Abstract
Individuals with autism spectrum disorder (ASD) experience a significant number of co-occurring medical conditions, yet little is known about these conditions beyond prevalence. Using large-scale deidentified medical records, we can use a novel phecode-based tool to characterize co-occurring conditions in ASD. We hypothesized that individuals with ASD experience an increased burden of co-occurring conditions as measured by presence, frequency, and duration of visits related to co-occurring conditions. Secondarily, we hypothesized that age at first encounter for ASD (early, <5; late, >5) would be associated with different co-occurring conditions. ICD-9 codes were extracted from a large anonymized electronic medical center database for 3097 individuals with ASD and 3097 matched controls. Co-occurring conditions were characterized using a novel tool (pyPheWAS) to examine presence, frequency, and duration of each condition. We identified several categories of co-occurring conditions in ASD: neurological (convulsions, sleep disorders); psychiatric (anxiety disorders, adjustment/conduct disorders), as well as constipation, hearing loss, and developmental delays. Our work confirms individuals with ASD are under a significant medical burden, with increased duration and frequency of visits associated with co-occurring conditions. Adequate management of these conditions could improve quality of life for individuals with ASD.
Lay Abstract
People with autism spectrum disorder often have a number of other medical conditions in addition to autism. These can range from constipation to epilepsy. This study uses medical record data to understand how frequently and how long people with autism have to be seen by a medical professional for these other medical conditions. This study confirmed that people with autism often have a number of other medical conditions and that they have to go see a medical professional about those conditions often. We also looked to see if children diagnosed with autism after age 5 might have different medical conditions compared to children diagnosed earlier. Children diagnosed later had more conditions like asthma, hearing loss, and mood disorders. This work describes how much medical care people with autism get for different medical conditions and the burden of seeking additional medical care for people with autism and their families.
Introduction
Autism spectrum disorder (ASD) is characterized by altered social communication as well as restrictive and repetitive behaviors. Patients with ASD often present with a wide and varied profile of co-occurring medical conditions1–6 that can greatly impact them and their families. Commonly reported co-occurring conditions in ASD include developmental delays7, gastrointestinal issues8, epilepsy1, and other psychiatric conditions9. Individuals with autism have an increased mortality risk compared to their peers 10; successful monitoring of complex co-occurring conditions could mitigate this risk.
Electronic medical records (EMR) are increasingly used in research as they provide large sample sizes that allow for assessment of highly heterogenous populations like ASD. Recent studies have used EMR to examine co-occurring conditions in ASD5,11–13, often corroborating previous findings from smaller cohorts3,9,14. In our study, we extend the characterization of co-occurring medical conditions in ASD in two important ways. First, we move beyond prevalence to examine first encounters, duration, and frequency of visits associated with each co-occurring condition. Characterizing medical burden in this way may help us understand medical care utilization in association with co-occurring conditions. Understanding medical care utilization due to co-occurring conditions is important as individuals with ASD report being unsatisfied with their healthcare experience, especially with management of long-term conditions15. Management of co-occurring medical conditions with ASD increases healthcare costs and burdens on family and other care-takers16, and many families describe difficulty in accessing appropriate care17. Compared to adults without ASD, individuals with ASD are more likely to visit the emergency department15, most often due to co-occurring conditions18, and, on average, incur higher costs18. Thus, understanding how and when co-occurring medical conditions present, and how they are managed, could reduce cost and mitigate burden on individuals and families.
Secondly, in this study, we used a newly developed tool for co-occurring condition assessment in EMR19. This tool uses an approach similar to genome-wide association studies, but focuses on the phenotypic condition; thus, using a phenome-wide association study (PheWAS) approach, we can identify co-occurring conditions related to a phenotypic condition like ASD. To do this, we utilize phecodes mapped from EMR derived ICD-9 codes. Phecodes mapping reduces ICD-9 codes down to 1865 conditions with improved phenotypic consistency, making phecodes ideal for ‘whole phenome’ analysis.
As a secondary analysis, we also examine co-occurring conditions in two potential subtypes of ASD. As patients with ASD are highly heterogeneous, subtyping can allow for earlier identification, better prognostication, and more personalized treatment. Previous work using EMR has characterized ASD subgroups by presence of co-occurring conditions11,20. We hypothesized that age at ASD diagnosis may differentiate profiles of co-occurring conditions. Later age of ASD diagnosis is associated with more co-occurring conditions7,21. While estimates of average age at ASD diagnosis range from 3.1–7.2 years22, there is a growing subset of individuals who may not receive an ASD diagnosis until adulthood23,24. It is not clear if individuals diagnosed in late childhood25, or even adulthood26, represent phenotypically distinct subtypes, missed/erroneous diagnoses, subtle presentation25,27, or some combination of other factors. Thus, we examined co-occurring conditions in those with an early (prior to age 5) and late ASD diagnosis (after age 5) to further characterize co-occurring conditions across different cohorts. We considered several factors in our diagnostic age split: the distribution of age at diagnosis in our data set, previous estimates of average age at diagnosis22, previous co-occurring condition studies21, the age 4–5 window specified by the Autism Diagnostic Interview, Revised28 as a likely peak of symptom emergence, and potential life-events (e.g., starting school) that could influence identification.
In this study, we characterize medical burden by the presence of these conditions, as well as the frequency and duration of visits associated with each condition using a novel phecode-dependent tool for EMR research. In this work, we replicate significant co-occurring conditions identified in previous studies and provide evidence for increased burden of co-occurring medical conditions in ASD, overall, as well as varying profiles of co-occurring condition burden based on age at ASD diagnosis.
Methods
Patient Sample
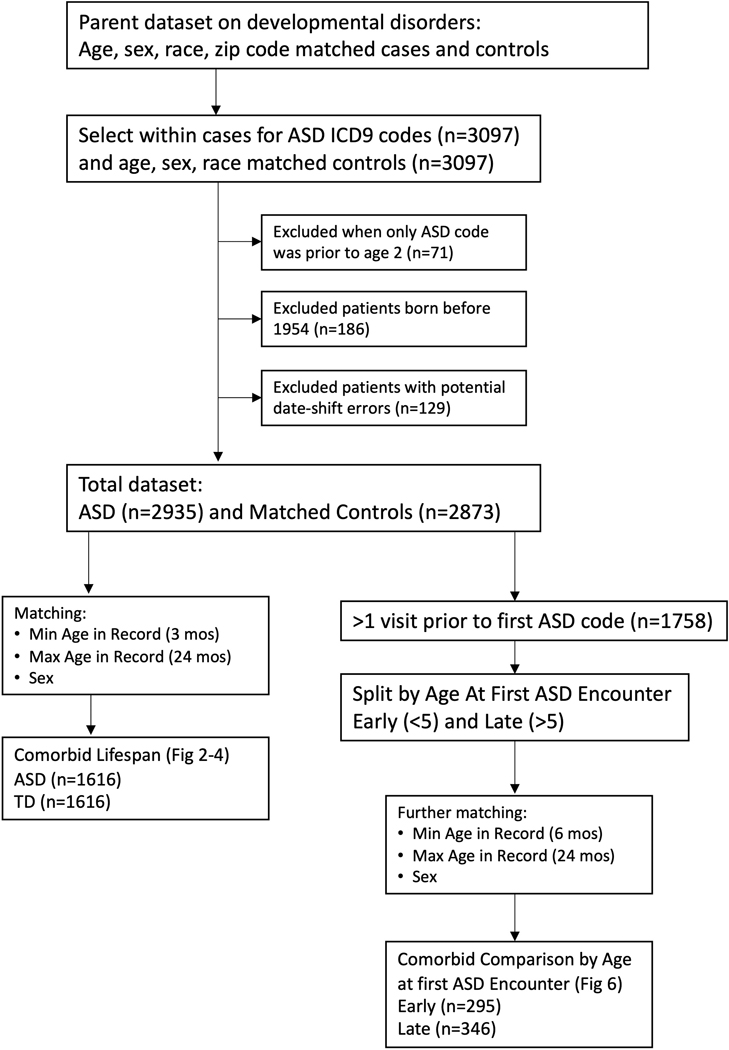
This study was approved by the Internal Review Board of Vanderbilt University Medical Center. We compared individuals with ASD (n=3097) to typically developing (TD) controls, matched on age, sex, and race (n=3097). Figure 1 describes the study flow. The complete International Classification of Diseases, Ninth Revision (ICD-9) history was obtained for each patient from de-identified electronic health records at Vanderbilt University Medical Center (1991–2015). Electronic health records included in the Vanderbilt University Medical Center system include ~2,000,000 visits per year in 120 inpatient and outpatient facilities across the Middle Tennessee region, including primary care as well as specialist visits. Demographics are reported in Table 1. Individuals with ASD had at least one phecode for ASD (313.3) which maps to the following ICD-9 codes for ASD (299, 299.0, 299.00, 299.01, 299.1, 299.10, 299.11, 299.8, 299.80, 299.81, 299.9, 299.90, 299.91), similar to previous work11. As some EMR studies in ASD have reported cohorts requiring at least two ICD-9 codes for the condition of interest5,12,13, we replicated the primary findings in this report in a smaller cohort that met this criterion (see Supplemental Table 1.)
Figure 1.
Overall study design.
Table 1.
Demographic information for the ASD and control cohorts.
| Total | Lifespan | ASD: Age At Dx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TD n=2873 | ASD n=2935 | p | TD n=1616 | ASD n=1616 | p | Age<5 n=295 | Age>5 n=346 | p | ||
| Sex | Female | 573 | 583 | 0.963 | 328 | 324 | 0.398 | 49 | 49 | 0.454 |
| Male | 2295 | 2346 | 1284 | 1291 | 246 | 297 | ||||
| Unknown | 5 | 6 | 4 | 1 | 0 | 0 | ||||
| Race | White | 1655 | 1504 | <0.001 | 907 | 910 | <0.001 | 197 | 214 | 0.682 |
| Black/African American | 414 | 228 | 222 | 149 | 30 | 43 | ||||
| Asian | 23 | 17 | 10 | 11 | 1 | 1 | ||||
| Hispanic | 139 | 97 | 70 | 59 | 15 | 24 | ||||
| American Indian or | 4 | 4 | 2 | 1 | 0 | 0 | ||||
| Alaska Native | ||||||||||
| Unknown | 638 | 1085 | 405 | 486 | 52 | 64 | ||||
| Death Status | Alive | 2853 | 2921 | 0.356 | 1604 | 1609 | 0.357 | 295 | 346 | - |
| Deceased | 20 | 14 | 12 | 7 | 0 | 0 | ||||
Exclusion criteria
We excluded patients who received an ASD code prior to age 2 and who never received another ASD code in their record. These cases suggested a spurious reason for the ASD code or that the diagnoses before age 2 was not stable29. We removed patients born before 1954 and records with potential date shift errors (usually related to short EMRs around the time of birth). Figure 1 describes the number of patients excluded for each reason.
PheDAS Analysis
EMR phenotypes called phecodes20,30 were identified from anonymized EMRs based on ICD-9 codes. EMR phenotypes are sets of characteristics/symptoms that consistently define clinical presentations of different conditions and can be mapped to specific ICD-9 or ICD-10 codes. Using a new python tool (pyPheWAS), significant conditions were identified using phenome-disease association study (PheDAS19), wherein associations between each EMR phenotype and the condition of interest (ASD) are tested. Individuals’ ICD-9 codes were mapped to phecodes and the incidence of each condition was determined relative to matched controls. Logistic regression, with ASD group membership as the dependent variable, was used to examine relative association of phecodes with the ASD cohort compared to the control cohort. We conducted three sets of logistic regressions where the phecode variable was treated in one of three ways: as presence (binary absence/presence of the code), count/frequency (number of incidences of the code), or duration (min-max dates the code appeared). For each set of regressions, PheDAS runs 1865 logistic regressions (one for each phecode) with each regression yielding a p-value indicating association between the condition and ASD. Multiple comparison correction was conducted with Bonferroni or False Discovery Rate (FDR).
Analysis by Age at First ASD Encounter
Age at first ASD encounter was explored as a potential factor in co-occurring condition profiles. Age at first ASD encounter was defined as the age at first ASD phecode in the EMR. The ASD cohort was split by age: first ASD code before age 5 (early diagnosis) and after age 5 (late diagnosis). For this analysis, we only included patients who had at least 1 visit prior to their first ASD code to increase confidence in the first ASD code as a diagnostic-like event. For these comparisons, we also added presence of intellectual disability (defined by presence of the mental retardation phecode, 315.3, in the record) as a covariate in the phecode analysis. See Table 1 for demographics for each cohort.
Additional Statistical Analyses
To determine differences in demographic variables between ASD and controls and between early and late diagnostic groups, we used Chi-square tests. Analyses were conducted using Python (v.2.7) and R (v.3.3.2).
Results
Burden of co-occurring medical conditions across the lifespan in ASD
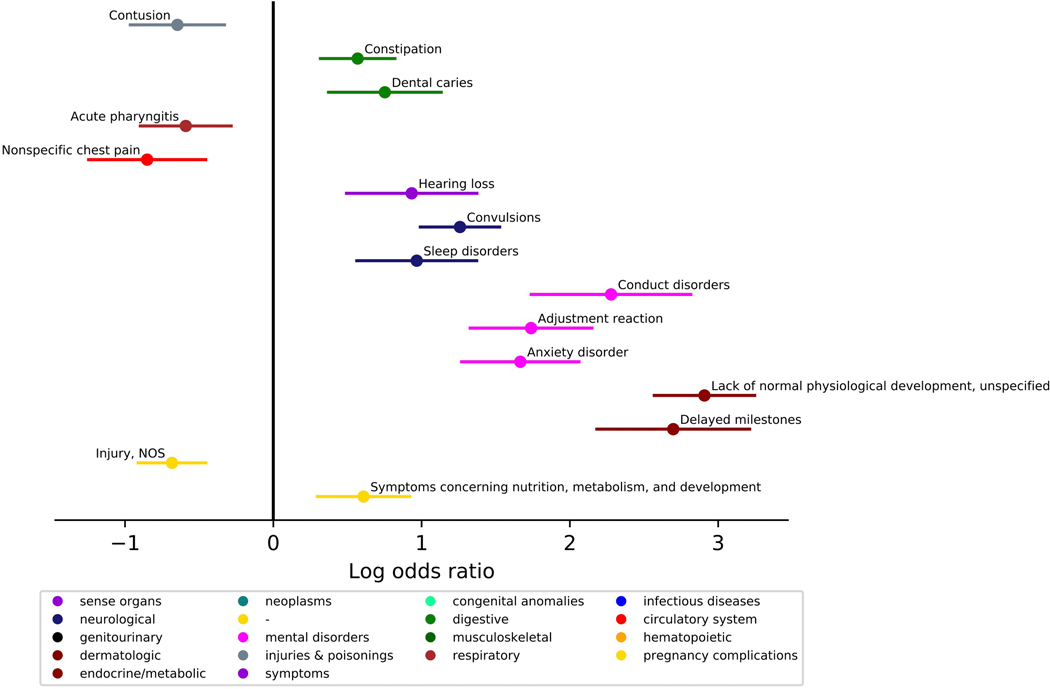
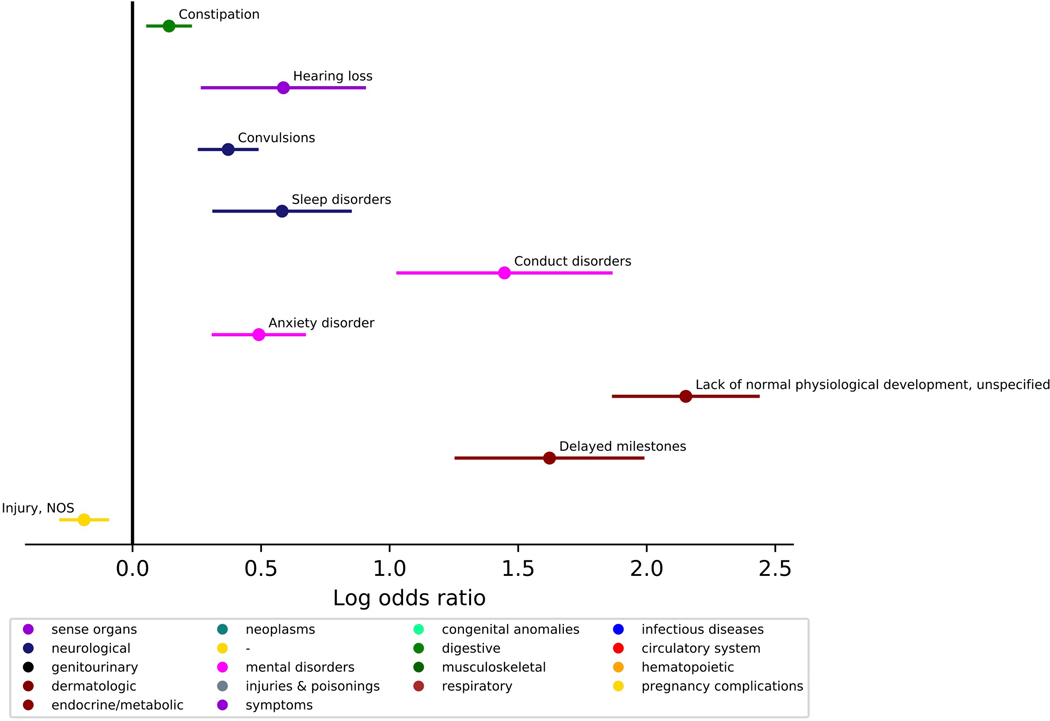
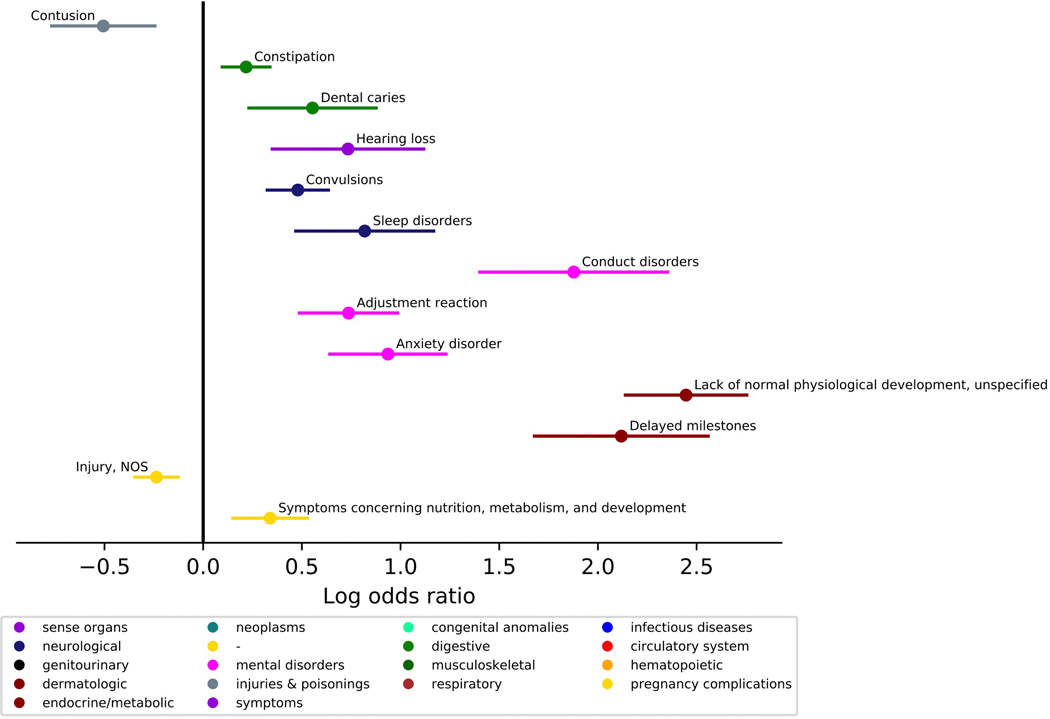
We investigated the presence of co-occurring conditions in ASD across the lifespan. The ASD group was matched to the TD group on age at first visit (within 6 months), age at last visit (within 24 months), and sex (n, ASD=1616, TD=1616, Lifespan Cohort, Table 1). Phecodes enriched in the ASD group included constipation, dental issues, hearing loss, convulsions, sleep disorders; psychiatric disorders including conduct disorders, adjustment reaction, anxiety disorders; as well as developmental delay and concerns. Several conditions were less prevalent in the ASD group: contusions, nonspecific chest pain, acute pharyngitis, and injuries (Figure 2, Table 2, see Supplemental Figure 1 for similar results in a cohort where two ASD ICD-9 codes were required). The ASD group had more visits for constipation, hearing loss, convulsions, sleep disorders, anxiety disorder, conduct disorders, delayed milestones, and lack of normal physiological development. The ASD group had fewer visits for injuries (Figure 3, Table 3). Several phecodes had longer durations in the ASD group: constipation, dental issues, hearing loss, convulsions, sleep disorders; psychiatric disorders including conduct disorders, adjustment reaction, anxiety disorder; as well as delayed milestones and lack of normal physiological development. The TD group had longer durations of contusions and injuries (Figure 4, Table 4).
Figure 2. Comparing presence of medical conditions co-occurring with ASD diagnosis across the lifespan.
(N, ASD=1616, Typical Comparison=1616). Phecodes with significant log odds ratios following a Bonferroni correction are displayed on the graph.
Table 2.
Regression models with the presence of co-occurring conditions associated with ASD.
| Phecode and Description | −log(p) | p-val | beta | 95% Conf-interval | |
|---|---|---|---|---|---|
| More Common in ASD | |||||
| 264.9 | Lack of normal physiological development, unspecified | 62.833 | 1.47E-63 | 2.907 | [2.569,3.246] |
| 264.3 | Delayed milestones | 23.950 | 1.12E-24 | 2.697 | [2.182,3.212] |
| 345.3 | Convulsions | 19.537 | 2.91E-20 | 1.259 | [0.991,1.526] |
| 312 | Conduct disorders | 15.971 | 1.07E-16 | 2.278 | [1.740,2.816] |
| 304 | Adjustment reaction | 15.968 | 1.08E-16 | 1.738 | [1.328,2.149] |
| 300.1 | Anxiety disorder | 15.805 | 1.57E-16 | 1.666 | [1.270,2.061] |
| 327 | Sleep disorders | 5.556 | 2.78E-06 | 0.967 | [0.563,1.372] |
| 563 | Constipation | 5.034 | 9.25E-06 | 0.569 | [0.317,0.820] |
| 389 | Hearing loss | 4.497 | 3.18E-05 | 0.933 | [0.493,1.3728] |
| 521.1 | Dental caries | 3.975 | 1.05E-04 | 0.752 | [0.372,1.133] |
| 1002 | Symptoms concerning nutrition, metabolism, and development | 3.891 | 1.29E-04 | 0.609 | [0.297,0.920] |
| Less Common in ASD | |||||
| 1009 | Injury, NOS | 8.320 | 4.78E-09 | −0.683 | [−0.912,−0.455] |
| 418 | Nonspecific chest pain | 4.615 | 2.43E-05 | −0.851 | [−1.245,−0.456] |
| 916 | Contusion | 4.182 | 6.58E-05 | −0.647 | [−0.964,−0.329] |
| 465.2 | Acute pharyngitis | 3.796 | 1.60E-04 | −0.590 | [−0.897,−0.289] |
Figure 3. Comparing count incidences of medical conditions co-occurring with ASD diagnosis across the lifespan.
(N, ASD=1616, Typical Comparison=1616). Phecodes with significant log odds ratios following a Bonferroni correction are displayed on the graph.
Table 3.
Regression models with the count of co-occurring conditions associated with ASD.
| Phecode and Description | −log(p) | p-val | beta | 95% Conf-interval | |
|---|---|---|---|---|---|
| Higher Count in ASD | |||||
| 264.9 | Lack of normal physiological development, unspecified | 50.131 | 7.39E-51 | 2.152 | [1.871,2.433] |
| 264.3 | Delayed milestones | 17.678 | 2.10E-18 | 1.622 | [1.259,1.985] |
| 312 | Conduct disorders | 11.113 | 7.71E-12 | 1.447 | [1.033,1.861] |
| 345.3 | Convulsions | 10.109 | 7.77E-11 | 0.372 | [0.260,0.484] |
| 300.1 | Anxiety disorder | 7.272 | 5.35E-08 | 0.491 | [0.314,0.668] |
| 327 | Sleep disorders | 4.770 | 1.70E-05 | 0.582 | [0.317,0.847] |
| 389 | Hearing loss | 3.583 | 2.61E-04 | 0.587 | [0.272,0.902] |
| 563 | Constipation | 3.102 | 7.90E-04 | 0.142 | [0.059,0.225] |
| Lower Count in ASD | |||||
| 1009 | Injury, NOS | 4.307 | 4.94E-05 | −0.188 | [−0.279,−0.097] |
Figure 4. Comparing duration (minimum age the code first appears to maximum age the code last appears in the medical record) of medical conditions co-occurring with ASD diagnosis across the lifespan.
(N, ASD=1616, Typical Comparison=1616). Phecodes with significant log odds ratios following a Bonferroni correction are displayed on the graph.
Table 4.
Regression models with the duration of co-occurring conditions associated with ASD.
| Phecode and Description | −log(p) | p-val | beta | 95% Conf-interval | |
|---|---|---|---|---|---|
| Longer Duration in ASD | |||||
| 264.9 | Lack of normal physiological development, unspecified | 54.119 | 7.61E-55 | 2.447 | [2.139,2.754] |
| 264.3 | Delayed milestones | 20.407 | 3.92E-21 | 2.119 | [1.679,2.559] |
| 312 | Conduct disorders | 13.963 | 1.09E-14 | 1.878 | [1.402,2.354] |
| 300.1 | Anxiety disorder | 9.344 | 4.52E-10 | 0.936 | [0.642,1.230] |
| 345.3 | Convulsions | 8.875 | 1.33E-09 | 0.480 | [0.325,0.635] |
| 304 | Adjustment reaction | 8.181 | 6.59E-09 | 0.737 | [0.488,0.986] |
| 327 | Sleep disorders | 5.355 | 4.41E-06 | 0.818 | [0.469,1.168] |
| 389 | Hearing loss | 3.747 | 1.79E-04 | 0.734 | [0.350,1.118] |
| 563 | Constipation | 3.371 | 4.25E-04 | 0.218 | [0.097,0.339] |
| 1002 | Symptoms concerning nutrition, metabolism, and development | 3.360 | 4.36E-04 | 0.340 | [0.150,0.529] |
| 521.1 | Dental caries | 3.115 | 7.67E-04 | 0.554 | [0.231,0.877] |
| Shorter Duration in ASD | |||||
| 1009 | Injury, NOS | 4.575 | 2.66E-05 | −0.237 | [−0.347,−0.126] |
| 916 | Contusion | 3.820 | 1.51E-04 | −0.506 | [−0.768,−0.244] |
Co-occurring medical condition profiles differ by age at diagnosis
The ASD group was split by age at diagnosis (<5, early; >5, late). Figure 5 shows the distribution of age at diagnosis by early and late ASD group before matching. The early and late groups were then matched to each other on age at first visit (within 6 mos), age at last visit, (within 24 months), and sex (N, Early=295, Late=346). We compared co-occurring medical conditions based on age at first encounter for ASD. Based on phecode presence in the record, phecodes with significant log odds ratios following an FDR correction are listed in Table 5. Phecodes more associated with the late ASD group included respiratory issues, nausea and vomiting, asthma, otitis media, GERD, psychiatric comorbidities, and convulsions, among others.
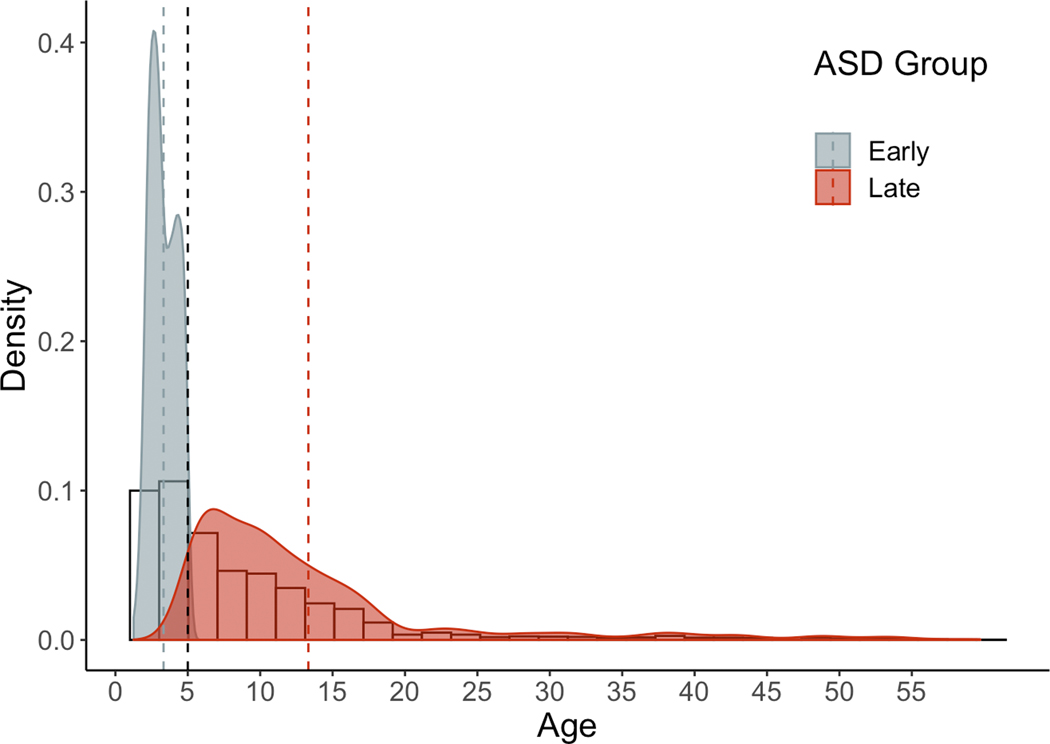
Figure 5. Histogram depicting age at first ASD diagnostic code.
Dashed black line represents split at age of first diagnosis, before and after age 5. Histogram represents age at ASD diagnosis for total cohort (n=2935). Density plots indicate age at ASD first encounter by early and late group; dashed lines designate mean age at diagnosis for each group.
Table 5.
Regression models comparing presence of co-occurring conditions based on age at first ASD encounter (before or after age 5).
| Phecode and Description | −log(p) | p-val | beta | 95% Conf-interval | |
|---|---|---|---|---|---|
| More Common in ASD | |||||
| 483 | Acute bronchitis and bronchiolitis | 6.601 | 2.51E-07 | 1.941 | [1.204,2.680] |
| 381.11 | Suppurative and unspecified otitis media | 4.978 | 1.05E-05 | 1.160 | [0.644,1.676] |
| 512.9 | Other dyspnea | 4.577 | 2.65E-05 | 1.617 | [0.863,2.372] |
| 789 | Nausea and vomiting | 4.267 | 5.40E-05 | 1.049 | [0.540,1.559] |
| 465 | Acute upper respiratory infections of multiple or unspecified sites | 4.001 | 9.97E-05 | 0.960 | [0.476,1.443] |
| 495 | Asthma | 3.990 | 1.02E-04 | 1.216 | [0.602,1.829] |
| 749 | Congenital anomalies of face and neck | 3.937 | 1.16E-04 | 1.854 | [0.912,2.797] |
| 276.5 | Hypovolemia | 3.842 | 1.44E-04 | 1.264 | [0.612,1.916] |
| 381.1 | Otitis media | 3.558 | 2.76E-04 | 1.317 | [0.607,2.026] |
| 389.2 | Conductive hearing loss | 3.374 | 4.22E-04 | 1.472 | [0.654,2.291] |
| 512.8 | Cough | 3.191 | 6.44E-04 | 0.913 | [0.389,1.438] |
| 783 | Fever of unknown origin | 3.180 | 6.62E-04 | 0.817 | [0.347,1.29] |
| 530.11 | GERD | 3.118 | 7.62E-04 | 1.070 | [0.447,1.693] |
| 79 | Viral infection | 2.994 | 0.00101 | 0.979 | [0.395,1.563] |
| 396 | Abnormal heart sounds | 2.945 | 0.00114 | 1.210 | [0.482,1.940] |
| 1001 | Foreign body injury | 2.841 | 0.00144 | 1.420 | [0.547,2.294] |
| 1009 | Injury, NOS | 2.666 | 0.00216 | 0.955 | [0.345,1.566] |
| 304 | Adjustment reaction | 2.618 | 0.00241 | 1.331 | [0.471,2.191] |
| 312 | Conduct disorders | 2.496 | 0.00319 | 0.809 | [0.271,1.346] |
| 512.1 | Wheezing | 2.470 | 0.00338 | 1.106 | [0.366,1.846] |
| 558 | Noninfectious gastroenteritis | 2.465 | 0.00342 | 1.060 | [0.352,1.771] |
| 656.8 | Perinatal jaundice | 2.446 | 0.00360 | 1.377 | [0.450,2.304] |
| 264.9 | Lack of normal physiological development, unspecified | 2.437 | 0.00365 | 0.561 | [0.183,0.939] |
| 306 | Other mental disorder | 2.303 | 0.00497 | 1.510 | [0.456,2.564] |
| 369.5 | Conjunctivitis, infectious | 2.101 | 0.00793 | 1.2721 | [0.333,2.211] |
| 656.3 | Endocrine and metabolic disturbances of fetus and newborn | 2.088 | 0.00817 | 1.332 | [0.345,2.320] |
| 381.2 | Eustachian tube disorders | 2.051 | 0.00888 | 1.001 | [0.251,1.751] |
| 296 | Mood disorders | 2.008 | 0.00982 | 2.665 | [0.642,4.688] |
| 345.3 | Convulsions | 1.951 | 0.0112 | 0.595 | [0.135,1.055] |
| 773 | Pain in limb | 1.894 | 0.01274 | 0.992 | [0.211,1.773] |
| 350.3 | Lack of coordination | 1.884 | 0.01304 | 0.981 | [0.207,1.756] |
Discussion
This study characterizes co-occurring medical conditions in ASD with a new tool (pyPheWAS), allowing us to understand the burden of these conditions in ASD through the presence, count, and duration of related visits. This work provides strong evidence of an increased medical burden on individuals with ASD and highlights the need for adequate management of co-occurring conditions. By characterizing this burden, we can build predictive models of co-occurring condition progression (eg. estimated diagnostic peaks), introduce targeted risk assessments across the lifespan, and move towards more personalized medicine, ultimately reducing burden.
This study confirms many previously reported co-occurring condition categories in ASD, including neurological1,7,21, psychiatric9,21, gastrointestinal8, hearing31, and developmental conditions7,21. Our finding of increased developmental delays in ASD acts as an internal control of the PheDAS tool; delayed milestones are often a first sign of ASD and lead to increased screening for ASD. A particularly burdensome co-occurring condition for individuals and their families is disordered sleep32,33, with reported rates of 50–80%34 in ASD. Sleep disturbances exacerbate core symptoms of ASD35, and seem to be related to other complaints like feeding issues in ASD6, which could explain increased visits and longer durations associated with sleep disorders in our study. Convulsions were also elevated in ASD. The prevalence of epilepsy in ASD (5–38%1,36) is significantly higher than the general population (1.2%37). Underlying biological phenomena may explain overlapping diagnostic peaks in epilepsy and ASD38. Yet, co-occurring diagnoses may also reflect increased medical care usage, increasing the likelihood of identification for ASD. We also identified increased presence of constipation in ASD, a treatable and commonly reported problem in ASD39. While it is hypothesized that constipation may be related to rigid, repetitive behaviors in ASD40, communication difficulties may increase the likelihood of an abnormal presentation gastrointestinal issues in ASD, thus a full-workup for gastrointestinal issues is often warranted41. We also observed hearing loss was more prevalent in ASD. While the prevalence of hearing impairment in ASD has been debated31, there is evidence of increased rates of acute otitis media and related complications (including increased rates of mastoidectomy and typanoplasty)42.
We observed significant co-occurrence of psychiatric conditions in ASD, consistent with previous reports9,43,44. Co-occurring psychiatric conditions represent a significant burden for individuals with ASD and their families as they are related to emergency department visits18,45, associated with parental stress46, and remain prevalent throughout adulthood47. Psychiatric disorders were also associated with more visits and longer durations in ASD. Our findings of increased presence of anxiety disorder in ASD is consistent with smaller studies of anxiety (11–84%48,49). In a larger, more recent study of anxiety disorders in the Stockholm Youth Cohort50, anxiety disorders were more common in ASD, as well as adjustment and stress disorders, similar to our work here. Adjustment disorders are associated with suicidal behaviors in ASD51,52, and thus, these are important associations to investigate further.
We also examined differences in co-occurring medical conditions by first encounter for ASD. Estimates of average age of ASD diagnosis22 suggest a peak around 3–4 years old, when stable diagnoses can be made, but as many as half of cases may not be identified until age six or later, and a significant number of individuals are not diagnosed until even later childhood or adulthood23,24,27. Many hypothesize these diagnostic peaks reflect ASD subtypes, while others suggest a “two-hit” hypothesis, where adolescence, with its increased social demands and biological changes, may overwhelm adaptive functioning53.
In our analysis, we describe co-occurring condition profiles by age at first ASD encounter that suggest a more complicated profile for later diagnosed individuals with ASD, including more respiratory, auditory, gastrointestinal, and psychiatric conditions. It is possible that some of these conditions may delay ASD evaluation as medical concerns could eclipse or mask ASD-related issues54. In one study, co-occurring psychiatric conditions were present in 77% of individuals with higher cognitive functioning and later ages of ASD diagnosis (~11 years), with up to ~20% of co-occurring conditions potentially mis-diagnosed44. It is not clear if these differences by diagnostic group reflect subtypes with different underlying etiologies or if later diagnoses represent more complicated cases. Later ASD diagnosis could be due to other factors (like socioeconomic status and geographic catchment area) that we could not test using EMR, but may contribute to misdiagnosis or delayed evaluation54. Unfortunately, there are a number of factors that may lead to a later ASD diagnosis that cannot be evaluated easily in EMR and could conflate our age by diagnostic findings. Importantly, we covaried for intellectual disability as it could affect age at diagnosis. While we cannot be sure the first encounter for ASD was diagnostic, we matched our cohorts in several ways that provide more confidence. Each record had at least 1 visit prior to the visit with the first ASD code, and our cohorts were matched by age at first visit to our facilities. Future studies will need to match individuals with ASD on minimum age at first visit and track individuals as they are diagnosed to increase confidence in age at diagnosis-related comorbidities.
We also identified phecodes that were less likely to be associated with ASD (see Table 2). These findings could suggest individuals with ASD are less likely to experience sore throats, injuries, and acute pain; yet, evidence suggests altered pain reporting, and expression in ASD55,56, possibly reducing identification of conditions that require pain reporting. Motivation to seek medical care may also be reduced in individuals with ASD as medical facilities can exacerbate sensory sensitivities and increase social demands, complicating assessment2. Alternatively, these conditions that are less present in the ASD group could reflect potential catchment effects of the control group.
There are some limitations to consider in this study. As ICD-9 codes are primarily used for billing purposes, they do not represent a definitive diagnosis57. However, our use of phecodes, which group relevant ICD-9 codes, increases our diagnostic confidence in each condition. Similarly, these data may not represent the larger population and may reflect some catchment artifacts. Specifically, as we cannot address SES in this study, SES could contribute to differences in catchment between controls and ASD. We have reduced the likelihood of these artifacts with rigorous control matching, but cannot rule out their effects. Similarly, these data only represent visits to this medical center, other visits prior to these or outside of the assessed medical center are not available to us. This study also has a number of strengths. The PheDAS tool allowed for a nuanced characterization of co-occurring medical conditions across the lifespan of individuals with ASD, beyond simply prevalence of each condition. The ability to examine count and duration of conditions allows us to better understand the burden of these conditions in ASD. This study confirms previous reports4,5,11, extending our understanding of medical issues in ASD. However, with the addition of frequency and duration information related to these conditions, this work highlights the urgency to reduce the burden of co-occurring conditions for people with ASD.
Supplementary Material
Acknowledgements
The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation Grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Dr. Failla was supported by a NIMH training grant (T32-MH18921) during completion of this work. This work was also supported in part by the National Institute of Biomedical Imaging and Bioengineering training grant T32-EB021937 and by Eunice Kennedy Shriver National Institute of Child Health & Human Development grant U54HD08321105.
References
- 1.Tuchman R. & Rapin I. Epilepsy in autism. The Lancet Neurology 1, 352–358 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Bauman ML Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics 7, 320–327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorrindo P. et al. Gastrointestinal Dysfunction in Autism: Parental Report, Clinical Evaluation, & Associated Factors. Autism Res 5, 101–108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohane IS et al. The Co-Morbidity Burden of Children and Young Adults with Autism Spectrum Disorders. PLOS ONE 7, e33224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croen LA et al. The health status of adults on the autism spectrum. Autism 19, 814–823 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Neumeyer AM et al. Identifying Associations Among Co-Occurring Medical Conditions in Children With Autism Spectrum Disorders. Acad Pediatr 19, 300–306 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Levy SE et al. Autism Spectrum Disorder and Co-occurring Developmental, Psychiatric, and Medical Conditions Among Children in Multiple Populations of the United States. Journal of Developmental & Behavioral Pediatrics 31, 267 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim SH, Voigt RG, Katusic SK, Weaver AL & Barbaresi WJ Incidence of Gastrointestinal Symptoms in Children With Autism: A Population-Based Study. Pediatrics 124, 680–686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonoff E. et al. Psychiatric Disorders in Children With Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. Journal of the American Academy of Child & Adolescent Psychiatry 47, 921–929 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Woolfenden S, Sarkozy V, Ridley G, Coory M. & Williams K. A systematic review of two outcomes in autism spectrum disorder – epilepsy and mortality. Developmental Medicine & Child Neurology 54, 306–312 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Doshi-Velez F, Ge Y. & Kohane I. Comorbidity Clusters in Autism Spectrum Disorders: An Electronic Health Record Time-Series Analysis. Pediatrics 133, e54–e63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexeeff SE et al. Medical Conditions in the First Years of Life Associated with Future Diagnosis of ASD in Children. J Autism Dev Disord 47, 2067–2079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davignon MN, Qian Y, Massolo M. & Croen LA Psychiatric and Medical Conditions in Transition-Aged Individuals With ASD. Pediatrics 141, S335–S345 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Amiet C. et al. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol. Psychiatry 64, 577–582 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Nicolaidis C. et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J Gen Intern Med 28, 761–769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kogan MD et al. A National Profile of the Health Care Experiences and Family Impact of Autism Spectrum Disorder Among Children in the United States, 2005–2006. Pediatrics 122, e1149–e1158 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Vohra R, Madhavan S, Sambamoorthi U. & St. Peter C. Access to services, quality of care, and family impact for children with autism, other developmental disabilities, and other mental health conditions. Autism (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vohra R, Madhavan S. & Sambamoorthi U. Emergency Department Use Among Adults with Autism Spectrum Disorders (ASD). J Autism Dev Disord 46, 1441–1454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaganti S. et al. Electronic Medical Record Context Signatures Improve Diagnostic Classification using Medical Image Computing. IEEE journal of biomedical and health informatics (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny JC, Bastarache L. & Roden DM Phenome-Wide Association Studies as a Tool to Advance Precision Medicine. Annu Rev Genomics Hum Genet 17, 353–373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M. & Schieve LA Prevalence of Co-occurring Medical and Behavioral Conditions/Symptoms Among 4- and 8-Year-Old Children with Autism Spectrum Disorder in Selected Areas of the United States in 2010. J Autism Dev Disord 48, 2663–2676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandell DS, Novak MM & Zubritsky CD Factors Associated With Age of Diagnosis Among Children With Autism Spectrum Disorders. Pediatrics 116, 1480–1486 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brugha TS et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch. Gen. Psychiatry 68, 459–465 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Happé FG et al. Demographic and Cognitive Profile of Individuals Seeking a Diagnosis of Autism Spectrum Disorder in Adulthood. J Autism Dev Disord 46, 3469–3480 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Ozonoff S. et al. Diagnosis of Autism Spectrum Disorder After Age 5 in Children Evaluated Longitudinally Since Infancy. J Am Acad Child Adolesc Psychiatry 57, 849–857.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott P, Happé FG & Charlton RA Exploratory Study of Executive Function Abilities Across the Adult Lifespan in Individuals Receiving an ASD Diagnosis in Adulthood. J Autism Dev Disord 48, 4193–4206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldrick RC, Maye MP & Carter AS Age at First Identification of Autism Spectrum Disorder: An Analysis of Two US Surveys. Journal of the American Academy of Child & Adolescent Psychiatry 56, 313–320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bildt A. et al. Autism Diagnostic Interview-Revised (ADI-R) Algorithms for Toddlers and Young Preschoolers: Application in a Non-US Sample of 1,104 Children. J Autism Dev Disord 45, 2076–2091 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landa RJ Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Reviews Neurology 4, 138–147 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Denny JC et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics 26, 1205–1210 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beers AN, McBoyle M, Kakande E, Dar Santos RC & Kozak FK Autism and peripheral hearing loss: A systematic review. International Journal of Pediatric Otorhinolaryngology 78, 96–101 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Hodge D, Hoffman CD, Sweeney DP & Riggs ML Relationship Between Children’s Sleep and Mental Health in Mothers of Children with and Without Autism. J Autism Dev Disord 43, 956–963 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Delahaye J. et al. The relationship between Health-Related Quality of Life and sleep problems in children with Autism Spectrum Disorders. Research in Autism Spectrum Disorders 8, 292–303 (2014). [Google Scholar]
- 34.Richdale AL & Schreck KA Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews 13, 403–411 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Mazurek MO, Dovgan K, Neumeyer AM & Malow BA Course and Predictors of Sleep and Co-occurring Problems in Children with Autism Spectrum Disorder. J Autism Dev Disord (2019) doi: 10.1007/s10803-019-03894-5. [DOI] [PubMed] [Google Scholar]
- 36.Fiest KM et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 88, 296–303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zack MM National and State Estimates of the Numbers of Adults and Children with Active Epilepsy — United States, 2015. MMWR Morb Mortal Wkly Rep 66, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BH, Smith T. & Paciorkowski AR Autism Spectrum Disorder and Epilepsy: disorders with a shared biology. Epilepsy Behav 47, 191–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuta GT et al. Management of Constipation in Children and Adolescents With Autism Spectrum Disorders. Pediatrics 130, S98–S105 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Peters B. et al. Rigid-compulsive behaviors are associated with mixed bowel symptoms in autism spectrum disorder. J Autism Dev Disord 44, 1425–1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buie T. et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals With ASDs: A Consensus Report. Pediatrics 125, S1–S18 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Adams DJ et al. Otitis Media and Related Complications Among Children with Autism Spectrum Disorders. J Autism Dev Disord 46, 1636–1642 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Leyfer OT et al. Comorbid Psychiatric Disorders in Children with Autism: Interview Development and Rates of Disorders. J Autism Dev Disord 36, 849–861 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Mazefsky CA et al. ASD, a Psychiatric Disorder, or Both? Psychiatric Diagnoses in Adolescents with High-Functioning ASD. J Clin Child Adolesc Psychol 41, 516–523 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalb LG, Stuart EA, Freedman B, Zablotsky B. & Vasa R. Psychiatric-related emergency department visits among children with an autism spectrum disorder. Pediatr Emerg Care 28, 1269–1276 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Yorke I. et al. The Association Between Emotional and Behavioral Problems in Children with Autism Spectrum Disorder and Psychological Distress in Their Parents: A Systematic Review and Meta-analysis. J Autism Dev Disord 48, 3393–3415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lever AG & Geurts HM Psychiatric Co-occurring Symptoms and Disorders in Young, Middle-Aged, and Older Adults with Autism Spectrum Disorder. J Autism Dev Disord 46, 1916–1930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White SW, Oswald D, Ollendick T. & Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review 29, 216–229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plana-Ripoll O. et al. Exploring Comorbidity Within Mental Disorders Among a Danish National Population. JAMA Psychiatry (2019) doi: 10.1001/jamapsychiatry.2018.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nimmo-Smith V. et al. Anxiety Disorders in Adults with Autism Spectrum Disorder: A Population-Based Study. J Autism Dev Disord 50, 308–318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikami K. et al. Frequency and clinical features of pervasive developmental disorder in adolescent suicide attempts. General Hospital Psychiatry 31, 163–166 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Kato K. et al. Clinical features of suicide attempts in adults with autism spectrum disorders. General Hospital Psychiatry 35, 50–53 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Picci G. & Scherf KS A Two-Hit Model of Autism: Adolescence as the Second Hit. Clin Psychol Sci 3, 349–371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels AM & Mandell DS Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism 18, 583–597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore DJ Acute pain experience in individuals with autism spectrum disorders: a review. Autism 19, 387–399 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Failla MD et al. Initially intact neural responses to pain in autism are diminished during sustained pain. Autism 22, 669–683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bush RA, Connelly CD, Pérez A, Barlow H. & Chiang GJ Extracting autism spectrum disorder data from the electronic health record. Appl Clin Inform 8, 731–741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.