0.041; Table 1, Figure 1). There was also a significantly increased risk for failure for first-time tubes in younger patients with a 23% reduction of failure with each 3-year increase in age (HR = 0.77, 95% CI: 0.61, 0.98; p = 0.034). No other factors were significantly associated with the risk of failure (Table 1). The single Molteno tube was excluded from statistical analysis of tube type comparisons due to low power.
Table 1.
Hazard Ratios (HR), 95% Confidence Intervals (CI), and P-values for first-time glaucoma drainage device (GDD) failures and complications as well as second-time GDD failures in pediatric eyes
| First-Time GDD Failure | First-Time GDD Complications | Second-Time GDD Failure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Groups Compared | Hazard Ratio | HR 95% CI | Hazard Ratio | HR 95% CI | Hazard Ratio | HR 95% CI | ||||||
| low | high | p - value | low | high | p - value | low | high | p - value | |||||
| Categorical Variables | HR is for a change from the first category to the second category | ||||||||||||
| Concurrent Procedure | NONE to Glaucoma-Related Surgery ± Anterior Segment Surgery | 1.2 | 0.18 | 7.5 | 0.88 | 0.50 | 0.066 | 3.7 | 0.50 | 13 | 1.9 | 93 | 0.0085** |
| Type of Glaucoma | Glaucoma associated with acquired condition vs. Glaucoma associated with non-acquired systemic disease or syndrome | 12 | 2.0 | 74 | 0.0063** | 0.71 | 0.18 | 2.9 | 0.63 | No Failures with diagnosis Glaucoma associated with acquired condition | |||
| Glaucoma associated with acquiredcondition vs. Glaucoma associated with non-acquired ocular anomalies | 12 | 2.0 | 68 | 0.0054** | 1.4 | 0.46 | 4.1 | 0.58 | No Failures with diagnosis Glaucoma associated with acquired condition | ||||
| Glaucoma associated with acquired condition vs. Primary Congenital Glaucoma | 5.4 | 1.1 | 27 | 0.041** | 1.2 | 0.43 | 3.2 | 0.77 | No Failures with diagnosis Glaucoma associated with acquired condition | ||||
| Type of Implant | Ahmed to Baerveldt | 0.82 | 0.33 | 2.0 | 0.66 | 1.4 | 0.62 | 3.4 | 0.40 | 1.6 | 0.13 | 20 | 0.72 |
| Age Less than 1 Year Old | Older to Younger Age | 1.6 | 0.67 | 3.8 | 0.29 | 1.7 | 0.89 | 3.3 | 0.10 | 27 | 2.7 | 27 × 10^1 | 0.0053** |
| Age Less than 2 Years Old | Older to Younger Age | 2.2 | 0.89 | 5.3 | 0.088 | 1.9 | 1.0 | 3.7 | 0.046** | 1.7 | 0.27 | 10 | 0.58 |
| Age Less than 3 Years Old | Older to Younger Age | 2.0 | 1.0 | 4.9 | 0.14 | 2.1 | 1.1 | 4.0 | 0.024** | 0.98 | 0.22 | 4.4 | 0.98 |
| Continuous Variables | Hazard Ratio is for this many units increase | ||||||||||||
| Age at Surgery | Unit = 3 Years | 0.77 | 0.61 | 0.98 | 0.034** | 0.87 | 0.73 | 1.0 | 0.14 | 0.94 | 0.61 | 1.5 | 0.78 |
| Pre-Operative IOP | Unit = 5 mmHg | 1.2 | 0.97 | 1.5 | 0.086 | 1.2 | 1.0 | 1.5 | 0.038** | 0.68 | 0.39 | 1.2 | 0.17 |
| Pre-Operative Number of Glaucoma Medications | Unit = 1 medication | 0.74 | 0.51 | 1.1 | 0.11 | 1.0 | 0.72 | 1.5 | 0.87 | 0.89 | 0.53 | 1.5 | 0.67 |
A p-value < 0.05 was considered statistically significant
The single Molteno tube in the study was removed from analyses comparing Ahmed to Baerveldt, but included elsewhere.
Figure 1.
Kaplan-Meier curve of failure rates in first-time glaucoma drainage device implant by Childhood Glaucoma Research Network diagnosis group.5
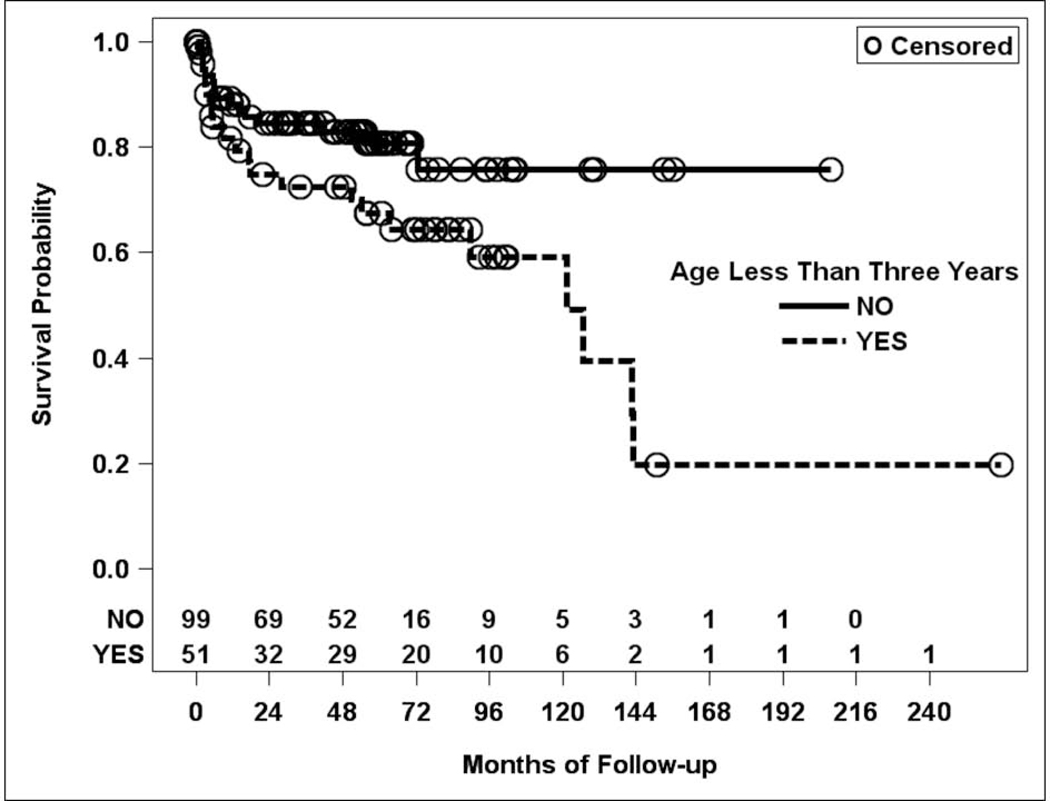
Of the 150 first-time GDD implants, 38 resulted in complications during the study period (25.3%). The mean time from implantation to complication was 31 +/− 42 months (Supplementary Table 1). The most common complication was tube malpositioning (10, 26.3%) followed by corneal decompensation (9, 23.7%), tube obstruction (7, 18.4%), tube exposure (4, 10.5%), and hypotony (4, 10.5%). Only one patient developed endophthalmitis (2.6%), which was sterile and non-infectious in etiology. The remaining complications were a flat anterior chamber (1, 2.6%), bleb complication (1, 2.6%), and tube exposure with concurrent supra choroidal hemorrhage (1, 2.6%). Higher preoperative IOP (every 5-mmHg increase) was associated with an increased risk of complication (HR = 1.2, 95% CI: 1.0, 1.28; p = 0.038). Similarly, being younger at the time of surgery was also associated with an increased risk of complications (younger than 3 years of age HR = 2.1, 95% CI: 1.1, 4.0; p = 0.024; younger than 2 years of age HR = 1.9, 95% CI: 1.0, 3.7; p = 0.046; Figure 2). No other factors were significantly associated with the risk of complication (a complete list of comparison is outlined in Supplementary Table 2).
Figure 2.
Kaplan-Meier curve of complication rates in first-time glaucoma drainage device implant by age group.
Second-time GDD failure survival analyses
There were 22 second-time GDD implants in the study. The mean follow-up duration was 5.5 +/−3.3 years. The mean time from implantation to failure was 35 +/− 30 months (Supplementary Table 1). There is a male (13, 59.1%) and self-reported “Caucasian, Hispanic” (10, 45.5%; Supplementary Table 1) predominance. The most commonly placed type of GDD was a Baerveldt (19, 86.4%), followed by an Ahmed (3, 13.6%). There were no Molteno tubes in this population. The most common location for tube placement was the anterior chamber (14, 63.6%) followed by pars plana (5, 22.7%), and then sulcus (3, 13.6%). Primary congenital glaucoma (8, 36.4%) and its subtypes were the most common form of glaucoma represented in this study sub-population followed by glaucoma associated with non-acquired systemic disease or syndrome (7, 31.8%; Supplementary Table 1).
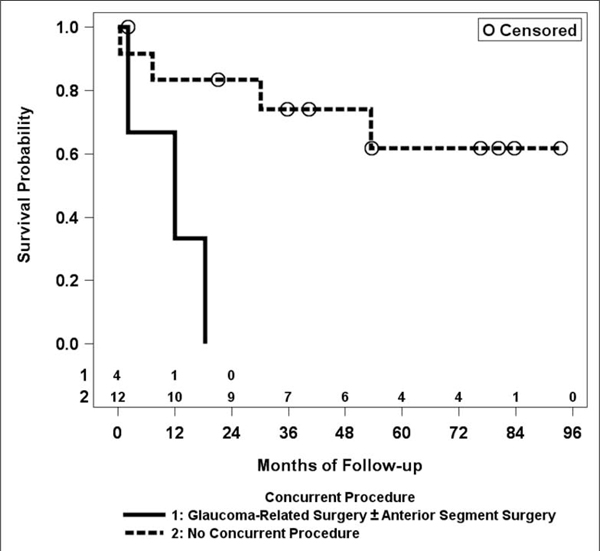
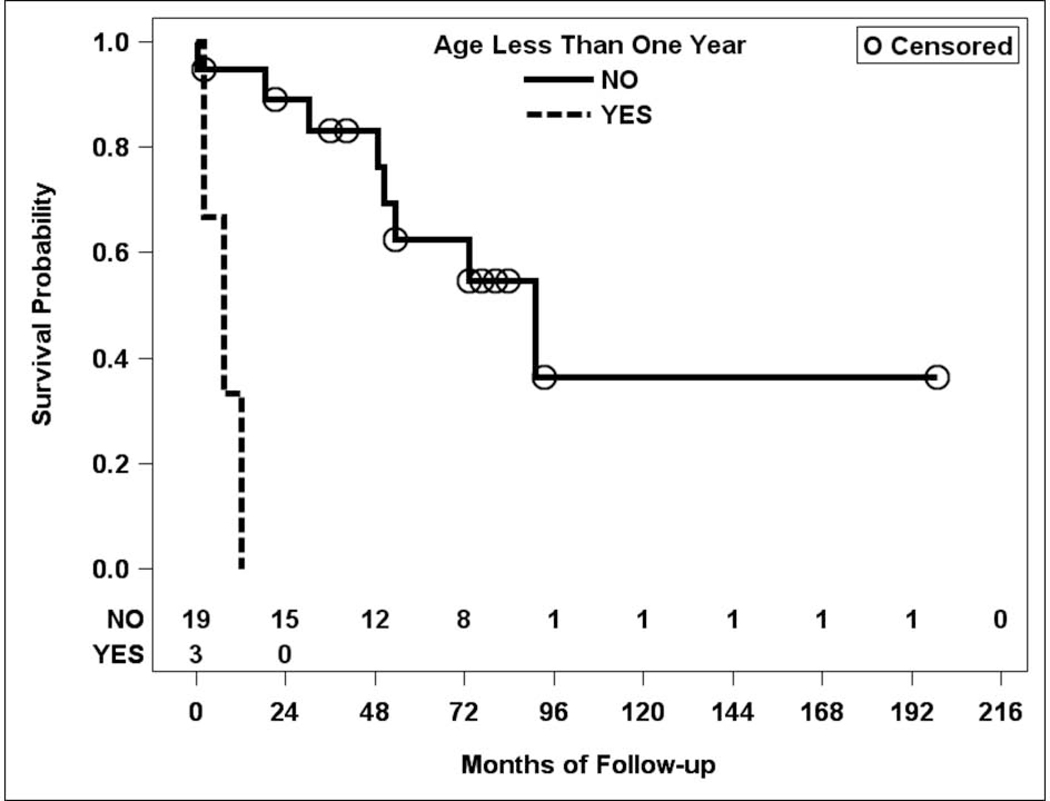
Of the 22 second-time GDD implants in the study, 11 failed (50%; Table 1). Sub-group analysis demonstrated an increased risk of failure if eyes had concurrent glaucoma-related surgery (such as repositioning another tube already present in the eye) +/− anterior segment surgery (such as cataract surgery) at the time of implantation, when compared to eyes that had no concurrent procedures at all (HR = 13, 95% CI: 1.9, 93; p = 0.0085, Figure 3). Younger age was also associated with an increased risk of failure (HR = 27 for age < 1 year, 95% CI: 2.6, 270; p =0.0053). No other factors were significantly associated with the risk of failure (Table 1, Figure 4). Due to low power, complication analysis was not performed for second-time GDD eyes.
Figure 3.
Kaplan-Meier curve for failure rate in second-time glaucoma drainage device (GDD) implant by whether concurrent glaucoma-related and/or other anterior segment surgeries were performed at the time of second GDD implantation.
Figure 4.
Kaplan-Meier curve for failure rate in second-time glaucoma drainage device implant by age group.
Concordance analysis between the two eyes of the same child
Thirty-three children had bilateral first tubes. There were 12 failures in the right eyes and 15 failures in the left eyes, with 11 children (33%) having failures in both eyes, 5 (15%) having a failure in one eye, and 17 (52%) having no failures (p = 0.0001, chi-square test), which indicates that there was significant association (concordance) between whether the eyes failed. Children with failure in one eye were significantly more likely to have failure in the other eye. There were 9 complications in the right eyes and 9 complications in the left eyes, with 4 (12%) children having complications in both eyes, 10 (30%) having a complication in one eye, and 19 (58%) having no complications (p = 0.2122, Fisher Exact test), so there was no significant association between whether the eyes had a complication.
Three children had bilateral second tubes. There were 2 failures in the right eyes and 1 failure in the left eyes. Neither children with failures in the right eye had failure in the left eyes, and the child with failure in the left eyes did not have failure in the right eye. There was no significant association between whether the eyes failed (p = 0.3333, Fisher Exact test).
Sensitivity analyses
Sensitivity analyses, in which the samples only included the initial tubes (i.e. inserted into the first eyes) of bilateral patients plus all tubes of the unilateral patients, were performed for the primary survival results. For the 117 initial, first-time GDD implants, the failure results were not significantly different from those in the primary analyses except that eyes aged less than two years (HR = 1.9; 95% CI:1.0, 3.6; p =0.046), eyes aged less than three years (HR = 2.0; 95% CI: 1.1, 3.9; p =0.031), and eyes with worse visual acuity at the time of surgery (HR = 2.0; 95% CI: 1.0, 4.1; p =0.048) had a significantly greater risk of failure. These age associations had been close to statistical significance in the primary analyses (see Supplemental Table 2).
For the 117 initial, first-time GDD implants, the complication results were not significantly different from those in the primary analyses except for eyes aged less than two years (HR = 1.9; 95% CI: 0.9, 4.1; p =0.083), eyes aged less than three years (HR = 1.8; 95% CI: 0.9, 3.8; p =0.117), and eyes with higher IOP at the time of surgery (HR = 1.2; 95% CI: 0.9, 1.5; p =0.079) no longer had a significantly greater risk of a complication. For the 19 initial, second-time GDD implants, the failure results were not significantly different from those in the primary analyses.
Discussion:
Surgeons rely on outcomes data to optimize their treatment strategies. In this study, we presented the outcomes data from a large cohort of pediatric patients with GDD. Our cohort receiving first-time GDD had similar outcomes compared to their adult counterparts at five years, with an average failure rate of 38% compared to 42.1% in the Ahmed Baerveldt Comparison (ABC) study at 5 years.7 There was a significantly lower risk of failure for patients with glaucoma associated with acquired conditions, like juvenile idiopathic arthritis and trauma compared to other etiologies. This failure rate was comparable to a large, prior retrospective study of pediatric uveitic glaucoma patients (119 eyes) treated with Ahmed Glaucoma Valves.8
The failure rate was higher in second-time GDD eyes compared to first-time GDD eyes in both our study and the ABC study at five years.7 Factors that may predispose GDD to failure, such as a propensity for inflammatory reaction and exuberant scar tissue formation, may have contributed to the failure of both the first-time and second-time GDD.9,10 This notion is particularly important for eyes that have multiple procedures at one time as demonstrated by an increased risk of failure in second-time GDD cases that had concurrent glaucoma-related procedures and/or anterior segment surgery during implantation (compared to eyes that did not have concurrent surgery). An alternative strategy may be to perform staged procedures to decrease the peak inflammatory drive in the postoperative period instead of combination procedures, although this potential benefit has to be weighed against the risks associated with repeated anesthetic exposure.11 Furthermore, in many instances the concurrent anterior segment procedures were necessary to widen the anterior chamber angle or to make the sulcus space available through lens surgery, and a staged approach may not have been feasible.
In both first-time and second-time GDD eyes, those who were younger at the time of GDD implantation were more likely to fail (Table 1), which is consistent with prior reports in the pediatric population.2,8,12 For example, a retrospective study of Ahmed valve implants in pediatric patients found a protective effect with increasing age when comparing patients less than 2 years of age to those that were 8 years or older of age at time of implantation,8 while another study found a similar outcome in patients treated with Baerveldt and Molteno implants.12 Since we have defined failure based partly on the differences between pre- and postoperative IOP levels, and it is conceivable that younger children may have falsely high IOP measurements due to poor cooperation in the postoperative period, we cannot exclude possible bias. However, since failure occurred approximately 47 months following implantation, as the patients mature and become more cooperative, systematic bias towards failure due to poor cooperation with IOP measurement would theoretically have diminished over time. If substantiated, younger age as a risk factor for GDD failure may support a strategy of exhausting non-GDD glaucoma procedures in younger children before considering GDD implantation, in order to maximize the likelihood of long-term IOP control.13 Similarly, given the high concordance for first-time GDD to fail between the two eyes, children who have failed GDD implantation in the first eye may benefit from GDD alternatives if the fellow eye needs bleb-forming glaucoma surgery. In particular, trabeculectomy augmented with mitomycin C may be a reasonable alternative to GDD implantation in children younger than two years of age.14
Surgical complications increase the burden of care, as they may require additional visits to the clinic and/or operating room. For first-time GDD eyes, younger age and higher preoperative IOP at the time of GDD implantation increased the risk of complication. Our cohort had a lower complication rate than what was reported in the ABC study (22% versus 56.9%) at five years,15 mostly attributed to a difference in definitions. Whereas ABC included transient hyphema, hypotony and shallow anterior chamber as complications, we limited our definition to events that required additional anesthetic induction (examinations or surgeries). Upon closer examination of the ABC data, 14.3 % (16/43) of the Ahmed group and 19.5% (24/133) of the Baerveldt group required complication-related surgery at 5 years, which is similar to the rates in this study. Based on these findings, the risk of a GDD-related complication requiring additional anesthetic exposure in children is perhaps similar to that of adults. Given the relative rarity of pediatric glaucoma, perhaps establishing a large prospective registry database with international contribution may increase the sample size and provide more granular data which can be used over time to provide high-quality evidence for the outcomes of a rare disease.
Our study had several limitations. First, it was retrospective and subject to inherent biases such as referral and selection biases. Next, we had a relatively small sample of second-time GDD patients, which reduced our power and made complication analysis in this group not feasible. Third, while the failure criteria relied heavily on IOP reduction (in accordance with the 2008 World Glaucoma Association consensus on glaucoma surgical trial outcomes), IOP measurements in infants and young children are often imprecise and challenging. Thus, an expert consensus on the definition of childhood glaucoma surgical success (based on the quality of IOP measurement) may be needed for future studies of childhood glaucoma surgery outcomes. Fourth, while anatomic variations as gleaned during prior angle surgeries (if tried), such as patency and continuity of the Schlemm canal and surgical effect, may be predictive of eventual GDD failure, our methodology did not allow this granular level of data collection. Finally, the study consisted of patients seen in a tertiary referral center with a population that was predominantly Hispanic, which may limit the results’ generalizability.
Despite these limitations, our study is one of the largest of its kind and adds valuable information to a sparse literature. Specifically, it supports the idea that failure in both first and second-time GDD is associated with younger age and that first-time GDD failure is related to glaucoma associated with acquired conditions. In addition, we demonstrated that second-time GDD failure was more likely when additional glaucoma-related procedures (like a tube repositioning) were performed at the time of implantation compared to no concurrent surgery. Finally, our data showed that complications in first-time tubes were associated with younger age and higher preoperative IOP. Future studies aimed at predicting the long-term surgical outcomes with early findings, such as angle configuration, comorbidities and angle surgery outcome, would be advantageous in helping to avoid GDD complications and avoid/delay GDD failures.
Supplementary Material
Acknowledgment –
the authors would like to acknowledge Dr. Robert Litowitz and Mrs. Donna Mae Balkan Litowitz for their philanthropic contribution to the research efforts at the Samuel & Ethel Balkan International Pediatric Glaucoma Center.
Financial Support: NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The University of Miami Institute for Advanced Study of the Americas 2019 Pilot Grant
Abbreviations:
- GDD
Glaucoma drainage device
- IOP
Intraocular pressure
Footnotes
Meeting Presentation: American Glaucoma Society Annual Meeting 2020
Conflict of Interest Statement:
No conflicting relationship exists for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Molteno AC. New implant for drainage in glaucoma. Animal trial. Br J Ophthalmol. 1969;53(3):161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giangiacomo A, Beck A. Pediatric glaucoma: review of recent literature. Curr Opin Ophthalmol. 2017;28(2):199–203. doi: 10.1097/ICU.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulos M, Edmunds B, Fenerty C et al. Childhood glaucoma surgery in the 21st Century. Eye 28, 931–943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen TC, Chen PP, Francis BA, et al. Pediatric glaucoma surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2014;121(11):2107–15. [DOI] [PubMed] [Google Scholar]
- 5.Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin AV. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol. 2018;29(5):385–394. [DOI] [PubMed] [Google Scholar]
- 6.Shaaraway TM, Sheroowd MB, Grehn F, eds. (2009) Guidelines on Design and Reporting of Glaucoma Surgical Trial. Amsterdam, The Netherlands: Kugler Publications. [Google Scholar]
- 7.Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A, Yu F, Law SK, Giaconi JA, Coleman AL, Caprioli J. Valved Glaucoma Drainage Devices in Pediatric Glaucoma: Retrospective Long-term Outcomes. JAMA Ophthalmol. 2015;133(9):1030–5. [DOI] [PubMed] [Google Scholar]
- 9.Dai Z, Yu X, Sun J, Sun X. Grooved Glaucoma Drainage Devices That Continuously Deliver Cyclosporine A Decrease Postsurgical Scar Formation in Rabbit Eyes. Invest Ophthalmol Vis Sci. 2017;58(3):1692–1701. [DOI] [PubMed] [Google Scholar]
- 10.Rotsos T, Tsioga A, Andreanos K, et al. Managing high risk glaucoma with the Ahmed valve implant: 20 years of experience. Int J Ophthalmol. 2018;11(2):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkden GJ, Pickering AE, Gill H. Assessing Long-term Neurodevelopmental Outcome Following General Anesthesia in Early Childhood: Challenges and Opportunities. Anesth Analg. 2019;128(4):681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autrata R, Helmanova I, Oslejskova H, Vondracek P, Rehurek J. Glaucoma drainage implants in the treatment of refractory glaucoma in pediatric patients. Eur J Ophthalmol. 2007;17(6):928–37. [DOI] [PubMed] [Google Scholar]
- 13.Green W, Lind JT, Sheybani A. Review of the Xen Gel Stent and InnFocus MicroShunt. Curr Opin Ophthalmol. 2018;29(2):162–170. [DOI] [PubMed] [Google Scholar]
- 14.Jayaram H, Scawn R, Pooley F, Chiang M, Bunce C, Strouthidis NG, Khaw PT, Papadopoulos M. Long-Term Outcomes of Trabeculectomy Augmented with Mitomycin C Undertaken within the First 2 Years of Life. Ophthalmology. 2015. November;122(11):2216–22. [DOI] [PubMed] [Google Scholar]
- 15.Budenz DL, Feuer WJ, Barton K, et al. Postoperative Complications in the Ahmed Baerveldt Comparison Study During Five Years of Follow-up. Am J Ophthalmol. 2016;163:75–82.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.