Abstract
Projections of ventral tegmental area dopamine (DA) neurons to the medial shell of the nucleus accumbens have been increasingly implicated as integral to the behavioral and physiological changes involved in the development of substance use disorders (SUDs). Recently, many of these nucleus accumbens-projecting DA neurons were found to also release the neurotransmitter glutamate. This glutamate co-release from DA neurons is critical in mediating the effect of drugs of abuse on addiction-related behaviors. Potential mechanisms underlying the role(s) of dopamine/glutamate co-release in contributing to SUDs are unclear. Nevertheless, an important clue may relate to glutamate’s ability to potentiate loading of DA into synaptic vesicles within terminals in the nucleus accumbens in response to drug-induced elevations in neuronal activity, enabling a more robust release of DA after stimulation. Here, we summarize how drugs of abuse, particularly cocaine, opioids and alcohol, alter DA release in the nucleus accumbens medial shell, examine the potential role of DA/glutamate co-release in mediating these effects, and discuss future directions for further investigating these mechanisms.
Keywords: dopamine, glutamate, substance use disorder, vesicular glutamate transporter 2, co-release, co-transmission, cocaine, opioids, alcohol
Introduction
Addictive substances including cocaine, opioids and alcohol, and the ensuing substance use disorders (SUDs) produced by these drugs, place high economic and medical burdens on society [1–4], and drug overdose deaths continue to rise [5]. Despite this enormous public health impact, there is still no clear understanding of the mechanisms by which these drugs of abuse work, particularly across cellular and behavioral levels. Additionally, most treatments are aimed at symptomatic relief rather than targeting the mechanisms responsible for the reinforcing aspects of drugs of abuse. This presents an urgent need to uncover the underlying neurological mechanisms behind SUDs so that more effective treatments can be created.
Drug-induced changes in dopamine (DA) neurotransmission have classically been the mainstay of our understanding of how drugs of abuse produce motivating and reinforcing properties [6–8]. Virtually all addictive drugs enhance DA neurotransmission [6, 9–11], either by: 1) enhancing presynaptic DA release, 2) reducing DA reuptake, 3) modifying presynaptic release probability, or via some combination of these presynaptic mechanisms [6, 12–14]. Additionally, the regional specificity of this DAergic neurotransmission is equally critical for the addictive properties of drugs. DA neurons which project from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) play a major role in SUDs [15–22]. Indeed, blocking DA neurotransmission in the NAc greatly reduces the reinforcing properties of addictive drugs [23–25]. Furthermore, recent improvements in imaging and optogenetic techniques have identified the medial shell of the nucleus accumbens (mNAcSh) to be crucial for the addictive behaviors associated with drugs like cocaine, opioids and alcohol [17, 22, 26–32]. These mNAcSh-projecting DA neurons are found in the medial portion of the VTA [27, 33], suggesting that the medial VTA-mNAcSh projection is fundamentally critical in drug reinforcement, motivation, and reward-related behaviors.
Medial VTA DA neurons: unique properties
mNAcSh-projecting medial VTA DA neurons possess unique properties that distinguish these cells from other DAergic neuron subpopulations. Unlike midbrain DA neurons projecting to the dorsal striatum which fire periodically at frequencies <10 Hz, these mNAcSh-projecting medial VTA DA neurons fire at high frequencies (>20 Hz) over extended periods of time [17, 34–36]. These neuron population-specific differences in firing frequency are correlated with higher basal firing rates, burst activity, and heightened vulnerability to drugs like cocaine [37]. Additionally, modulatory afferent inputs and gene expression patterns in medial VTA DA neurons are different from other DAergic areas [17, 34, 38]. Perhaps most importantly, a subset of medial VTA DA neurons co-release the neurotransmitter glutamate in addition to DA [39, 40]. A majority of these medial VTA DA/glutamate co-releasing neurons project their terminals to the mNAcSh [15, 41–44]. This is based on a growing body of evidence from multiple approaches. First, stimulation of medial VTA DA neurons evokes excitatory postsynaptic potentials in the mNAcSh [17, 45]. Second, the majority of medial VTA DA neurons projecting to the mNAcSh express vesicular glutamate transporter 2 (VGLUT2), which packages glutamate into vesicles for synaptic release [17, 39, 44, 46, 47]. In particular, studies have identified the rostral linear (RLi) and interfascicular (IF) nuclei of the VTA in rodent midbrain [43, 46] and the parabrachial pigmented (PBP) nucleus in humans [48] as areas containing the majority of VGLUT2-expressing DA neurons (Fig. 1). Finally, retrograde tracing of the mNAcSh shows that labeled VTA neurons almost exclusively express tyrosine hydroxylase (TH), the rate-limiting enzyme of DA synthesis [17, 27]. Nevertheless, apparent discrepancies remain in the precise numbers of medial VTA TH+/VGLUT2+ neurons. Immunocytochemistry studies by Chuhma and colleagues demonstrated that 79% of all medial VTA neurons were TH+/VGLUT2+ in juvenile mice, with the number of double-positive neurons declining to 48% by adulthood [17]. This result is consistent with earlier work in isolated postnatal rat mesencephalic DA neurons where ~80% of the neurons expressed VGLUT2 protein [39, 47]. In contrast, only ~40% of TH+ VTA DA neurons in P25 juvenile mice express VGLUT2, at least at the mRNA level [41]. In contrast, other studies have reported significantly lower numbers of VGLUT2+ DA neurons in the VTA ranging from 0.1–25% [39, 40, 49]. Such variability in VTA TH+/VGLUT2+ neuron numbers may depend on the age/developmental stage sampled, the approaches used and potential differences between mRNA versus protein expression of VGLUT2 and TH markers. Despite this potential variability in the neuron number, there is substantial evidence that glutamate co-release from this unique subset of medial VTA DA neurons plays a critical role in mediating the cellular response to addictive drugs (Fig. 2).
Figure 1. Spatial distribution of VTA TH+/VGLUT2+ neurons.
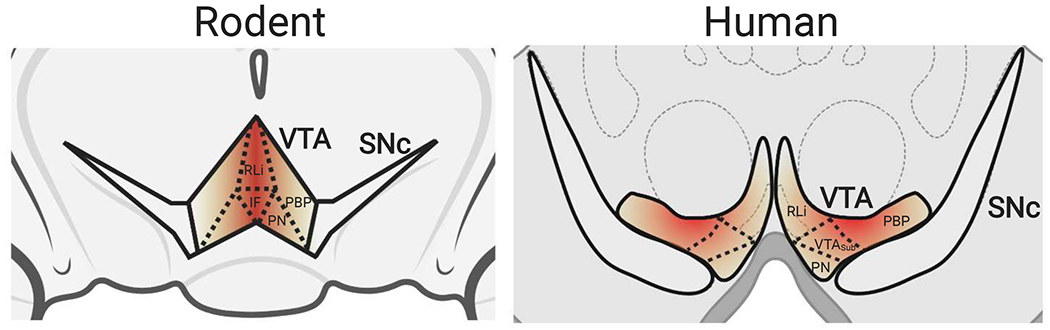
In rodents (left panel), the highest concentration of VGLUT2-expressing DA neurons (TH+/VGLUT2+) are in the medial regions of the VTA (darker shading), including the RLi and IF nuclei [43, 46]. In humans (right panel), most TH+/VGLUT2+ co-expressing neurons are found in the PBP [48]. RLi: rostral linear nucleus, IF: interfascicular nucleus, PN: paranigral nucleus, PBP: parabrachial pigmental nucleus, VTASub: VTA subdivision.
Figure 2. A novel role for TH+/VGLUT2+ neurons in drug reinforcement.
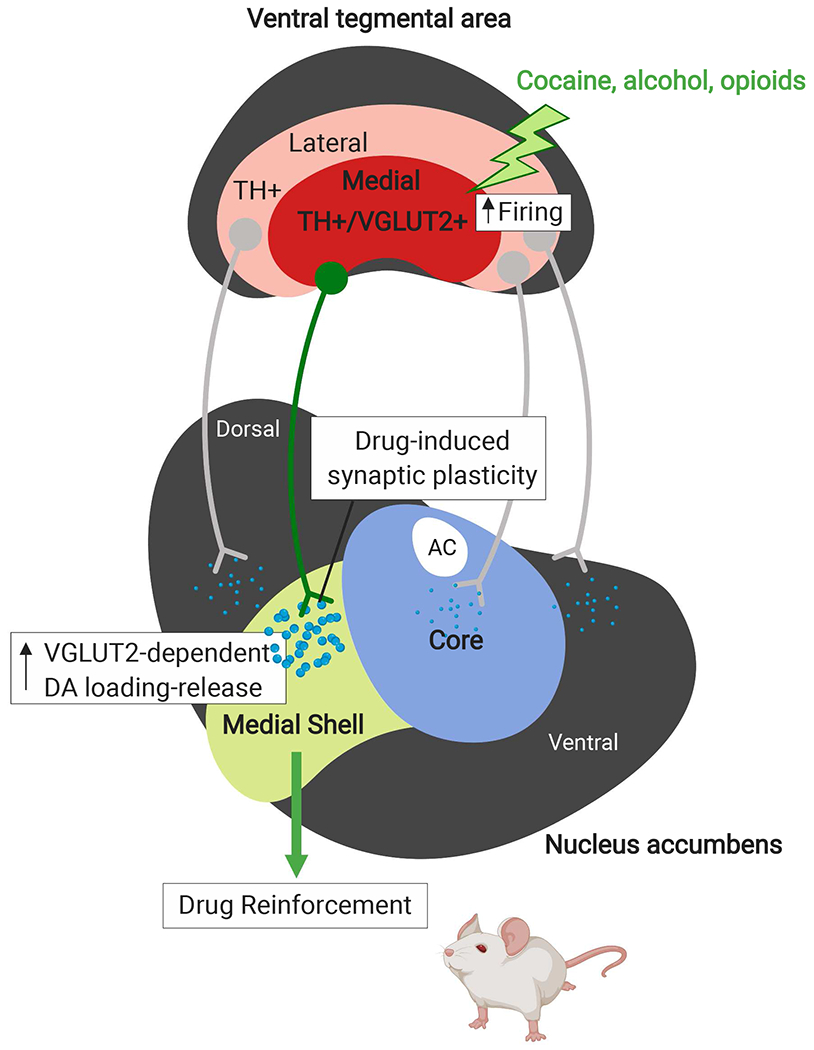
Drugs of abuse including cocaine, opioids and alcohol preferentially increase vesicular DA loading and release in a distinct subpopulation of DA neurons that co-transmit both DA and glutamate and express both TH and VGLUT2 (TH+/VGLUT2+). The cell bodies of these DA/glutamate co-transmitting neurons predominantly localize to the medial VTA and project to the medial nucleus accumbens shell (mNAcSh). In response to drug-induced increases in cell firing, these medial VTA TH+/VGLUT2+ DA neurons that project to the mNAcSh have functional features unique from other midbrain DA neurons including the DA-only neurons that project to the nucleus accumbens (NAc) core, including: distinct modulatory afferent inputs and high-frequency firing (>20 Hz). Moreover, DA/glutamate co-transmission by this distinct TH+/VGLUT2+ subpopulation of DA neurons may be a critical mechanism for regulating reward-related behaviors and associated with vulnerability to substance use disorders.
While the role of DA release from medial VTA DA neurons in SUDs has been well-studied, new research is beginning to investigate the role of glutamate co-release from these same neurons, presenting a potentially novel mechanism of addictive drug action. Here, we review recent findings on relationships between addictive drugs with an emphasis on cocaine, opioids and alcohol and their actions on mNAcSh DA release, the potential role(s) of glutamate co-release in mediating this relationship, and future areas of research to further uncover the intertwined roles of DA and glutamate co-transmission from DA neurons in SUDs and their potential as novel therapeutic strategies for treating addiction. To date, there has been very little investigation of the postsynaptic effects of DA and glutamate co-transmission from VTA terminals and the possible impact of co-transmission on reward-related behaviors. There are hints that the integration of DA and glutamate may be necessary for reward-related learning. For example, cue-evoked food learning induces a greater extracellular signal-related kinase (ERK) and phosphoERK response in the NAc via the coordinated activation of D1 and NMDA receptors [50]. In striatum, ERK acts to integrate separate DA and glutamate inputs that converge on medium spiny neurons involved in drug-induced synaptic and behavioral plasticity [51]. However, recent findings suggest DA and glutamate can be co-released from neuronal subpopulations in the midbrain, which may be used to further tune DA neurotransmission in response to drugs and other reward-related stimuli. This opens the possibility that integration of DA and glutamate neurotransmission may similarly potentiate the reinforcing actions of drugs of abuse (Fig. 3). Future studies will elucidate the precise signaling mechanisms for this integration, particularly in the context of different SUDs.
Figure 3. Model for drug-induced synaptic plasticity via signal integration of DA and glutamate co-transmission from VTA to NAc.
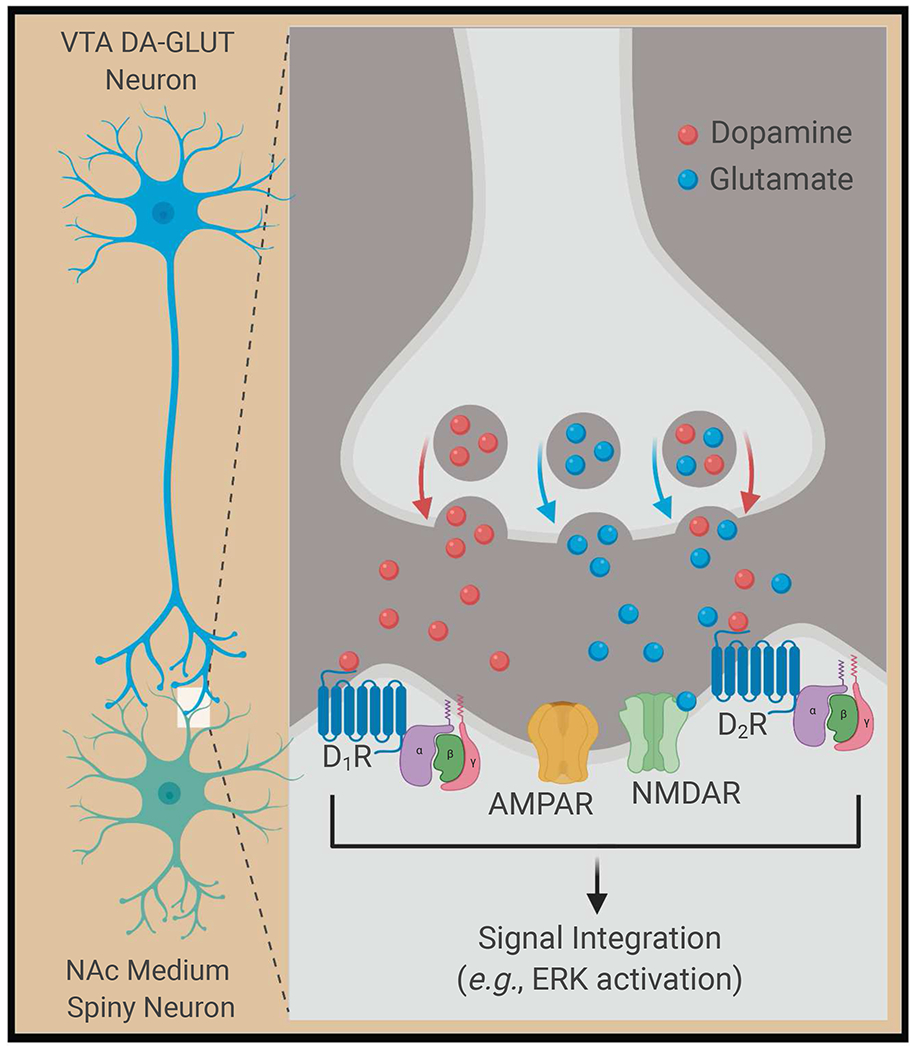
Drugs of abuse increase DA cell firing in the VTA and induce the co-release of DA and glutamate from presynaptic medial VTA DA neurons (left panel). DA and glutamate signaling is integrated by postsynaptic medium spiny neurons in the NAc via activation of DA receptors (D1 and D2), and glutamatergic AMPA receptors (AMPAR) and NMDA receptors (NMDAR) (right panel); all four receptor subtypes (D1, D2, AMPAR, NMDAR) are shown on the same cell for simplicity. Concurrent activation of postsynaptic DA and glutamate receptors in the NAc integrates signaling from these different receptors that may be unique from signaling induced by stimulation of either DA or glutamate receptors alone. For drugs of abuse, this DA/glutamate receptor co-stimulation can lead to induction of ERK-dependent intracellular signaling pathways events involved in regulating synaptic function and plasticity critical for drug reward and reinforcement.
1. Medial VTA-mNAcSh DA release and SUDs
1.1. Cocaine
Cocaine is known to elevate DA neurotransmission in the striatum by inhibiting DA reuptake via dopamine transporter (DAT) blockade [6, 7, 13, 52, 53]. Recent evidence suggests that cocaine also acts independently of DAT to increase striatal DA neurotransmission by increasing VTA DA neuron firing, which is consistent with cocaine’s continued reinforcing properties in self-administration and place paradigms in DAT knockout mice [11, 54]. Cocaine can also alter the timing and magnitude of DA release by enhancing both tonic and phasic DA neuron activity, uncoupling the relative contributions of both firing modes to DA-modulated behaviors [6, 11]. Within the striatum, the NAc shell is particularly important for cocaine-related behaviors, as the NAc shell exhibits greater increases in extracellular DA relative to other striatal regions after cocaine administration [7, 33, 55–58]. Additionally, NAc shell-projecting VTA DA neurons are important in cocaine-related behaviors such as locomotor sensitization and conditioned place preference [17, 26]. The mNAcSh, more specifically, shows larger transient increases in DA release in response to cocaine [6, 59, 60] and is crucial for cocaine reinforcement and related behaviors [17, 26, 27]. The medial VTA-mNAcSh projection has also been shown to encode aversive behaviors [61] and plays a role in incentive salience to environmental stimuli predictive of behaviorally relevant events [17, 27, 62]. Overall, these findings highlight that the medial VTA-mNAcSh projection is a critical location of cocaine action, where cocaine leads to further increases in DA neurotransmission via the inhibition of DA reuptake and the increased firing frequency of medial VTA DA neurons relative to other subpopulations of midbrain DA neurons.
1.2. Opioids
Opioids including morphine and heroin enhance DA neurotransmission by stimulating burst firing of VTA DA neurons that project to the NAc [6, 22, 56, 63, 64], which is especially critical for drug self-administration [11]. Opioids also block the actions of local inhibitory GABA neurons, leading to increased VTA DA neuron activity [65, 66] and enhance presynaptic release probability in a stimulus frequency-dependent manner [67]. In the mNAcSh, heroin increases DA release by activating a subset of medial VTA DA neurons [22]. Thus, while opioids may not act mainly through DAT inhibition like cocaine, these drugs induce a similar increase in medial VTA-derived DA neurotransmission in the mNAcSh.
1.3. Alcohol
Like cocaine and opioids, DA release has been heavily implicated in the reinforcing effects of alcohol [68–70]. Though the precise mechanisms remain poorly understood, there is evidence suggesting that alcohol increases DA neurotransmission by increasing the activity of VTA DA neurons [68, 71–73]. Interestingly, while this increased activity of VTA DA neurons in response to alcohol leads to large DA release in the NAc, the NAc shell exhibits larger transient increases in DA compared to the core, consistent with other drugs of abuse [30, 32, 59, 60]. In contrast, one study found that chronic ethanol exposure has no effect on DA reuptake in the mNAcSh [74] and other recent work showed that optogenetically activating NAc-projecting VTA neurons reduced ethanol consumption in a subset of mice exhibiting high ethanol consumption [75]. Clearly, many unanswered questions remain. For example, to date, there are no known studies of the effect of ethanol on presynaptic DA release probability in the mNAcSh. Together, these findings suggest that alcohol increases DAergic neurotransmission, particularly in the mNAcSh, via an upregulation of VTA DA neuron firing, and that alcohol may produce additional effects on DA release via unexplored mechanisms.
Overall, comparing the actions of cocaine, opioids and alcohol, all three types of drugs increase DAergic neurotransmission in the NAc, and even more specifically in the mNAcSh, which plays a number of important roles in the addictive, reinforcing properties of these drugs (Fig. 2). In all three cases, the neurons, which are built to fire rapidly and over prolonged periods, are especially well-suited to potentiate a drugs’ behavioral effects. Since a large proportion of these medial VTA DA neurons release glutamate, this leads us to ask: what role does glutamate co-release from this special population of DA neurons play in potentiating the actions of drugs of abuse?
2. Glutamate co-release from medial VTA DA neurons projecting to mNAcSh mediates the effects of addictive drugs
An important clue concerning the role of glutamate in DA neurons and its potential relevance to DA/glutamate co-transmission from medial VTA DA neurons stems from findings showing that glutamate and its vesicular transporter VGLUT modulate activity-dependent DA loading in NAc terminals [76]. This concept of “vesicular synergy”, where one neurotransmitter synergistically enhances vesicular packaging and release of another neurotransmitter, was first demonstrated in striatal cholinergic interneurons and subsequently extended to serotonergic neurons [77, 78]. Hnasko and colleagues were first to report vesicular synergy between DA and glutamate, resulting in glutamate-induced acidification of DA synaptic vesicles [16]. Additional work showed that DA/glutamate vesicular synergy was attributed to expression of VGLUT2 in these subsets of DA/glutamate co-releasing neurons [16, 79]. Consistent with these earlier findings, we recently demonstrated that VGLUT2 expression in NAc DA terminals is especially important for tuning vesicular DA loading and release in response to high neuronal activity [76]. Specifically, we found that VGLUT2 and glutamate are necessary to increase acidification of synaptic vesicles during periods of heightened neuronal activity. Since the vesicular pH gradient (ΔpH) is the primary driving force for DA loading [80–83], activity-dependent vesicular hyperacidification enables tuning of vesicular DA loading and release in response to firing frequency [76, 79]. These findings place presynaptic glutamate into a unique physiological context within DA neurons as facilitators of neurotransmitter loading through effects on vesicular pH regulation, rather than strictly as a neurotransmitter acting on postsynaptic receptors. Moreover, these data suggest that glutamate enables DA neurons to meet the demands of high frequency burst firing and therefore sustain adequate vesicular DA loading and release over time during exposure to drugs of abuse, enabling these drugs’ reinforcing properties at the molecular level (Fig. 4). Lastly, we and others showed that VGLUT2 expression can be modified in response to cell stress, particularly in VTA DA neurons, emphasizing the importance of glutamate and VGLUT2 in the plasticity of these DA neurons [84–87]. Furthermore, we find that DA neuron VGLUT expression is similarly altered in response to agents that change synaptic DA levels such as psychostimulants like amphetamine or reserpine (unpublished data), further suggesting that DA neuron VGLUT2 expression may be a critical component of the machinery for neuronal plasticity by maintaining homeostatic control over synaptic DA levels.
Figure 4. Model for VGLUT2-mediated synaptic vesicle hyperacidification and increased DA loading.
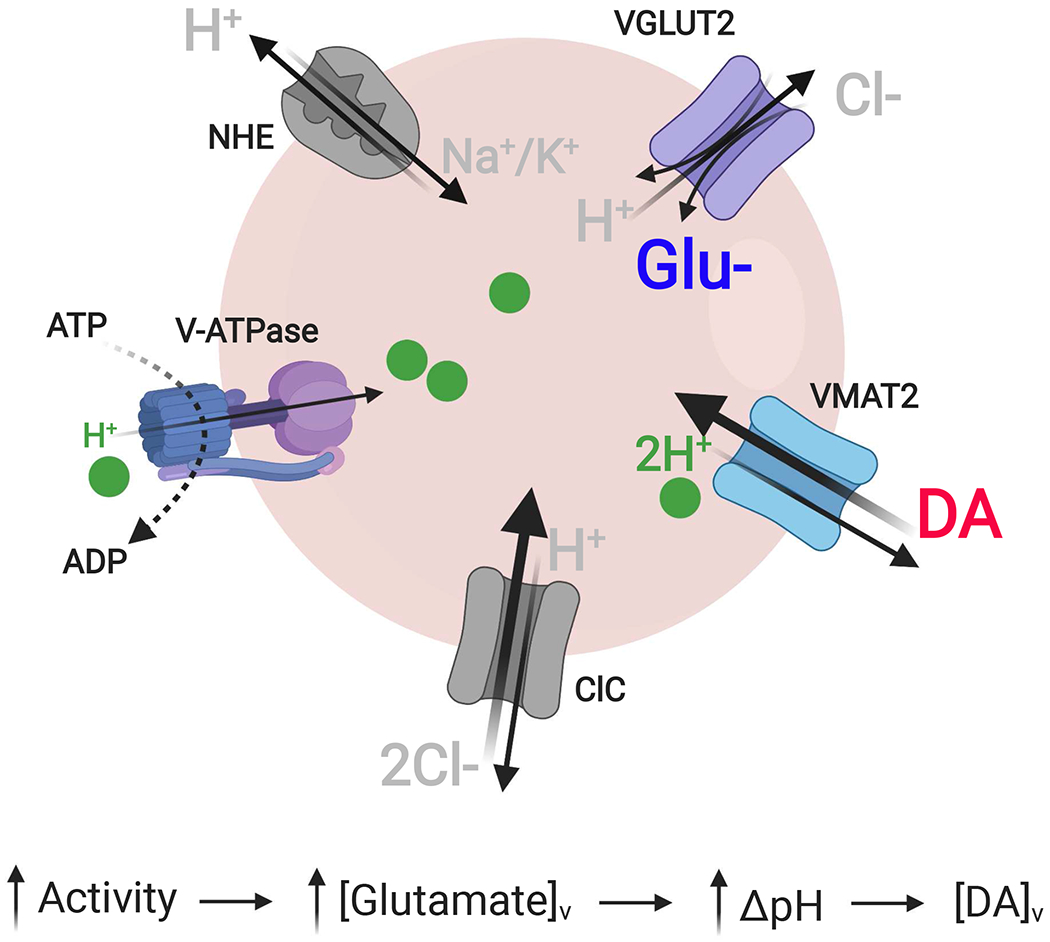
We propose a model for VGLUT2- and glutamate-mediated increases in synaptic vesicle increases in intraluminal acidification and content in DA terminals in response to neuronal stimulation, as follows: (1) Increased neuron activity causes a rise in cytoplasmic Na+. (2) Influx of Na+ into vesicles via Na+/K+ exchangers (NHE) increases the vesicle membrane potential (ΔΨ), the main driving force for glutamate entry. (3) The resulting buildup of intraluminal negative charge from the increased vesicular glutamate ([glutamate]v) increases the vesicular proton-motive force, causing the V-ATPase to pump more H+ into the vesicle, increasing the vesicular pH gradient (ΔpH). (5) The elevated ΔpH increases the driving force for vesicular DA loading via VMAT2, increasing overall DA vesicle content ([DA]v).
Despite the above findings, DA/glutamate vesicular synergy remains controversial. There are currently conflicting reports on the extent to which 1) DA and glutamate are released from the same presynaptic site within a neuron [88, 89], and 2) VGLUT2 and vesicular monoamine transporter 2 (VMAT2), the vesicular DA transporter, are localized to the same vesicle [16, 76, 90–92]. Support for vesicular co-localization of VMAT2 and VGLUT2 primarily comes from earlier biochemical and functional data. Hnasko and colleagues showed that VGLUT2+ synaptic vesicles isolated from rat ventral striatum are also VMAT2+ and vice versa via reciprocal immunoprecipitation [16]. Additional electrophysiological data also demonstrated that VGLUT2 knockdown in DA neurons decreases evoked DA release according to ex vivo fast-scan cyclic voltammetry, and that glutamate stimulates vesicular DA uptake [16]. Similarly, VGLUT2 knockdown eliminates activity-dependent vesicular DA loading, further suggesting a direct role for VGLUT2 in DA vesicle function [76]. Nevertheless, to date, there is no ultrastructural evidence demonstrating co-localization of VMAT2 and VGLUT2 to the same synaptic vesicles. Instead, several studies have shown that VGLUT2 and VMAT2 segregate in discrete subpopulations of vesicles in the same axons of mesoaccumbens fibers, including at separate sites of release unique to DA versus glutamate vesicles [39, 40, 89, 90, 93, 94]. Consistent with this, recent work showed that midbrain DA neurons release DA and glutamate with different properties (i.e., release probabilities, modes of release), reflecting storage in different DAergic and glutamatergic synaptic vesicle populations [90].
It may still be possible that a subset of DA neurons that co-transmit glutamate release both glutamate and DA from the same synaptic site and/or contain VGLUT2 and VMAT2 on the same vesicle. Because VGLUT2 is specifically required for activity-dependent changes in DA vesicle loading, the above differences in findings may reflect activity-dependent changes in vesicular co-release and co-localization of VGLUT2 and VMAT2. We propose that changes in neuronal activity may alter the trafficking of VMAT2 or VGLUT2 to synaptic vesicles to facilitate vesicular synergy and co-release. Indeed, work suggests that VGLUT2 expression and trafficking in neurons is altered in response to changes in activity [95, 96]. Further work is required to clarify these outstanding issues and their relevance to mechanisms of action for drugs of abuse. Together, these findings point to a role of DA neuron glutamate release in mediating the effects of drugs of abuse. Below, we review the current findings on DA neuron glutamate release in mediating cocaine-, opioid-, and alcohol-induced changes in DA neuron function and in the behaviors associated with these drugs.
2.1. Psychostimulants: cocaine and amphetamine
Recent studies have increasingly linked glutamate release from medial VTA DA neurons to the cellular and behavioral effects of cocaine. Conditional knockout of VGLUT2 in DA neurons, which eliminates the ability of DA neurons to release glutamate, blunts locomotor sensitization to cocaine [16, 21, 76] and disrupts reward consumption in response to cocaine [20]. At the postsynaptic level, conditional knockout of VGLUT2 in presynaptic DA neurons, which reduces glutamate co-release from DAergic terminals in the NAc, leads to an upregulation of AMPA/NMDA receptor ratios on accumbal D1 receptor-expressing medium spiny neurons [97]. VGLUT2 conditional knockout also occludes cocaine-induced upregulation of cell excitability mediated by AMPA/NMDA receptors [97]. In parallel, the coordinated release of DA alongside glutamate seems to participate in integrating cocaine-associated synaptic plasticity. These are the precisely the types of synaptic changes that have been linked previously to cocaine reward-related behaviors [51, 98, 99].
DA/glutamate co-release is also important for the behavioral effects of other psychostimulants including amphetamines. Conditional VGLUT2 knockout in mice reduces amphetamine-induced locomotion [21, 97]. Similar deficits in amphetamine sensitization have also been described after DA neuron-specific conditional heterozygous reduction of phosphate-activated glutaminase expression, the enzyme responsible for glutamate recycling [41]. Likewise, in Drosophila, RNAi-mediated VGLUT knockdown in DA neurons decreases amphetamine-stimulated hyperlocomotion [76]. Together, these findings indicate that glutamate release from medial VTA DA neurons in the mNAcSh is a critical regulator of the behavioral effects of psychostimulants.
2.2. Opioids
The precise contributions of glutamate co-released with DA to opioid-related behaviors remain poorly characterized. However, recent work by Luscher and colleagues showed that, in mice, reinforcement by optogenetic VTA GABA self-inhibition and reinforcement by heroin self-administration share underlying neural circuits and are compatible with a disinhibitory mechanism where heroin targets GABA neurons. This leads to an increase in activity of medial VTA DA neurons that project to the mNAcSh [22]. In fact, a single heroin injection can obstruct this optogenetically-driven VTA GABA self-inhibition mechanism and increase DA activity [22]. To date, no studies have specifically manipulated DA neuron glutamate co-release in the context of opioid-related DA neuron function or behavior. However, recent studies, including those using intersectional genetic strategies, confirmed that the majority of medial VTA-mNAcSh projecting DA neurons significantly express VGLUT2 and co-release glutamate [15, 41–44]. Because virtually all DA neurons transiently express VGLUT2 during development [39, 84, 100], we cannot rule out that the joint expression of Cre and Flp recombinases in VGLUT2+ DA neurons (required for intersectional genetic strategies) may non-physiologically modify DA neuron VGLUT2 expression; additional studies are required to validate these results. Nevertheless, the above data strongly suggest that the co-release of glutamate from these DA neurons specifically in the mNAcSh plays a critical role in mediating the effects of opioids. Future experiments are highly warranted to test this intriguing hypothesis.
2.3. Alcohol
Glutamate release plays a pivotal role in alcohol reinforcement and motivation. Ethanol administration elevates extracellular glutamate in the NAc in a dose-dependent manner [25], and these changes are critical for establishing and maintaining learning and motivation events associated with alcohol dependence and relapse behaviors [101–105]. Furthermore, genetic vulnerability to alcohol use disorders involves alterations in the glutamatergic system, including those involving VGLUT2 expression and function [25, 106]. Indeed, VGLUT2 expression changes in ethanolconsuming rats that were previously exposed to early life stress [107]. Moreover, chronic stress leads to altered expression of glutamatergic receptors in the NAcSh and increases both the locomotor activating effects and motivation for alcohol. These data suggest that glutamate-related plasticity and potentially glutamate release from VTA DA neurons underlie vulnerability to the effects of alcohol [108]. While the direct role of DA neuron glutamate release on alcohol-related neurological and behavioral changes has not been investigated, these findings provide indirect evidence of the importance of glutamate release in the NAc, from DA neurons and other inputs, on mediating the effects of alcohol.
3. Future Directions and Conclusions
There are many outstanding questions around the roles of DA/glutamate co-release that need to be resolved. First, some medial VTA neurons that project to the mNAcSh may be purely glutamatergic [42, 44, 109] and play a role either complementary to or separate from DA/glutamate co-releasing neurons. For example, the NAc shell receives glutamatergic projections from other brain regions including the ventromedial prefrontal cortex which has been implicated in the reinstatement of opioidseeking behaviors [110–113]. Second, since VGLUT2 function and expression may be critical for tuning DA vesicle loading and release in the context of neuronal activity, there is the intriguing possibility that VGLUT2 expression in presynaptic DA neurons may serve as a rheostat of DA neuron function. Thus, when synaptic DA levels diminish, DA/glutamate neurons may upregulate VGLUT2 expression and/or function to facilitate activity-dependent DA loading and release to maintain homeostasis of synaptic DA levels. Additionally, at the presynaptic level, besides the respective single and combined roles of DA and glutamate from the medial VTA-mNAcSh-projecting neurons, the mechanisms regulating the synthesis and loading of both neurotransmitters remain poorly understood. Furthermore, the local circuitry responsible for direct and indirect control over release from these co-transmitting terminals, and how these regulatory circuits respond to drugs of abuse, are relatively unknown. For example, Rayport and colleagues have identified mNAcSh cholinergic interneurons as a preferential target of glutamate release from DA neurons, as glutamate co-release from DA neurons drives burst firing of mNAcSh cholinergic interneurons [15, 114]. Cholinergic interneurons in the mNAcSh have also been implicated in reward processing and aversive learning, and they are activated by cocaine self-administration [115–119]. Together, these findings suggest glutamate release from DA neurons may mediate some actions of drugs of abuse by activating cholinergic interneurons in the mNAcSh. Future studies should investigate this possibility as well as potential downstream effects of cholinergic interneuron activation by DA/glutamate co-release.
At the postsynaptic level, the specific functional relevance of DA/glutamate co-transmission remains unclear. Just as glutamate may facilitate tuning of activity-dependent vesicular DA loading and release presynaptically, we propose that concurrent stimulation of DA and glutamate receptors may similarly tune postsynaptic signaling in the striatum, particularly in the mNAcSh. Co-stimulation of different populations of DA and glutamate receptors would therefore enable an integration of different intracellular signaling pathways. Importantly, this raises the possibility that different receptor combinations (i.e., D1/AMPA versus D2/AMPA versus D3/NMDA) give rise to unique postsynaptic responses (Fig. 3). Such a model would provide a broad palette of potential postsynaptic responses in keeping with the dynamic nature of the striatal circuitry. Just as importantly, such a model may enable further tuning of the postsynaptic signal based on the different frequencies of presynaptic firing – i.e., phasic versus tonic DA release, particularly in the context of different drugs of abuse. For example, do different opioids differentially modulate the relative amounts of DA and glutamate co-released in the mNAcSh based on their distinct effects on firing and release from the nerve terminals? These drug-specific effects on frequency and duration of firing could have vast implications with regards to different combinations of receptors being stimulated in different sequences and at different temporal intervals. This may ultimately explain drug-specific differences in time-scale and magnitude of changes to the DA system, including among drugs of the same class.
In summary, the discovery of DA/glutamate co-releasing neurons within the last two decades has opened up a wide range of speculation of these cells’ possible roles, from reward and SUDs [15, 21, 41, 97, 120] to DA neuron degeneration and Parkinson’s disease [85–87, 121]. Research has established that the majority of these co-transmitting neurons project to the mNAcSh, where DA release is modulated by drugs of abuse. Indeed, glutamate release from DA neurons has been directly linked to cocaine consumption and locomotor sensitization, as well as indirectly linked to opioid- and alcohol-related changes in behavior. The role of DA neuron glutamate release on mediating the effects of drugs of abuse appears to be linked to its excitatory action on cholinergic interneurons in the mNAcSh. Ultimately, we propose that these medial VTA neurons that co-release both DA and glutamate act as “integrators” of these two signals. We also introduce the idea that there may be “emergent” or synergistic properties that arise at the postsynaptic level in the targeted postsynaptic neurons that either transmitter alone could not accomplish. Might this foster increased synaptic plasticity that gives rise to the learned or reinforced behaviors associated with drugs of abuse? In all, current research points to DA neuron glutamate release as an essential aspect of how drugs of abuse exert their effects, opening up a new therapeutic avenue for treating SUDs.
Acknowledgements
The present study was supported in part by the John F. and Nancy A. Emmerling Fund of the Pittsburgh Foundation (Z.F.); NIDA R33DA041872 and NHLBI R01HL150432 (R.W.L.); NIDA R01DA042029, NIAAA R01AA028145, and NIAAA R21AA025547 (M.M.T.). Figures created with Biorender.com.
Abbreviations
- DA
Dopamine
- SUD
Substance use disorder
- VTA
Ventral tegmental area
- NAc
Nucleus accumbens
- mNAcSh
Medial nucleus accumbens shell
- VGLUT2
Vesicular glutamate transporter 2
- RLi
Rostral linear nucleus
- IF
Interfascicular nucleus
- PBP
Parabrachial pigmented nucleus
- TH
Tyrosine hydroxylase
- ERK
Extracellular signal-related kinase
- DAT
Dopamine transporter
- VMAT2
Vesicular monoamine transporter 2
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/FEBS.15496
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M & Degenhardt L (2018) Global statistics on alcohol, tobacco and illicit drug use: 2017 status report, Addiction (Abingdon, England). [DOI] [PubMed] [Google Scholar]
- 2.Scholl L, Seth P, Kariisa M, Wilson N & Baldwin G (2018) Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017, MMWR Morbidity and mortality weekly report. 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho AF, Heilig M, Perez A, Probst C & Rehm J (2019) Alcohol use disorders, Lancet. 394, 781–792. [DOI] [PubMed] [Google Scholar]
- 4.Axley PD, Richardson CT & Singal AK (2019) Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease, Clinics in liver disease. 23, 39–50. [DOI] [PubMed] [Google Scholar]
- 5.Jones CM, Einstein EB & Compton WM (2018) Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016, JAMA : the journal of the American Medical Association. 319, 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission, Neuron. 69, 628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Chiara G & Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats, Proc Natl Acad Sci U S A. 85, 5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diana M (2011) The dopamine hypothesis of drug addiction and its potential therapeutic value, Frontiers in psychiatry. 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira FA & Dalley JW (2015) Dopamine receptor partial agonists and addiction, Eur J Pharmacol. 752, 112–5. [DOI] [PubMed] [Google Scholar]
- 10.Baik JH (2013) Dopamine signaling in reward-related behaviors, Frontiers in neural circuits. 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V & Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection, Neuropharmacology. 47 Suppl 1, 227–41. [DOI] [PubMed] [Google Scholar]
- 12.Pereira DB & Sulzer D (2012) Mechanisms of dopamine quantal size regulation, Front Biosci (Landmark Ed). 17, 2740–67. [DOI] [PubMed] [Google Scholar]
- 13.Rice ME, Patel JC & Cragg SJ (2011) Dopamine release in the basal ganglia, Neuroscience. 198, 112–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice ME & Cragg SJ (2008) Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway, Brain research reviews. 58, 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingote S, Amsellem A, Kempf A, Rayport S & Chuhma N (2019) Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching, Neurochemistry international. 129, 104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S & Edwards RH (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo, Neuron. 65, 643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuhma N, Choi WY, Mingote S & Rayport S (2009) Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection, Neuroscience. 164, 1068–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulzer D & Rayport S (1990) Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action, Neuron. 5, 797–808. [DOI] [PubMed] [Google Scholar]
- 19.Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S & Sulzer D (2014) The multilingual nature of dopamine neurons, Prog Brain Res. 211, 141–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsio J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LE, Kullander K, Levesque D & Wallen-Mackenzie A (2011) Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice, J Neurosci. 31, 12593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K & Wallen-Mackenzie A (2010) VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation, Proc Natl Acad Sci U S A. 107, 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V & Luscher C (2018) Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement, eLife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldonado R, Robledo P, Chover AJ, Caine SB & Koob GF (1993) D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat, Pharmacol Biochem Behav. 45, 239–42. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DC, Corcoran ME & Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine, Pharmacol Biochem Behav. 6, 615–20. [DOI] [PubMed] [Google Scholar]
- 25.Alasmari F, Goodwani S, McCullumsmith RE & Sari Y (2018) Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence, Prog Neurobiol. 171, 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin JH, Adrover MF & Alvarez VA (2017) Distinctive Modulation of Dopamine Release in the Nucleus Accumbens Shell Mediated by Dopamine and Acetylcholine Receptors, J Neurosci. 37, 11166–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex, Brain research reviews. 56, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RI, Becker HC, Adams BL, Jesudason CD & Rorick-Kehn LM (2014) Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models, Front Neurosci. 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhri N, Sahuque LL, Schairer WW & Janak PH (2010) Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking, Neuropsychopharmacology. 35, 783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbit LH & Balleine BW (2016) Learning and Motivational Processes Contributing to Pavlovian-Instrumental Transfer and Their Neural Bases: Dopamine and Beyond, Curr Top Behav Neurosci. 27, 259–89. [DOI] [PubMed] [Google Scholar]
- 31.Marchant NJ, Kaganovsky K, Shaham Y & Bossert JM (2015) Role of corticostriatal circuits in context-induced reinstatement of drug seeking, Brain Res. 1628, 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saddoris MP, Sugam JA, Cacciapaglia F & Carelli RM (2013) Rapid dopamine dynamics in the accumbens core and shell: learning and action, Front Biosci (Elite Ed). 5, 273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM & Carelli RM (2009) Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell, The European journal of neuroscience. 30, 1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roeper J (2013) Dissecting the diversity of midbrain dopamine neurons, Trends Neurosci. 36, 336–42. [DOI] [PubMed] [Google Scholar]
- 35.Liss B & Roeper J (2008) Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease, Brain research reviews. 58, 314–21. [DOI] [PubMed] [Google Scholar]
- 36.Grace AA & Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: burst firing, J Neurosci. 4, 2877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinelli M & White FJ (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons, J Neurosci. 20, 8876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brischoux F, Chakraborty S, Brierley DI & Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli, Proc Natl Acad Sci U S A. 106, 4894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Descarries L, Berube-Carriere N, Riad M, Bo GD, Mendez JA & Trudeau LE (2008) Glutamate in dopamine neurons: synaptic versus diffuse transmission, Brain Res Rev. 58, 290–302. [DOI] [PubMed] [Google Scholar]
- 40.Trudeau LE & El Mestikawy S (2018) Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine Systems: Contrasts and Commonalities, Frontiers in neural circuits. 12, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mingote S, Chuhma N, Kalmbach A, Thomsen GM, Wang Y, Mihali A, Sferrazza C, Zucker-Scharff I, Siena AC, Welch MG, Lizardi-Ortiz J, Sulzer D, Moore H, Gaisler-Salomon I & Rayport S (2017) Dopamine neuron dependent behaviors mediated by glutamate cotransmission, eLife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Wang HL, Li X, Ng TH & Morales M (2011) Mesocorticolimbic glutamatergic pathway, J Neurosci. 31, 8476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K & Awatramani R (2018) Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches, Nat Neurosci. 21, 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY & Rayport S (2015) Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions, J Neurosci. 35, 16259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R & Rayport S (2004) Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses, J Neurosci. 24, 972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H & Hisano S (2006) Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain, J Comp Neurol. 498, 581–92. [DOI] [PubMed] [Google Scholar]
- 47.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M & Trudeau LE (2004) Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine, J Neurochem. 88, 1398–405. [DOI] [PubMed] [Google Scholar]
- 48.Root DH, Wang HL, Liu B, Barker DJ, Mod L, Szocsics P, Silva AC, Magloczky Z & Morales M (2016) Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans, Sci Rep. 6, 30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi T, Sheen W & Morales M (2007) Glutamatergic neurons are present in the rat ventral tegmental area, The European journal of neuroscience. 25, 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirschmann EK, Mauna JC, Willis CM, Foster RL, Chipman AM & Thiels E (2014) Appetitive cue-evoked ERK signaling in the nucleus accumbens requires NMDA and D1 dopamine receptor activation and regulates CREB phosphorylation, Learning & memory (Cold Spring Harbor, NY). 21, 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cahill E, Salery M, Vanhoutte P & Caboche J (2014) Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse, Frontiers in pharmacology. 4, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulzer D, Cragg SJ & Rice ME (2016) Striatal dopamine neurotransmission: regulation of release and uptake, Basal ganglia. 6, 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carboni E, Imperato A, Perezzani L & Di Chiara G (1989) Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats, Neuroscience. 28, 653–61. [DOI] [PubMed] [Google Scholar]
- 54.Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B & Di Chiara G (2001) Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene, J Neurosci. 21, Rc141: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM & Wightman RM (2008) Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events, J Neurosci. 28, 8821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pontieri FE, Tanda G & Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens, Proc Natl Acad Sci U S A. 92, 12304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuber GD, Wightman RM & Carelli RM (2005) Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens, Neuron. 46, 661–9. [DOI] [PubMed] [Google Scholar]
- 58.Stuber GD, Roitman MF, Phillips PE, Carelli RM & Wightman RM (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration, Neuropsychopharmacology. 30, 853–63. [DOI] [PubMed] [Google Scholar]
- 59.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F & Baler R (2010) Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit, BioEssays : news and reviews in molecular, cellular and developmental biology. 32, 748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luscher C & Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling, Neuron. 69, 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K & Lammel S (2019) A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System, Neuron. 101, 133–151.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Everitt BJ & Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion, Nat Neurosci. 8, 1481–9. [DOI] [PubMed] [Google Scholar]
- 63.Gysling K & Wang RY (1983) Morphine-induced activation of A10 dopamine neurons in the rat, Brain Res. 277, 119–27. [DOI] [PubMed] [Google Scholar]
- 64.Nowycky MC, Walters JR & Roth RH (1978) Dopaminergic neurons: effect of acute and chronic morphine administration on single cell activity and transmitter metabolism, J Neural Transm. 42, 99–116. [DOI] [PubMed] [Google Scholar]
- 65.Johnson SW & North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons, J Neurosci. 12, 483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grace AA & Bunney BS (1979) Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons, Eur J Pharmacol. 59, 211–8. [DOI] [PubMed] [Google Scholar]
- 67.Britt JP & McGehee DS (2008) Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens, J Neurosci. 28, 1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morel C, Montgomery S & Han MH (2019) Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement, The European journal of neuroscience. 50, 2180–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gessa GL, Muntoni F, Collu M, Vargiu L & Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area, Brain Res. 348, 201–3. [DOI] [PubMed] [Google Scholar]
- 70.Brodie MS, Pesold C & Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons, Alcoholism, clinical and experimental research. 23, 1848–52. [PubMed] [Google Scholar]
- 71.You C, Vandegrift B & Brodie MS (2018) Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons, Psychopharmacology (Berl). 235, 1711–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.di Volo M, Morozova EO, Lapish CC, Kuznetsov A & Gutkin B (2019) Dynamical ventral tegmental area circuit mechanisms of alcohol-dependent dopamine release, The European journal of neuroscience. 50, 2282–2296. [DOI] [PubMed] [Google Scholar]
- 73.Gibson GD, Prasad AA, Jean-Richard-Dit-Bressel P, Yau JOY, Millan EZ, Liu Y, Campbell EJ, Lim J, Marchant NJ, Power JM, Killcross S, Lawrence AJ & McNally GP (2018) Distinct Accumbens Shell Output Pathways Promote versus Prevent Relapse to Alcohol Seeking, Neuron. 98, 512–520.e6. [DOI] [PubMed] [Google Scholar]
- 74.Melchior JR & Jones SR (2017) Chronic ethanol exposure increases inhibition of optically targeted phasic dopamine release in the nucleus accumbens core and medial shell ex vivo, Mol Cell Neurosci. 85, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, Gregoire H, Ribeiro E, Crumiller M, Roman-Ortiz C, Walsh JJ, Jackson K, Croote DE, Zhu Y, Zhang S, Vendruscolo LF, Edwards S, Roberts A, Hodes GE, Lu Y, Calipari ES, Chaudhury D, Friedman AK & Han MH (2017) Midbrain circuit regulation of individual alcohol drinking behaviors in mice, Nature communications. 8, 2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aguilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, Choi SJ, Grygoruk A, Zhang Y, Cela C, Choi BJ, Flores J, Freyberg RJ, McCabe BD, Mosharov EV, Krantz DE, Javitch JA, Sulzer D, Sames D, Rayport S & Freyberg Z (2017) Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT, Neuron. 95, 1074–1088.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B & El Mestikawy S (2008) The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone, Nat Neurosci. 11, 292–300. [DOI] [PubMed] [Google Scholar]
- 78.Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B & El Mestikawy S (2010) VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety, J Neurosci. 30, 2198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stuber GD, Hnasko TS, Britt JP, Edwards RH & Bonci A (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate, J Neurosci. 30, 8229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson RG & Scarpa A (1979) Protonmotive force and catecholamine transport in isolated chromaffin granules, J Biol Chem. 254, 3750–60. [PubMed] [Google Scholar]
- 81.Knoth J, Zallakian M & Njus D (1981) Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts, Biochemistry. 20, 6625–9. [DOI] [PubMed] [Google Scholar]
- 82.Moriyama Y, Amakatsu K, Yamada H, Park MY & Futai M (1991) Inhibition of neurotransmitter and hormone transport into secretory vesicles by 2-(4-phenylpiperidino)cyclohexanol and 2-bromo-alpha-ergocryptine: both compounds act as uncouplers and dissipate the electrochemical gradient of protons, Arch Biochem Biophys. 290, 233–8. [DOI] [PubMed] [Google Scholar]
- 83.Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, Pizzo AB, Zhang Y, Farino ZJ, Chen A, Martin CA, Kopajtic TA, Fei H, Hu G, Lin YY, Mosharov EV, McCabe BD, Freyberg R, Wimalasena K, Hsin LW, Sames D, Krantz DE, Katz JL, Sulzer D & Javitch JA (2016) Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain, Nature communications. 7, 10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steinkellner T, Zell V, Farino ZJ, Sonders MS, Villeneuve M, Freyberg RJ, Przedborski S, Lu W, Freyberg Z & Hnasko TS (2018) Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons, The Journal of Clinical Investigation. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen H, Marino RAM, McDevitt RA, Bi GH, Chen K, Madeo G, Lee PT, Liang Y, De Biase LM, Su TP, Xi ZX & Bonci A (2018) Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice, Proc Natl Acad Sci U S A. 115, E11532–e11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE & Descarries L (2009) The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain, The Journal of comparative neurology. 517, 873–91. [DOI] [PubMed] [Google Scholar]
- 87.Dal Bo G, Berube-Carriere N, Mendez JA, Leo D, Riad M, Descarries L, Levesque D & Trudeau LE (2008) Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion, Neuroscience. 156, 59–70. [DOI] [PubMed] [Google Scholar]
- 88.Fortin GM, Ducrot C, Giguere N, Kouwenhoven WM, Bourque MJ, Pacelli C, Varaschin RK, Brill M, Singh S, Wiseman PW & Trudeau LE (2019) Segregation of dopamine and glutamate release sites in dopamine neuron axons: regulation by striatal target cells, Faseb j. 33, 400–417. [DOI] [PubMed] [Google Scholar]
- 89.Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR & Morales M (2015) Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons, Nat Neurosci. 18, 386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, Newman AH, Ford CP & Edwards RH (2019) Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties, Neuron. 102, 786–800.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morales M & Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour, Nat Rev Neurosci. 18, 73–85. [DOI] [PubMed] [Google Scholar]
- 92.Deshpande SA, Freyberg Z, Lawal HO & Krantz DE (2020) Vesicular neurotransmitter transporters in Drosophila melanogaster, Biochimica et biophysica acta Biomembranes, 183308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barker DJ, Root DH, Zhang S & Morales M (2016) Multiplexed neurochemical signaling by neurons of the ventral tegmental area, Journal of chemical neuroanatomy. 73, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M & Parnavelas JG (2002) Postnatal development of the dopaminergic system of the striatum in the rat, Neuroscience. 110, 245–56. [DOI] [PubMed] [Google Scholar]
- 95.Erickson JD, De Gois S, Varoqui H, Schafer MK & Weihe E (2006) Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size, Neurochemistry international. 48, 643–9. [DOI] [PubMed] [Google Scholar]
- 96.De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H & Erickson JD (2005) Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits, J Neurosci. 25, 7121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papathanou M, Creed M, Dorst MC, Bimpisidis Z, Dumas S, Pettersson H, Bellone C, Silberberg G, Luscher C & Wallen-Mackenzie A (2018) Targeting VGLUT2 in Mature Dopamine Neurons Decreases Mesoaccumbal Glutamatergic Transmission and Identifies a Role for Glutamate Co-release in Synaptic Plasticity by Increasing Baseline AMPA/NMDA Ratio, Frontiers in neural circuits. 12, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parekh PK, Logan RW, Ketchesin KD, Becker-Krail D, Shelton MA, Hildebrand MA, Barko K, Huang YH & McClung CA (2019) Cell-Type-Specific Regulation of Nucleus Accumbens Synaptic Plasticity and Cocaine Reward Sensitivity by the Circadian Protein, NPAS2, J Neurosci. 39, 4657–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dobi A, Seabold GK, Christensen CH, Bock R & Alvarez VA (2011) Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal, J Neurosci. 31, 1895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC & Trudeau LE (2008) Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons, J Neurosci. 28, 6309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Das SC, Yamamoto BK, Hristov AM & Sari Y (2015) Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats, Neuropharmacology. 97, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding ZM, Engleman EA, Rodd ZA & McBride WJ (2012) Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats, Alcohol Clin Exp Res. 36, 633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK & McBride WJ (2013) Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats, Addict Biol. 18, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gass JT, Sinclair CM, Cleva RM, Widholm JJ & Olive MF (2011) Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors, Addict Biol. 16, 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Melendez RI, Hicks MP, Cagle SS & Kalivas PW (2005) Ethanol exposure decreases glutamate uptake in the nucleus accumbens, Alcohol Clin Exp Res. 29, 326–33. [DOI] [PubMed] [Google Scholar]
- 106.Vrettou M, Nilsson KW, Tuvblad C, Rehn M, Aslund C, Andershed AK, Wallen-Mackenzie A, Andershed H, Hodgins S, Nylander I & Comasco E (2019) VGLUT2 rs2290045 genotype moderates environmental sensitivity to alcohol-related problems in three samples of youths, European child & adolescent psychiatry. 28, 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vrettou M, Granholm L, Todkar A, Nilsson KW, Wallen-Mackenzie A, Nylander I & Comasco E (2017) Ethanol affects limbic and striatal presynaptic glutamatergic and DNA methylation gene expression in outbred rats exposed to early-life stress, Addiction biology. 22, 369–380. [DOI] [PubMed] [Google Scholar]
- 108.Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R & Szumlinski KK (2016) Homer2 regulates alcohol and stress cross-sensitization, Addiction biology. 21 , 613–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamaguchi T, Qi J, Wang HL, Zhang S & Morales M (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area, The European journal of neuroscience. 41, 760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sesack SR, Deutch AY, Roth RH & Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin, J Comp Neurol. 290, 213–42. [DOI] [PubMed] [Google Scholar]
- 111.Groenewegen HJ, Wright CI, Beijer AV & Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs, Ann N Y Acad Sci. 877, 49–63. [DOI] [PubMed] [Google Scholar]
- 112.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M & Shaham Y (2012) Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking, J Neurosci. 32, 4982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT & Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin, Nat Neurosci. 14, 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chuhma N, Mingote S, Moore H & Rayport S (2014) Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling, Neuron. 81, 901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL & Alcantara AA (2003) Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine, Neuroscience. 120, 1149–56. [DOI] [PubMed] [Google Scholar]
- 116.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM & Cheer JF (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing, Cell Rep. 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng J, Umschweif G, Leung J, Sagi Y & Greengard P (2019) HCN2 Channels in Cholinergic Interneurons of Nucleus Accumbens Shell Regulate Depressive Behaviors, Neuron. 101, 662–672 e5. [DOI] [PubMed] [Google Scholar]
- 118.Hanada Y, Kawahara Y, Ohnishi YN, Shuto T, Kuroiwa M, Sotogaku N, Greengard P, Sagi Y & Nishi A (2018) p11 in Cholinergic Interneurons of the Nucleus Accumbens Is Essential for Dopamine Responses to Rewarding Stimuli, eNeuro. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C & Deisseroth K (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning, Science. 330, 1677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Root DH, Estrin DJ & Morales M (2018) Aversion or Salience Signaling by Ventral Tegmental Area Glutamate Neurons, iScience. 2, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steinkellner T, Zell V, Farino ZJ, Sonders MS, Villeneuve M, Freyberg RJ, Przedborski S, Lu W, Freyberg Z & Hnasko TS (2018) Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons, J Clin Invest. 128, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]


