Abstract
Objectives
There is debate regarding the timing of procedural sedation and analgesia (PSA) in relation to fasting status. Point-of-care ultrasound (POCUS) provides the ability to measure gastric content and is being used as a surrogate for aspiration risk in anesthesia. We sought to evaluate the gastric content of Pediatric Emergency Department (PED) patients undergoing PSA using POCUS.
Methods
We performed a prospective observational study using a convenience sample of pediatric patients undergoing PSA between July 1, 2018 and June 30, 2019. Following a brief history, gastric content was measured using POCUS in both supine and right lateral decubitus positions at 2-hour intervals until the time of PSA. Qualitative content and calculated volume were classified based on the Perlas Model of anesthesia ‘Risk’ assessment.
Results
93 patients were enrolled with 61.3% male and mean age of 6.5 years. Gastric content was determined in 92 patients. 79.3% had ‘High Risk’ content at the time of PSA, with a median fasting time of 6.25 hours and no serious adverse events. Fasting duration had a weak to moderate ability to predict ‘Risk’ category (AUC = 0.73), with no patient (n=17) who underwent multiple evaluations awaiting PSA progressing from ‘High’ to ‘Low Risk.’
Conclusion
The majority of PED patients undergoing PSA at our institution had ‘High Risk’ gastric content with no clinically-significant change occurring during serial evaluations. This calls into question the utility of delaying PSA based upon fasting status, and lends support to a more comprehensive risk-benefit approach when planning pediatric PSA.
Keywords: Point-of-care ultrasound, fasting, procedural sedation, gastric content
Introduction
Procedural sedation and analgesia (PSA) is a common procedure in the Pediatric Emergency Department (PED) for patients requiring urgent noxious interventions. It allows both increased procedural success as well as minimizes the short and long term physical and psychological detrimental effects that can be incurred from uncontrolled pain and anxiety.1–3 Reported PSA outcomes are overwhelmingly favorable; however, adverse events can occur. Much work has been done to characterize these events and identify factors contributing to them.4–9
One particular adverse event deserving attention involves aspiration of gastric contents. Pulmonary aspiration carries risk of serious morbidity and mortality and has been estimated to account for 9% of all general anesthesia-related deaths.10,11 While potentially causing serious harm, the actual incidence of aspiration is overall quite low, occurring in 3.8–10.2 per 10,000 pediatric general anesthesia patients,12,13 and only 0.9 per 10,000 pediatric PSA patients.14
In general anesthesia, which typically involves relaxation of protective airway reflexes, a major contributor to aspiration risk is the quality and quantity of stomach contents. To address this risk, the American Society of Anesthesiologists (ASA) provides fasting guidelines, also known as nil per os (NPO) guidelines, for patients prior to elective procedures.15 While these guidelines are not meant for application to non-elective, emergent situations, they have historically been applied to PSA despite the fact that, in contrast to operative anesthesia, fasting status has been shown repeatedly to portend no increase in adverse events in pediatric PSA.14,16–22 More consistent with this data, multiple guidelines from the fields of emergency medicine23 and pediatrics24 advise a balanced risk-benefit approach to NPO guidelines and PSA. In its 2014 clinical policy on procedural sedation, the American College of Emergency Physicians (ACEP) included a Level B recommendation, “Do not delay procedural sedation in adults or pediatrics in the ED based on fasting time,”25 which they reiterated in their recent 2019 unscheduled procedural sedation guidelines.21 While these competing guidelines can be a source of consternation, the Centers for Medicare and Medicaid Services (CMS) endorses a system in which both ASA and ACEP guidelines are used in their respective arenas.26
Central to the debate regarding aspiration risk and gastric content is the lack of a standardized non-invasive method for easily and reproducibly assessing gastric content in real-time. Current standard of care relies on patient/parent report of last oral intake, which is open to any number of inaccuracies and requires an alert and communicative patient. Further, studies have shown parental recall of medical visits and perception of time during stressful situations to be poor,27,28 which raises question as to the accuracy of this data in the emergency room setting.
Ultrasound (US) has been used since the mid 1980s to assess gastric volume and emptying within the field of gastroenterology.29,30 Over the past decade the field of general anesthesia has evaluated the use of point-of-care ultrasound (POCUS) to assess gastric content in both adult and pediatric patients.31–42 This work has resulted in development of a model (the Perlas Model) to define the ‘at-risk’ stomach for purposes of pre-operative general anesthesia planning,34,35 which is now being used to better understand peri-operative gastric content43–46 and to guide anesthesia airway management.47–51 Additionally, gastric POCUS has been used to assess stomach content in critically-ill ICU patients52,53 and more recently in emergency department patients.54–58 Importantly, while the Perlas Model is now being used to evaluate gastric content in these settings, the applicability and implication of the model’s assigned ‘Risk’ categories outside of the anesthesia arena are entirely unknown, although for the sake of consistency with prior literature we will continue to use the model’s terminology.
The use of POCUS to better understand the gastric content of pediatric patients undergoing PSA is intriguing and could help to better inform decisions and debate surrounding NPO timing and PSA. We sought to use POCUS to assess the stomach content of PED patients undergoing PSA using the Perlas Model. Our primary goals were to determine the gastric content of pediatric patients at the time of PSA, and to describe how gastric content changed over time in patients awaiting PSA. We hypothesized that PED patients frequently have ‘non-empty’ stomachs at the time of PSA even when meeting ASA guidelines, and that patients awaiting PSA have little change in their gastric content.
Materials and Methods
Study Design and Setting
This was a prospective observational study using a convenience sample of pediatric patients presenting to the PED at our single tertiary care pediatric hospital which sees approximately 28,000 PED patients and performs about 500 PSA procedures annually. The institutional review board at the Medical University of South Carolina approved this study.
Study Population
Pediatric patients undergoing intravenous PSA between July 1, 2018 and June 30, 2019 were recruited for the study. Inclusion criteria included: (1) age 6 months or greater; (2) English speaking; and (3) ASA physical status I-III. Patients were excluded if they: (1) had known abnormal upper gastrointestinal anatomy or prior upper gastrointestinal surgery; (2) were pregnant (by self-reported or PED tested); (3) had vomited since most recent oral intake; (4) had wounds of the epigastrium precluding probe placement; (5) were deemed unable to tolerate US positioning; and (6) were deemed critically ill by the treating team. Potential participants were identified by the PED staff during hours when then primary investigator (PI – Dr. MMM) was present in the PED or could be in the PED prior to planned PSA. The PI performed all study enrollment and consent procedures in person with families in the PED prior to any study data collection. All patient care decisions were made by the treating team with no involvement or influence by study personnel. The treating team was kept blinded to all POCUS results.
Study Protocol and Data Collection
After enrollment and informed consent, a brief history was obtained which included age, gender, height, presenting complaint, past medical/surgical history, medications and timing of last administration, allergies, current and worst level of pain (0–10 FACES scale), and timing of most recent oral intake. Timing of last oral intake was determined separately for clears, thick liquid, breast milk, and solids. All times were recorded to the nearest quarter hour. Timing of planned PSA, and whether PSA was being delayed due specifically to concerns over NPO timing, were obtained from the attending physician. For patients anticipated to undergo PSA within 2 hours of enrollment, a single POCUS study was performed at the time of PSA (30 minutes before or after). In patients anticipated to undergo PSA beyond 2 hours from enrollment, serial measurements were made at 2-hour intervals (+/− 30 minutes) until the time of PSA. Following patient disposition from the PED, review of the electronic medical encounter was performed to record measured weight, final diagnosis, disposition, details of medications received, and relevant sedation data, including indication, timing, medications used, duration, outcome, and adverse events. All data were recorded using a standardized data collection instrument (Supplemental Figure 1).
All ultrasound studies were performed by the PI, a combined Pediatric Emergency Medicine and Emergency Ultrasound Fellow who had previously completed over 30 gastric POCUS scans and attended a full day in-person training seminar with Dr. Perlas prior to study enrollment. A Sonosite Edge (Sonosite; Bothell, WA) portable ultrasound machine equipped with both low-frequency curvilinear and high-frequency linear probes was used for all studies. Patients were imaged in the sagittal plane first in the supine position followed by the right lateral decubitus (RLD) position (Figure 1) following the technique described by Perlas31–35 and previously reviewed by our group.42 All patients were imaged with the curvilinear probe to establish a wide view of abdominal anatomy, with addition of the high-frequency linear probe to allow for increased resolution of gastric anatomy and content when patient habitus was amenable. A minimum of one live clip and three still images were obtained in each position. Stomach content was assigned as empty, clear liquid, or solid/thick liquid as shown in Figure 2. Antral cross-sectional area (CSA) at the level of the aorta was measured in the RLD position using a free-traced circumference. Gastric volume was determined via average of three individual CSA measurements using the pediatric equation:38 Volume = −7.8 + (3.5 × RLD CSA) + (0.127) × age (months). Final ‘Grade’ and ‘Risk’ category were assigned for all studies per the Perlas Model34,35,42 (Figure 3) using a volume cutoff of 1.25 ml/kg. All POCUS images were saved to the HIPAA-compliant web-based archiving system QPath (Telexy Healthcare; Maple Ridge, British Columbia, Canada).
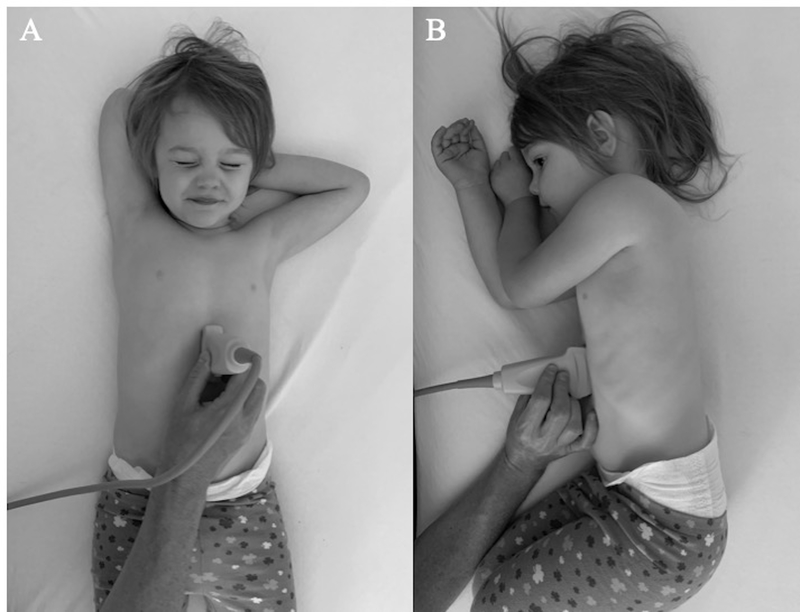
Figure 1.
Patient positioning and probe placement for gastric POCUS. A curvilinear or linear probe is placed in the midline sagittal plane inferior to the xiphoid process with the probe marker towards the patient’s head. Imaging is performed first in the supine position (A) and then the right lateral decubitus position (B).
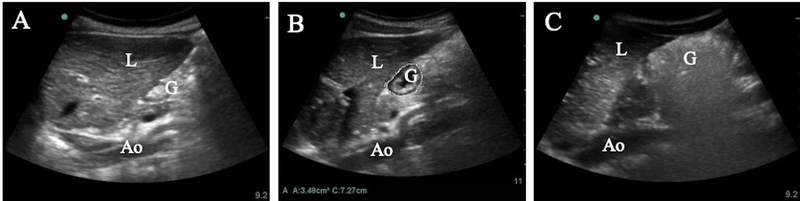
Figure 2.
Gastric content and volume assessment. Using the curvilinear probe, gastric content can be seen as (A) empty with opposing hyperechoic layers of antral mucosal, (B) hypoechoic clear fluid, or (C) heterogeneous hyperechoic solid/thick liquid with additional air artifact obscuring the posterior antral wall. Antral cross-sectional area can be calculated as shown in (B) using the free-tracing caliper (dotted line) to outline the circumference of the gastric antrum. G = Gastric antrum; L = Liver; Ao = Aorta.
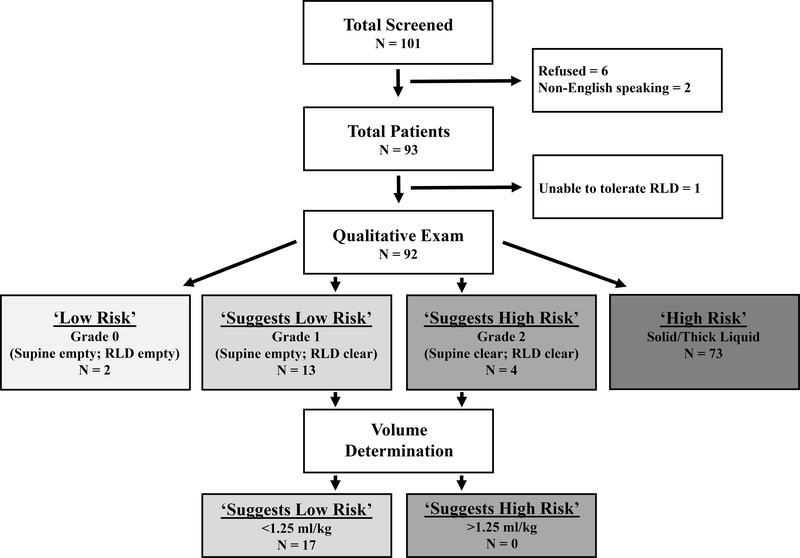
Figure 3.
Study flow diagram and Perlas Model ‘Risk’ stratification. Patient gastric content was assigned a ‘Grade’ (0, 1, 2, solid/thick) based on qualitative content seen in the supine and right lateral decubitus (RLD) positions. This was further refined based on qualitative volume measurements in patients with clear liquid content (‘Grades’ 1 and 2). Risk status was then assigned, with ‘Low Risk’ being those with empty content or clear liquid content < 1.25 ml/kg, and ‘High Risk’ being those with solid/thick liquid content or clear liquid content > 1.25 ml/kg.
Data Analysis
At the outset of this study, there were no reported studies evaluating gastric content in the PED context to guide sample size calculations. Prior studies from operative anesthesia had reported approximately 50% of non-elective surgery patients to have ‘High Risk’ content.45,51 With a total population of roughly 500 PSA patients annually, we calculated that an enrollment of 93 patients would allow us to estimate a 95% confidence interval for the proportion of ‘High Risk’ patients with a width of ± 10%. We estimated that we could successfully enroll 20% of our annual PSA volume for a total of 100 patients, which would allow for patients with indeterminate gastric content.
Descriptive statistics were calculated to describe the study population’s demographic and clinical characteristics. Categorical variables were described using frequencies and proportions, and continuous variables were described using either means with standard deviations (SD) or medians with interquartile ranges (IQR) as appropriate. The primary outcome of interest was the proportion of `High Risk’ patients and the 95% confidence interval for the proportion was estimated using Clopper-Pearson exact confidence intervals. Association between length of stay and delayed PSA based upon NPO status was evaluated using a Wilcoxon rank sum test. As an exploratory analysis, we also examined the association between fasting duration of solids and whether or not a patient was considered `High Risk’ using a univariate logistic regression model. The predictive ability of fasting duration was assessed using the 10-fold cross-validated area under the receiver operating characteristics curve (cvAUC) calculated using the predicted probabilities from the validation data excluded in fitting the 10-fold models. The 95% confidence intervals for the cvAUC were calculated using the method proposed by LeDell et al.59
POCUS images for all PSA patients were independently evaluated via QPath by two additional sonographers (BCP, Director of Emergency Ultrasound, and JGH, Director of Pediatric Radiology), to determine inter-rater agreement for gastric content interpretation using Fleiss’s kappa. Analyses were conducted in SAS v.9.4 (SAS Institute; Cary, NC) and R v. 3.5.2 (The Comprehensive R Archive Network, https://cran.r-project.org/).
Results
Patient & PSA Characteristics
A total of 101 patients were screened and 93 enrolled (Figure 3). During our 1-year enrollment period our department performed 339 total intravenous procedural sedations, and thus we captured 27.4% of all PSA patients during that window. Table 1 highlights major patient demographics and clinical characteristics of the study cohort. The overall population was predominantly male (61.3%) with a mean age of 6.5 ± 4.1 years. All patients were ASA class I (90.3%) or II (9.7%). The median worst pain score was 10/10, with 81.7% of patients endorsing severe pain (≥ 7/10). 47.3% of patients received narcotics prior to PSA.
Table 1.
Population Demographic and Clinical Characteristics.
| Total patients, n | 93 |
| Sex, male, n (%) | 57 (61.3) |
| Age, years, mean (SD) | 6.54 (4.14) |
| Height, m, mean (SD) | 1.20 (0.28) |
| Weight, kg, mean (SD) | 26.6 (14.6) |
| BMI, mean (SD) | 17.2 (3.08) |
| GERD, n (%) | 2 (2.15) |
| ASA Score, n (%) | |
| I | 84 (90.3) |
| II | 9 (9.7) |
| ESI, n (%) | |
| 2 | 3 (3.23) |
| 3 | 89 (95.7) |
| 4 | 1 (1.08) |
| Worst pain, median (IQR) | 10 (3) |
| Pain at PSA, median (IQR) | 2 (6) |
| Pain score ≥ 7, yes, n (%) | 76 (81.7) |
| Narcotics given prior to ED, yes, n (%) | 13 (14.0) |
| Narcotics given in ED, yes, n (%) | 39 (41.9) |
| Any Pre-PSA narcotics, yes, n (%) | 44 (47.3) |
| Admitted, n (%) | 10 (10.8) |
| Length of stay, minutes, median (IQR) | 307 (108) |
| ASA fasting guidelines met, n (%) | 37 (39.8) |
| Fasting duration, hours, median (IQR) | |
| Clears | 5.75 (3.25) |
| Solids | 6.25 (4) |
| Sedation delayed for fasting status, n (%) | 15 (16.1) |
| Sedation indication, n (%) | |
| Orthopedic reduction | 57 (61.3) |
| Laceration | 22 (23.6) |
| Incision & drainage | 7 (7.5) |
| Burn | 3 (3.2) |
| Other | 4 (4.3) |
| Sedation agent, n (%) | |
| Ketamine | 68 (73.1) |
| Ketamine & propofol | 23 (24.7) |
| Other | 2 (2.2) |
| Sedation complication, n (%) | |
| Post-PSA emesis | 3 (3.2) |
| Emergence agitation | 2 (2.2) |
| Aspiration | 0 (0) |
| Ondansetron given, n (%) | 19 (20.4) |
Data are reported as number (n) and percentage (%), mean and standard deviation (SD), or median and interquartile range (IQR). BMI = body mass index; ESI = emergency severity index; GERD = gastroesophageal reflux disease; ASA = American Society of Anesthesiologists; PSA = procedural sedation and analgesia.
39.8% of patients were considered fasting by ASA guidelines (>8 hours for solids and >2 hours for clears) at the time of sedation, with median fasting times of 6.25 and 5.75 hours for solids and liquids, respectively. 15 patients had their PSA delayed specifically due to provider concerns over NPO timing. Median LOS was 307 minutes overall. Patients whose PSA was delayed due to NPO concerns had an overall longer median LOS relative to those who did not (338 (IQR = 128; range: 244–712) versus 301 (IQR = 110; range 152–905) minutes, respectively; p = 0.039).
The most common indications for PSA were orthopedic reduction (61.2%) and laceration repair (23.7%). PSA was accomplished primarily with ketamine alone (73.1%) or combination of ketamine and propofol (24.7%). 3 patients had emesis during recovery, 19 patients received ondansetron, 2 had emergence agitation, and 1 patient sedated with ketamine and propofol had mild hypoxia to 89% that resolved with airway adjustment as well as mild hypotension that did not require intervention. There were no cases of aspiration.
Gastric Content at the Time of and Awaiting Sedation
A definitive Perlas ‘Risk’ category was successfully assigned in 92 of 93 patients at the time of PSA, with 1 patient failing to tolerate awake RLD positioning and subsequently refusing any further attempts. Overall, 79.3% (73/92; 95% CI = 69.6%−87.1%) of evaluable patients were classified as ‘High Risk,’ all due to the presence of solid content, 17 were classified as ‘Suggests Low Risk,’ and 2 were classified as ‘Low Risk.’ While 4 patients were classified as ‘Suggests High Risk’ based on Perlas ‘Grade’ alone, no patient was classified as such after evaluation of CSA, with all 4 having volumes < 1.25 ml/kg. Figure 3 outlines the breakdown of PSA patients based upon their Perlas ‘Grade’ and ‘Risk’ categories. Amongst patients who were fasting by ASA guidelines, 67.6% (25/37) were found to have ‘High Risk’ content. Of the 15 patients who had their PSA specifically delayed due to concerns over NPO timing, 13 (86.7%) had ‘High Risk’ content. All three patients with emesis during recovery had ‘High Risk’ content on US.
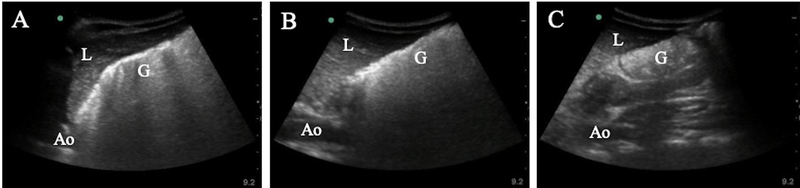
In addition to assessment of gastric content at the time of PSA, 17 patients were imaged 2 hours prior to sedation, and another 2 of these patients were imaged 4 hours prior. While in some cases a clearing of air artifact and/or decrease in gastric content could be appreciated, no patient had a transition in gastric content from ‘High Risk’ to ‘Low Risk.’ 4 patients did transition from an ASA classification of non-fasting to fasting during this time frame (Table 2). Figure 4 displays the gastric content progression for one such case, a 7-year old boy who presented for evaluation of a forearm fracture approximately 4 hours after eating “a lot of pizza.” POCUS demonstrated solid/thick liquid content with heavy air artifact at 4.25 and 6.25 hours NPO, and at 8.5 hours, when he underwent PSA, demonstrated clearing of air artifact but persistent solid/thick liquid content. A 1-hour increase in solid fasting duration was associated with a 14% decrease in the odds of being categorized as ‘High Risk’ at initial evaluation (OR: 0.86, 95% CI: 0.78–0.96; p = 0.005). The cvAUC for fasting duration was 0.73 (95% CI: 0.61–0.84) suggesting weak to moderate ability of fasting duration to predict ‘Risk’ category.
Table 2.
Serial Ultrasound Studies for Patients undergoing Procedural Sedation.
| Clinical Characteristics | Gastric Content 4 Hours Before PSA | Gastric Content 2 Hours Before PSA | Gastric Content at PSA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Gender | BMI | Worst Pain (0–10) | Pre-PSA Narcotics | LOS (minutes) | Sedation Indication | Fasting Solids (h) | Perlas Grade | Volume (ml/kg) | Risk Assessment | Fasting Solids (h) | Perlas Grade | Volume (ml/kg) | Risk Assessment | Fasting Solids (h) | Perlas Grade | Volume (ml/kg) | Risk Assessment |
| PSA12 | 14 | M | 18.92 | 10 | Yes | 278 | Ortho | 3.25 | Solid | UTD | High | 5.50 | Solid | 0.71 | High | ||||
| PSA17 | 7 | M | 16.49 | 9 | Yes | 439 | Ortho | 4.25 | Solid | UTD | High | 6.25 | Solid | UTD | High | 8.50 | Solid | 1.40 | High |
| PSA21 | 15 | F | 18.22 | 9 | Yes | 366 | Ortho | 3.00 | Solid | 0.74 | High | 6.00 | Solid | 0.73 | High | ||||
| PSA22 | 3 | F | 16.07 | 10 | No | 408 | Ortho | 3.00 | Solid | UTD | High | 6.00 | Solid | 0.60 | High | ||||
| PSA24 | 8 | F | 17.57 | 10 | Yes | 324 | Ortho | 4.00 | Solid | UTD | High | 7.50 | Solid | UTD | High | ||||
| PSA27 | 4 | M | 14.15 | 10 | No | 204 | Ortho | 7.50 | Solid | UTD | High | 9.50 | Solid | 0.50 | High | ||||
| PSA28 | 8 | F | 15.32 | 10 | Yes | 318 | Ortho | 2.50 | Solid | UTD | High | 4.75 | Solid | 0.63 | High | ||||
| PSA41 | 9 | M | 15.55 | 10 | Yes | 712 | Ortho | 3.50 | Solid | UTD | High | 5.75 | Solid | 1.31 | High | 7.75 | Solid | 0.81 | High |
| PSA43 | 3 | F | 15.84 | 10 | No | 331 | Laceration | 5.25 | Solid | 0.92 | High | 8.00 | Solid | 1.25 | High | ||||
| PSA44 | 13 | M | 16.92 | 9 | No | 327 | Ortho | 4.25 | Solid | UTD | High | 6.25 | Solid | 0.84 | High | ||||
| PSA46 | 6 | M | 14.23 | 9 | Yes | 368 | Ortho | 3.00 | Solid | UTD | High | 5.25 | Solid | UTD | High | ||||
| PSA48 | 8 | M | 19.55 | 8 | No | 325 | Ortho | 2.00 | Solid | UTD | High | 4.00 | Solid | UTD | High | ||||
| PSA50 | 1 | M | 13.66 | 6 | No | 290 | Laceration | 2.25 | Solid | 0.75 | High | 4.25 | Solid | 0.33 | High | ||||
| PSA50 | 0.83 | F | 20.63 | 10 | No | 379 | Laceration | 5.75 | Solid | 0.16 | High | 7.75 | Solid | 0.10 | High | ||||
| PSA53 | 13 | M | 17.54 | 9 | Yes | 362 | Ortho | 6.25 | Solid | 0.68 | High | 8.25 | Solid | 0.87 | High | ||||
| PSA85 | 8 | F | 18.77 | 10 | No | 244 | Ortho | 2.75 | Solid | UTD | High | 4,75 | Solid | UTD | High | ||||
| PSA86 | 7 | F | 14.62 | 10 | No | 293 | Ortho | 8.00 | Solid | UTD | High | 10.00 | Solid | 1.35 | High | ||||
Shown here are the measurements for the 17 patients who underwent serial ultrasound studies prior to PSA. M = male; F = female; BMI = body mass index; PSA = procedural sedation and analgesia; LOS = length of stay; UTD = unable to determine.
Figure 4.
Serial gastric content measurements in a procedural sedation (PSA) patient. Serial ultrasound (US) examinations were performed on a healthy 7-year-old male awaiting PSA to reduce a forearm fracture. US demonstrated solid/thick liquid content with heavy air artifact at (A) 4.25 and (B) 6.25 hours fasting, and at (C) 8.5 hours, when he underwent PSA, demonstrated clearing of air artifact but persistent solid/thick liquid content. G = Gastric antrum; L = Liver; Ao = Aorta.
Review of all PSA studies by three independent physicians yielded an exact agreement for Perlas Risk category of 87.4% with a Fleiss’s kappa for inter-rater agreement of 0.75, suggesting good agreement. 52 of 93 patients were scanned after PSA per parent request. Total scan time was not directly measured but was approximately 5 minutes.
Discussion
In this study we investigated the use of gastric POCUS to evaluate stomach content in pediatric PSA patients both at the time of PSA and over time while awaiting PSA. We identified nearly 80% of patients undergoing PSA in our department to have gastric content that would be classified as ‘High Risk’ using the Perlas Model of anesthesia risk-stratification34,35,42 despite a median fasting time of over 6 hours. Even in patients who were conservatively (>8 hours solid intake) considered fasting per ASA guidelines,15 over 60% had ‘High Risk’ content, indicating that fasting by NPO time is a poor prognosticator of ‘Low Risk’ gastric content in the PED PSA setting. This is further supported by our regression model showing only a weak to moderate ability of fasting duration to predict ‘Risk’ category. These findings resemble those of the recently published study by Leviter and colleagues56 who reported 69% of 107 pediatric patients to have a ‘Full Stomach’ at the time of PSA with a median fasting time of 5.8 hours and a weak predictive ability of fasting duration in discriminate amongst ‘Risk’ categories (C-index 0.66). In addition to gastric content at the time of PSA, we were able to perform repeat evaluations in 17 patients. Recognizing that these represent a relatively small population, none of these patients demonstrated a transition in ‘Risk’ category, all remaining ‘High Risk’ throughout their serial evaluation. To our knowledge this is the first study to serially evaluate gastric content in the PED or PSA contexts.
In contrast to our data, studies from the elective surgery arena consistently show fasting elective surgical patients to have ‘High Risk’ content in less than 5% of both pediatric and adult patients,32,44,46 including rates of only 1% in 200 pediatric patients presenting for non-GI related elective surgical procedures46 and 0% in 75 pediatric patients presenting for elective ear, nose, and throat procedures.44 Even fasting pediatric patients presenting for scheduled upper endoscopy, a population with an increased incidence of gastrointestinal pathology, had a rate of only 9% ‘High Risk’ content.38 Two small studies have evaluated serial measurements of gastric content in adult elective surgery patients who endorsed fasting non-compliance and demonstrated rapid gastric emptying in this context.48,49 The larger of these two studies reported 18 of 22 patients transitioning from ‘High Risk’ to ‘Low Risk’ following repeat POCUS measurements performed 1–4 hours later with a median fasting time of 6 hours for the second exam.49 However, in non-elective surgical patients, studies have shown that 56% of adult45 and 46% of pediatric51 patients had ‘High Risk’ gastric content despite average fasting times of 18 and 11 hours, respectively; numbers much more akin to those of our study and the study by Leviter.56 Thus, there seems to be something inherent about patients in the emergent setting that causes gastric emptying to be very different from the elective setting. Multiple factors likely explain this. Prior studies have identified pain, stress, trauma, diabetes, obesity, increasing ASA status, and pre-operative opioid administration to delay gastric emptying.13,45,60,61 In our ongoing work we hope to further evaluate some of these factors using a larger cohort of PED patients.
The incidence of recovery emesis in our study is on par with prior reported studies.4–9 The fact that these occurred in patients with ‘High Risk’ content is not surprising given the vast majority of our cohort had such content. Larger studies would be needed to determine if there is a link between ‘High Risk’ content and recovery emesis. While there were no significant adverse events in our study, it was vastly underpowered to evaluate this, nor was that our intention. The overwhelming preponderance of evidence already indicates that fasting status does not have implication on adverse events in pediatric PSA, and that pediatric PSA is incredibly safe.14,16–22 Our data shows that the majority of patients undergoing standard of practice PSA do so with ‘High Risk’ gastric content, and that this content is unlikely to change significantly during the PED encounter. While perhaps counterintuitive, we believe this fact adds further support against delaying PSA in order to meet operative fasting guidelines. Such delays will likely provide little decrease in gastric content and perhaps give providers a false sense of reassurance when a patient is fasting, while at the same time have been shown to have negative consequences including procedural delays, decreased sedation efficacy, and increased anxiety, resource utilization, length of stay, and sedative dosing.62–65 Providers should instead balance patient risk factors, procedural urgency, and sedative technique to guide appropriate PSA timing as recommended by multiple recent consensus statements.21,22
Further studies are needed to expand upon the generalizability of these findings and to determine what role gastric US can provide in the clinical and research settings. Prior studies have utilized this technique to guide use of pre-intubation gastric decompression in controlled surgical50 and intensive care53 settings. This is unlikely to be of utility in the emergent setting of most PED intubations, but may be of benefit in the rare cases when advanced airway management is adequately anticipated. In these same instances, knowledge of stomach content may also help guide selection of induction agent or preoxygenation method, favoring those agents with retained airway protective reflexes and avoiding/minimizing bag-mask ventilation in patients with ‘High Risk’ content, respectively. Regarding pediatric PSA, the extremely low rate of adverse events in current practice likely precludes benefit of incorporating gastric US into routine use. It may have a role in augmenting recent clinical decision-making algorithms22 for select patients with identified patient and procedural risk factors such as severe systemic disease, obesity, procedures involving the upper airway, or use of airway relaxing agents such as propofol. Gastric US may further have a role in helping to identify additional risk factors, such as severe pain, trauma, and opioid analgesia, to aid in pre-sedation patient assessment.
Limitations
This study is subject to a number of limitations. First, we utilized a convenience sample of patients at a single institution, and as such our study population may not be representative of our own full population or those at other institutions. While we did not reach our enrollment goal by number (93 versus 100), due to an overall decrease in intravenous PSA volume, we were able to exceed our estimated catchment fraction (27.4 % versus 20%), making our own internal generalizability good. The causes for our decreased PSA volumes are not entirely known, but likely include increased patient volume at a competing private hospital as well as our own recent introduction of inhaled nitrous oxide. Our data as a whole resemble those from recently published studies from other institutions,55,56 supporting perhaps a broader generalizability.
We relied on patient and/or parent report of last PO intake. This is likely fraught with inaccuracy as parental recall of medical visits and perception of time during stressful situations has been shown to be poor,27,28 and the quality and quantity of routine PO intake is highly subjective. This limitation is, however, currently the standard of care for both operative anesthesia and PSA planning and not a specific limitation to this study. We did not attempt to discriminate a ‘light’ versus ‘full’ meal in this study, opting to assign all solid intake as a ‘full’ meal requiring 8 hours to be considered fasting. This simplification was chosen to provide as conservative an estimate of fasting as possible. Use instead of a 6-hour fasting definition for light meals would result in an increase in fasting patients with ‘High Risk’ gastric content in our study.
A single investigator with significant US experience performed all POCUS examinations, and our inter-rater reliability was assessed on saved digital images, both limiting broader applicability to general PEM providers. However, training on this specific modality was consistent with standard ACEP and PEM recommendations for POCUS competency.66–68 Prior studies have demonstrated learning curves similar to other POCUS modalities69 as well as high intra- and inter-rater reliability.70 For the sake of study consistency, we utilized both curvilinear and linear probes when patient body habitus was amenable. In many patients no additional information was gained with this approach, and future studies or applications could likely forego use of both probes to save scan time. 52 of 93 PSA patients were scanned a max of 30 minutes after PSA. This delay may have allowed for a small change in gastric content that likely would have had little implication on ‘Risk’ categorization. Importantly, the option for post-PSA POCUS was presented to families during study enrollment and does not represent patient intolerance of the POCUS protocol.
There is no known safe aspiration volume. Prior studies have debated this cutoff and used anywhere between 0.8 and 1.5 ml/kg.33–35,37–38,42,44,46,56,71,72 We chose our cutoff of 1.25 ml/kg based on data by Cook-Sather71 in which 95% of 611 fasting pediatric patients undergoing elective surgery demonstrated a volume <1.25 ml/kg. Implications of various quality and quantity of solid content have also not been quantified. The Perlas Model assigns the presence of any solid content as being ‘High Risk’ regardless of volume. This binary classification of ‘High Risk’ versus ‘Low Risk’ is convenient but overly simplistic of content that exists on a continuum.73 Further work may allow finer delineation of content classification and ‘Risk’ implications beyond the model utilized here.
Conclusions
The majority of PED patients undergoing PSA at our institution had ‘High Risk’ gastric content based on the Perlas Model of pre-operative anesthesia ‘Risk’ assessment, even in those meeting ASA fasting guidelines, with no clinically significant change occurring during serial evaluations. This calls into question the utility of delaying PSA based upon fasting status, and lends further support to a more comprehensive risk-benefit approach when planning pediatric PSA.
Supplementary Material
Supplemental Figure 1. Standardized study data collection sheet.
Acknowledgements
The authors thank Dr. Anahi Perlas for her training on the POCUS technique. This project was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UL1 TR001450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grants & Funding: This project was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UL1 TR001450.
Footnotes
Conflict of Interest: None
References
- 1.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Eng J Med. 1987;317(21):1321–1329. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Manno M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann Emerg Med. 2003;41(5):617–622. [DOI] [PubMed] [Google Scholar]
- 3.Drendel AL, Kelly BT, Ali S. Pain assessment for children: overcoming challenges and optimizing care. Pediatr Emerg Care. 2011;27(8):773–781. [DOI] [PubMed] [Google Scholar]
- 4.Roback MG, Wathen JE, Bajaj L, et al. Adverse events associated with preprocedural sedation and analgesia in a pediatric emergency department: A comparison of common parenteral drugs. Acad Emerg Med. 2005;12(6):508–513. [DOI] [PubMed] [Google Scholar]
- 5.Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: Report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118(3):1087–1096. [DOI] [PubMed] [Google Scholar]
- 6.Green SM, Roback MG, Krauss B, et al. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54(2):158–168. [DOI] [PubMed] [Google Scholar]
- 7.Green SM, Roback MG, Krauss B, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54(2):171–180. [DOI] [PubMed] [Google Scholar]
- 8.Bellolio MF, Puls HA, Anderson JL, et al. Incidence of adverse events in paediatric procedural sedation in the emergency department: a systematic review and meta-analysis. BMJ Open. 2016;6(6):e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt M, Johnson DW, Chan J, et al. Risk factors for adverse events in emergency department procedural sedation for children. JAMA Pediatr. 2017;171(10):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lienhart A, Auroy Y, Pequignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105(6):1087–1097. [DOI] [PubMed] [Google Scholar]
- 11.Landreau B, Odin I, Nathan N. Pulmonary aspiration: epidemiology and risk factors. Ann Fr Anesth Reanim. 2009;28(3):206–210. [DOI] [PubMed] [Google Scholar]
- 12.Borland LM, Sereika SM, Woelfel SK, et al. Pulmonary aspiration in pediatric patients during general anesthesia: incidence and outcomes. J Clin Anesth. 1998;10(2):95–102. [DOI] [PubMed] [Google Scholar]
- 13.Warner MA, Warner ME, Warner DO, et al. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90(1):66–71. [DOI] [PubMed] [Google Scholar]
- 14.Beach ML, Cohen DM, Gallagher SM, et al. Major adverse events and relationship to nil per os status in pediatric sedation/anesthesia outside the operating room: a report of the Pediatric Sedation Research Consortium. Anesthesiology. 2016;124(1):80–88. [DOI] [PubMed] [Google Scholar]
- 15.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376–393. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal D, Manzi SF, Gupta R, et al. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 2003;42(5):636–646. [DOI] [PubMed] [Google Scholar]
- 17.Roback MG, Bajaj L, Wathen JE, et al. Preprocedural fasting and adverse events in procedural sedation and analgesia in a pediatric emergency department: are they related? Ann Emerg Med. 2004;44(5):454–459. [DOI] [PubMed] [Google Scholar]
- 18.Green SM, Mason KP, Krauss BS. Pulmonary aspiration during procedural sedation: a comprehensive systematic review. Br J Anaesth. 2017;118(3):344–354. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt M, Johnson DW, Taljaard M, et al. Association of preprocedural fasting with outcomes of emergency department sedation in children. JAMA Pediatr. 2018;172(7):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chumpitazi CE, Camp EA, Bhamidipati DR, et al. Shortened preprocedural fasting in the pediatric emergency department. Am J Emerg Med. 2018;36(9):1577–1580. [DOI] [PubMed] [Google Scholar]
- 21.Green SM, Roback MG, Krauss BS, et al. Unscheduled procedural sedation: a multidisciplinary consensus practice guideline. Ann Emerg Med. 2019;73(5):e51–e65. [DOI] [PubMed] [Google Scholar]
- 22.Green SM, Leroy PL, Roback MG, et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children. Anaesthesia. 2020;75(3):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green SM, Roback MG, Miner JR, et al. Fasting and emergency department procedural sedation and analgesia: a consensus-based clinical practice advisory. Ann Emerg Med. 2007;49(4):454–461. [DOI] [PubMed] [Google Scholar]
- 24.Cote CJ, Wilson S, American Academy of Pediatrics, et al. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures: update 2016. Pediatrics. 2016;138(1):e1–33. [DOI] [PubMed] [Google Scholar]
- 25.Godwin SA, Burton JH, Gerardo CJ, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2014;63(2):247–258. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare & Medicaid Services (CMS). Revised Hospital Anesthesia Services Interpretive Guidelines – State Operations Manual (SOM) Appendix A. 2011. Available at https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCLetter11_10.pdf. Accessed February 19, 2018.
- 27.Grover G, Berkowitz CD, Lewis RJ. Parental recall after a visit to the emergency department. Clin Pediatr (Phila). 1994;33:194–201. [DOI] [PubMed] [Google Scholar]
- 28.Kelly C, Shulman V, Khine H, et al. Parental perception of the passage of time during a stressful event. Pediatr Emerg Care. 2007;23(6):376–379. [DOI] [PubMed] [Google Scholar]
- 29.Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89(4):752–759. [DOI] [PubMed] [Google Scholar]
- 30.Holt S, Cervantes J, Wilkinson AA, et al. Measurement of gastric emptying rate in humans by real-time ultrasound. Gastroenterology. 1986;90(4):918–923. [DOI] [PubMed] [Google Scholar]
- 31.Perlas A, Chan VW, Lupu CM, et al. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111(1):82–89. [DOI] [PubMed] [Google Scholar]
- 32.Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113(1):93–97. [DOI] [PubMed] [Google Scholar]
- 33.Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116(2):357–363. [DOI] [PubMed] [Google Scholar]
- 34.Perlas A, Van de Putte P, Van Houwe P, et al. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116(1):7–11. [DOI] [PubMed] [Google Scholar]
- 35.Perlas A, Arzola C, Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anaesth. 2018;65(4):437–448. [DOI] [PubMed] [Google Scholar]
- 36.Bouvet LM, Miquel A, Chassard D, et al. Could a single standardized ultrasonographic measurement of antral area be of interest for assessing gastric contents? A preliminary report. Eur J Anaesthesiol. 2009;26(12):1015–1019. [DOI] [PubMed] [Google Scholar]
- 37.Bouvet L, Mazoit JX, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114(5):1086–1092. [DOI] [PubMed] [Google Scholar]
- 38.Spencer AO, Walker AM, Yeung AK, et al. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: development of a predictive model using endoscopically suctioned volumes. Paediatr Anaesth. 2015;25(3):301–308. [DOI] [PubMed] [Google Scholar]
- 39.Moser JJ, Walker AM, & Spencer AO. Point-of-care paediatric gastric sonography: can antral cut-off values be used to diagnose an empty stomach? Br J Anaesth. 2017;119(5); 943–947. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz A, Thomas S, Melanie F, et al. Ultrasonographic gastric antral area and gastric contents volume in children. Paediatr Anaesth. 2012:22(2):144–149. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz A, Schmidt AR, Buelher PH, et al. Gastric ultrasound as a preoperative bedside test for residual gastric contents volume in children. Paediatr Anaesth. 2016;26(12):1157–1164. [DOI] [PubMed] [Google Scholar]
- 42.Moake MM, Jackson BF, Presley BC. Point-of-care ultrasound to assess gastric content. Pediatr Emerg Care. Epub ahead of print 11.13.19. [DOI] [PubMed] [Google Scholar]
- 43.Van de Putte P, Vernieuwe L, Jerjir A, et al. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118(3):363–371 [DOI] [PubMed] [Google Scholar]
- 44.Desgranges FP, Gagey Riegel AC, Aubergy C, et al. Ultrasound assessment of gastric contents in children undergoing elective ear, nose, and throat surgery: a prospective cohort study. Anaesthesia. 2017;72(11):1351–1356. [DOI] [PubMed] [Google Scholar]
- 45.Bouvet L, Desgranges FP, Aubergy C, et al. Prevalence and factors predictive of full stomach in elective and emergency surgical patients: a prospective cohort study. Br J Anaesth. 2017;118(3):372–379. [DOI] [PubMed] [Google Scholar]
- 46.Bouvet L, Bellier N, Gagey-Riegel AC, et al. Ultrasound assessment of the prevalence of increased gastric contents and volume in elective pediatric patients: a prospective cohort study. Paediatr Anaesth. 2018;28(10):906–913. [DOI] [PubMed] [Google Scholar]
- 47.Van de Putte P Bedside gastric ultrasonography to guide anesthetic management in a nonfasted emergency patient. J Clin Anesth. 2013;25(2):165–166. [DOI] [PubMed] [Google Scholar]
- 48.Van de Putte P, van Hoonacker J, Perlas A. Gastric ultrasound to guide anesthetic management in elective surgical patients non-compliant with fasting instructions: a retrospective review. Minerva Anestesiol. 2018;84(7):787–795. [DOI] [PubMed] [Google Scholar]
- 49.Alakkad H, Kruisselbrink R, Chin KJ, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anaesth. 2015;62(11):1188–1195. [DOI] [PubMed] [Google Scholar]
- 50.Gagey AC, de Queiroz Siqueira M, Desgranges FP, et al. Ultrasound assessment of the gastric contents for the guidance of the anaesthetic strategy in infants with hypertrophic pyloric stenosis: a prospective cohort study. Br J Anaesth. 2016;116(5):649–654. [DOI] [PubMed] [Google Scholar]
- 51.Gagey AC, de Queiroz Siqueira M, Monard C, et al. The effect of pre-operative gastric ultrasound examination on the choice of general anaesthetic induction technique for non-elective paediatric surgery. A prospective cohort study. Anaesthesia. 2018;73(3):304–312. [DOI] [PubMed] [Google Scholar]
- 52.Hamada SR, Garcon P, Ronot M, et al. Ultrasound assessment of gastric volume in critically ill patients. Intensive Care Med. 2014;40(7):965–972. [DOI] [PubMed] [Google Scholar]
- 53.Koenig SJ, Lakticova V, Mayo PH. Utility of ultrasonography for detection of gastric fluid during urgent endotracheal intubation. Intensive Care Med. 2011;37(4):627–631. [DOI] [PubMed] [Google Scholar]
- 54.Jacoby J, Smith G, Eberhardt M, et al. Bedside ultrasound to determine prandial status. Am J Emerg Med. 2003;21(3):216–219. [DOI] [PubMed] [Google Scholar]
- 55.Azad AM, Al Madi HA, Abdull Wahab SF, et al. Gastric ultrasonography in evaluating NPO status of pediatric patients in the emergency department. Am J Emerg Med. 2019;37(2):355–356. [DOI] [PubMed] [Google Scholar]
- 56.Leviter J, Steele DW, Constantine E, et al. “Full stomach” despite the wait: point-of-care gastric ultrasound at the time of procedural sedation in the pediatric emergency department. Acad Emerg Med. 2019;26(7):752–760. [DOI] [PubMed] [Google Scholar]
- 57.Mackenzie DC, Azad AM, Noble VE, et al. Test performance of point-of-care ultrasound for gastric content. Am J Emerg Med. 2019;37(1):123–126. [DOI] [PubMed] [Google Scholar]
- 58.Miller AF, Levy JL, Krauss BS, et al. Does point-of-care ultrasound correlate with reported fasting time? Pediatr Emerg Care. Epub ahead of print 1.6.20. [DOI] [PubMed] [Google Scholar]
- 59.LeDell E, Petersen ML, van der Laan MJ. Computationally efficient confidence intervals for cross-validated area under the ROC curve estimates. UCBerkeley Division of Biostatistics Working Paper Series. December 2012; Working Paper 304: http://biostats.bepress.com/ucbbiostat/paper304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouvet L, Duflo F, Bleyzac N, et al. Erythromycin promotes gastric emptying during acute pain in volunteers. Anesth Analg. 2006;102(6):1803–1808. [DOI] [PubMed] [Google Scholar]
- 61.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56–62. [DOI] [PubMed] [Google Scholar]
- 62.Green SM, Krauss B. Pulmonary aspiration risk during emergency department procedural sedation – an examination of the role of fasting and sedation depth. Acad Emerg Med. 2002;9(1):35–42. [DOI] [PubMed] [Google Scholar]
- 63.Hoffman GM, Nowakowski R, Troshynski TJ, et al. Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics. 2002;109(2):236–243. [DOI] [PubMed] [Google Scholar]
- 64.Ghaffar S, Haverland C, Ramaciotti C, et al. Sedation for pediatric echocardiography: evaluation of preprocedure fasting guidelines. J Am Soc Echocardiogr. 2002;15(9):980–983. [DOI] [PubMed] [Google Scholar]
- 65.Keidan I, Gozal D, Minuskin T, et al. The effect of fasting practice on sedation with chloral hydrate. Pediatr Emerg Care. 2004;20(12):805–807. [DOI] [PubMed] [Google Scholar]
- 66.American College of Emergency Physicians (ACEP). Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27–e54. [DOI] [PubMed] [Google Scholar]
- 67.Vieira RL, Hsu D, Nagler J, et al. Pediatric emergency medicine fellow training in ultrasound: consensus educational guidelines. Acad Emerg Med. 2013;20(3):300–306. [DOI] [PubMed] [Google Scholar]
- 68.Abo AA, Alade KH, Rempell RG, et al. Credentialing pediatric emergency medicine faculty in point-of-care ultrasound. Pediatr Emerg Care. Epub ahead of print 1.7.19. [DOI] [PubMed] [Google Scholar]
- 69.Arzola C, Carvalho JC, Cubillos J, et al. Anesthesiologists’ learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anaesth. 2013;60(8):771–779. [DOI] [PubMed] [Google Scholar]
- 70.Kruisselbrink R, Arzola C, Endersby R, et al. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121(1):46–51. [DOI] [PubMed] [Google Scholar]
- 71.Cook-Sather SD, Liacouras CA, Previte JP, et al. Gastric fluid measurement by blind aspiration in paediatric patients: a gastroscopic evaluation. Can J Anaesth. 1997;44(2):168–172. [DOI] [PubMed] [Google Scholar]
- 72.Van de Putte, Perlas A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anesthesia. 2018;73(3):274–279. [DOI] [PubMed] [Google Scholar]
- 73.Kinsella SM. The ‘full stomach’: full time for sloppy terminology? Anesthesia. 2018;73(10):1189–1190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Standardized study data collection sheet.