Abstract
OBJECTIVE:
We compared intrinsic network connectivity in symptomatic youth at high risk (HR) for bipolar disorder (BD) and healthy comparison (HC) youth, and, in HR youth, investigated treatment-related changes in intrinsic connectivity following family focused therapy for high-risk youth (FFT-HR) versus standardized family psychoeducation.
METHOD:
HR youth (N=34; age 9–17 years; mean 14 years, 56% girls) with depressive and/or hypomanic symptoms and at least one first- or second-degree relative with BD I or II were randomly assigned to 4 months of FFT-HR (12 sessions of psychoeducation, communication, and problem-solving skills training) or Enhanced Care (EC; 3 family and 3 individual psychoeducation sessions). Before and after 4-months of treatment, participants underwent resting state functional magnetic resonance imaging (rs-fMRI). A whole brain independent component analysis compared rs-fMRI networks in HR youth and 30 age-matched HC youth at a pretreatment baseline. Then, we identified pretreatment to post-treatment (4-month) changes in network connectivity in HR youth receiving FFT-HR (n=16) or EC (n=18) and correlated these changes with depression improvement.
RESULTS:
At baseline, HR youth had greater connectivity between the ventrolateral prefrontal cortex (VLPFC) and the anterior default mode network (aDMN) than did HCs (p=.004). Over 4 months of treatment, FFT-HR-assigned HR youth had increased VLPFC-aDMN connectivity from pre- to post-treatment (p=.003), whereas HR youth in EC showed no significant change over time (p=.11) (treatment by time interaction, [t(31)=3.33, CI 95% [0.27, 1.14], p=.002]. Reduction in depression severity over 4 months inversely correlated with enhanced anterior DMN (r= −.71) connectivity in the FFT-HR but not in the EC (r=−.07) group (z=−2.17, p=.015).
CONCLUSION:
Compared to standard psychoeducation, FFT-HR is associated with stronger connectivity between the VLPFC and aDMN, suggesting possible enhancements of self- and illness awareness and emotion regulation.
Clinical trial registration information:
Early Intervention for Youth at Risk for Bipolar Disorder; https://clinicaltrials.gov/; NCT01483391.
Keywords: family focused therapy, bipolar disorder, familial risk, depression, resting state functional connectivity
INTRODUCTION
Twin and family studies provide compelling evidence that having a parent with bipolar disorder (BD) is associated with a dramatically increased risk for the development of mood disorders in offspring.1 Compared to healthy offspring of parents with no psychopathology, offspring of parents with BD are chronically exposed to stress,2 to conflictual family environments,3 and have higher rates of mood and other psychiatric disorders,4 often with significant neural, cognitive, and biological consequences.5,6 Given the enormous personal and societal costs of mood disorders, and the difficulty in treating its complications once they have developed, there is a pressing need to identify and treat youth at high risk for BD and prevent them from developing adverse outcomes that can persist over the life course.
Studies by our research group and others suggest that early disruptions in key prefrontal and striatolimbic networks are significant risk factors for developing mood and other psychiatric disorders in youth at high risk (HR) for BD. Neuroimaging studies in offspring of parents with BD suggest risk phenotypes including decreases in frontal and temporal gray7 and white8 matter volumes and cortical thinning,9 as well as greater prefrontal functional activations during a number guessing reward task,10 greater prefrontal and lower temporal activations during face emotion labeling,11 and impaired prefrontal-striatal-temporal network controllability.12 The ventrolateral prefrontal cortex (VLPFC), executive control (ECN), and default mode networks (DMN) stand out as particularly relevant to bipolar disorder as prominent markers of risk (indicated by decreases in VLPFC-striatal connectivity)13 and disease burden (indicated by lower affective network activity in patients with a higher number of episodes),14 but also as markers of recovery (indicated by increased or stable network connectivity, similar to what is observed in healthy controls).15 Because aberrant neural network findings in prefrontal, striatal, and limbic regions have been consistently observed in offspring prior to the onset of any mood symptoms,13 there is a critical need to target symptoms and neural circuit vulnerability through early intervention. A few studies are beginning to prospectively examine changes in prefrontal and striatolimbic network trajectories in high-risk offspring, reporting on distinct neural findings associated with symptomatic16 and resilient17 outcomes. These studies suggest the plasticity of neural networks toward more adaptive function. However, few studies have tested this adaptability directly through an investigation of the effects of a randomized psychosocial intervention on neural networks implicated in risk for BD.
Depending on the level of impairment and symptoms, youth at risk for BD may benefit from a combination of psychosocial and pharmacological treatments.18 An evidence-based intervention is family focused therapy for high-risk youth (FFT-HR), which consists of 12 sessions of family psychoeducation, communication skills training, and problem solving skills training. FFT-HR is associated with more rapid recovery from mood episodes, increased time in remission, and more favorable hypomania symptom trajectories in high-risk youth when compared to a “treatment as usual” brief family educational treatment (“Enhanced Care,” EC), 19 and has been demonstrated by randomized controlled trials to improve the course of BD in adults and adolescents.20
In a pilot study of 12 youth offspring of bipolar parents who received FFT-HR or EC, we previously reported increased dorsolateral prefrontal cortex activation during face emotion processing over a 4-month treatment period, which corresponded with amount of mood symptom improvement.21 However, due to the small sample size, the study was not able to disentangle differential neural effects by treatment condition, or investigate a broader array of intrinsic neural networks supporting cognitive and emotional function that may change while youth are responding to FFT-HR.
In this study, we used a network based approach to identify early neural risk factors that could help to 1) differentiate high-risk (HR) youth from typically developing healthy comparison (HCs) youth; and 2) compare treatment-related changes in network connectivity in HR youth randomized to either 12 sessions in 4 months of family focused therapy (FFT-HR) or 6 sessions in 4 months of enhanced care (EC) only. To date, no study has examined changes in intrinsic functional connectivity in youth with a familial vulnerability for BD who receive a psychosocial intervention. Drawing on findings from resting state fMRI in youth at risk for BD,6,13,22 we predicted that, compared with healthy offspring of parents with no history of any psychiatric disorders, youth of parents with BD would show patterns of reduced intrinsic functional connectivity between VLPFC, ECN, and DMN networks. In addition, we hypothesized that over 4 months of intervention, FFT-HR would increase prefrontal-limbic connectivity to baseline levels observed in HC youth to a greater extent than EC. Finally, we predicted that treatment-related changes in connectivity would correlate with pre-/post-treatment improvements in mood symptom severity.
METHOD
Participants
The study was approved by the medical institutional review boards of Stanford University’s School of Medicine (Stanford) and the University of California, Los Angeles (UCLA) Semel Institute After receiving a full explanation of the study procedures, participants and their parent(s) gave written informed assent and consent to participate. Participants were recruited from clinical referrals to outpatient clinics at Stanford and UCLA or learned of the study through online, radio, or print advertisements or public presentations. A third study site in the larger trial, University of Colorado, did not have the necessary infrastructure at the beginning of the trial to conduct neuroimaging procedures. A full description of the larger treatment trial has been published previously.23,24
Eligibility criteria for HR youth included: (1) age between 9 years, 0 months and 17 years, 11 months; (2) meets DSM-IV criteria for major depressive disorder (MDD) or BD-Not Otherwise Specified (BD-NOS), the latter adapted to follow the COBY protocol 25 as distinct periods of abnormally elevated, expansive, or irritable mood plus two (three, if irritable mood only) DSM symptoms of mania that caused a change in functioning, lasted ≥ 4 hours in a day, and occurred for a total of 10 or more days in the child’s lifetime; (3) at least one first or second-degree relative has a lifetime history of BD I or II, based on the MINI International Neuropsychiatric Interview and DSM-IV criteria26; (4) based on a consensus between child and parent interviews, a current (prior week) Young Mania Rating Scale (YMRS)27 score >11 or a prior 2-week Children’s Depression Rating Scale, Revised (CDRS-R)28 score >29 (both indicating moderate symptoms) in the child; and (5) no prior syndromal manic episode. Exclusion criteria included any developmental disorders, neurological conditions or major medical illnesses, substance use disorders, IQ less than 80, MRI contraindications (metal in the body), orthodontic braces, or current hospitalization. In addition to these exclusion criteria, HC youth could not meet criteria for any current or lifetime psychiatric disorder (including depression, anxiety, or any other psychopathology) in themselves or in their first-degree relatives.
Clinical Assessments
Mood symptom severity was rated at pre- and post-treatment (4 months) and administered separately to parents and youth by an independent evaluator with established interrater reliability. Interrater reliability for KSADS Depression and Mania Rating scales had means of 0.74 and 0.84 (intraclass correlations) across sites.24 The YMRS was used to measure subthreshold symptoms of mania in the past week and the CDRS-R was used to measure depressive symptoms in the preceding 2 weeks. The “Kiddie” Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (KSADS-PL)29 semi-structured interview was conducted with the child and at least one parent (regarding the child) to determine diagnoses, with final ratings based on a clinician-rated consensus of the two reports. Each child’s biological parent was interviewed about their own psychiatric history using the MINI.26 First- or second-degree relatives who could not be interviewed were diagnosed from secondary information provided by one or both biological parents using the Family History Screening Instrument.30
Family Intervention
Once baseline eligibility data was established, the site’s PI randomly assigned eligible participants in a 1:1 ratio by a computer generated algorithm to either Family Focused Therapy for High-Risk Youth (FFT-HR) or Enhanced Care (EC). The algorithm balanced the groups within sites on primary diagnosis (MDD or BD-NOS), age (<13 or ≥13 years), and initial medications (mood stabilizers/antipsychotics vs. neither). All study therapists administered both treatments, whereas outcome assessments were conducted by an independent evaluator who was blind to treatment condition. Both treatment protocols have been previously described.31 FFT-HR involved the high-risk child, all adult caregivers (e.g., parents/stepparents or grandparents), and when possible, siblings. FFT-HR consisted of didactic psychoeducation, communication enhancement training (e.g., practicing active listening or how to constructively express positive or negative feelings), and problem-solving skills training in 12 sixty minute sessions over 4 months (8 weekly, 4 biweekly). EC consisted of 3 weekly standard family psychoeducational sessions with the child, caregiving adults and siblings, followed by 3 monthly individual psychoeducation sessions that focused on developing and implementing a mood management plan. Thus, the two groups differed in the frequency of sessions and in the provision of skills training (communication and problem-solving), which occurred in FFT-HR but not EC. Children who required medications received medication management sessions from their assigned study psychiatrist, though they were not required to take psychiatric medications to be in the trial. Study physicians followed a written pharmacotherapy algorithm previously described.32
Multi-site fMRI data acquisition and reliability
HR subjects at the Stanford and UCLA sites underwent neuroimaging scans at baseline and again at 4 months (the length of the psychosocial treatment interval) and HC youth were scanned once at baseline. The 1-hour scan session included three fMRI scans and a high-resolution T1-weighted anatomical scan. Participants were first familiarized with the scanning environment in an MRI simulator. CDRS-R and YMRS ratings were obtained within 1 week of each scan.
fMRI data were collected at Stanford’s Richard M. Lucas Center for Imaging and at UCLA’s Center for Cognitive Neuroscience. Scan parameters were optimized for comparability across sites using recommendations from the Functional Biomedical Informatics Research Network for multi-site fMRI studies.33 Three subjects were scanned on 2 different days at each site and showed high test-retest consistency between sites and good comparability of fMRI results across sites.31 Nevertheless, scanner site was conservatively included as a covariate in all imaging analyses. Site specific acquisition parameters and scanner type are provided in Supplement 1, available online.
fMRI data preprocessing
Image preprocessing was performed using the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library (FSL version 5.0.10; www.fmrib.ox.ac.uk/fsl) 34,35. The following standard preprocessing steps were used: (a) first five volumes were discarded to allow for signal stabilization; (b) head motion correction was performed using the Motion Correction FMRIB’s Linear Image Registration Tool (MCFLIRT) 36; (c) non-brain tissue was extracted using the Brain Extraction Tool (BET)37; and (d) spatial smoothing was conducted using a Gaussian kernel of 5-mm full width half maximum. Functional data were registered to each individual’s 3D FSPGR or MPRAGE, followed by registration to the MNI152 template with resolution 2×2×2mm standard-space by affine linear registration using FMRIB’s Linear Image Registration Tool (FLIRT)36,38.
Subsequently, to remove motion-related spurious noise and artifacts and to improve sensitivity to group-level connectivity differences, we employed an advanced ICA-based strategy for automatic detection and removal of motion-related artifacts (ICA-AROMA)39. Global signal regression was not used. Advantages of ICA-AROMA include the preservation of the autocorrelation structure of the fMRI time-series, little impact on the temporal degrees of freedom, and increased sensitivity to signals of interest. Further, this denoising approach identifies motion-related and noise-related components with high discrimination from resting state networks, and the resulting resting state networks do not overlap with white matter or cerebrospinal fluid signal. Prior research has demonstrated that this approach removes motion-related spurious noise and artifacts to a greater extent than regression using 24 motion parameters or spike regression.39
Group independent component analysis (ICA) and dual regression
The denoised outputs from ICA-AROMA were inputted into a group-concatenated Independent Components Analysis (ICA) using the MELODIC toolbox, part of the FSL software library (FMRIB Software Library, http://www.fmrib.ox.uk/fsl)40, to identify resting state networks for the group of all subjects combined. Temporal concatenation was then completed after voxel-wise variance normalization to create 20 spatial components masked at Z = 4, of which 7 were attributed to physiological, movement related, or imaging artifacts, and an additional 6 contained primarily visual or motion regions. Thus, these 13 networks were removed and the remaining 7 networks were identified for inclusion in analyses because they exhibited peak activations in grey matter, showed low spatial overlap with known vascular, ventricular, motion, edges, and susceptibility artifacts, and were relevant to the BD and BD risk literature.13,41,42 These remaining 7 network components were entered into the fslcc utility in FSL to spatially correlate them to canonical components from http://findlab.stanford.edu/functional_ROIs.html. Component labels were additionally confirmed by referencing network templates published in the literature.43 Next, we ran dual regression (first temporal, then spatial)44 to estimate subject specific spatial maps for each component.44 These subject specific spatial maps were used for HR vs HC group and FFT-HR vs EC treatment group analyses.
Selection of networks and region-of-interests (ROI)
The seven out of the twenty group functional network components for our group MELODIC ICA (including HC scans) in default mode, executive control, and salience networks, were included in multivariate voxel based analyses based on their relevance to the BD and BD risk literature.41,45 Based on our prior work in offspring of parents with BD,13 we investigated a priori connectivity between these components and the VLPFC region of interest (BA 45) created from the Harvard–Oxford Cortical atlas in FSL (see Figure S1, available online). This region was selected because of its consistent representation in resting state and task-related fMRI studies in BD and BD risk, and because it subserves emotion regulatory functions postulated to be engaged during family focused therapy.20
HR vs HC Group Analyses
Whole-brain voxel-wise risk group analyses to compare HR versus HCs were conducted using a multivariate ANOVA (MANOVA) using the Multivariate and Repeated Measures (MRM) MATLAB Toolbox.46 We tested for group differences in connectivity within our a priori MELODIC components, covarying for scanner site, age, and sex.
FFT vs EC Treatment Group Analyses
The MRM toolbox also allows advanced statistical modeling of repeated measures mixed effects designs using a multivariate form of the general linear model.46 Thus, we tested for longitudinal changes in connectivity within our a priori MELODIC components from pre to post treatment using a group (FFT-HR vs EC) x time (pre vs post) using a mixed effect regression model, covarying for scanner site, age, and sex. Thresholding and correction for multiple comparisons for both analyses were achieved using nonparametric permutation testing with 5000 iterations, with a cluster-setting threshold of p=.001 and family wise error (FWE) correction of p < .05 at the cluster level.
Mean connectivity estimates between a priori components and bilateral VLPFC were extracted for each subject in order to examine 1) treatment differences in connectivity and 2) how post treatment connectivity values compared to HC baseline connectivity values. To test for group differences in pre and post treatment change, a mixed-effects regression using SPSS Version 25 was used,47 with group as fixed factor and time as repeated factor (pre vs post). Univariate analyses were conducted to assess whether diagnosis or medication class predicted any treatment-related differences in mean connectivity results. ANOVAs were conducted to compare post treatment connectivity values for the FFT-HR and EC groups and baseline HCs, followed by Tukey post hoc t-tests to further examine group differences.
Symptom Correlations
We conducted Pearson correlations, adjusting for age, sex, and site, to examine between connectivity estimates and change in CDRS-R and YMRS scores from baseline to end of treatment within each group. We then conducted Fisher’s r-to-z transformations to determine whether the FFT-HR and EC groups differed significantly with respect to these within-group correlations.
RESULTS
Inclusion of Participants
Seventy-two participants were scanned for this study. Forty HR participants completed resting state scans before and after psychosocial treatment. Three HR participants were excluded due to high motion, two for scan acquisition issues, and one participant was excluded due to have fallen asleep during resting state scan. Thus, 34 HR participants (17 at Stanford, 17 at UCLA) all of whom had usable pre- and post-treatment scans were included in the analysis (for Consort Diagram, see Figure S2, available online). Thirty-two HC participants (22 at Stanford, 10 at UCLA) were also scanned with one at each site excluded due to motion (UCLA) or poor scan acquisition (Stanford), leaving the final sample of 30 usable HC scans at baseline (21 at Stanford, 9 at UCLA). There were no statistically significant HR vs HC or FFT-HR vs EC group differences in motion artifacts before (Table 1) or after ICA-AROMA [HR vs HC (p=0.134) or FFT-HR vs EC (p=.678)].
Table 1.
Demographic and Clinical Variables for High-Risk and Healthy Comparison Youth
| Group Analysis | Healthy Comparison (HC) (N=30) | High Risk (HR) (N=34) | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Site (Stanford/UCLA) | 21/9 | 17/17 | χ2(1)=2.64; p=.10 | ||||
| Age (SD) | 14.41 (2.27) | 13.98 (2.64) | t(62)=.70; p=.48 | ||||
| Sex (F/M) | 16/14 | 19/15 | χ2(1)=0.04; p=.84 | ||||
| Motion: absolute mean displacement (mm) (mean, SD, range) | .22 (.34), [.04–1.95] | .33 (.29), [.07–1.17] | t(62)=1.37; p=.18 | ||||
| Treatment Group Analysis | HR FFT-HR (n=16) | HR EC (n=18) | Statistics | ||||
| pre | post | pre | post | Pre | post | ||
| Site (Stanford/UCLA) | 7/9 | -- | 10/8 | -- | χ2(1)=0.47; p=.49 | -- | |
| Age (SD) | 14.57 (2.39) | 15.02 (2.37) | 13.45 (2.80) | 13.84 (2.79) | t(32)=1.24; p=.22 | t(32)=1.30; p=.20 | |
| Sex (F/M) | 8/8 | -- | 7/11 | -- | χ2(1)=0.42; p=.52 | -- | |
| Motion: absolute mean displacement (mm) (mean, SD, range) | .31 (.25), [.07–.77] | .36 (.29), [.10–1.22] | .35 (.33), [.08–1.17] | .30 (.33), [.07–1.17] | t(32)=−.33; p=.74 | t(32)=.50 p=.62 | |
| CDRS-R mean (SD) | 46.87 (10.31) | 39.56 (13.07) | 50.67 (17.67) | 36.83 (15.99) | t(32)=−.75; p=.46 | t(32)=.54; p=.59 | |
| YMRS mean (SD) | 10.00 (7.73) | 8.25 (6.12) | 11.78 (6.23) | 9.28 (5.54) | t(32)=−.74; p=.46 | t(32)=−.51; p=.61 | |
| Primary Diagnosis | |||||||
| MDD | 11 (69%) | 9 (50%) | χ2(1)=1.23; | ||||
| BD-NOS | 5 (31%) | 9 (50%) | p=.27 | ||||
| Medications at Baseline | |||||||
| Unmedicated | 4 (25%) | 10 (55%) | χ2(1)=3.26; p=.07 | ||||
| Antidepressant | 8 (50%) | 2 (11%) | χ2(1)=6.17; p=.01 | ||||
| Antipsychotic | 3 (19%) | 7 (39%) | χ2(1)=2.06; p=.36 | ||||
| Anticonvulsant | 4 (25%) | 2 (11%) | χ2(1)=1.12; p=.29 | ||||
| Anxiolytic | 0 (0%) | 1 (6%) | χ2(1)=.92; p=.34 | ||||
| Stimulant | 4 (25%) | 4 (22%) | χ2(1)=.04; p=.85 | ||||
Note: BD-NOS =Bipolar Disorder, Not Otherwise Specified; CDRS-R = Children’s Depression Rating Scale-Revised; EC = Enhanced Care; FFT-HR = Family Focused Therapy for High-Risk Youth; MDD = Major Depressive Disorder; YMRS = Youth Mania Rating Scale; values indicate mean ± SD = standard deviation.
Demographics and Clinical Characteristics
Demographic information is presented in Table 1. HR and HC groups were balanced for age and sex. There were no significant differences in the demographics of HR and HC youth across the Stanford and UCLA sites. In addition, there were no differences in CDRS-R or YMRS scores or primary diagnoses between the FFT-HR and EC groups (all ps>0.05). Eight youth in the FFT-HR group compared to two youth in the EC group received antidepressants at baseline (χ2(1)=6.17; p=.013), but no other medication exposure was significantly different between the groups (all ps>0.05), nor were the distributions of unmedicated to medicated youth significantly different across treatment groups (χ2(1)=3.26; p=.07).
Confirmation of ICA Components
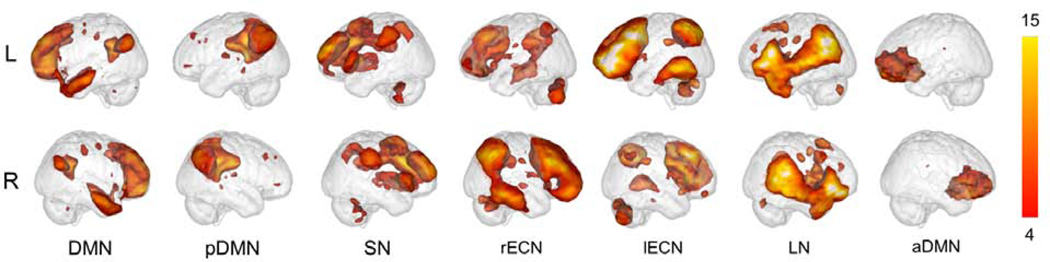
Correlations with canonical components showed that Component 2 was significantly correlated with a DMN template (r=.55) so was labeled as DMN and was comprised of the medial prefrontal cortex (mPFC), inferior parietal lobe (IPL), and precuneus. Component 4 was labeled as the posterior DMN (pDMN), with significant correlates to precuneus (r=.36), ventral DMN (r=.31), dorsal DMN (r=.23) templates. Component 6 significantly correlated with the salience network (SN) template (r=.47), so was thus labeled. Component 8 significantly correlated with the right executive control network (rECN) template (r=.50) and consisted of the right ventrolateral prefrontal cortext (VLPFC), right dorsolateral prefrontal cortex (DLPFC), right angular gyrus, and right middle frontal gyrus (MFG). Component 11 significantly correlated with the left executive control network (lECN) template (r=.45), so was thus labeled, and was comprised primarily of the left paracingulate gyrus, left superior frontal gyrus, and left VLPFC. Component 14 significantly correlated with the language network (LN) template (r=.40), so was thus labeled. Finally, component 17 significantly correlated with the dorsal DMN template (r=.28) and was labeled the anterior DMN (aDMN), comprised mainly of the mPFC.
Whole-sample ICA Components
Figure 1 displays the spatial maps of the 7 components identified from group level ICA. Based on their known structural and functional properties, networks included the default mode (DMN), posterior DMN, salience (SN), left and right executive control (lECN, rECN), language (LN), and anterior DMN (aDMN) (for regional anatomical labels and spatial correlates, see Supplement 1, available online). These components were spatially correlated to individual network functional ROI atlases, and were similar to those observed in published ICA analyses in the BD literature13,15,22 and referenced brain network templates.43
Figure 1: Whole-sample Components.
Note: Seven components resulting from our Independent Components Analysis (ICA). DMN, default mode network; aDMN, anterior default mode network; lECN, left executive control network; LN, language network; pDMN, posterior default mode network; rECN, right executive control network; SN, salience network.
HR vs HC Results
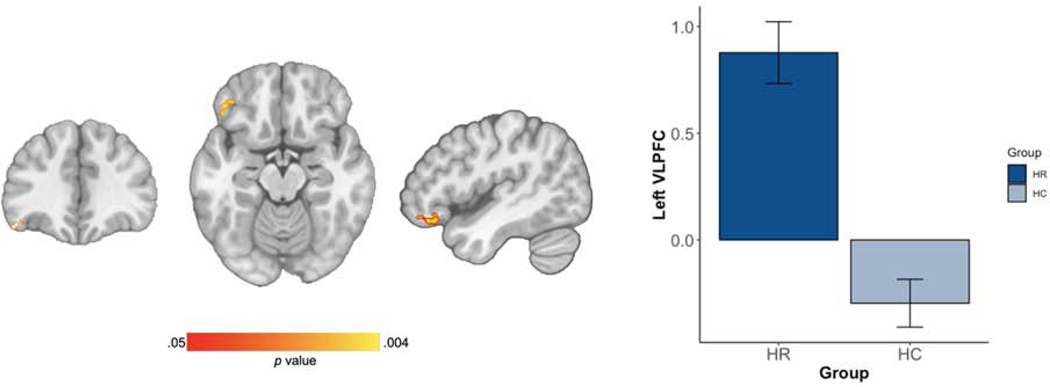
Voxel-wise results of MANOVA showed that among the seven networks of interest, the HR group had greater connectivity between left VLPFC in the anterior default mode network (aDMN) at baseline than did HCs (Figure 2) [peak X=−44, Y=30, Z=−12; cluster size = 104; BA 11/47; p = .004]. The two groups did not differ in connectivity in any of the other seven networks (all ps>0.05). When comparing baseline connectivity between HR youth and HCs, with the HR youth separated out by FFT-HR and EC treatment groups, the ANOVA similarly showed a significant group effect [F(61,2)=21.14; p < .01], driven post-hoc by FFT-HR youth having greater VLPFC-aDMN connectivity than the HCs (p <.01) and EC youth having greater VLPFC-aDMN connectivity than the HCs (p <.01). There were no significant differences in VLPFC-aDMN connectivity between the FFT-HR and EC youth at baseline (p=.20) (Figure 3).
Figure 2. Group Independent Component Analysis (ICA) Results in the High-Risk (HR) and Healthy Comparison (HC) Groups.
Note: (A) Cluster in the ventrolateral prefrontal cortex (VLPFC) [peak X=−44, Y=30, Z=−12; cluster size = 104; BA 11/47; p = .004] showing HR > HC connectivity differences (B) Extracted connectivity measures from this network show HR participants at baseline have increased connectivity within these regions compared to HC youth at baseline (p < .001).
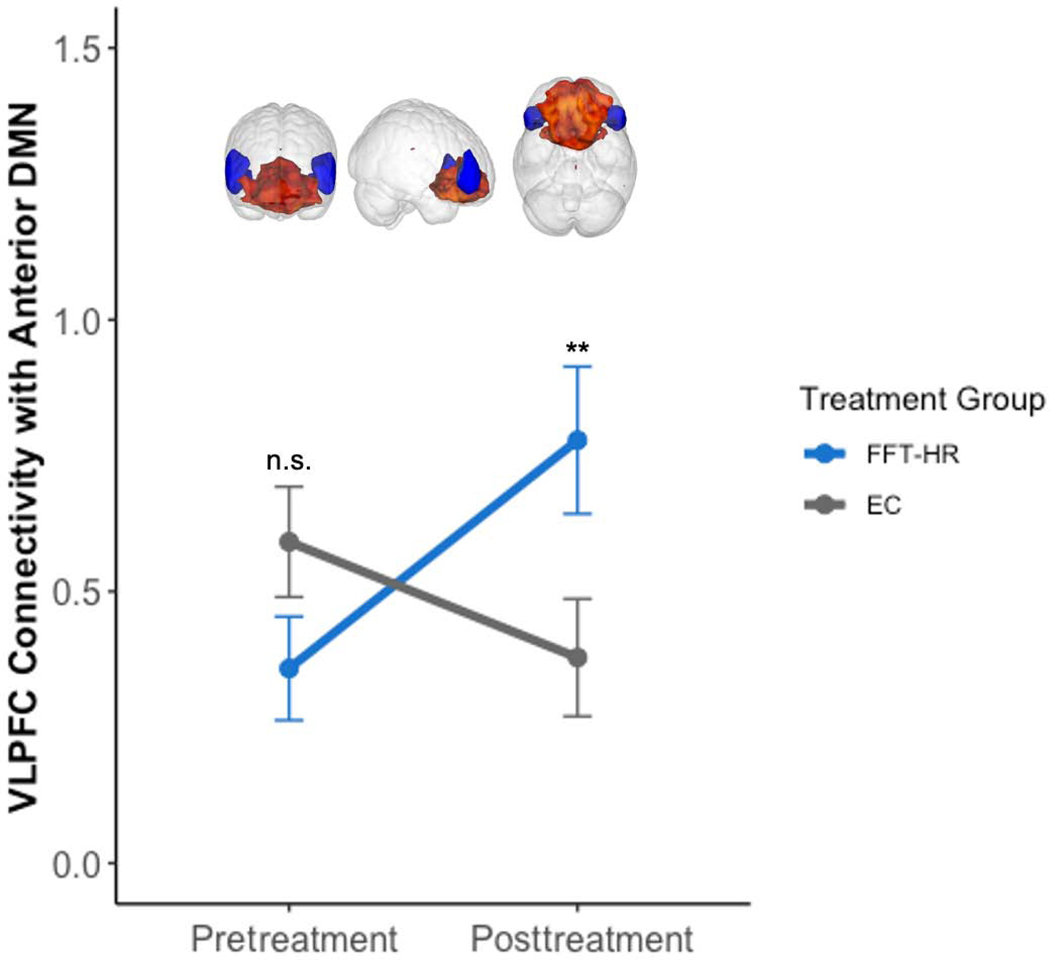
Figure 3. Significant Treatment Group x Time Interaction.
Note: In ventrolateral prefrontal cortex (VLPFC) and anterior default mode network (DMN) connectivity from pre- to posttreatment in Family Focused Therapy (FFT-HR) versus Enhanced Care (EC) for high-risk youth [(t(31)=3.33, CI 95% [0.27, 1.14], p=.002)]; n.s. = not significant; ** p < .01.
FFT vs EC Treatment Group x Time Interaction analysis
Using MRM to test for longitudinal changes in connectivity within our 7 network components of interest from pre- to post-treatment using a group (FFT vs EC)-by-time (pre vs post) analysis, after covarying scanner site, age, and sex, did not yield any significant clusters. However, a mixed-effects regression with group as a fixed factor and time as a repeated factor (pre versus post), covarying for scanner site, age, and sex was conducted to investigate connectivity between VLPFC and each of the 7 network components of interest (Figure 3). Results showed significant group-by-time interaction on the connectivity between VLPFC and aDMN ([(t(31)=3.33, CI 95% [0.27, 1.14], p=.002]). In this interaction, the FFT-HR group showed a significant increase in connectivity between VLPFC and aDMN from pre- to post-treatment ([(t(68)=3.04, CI 95% [.16, .76], p=.003]), whereas the EC group showed no significant change in connectivity over time ([(t(68)=−1.63, CI 95% [−.50, .05], p=.11]) (Figure 3 and Figure S3, available online). Neither primary diagnosis nor medication class exposures at baseline predicted treatment-related changes in VLPFC-aDMN connectivity (all ps>0.05).
Comparison of post treatment HR connectivity values to baseline HC
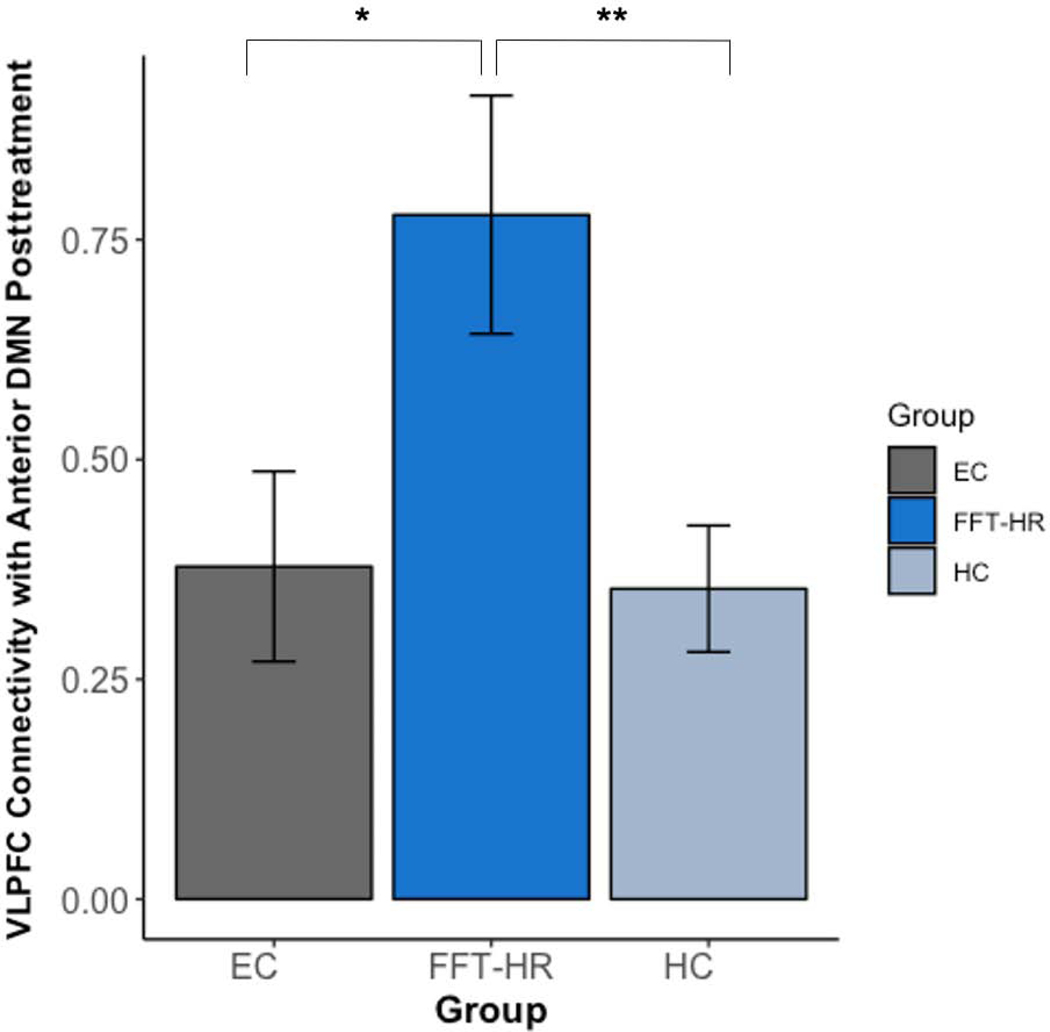
Using a one-way ANOVA, we investigated how connectivity observed in the FFT-HR and EC high-risk groups at post-treatment compared to connectivity among HCs at baseline (Figure 4). Post-treatment connectivity significantly differed between the three groups in the VLPFC and aDMN connectivity (F(63)=5.09, p=.009). Specifically, FFT-HR group had significantly stronger VLPFC to aDMN connectivity compared to the EC (t(32)=2.33, p=.026) and HC (t(44)=3.06, p=.004) groups.
Figure 4. Connectivity Estimates Posttreatment.
Note: Posttreatment connectivity values differed significantly among the Family Focused Therapy for High-Risk Youth (FFT-HR) and Enhanced Care (EC) for high-risk youth and baseline connectivity in healthy comparison (HC) youth between the ventrolateral prefrontal cortex (VLPFC) and anterior default mode network (DMN). * p < .05 ** p < .01
Correlations with symptom improvement
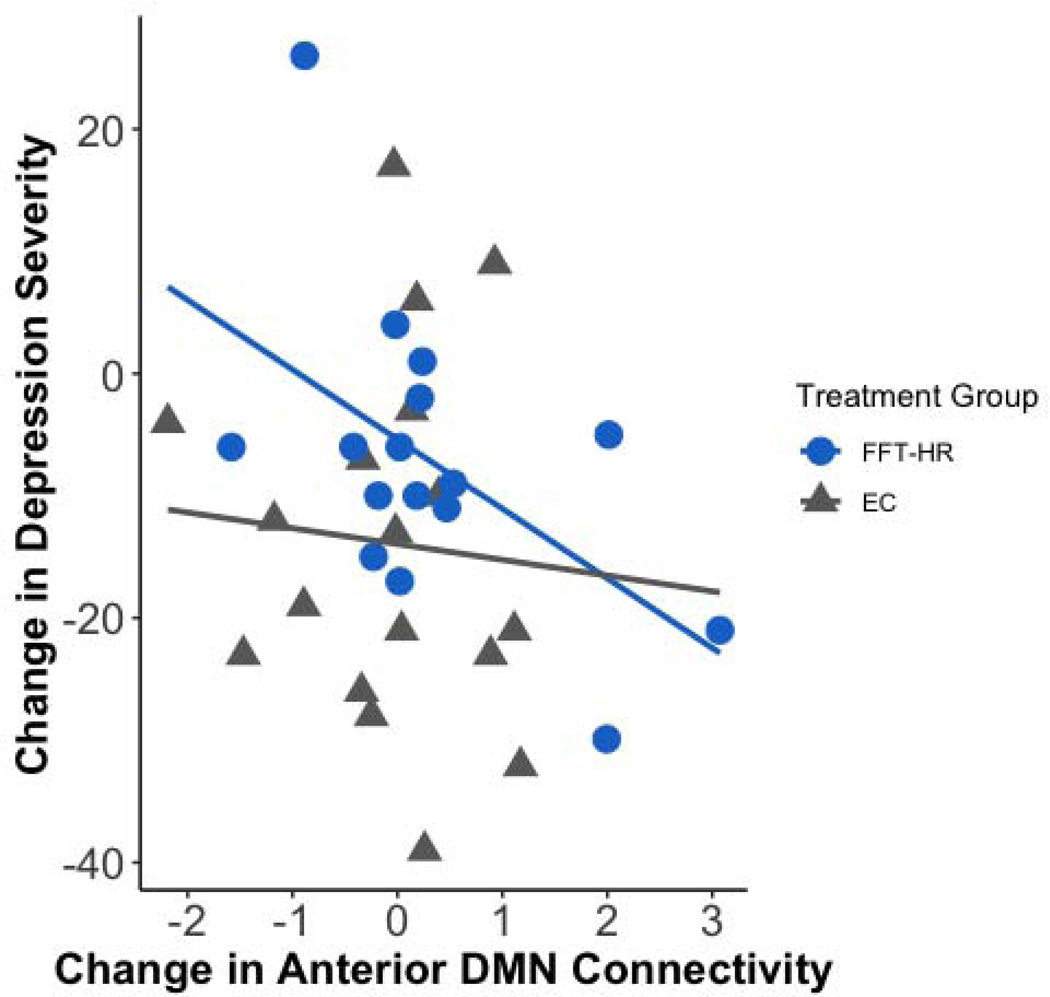
After correcting for age, sex, and scanner site, post- minus pre-treatment changes in anterior DMN connectivity were significantly correlated with post- minus pretreatment changes in CDRS-R scores in the FFT-HR group (r(13)=−.71 p =.006) but not in the EC group (r(15)=−.065; p=.82 Fisher’s r-to-z, z=−2.17, p =.015). This correlation was significant after correcting for multiple comparisons (Figure 5). There were no significant correlations between any connectivity changes and YMRS mania scores within the FFT-HR or EC groups (all ps>.05). Additional trend-level correlations are described in Supplement 1 (see Figures S4 and S5), available online.
Figure 5. Change in Depression Severity x Change in Network Connectivity Over 4 Months.
Note: Post- minus pretreatment change in anterior default mode network (DMN) connectivity was correlated with post- minus pretreatment with Family Focused Therapy (FFT-HR) and Enhanced Care (EC) changes in depression severity on the Children’s Depression Rating Scale-Revised (CDRS-R) for high-risk youth after covarying for age, sex, and site (Fisher’s r-to-z transformation, z=−2.17, p =.015).
DISCUSSION
In this randomized controlled trial of family intervention, we found that symptomatic youth with first or second-degree relatives with BD had greater intrinsic connectivity between the VLPFC and anterior DMN than did healthy comparison youth. Within this HR group, youth receiving FFT-HR had increased connectivity from pre- to post-treatment in VLPFC-aDMN connectivity, whereas the EC group showed no significant change in connectivity over time. Further, when comparing post-treatment network connectivity in HR youth to HC network connectivity at baseline, there were stronger connectivities between VLPFC-aDMN in FFT-HR compared to HC youth or HR youth who received EC. Finally, enhanced anterior DMN connectivity inversely correlated with improvement in depression severity in FFT-HR but not in EC youth, suggesting network adaptations that may be related to improved self-awareness and emotion regulation learned during FFT-HR.
Using a network-based analysis, the only significant network difference found between HR and HC youth was an increase between the VLPFC and anterior DMN at baseline. The veracity of this finding is supported by results surviving stringent correction for multiple comparisons and by the robust ICA-AROMA approach implemented to reduce motion-induced signal variations in fMRI data.39 Network connectivity associated with BD risk and disorder phenotypes depend on multiple factors including stage of risk, predominant mood state, and treatment exposure. Increases in VLPFC and anterior DMN connectivities have been observed in adults with partially15 or fully48 remitted symptoms, and have, in some cases, been proposed as a compensatory mechanism to maintain euthymia.15 In youth with BD, increased DMN and salience network connectivity has been proposed as a trait biomarker or sequelae of altered executive function.41 Baseline neural factors putatively associated with treatment response for depression across mood spectrum disorders include greater white matter integrity, increased prefrontal and DMN resting state connectivity, and greater prefrontal gray matter volume.49 However, a preponderance of studies of individuals with BD have reported deficits in emotion regulation, excessive self-referential thinking and rumination in depressed states, and reduced volumes and connectivities in prefrontal and limbic networks. These deficits have also been consistently implicated as risk factors for predicting the onset of BD in structural MRI,12 task-based fMRI,50–52 and resting state fMRI53 studies of high-risk youth. It may be that the high-risk youth in our study were more depressed, treatment-seeking, and at a different stage of illness than those in prior studies, showing increases in VLPFC-aDMN connectivity rather than the expected decreases in prefrontal and limbic connectivity that have been observed in youth with and at risk for BD. Indeed, improvement in depressive (but not manic) symptoms correlated with increases in aDMN network connectivity, suggesting that these intrinsic networks are altered with changes in depressed mood states in high-risk youth. Studies comparing intrinsic connectivity in patients with unipolar and bipolar depression (or those at risk) support this finding, suggesting that increased DMN connectivity represents both a predictor of depression treatment response across mood disorders,49 and a feature that distinguishes unipolar from bipolar risk in young adults with remitted depression.54
Other studies have suggested that hyperactivity of VLPFC and stronger connectivity to other high order networks important for emotion regulation and executive control may represent a biomarker of adaptation toward more resilient outcomes, buffering high-risk offspring from developing BD.13,55 It is the interpretation of increased strength of VLPFC connectivity as compensatory for early mood symptoms in these HR youth that brings the treatment group by time interaction finding into context. A few studies investigating neural correlates of treatment response have posited greater improvements in emotion regulation after cognitive behavioral therapy56 and in depressive symptoms after pharmacological treatment with quetiapine57 and other psychotropic medications58 that were VLPFC connectivity-dependent. Importantly, post-treatment VLPFC to aDMN connectivity and salience network estimates in HR youth randomized to FFT-HR did not decrease toward HC values, but rather increased in strength, suggesting a compensatory intrinsic connectivity that is enhanced with treatment. As mentioned above, increased anterior DMN connectivity was selectively associated with improved depressive but not with improved mania symptoms. Correspondingly, increased DMN integration has been described as a key feature to promote resilience in unaffected siblings of individuals with BD,59 and together with enhanced prefrontal connectivity, likely represents an adaptive change to intrinsic neural networks suggestive of increased neural reserve.60 Moreover, correlations with depression improvement were observed only with changes within aDMN connectivity after FFT, but not with changes between VLPFC-aDMN connectivity. This may reflect an early and specific compensatory response within the DMN component of the network, that may be necessary to counteract increases in VLPFC connectivity that might be symptom-driven or maladapative.61 It is also possible that that extrinsic effects of FFT-HR on network connectivity take time to promote plasticity and any mechanisms of treatment response intrinsic to high-risk youth are working in concert with FFT-HR to promote adaptive outcomes that may only be observed further out after treatment.24
We wish to acknowledge a few limitations of our study. First, we had a modest sample size; nevertheless, we found robust and consistent results after adjusting for multiple comparisons and accounted for site, developmental variance, and sexual dimorphism by controlling for site, age, and sex. Second, high-risk youth in this study were clinically heterogeneous, and were on a variety of medication regimens, with random assignment stratified on whether the participant was taking a mood stabilizing medication. HR youth in this study had diagnostic distributions typical of bipolar offspring samples who are frequently treated with a combination of psychosocial and pharmacological interventions. Treatment groups were not significantly different in their distributions of diagnoses or medication exposure status at baseline or follow-up, and univariate analyses did not find primary diagnosis or medication class to predict treatment-related differences in intrinsic network connectivity results. Third, the EC condition was matched to the FFT-HR condition in duration (4 months), but not in the number of sessions (12 vs. 6). Thus, group differences in network connectivity could have been due to nonspecific clinical contact or epiphenomena. However, symptom correlations and networks relevant to skills targeted in FFT make this possibility less likely. Fourth, scans were obtained from two sites, with more healthy controls scanned at Stanford than at UCLA. The group differences, however, were not attributable to site differences or group x site interactions. Ideally, future studies would replicate these comparisons with equal proportions of control subjects across study sites. Finally, we did not disentangle specific components of FFT-HR that map onto network connectivity results. Given the results of this analysis, future studies can confirm, for example, whether psychoeducation strengthens emotion regulatory networks or whether communication skills improve language network connectivity.
Despite these limitations, our study provides novel insights about how intrinsic neural networks may be strengthened with FFT-HR in youth at high risk for BD. Based on the extant literature and our findings, it is clear that for youth at risk for bipolar disorder, VLPFC and DMN connectivity may represent viable biological targets to delay or prevent mood episodes and recurrence. Our study also suggests that changes in the brain in response to FFT-HR may protect youth from earlier or more rapid recurrences, especially if neural changes are correlated with acquisition of communication or problem-solving skills. Measuring brain plasticity as a measure of change in family or other psychosocial interventions should be a focus of research in high-risk populations. Additional work is needed to determine how increasing VLPFC to anterior DMN connectivity can be leveraged to develop more targeted early interventions for high-risk youth. Rational future directions for this work would be to investigate brain network connectivity within psychoeducation, communication skill, and problem-solving skill modules, and to relate brain network connectivity to skill development, family functioning, and to bipolar versus unipolar long-term outcomes in high-risk youth.
Supplementary Material
Acknowledgements (with written permission obtained):
We wish to thank the following individuals for providing administrative support, diagnostic intake interviews., family interviews, independent evaluations, psychological or pharmacological treatment, or preprocessing of neuroimaging data: Christopher, Schneck, M.D., Aimee Sullivan, Ph.D., Brittany Matkevich, B.A., Antonio Hardan, M.D., Casey Armstrong, M.A., Jennifer G. Pearlstein, M.A., Kathryn Goffin, B.A., Daniella DeGeorge, B.A., and Corrina Fonseca, B.S. The following are acknowledged for providing study treatments: Sarah Marvin, PhD., Danielle Keenan-Miller, Ph.D., Alissa Ellis PhD, Victoria Cosgrove, PhD, Meghan Howe, LCSW, MSW, Amy C. Friedman, LMFT, Tenah Acquaye, PhD MPH. The independent fidelity ratings of therapy sessions were provided by Eunice Y. Kim, Ph.D. The following are acknowledged for providing consultation on study tasks, procedures, and statistical analyses: David A. Axelson, M.D., Melissa DelBello, MD, MS, and Boris Birmaher, MD. We thank the members of the Data Safety Monitoring Board: Howard J. Markman, PhD, Frederick S. Wamboldt, M.D., and Charles M. Judd, Ph.D. Members of the board were financially compensated annually for their contributions.
MSF Section 3: Funding, Acknowledgements, Financial Disclosures Funding/Support. This study was supported in part by grants R01MH093676 (Miklowitz), R01MH093666 (Chang), and the Brain and Behavior Research Foundation (Chang).
Role of the Funding/Sponsor: The funding sources had no role in the design or conduct of the study: collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This study was supported in part by grants R01MH093676 (Miklowitz), R01MH093666 (Chang), and the Brain and Behavior Research Foundation (Chang). The funding sources had no role in the design or conduct of the study: collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors wish to thank the following individuals for providing administrative support, diagnostic intake interviews, family interviews, independent evaluations, psychological or pharmacological treatment, or preprocessing of neuroimaging (affiliations listed were at the time of the study): Christopher Schneck, MD, and Aimee Sullivan, PhD, of the University of Colorado, Brittany Matkevich, BA, of the University of California-Los Angeles, Antonio Hardan, MD, of Stanford University, Casey Armstrong, MA, of the University of California-Los Angeles, Jennifer G. Pearlstein, MA, Kathryn Goffin, BA, Daniella DeGeorge, BA, and Corrina Fonseca, BS, of Stanford University. The following are acknowledged for providing study treatments: Sarah Marvin, PhD, Danielle Keenan-Miller, PhD, and Alissa Ellis, PhD, of the University of California-Los Angeles, Victoria Cosgrove, PhD, Meghan Howe, LCSW, MSW, Amy C. Friedman, LMFT, and Tenah Acquaye, PhD, MPH, of Stanford University. The independent fidelity ratings of therapy sessions were provided by Eunice Y. Kim, PhD, of the University of California-Los Angeles. The following are acknowledged for providing consultation on study tasks, procedures, and statistical analyses: David A. Axelson, MD, of Nationwide Children’s Hospital and The Ohio State University, Melissa DelBello, MD, MS, of the University of Cincinnati, and Boris Birmaher, MD, of the University of Pittsburgh. The authors thank the members of the Data Safety Monitoring Board: Howard J. Markman, PhD, with the University of Denver, Frederick S. Wamboldt, MD, with the National Jewish Medical and Research Center, and Charles M. Judd, PhD, with the University of Colorado-Boulder. Members of the board were financially compensated annually for their contributions.
Disclosure: Dr. Singh has received research support from Stanford’s Department of Psychiatry and Maternal Child Health Research Institute, the National Institute of Mental Health (NIMH), the National Institute of Aging, the Patient-Centered Outcomes Research Institute, Johnson and Johnson, Allergan, and the Brain and Behavior Research Foundation. She has served on the advisory board for Sunovion and as a consultant for Google X and Limbix and has received royalties from American Psychiatric Association Publishing. Dr. Chang has served as a consultant and on the speaker’s bureau for Sunovion. Dr. Miklowitz has received grant funding from NIMH, the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, the American Foundation for Suicide Prevention, and the Max Gray Fund. He has received book royalties from Guilford Press and John Wiley and Sons. Drs. Garrett, Roybal, Walshaw, Ms. Nimarko, and Mr. Gorelik have reported no biomedical financial interests or potential conflicts of interest.
Conflict of Interest Disclosures.
Dr. Singh has received research support from Stanford’s Department of Psychiatry and Maternal Child Health Research Institute, National Institute of Mental Health, National Institute of Aging, Johnson and Johnson, Allergan, and the Brain and Behavior Research Foundation. She is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from the American Psychiatric Association Publishing.
Dr. Miklowitz received grant funding from the National Institute of Mental Health (NIMH), the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, American Foundation for Suicide Prevention, and the Max Gray Fund; and book royalties from Guilford Press and John Wiley and Sons.
Dr. Chang is a consultant and on the speaker’s bureau for Sunovion.
Footnotes
All authors had full access to the data and take full responsibility for the integrity of the data and the accuracy of the data analysis. All individuals listed as authors meet authorship criteria and have participated sufficiently to take public responsibility for the content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RH = Intrinsic Connectivity Changes With FFT
Contributor Information
Manpreet K. Singh, Stanford University School of Medicine, California..
Akua F. Nimarko, Stanford University School of Medicine, California..
Amy S. Garrett, University of Texas, Health Science Center at San Antonio..
Aaron J. Gorelik, University of California-Davis..
Donna J. Roybal, University of Texas, Health Science Center at San Antonio..
Patricia D. Walshaw, University of California, Los Angeles School of Medicine..
Kiki D. Chang, private practice, Menlo Park, California..
David J. Miklowitz, University of California, Los Angeles School of Medicine..
REFERENCES
- 1.Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013;381(9878):1654–1662. [DOI] [PubMed] [Google Scholar]
- 2.Ostiguy CS, Ellenbogen MA, Linnen A-M, Walker EF, Hammen C, Hodgins S. Chronic stress and stressful life events in the offspring of parents with bipolar disorder. J Affect Disord. 2009;114(1–3):74–84. [DOI] [PubMed] [Google Scholar]
- 3.Stapp EK, Musci RJ, Fullerton JM, et al. Patterns and predictors of family environment among adolescents at high and low risk for familial bipolar disorder. J Psychiatr Res. 2019;114:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau P, Hawes DJ, Hunt C, Frankland A, Roberts G, Mitchell PB. Prevalence of psychopathology in bipolar high-risk offspring and siblings: a meta-analysis. Eur Child Adolesc Psychiatry. 2018;27(7):823–837. [DOI] [PubMed] [Google Scholar]
- 5.Duffy A, Horrocks J, Doucette S, et al. Immunological and neurotrophic markers of risk status and illness development in high-risk youth: understanding the neurobiological underpinnings of bipolar disorder. Int J Bipolar Disord. 2014;2(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin K, Shao R, Lu R, et al. Resting-state fMRI signals in offspring of parents with bipolar disorder at the high-risk and ultra-high-risk stages and their relations with cognitive function. J Psychiatr Res. 2018;98:99–106. [DOI] [PubMed] [Google Scholar]
- 7.Hanford LC, Hall GB, Minuzzi L, Sassi RB. Gray matter volumes in symptomatic and asymptomatic offspring of parents diagnosed with bipolar disorder. Eur Child Adolesc Psychiatry. 2016;25(9):959–967. [DOI] [PubMed] [Google Scholar]
- 8.Nery FG, Norris M, Eliassen JC, et al. White matter volumes in youth offspring of bipolar parents. J Affect Disord. 2017;209:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanford LC, Sassi RB, Minuzzi L, Hall GB. Cortical thickness in symptomatic and asymptomatic bipolar offspring. Psychiatry Research: Neuroimaging. 2016;251:26–33. [DOI] [PubMed] [Google Scholar]
- 10.Manelis A, Ladouceur CD, Graur S, et al. Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychol Med. 2016;46(1):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiggins JL, Brotman MA, Adleman NE, et al. Neural Markers in Pediatric Bipolar Disorder and Familial Risk for Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeganathan J, Perry A, Bassett DS, Roberts G, Mitchell PB, Breakspear M. Fronto-limbic dysconnectivity leads to impaired brain network controllability in young people with bipolar disorder and those at high genetic risk. Neuroimage Clin. 2018;19:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh MK, Chang KD, Kelley RG, Saggar M, Reiss AL, Gotlib IH. Early signs of anomalous neural functional connectivity in healthy offspring of parents with bipolar disorder. Bipolar Disord. 2014;16(7):678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgelt L, Strakowski SM, DelBello MP, et al. Neurophysiological effects of multiple mood episodes in bipolar disorder. Bipolar Disord. April 2019. [DOI] [PubMed] [Google Scholar]
- 15.Syan SK, Smith M, Frey BN, et al. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J Psychiatry Neurosci. 2018;43(5):298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acuff HE, Versace A, Bertocci MA, et al. Baseline and follow-up activity and functional connectivity in reward neural circuitries in offspring at risk for bipolar disorder. Neuropsychopharmacology. 2019;44(9):1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimarko AF, Garrett AS, Carlson GA, Singh MK. Neural correlates of emotion processing predict resilience in youth at familial risk for mood disorders. Dev Psychopathol. 2019;31(3):1037–1052. [DOI] [PubMed] [Google Scholar]
- 18.Zalpuri I, Singh MK. Treatment of psychiatric symptoms among offspring of parents with bipolar disorder. Curr Treat Options Psychiatry. 2017;4(4):341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miklowitz DJ, Schneck CD, Singh MK, et al. Early intervention for symptomatic youth at risk for bipolar disorder: a randomized trial of family-focused therapy. J Am Acad Child Adolesc Psychiatry. 2013;52(2):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miklowitz DJ, Chung B. Family-Focused Therapy for Bipolar Disorder: Reflections on 30 Years of Research. Fam Process. 2016;55(3):483–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett AS, Miklowitz DJ, Howe ME, et al. Changes in brain activation following psychotherapy for youth with mood dysregulation at familial risk for bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafeman DM, Chase HW, Monk K, et al. Intrinsic functional connectivity correlates of person-level risk for bipolar disorder in offspring of affected parents. Neuropsychopharmacology. 2019;44(3):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miklowitz DJ, Schneck CD, Walshaw PD, et al. Early intervention for youth at high risk for bipolar disorder: A multisite randomized trial of family-focused treatment. Early Interv Psychiatry. 2019;13(2):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miklowitz DJ, Schneck CD, Walshaw PD, et al. Effects of Family-Focused Therapy vs Enhanced Usual Care for Symptomatic Youths at High Risk for Bipolar Disorder: A Randomized Clinical Trial. JAMA Psychiatry. January 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- 27.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 28.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23(2):191–197. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 30.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–682. [DOI] [PubMed] [Google Scholar]
- 31.Miklowitz DJ, Schneck CD, Walshaw PD, et al. Early intervention for youth at high risk for bipolar disorder: A multisite randomized trial of family-focused treatment. Early Interv Psychiatry. August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneck CD, Chang KD, Singh MK, DelBello MP, Miklowitz DJ. A Pharmacologic Algorithm for Youth Who Are at High Risk for Bipolar Disorder. J Child Adolesc Psychopharmacol. July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glover GH, Mueller BA, Turner JA, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012;36(1):39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. [DOI] [PubMed] [Google Scholar]
- 39.Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. [DOI] [PubMed] [Google Scholar]
- 40.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond, B, Biol Sci. 2005;360(1457):1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Larson MP, Shah LM, Weeks HR, et al. Abnormal Functional Connectivity Between Default and Salience Networks in Pediatric Bipolar Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nickerson LD, Smith SM, Öngür D, Beckmann CF. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front Neurosci. 2017;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarquhar M, McKie S, Emsley R, Suckling J, Elliott R, Williams S. Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group-level neuroimaging data. Neuroimage. 2016;132:373–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connell NS, Dai L, Jiang Y, et al. Methods for Analysis of Pre-Post Data in Clinical Research: A Comparison of Five Common Methods. J Biom Biostat. 2017;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torrisi S, Moody TD, Vizueta N, et al. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord. 2013;15(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeberg I, Kjaerstad HL, Miskowiak KW. Neural and Behavioral Predictors of Treatment Efficacy on Mood Symptoms and Cognition in Mood Disorders: A Systematic Review. Front Psychiatry. 2018;9:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Oliveira L, Portugal LCL, Pereira M, et al. Predicting Bipolar Disorder Risk Factors in Distressed Young Adults From Patterns of Brain Activation to Reward: A Machine Learning Approach. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(8):726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acuff HE, Versace A, Bertocci MA, et al. Association of Neuroimaging Measures of Emotion Processing and Regulation Neural Circuitries With Symptoms of Bipolar Disorder in Offspring at Risk for Bipolar Disorder. JAMA Psychiatry. 2018;75(12):1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang K, Garrett A, Kelley R, et al. Anomalous prefrontal-limbic activation and connectivity in youth at high-risk for bipolar disorder. J Affect Disord. 2017;222:7–13. [DOI] [PubMed] [Google Scholar]
- 53.Roberts G, Lord A, Frankland A, et al. Functional Dysconnection of the Inferior Frontal Gyrus in Young People With Bipolar Disorder or at Genetic High Risk. Biol Psychiatry. 2017;81(8):718–727. [DOI] [PubMed] [Google Scholar]
- 54.Kling LR, Bessette KL, DelDonno SR, et al. Cluster analysis with MOODS-SR illustrates a potential bipolar disorder risk phenotype in young adults with remitted major depressive disorder. Bipolar Disord. 2018;20(8):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welge JA, Saliba LJ, Strawn JR, et al. Neurofunctional Differences Among Youth With and at Varying Risk for Developing Mania. J Am Acad Child Adolesc Psychiatry. 2016;55(11):980–989. [DOI] [PubMed] [Google Scholar]
- 56.Ellard KK, Gosai AG, Bernstein EE, et al. Intrinsic functional neurocircuitry associated with treatment response to transdiagnostic CBT in bipolar disorder with anxiety. J Affect Disord. 2018;238:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang K, DelBello M, Garrett A, et al. Neurofunctional Correlates of Response to Quetiapine in Adolescents with Bipolar Depression. J Child Adolesc Psychopharmacol. 2018;28(6):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diler RS, Segreti AM, Ladouceur CD, et al. Neural correlates of treatment in adolescents with bipolar depression during response inhibition. J Child Adolesc Psychopharmacol. 2013;23(3):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The Role of Intrinsic Brain Functional Connectivity in Vulnerability and Resilience to Bipolar Disorder. Am J Psychiatry. 2017;174(12):1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frangou S. Neuroimaging Markers of Risk, Disease Expression, and Resilience to Bipolar Disorder. Curr Psychiatry Rep. 2019;21(7):52. [DOI] [PubMed] [Google Scholar]
- 61.Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.