Abstract
Acute Respiratory Distress Syndrome (ARDS) is defined as the rapid onset of non-cardiogenic pulmonary edema resulting in respiratory failure and hypoxemia. Efforts over the past 25 years, such as those of the ARDS and Prevention and Early Treatment of Acute Lung Injury (PETAL) Networks, have demonstrated a praiseworthy collaboration to further optimize the management of ARDS. However, improvements have been only moderate and ARDS remains a leading cause of mortality in the perioperative and critical care setting. Recently, the significant morbidity and mortality of ARDS have been emphasized by its high incidence in Coronavirus Disease 2019 (COVID-19) patients. A major hurdle to reducing ARDS mortality is that current treatment is limited to preventive measures – such as the use of lung-protective ventilation. Therapeutic approaches targeting the underlying inflammatory lung disease are areas of intensive research, but have not been clinically implemented. Nevertheless, basic science and clinical research efforts that are aimed at identifying novel treatment approaches and further improving outcomes for ARDS are ongoing. Here, we review evidence-based management approaches for ARDS, while highlighting those being investigated or heavily utilized in ARDS associated with COVID-19.
Summary Statement:
Acute Respiratory Distress Syndrome remains a condition that carries a high mortality. Evidence-based clinical management and emerging concepts for new therapies for COVID-19 are reviewed.
Introduction
The acute respiratory distress syndrome (ARDS) is defined as hypoxemia secondary to a rapid onset of noncardiogenic pulmonary edema.1 Etiologic risk factors for ARDS encompass both direct- and indirect-lung injuries including, but not limited to pneumonia, sepsis, non-cardiogenic shock, aspiration, trauma, contusion, transfusion, and inhalation injuries. Though clinical recognition and management of ARDS have improved significantly over the past 25 years, it is still a leading cause of death in critically ill patients, with mortality rates consistently reported around 30% – 40%.2 An important factor in the high mortality rate in ARDS is that treatment is mainly focused on clinical management and no targeted therapies currently exist. Furthermore, ARDS management is often challenging as it commonly occurs in a clinical setting of multiple organ failure and can also lead to the development of nonpulmonary organ injury, such as acute kidney injury.3 Recently, the pandemic caused by coronavirus disease-2019 (COVID-19), which results from infection by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has led to a dramatic incidence in COVID-19 related ARDS. 30–40% of COVID-19 hospitalized patients develop ARDS and is associated with 70% of fatal cases.4,5 At the time of this writing (July 31st, 2020) there are over 4.5 million COVID-19 cases and 152,000 related deaths in the United States.6 Here, we describe select management strategies that have become foundations of ARDS clinical management and provide an update of emerging approaches for the treatment of ARDS related with COVID-19.
Clinical Treatment Concepts
Nationally Organized Research Consortia to Study ARDS
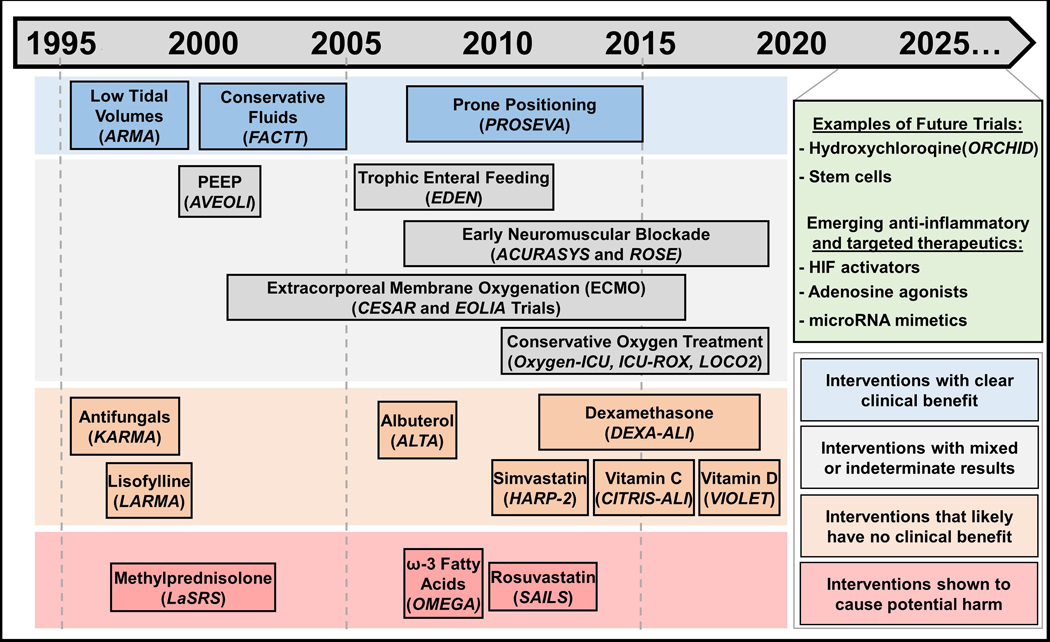
In order to improve outcomes and develop treatment protocols for ARDS, the National Heart, Lung, and Blood Institute (NHLBI) of the NIH funded a series of multi-center clinical trials, which formed a research collaboration called the ARDS Network (http//:ardsnet.org).7 Beginning in 1994, the network studies enrolled over 5,500 patients, included 10 clinical trials and 1 observational study, led to the development of new clinical parameters such as ventilator free days (VFD)8 and resulted in seminal advances that have helped to shape current ARDS management. NHLBI-funded clinical trials continue presently under the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network (http://petalnet.org).. Figure 1 and Table 1 briefly summarize the results and implications of the results for ARDS and PETAL Network trials, along with other important trials performed internationally.
Figure 1:
A summary of 25 years of Acute Respiratory Distress Syndrome (ARDS) intervention trials. Interventions are chronologically displayed with corresponding clinical trials italicized underneath and color-coded to denote clinical efficacy. Interventions that have clear clinical efficacy, in blue boxes, include the use of small tidal volumes,9 prone positioning,20 and restrictive fluid administration,37 which have demonstrated clear mortality or ventilator-free days benefits. Interventions in grey boxes include those that have mixed results from different trials, as is the case for conservative oxygen treatment32,33,75 and early neuromuscular blockade.38,39 This category (grey boxes) also includes interventions with indeterminate results, such as the case for Positive End Expiratory Pressure (PEEP)15 – itself is a component of lung-protective ventilation, but the appropriate amount to use is still contended – or those that have value in ARDS patients aside from improving ARDS outcomes, such as early trophic enteral nutrition to prevent gastric intolerance40 and Extracorporeal Membrane Oxygenation (ECMO) as a rescue therapy.35,36 In orange boxes are interventions that failed to demonstrate improvements in ARDS outcomes, such as antifungals, lisofylline, albuterol, simvastatin, vitamin C and vitamin D.41–47 Dexamethasone is also listed in this category given that the DEXA-ALI trial was conducted in an unblinded fashion28 and prior randomized trials showed no clinical efficacy for steroid administration in ARDS. Methylprednisolone,27 rosuvastatin49 and ω−3 Fatty Acids,48 listed in red boxes, have shown to cause potential harm in randomized controlled trials. Currently, ongoing or planned trials and emerging therapeutic targets are displayed in green.
Table 1.
Summarized results of select, large-scale intervention trials aimed at improving outcomes in patients with Acute Respiratory Distress Syndrome.
| Clinical Intervention | Trial Name | Study Groups | Outcomes |
|---|---|---|---|
| Small Tidal Volumes | The 2000 Acute Respiratory Distress Syndrome Network trial (ARMA)9 | Low Tidal Volume (6 ml/kg of predicted body weight) or Traditional Tidal Volume (12 ml/kg of predicted body weight) | Reduction in 180-day mortality 31.0% vs. 39.8% |
| Positive End Expiratory Pressure (PEEP) | Higher vs Lower PEEP (ALVEOLI)15 | Low PEEP or High PEEP (inspiratory plateau pressure of 28–30) | No change in 28-day mortality 31.2% vs. 27.8 (p=0.31) |
| Prone Positioning | Proning Severe ARDS Patients (PROSEVA) trial20 | Supine Position or Prone position (at least 16 hours/day) | Reduction in 28-day mortality 16.0% vs. 32.8% |
| Steroids | Late Steroid Rescue Study (LaSRS)27 | In patients 7–28 days after onset of ARDS: Placebo or methylprednisolone | No change in 60 day mortality 28.6% vs. 29.2% and Increased mortality in patients receiving methylprednisolone at least 14 days after ARDS diagnosis |
| Dexamethasone Treatment for the Acute Respiratory Distress Syndrome (DEXA-ARDS)28 | Standard of Care or Dexamethasone | Increase in ventilator free days 12.3 vs 7.5 days (p<0.0001) and Reduction in all-cause mortality at day 60 21% vs. 36% | |
| Conservative Oxygenation | OXYGEN-ICU trial32 | Conventional oxygen: PaO2 up to 150 mmHg or SaO2 up to 97% to 100% or Conservative Oxygen: PaO2 70 to 100 or SaO2 of 94% to 98% | Reduction in ICU mortality 11.6% vs. 20.2% (p=0.01) |
| Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU-ROX)64 | Usual Oxygen Therapy: no upper limit to FIO2 or SaO2 Or Conservative Oxygen Therapy: SaO2 between 90% and 97% | No change in ventilator free days 21.3 vs. 22.1 and No change in 180 day mortality 35.7% vs. 34.5% | |
| Liberal or Conservative Oxygen Therapy for ARDS (LOCO2)33 | Liberal Oxygenation: Target PaO2 90–105 mmHg; SaO2 > 96% or Conservative Oxygenation: Target PaO2 55–70 mmHg; SaO2 88%−92% | Increased mortality in conservative oxygen group 34.3% vs. 26.5% | |
| Extracorporeal Membrane Oxygenation (ECMO) | Conventional ventilatory support vs. ECMO for severe adult respiratory failure (CESAR)35 | Conventional Management or ECMO | Increased survival without severe disability at 6 months 63% vs. 47% |
| Rescue Lung Injury in Severe ARDS (EOLIA)36 | Early ECMO or Conventional mechanical ventilation with ECMO as a rescue therapy | Non-statistically significant reduction in mortality 35% vs. 46% (p=0.09) | |
| Fluid Restriction | Fluids and Catheters Treatment Trial (FACTT)37 | Liberal Fluids (CVP 10–14) or Conservative Fluids (CVP <4) | No change in all-cause mortality at 60 days 25.5% vs. 28.4% (p=0.30) |
| Early Neuromuscular (NM) Blockade | ARDS et Curarisation Systematique (ACURASYS)38 | Patients first sedated to a Ramsay sedation score of 6, then given: Placebo or Cisatracurium | Reduction in 90 day mortality 31.6% vs. 40.7% |
| Reevaluation of Systemic Early Neuromuscular Blockade (ROSE)39 | Usual care: lightly sedated or Early NM blockade: deep sedation and cisatracurium | No change in 90-day mortality 41.5% vs. 42.8% | |
| Statin Treatment | Simvastatin in the Acute Respiratory Distress Syndrome (HARP-2)47 | Placebo or Simvastatin for maximum 28 days | No significant change in ventilator free days 12.6 vs. 11.5 or 28-day mortality 22% vs. 26.8% |
| Statins for Acutely Injured Lungs from Sepsis (SAILS)49 | Placebo Or Rosuvastatin for maximum 28 days | No change in 60-day mortality 28.5% vs. 24.9%) and fewer days free of renal or hepatic failure | |
| Vitamins, Nutrition, and Supplements | Early vs. Delayed Enteral Nutrition (EDEN)40 | Trophic enteral feeding: 10–20 kcal/hour or Full enteral feeding: 25 to 30 kcal/kg per day of nonprotein calories and 1.2 to 1.6 g/kg per day of protein | No change in ventilator free days 14.9 vs. 15 and No change in 60-day mortality 23.2% vs. 22.2% |
| Omega Nutrition Supplement Trial (Omega)48 | Enteral supplementation of n-3 fatty acids, γ-linolenic acid, and antioxidants or An isocaloric control | Reduction in ventilator free days 14.0 vs. 17.2 and Nonstatistical increase in mortality 26.6% vs. 16.3% (p=0.054) | |
| Vitamin C Infusion for Treatment in Sepsis Induced Acute Lung Injury (CITRINS-ALI) 80 | Matched placebo (5% dextrose in water) or Vitamin C 50mg/kg total body weight every 6 hours for 96 hours | No change in SOFA score 3 vs. 3.5 and improved 28-day mortality 29.8% vs. 43.6% | |
| Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET)81 | Placebo or Vitamin D3 | No difference in 90-day mortality 23.5% vs. 20.6% | |
| β2-agonist | Albuterol for the Treatment of ALI (ALTA)42 | Aerosolized albuterol (5 mg) or Placebo (aerosolized saline) | No difference in ventilator free days 14.4 and 16.6 and No difference in mortality before hospital discharge 23% vs. 17.7% |
| Antifungals | Ketoconazole for ALI/ARDS (KARMA)46 | Ketoconazole, 400 mg/day or Placebo | No difference in in-hospital mortality 34.1% vs. 35.2% |
| Lisofylline | Lisofylline for ALI/ARDS (LARMA)45 | Lisofylline (3 mg/kg with a maximum dose of 300 mg) or Placebo | No difference in mortality 31.9% vs. 24.7% (p=0.215) |
Small tidal volumes
Amongst the best-established guidelines in managing ARDS patients is the use of small tidal volumes during mechanical ventilation (fig. 1). In 2000, Investigators from the ARDSNet Lower Tidal Volume Trial (ARMA) trial reported significantly decreased rates of mortality (31.0% vs. 39.8 %) in ARDS patients ventilated with 6ml/kg of predicted body weight (kgPBW) tidal volumes versus those with 12 ml/kgPBW.9 While small tidal volume ventilation remains a tenant of lung-protective ventilation during ARDS, recent efforts have sought to determine whether small tidal volumes play a lung-protective role more broadly in all critically-ill ventilated patients. In 2018 the Protective Ventilation in Patients Without ARDS (PReVENT) trial demonstrated that ventilation with low tidal volumes may not be more effective than intermediate volumes in non-ARDS ICU patients.10
Positive End Expiratory Pressure (PEEP)
In their seminal 1967 report of ARDS cases, Ashbaugh et al reported that improvement of hypoxemia and atelectasis was achieved by the implementation of PEEP.11 Since then, PEEP continues to be employed in ARDS management and remains the focus of many clinical research efforts. Conceptually, PEEP is administered in order to reduce atelectrauma (repetitive opening and closing of alveoli) by recruiting collapsed alveoli.12 Much attention has been directed at the levels at which PEEP is applied, with clinical evidence yielding mixed results (fig. 1). Several trials that report protective benefits from higher versus lower targets of PEEP employed higher tidal volumes in their control (lower PEEP) groups, which perhaps introduced bias in their conclusions.13,14 Trials that have controlled for low tidal volumes (6 ml/kg), including the 2004 ARDS Network ALVEOLI trial, have failed to demonstrate a survival benefit for higher PEEP.15,16 Subgroup analysis does, however, suggest that higher PEEP is associated with improved survival among the subgroup of patients with ARDS who objectively respond to increased PEEP (patients who show improved oxygenation in response to increased PEEP).17 Still, it has yet to be demonstrated whether survival in selected patients improves with increased PEEP in large randomized trials.
Prone positioning
Beneficial effects of prone positioning during mechanical ventilation of ARDS patients are considered in order to establish a more even distribution of gravitational force in pleural pressure allowing for improved ventilation of the dorsal lung space18 and to limit over-distention of alveoli.19 In 2013, Guérin and colleagues reported the results of the Proning Severe ARDS Patients (PROSEVA) trial in which severe ARDS patients (PaO2/FiO2 less than 150 on FiO2 of at least 0.6) were randomized to prone positioning for a minimum of 16 hours a day. Patients randomized to prone positioning had a 50% reduction in mortality (16% versus 32.8%) at 28 days (fig. 1).20 A recent meta-analysis corroborates these results and supports the survival benefits of prolonged prone positioning (greater than 12 hours) in patients with severe ARDS.21 Despite these encouraging results in reducing mortality with the use of prone positioning, data from a large, multinational prospective observational study indicate that the maneuver was employed in only 16.3% of severe ARDS patients.2 Possible reasons for this low implementation could be attributed to the relative complexity and logistic considerations of prone positioning (e.g. multiple persons required for the maneuver, increased workloads, management of secretions, and nutrition) or due to the inherent risks of the procedure such as endotracheal tube and vascular line displacement. Nonetheless, the use of prone positioning for more than 12 hours per day remains a strong recommendation for patients with severe ARDS.22
Although the efficacy of prone positioning is almost exclusively suggested in patients with PaO2/FiO2 ratios of 150 or less, trials that failed to show efficacy in mild and moderate ARDS are largely underpowered and failed to administer prone positioning for recommended lengths of time.23 As such, randomized trials implementing early prone positioning in mild to moderate cases of ARDS are necessary in order to determine any survival benefits and to make recommendations for clinical implementation.
Steroids in non-COVID ARDS
In the report of ARDS patients by Ashbaugh et al. in 1967, it was suggested that corticosteroids appeared to have clinical value in cases associated with fat emboli and viral pneumonia.11 Randomized control trials conducted in the 1980s have since demonstrated that early administration of methylprednisolone did not result in improved ARDS survival.24,25 However, in 1998 a prospective trial by Meduri and colleagues showed an improved outcome in ARDS patients treated with prolonged methylprednisolone.26 The results of the study were subject to scrutiny due to the small sample size (n=8) of the control group, significant cross-over into the methylprednisolone group (all of which died) and a relatively large mortality rate of 60%. Subsequently, in 2006 the ARDS Network addressed the role of corticosteroid administration late in ARDS with the Late Steroid Rescue Study (LaSRS) in which 180 patients were randomized to methylprednisolone administration 7–28 days after diagnosis of ARDS. Administration of methylprednisolone was associated with no significant reduction in mortality (fig. 1).27 Furthermore, patients who started steroid treatment after 14 days of diagnosis experienced increased mortality.
Based on the postulate that, compared to other corticosteroids, dexamethasone has an improved potency, lengthened duration of action, and weak mineralocorticoid effect, Villar and colleagues performed a prospective trial randomizing ARDS patients to receive either dexamethasone or placebo.28 Compared to Patients in the control group, dexamethasone treatment group showed a reduced time on mechanical ventilation and 60-day mortality, however, drug allocation and data analysis were performed in an unblinded fashion, potentially leading to bias. Furthermore, 250 patients were excluded for already receiving steroids prior to randomization, indicating that participating physicians already favored the use of corticosteroids which might have influenced clinical decisions to modify mechanical ventilation duration. In summary, guidelines supporting routine glucocorticoid administration in ARDS based on rigorously performed RCTs are currently not supporting their use. However, as discussed later in this review, dexamethasone treatment has been the first therapy to show mortality improvement in mechanically ventilated COVID-19 patients.29
Conservative Oxygenation
Among the most common therapies implemented in critically ill patients and nearly all ARDS patients is the supplemental provision of oxygen. Oxygen is frequently delivered generously in order to increase PaO2 and oftentimes patients become hyperoxic while attempting to reverse tissue hypoxia. However, evidence indicates that liberal oxygen use is associated with vasoconstriction, decreased cardiac output, absorption atelectasis, increased pro-inflammatory responses, and increased mortality.30,31 As such, establishing a protocol of oxygen treatment that balances essential delivery to organs while preventing excessive harmful effects of hyperoxia has been an important subject of recent investigations (fig. 1). In a single-center randomized trial published in 2016, critically ill ICU patients with a length of stay of 3 days or longer who were assigned to receive conservative oxygen therapy (PaO2 between 70 and 100 mm Hg) had lower mortality than those who received more conventional care (PaO2 up to 150 mm Hg).32 A more recent study, the 2020 Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome (LOCO2) trial, Barrot et al. recruited ARDS patients to conservative (SpO2 between 88 and 92%) or liberal (SpO2 greater than 96%) oxygen treatment arms. The trial was terminated early due to an associated increase in mortality at 28 days and five episodes of mesenteric ischemia in the conservative oxygen treatment group.33 Worse outcomes in conservative oxygenation may be attributed to the deteriorated gas exchange in ARDS patients, making them more prone to hypoxemia in the conservative oxygen treatment arm. Going forward, trials will need to carefully assess how to determine target oxygenation levels (e.g. SpO2 and PaO2 targets, measurements from mixed venous blood, different targets for different organ injuries) to better answer how oxygen concentrations are selected.
Extracorporeal Membrane Oxygenation (ECMO)
Extracorporeal membrane oxygenation (ECMO) is a rescue therapy that has been employed in ARDS patients that fail to improve on mechanical ventilation management and as a means to avoid potential injurious aspects of ventilator associated lung injury. Advances in ECMO delivery have been associated with an increase in the number of centers and cases using it, particularly since the 2009 H1N1 influenza pandemic.34 Investigators from the 2009 Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial group sought to answer whether the use of ECMO during severe ARDS would povide a survival benefit when compared to conventional support by mechanical ventilation (fig. 1).35 The results of the trial indicated that there was a survival benefit in favor of patients being randomized to ECMO treatment, but this difference was not statistically significant. Furthermore, the study was impaired by the use of heterogeneous mechanical ventilation strategies in the control group (including the use of large tidal volumes). Additionally, a large percentage of patients in the ECMO group who were transferred to ECMO-capable hospitals never received ECMO, allowing for the potential confounding effects attributed to the fact that ECMO-capable hospitals may attain enhanced ARDS survival regardless of whether patients actually received ECMO. A subsequent international multi-center study was conducted to specifically address weaknesses of previous trials implementing ECMO in early severe ARDS, the 2018 ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial.36 Despite achieving a high quality of control for ventilation strategies in both groups and nearly universal implementation of ECMO in patients randomized to receive it, the results demonstrated that there was no significant difference in mortality between the ECMO group and the control group. Given the lack of strong evidence supporting the use of ECMO as a routine early treatment for ARDS, it is recommended that ECMO is reserved as rescue therapy in patients that remain hypoxemic despite conventional evidence-based approaches.
Other Investigated Therapeutic Approaches
A large number of pharmacologic approaches have been tested in large, randomized controlled trials in order to improve clinical outcomes in patients with ARDS. These trials have included approaches such as the use of β2-adrenergics, ketoconazole, lisofylline, vitamin C and D, omega fatty acids, restrictive fluid administration and statins (fig. 1 and table 1). 37–47,48,49 Though none of these trials have demonstrated a mortality benefit in ARDS patients, it should be highlighted that recent advancements in our understanding of ARDS pathophysiology indicate that there are likely important subtypes of injury that predict beneficial response to particular therapies.50 Appropriate identification and selection of patients with specific subphenotypes of ARDS may allow for a targeted approach to effective treatments and more efficient clinical trials.
ARDS in COVID-19
ARDS in COVID-19 patients (fig. 2) presents with several unique characteristics that are not regularly described in non-COVID-19 associated ARDS. Among these characteristics is the significant development of microvascular thrombosis within the lung vasculature that contributes to ventilation-perfusion mismatch and right ventricular stress.5,51,52 Though the cause for widespread activation of the coagulation cascade is not yet fully understood, dysregulated inflammation and direct injury to endothelial cells by SARS-CoV-2 contribute to the development of microthrombotic immunopathology.51–53 Additionally, endothelial cell damage in SARS-CoV-2 infection impairs pulmonary vasoconstriction that normally occurs in response to hypoxia to restrict blood flow to poorly ventilated areas of the lung. Disruption in this physiologic adaptation in COVID-19 patients results in shunting of blood. To this end, treatment for COVID-19 related ARDS has been focused on mitigation of these drivers of disease pathophysiology through the use of antivirals, steroids, anticoagulants, and prone positioning.
Figure 2.
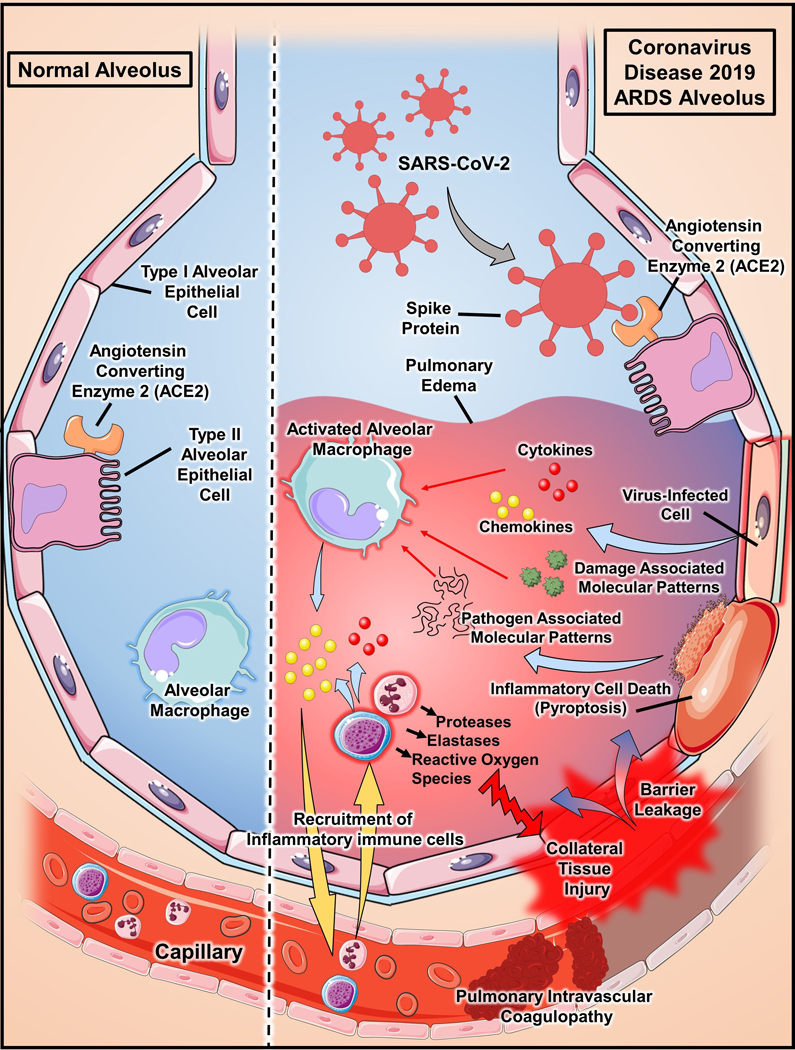
Pathophysiology of Acute Respiratory Distress Syndrome (ARDS) in Coronaviruas Disease 2019 (COVID-19). SARS-CoV-2 infection is mediated by virus spike binding to angiotensin converting enzyme-2 (ACE2) on type 2 alveolar epithelial cells.76,77 Viral infection prompts cells to react by releasing chemokines and cytokines.78 Infection can also overwhelm epithelial cells and cause them to die via pyroptosis, which results in the release of inflammatory Damage and Pathogen Associated Molecular Patterns (DAMPs/PAMPs). Recognition of PAMPs/DAMPs and cytokines activates alveolar macrophages and chemokines act to recruit inflammatory immune cells to the lung. Excessive immune cell release of antimicrobial effectors, such as metallomatrix proteases (MMPs), elastases, and reactive oxygen species (ROS), induce collateral tissue injury that results in loss of epithelial and endothelial barrier integrity and infiltration of proteinaceous fluid into the alveolar airspace.78 Furthermore, increasing evidence supports the important role of endothelial cells in the initiation of inflammation and the development of extensive pulmonary intravascular coagulopathy that is common in COVID-19 patients.51–53 In severe cases, patients with COVID-19 have developed disseminated intravascular coagulopathy.79
Antiviral therapy
The use of antiviral therapeutics in COVID-19 related ARDS is an approach that has gained tremendous effort and attention. Their mechanisms of action are directed at specific viral components that are necessary for SARS-CoV-2 replication and pathogenicity. In this way antivirals are unique in that they target the inciting virus instead of host-related factors, such as tissue inflammation and immune cell functions, to prevent lung injury and subsequent excessive inflammation. Remdesivir, an inhibitor of viral RNA-dependent RNA polymerase, is perhaps the most noted antiviral currently under investigation.54 In mere months after the emergence of SARS-CoV-2, Beigel et al. published the preliminary results of the Adaptive COVID-19 Treatment Trial (ACTT-1), a large randomized, placebo-controlled trial for the anti-viral drug, remdesivir.55 The results demonstrate a statistically significant reduction in time to recovery in severe COVID-19 patients that received remdesivir. The shortened time to recovery effect was strongest in the early severe disease group (patients requiring oxygen, but not yet intubated), which likely indicates that the timing of administration will be critical for future use. Unfortunately, the trial did not demonstrate efficacy for remdesivir in patients who began treatment after already requiring mechanical ventilation. Indeed, the follow-up time may have been too short to evaluate these patients and the results for the complete cohort are still pending.
Additional antiviral treatments that have been proposed for the treatment of hospitalized COVID-19 patients include the hydroxychloroquine, an anti-malarial drug, and lopinavir-ritonavir, a protease inhibitor cocktail used for treating HIV. Indeed both drugs have demonstratable efficacy in reducing SARS-CoV-2 infection in vitro, but have both failed to translate into therapeutic results in COVID-19 patients.56–58 On June 29th, 2020, the RCOVERY trial terminated its lopinavir-ritonavir arm due to lack of clinical benefit (www.recoverytrial.net/results/lopinavar-results). Similarly, on June 20th, the NIH PETAL Network halted its trial investigating hydroxychloroquine use (www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine).
Anticoagulation and thrombolytics
Given that a key pathologic finding in COVID-19 is the prevalence of thrombotic coagulopathy within lung vasculature, a great deal of attention has been directed at whether anticoagulation or thrombolytic therapy may provide therapeutic efficacy in COVID-19 ARDS. Indeed, a French multicenter prospective study found that there was a statistically significant increase in thromboses in COVID-19 related ARDS when compared with a historic cohort in non-COVID-19 ARDS.59 Though there is currently a lack of evidence from randomized control trials that support the use of intermediate or treatment-level doses of prophylactic anticoagulation, some centers have adopted the use of such strategies. In an early Chinese retrospective analysis of severe COVID-19, anticoagulation therapy was associated with reduced 28-day mortality.60 Furthermore, in another retrospective observational study of 2,773 patients hospitalized for COVID-19 in New York City, patients receiving mechanical ventilation (n = 395) had significantly reduced in-hospital mortality when treated with treatment-dose levels of anticoagulation (29.1% vs. 62.7%).61 In light of these observations and the current recognition for the pathophysiological role for coagulopathy in SARS-CoV-2 infection, a number of clinical trials aimed at ascertaining the role of empiric therapeutic dosing with anticoagulation in COVID-19 ARDS have been initiated.
In addition to anticoagulation, thrombolytic treatment in COVID-19 ARDS patients has been proposed as a salvage therapy. Current evidence for the use of thrombolytic treatment in ARDS is limited to a 2001 phase I trial in which 20 patients with severe ARDS were treated with urokinase, which demonstrated improved oxygenation and no risk of bleeding.62 Indeed, some groups have published case series for patients with COVID-19 ARDS that were treated with salvage anti-thrombolytic agents. 63–65 All patients had some level of improvement in oxygenation and/or hemodynamics after the administration of tissue plasminogen activator, but in most cases, patients ultimately died. Nonetheless, the scientific rationale for using fibrinolytic therapy in COVID-19 ARDS – namely the consistent findings of pulmonary microvascular thrombosis – has resulted in the initiation of urgently needed clinical trials studying the role of anti-thrombotic agents in COVID-19 ARDS.66
Prone positioning in COVID-19 ARDS
Based on the significant prevalence for ventilation-perfusion mismatch as a result of microvascular thromboses in COVID-19 patients, prone positioning mechanically ventilated patients is recommended in order to improve lung recruitability and oxygenation.67–70 In a detailed characterization of mechanically ventilated COVID-19 patients in two Boston, Massachusetts hospitals, patients who underwent prone positioning had increased median PaO2:FiO2 ratios from 150 to 232, an improvement that persisted 72 hours later when PaO2:FiO2 ratios of 233 were measured while patients were supine.71 Though there is currently not enough data to conclude that prone positioning improves long term outcomes and mortality in mechanically ventilated patients, the NIH COVID-19 treatment guidelines currently suggest its use.72
Steroids in COVID-19 ARDS
Recent data from the UK RECOVERY (Randomised Evaluation of COVid-19 thERapY) trial investigating the use of dexamethasone in hospitalized COVID-19 patients have demonstrated that dexamethasone is the first drug to improve mortality.29 Mechanically ventilated patients that were randomized to receive 6 mg once per day for 10 days were found to have a reduction of mortality by one third when compared to patients who underwent usual care. Interestingly, this mortality benefit was not observed in patients who did not require respiratory support. In response to these findings, current COVID-19 treatment guidelines from the National Institutes of Health recommend its use in patients that are mechanically ventilated or require oxygen supplementation.72 Moreover, similar to ARDS and PETAL Network studies, the RECOVERY trial provides an example for the power of organized multicenter investigations for new treatment approaches in critically-ill patients, especially those with ARDS. Moving forward, data from the dexamethasone arm are likely to reinvigorate studies for its use in non-COVID-19 ARDS patients that may support the open-label dexamethasone studies previously mentioned.28
Conclusions
The past 25 years of large, randomized clinical trial efforts have contributed a tremendous amount of insight that has advanced the clinical practice of lung-protective mechanical ventilation. Indeed, implementation of clinically proven management interventions, such as the use of low tidal volumes and prone positioning, have dramatically improved the outcomes for ARDS. However, mortality remains high and there is a lack of targeted treatment options. Nonetheless, emerging basic science research has resulted in novel therapeutic targets, such as hypoxia, adenosine, and microRNA signaling, that might pave the way for new pharmacologic ARDS treatments. Advancements in our appreciation for pathological and clinical subtypes of ARDS will likely also play a critical role in designing clinical trials to identify efficacy for treatments in specific cohorts of ARDS patients.50 Furthermore, the recent COVID-19 pandemic has stimulated the rapid initiation of clinical trials aimed at targeting ARDS. At the time of this writing, there are over 100 registered controlled trials for COVID-19 ARDS listed on ClinicalTrials.gov. Potential interventions that demonstrate clinical efficacy in COVID-19 ARDS could also provide usefulness in treating ARDS patients independent of SARS-CoV-2 infection. It is important to note, however, that insights gained from proven therapies for COVID-19 ARDS could translate to non-COVID-19 ARDS subtypes that share pathophysiological components with COVID-19 cases. For example, the efficacy reported with dexamethasone could indicate specific use for patients with viral-associated ARDS that are characterized by immune profiles similar to what is seen in COVID-19 and not for patients with other etiologic types of ARDS. Additional clinical studies will be required to carefully address such hypotheses. Lastly, to establish efficacy for novel ARDS interventions, collaborative efforts, such as the multicenter trials ongoing in the PETAL Network, will continue to be vital for the successful improvement of ARDS outcomes. In addition to these large scale studies, a network of smaller clinical trials investigating the efficacy of novel treatment concepts73,74 may be required to identify new approaches for ARDS therapy. Channeling enthusiasm for new trials targeting COVID-19 ARDS may provide a catalyst and framework for these important collaborations going forward.
Acknowledgments
Funding: National Institute of Health Grant T32GM120011 to NKB; 2T32HL007747-21, the American Thoracic Society Unrestricted Grant, American Heart Association Career Development Award (19CDA34660279), American Lung Association Catalyst Award (CA-622265), the Center for Clinical and Translational Sciences, McGovern Medical School Pilot Award (1UL1TR003167-01), and Parker B. Francis Fellowship to XY; R01-DK097075, R01-HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE
Footnotes
Conflicts of Interest: GWW: Merck Pharmaceuticals- Scientific Speaker for Sugammadex
References:
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, Force ADT: Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–33 [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET: Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016; 315: 788–800 [DOI] [PubMed] [Google Scholar]
- 3.Gumbert SD, Kork F, Jackson ML, Vanga N, Ghebremichael SJ, Wang CY, Eltzschig HK: Perioperative Acute Kidney Injury. Anesthesiology: The Journal of the American Society of Anesthesiologists 2020; 132: 180–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Chen X, Cai Y, Ja Xia, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y: Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong E, Du H, Gardner L: An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20: 533–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson BT, Bernard GR: ARDS Network (NHLBI) studies: successes and challenges in ARDS clinical research. Critical care clinics 2011; 27: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenfeld DA, Bernard GR: Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical care medicine 2002; 30: 1772–1777 [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Network ARDS: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8 [DOI] [PubMed] [Google Scholar]
- 10.Investigators WGftP: Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA 2018; 320: 1872–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashbaugh D, Boyd Bigelow D, Petty T, Levine B: ACUTE RESPIRATORY DISTRESS IN ADULTS. The Lancet 1967; 290: 319–323 [DOI] [PubMed] [Google Scholar]
- 12.Tremblay LN, Slutsky AS: Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006; 32: 24–33 [DOI] [PubMed] [Google Scholar]
- 13.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR: Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338: 347–54 [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A: A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical care medicine 2006; 34: 1311–1318 [DOI] [PubMed] [Google Scholar]
- 15.Higher versus Lower Positive End-Expiratory Pressures in Patients with the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2004; 351: 327–336 [DOI] [PubMed] [Google Scholar]
- 16.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE, Lung Open Ventilation Study Investigators ft: Ventilation Strategy Using Low Tidal Volumes, Recruitment Maneuvers, and High Positive End-Expiratory Pressure for Acute Lung Injury and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 2008; 299: 637–645 [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Xie J, Huang Y, Pan C, Yang Y, Qiu H, Liu L: Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiology 2018; 18: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamm WJ, Graham MM, Albert RK: Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med 1994; 150: 184–93 [DOI] [PubMed] [Google Scholar]
- 19.Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ: Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Critical care medicine 2000; 28: 295–303 [DOI] [PubMed] [Google Scholar]
- 20.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–68 [DOI] [PubMed] [Google Scholar]
- 21.Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, Uleryk E, Mancebo J, Pesenti A, Ranieri VM, Fan E: Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2017; 14: S280–S288 [DOI] [PubMed] [Google Scholar]
- 22.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ: An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017; 195: 1253–1263 [DOI] [PubMed] [Google Scholar]
- 23.Albert RK: Prone Ventilation for Patients with Mild or Moderate Acute Respiratory Distress Syndrome. Annals of the American Thoracic Society 2020; 17: 24–29 [DOI] [PubMed] [Google Scholar]
- 24.Weigelt JA, Norcross JF, Borman KR, Snyder WH: Early steroid therapy for respiratory failure. Archives of surgery 1985; 120: 536–540 [DOI] [PubMed] [Google Scholar]
- 25.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA: High-dose corticosteroids in patients with the adult respiratory distress syndrome. New England Journal of Medicine 1987; 317: 1565–1570 [DOI] [PubMed] [Google Scholar]
- 26.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA: Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. Jama 1998; 280: 159–165 [DOI] [PubMed] [Google Scholar]
- 27.Efficacy and Safety of Corticosteroids for Persistent Acute Respiratory Distress Syndrome. New England Journal of Medicine 2006; 354: 1671–1684 [DOI] [PubMed] [Google Scholar]
- 28.Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, Martin-Rodriguez C, Diaz-Dominguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Anon JM, Fernandez RL, Gonzalez-Martin JM: Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020; 8: 267–276 [DOI] [PubMed] [Google Scholar]
- 29.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ: Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmerhorst HJ, Roos-Blom M-J, van Westerloo DJ, de Jonge E: Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Critical care medicine 2015; 43: 1508–1519 [DOI] [PubMed] [Google Scholar]
- 31.Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, Grimaldi D, Grelon F, Anguel N, Lasocki S, Henry-Lagarrigue M, Gonzalez F, Legay F, Guitton C, Schenck M, Doise JM, Devaquet J, Van Der Linden T, Chatellier D, Rigaud JP, Dellamonica J, Tamion F, Meziani F, Mercat A, Dreyfuss D, Seegers V, Radermacher P: Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. The Lancet Respiratory Medicine 2017; 5: 180–190 [DOI] [PubMed] [Google Scholar]
- 32.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M: Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. Jama 2016; 316: 1583–1589 [DOI] [PubMed] [Google Scholar]
- 33.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G, Investigators L, Network RR: Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 2020; 382: 999–1008 [DOI] [PubMed] [Google Scholar]
- 34.April I: Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. Jama 2009; 302: 1888–1895 [DOI] [PubMed] [Google Scholar]
- 35.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374: 1351–63 [DOI] [PubMed] [Google Scholar]
- 36.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, EOLIA Trial Group REVA, and ECMONet: Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018; 378: 1965–1975 [DOI] [PubMed] [Google Scholar]
- 37.Wiedemann H: National Heart, Lung, and Blood Institute acute respiratory distress syndrome (ARDS) clinical trials network, comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575 [DOI] [PubMed] [Google Scholar]
- 38.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A, Investigators AS: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363: 1107–16 [DOI] [PubMed] [Google Scholar]
- 39.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC, National Heart Ln, and Blood Institute PETAL Clinical Trials Network: Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 2019; 380: 1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P: Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama 2012; 307: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT: Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184: 561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranieri VM, Pettila V, Karvonen MK, Jalkanen J, Nightingale P, Brealey D, Mancebo J, Ferrer R, Mercat A, Patroniti N, Quintel M, Vincent JL, Okkonen M, Meziani F, Bellani G, MacCallum N, Creteur J, Kluge S, Artigas-Raventos A, Maksimow M, Piippo I, Elima K, Jalkanen S, Jalkanen M, Bellingan G, Group IS: Effect of Intravenous Interferon beta-1a on Death and Days Free From Mechanical Ventilation Among Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2020 [DOI] [PubMed] [Google Scholar]
- 43.Miller AC, Elamin EM, Suffredini AF: Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit Care Med 2014; 42: 413–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmoodpoor A, Hamishehkar H, Shadvar K, Ostadi Z, Sanaie S, Saghaleini SH, Nader ND: The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome. Immunol Invest 2019; 48: 147–159 [DOI] [PubMed] [Google Scholar]
- 45.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med 2002; 30: 1–6 [DOI] [PubMed] [Google Scholar]
- 46.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. Jama 2000; 283: 1995–2002 [DOI] [PubMed] [Google Scholar]
- 47.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, Johnston P, Hopkins PA, Johnston AJ, McDowell C, McNally C: Simvastatin in the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2014; 371: 1695–1703 [DOI] [PubMed] [Google Scholar]
- 48.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P, Investigators NNARDSNo: Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011; 306: 1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome. New England Journal of Medicine 2014; 370: 2191–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson JG, Calfee CS: ARDS Subphenotypes: Understanding a Heterogeneous Syndrome. Critical Care 2020; 24: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C: Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. The Lancet Rheumatology 2020; 2: e437–e445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teuwen LA, Geldhof V, Pasut A, Carmeliet P: COVID-19: the vasculature unleashed. Nat Rev Immunol 2020; 20: 389–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao W, Li T: COVID-19: towards understanding of pathogenesis. Cell Res 2020; 30: 367–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pruijssers AJ, George AS, Schäfer A, Leist SR, Gralinksi LE, Dinnon KH, Yount BL, Agostini ML, Stevens LJ, Chappell JD, Lu X, Hughes TM, Gully K, Martinez DR, Brown AJ, Graham RL, Perry JK, Du Pont V, Pitts J, Ma B, Babusis D, Murakami E, Feng JY, Bilello JP, Porter DP, Cihlar T, Baric RS, Denison MR, Sheahan TP: Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Reports 2020: 107940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-d, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC: Remdesivir for the Treatment of Covid-19 — Preliminary Report. New England Journal of Medicine 2020 [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M: Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M: Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID‐19: initial assessment. Journal of the International AIDS Society 2020; 23: e25489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C: A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. New England Journal of Medicine 2020; 382: 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes P-M, Meziani F, Group CT: High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine 2020; 46: 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z: Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis 2020; 18: 1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN: Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. Journal of the American College of Cardiology 2020; 76: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF: Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. The American surgeon 2001; 67: 377–382 [PubMed] [Google Scholar]
- 63.Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, Dangayach NS, Olin J, Kohli-Seth R, Powell CA: COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clinical and translational medicine 2020: 10.1002/ctm2.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD: Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost 2020; 18: 1752–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett CD, Oren-Grinberg A, Chao E, Moraco AH, Martin MJ, Reddy SH, Ilg AM, Jhunjhunwala R, Uribe M, Moore HB, Moore EE, Baedorf-Kassis EN, Krajewski ML, Talmor DS, Shaefi S, Yaffe MB: Rescue Therapy for Severe COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS) with Tissue Plasminogen Activator (tPA): A Case Series. J Trauma Acute Care Surg 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrett CD, Moore HB, Moore EE, McIntyre RC, Moore PK, Burke J, Hua F, Apgar J, Talmor DS, Sauaia A: Fibrinolytic therapy for refractory COVID‐19 Acute Respiratory Distress Syndrome: Scientific rationale and review. Research and Practice in Thrombosis and Haemostasis 2020; 4: 524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tobin MJ: Basing Respiratory Management of COVID-19 on Physiological Principles. American Journal of Respiratory and Critical Care Medicine 2020; 201: 1319–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, Li MD, Witkin A, Rodriguez-Lopez JM, Shepard JO, Little BP: Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, Tubiolo D, Tagliabue P, Zanella A, Grasselli G, Pesenti A: Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients With the Acute Respiratory Distress Syndrome From Coronavirus Disease 2019. Critical care medicine 2020: 10.1097/CCM.0000000000004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan C, Chen L, Lu C, Zhang W, Xia J-A, Sklar MC, Du B, Brochard L, Qiu H: Lung Recruitability in COVID-19-associated Acute Respiratory Distress Syndrome: A Single-Center Observational Study. American journal of respiratory and critical care medicine 2020; 201: 1294–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC: Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. American Journal of Respiratory and Critical Care Medicine 2020; 201: 1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed July 12, 2020. [PubMed] [Google Scholar]
- 73.Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH, Kaur B, Eltzschig HK: Strategies to Modulate MicroRNA Functions for the Treatment of Cancer or Organ Injury. Pharmacological Reviews 2020; 72: 639–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O’Neill LA, Eltzschig HK, Masters SL, McNamee EN: Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med 2017; 214: 1737–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N: Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. The New England journal of medicine 2019 [Google Scholar]
- 76.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP: The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20: 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang N, Li D, Wang X, Sun Z: Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M: Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019; 322: 1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.National Heart L, Blood Institute PCTN, Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, Hayden D, Hough CL, Hyzy RC, Khan A, Levitt JE, Park PK, Ringwood N, Rivers EP, Self WH, Shapiro NI, Thompson BT, Yealy DM, Talmor D: Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N Engl J Med 2019; 381: 2529–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]