Abstract
Rationale & Objective:
Remission of proteinuria has been shown to be associated with lower rates of kidney disease progression among people with focal segmental glomerulosclerosis (FSGS). The goal of this study was to evaluate if reductions in proteinuria following treatment are associated with greater kidney survival.
Study Design:
Cohort analysis of clinical trial participants
Setting & Participants:
Patients with steroid resistant FSGS enrolled in a randomized treatment trial that compared cyclosporine to mycophenolate mofetil plus dexamethasone.
Predictors:
Reduction in proteinuria measured during 26 weeks after initiating treatment
Outcomes:
Repeated measures of estimated glomerular filtration rate (eGFR) and, time to end-stage kidney disease (ESKD) or death assessed between 26 weeks and 54 months after randomization
Analytic Approach:
Multivariable, linear-mixed effects models with subject-specific slope and intercept to estimate the association of change in proteinuria over 26 weeks while receiving treatment with the subsequent slope of change in eGFR. Multivariable, time-varying Cox-proportional hazards models were used to estimate the association of changes in proteinuria with time to ESKD or death.
Results:
138 of 192 trial participants were included. Changes in proteinuria over 26 weeks were significantly related to eGFR slope. A 1 unit reduction in log-transformed urinary protein:creatinine ratio was associated with a 3.90 ml/year rise in eGFR (95% CI=2.01 to 5.79). This difference remained significant after adjusting for complete remission. There was an analogous relationship between time-varying proteinuria and time to end-stage kidney disease: the HR for ESKD or death per 1 unit reduction in log-transformed urinary protein-creatinine ratio was 0.23 (95% CI=0.12 to 0.44).
Limitations:
Limited to individuals with steroid-resistant FSGS followed for a maximum of five-years.
Conclusions:
These findings provide evidence for the benefit of urinary protein reduction in FSGS. Reductions in proteinuria warrant further evaluation as a potential surrogate for preservation of kidney function that may inform the design of future clinical trials.
Keywords: FSGS, clinical trials, proteinuria, renal failure, remission, eGFR slope, clinical outcome assessment
Plain-language summary:
Remission of proteinuria has been shown to be associated with a decreased risk of kidney failure in FSGS. This study examined the relationship between changes in proteinuria and progression of kidney disease that occurred in participants of a treatment trial comparing two different immunosuppressant regimens. After controlling for other clinical factors, a reduction in proteinuria occurring over the first 26 weeks of treatment was significantly associated with a less steep slope of decline in kidney function measured by the eGFR and a lower rate of kidney failure or death. These associations were observed even when complete remission of proteinuria was not achieved. These findings provide evidence for the benefit of urinary protein reduction in FSGS. Reductions in proteinuria warrant further evaluation as a surrogate for preservation of kidney function that may inform the design of future clinical trials.
Introduction
Focal segmental glomerulosclerosis (FSGS) represents an important cause of end stage kidney disease (ESKD) among children and adults.1 There are currently no proven treatments specifically targeting FSGS, and there is a pressing need for clinical trials of novel therapies.2, 3 Most FSGS clinical trials rely upon changes in proteinuria as an indicator of efficacy.4–6 The advantage of this approach is that assessments of changes in proteinuria can be documented in a period of time and sample size that makes trials feasible.
Using changes in proteinuria as an early or intermediate clinical endpoint is based on evidence that normalization of proteinuria predicts kidney survival. Among chronic kidney diseases in general, there has been increased enthusiasm from both the scientific community and from regulatory agencies in accepting changes in proteinuria as a clinical trial endpoint that predicts progression to kidney failure.7–9 For FSGS specifically, a combined analysis of four prospective studies and one clinical trial identified that reaching a complete remission or reaching a urine protein: creatinine ratio of <1.5g/g was associated with improved kidney survival.10
However, in the context of a clinical trial, depending on the population enrolled, reaching either a complete remission or the threshold of proteinuria <1.5g/g may still be too restrictive of an endpoint to capture clinically important responses. Few participants might meet this threshold in response to treatment and it may be difficult to detect statistically significant and clinically relevant differences in categorical endpoints between study arms in clinical trials. Statistical power is gained by instead examining continuous changes in proteinuria. However, it is critical to understand if there is a long-term kidney survival benefit associated with reductions in proteinuria on a continuous scale even if proteinuria remains persistently elevated. For example, if a change in a participant’s proteinuria from 10g/g to 5g/g is not associated with a clinically meaningful improvement in kidney survival, then FSGS trials must show larger reductions in proteinuria in order to infer a clinical benefit. However, if this difference is clinically meaningful, then this would support the use of continuous reduction in proteinuria as a clinically meaningful trial endpoint. It is also important for the purposes of clinical trial design to quantify the magnitude of the relationship between proteinuria reduction and long-term kidney function in order to project the extended preservation of kidney function associated with proteinuria reduction, as has been demonstrated for IgA and diabetic nephropathies.11, 12
The goal of this study was to determine if reduction in proteinuria in response to treatment was associated with better kidney survival.
Methods
Participants and Data Source
The National Institutes of Health-funded NIDDK FSGS Clinical Trial (FSGS-CT) was a multicenter randomized clinical trial of children and adults with steroid-resistant primary FSGS.6 Eligible participants had primary FSGS confirmed by pathology review, age of proteinuria onset between 2 and 40 years old, eGFR ≥40mL/min/1.73m2, urine protein: creatinine ratio (UP:C) >1 g/g, and steroid resistance defined as persistent proteinuria after at least 4 weeks of steroid therapy. Participants were treated at clinical centers in the United States and were randomized between November 2004 and May 2008. Of the 192 participants enrolled, 138 were randomized. The efficacy of treatment with cyclosporine for 26 weeks (72 subjects) was compared to the efficacy a combination of oral dexamethasone and mycophenolate mofetil for 26 weeks (66 subjects). All participants received a maximally tolerated dose of an angiotensin converting enzyme inhibitor (Lisinopril) or an angiotensin receptor blocker (losartan). Follow-up laboratory testing was performed at weeks 2, 4, 6, 8, 14, 20, 26, 32, 38, 44, 52, 65, 78, and months 24, 30, 36, 42, 48, and 54. If there was no response by week 26, participants were allowed to switch to alternative therapies. The study protocol was reviewed and approved by the Institutional Review Board at each participating site. Informed consent and, when appropriate, assent was obtained before enrollment.
General Approach
The primary question of this analysis is if early reductions in proteinuria are associated with better long-term kidney function in participants with FSGS. To assess this, in our primary analyses, we examined if reduction in proteinuria from baseline (i.e., when participants entered the trial and were randomized) to week 26 was associated with slope of eGFR from week 26 to a maximum of 54 months. We also assessed if time-varying reductions in proteinuria (during the first 26 weeks) were associated with time to a composite endpoint of ESKD/death (28 events, 26 ESKD, 2 deaths without ESKD).
Primary Predictor: Reduction in Proteinuria
UP:C was analyzed on a natural log scale due to right-skewedness. Reduction in UP:C was analyzed as differences in log values, and primary analyses focused on reductions from baseline to week 26 (i.e., log(baseline UP:C)- log(week 26 UP:C). “Proteinuria” and “UP:C” are used interchangeably throughout. We hypothesized that reduction in UP:C would be associated with a slower rate of decline in eGFR over time, and with a decreased risk of kidney failure..
Descriptive Statistics
The cohort was described using frequencies and percentages for categorical variables and medians with interquartile ranges for continuous variables. Summary descriptions were provided by treatment arm. The original published efficacy results of this trial showed no difference between treatment arms on the intervention-associated effect on proteinuria.6 Therefore, all analyses combine the cyclosporine and mycophenolate arms as a single group with randomization arm included as a covariate in multivariable modeling.
Winsorization and Multiple Imputation
In this sample of mostly child, adolescent, and young adult participants, hyperfiltration is not uncommon, and eGFR values are known to fall outside expected ranges. A fall in eGFR from 200 to 100 ml/min/1.73m2 indicates a normalization, rather than a loss of kidney function. These analyses accounted for this by winsorizing the eGFR distribution above 120 in all analyses (i.e., values over 120 were imputed to 120). For participants who did not have UP:C data at week 26 (17 of 138 participants), multiple imputation by fully conditional specification was used.13 Twenty-five imputations were made based on age, sex, race, baseline UP:C, baseline eGFR, treatment arm, systolic blood pressure index at baseline, and all available UP:C and blood pressure measurements between baseline and week 26 (i.e., weeks 2, 4, 6, 8, 14, and 20). Multiple imputation based on baseline characteristics was also used for participants missing APOL1 genotype.
Primary Analyses: Proteinuria and eGFR slope
eGFR was calculated using the creatinine-based modified CKiD formula in children and the CKD-EPI equation in adults.14, 15 These updated equations were published after the trial, and thus eGFR values from this analysis differ from previously published analyses using the 1976 Schwartz and Cockcroft-Gault formulas. For the 18 participants who reached adulthood (i.e., age 18) during the study, the CKiD formula was used throughout follow-up to avoid a discontinuous jump in eGFR at age 18. eGFR-slope post-week 26 was estimated using linear-mixed effects models with random slope (time) and intercept (participant) terms in the model. Models included predicted eGFR as the outcome with time and reduction in UP:C as covariates, and an interaction term between time and reduction in UP:C to determine if trajectories of eGFR differed by reduction in UP:C from baseline to week 26. We hypothesized that reduction in UP:C would be associated with a positive regression coefficient of the interaction.
Models were adjusted for age, sex, race, APOL1 genotype, log-baseline UP:C, baseline eGFR, treatment arm, systolic blood pressure index, and change in systolic blood pressure index from baseline to week 26. Systolic blood pressure index was calculated as systolic blood pressure divided by the threshold for a clinical diagnosis of hypertension (i.e., 95th percentile value for age, sex, height in children <13 years old, minimum of either 95th percentile value for age, sex, height or 130 mm Hg for adolescents aged 13–17, and 130 mm Hg for adults).16, 17 Interactions for each of these factors by time were tested and any factors significant at α=0.05 were retained. Factors (main effects or interactions) that did not reach statistical significance at α=0.05 were removed from the models (however, non-significant main-effects that were a part of a significant interaction were retained to ensure the model was hierarchically well-formulated).
The analysis was repeated after adjusting for complete remission at week 26 (UP:C<0.3g/g). A significant finding after adjusting for complete remission would support that reductions in UP:C are beneficial even if this endpoint is not attained.
While, the primary analyses described above use all follow-up eGFR data from week 26 to a maximum of 54 months, this length of follow-up is not feasible for efficacy assessment in clinical trials. Also, clinical trials are more likely to measure changes in UP:C and eGFR from a common baseline. While findings from the primary analysis may help inform clinical care and have a stricter control of temporality, they are less likely to reflect the observable impact that proteinuria reduction would have in eGFR in a one to two-year trial. To account for this, additional sensitivity analyses also explored limiting eGFR data from baseline to 24 months, and from baseline to 12 months. These analyses may more accurately demonstrate the relationship between reduction in proteinuria and eGFR within the likely duration of a trial, and could aid in power and sample size calculations of future trials by projecting eGFR trajectories from baseline based on various effect sizes of treatment-associated reductions in proteinuria.
Finally, we also performed a series of sensitivity analyses to examine if changes in UP:C assessed at earlier time points (e.g., weeks 8, 4, and 2) were also associated with differences in slope of eGFR from that time point onward.
Secondary Analyses: Proteinuria and Progression to ESKD or death
Secondary analyses examined the impact of continuous reductions in log proteinuria on time to ESKD/death, censored at last follow-up. These analyses used time-varying reduction in proteinuria (assessed at weeks 2, 4, 6, 8, 14, 20 and 26) as a predictor of survival in time-varying Cox-proportional hazards models.18 These models also adjusted for age, sex, race, APOL1 genotype, log-baseline UP:C, baseline eGFR, treatment arm, systolic blood pressure index, and change in systolic blood pressure index from baseline. We hypothesized that the regression coefficient for reduction in UP:C would be negative. Analyses were repeated after controlling for complete remission.
Effect modification
Unadjusted models of eGFR slope and time to ESKD/death were tested for effect modification using interaction terms for: age, sex, race (black vs. non-black), APOL1 genotype (2 alleles vs. 0–1 alleles), baseline UP:C, baseline eGFR, treatment arm (CSA vs. MMF), and hypertension at baseline. Stratified sub-group estimates were reported for characteristics with statistically significant evidence of effect modification. Adjusted subgroup analyses were not possible due to small sample sizes and outcome frequencies in subgroups.
All analyses were conducted in SASv9.4. α=0.05 was used to assess statistical significance and all tests were two-sided.
Results
Description of Sample
Of the 192 participants enrolled, 138 were randomized. The most frequent reasons for exclusion were lack of FSGS lesion on biopsy (n=18), UP:C<1g/g (n=20), and eGFR<40ml/min/1.73m2 (n=5).6 The baseline characteristics of the 138 participants randomized in the FSGS-CT used in these analyses are shown in Table 1. Two-thirds were children (age<18), and 38% identified as Black/African American race. All participants had UP:C>1.0 at baseline, and median UP:C at baseline was 4.0g/g (IQR=2.2 to 8.3 g/g). There were no significant differences in participant characteristics by treatment arm. APOL1 genotype was available on 94 participants: 27 had high risk genotype (two risk alleles) vs. 67 with low risk genotype (0 or 1 risk allele).19
Table 1.
Baseline characteristics of participants by treatment arm, FSGS-CT (n=138).
| All participants (n=138) | CSA (n=72) | MMF (n=66) | |
|---|---|---|---|
| Age, median (IQR) | 15(11,23) | 15(11,25) | 15(11,19) |
| Age≤12, n(%) | 43(31) | 23 (32) | 20 (30) |
| Age 13–17, n(%) | 50 (36) | 25 (35) | 25 (38) |
| Age≥18, n(%) | 45 (33) | 24 (33) | 21 (32) |
| Female, n (%) | 65 (47) | 32 (44) | 33 (50) |
| Black Race, n (%) | 53(38) | 27 (38) | 26 (39) |
| UP:C (g/g), median (IQR) | 4.0 (2.2, 8.3) | 4.4(1.9,8.4) | 3.7 (2.6, 8.3) |
| 1.0–1.4, n(%) | 22(16) | 14(19) | 8(12) |
| 1.5–2.9, n(%) | 31(22) | 14(19) | 17(26) |
| 3.0–4.9, n(%) | 29(21) | 14(19) | 15 (23) |
| 5–10, n(%) | 30(22) | 15(21) | 15 (23) |
| >10, n(%) | 26(19) | 15(21) | 11(17) |
| eGFR (ml/min/1.73m2), median (IQR) | 80(60,113) | 85(60, 117) | 78(60, 111) |
| <30, n (%) | 2(1) | 1(1) | 1(2) |
| 30–59, n (%) | 33 (24) | 17(24) | 16(24) |
| 60–89, n (%) | 42 (30) | 21 (29) | 21 (32) |
| 90–119, n(%) | 30(22) | 15(21) | 15 (23) |
| 120–150, n(%) | 15(11) | 7(10) | 8(12) |
| >150, n(%) | 16(12) | 11(15) | 5(8) |
| Serum albumin (g/dl), median (IQR) | 2.9(2.1,3.7) | 3.0(2.3,3.7) | 2.8(2.1,3.7) |
| Hypertension at baseline, n (%) | 80(58) | 41(57) | 39 (59) |
| APOL1 genotype, n (%) | |||
| High risk (2 risk alleles) | 27(20) | 15(21) | 12(18) |
| Low risk (0–1 risk alleles) | 67 (49) | 35 (49) | 32 (48) |
| Not genotyped/non-Black Race | 23 (17) | 11(15) | 12(18) |
| Not genotyped/Black Race | 21(15) | 11(15) | 10(15) |
Abbreviations: APOL1, Apolipoprotein L1; CSA, cyclosporine; eGFR, estimated glomerular filtration rate; FSGS-CT, National Institutes of Health-funded National Institute of Diabetes and Digestive and Kidney Diseases FSGS Clinical Trial; IQR, interquartile range; MMF, mycophenolate mofetil; SD, standard deviation, UP:C, urine protein: creatinine ratio
Changes in proteinuria from baseline to week 26 (among those with proteinuria at week 26) are shown in Figure S1. The median percentage reduction in proteinuria was 67% (IQR=35 to 85%); the median absolute reduction in proteinuria was −2.3 g/g (IQR −4.2 to −1.0); 21% were in a complete remission and 49% had reached either a complete or partial remission (UP:C<1.5g/g and ≥40% reduction in UP:C).10 (Figure S1 and the above estimates are based on 121 participants with week 26 proteinuria data, but multiple imputation was used to allow inclusion of all participants despite missing data).Participants had a median of 13 (IQR=10 to 16) eGFR assessments over a median of 24 months (IQR=14 to 37).
Proteinuria and eGFR slope
Unadjusted linear-mixed effects model parameters are shown in Table S1. Change in UP:C, baseline UP:C, change in systolic blood pressure, and complete remission at week 26 each had significant unadjusted interactions with eGFR over time. The multivariable adjusted model identified a significant interaction with a positive coefficient between reduction in UP:C (from baseline to week 26) and eGFR beyond week 26, indicating greater reduction in proteinuria was associated with a slower rate of decline in eGFR (+3.9 ml/year in eGFR per 1 unit increase in the reduction in log UP:C, 95% CI=2.0 to 5.8) (Table S2). APOL1 genotype, baseline UP:C, baseline eGFR and treatment arm were also significant main effects predictors of eGFR, but were not associated with change in eGFR over time beyond week 26 (i.e., non-significant interactions with time). Estimated eGFR slopes derived from the model for several levels of proteinuria reduction are shown in a forest plot in Figure 1. Differences in logs were expressed as percent reduction for the purpose of data visualization using the following formula:
Figure 1. Reduction in proteinuria from baseline to week-26 associated with slower rate of eGFR decline from week-26 onward. Results of adjusted linear mixed effects models (n=138 participants; n=752 observations).

Model adjusted for APOL1 genotype, log-baseline UP:C, baseline eGFR, and treatment arm. Non-significant covariates removed during model selection include age, sex, race, systolic blood pressure index, and reduction in systolic blood pressure index (baseline - week 26). “Persistent proteinuria” refers to 0% reduction in UP:C. Multiple imputation by fully conditional specification was used to handle missing data. Observation “time” for eGFR outcome measurements=week 26 to last follow-up (maximum of month 54 from baseline).
Abbreviations: 95% CI, 95% confidence interval; APOL1, Apolipoprotein L1; eGFR, estimated glomerular filtration rate; UP:C, urine protein: creatinine ratio.
For example, a reduction from 10g/g at baseline to 7g/g at week 26: log(10)=2.30026, log(7)=1.9459, (1 – [1/e(2.30026−1.9459)]) × 100%) = 30% reduction; a reduction from 10g/g at baseline to 4g/g at week 26: log(10)=2.30026, log(4)=1.3863, 1 – [1/e(2.30026−1.3863)]) × 100%) = 60% reduction. Estimates from Figure 1 show, participants with no change in UP:C had an estimated slope of −6.7 ml/year (95% CI=−10.0 to −3.4), participants with a 30% decrease in UP:C had a slope of −5.3 ml/year (95% CI=−8.1 to −2.5), and participants with a 60% decrease had a slope of −3.1 ml/year (95% CI=−5.3 to −0.9). All adjusted figures for levels of change in UP:C were reported using mean values for other variables with significant interactions over time (i.e., mean Log-baseline UP:C=1.26; complete remission=21%; CSA treatment arm=52%.)
Analyses were repeated by adjusting for complete remission status at week 26 (i.e., UP:C<0.3g/g). Results are shown in Figure 2 and Table S3. Change in proteinuria, on a continuous scale, predicted eGFR slope (p<0.001). This adjusted model with terms for both continuous change in proteinuria and complete remission did not find a significant additive relationship for reaching a complete remission (Table S3) beyond the benefit associated with continuous reduction in proteinuria, despite complete remission showing a strong unadjusted relationship with eGFR slope (+8.5ml/year in eGFR, 95% CI=3.8 to 13.2 Table S1). Also, there was still a significant relationship between reduction in proteinuria and higher eGFR after excluding participants who reached complete remission at week 26 (+3.8ml/year, 95% CI=0.8 to 6.8 p=0.01, Table S4).
Figure 2. Reduction in proteinuria from baseline to week-26 associated with slower rate of eGFR decline from week-26 onward after adjusting for reaching complete remission. Results of adjusted linear mixed effects models (n=138 participants; n=752 observations).
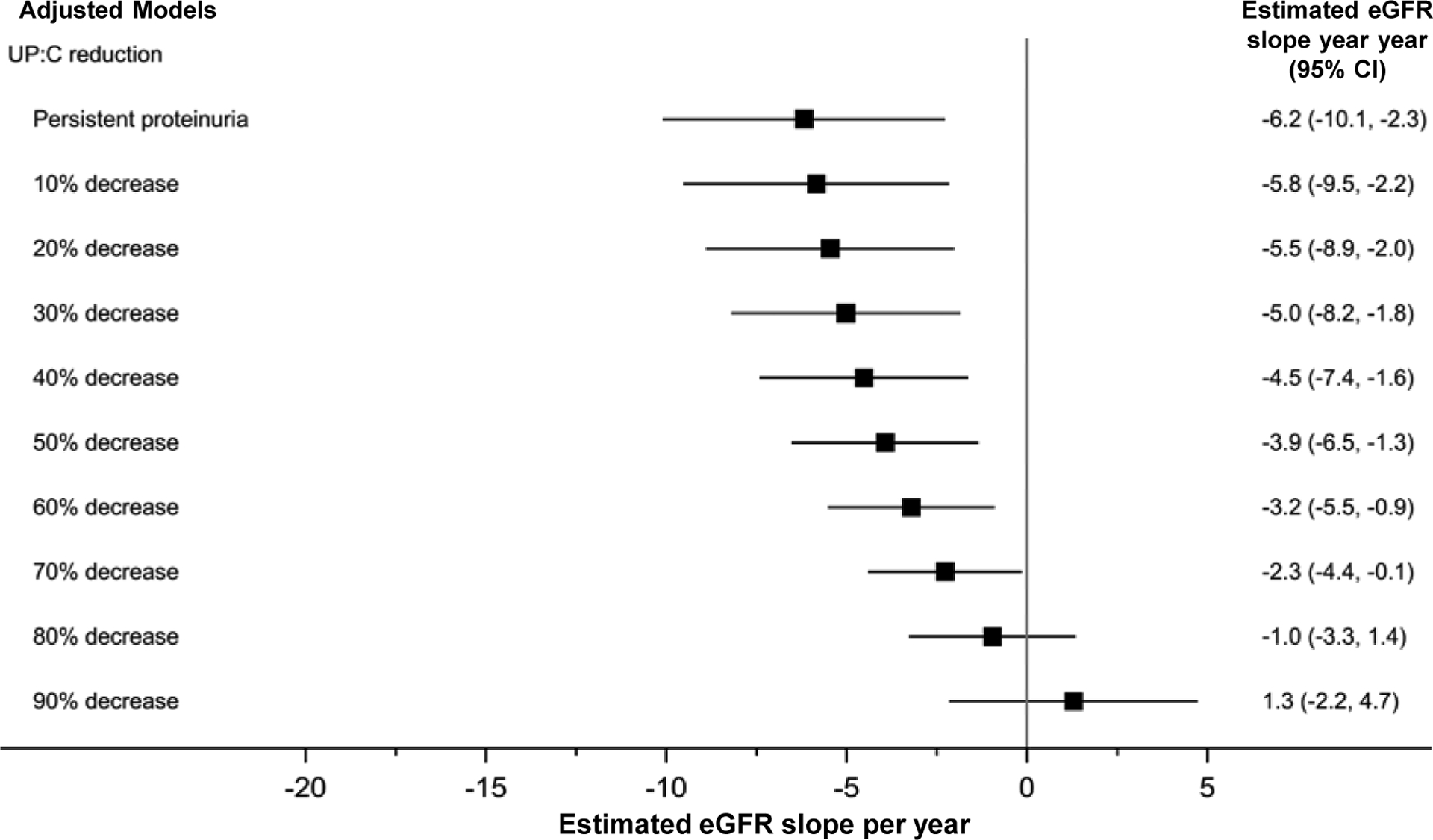
Model adjusted for complete remission, APOL1 genotype, log-baseline UP:C, baseline eGFR, and treatment arm. Non-significant covariates removed during model selection include age, sex, race, systolic blood pressure index, and reduction in systolic blood pressure index (baseline - week 26). “Persistent proteinuria” refers to 0% reduction in UP:C. Multiple imputation by fully conditional specification was used to handle missing data. Due to an included interaction between complete remission and time, slopes above are for average complete remission at week 26 (21%). Observation “time” for eGFR outcome measurements=week 26 to last follow-up (maximum of month 54 from baseline). Abbreviations: 95% CI, 95% confidence interval; APOL1, Apolipoprotein L1; eGFR, estimated glomerular filtration rate; UP:C, urine protein: creatinine ratio.
Additional analyses examined eGFR slope from study baseline and limited eGFR follow-up to 24- and 12-months after baseline. The goal of these analyses was quantify the relationship between early changes in proteinuria and longer term changes in eGFR in a clinical trial using a common baseline. The 24-month follow-up analyses are shown in Figure 3 and Table S5; 12-month analyses are found in Figure 4 and Table S6. Reduction in UP:C was a robust predictor of slope in both analyses. In the 24-month analyses, a 1 unit increase in the reduction in log UP:C was associated with a 6.2 ml/year increase in eGFR slope (95% CI=3.6 to 8.9); in 12-month analyses a 1 unit increase in the reduction in log UP:C was associated with a 6.4 ml/year increase in eGFR slope (95% CI=2.1 to 10.6).
Figure 3. Reduction in proteinuria from baseline to week-26 associated with slower rate of eGFR decline. Results of adjusted linear mixed effects models with eGFR data restricted to first 24 months after baseline (n=138 participants; n=1,595 observations).
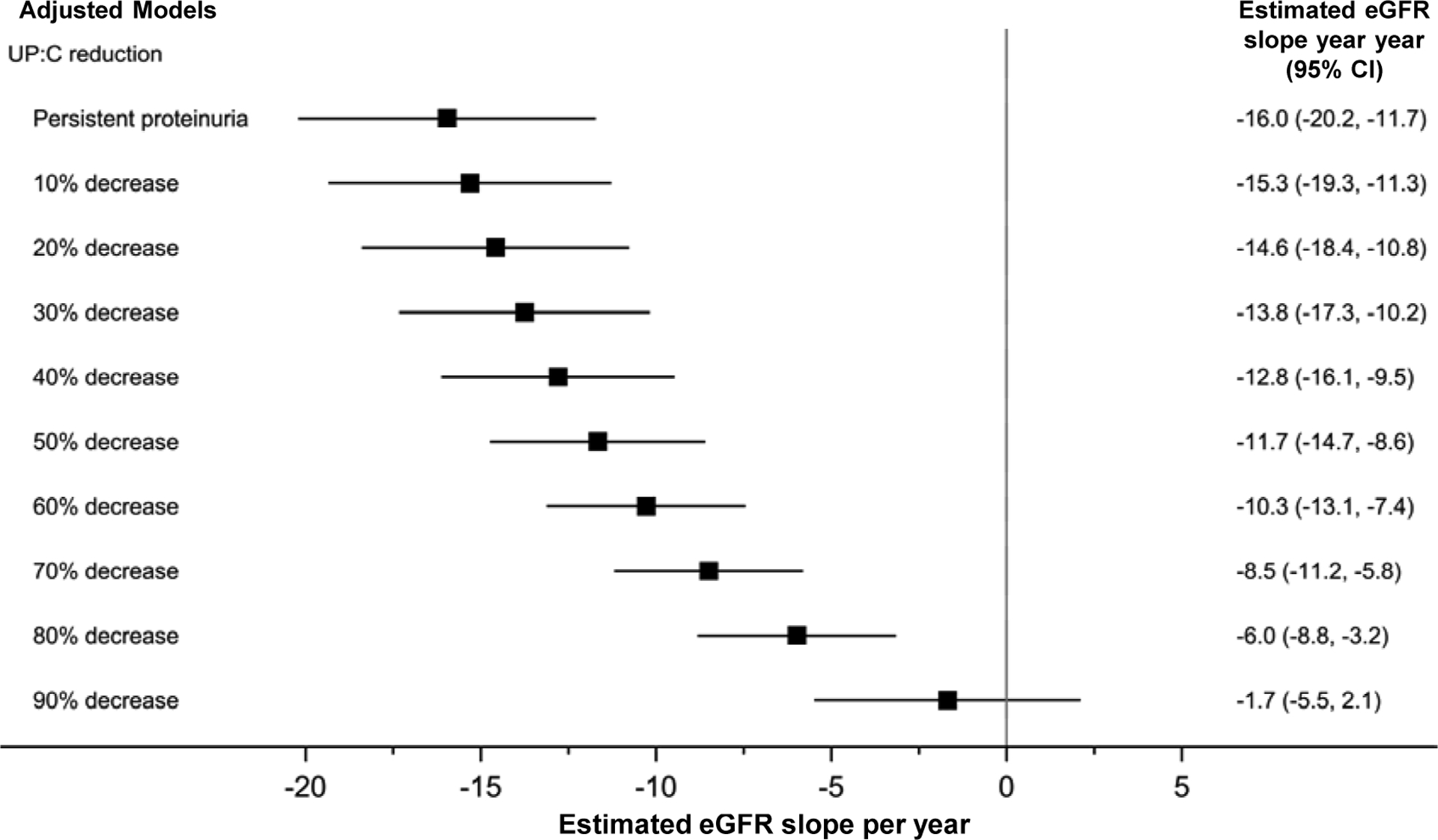
Model adjusted for age, APOL1 genotype, log-baseline UP:C, baseline eGFR, and treatment arm. Non-significant covariates removed during model selection include sex, race, systolic blood pressure index, and reduction in systolic blood pressure index (baseline - week 26). “Persistent proteinuria” refers to 0% reduction in UP:C. Multiple imputation by fully conditional specification was used to handle missing data. Observation “time” for eGFR outcome measurements=baseline to 24 months. Abbreviations: 95% CI, 95% confidence interval; APOL1, Apolipoprotein L1; eGFR, estimated glomerular filtration rate; UP:C, urine protein: creatinine ratio.
Figure 4. Reduction in proteinuria from baseline to week-26 associated with slower rate of eGFR decline. Results of adjusted linear mixed effects models with eGFR data restricted to first 12-months after baseline (n=138 participants; n=1,332 observations).
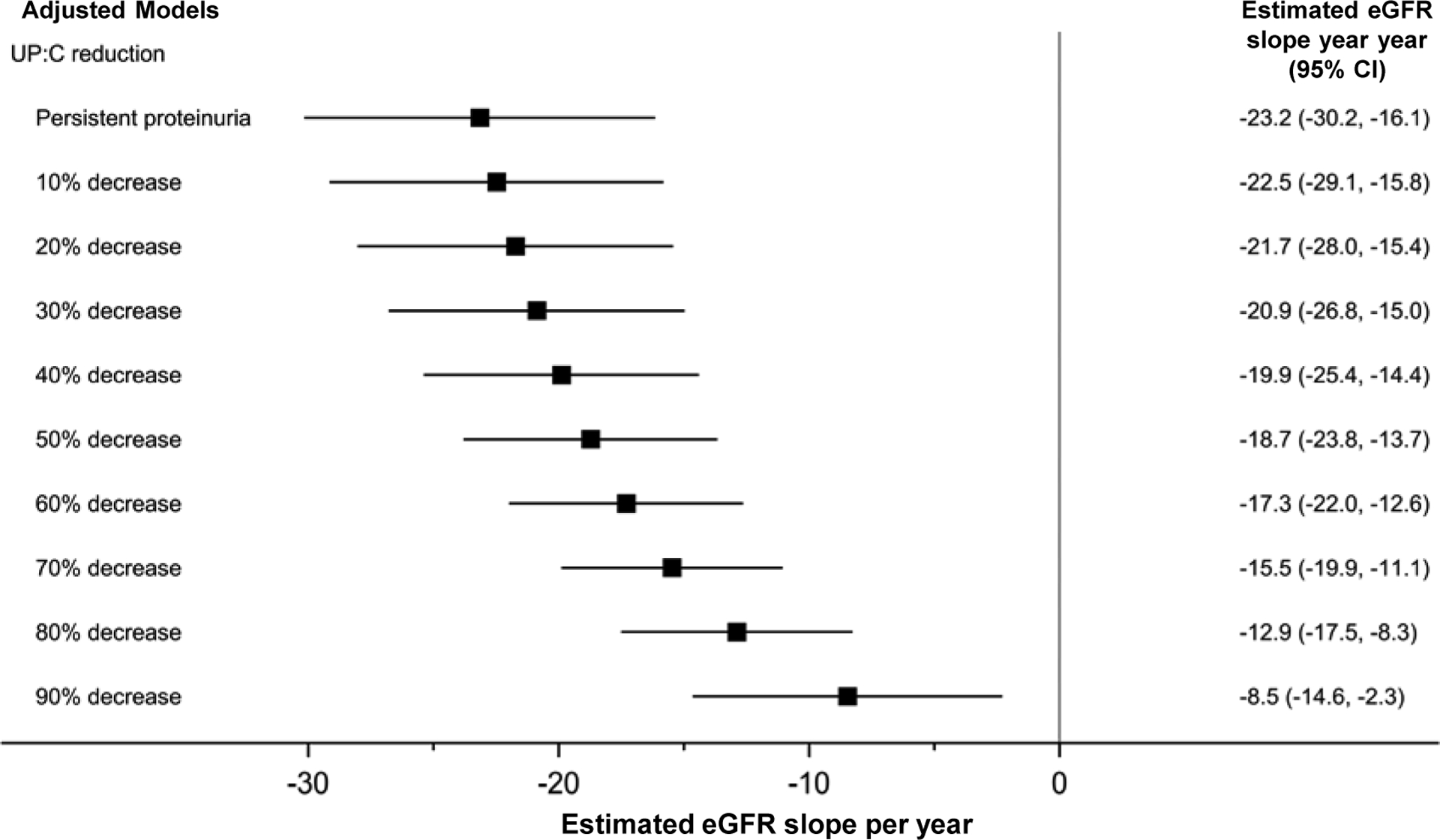
Model adjusted for age, APOL1 genotype, log-baseline UP:C, baseline eGFR, and treatment arm. Non-significant covariates removed during model selection include sex, race, systolic blood pressure index, and reduction in systolic blood pressure index (baseline - week 26). “Persistent proteinuria” refers to 0% reduction in UP:C. Multiple imputation by fully conditional specification was used to handle missing data. Observation “time” for eGFR outcome measurements=baseline to 12 months.
Abbreviations: 95% CI, 95% confidence interval; APOL1, Apolipoprotein L1; eGFR, estimated glomerular filtration rate; UP:C, urine protein: creatinine ratio.
Finally, sensitivity analyses using reduction of UP:C by week 8 also identified a significant impact on eGFR slope from week 8 onward (+5.4 ml/year per 1 unit increase in the reduction in log proteinuria 95% CI=3.1 to 7.8 p<0.001). Analogous analyses of UP:C reduction by week 4 found a similar relationship (+4.3 ml/year,95% CI =1.4 to 7.1, p=0.008). Proteinuria reduction at week 2 were not associated with differences in eGFR (+2.7ml/year, 95% CI =−0.6 to 6.0 p=0.11).
Proteinuria and Progression to ESKD or death
Similar to the above eGFR slope analyses, analyses were adjusted for age, sex, race, APOL1 genotype, log-baseline UP:C, baseline eGFR, treatment arm, systolic blood pressure index, and change in systolic blood pressure index from baseline. Unadjusted time-varying Cox-models of reduction in proteinuria are shown in Table S7; the adjusted model is reported in Table 2. The adjusted model showed a continuous relationship with reduction in UP:C and a decreased likelihood of progression to ESKD/death (Hazard ratio per 1 unit increase in the reduction in log UP:C=0.23, 95% CI=0.12 to 0.44). Results did not differ when adjusting for reaching a complete remission (Table S8).
Table 2.
Reduction in proteinuria from baseline to week-26 inversely associated with time to ESKD/death. Results of time-varying cox-proportional hazards (n=138 participants, n=28 events)
| Model | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Reduction in proteinuria (per 1 unit increase in the reduction in log proteinuria) | 0.23 | 0.12 to 0.44 | <0.001 |
| Baseline log-UP:C (per 1 log) | 5.17 | 2.93 to 9.13 | <0.001 |
| Baseline eGFR (per 30ml/min/1.73m2) | 0.31 | 0.19 to 0.49 | <0.001 |
| Treatment arm (CSA vs. MMF) | 2.98 | 1.31 to 6.78 | 0.01 |
Non-significant covariates removed during model selection include sex, race, systolic blood pressure index, and reduction in systolic blood pressure index. Multiple imputation by fully conditional specification was used to handle missing data.
Abbreviations: 95% CI, 95% confidence interval, CSA, cyclosporine; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; MMF, mycophenolate mofetil; UP:C, urine protein: creatinine ratio.
Effect modification and confirmation of functional form
Significance tests for effect modification are reported in Table S9. There was no evidence of effect modification in the relationship between reduction in UP:C and eGFR slope from week 26 onward by age, sex, race, APOL1 genotype, baseline UP:C or eGFR, treatment arm or hypertension at baseline. However, these was significance evidence of effect modification in the time-varying Cox-proportional hazards model of time to ESKD/death by sex (p of interaction=0.02; female specific stratum n=65 participants, n=13 events HR=0.56 95% CI=0.43 to 0.72 p <0.001; male specific stratum n=73 participants, n=15 events HR=0.80 95% CI=0.63 to 0.98 p =0.04).
Finally, while proteinuria was analyzed on a log scale, due to expected right skewedness, post-hoc exploratory analyses were performed to compare model fit of alternative functional forms. For both the eGFR slope and time to event analyses, change in log proteinuria showed stronger fit than absolute or percentage change in proteinuria (Figure S2, Table S10) and categorical analyses of change in UP:C confirmed modest reductions in UP:C were associated with slower rate of eGFR decline (Figure S3).
Discussion
These results provide additional evidence to a growing literature of the benefit of proteinuria reduction on kidney survival in patients with FSGS,10, 20–22 and the utility of proteinuria as a clinically meaningful endpoint glomerular disease.23–26 These novel findings expand on previous reports showing that complete and partial proteinuria remissions are associated with large clinical benefit, and also suggest that reduction in proteinuria—even if not to a complete remission—is still associated with improved survival. Finally, this study provides estimated effect sizes on eGFR preservations and survival at various levels of proteinuria reduction for different lengths of follow-up. These can aid in delineating prognosis, assessing the impact of treatment, clinical decision making, and in the design of clinical trials.
Remission of proteinuria has consistently been established as a strong predictor of kidney survival in FSGS. Several observational studies have shown that participants reaching and maintaining a complete remission of proteinuria rarely progress to ESKD.10, 20, 27 This study suggests that incremental proteinuria reductions are also important. Even modest reductions in proteinuria (i.e., 20–30% reduction) translated to clinically meaningful differences in eGFR slope of >1–2ml/year. While participants with elevated proteinuria still experienced disease progression, we consider the magnitude of these differences to be clinically meaningful. Reduction in proteinuria—even if not to complete remission—could still yield years of preserved native kidney function and delay the onset of end-stage kidney disease, with attendant morbidity and mortality, and need for renal replacement therapy.
Additionally, within-participant reduction in proteinuria was a significant predictor of kidney disease progression after accounting for baseline proteinuria. Lower proteinuria during follow-up was associated with better long-term outcomes regardless of proteinuria at the time of randomization. Baseline proteinuria was also independently significantly associated with kidney survival in the time to ESKD/death analyses. High UP:C at baseline was associated with more rapid progression to ESKD/death (Table 2). This indicates that lower achieved proteinuria (driven by either larger reduction in proteinuria or lower proteinuria at baseline) predicts better kidney function outcomes, and suggests that in steroid resistant FSGS, incrementally reducing proteinuria to as low as a level as possible may attenuate the underlying disease process.
These analyses identified long-term benefit on eGFR of continuous reduction in proteinuria for 26 weeks after adjusting for complete remission or limiting analyses to those who did not reach complete remission. This suggests reduction in proteinuria could be used as an early clinical predictor of long-term kidney function for those who do and do not go to remission. Additionally, we did not observe effect modification by baseline proteinuria, indicating a similar benefit in proteinuria reduction found for those with nephrotic or subnephrotic range proteinuria, nor did we observe effect modification by APOL1 genotype. This is important for the use of reduction in proteinuria as a clinical trial endpoint as sub-nephrotic patients are a non-negligible portion of the FSGS population (e.g., 38% of this study; 46% of FSGS in the Nephrotic Syndrome Study Network enrolled at initial kidney biopsy).28
Our sensitivity analyses revealed similar effect sizes for the impact of proteinuria on eGFR regardless of follow-up duration. Additionally, proteinuria reduction assessed at weeks 4 and 8 were also associated with preserved eGFR slope. These findings may support efficacy assessment after shorter treatment periods in clinical trials. For example, if a novel agent demonstrates an effect on proteinuria reduction by week 8 these data suggest that reduction is associated with better kidney function over time. Moreover, the differences could be observed at 12–24 months of eGFR follow-up.
A limitation of this study is its relatively modest sample size and lower power, particularly for tests of effect modification. While this did not impact our ability to detect statistical significance, future clinical trial simulations of these studies may be limited by wide 95% confidence intervals for expected eGFR slopes for various proteinuria levels. The wide confidence intervals for the eGFR slopes preclude precise predictions of the timing of key events in the disease course. However, these estimates could be useful in formulating an estimated general prognosis for an individual participant with regard to propensity to progressive loss of eGFR, though precision is limited. Other limitations are that these data are limited to steroid-resistant FSGS, and did not include longer-term follow-up beyond 5 years. Thus, we cannot be certain that the benefit of graded reductions in proteinuria is sustained. Counterbalancing strengths include centrally collected longitudinal laboratory measurements performed under randomized clinical trial conditions in a rare disease population. In addition, the study cohort was a valid representation of the population affected by the disease and the patients received standard of care of FSGS.
These findings suggest that efficacy of treatments for FSGS may be gauged based on the continuous reduction in proteinuria in addition to pre-defined cut-off values. In addition, these findings demonstrate that reductions in proteinuria warrant further evaluation as a potential surrogate for preservation of kidney function that may inform the design of future clinical trials.
Supplementary Material
Figure S1. Changes in proteinuria during interventional randomized treatment phase from baseline to week 26 (n=121)
Figure S2. Fit indices for alternative functional forms of reduction in proteinuria. Results of unadjusted linear mixed effects models (n=138 participants; n=752 observations). Distributions for fit indices across all 25 imputations are shown. Lower values indicate stronger fit.
Figure S3. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward. Results of unadjusted linear mixed effects models of categories of proteinuria reduction (n=121 participants; n=702 observations)
Table S1. Results of unadjusted linear mixed effects models of eGFR from week-26 onward (n=138 participants; n=752 observations)
Table S2. Multivariable mixed effects model of change in eGFR week 26–54 (n=138 participants; n=752 observations)
Table S3. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward after adjusting for reaching complete remission. Results of adjusted linear mixed effects models (n=138 participants; n=752 observations)
Table S4. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward among those not in complete remission by week-26. Results of adjusted linear mixed effects models. (n=101 participants; n=527 observations)
Table S5. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR. Results of adjusted linear mixed effects models with eGFR data restricted to first 24 months after baseline (n=138 participants; n=1,595 observations)
Table S6. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR. Results of adjusted linear mixed effects models with eGFR data restricted to first 12-months after baseline (n=138 participants; n=1,332 observations)
Table S7. Results of unadjusted time-varying cox-proportional hazards model (n=138 participants, n=28 events)
Table S8. Reduction in proteinuria from baseline to week-26 inversely associated with time to ESKD/death after adjusting for reaching complete remission. Results of time-varying cox-proportional hazards (n=138 participants, n=28 events)
Table S9. P-value results of tests of subgroup variation in linear mixed effects models of reduction in proteinuria on eGFR slope and time-varying cox-proportional hazards models of reduction in proteinuria on ESKD/death.
Table S10. Fit indices for alternative functional forms of reduction in proteinuria. Results of unadjusted time-varying cox-proportional hazards unadjusted linear mixed effects models (n=138 participants, n=28 events). Bolded values indicate strongest fit.
Acknowledgements:
We are indebted to the patients and families who graciously participated in this research.We express our thanks to all the site coordinators who assisted with patient identification, enrollment, treatment, and follow-up. We would also like to acknowledge Norman Siegel (deceased) for his leadership as the first as the FSGS-CT Steering Committee Chair. We would also like to thank members of the United States Food and Drug Administration for their review and advice on content.
Support: This study was supported by the National Center for Advancing Translational Sciences (NCATS Grant Number: UL1TR002240) for the Michigan Institute for Clinical and Health Research. The FSGS Clinical Trial was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) Grants U01- DK063385, DK063490, DK063455, and DK063549. Jeffrey Kopp. was supported by the NIDDK Intramural Research Program. This study was supported by many Clinical and Translational Science Award/NIH-funded institutions for the conduct of study visits, nursing, laboratory, and outpatient research facilities throughout the trial. The NIH had a role in the original study design and data collection, but no funders had a role in the analyses, reporting, or the decision to submit these analyses for publication.
Financial Disclosure: These analyses are investigator initiated. Several investigators on this study serve on scientific advisory boards and have funded clinical trials consulting agreements for several companies with active or in development FSGS trials: Pfizer Inc., Complexa, Goldfinch Bio, Vertex. Jonathan Troost and Debbie Gipson have research funding through the University of Michigan with Complexa Inc., Retrophin Inc. and Goldfinch Bio; Debbie Gipson has past research funding through the University of Michigan with Bristol-Meyers Squibb and Zyversa; Jonathan Troost through the University of Michigan with Vertex Pharmaceuticals and Pfizer Inc.. Howard Trachtman has research funding through the NYU with Retrophin Inc. and Goldfinch Bio. Liron Walsh is an employee of Goldfinch Bio. Rong Wang is an employee of Pfizer Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hruska K, Mathew S, Lund R, Fang Y, Sugatani T. Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl. 2011(121): S9–13. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney inter., Suppl. 2012; 2: 139–274. [Google Scholar]
- 3.Han KH, Kim SH. Recent Advances in Treatments of Primary Focal Segmental Glomerulosclerosis in Children. BioMed research international. 2016;2016: 3053706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trachtman H, Nelson P, Adler S, et al. DUET: A Phase 2 Study Evaluating the Efficacy and Safety of Sparsentan in Patients with FSGS. J Am Soc Nephrol. 2018;29(11): 2745–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincenti F, Fervenza FC, Campbell KN, et al. A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int Rep. 2017;2(5): 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80(8): 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1): 84–104. [DOI] [PubMed] [Google Scholar]
- 8.Carrero JJ, Grams ME, Sang Y, et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91(1): 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in Albuminuria and Subsequent Risk of Incident Kidney Disease. Clin J Am Soc Nephrol. 2017;12(12): 1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troost JP, Trachtman H, Nachman PH, et al. An Outcomes-Based Definition of Proteinuria Remission in Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13(3): 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson A, Carroll K, L AI, et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clin J Am Soc Nephrol. 2019;14(3): 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2): 128–139. [DOI] [PubMed] [Google Scholar]
- 13.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3): 219–242. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3): 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17): e426–e483. [DOI] [PubMed] [Google Scholar]
- 18.Barbour SJ, Cattran DC, Espino-Hernandez G, Hladunewich MA, Reich HN. Identifying the ideal metric of proteinuria as a predictor of renal outcome in idiopathic glomerulonephritis. Kidney Int. 2015;88(6): 1392–1401. [DOI] [PubMed] [Google Scholar]
- 19.Kopp JB, Winkler CA, Zhao X, et al. Clinical Features and Histology of Apolipoprotein L1-Associated Nephropathy in the FSGS Clinical Trial. J Am Soc Nephrol. 2015;26(6): 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry G. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4): 1061–1068. [DOI] [PubMed] [Google Scholar]
- 21.Korbet SM, Schwartz MM, Lewis EJ. Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis. 1994;23(6): 773–783. [DOI] [PubMed] [Google Scholar]
- 22.Banfi G, Moriggi M, Sabadini E, Fellin G, D’Amico G, Ponticelli C. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clinical nephrology. 1991;36(2): 53–59. [PubMed] [Google Scholar]
- 23.Inker LA, Mondal H, Greene T, et al. Early Change in Urine Protein as a Surrogate End Point in Studies of IgA Nephropathy: An Individual-Patient Meta-analysis. Am J Kidney Dis. 2016;68(3): 392–401. [DOI] [PubMed] [Google Scholar]
- 24.Thompson A, Cattran DC, Blank M, Nachman PH. Complete and Partial Remission as Surrogate End Points in Membranous Nephropathy. J Am Soc Nephrol. 2015;26(12): 2930–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54(2): 205–226. [DOI] [PubMed] [Google Scholar]
- 26.Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. British journal of clinical pharmacology. 2013;76(4): 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21(3): 344–349. [DOI] [PubMed] [Google Scholar]
- 28.Gipson DS, Troost JP, Lafayette RA, et al. Complete Remission in the Nephrotic Syndrome Study Network. Clin J Am Soc Nephrol. 2016;11(1): 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes in proteinuria during interventional randomized treatment phase from baseline to week 26 (n=121)
Figure S2. Fit indices for alternative functional forms of reduction in proteinuria. Results of unadjusted linear mixed effects models (n=138 participants; n=752 observations). Distributions for fit indices across all 25 imputations are shown. Lower values indicate stronger fit.
Figure S3. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward. Results of unadjusted linear mixed effects models of categories of proteinuria reduction (n=121 participants; n=702 observations)
Table S1. Results of unadjusted linear mixed effects models of eGFR from week-26 onward (n=138 participants; n=752 observations)
Table S2. Multivariable mixed effects model of change in eGFR week 26–54 (n=138 participants; n=752 observations)
Table S3. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward after adjusting for reaching complete remission. Results of adjusted linear mixed effects models (n=138 participants; n=752 observations)
Table S4. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR from week-26 onward among those not in complete remission by week-26. Results of adjusted linear mixed effects models. (n=101 participants; n=527 observations)
Table S5. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR. Results of adjusted linear mixed effects models with eGFR data restricted to first 24 months after baseline (n=138 participants; n=1,595 observations)
Table S6. Reduction in proteinuria from baseline to week-26 associated with slower rate of decline in eGFR. Results of adjusted linear mixed effects models with eGFR data restricted to first 12-months after baseline (n=138 participants; n=1,332 observations)
Table S7. Results of unadjusted time-varying cox-proportional hazards model (n=138 participants, n=28 events)
Table S8. Reduction in proteinuria from baseline to week-26 inversely associated with time to ESKD/death after adjusting for reaching complete remission. Results of time-varying cox-proportional hazards (n=138 participants, n=28 events)
Table S9. P-value results of tests of subgroup variation in linear mixed effects models of reduction in proteinuria on eGFR slope and time-varying cox-proportional hazards models of reduction in proteinuria on ESKD/death.
Table S10. Fit indices for alternative functional forms of reduction in proteinuria. Results of unadjusted time-varying cox-proportional hazards unadjusted linear mixed effects models (n=138 participants, n=28 events). Bolded values indicate strongest fit.


