Abstract
Background:
Attaining pregnancy is conditional upon a series of complex processes, including adequately timed intercourse, ovulation, fertilization, and implantation. Anovulation is a first line treatment target for couples with difficulty conceiving and is frequently examined in studies of fecundability.
Objectives:
To identify whether sporadic anovulation is an important determinant of cumulative pregnancy rates and time to pregnancy among fertile women with regular menstrual cycles.
Methods:
We simulated cumulative pregnancy rates and time to pregnancy for 12 consecutive menstrual cycles among 100,000 women based on data-driven probabilities of implantation, fertilization, ovulation, and intercourse occurring in the fertile window. We assumed anovulation probabilities of 1%, 8%, or 14.5% and intercourse averaging once per week, every other day, and daily. The model incorporated reductions in implantation and fertilization rates for successive cycles of non-pregnancy.
Results:
After 12 cycles, a reduction in the per-cycle incidence of anovulation from 14.5% to 1% resulted in a 4.0% higher cumulative pregnancy rate (86.7 versus 90.7%) and similar time to pregnancy (1-cycle median difference). In contrast, increasing mean unscheduled sexual intercourse frequency from weekly to every other day was associated with a 5-cycle median reduction in time to pregnancy (weekly: 7 cycles; every other day or daily: 2 cycles) and a 28.9% increase in the cumulative pregnancy rate (weekly: 59.9%, every other day: 88.8%; daily: 91.6%).
Conclusions:
In presumed fertile women with regular menstrual cycles, routine investigation of anovulation may not be an informative outcome in studies of fecundability, and routine testing to ensure ovulation and treatment of anovulation are unlikely to be medically necessary. While biomarkers or cervical fluid may help time intercourse to the fertile window, time to pregnancy can also be improved through increasing the frequency of unscheduled intercourse. These findings need corroboration in large preconception time to pregnancy studies.
Keywords: ovulation, embryo implantation, coitus, pregnancy rate, humans, menstrual cycle
BACKGROUND
Pregnancy is conditional upon a series of reproductive events starting with adequately timed intercourse, ovulation, fertilization of the ovum, and culminates in implantation of an embryo into a receptive endometrium. In seeking to conceive, couples frequently utilize over the counter urine luteinizing hormone (LH) tests, “ovulation predictor kits,” or other fertility indicators, such as cervical fluid, to identify ovulation and to time intercourse to conceive. Recent data suggest that these efforts may result in modestly increased fecundability (shorter times to conceive) in couples with normal fecundity.
Chronic anovulation is a common cause of infertility and may be due to polycystic ovarian syndrome or other causes. In the context of the reproductive events required for pregnancy, anovulation is often the first line treatment target for couples with difficulty conceiving1, 2 even among women with regular menstrual cycles, and is frequently examined as an outcome of interest in studies of fecundability. However, among women who menstruate regularly and have no known subfertility, the reported per-cycle incidence of sporadic anovulation is highly variable, ranging from 1.0 to 14.5%3–6, defined based on LH surge or luteal progesterone. Furthermore, it is unclear whether and how sporadic anovulation may impact cumulative pregnancy rates for regularly menstruating couples seeking pregnancy. To address this question, we performed a simulation study examining the impact of anovulation incidence on cumulative pregnancy rates and time to pregnancy. We compared the impact on pregnancy rates of sporadic anovulation to the impact of intercourse frequency, with the latter being arguably the most easily modifiable factor in the reproductive process for couples attempting to conceive without the use of assisted reproductive technologies.
METHODS
To examine the impact of sporadic anovulation and intercourse frequency on cumulative pregnancy rates, distributions of time to pregnancy were simulated over 12 menstrual cycles among a population of 100,000 women attempting to conceive. To do this, we first constructed a mathematical model that characterized the underlying biological processes (e.g. intercourse, ovulation, fertilization, implantation) and then used estimates from the literature to derive reasonable estimates for each parameter involved in the pathway to pregnancy. We then simulated pregnancy rates after 6 and 12 months based on these data-driven parameters.
Relationships among parameters required for pregnancy
Attainment of a pregnancy is conditional upon a series of reproductive events starting with adequately timed intercourse, ovulation, fertilization of the ovum, and culminating in implantation of an embryo into the uterine lining, which is observable via the embryo’s secretion of human chorionic gonadotropin (hCG) into maternal circulation. The reproductive milestones required for pregnancy each have a probability of success, some being conditional on prior events7, and can be represented using the following equations as a simple model of this series of complex biological processes.
Since
We can obtain:
| Equation 1 |
Adequately timed intercourse, its probability represented by P(sex) in Equation 1, is defined as a female-male couple having intercourse within the approximate 6-day fertile window8, 9, beginning ~5 days prior to ovulation and ending on the day of ovulation10. Ovulation, its probability represented by P(ovulation), consists of maturation of at least one ovarian follicle, with release of viable oocyte into the oviduct11, 12. The release of a viable oocyte will be approximated using hormonal assessment of ovulation detection (LH surge or luteal progesterone). We describe the implications of this approximation in the Comment. Fertilization of the ovum can only occur if there is ovulation and adequately timed intercourse with a fertile male, the conditional probability denoted by P(fertilization|sex=1, ovulation=1). Similarly, implantation of the embryo into the endometrium can only happen if there is fertilization, represented as P(implantation|fertilization=1). For the purposes of this paper, the event of a new pregnancy is synonymous with implantation, clinically observable by the detection of hCG in maternal serum or urine. The probability of pregnancy (i.e., implantation) is modeled as the product of implantation given fertilization, fertilization given sex and ovulation, sex, and ovulation, shown in Equation 1. For the purposes of this simulation, we assume that each of the mathematical probabilities in Equation 1 are statistically and empirically independent of one another13. In a fecund population, this assumption likely holds, though we describe the implications of this assumption in the Discussion.
Simulation setup: parameter derivation
Probabilities used for parameters on the right-hand side of Equation 1 were based on existing literature14 and the relations required by the equation (Table 1 - Derivation).
Table 1.
Parameters used in derivation of Fertilization | sex & ovulation and parameters used in analyses
| Derivation | Analysis | ||||
|---|---|---|---|---|---|
| Varying sporadic anovulation | Varying intercourse frequency | ||||
| Parameter | Scenario 115 | Scenario 216 | Scenario 115 | Scenario 216 | |
| Implantation | 38% | 30% | Simulated | Simulated | Simulated |
| Anovulation | 8% | 8% | 1, 8, 14.5% | 1, 8, 14.5% | 8% |
| Implantation / fertilization | 70% | 70% | 70% | 70% | 70% |
| Fertilization / sex & ovulation | Calculated 59% | Calculated 49% | 59% | 49% | 59% |
| Sex | 100% | 95% | 87% | 87% | 38, 87, 97% |
Anovulation
Given the previously stated range of sporadic anovulation among reproductive aged women (1–14.5%3–6), we performed simulations across this range, at 1% and 14.5%, as well as at the approximate mid-point of this range (8%).
Sex and implantation
Among couples assumed to be fertile, the maximum probability of attaining pregnancy is approximately 38% from intercourse occurring on peak day fertility (i.e., sexual intercourse that always occurs in the fertile window), as documented by Stanford et al15. Meanwhile, the usual probability of pregnancy has been shown to be approximately 30% among fertile couples attempting conception (i.e., when intercourse occurs within the 6-day fertile window in 95% of cycles), as reported by Wilcox et al16. We used both relations in our simulations to examine the robustness of our findings.
Implantation given fertilization
Implantation given fertilization and fertilization given sex and ovulation are not directly observable outside of assisted reproductive technologies. However, the probability of implantation given fertilization in the setting of embryos transferred after IVF for women less than 35 years of age has been reported to be in the range of 45–70%17. We expected this probability for natural (i.e. in vivo) conceptions to be on the higher end of these ranges (e.g., 70%) due to the population being of normal fecundity and fertilization occurring in the more natural environment of the oviduct.
Fertilization given sex and ovulation
In Scenario 1, we used parameters from Stanford et al15 relating the relationship between sex occurring in the fertile window (100%) and implantation (38%) and plausible values of the other parameters (anovulation = 8%, and implantation given fertilization = 70%) to calculate the probability of fertilization given sex and ovulation as 59% using Equation 1. In Scenario 2, we used parameters based on Wilcox et al (sex = 95%, implantation = 30%)16 to calculate the probability of fertilization given sex and ovulation using Equation 1, resulting in 49% (Table 1 - Derivation). A meta-analysis of IVF cycles without ICSI (i.e., oocyte immersed in media with sperm) for unexplained infertility reported a 75–95% oocyte fertilization rate (fertilization given sex and ovulation)18. Fertilization rates are expected to be lower in the setting of attempted natural conception, given the need for successful sperm transport and capacitation that are bypassed in IVF, so the calculated probabilities of 49% and 59% were assumed to be plausible in the setting of natural conceptions.
Intercourse frequency and sex
Assuming the fertile window is unknown, intercourse averaging overall as once per week, every other day, and every day has been estimated to result in pregnancy probabilities of 15%, 33%, and 37% respectively16. Using Equation 1 with anovulation = 8%, fertilization given sex and ovulation = 59%, and implantation given fertilization = 70%), these overall average intercourse frequencies translated to a probability of sex occurring in the fertile window of 39%, 87%, and 97%, respectively. Here, we see that intercourse occurring on average, every other day and daily did not result in a probability of sex = 1.0 (e.g. less than 100% probability), as frequency is averaged across the menstrual cycle, and may be impacted by biology (e.g., variations in numbers of viable sperm in an ejaculation) and/or social and behavioral influences on the probability of intercourse19.
Statistical Analysis
We simulated the presence or absence of each of the events required for pregnancy described above. For each cycle, if all requisite events were simulated to have occurred, and accordingly pregnancy was calculated as having occurred, then individuals attaining pregnancy were removed from remaining menstrual cycles of observation.
For our primary analysis examining the impact of the varying reported incidence of sporadic anovulation, three sets of time to pregnancy distributions were simulated based on anovulation = 1%, 8%, or 14.5%, implantation given fertilization = 70%, sex = 87%, the latter corresponding to intercourse occurring every other day. Fertilization given sex and ovulation was set to either 59% or 49%, derived from Stanford15 or Wilcox16, respectively. For simulations examining the secondary comparative impact of intercourse frequency, three distributions were calculated based on sex = 39%, 87%, or 97% and anovulation = 8%, fertilization given sex and ovulation = 59%, and implantation given fertilization = 70% (Table 1 - Analysis). To reflect the lower fecundability among the remaining pool of non-pregnant women with each successive cycle of observation, relative reductions of 7.5% were implemented for implantation given fertilization and fertilization given sex and ovulation for each successive cycle of non-pregnancy, which yielded cumulative pregnancy rates consistent with reported infertility rates of 7.420 to 15%21 in the U.S. For each of these time to pregnancy distributions, cumulative pregnancy rates were quantified after 6 and 12 menstrual cycles of attempting pregnancy. For analyses examining the impact of sporadic anovulation, fecundability odds ratios (FORs) were calculated for 1% and 8% anovulation relative to 14.5% anovulation, and for analyses examining the impact of intercourse frequency, FORs for intercourse averaging every other day and daily were calculated relative to weekly intercourse.
Ethics Approval
As data were simulated, the current data are not based on a clinical study protocol and Institutional Review Board approvals do not apply.
RESULTS
Impact of anovulation on cumulative pregnancy rates and time to pregnancy
Changing per-cycle anovulation incidence between 1% and 14.5% had no appreciable impact on cumulative pregnancy rates in Scenario 115 or Scenario 216. Cumulative pregnancy rates were slightly lower under Scenario 2, but overall patterns were similar. Time to pregnancy survival curves remained close over the 12 cycles of follow-up (Figure 1 and eFigure 1).
Figure 1.
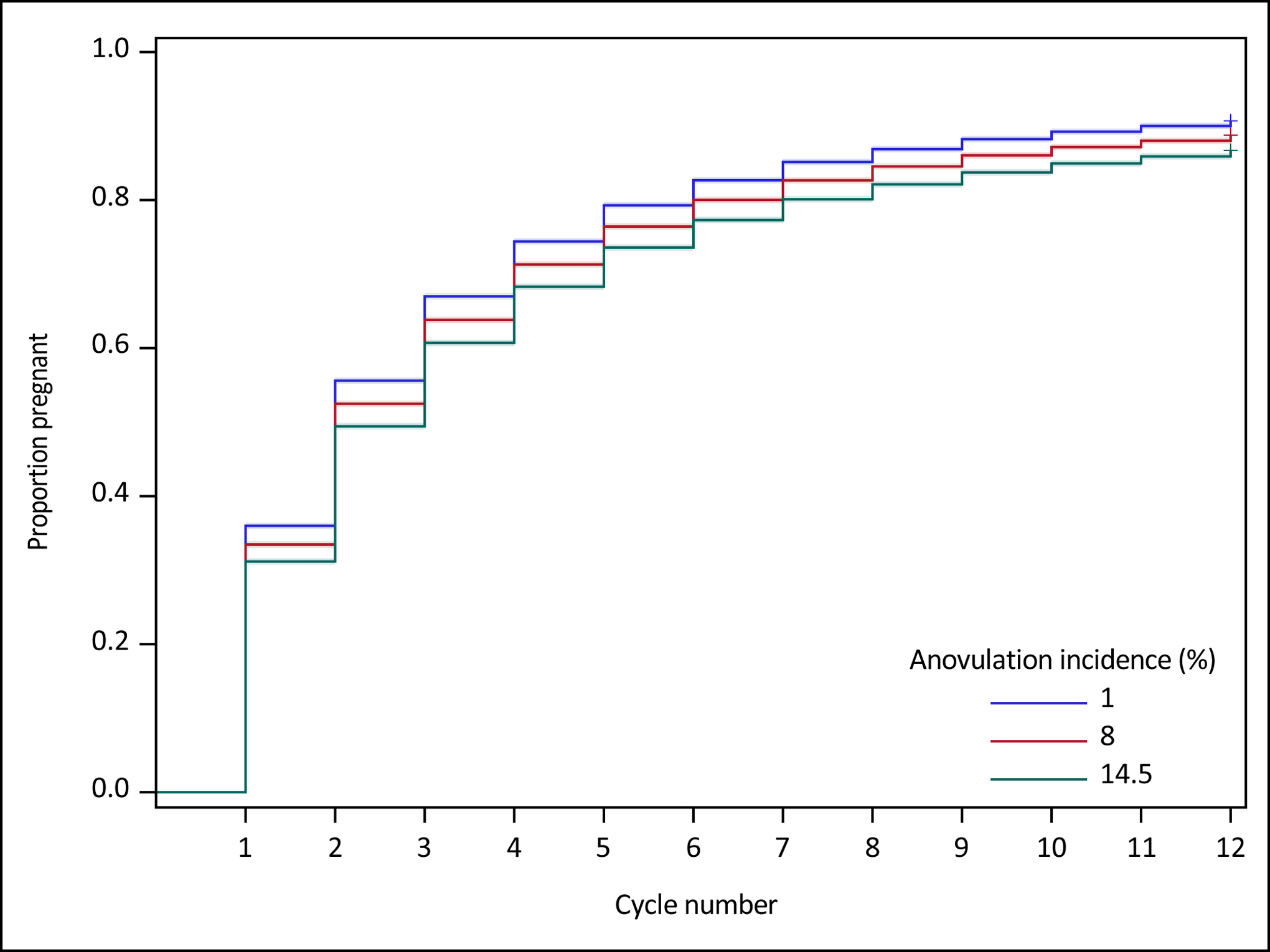
Survival curves and 95% Hall-Wellner confidence bands comparing sporadic anovulation rates of 1%, 8%, and 14.5% on simulated time to pregnancy over 12 cycles of follow-up among 100,000 womena,b
a Sex in the fertile window set equal to 87% for each cycle of observation; implantation given fertilization = 70% and fertilization given sex and ovulation = 59% for the first cycle of observation, followed by a 7.5% reduction per cycle for implantation given fertilization and fertilization given sex and ovulation; probability of implantation calculated using Equation 1.
b Scenario 1 relationship between sex and implantation: among fecund couples attempting conception, the maximum probability of attaining pregnancy is ~38% when sexual intercourse always occurs in the fertile window (sex = 1.0)15.
In Scenario 115, cumulative pregnancy rates after 12 cycles for 1%, 8%, and 14.5% anovulation incidence were 90.7%, 88.8%, and 86.7%, respectively, for an absolute difference of 4.0% between 1% to 14.5% anovulation (Table 2). The difference in cumulative 6-cycle pregnancy rate between 1% and 14.5% anovulation was expectedly slightly higher, at 5.4% (82.7% vs 77.3%). Anovulation incidence of 14.5% was associated with a 1-cycle median reduction in time to pregnancy compared to 8% or 1% incidence (14.5%: 3 cycles; 8% or 1%: 2 cycles). Compared to 14.5% anovulation, FORs for 8% and 1% anovulation were 1.21 and 1.49, respectively, after 12 cycles of follow-up.
Table 2.
Comparison of sporadic anovulation rates of 1%, 8% and 14.5% on simulated time to pregnancy and fecundability odds ratiosa over 12 cycles of follow-up in a cohort of 100,000 womenb (using Scenario 1c relationship between sex and implantation)
| anovulation = 14.5% | anovulation = 8% | anovulation = 1% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | FORd | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | FORd |
| 1 | 100,000 | 31.2 | 31.2 | 100,000 | 33.5 | 33.5 | 1.11 | 100,000 | 36.0 | 36.0 | 1.24 |
| 2 | 68,827 | 26.5 | 49.4 | 66,544 | 28.6 | 52.5 | 1.13 | 64,008 | 30.6 | 55.6 | 1.28 |
| 3 | 50,559 | 22.3 | 60.7 | 47,526 | 23.9 | 63.8 | 1.14 | 44,399 | 25.6 | 67.0 | 1.31 |
| 4 | 39,301 | 19.3 | 68.3 | 36,190 | 20.7 | 71.3 | 1.15 | 33,015 | 22.5 | 74.4 | 1.35 |
| 5 | 31,705 | 16.7 | 73.6 | 28,699 | 17.9 | 76.4 | 1.16 | 25,598 | 19.1 | 79.3 | 1.37 |
| 6 | 26,408 | 14.1 | 77.3 | 23,572 | 15.2 | 80.0 | 1.18 | 20,698 | 16.4 | 82.7 | 1.40 |
| 7 | 22,690 | 12.3 | 80.1 | 19,982 | 13.2 | 82.7 | 1.18 | 17,312 | 14.2 | 85.1 | 1.42 |
| 8 | 19,901 | 10.1 | 82.1 | 17,343 | 10.9 | 84.6 | 1.19 | 14,857 | 11.7 | 86.9 | 1.44 |
| 9 | 17,883 | 9.1 | 83.7 | 15,455 | 9.7 | 86.0 | 1.20 | 13,126 | 10.4 | 88.2 | 1.46 |
| 10 | 16,261 | 7.4 | 85.0 | 13,963 | 8.0 | 87.2 | 1.20 | 11,761 | 8.5 | 89.2 | 1.47 |
| 11 | 15,051 | 6.3 | 85.9 | 12,842 | 6.7 | 88.0 | 1.21 | 10,761 | 7.2 | 90.0 | 1.48 |
| 12 | 14,102 | 5.7 | 86.7 | 11,977 | 6.3 | 88.8 | 1.21 | 9,985 | 6.9 | 90.7 | 1.49 |
Fecundability odds ratios for 1% and 8% anovulation calculated relative to 14.5% anovulation
Sex in the fertile window set equal to 87% for each cycle of observation; implantation given fertilization = 70% and fertilization given sex and ovulation = 59% for the first cycle of observation, followed by a 7.5% reduction per cycle for implantation given fertilization and fertilization given sex and ovulation; probability of implantation calculated using Equation 1.
Scenario 1: among fecund couples attempting conception, the maximum probability of attaining pregnancy is ~38% when sexual intercourse always occurs in the fertile window (sex = 1.0)15.
Fecundability Odds Ratio
Assessing the robustness of these findings to a different relationship between sex and implantation16, Scenario 2 produced 12-cycle cumulative pregnancy rates of 85.5%, 83.2%, and 80.7% for anovulation incidence of 1%, 8%, and 14.5%, respectively, leading to an absolute difference of 5.2% between 1% and 14.5% anovulation (eTable 1 and eFigure 1). The difference in cumulative 6-cycle pregnancy rate between 1% and 14.5% anovulation was similar, at 5.8% (75.7% vs 69.9%). Anovulation incidence of 14.5%, 8%, and 1% were each associated with a median time to pregnancy of 3 cycles. Compared to 14.5% anovulation, FORs for 8% and 1% anovulation were 1.18 and 1.41, respectively, after 12 cycles of follow-up.
Impact of intercourse on cumulative pregnancy rates and time to pregnancy
In contrast, cumulative pregnancy rates varied widely by frequency of unscheduled intercourse. Specifically, cumulative pregnancy rates after 12 cycles were 59.9% for untimed weekly intercourse, 88.8% for every other day, and 91.6% for daily, contributing to an absolute difference of 28.9% when average intercourse pattern increased from weekly to every other day (Table 3). The difference in cumulative 6-cycle pregnancy rate between intercourse occurring weekly to every other day was 30.3% (80.0% vs 48.7%). Time to pregnancy survival curves for every other day and daily intercourse tracked closely, while the survival curve for weekly intercourse tracked noticeably lower (Figure 2). Intercourse averaging weekly was associated with a 5-cycle median reduction in time to pregnancy compared to intercourse averaging every other day or daily (weekly: 7 cycles; every other day or daily: 2 cycles).
Table 3.
Comparison of intercourse averaging weekly, every other day, and daily on simulated time to pregnancy and fecundability odds ratiosa over 12 cycles of follow-up in a cohort of 100,000 womenb (using Scenario 1c relationship between sex and implantation)
| Weekly | Every other day | Daily | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | FORd | Population at risk | % becoming pregnant | Cumulative pregnancy rate (%) | FORd |
| 1 | 100,000 | 15.1 | 15.1 | 100,000 | 33.5 | 33.5 | 2.83 | 100,000 | 37.1 | 37.1 | 3.32 |
| 2 | 84,900 | 12.7 | 25.9 | 66,544 | 28.6 | 52.5 | 3.16 | 62,873 | 31.8 | 57.1 | 3.80 |
| 3 | 74,083 | 10.9 | 34.0 | 47,526 | 23.9 | 63.8 | 3.42 | 42,900 | 26.6 | 68.5 | 4.22 |
| 4 | 66,000 | 9.3 | 40.1 | 36,190 | 20.7 | 71.3 | 3.71 | 31,499 | 23.1 | 75.8 | 4.67 |
| 5 | 59,868 | 7.9 | 44.9 | 28,699 | 17.9 | 76.4 | 3.98 | 24,222 | 20.1 | 80.6 | 5.12 |
| 6 | 55,132 | 6.9 | 48.7 | 23,572 | 15.2 | 80.0 | 4.22 | 19,364 | 17.0 | 83.9 | 5.51 |
| 7 | 51,335 | 5.8 | 51.7 | 19,982 | 13.2 | 82.7 | 4.46 | 16,070 | 14.7 | 86.3 | 5.89 |
| 8 | 48,352 | 4.9 | 54.0 | 17,343 | 10.9 | 84.6 | 4.66 | 13,713 | 12.2 | 88.0 | 6.22 |
| 9 | 45,974 | 4.3 | 56.0 | 15,455 | 9.7 | 86.0 | 4.84 | 12,035 | 10.7 | 89.3 | 6.52 |
| 10 | 44,004 | 3.6 | 57.6 | 13,963 | 8.0 | 87.2 | 5.00 | 10,749 | 8.8 | 90.2 | 6.78 |
| 11 | 42,401 | 3.0 | 58.9 | 12,842 | 6.7 | 88.0 | 5.14 | 9,799 | 7.4 | 90.9 | 7.01 |
| 12 | 41,141 | 2.6 | 59.9 | 11,977 | 6.3 | 88.8 | 5.28 | 9,069 | 7.2 | 91.6 | 7.28 |
Fecundability odds ratios for every other day and daily calculated relative to weekly intercourse
Anovulation set equal to 8% for each cycle of observation; probability of implantation|fertilization = 70% and probability of fertilization|sex & ovulation = 59% for the first cycle of observation, followed by a 7.5% reduction per cycle for implantation|fertilization and fertilization|sex & ovulation; probability of implantation calculated using Equation 1.
Scenario 1: among fecund couples attempting conception, the maximum probability of attaining pregnancy is ~38% when sexual intercourse always occurs in the fertile window (sex = 1.0)15.
Fecundability Odds Ratio
Figure 2.
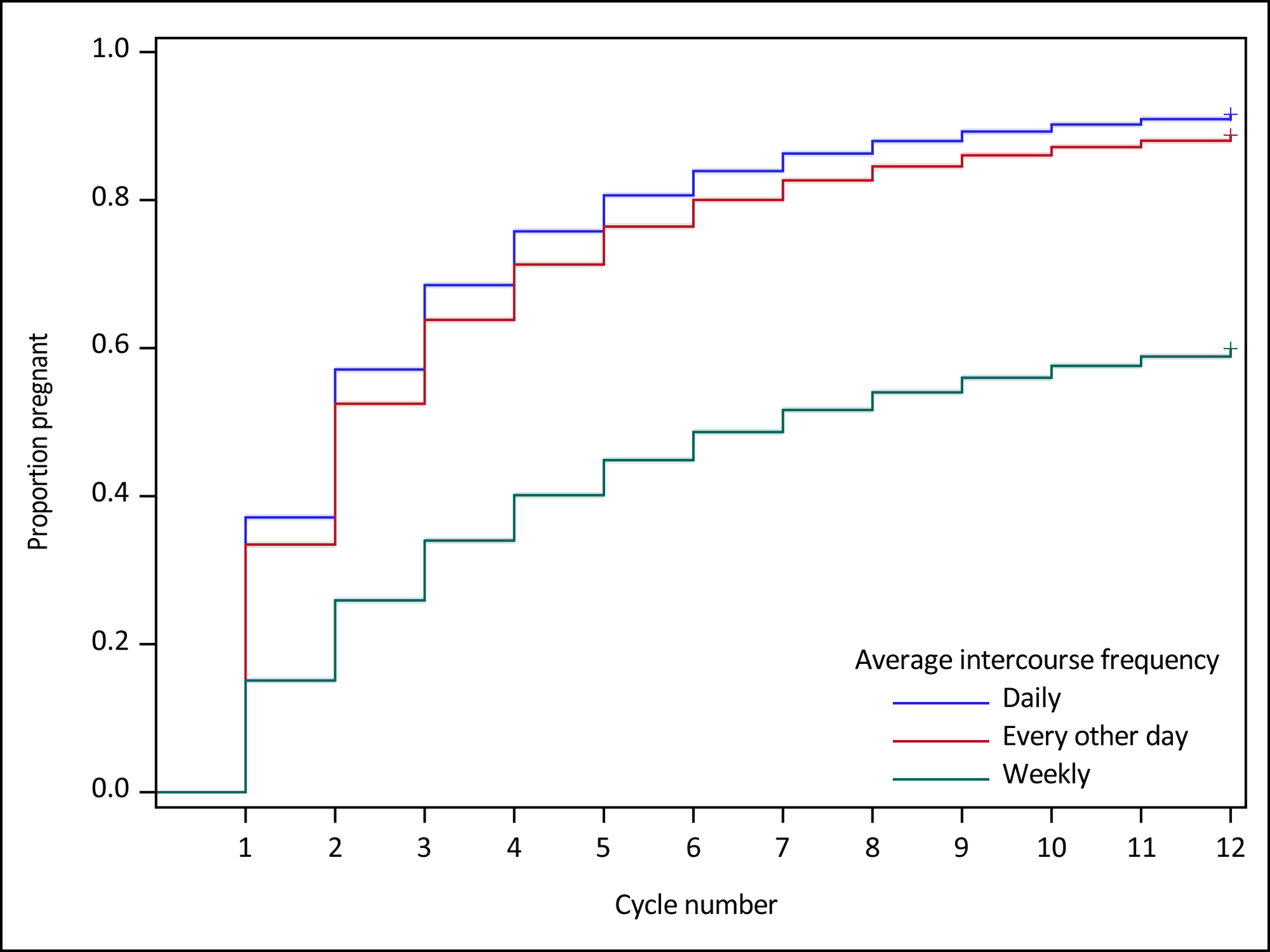
Survival curves and 95% Hall-Wellner confidence bands comparing weekly, every other day, and daily intercourse on simulated time to pregnancy over 12 cycles of follow-up among 100,000 womena,b
a Anovulation set equal to 8% for each cycle of observation; probability of implantation|fertilization = 70% and probability of fertilization|sex & ovulation = 59% for the first cycle of observation, followed by a 7.5% reduction per cycle for implantation|fertilization and fertilization|sex & ovulation; probability of implantation calculated using Equation 1
b Scenario 1 relationship between sex and implantation: among fecund couples attempting conception, the maximum probability of attaining pregnancy is ~38% when sexual intercourse always occurs in the fertile window (sex = 1.0)15.
Compared to intercourse averaging weekly, FORs for intercourse averaging every other day and daily were 5.28 and 7.28, respectively, after 12 cycles of follow-up.
We observed similar patterns across ranges of all parameters reported in the literature (eTable 2). In particular, we see that cumulative pregnancy rates are nearly identical whether we assume that fertilization | sex & ovulation is higher or lower in the setting of attempted natural conceptions, as an increase in fertilization | sex & ovulation requires implantation | fertilization to be proportionally lower.
COMMENT
Principal findings
Large differences in the probability of sporadic anovulation as has been reported in prior literature resulted in small changes in cumulative pregnancy rates and time to pregnancy, whereas intercourse frequency produced substantially larger changes. While anovulation is frequently examined in studies of fecundability and is a first line treatment target for couples with difficulty conceiving, these simulations highlight that sporadic anovulation in regularly menstruating women is not an important determinant of time to pregnancy. Given the similar cumulative pregnancy rates observed across the simulated range of sporadic anovulation incidence, previously reported estimates for its incidence are likely similarly plausible.
Strengths of the study
For each event requisite to pregnancy, we used empirical probabilities to understand the impact of anovulation across its reported spectrum of incidence on cumulative pregnancy rates and time to pregnancy. Our finding that anovulation does not meaningfully impact time to pregnancy or cumulative pregnancy rate was consistent across ranges of plausible values for each event requisite to pregnancy (Equation 1), within the bounds of literature-based rates for fecund couples. Since the probabilities of implantation given fertilization and fertilization given sex and ovulation are not directly observable among in vivo conceptions in humans or animal models22, we have benchmarked with observed measurements from IVF. We can logically expect that the higher end of the range for implantation and the lower end of the range for fertilization in couples receiving IVF may reflect their values in fecund couples. However, as shown in eTable2, findings are not contingent upon this assumption, as the joint probability of implantation given fertilization and fertilization given sex and ovulation is fixed by Equation 1 given implantation and sex (relationship prescribed in Scenario 115 or in Scenario 216), and anovulation (1–14.5%3–6).
Limitations of the data
Since we assumed sporadic anovulation within fecund populations to be representative of normal reproductive function, we believe the assumption of independence of all probabilities in Equation 1 to be a reasonable and likely approximation for the purposes of this simulation. For example, a prior study found no relationship between intercourse frequency and anovulation among sexually active women23, justifying the independence between sex and ovulation. While independence of all parameters may be a simplification of the underlying biological mechanism, it is reassuring that our model generates results congruent with observed cohort data for time to pregnancy studies24–26.
This simulation is only generalizable to fecund couples with regularly menstruating women since the etiology of sporadic anovulation likely differs from chronic disorders such as polycystic ovarian syndrome. Specifically, there is likely dependence between probabilities in Equation 1: anovulation could be correlated with suboptimal fertilization or endometrial receptivity for implantation27, and common comorbidities such as obesity and hyperandrogenism may impact pregnancy rates28–30. Further, polycystic ovarian syndrome is characterized by much higher anovulation incidence than examined in this simulation as a result of long or absent menstrual cycles, which is distinct from sporadic anovulation with typical menstrual cycle length and regular menstruation.
Interpretation
While we have defined ovulation as “maturation of at least one ovarian follicle, with release of viable oocyte into the oviduct”, we utilized anovulation incidence estimates based hormonal assessment rather than direct assessment using transvaginal ultrasonography. Using hormonal assessment, reported incidence of sporadic anovulation among women who menstruate regularly and without known subfertility has ranged from 1.0 to 14.5%3–6. These estimates are impacted by normal variation in the strength of the endocrine signals of the ovulation stimulus that are relied upon for ovulation detection (i.e. LH surge or luteal progesterone)31, and are impacted by the algorithm used to interpret the available data32. Hormonal assessment is more prevalent than transvaginal sonographic assessment due to practical and cost considerations associated with using transvaginal sonography in large cohorts of women, and over consecutive menstrual cycles. However, LH surge or luteal progesterone assessment may overestimate the incidence of true anovulation by including suboptimal ovulation. There may be a continuum in the quality of ovulation, whereby sporadic anovulation may be associated with altered and diminished ovulatory quality when ovulation does occur31. For instance, a study examining 250 women each for 2 menstrual cycles reported lower serum sex steroids in the ovulatory cycles of women who also had one anovulatory cycle compared to women with two ovulatory cycles3. A dose–response was reported in progesterone levels, the highest among women with two ovulatory cycles, followed by those with one ovulatory and one anovulatory cycle, followed by those with two anovulatory cycles. The incidence of true anovulation may be on the lower end of the reported range of 1.0 to 14.5%, as evidenced by a single anovulatory cycle detected by transvaginal sonography among 150 cycles in 53 women33, while higher estimates up to 14.5% may have an increased propensity for including suboptimal ovulation. Nevertheless, sporadic anovulation estimates of 1% produced similar findings regarding the impact of intercourse frequency (eTable 2). Further, we find that anovulation incidence across this broad reported range are likely similarly plausible among fecund couples given reported rates of other steps of the reproductive process.
While sporadic anovulation did not produce remarkable differences in cumulative pregnancy rates, substantial differences did emerge by frequency of intercourse, underscoring the importance of frequency and its relationship to timing within the fertile window. Without having knowledge of the fertile window, intercourse averaging every day or every other day both translated to sex occurring within the fertile window for most cycles (sex = 0.97 and 0.87, respectively). Since these are average intercourse frequencies, these probabilities are relevant for a variety of specific intercourse patterns. These probabilities do not require a constant frequency of intercourse throughout the menstrual cycle, as intercourse may naturally occur more frequently within the fertile window34, even without specific knowledge of when it occurs.
Though the body of existing data on the impact of timed intercourse are insufficient to draw conclusions35, recent observational studies and a trial in couples trying to conceive suggest that timing of intercourse to the fertile window using fertility indicators, results in higher fecundability independent of coital frequency, further highlighting the importance of intercourse timing36–38. A recent study that found that double intrauterine insemination with donor sperm did not increase ongoing pregnancy rates in women without a history of infertility provides additional evidence supporting that increasing frequency matters to cover the fertile window, but does not improve pregnancy rates once the fertile window is already attained39.
Our simulation supports the principle that unscheduled regular intercourse, averaging every other day, will greatly increase the chances of intercourse occurring within the fertile window, which will in turn improve time to pregnancy. However, for a variety of personal reasons, some couples of normal fecundity35, 37, 38 may find timing of intercourse to the fertile window a more acceptable approach to increase fecundability, e.g., among women who experience pain with intercourse. An additional caveat for the strategy of frequent unscheduled intercourse is that one study has suggested that frequent intercourse in the peri-implantation time window may reduce fecundability40, though this finding has not yet been confirmed.
CONCLUSIONS
This study provides evidence that sporadic anovulation does not seem to be an important determinant of time to pregnancy in healthy, regularly cycling populations of women attempting to conceive. Accordingly, routine investigation of sporadic anovulation in regularly cycling women may not be warranted in studies of fecundability, though previously reported estimates for its incidence are likely similarly plausible. Similarly, routine testing to ensure ovulation and treatment of anovulation are unlikely to be medically necessary among women with regular menstrual cycles and without a history of subfertility. While biomarkers such as urinary luteinizing hormone or cervical fluid may be useful for precise timing of intercourse to the fertile window, time to pregnancy can also be improved through unscheduled frequent intercourse. We encourage large preconception time to pregnancy studies to seek validation of the current findings.
Supplementary Material
eFigure 1. Survival curves comparing sporadic anovulation rates of 1%, 8% and 14.5% on simulated time to pregnancy over 12 cycles of follow-up among 100,000 womena (using Scenario 2b relationship between sex and implantation)
a Sex in the fertile window = 87% and probabilities of anovulation specified in header; probability of implantation given fertilization = 70% and probability of fertilization given sex and ovulation = 49% for the first cycle of observation; 7.5% relative reductions in probabilities per cycle implemented for implantation given fertilization and fertilization given sex and ovulation; probability of implantation calculated using Equation 1.
b Scenario 2: among fecund couples attempting conception, the usual probability of pregnancy is ~30% when intercourse occurs within the fertile window in 95% of cycles16
Social media quote:
In presumed fertile women with regular menstrual cycles, routine investigation of anovulation may not be an informative outcome in studies of fecundability, and routine testing to ensure ovulation and treatment of anovulation are unlikely to be medically necessary.
Synopsis.
Study question:
Is sporadic anovulation an important contributor to cumulative pregnancy rates and time to pregnancy among fertile women with regular menstrual cycles?
What’s already known:
Anovulation is a first line treatment target for couples with difficulty conceiving and is frequently examined in studies of fecundability.
What this study adds:
In a data-driven simulation of 12 consecutive menstrual cycles, large differences in sporadic anovulation did not produce important differences in cumulative pregnancy rates or time to pregnancy. This study provides evidence that in presumed fertile women with regular menstrual cycles, routine investigation of anovulation may not be an informative outcome in studies of fecundability, and routine testing to ensure ovulation and treatment of anovulation are unlikely to be medically necessary.
FUNDING
Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (National Institutes of Health, Bethesda, MD, USA; contract numbers HHSN267200603423, HHSN267200603424, HHSN267200603426)
Footnotes
Data sharing plan: Simulated data available upon request.
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.American Society for Reproductive Medicine, Committee on Gynecologic Practice. Infertility workup for the women’s health specialist. ACOG Committee Opinion No. 781. Obstet Gynecol. 2019; 133:e377–384. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health Care and Excellence. Fertility problems: assessment and treatment (clinical guideline CG156). 2013. [updated September 2017; cited 2020 March 18, 2020]; Available from: https://www.nice.org.uk/guidance/cg156/chapter/Recommendations#investigation-of-fertility-problems-and-management-strategies.
- 3.Hambridge H, Mumford S, Mattison D, Ye A, Pollack A, Bloom M, et al. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Human Reproduction. 2013; 28:1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radin RG, Sjaarda LA, Perkins NJ, Silver RM, Chen Z, Lesher LL, et al. Low-dose aspirin and sporadic anovulation in the EAGeR randomized trial. The Journal of Clinical Endocrinology & Metabolism. 2016; 102:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radin RG, Sjaarda LA, Silver RM, Nobles CJ, Mumford SL, Perkins NJ, et al. C-Reactive protein in relation to fecundability and anovulation among eumenorrheic women. Fertility and Sterility. 2018:240–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird DD, McConnaughey DR, Weinberg CR, Musey PI, Collins DC, Kesner JS, et al. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995:547–550. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox AJ, Weinberg CR, O’connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988; 319:189–194. [DOI] [PubMed] [Google Scholar]
- 8.Stanford JB. Revisiting the fertile window. Fertility and Sterility. 2015; 103:1152–1153. [DOI] [PubMed] [Google Scholar]
- 9.Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Human Reproduction. 2002; 17:1399–1403. [DOI] [PubMed] [Google Scholar]
- 10.Dunson DB, Baird DD, Wilcox A, Weinberg C. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Human Reproduction. 1999; 14:1835–1839. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie W Ultrasound in the evaluation of normal and induced ovulation. Fertility and Sterility. 1985; 43:167–181. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczyk RT, Stanford JB, Ecochard R. Multilevel model to assess sources of variation in follicular growth close to the time of ovulation in women with normal fertility: a multicenter observational study. Reproductive Biology and Endocrinology. 2008; 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavličev M, Wagner G. The evolutionary origin of female orgasm. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2016; 326:326–337. [DOI] [PubMed] [Google Scholar]
- 14.Wood JW. Conception, implantation, and pregnancy In: Dynamics of human reproduction: biology, biometry, demography. New York: Aldine De Gruyter, 1994; pp. 185–237. [Google Scholar]
- 15.Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstetrics & Gynecology. 2003; 101:1285–1293. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation—effects on the probability of conception, survival of the pregnancy, and sex of the baby. New England Journal of Medicine. 1995; 1995:1517–1521. [DOI] [PubMed] [Google Scholar]
- 17.IVF Success Rates with 5 Day Blastocyst Transfers at the Advanced Fertility Center of Chicago. [November 27, 2019]; Available from: https://www.advancedfertility.com/blastocystpregnancyrates.htm.
- 18.Tournaye H, Verheyen G, Albano C, Camus M, Van Landuyt L, Devroey P, et al. Intracytoplasmic sperm injection versus in vitro fertilization: a randomized controlled trial and a meta-analysis of the literature. Fertility and Sterility. 2002; 78:1030–1037. [DOI] [PubMed] [Google Scholar]
- 19.Heuchel V, Schwartz D, Price W. Within‐subject variability and the importance of abstinence period for sperm count, semen volume and pre‐freeze and post‐thaw motility. Andrologia. 1981; 13:479–485. [DOI] [PubMed] [Google Scholar]
- 20.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertility and Sterility. 2006; 86:516–523. [DOI] [PubMed] [Google Scholar]
- 21.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Hormones. 2002; 77:873–882. [DOI] [PubMed] [Google Scholar]
- 22.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Molecular reproduction and development. 2002; 61:234–248. [DOI] [PubMed] [Google Scholar]
- 23.Prasad A, Mumford SL, Louis GMB, Ahrens KA, Sjaarda LA, Schliep KC, et al. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Hormones. 2014; 66:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL, Silver RM, Stanford JB, et al. Is anti-Müllerian hormone associated with fecundability? Findings from the EAGeR trial. The Journal of Clinical Endocrinology. 2015; 100:4215–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch C, Sundaram R, Maisog J, Sweeney A, Buck Louis G. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study—the LIFE study. Human Reproduction. 2014; 29:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology. 2013:871–879. [DOI] [PubMed] [Google Scholar]
- 27.ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility. 2004; 81:19–25. [DOI] [PubMed] [Google Scholar]
- 28.Schulte MM, Tsai J-h, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reproductive Sciences. 2015; 22:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jungheim ES, Travieso JL, Carson KR, Moley KH. Obesity and reproductive function. Obstetrics and Gynecology Clinics. 2012; 39:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vos M, Pareyn S, Drakopoulos P, Raimundo JM, Anckaert E, Santos-Ribeiro S, et al. Cumulative live birth rates after IVF in patients with polycystic ovaries: phenotype matters. Reproductive biomedicine online. 2018; 37:163–171. [DOI] [PubMed] [Google Scholar]
- 31.Legro RS. The quality of ovulation is strained in normal women. Human Reproduction. 2013; 28:1446–1447. [DOI] [PubMed] [Google Scholar]
- 32.Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertility and Sterility. 2014; 102:511–518. e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HPG, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan (R) Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Human Reproduction. 2000; 15:2478–2482. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox A, Day Baird D, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Human Reproduction. 2004; 19:1539–1543. [DOI] [PubMed] [Google Scholar]
- 35.Manders M, McLindon L, Schulze B, Beckmann MM, Kremer JA, Farquhar C. Timed intercourse for couples trying to conceive. Cochrane Database of Systematic Reviews. 2015:1–43. [DOI] [PubMed] [Google Scholar]
- 36.Evans-Hoeker E, Pritchard DA, Long DL, Herring AH, Stanford JB, Steiner AZ. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertility and Sterility. 2013; 100:1033–1038. e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanford JB, Willis SK, Hatch EE, Rothman KJ, Wise LA. Fecundability in relation to use of fertility awareness indicators in a North American preconception cohort study. Fertility and Sterility. 2019; 112:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson S, Stanford JB, Warren G, Bond S, Bench-Capon S, Zinaman MJ. Increased Likelihood of Pregnancy Using an App-Connected Ovulation Test System: A Randomized Controlled Trial. Journal of Women’s Health. 2019:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monseur BC, Franasiak JM, Sun L, Scott RT, Kaser DJ. Double intrauterine insemination (IUI) of no benefit over single IUI among lesbian and single women seeking to conceive. Journal of assisted reproduction and genetics. 2019; 36:2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner AZ, Pritchard DA, Young SL, Herring AH. Peri-implantation intercourse lowers fecundability. Fertility and Sterility. 2014; 102:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Survival curves comparing sporadic anovulation rates of 1%, 8% and 14.5% on simulated time to pregnancy over 12 cycles of follow-up among 100,000 womena (using Scenario 2b relationship between sex and implantation)
a Sex in the fertile window = 87% and probabilities of anovulation specified in header; probability of implantation given fertilization = 70% and probability of fertilization given sex and ovulation = 49% for the first cycle of observation; 7.5% relative reductions in probabilities per cycle implemented for implantation given fertilization and fertilization given sex and ovulation; probability of implantation calculated using Equation 1.
b Scenario 2: among fecund couples attempting conception, the usual probability of pregnancy is ~30% when intercourse occurs within the fertile window in 95% of cycles16


