Abstract
Growing evidence during the last 15 years has implicated epigenetic mechanisms in the behavioral effects of addictive drugs. The main focus of these studies has been on epigenetic mechanisms of psychomotor sensitization and drug reinforcement, as assessed by the conditioned place preference and drug self-administration procedures. Some of these studies documented long-lasting changes in expression of epigenetic enzymes and molecules that persisted for weeks after the last drug exposure. These observations inspired recent investigations on epigenetic mechanisms of relapse to drug seeking after prolonged abstinence.
Here, we review studies examining epigenetic mechanisms (e.g., histone modifications, chromatin remodeler-associated modifications and DNA methylation) that contribute to relapse to cocaine, amphetamine, methamphetamine, morphine, heroin, nicotine and alcohol seeking, as assessed in rodent models. We first provide a brief overview of studies examining persistent epigenetic changes in the brain after prolonged abstinence from non-contingent drug exposure or drug self-administration. Next, we review studies on the effect of either systemic or brain-site specific epigenetic manipulations on reinstatement of drug-conditioned place preference after extinction of the learned preference, reinstatement of drug seeking after operant drug self-administration and extinction of the drug-reinforced responding, and incubation of drug craving (the time-dependent increase in drug seeking after cessation of drug self-administration). We conclude by discussing the implications of these studies to understanding mechanisms contributing to persistent relapse vulnerability after prolonged abstinence. We also discuss the implications of these results to translational research on the potential use of systemically administered epigenetic enzyme inhibitors for relapse prevention in human drug users.
Keywords: addiction, conditioned place preference, relapse, reinstatement, incubation of craving, epigenetics
Introduction
In 2005, Kumar et al. (1) published a paper entitled “Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum.” They reported that a single non-contingent cocaine injection acutely increases histone 4 (H4) acetylation at cFos (Table S1 for glossary of terms) promoter in rat striatum. They also showed that repeated cocaine injections increase striatal histone 3 (H3) acetylation at Bdnf and Cdk5 promoters 24 h after the last cocaine injection. The results of this study suggest that epigenetic mechanisms (i.e., regulation of gene expression through non-DNA encoded mechanisms) contribute to cocaine’s physiological and potentially behavioral effects. This publication inspired many studies on epigenetic mechanisms, primarily in the form of histone modifications (Fig. 1), in physiological and behavioral effects of addictive drugs (2–11).
Figure 1. Histone modifications associated with drug relapse.
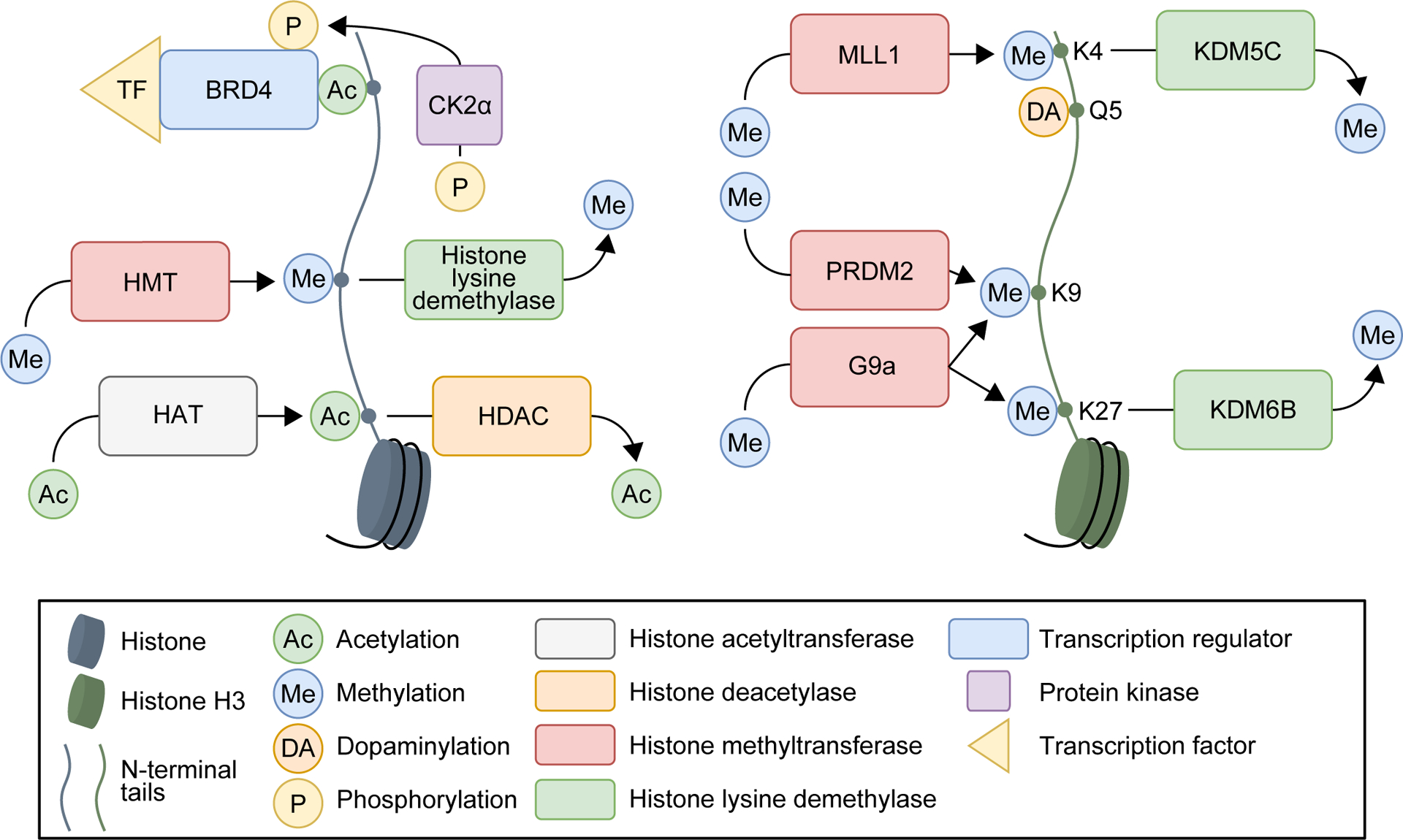
General histone (left) and examples of histone H3-specific (right) modifications associated with drug relapse in preclinical models.
At the molecular level, many studies observed epigenetic changes within 24 h after non-contingent drug exposure or drug self-administration (7–9). Some studies also documented persistent epigenetic changes several days to weeks after the last drug exposure; we summarize these findings in Tables S2–S31. These observations suggested that epigenetic mechanisms contribute to relapse to drug-seeking behaviors that are either sustained or emerge during prolonged abstinence. Due to space limitations, we do not discuss these correlational studies. Briefly, these studies reported persistent changes of various histone modifications (Fig. 1), histone modifying enzymes (expression and activities; Fig. 1), DNA methylation, DNA methylation-related enzymes (expression and activities), or chromatin remodeling proteins from 2 days to ~1 month after last drug exposure across drug classes and brain regions (see Tables S2–S3).
At the behavioral level, the main focus has been on epigenetic mechanisms in the development and expression of psychomotor sensitization (the progressive increase in locomotor activity after repeated drug injections) and drug reinforcement, assessed by conditioned place preference (CPP) and drug self-administration (7–10). In contrast, fewer studies examined causal roles of epigenetic changes in drug relapse. Preventing relapse is a key challenge for treating addiction (12, 13), and in the present review, we discuss rodent studies examining the causal roles of epigenetic mechanisms in relapse to different addictive drugs. We focus on both systemic and brain-site specific epigenetic manipulations in three models: resumption of drug preference in the CPP procedure (including drug-priming-induced reinstatement of drug CPP after extinction of the learned preference or memory retrieval and reconsolidation during abstinence), reinstatement of drug seeking after operant drug self-administration and extinction of the drug-reinforced responding, and incubation of drug craving (the time-dependent increase in drug seeking after cessation of drug self-administration). Due to space limitations, we do not discuss studies on noncoding RNAs and refer readers to recent reviews (14–16). We provide a glossary of epigenetic and physiological terms used in this review (blue font) in Table S1.
Epigenetic mechanisms in animal models of relapse
Conditioned place preference (CPP)
Drug-induced CPP has been used to evaluate the reinforcing effects of addictive drugs (17). In 2000, the model was adapted to evaluate drug priming-induced reinstatement of drug preference after extinction of the learned preference (18, 19). However, the model’s relevance to human addiction, characterized by prolonged volitional drug use that escalates over time, is questionable, because rodents are non-contingently exposed to low drug amounts over several days. Below, we briefly discuss studies using CPP to study epigenetic mechanisms, primarily histone acetylation, in reinstatement of drug CPP after extinction, and retrieval and reconsolidation of drug-associated memories after CPP training during abstinence (Table S4).
Systemic drug injection studies
Malvaez et al. (20) assessed the effect of a non-specific histone deacetylase (HDAC; Fig. 1) inhibitor (sodium butyrate, NaBut) on extinction of cocaine CPP and cocaine priming-induced reinstatement in mice. They reported that daily systemic NaBut injections immediately after each extinction session facilitate extinction and decrease reinstatement. Their findings were extended using a specific HDAC inhibitor against HDAC3 (21). Wang et al. (22) and Zhu et al. (23) reported that daily NaBut injections immediately after extinction sessions facilitate extinction and decrease drug priming-induced reinstatement of morphine and methamphetamine (Meth) CPP. In contrast, NaBut has no effect on extinction or cocaine priming-induced reinstatement of CPP when injected daily without extinction sessions, or injected daily 10 h after the extinction sessions (20). NaBut also has no effect on Meth priming-induced reinstatement when injected acutely 30 min before Meth priming (23). Together, results indicate that repeated HDAC inhibition following extinction suppresses drug priming-induced reinstatement of CPP across drug classes.
In contrast, Itzhak et al. (24) reported that daily NaBut injections immediately after the extinction sessions increase resistance to extinction and has no effect on cocaine priming-induced reinstatement in mice. Differences in the CPP procedures across studies (20, 24) may account for these discrepant results.
Three studies examined the effect of inhibiting other epigenetic modifications on reinstatement of drug CPP. Zhang et al. (25) reported that repeated injections of a KDM6B (a histone lysine demethylase; Fig. 1) inhibitor after cocaine CPP training decrease expression of cocaine CPP and cocaine priming-induced reinstatement in mice. Guo et al. (26) reported that daily injections of a BRD4 (a chromatin remodeling protein; Fig. 1) inhibitor prior to extinction session decrease cocaine priming-induced reinstatement in mice. Lastly, Lax et al. (27) reported that injections of a PARP-1 (a DNA damage response enzyme and chromatin remodeler) inhibitor 50 min before the first CPP test decrease subsequent expression of cocaine-induced CPP for up to 14 days later. Guo et al, (26) and Lax et al. (27) also identified specific brain sites for the action of systemic epigenetic manipulations (see below).
Brain-site specific studies
Nucleus accumbens
Guo et al. (26) reported that daily NAc injections of CK2α (responsible for activating chromatin remodeling protein, BRD4; Fig. 1) inhibitor prior to the extinction sessions facilitate extinction of cocaine CPP and decrease cocaine priming-induced reinstatement in mice. Aguilar-Valles et al. (28) assessed the role of histone methylation in NAc on retrieval and reconsolidation of Meth-associated memories in mice. They reported that decreasing Mll1 (a histone methyltransferase [HMT] Fig.1) or KDM5C (a histone lysine demethylase; Fig. 1) expression by siRNA in NAc decreases Meth-induced CPP expression on abstinence days 2 and 6.
Other brain regions
Lax et al. (27) determined the role of amygdala PARP-1 in retrieval and reconsolidation of cocaine-associated memories in rats. They found that injection of a PARP-1 inhibitor into central amygdala (CeA), but not basolateral amygdala (BLA), prior to the first CPP test decreases CPP expression for up to 14 days after CPP training. Additionally, CeA overexpression of D3ZLJ1 (a downstream target of PARP-1) decreases CPP expression for up to 14 days after training. Wang et al. (29) reported that daily BLA injections of a nonspecific HDAC inhibitor (Trichostatin A [TsA]) facilitate extinction of morphine CPP and decrease morphine priming-induced reinstatement. Lastly, Lopez et al. (30) reported that HDAC3 overexpression in the cholinergic neurons in medial habenula (MHb) in mice has no effect on cocaine priming-induced reinstatement.
Summary
Studies using systemic injections of HDAC inhibitors show a causal role of histone acetylation in extinction and drug priming-induced reinstatement across drug classes, but effects vary across different CPP procedures and different injection schedules. Brain-site specific studies suggest that the site of HDAC action is BLA for morphine but not MHb for cocaine. Other studies show causal roles of two chromatin remodeling proteins in cocaine priming-induced reinstatement and retrieval and reconsolidation of cocaine-associated memories after CPP training. Finally, evidence supports a causal role of histone methylation in NAc in retrieval and reconsolidation of Meth-associated CPP memories. Overall, studies using the CPP procedure provided evidence supporting the role of various epigenetic mechanisms in reinstatement of drug preference and reconsolidation of drug-associated memories.
Reinstatement of drug seeking after extinction
Examination of epigenetic mechanisms underlying operant relapse began with the reinstatement model. In this model, resumption of drug seeking (reinstatement) after exposure to drug priming injections, drug cues and/or context, and/or different stressors is determined after drug self-administration training and extinction of the drug-reinforced responding (31–34). It is important to note that extinction training induces plasticity in brain areas involved in reinstatement of drug seeking [e.g., NAc (35)], which can be difficult to dissociate from plasticity induced by prior drug exposure and abstinence duration (36). Below, we discuss the roles of epigenetic mechanisms in reinstatement of drug seeking (see Table S5 and Fig. 2 for summary of findings).
Figure 2. Epigenetic mechanisms underlying operant drug relapse.
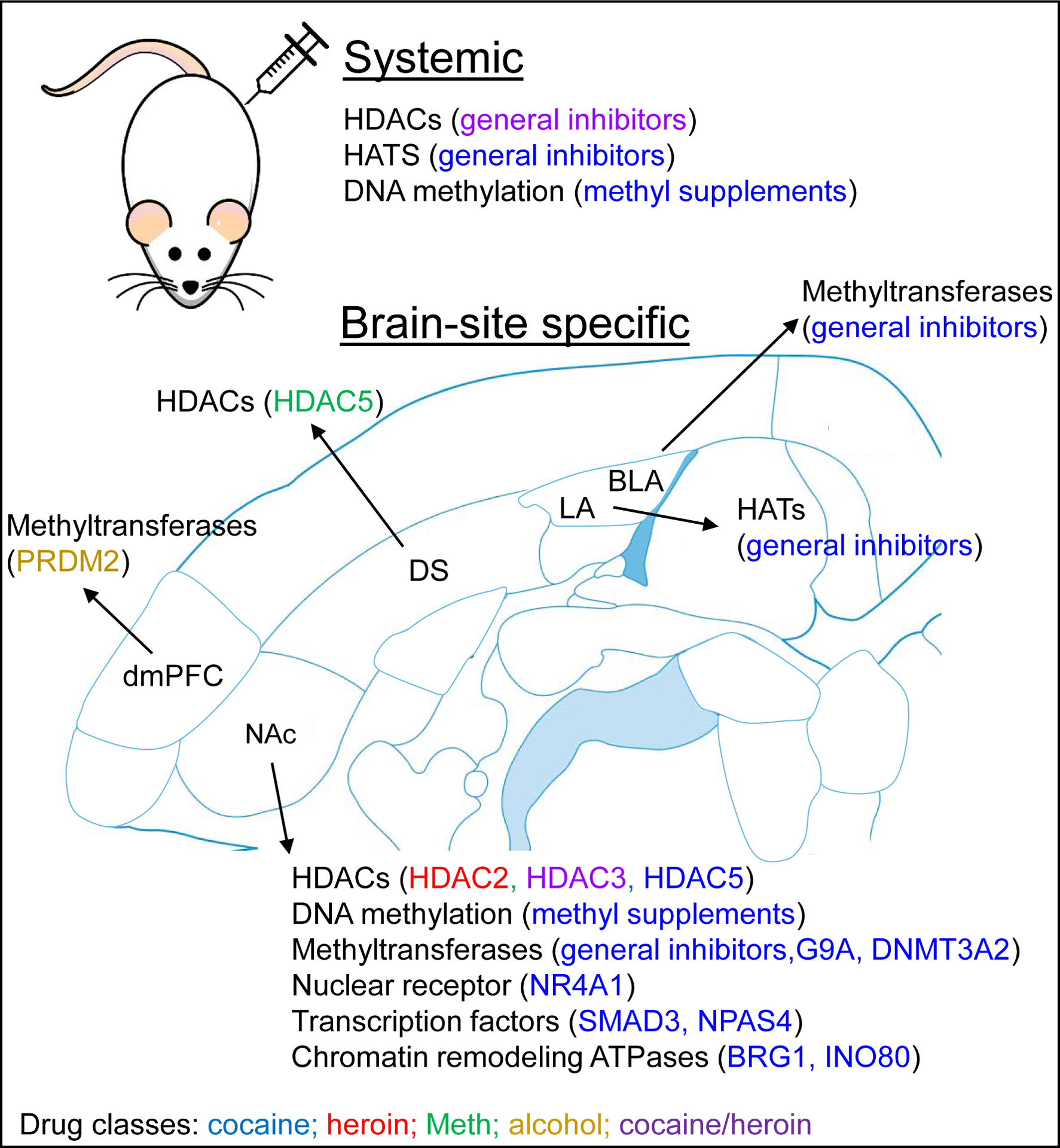
The figure summarizes results from Table S4 listing systemic and brain-site specific epigenetic manipulations that play a role in operant drug relapse. Mechanisms related to cocaine are in blue, heroin are in red, Meth are in green, alcohol are in yellow, and both cocaine and heroin are in purple. Abbreviations for brain regions: NAc, nucleus accumbens; dmPFC, dorsal medial prefrontal cortex; BLA, basolateral amygdala; LA, lateral amygdala; DS, dorsal striatum. Abbreviations for epigenetic terms are listed in Table S1.
Systemic drug injection studies
Systemic injections of non-specific HDAC (Fig. 1) inhibitors were reported to affect reinstatement of drug seeking across drugs and reinstating stimuli. Romieu et al. (37) reported that the HDAC inhibitors TsA or PhB injected daily for 4 d prior to testing decrease cocaine priming-induced reinstatement. Chen et al. (38) reported that NaBut injections 6 h (but not 12 h) prior to testing decrease heroin priming-induced reinstatement. Peterson et al. (39) examined neural mechanisms underlying the protective effect of exercise on cocaine seeking. They reported that daily NaBut injections for 14 d during abstinence reduce cue-induced reinstatement of cocaine seeking but have no effect on extinction responding (39). Arndt et al. (40) reported that injections of HDAC inhibitor TsA 30 min prior to extinction and reinstatement sessions decrease cue-induced reinstatement of amphetamine seeking, but not extinction or amphetamine priming-induced reinstatement.
Studies have also examined the role of HDACs in retrieval and reconsolidation of drug-associated memories. In these studies, extinction training is followed by a cue-exposure session where drug-associated cues are presented to reactivate drug-cue memories. Reinstatement is then tested the following day or after longer abstinence periods. Monsey et al. (41) reported that TsA injection 45 min after a cue-exposure session increases subsequent cue-induced reinstatement of cocaine seeking, suggesting that HDAC inhibition enhances reconsolidation of cocaine-cue memories. In contrast, injections of garcinol, a histone acetyltransferase (HAT; Fig. 1) inhibitor, 30 min after the cue-exposure session decrease cue-induced reinstatement (41, 42). TsA injections after garcinol injection prevent garcinol-induced decrease in cue-induced reinstatement (41).
Castino et al. (43) reported that NaBut injection immediately (but not 6-h later) after extinction sessions decreases nicotine- and nicotine+cue-induced reinstatement, but not extinction or cue-induced reinstatement of nicotine seeking. The authors hypothesized that HDAC inhibition facilitates consolidation of extinction memory that was not observable due to low levels of responding during extinction sessions. Therefore, they performed a subsequent experiment in which cues were presented during extinction sessions to increase responding. NaBut injections immediately after extinction sessions enhance extinction learning, suggesting that HDAC inhibition facilitates consolidation of extinction memories (43).
Wright et al. (44) examined the role of DNA methylation in reinstatement of cocaine seeking. They found that daily injections of exogenous L-methionine (MET) 1–2 h prior to all operant sessions decrease cocaine priming-induced reinstatement but not self-administration, extinction, or cue-induced reinstatement.
Brain site-specific studies
Nucleus accumbens
In contrast to studies using systemic injections of non-specific HDAC inhibitors, investigators manipulated specific HDAC isoforms in brain site-specific studies. Hitchcock et al. (45) reported that NAc injection of the HDAC3 inhibitor RGFP966 20 min prior to the first extinction session has no effect on extinction responding but decreases responding during subsequent extinction sessions. NAc injections of RGFP966 also decrease context-and cue-induced reinstatement but not cocaine priming-induced reinstatement. Martin et al. (46) reported that NAc injections of HDAC2/3 inhibitor MI-192 for 3 days following extinction training decrease heroin priming-induced reinstatement on the following day.
Taniguchi et al. (47) reported that NAc injection of a virus expressing a nuclear localized mutant HDAC5 21 d prior to cocaine self-administration training decreases cue- and cocaine priming-induced reinstatement, but not cocaine self-administration, extinction, or stress-induced reinstatement. HDAC5 regulates the expression of NPAS4, an activity-dependent immediate early gene, but NPAS4 knockdown in NAc has no effect on cue-induced reinstatement in mice (47).
Anderson et al. (48) reported that transient viral-mediated expression of the HMT G9a in NAc during cocaine self-administration training increases stress-induced reinstatement (but not cue- or drug-induced reinstatement) weeks after viral expression subsides. The authors also reported that G9A knockdown in NAc 3–7 d prior to self-administration training decreases stress-induced reinstatement (49). These studies suggest that G9a bidirectionally regulates stress-induced reinstatement of cocaine seeking.
Two studies examined the role of TGF-β signaling in reinstatement of cocaine seeking. Gancarz et al. (50) reported that transient viral-mediated expression of SMAD3 in NAc increases cocaine priming-induced reinstatement, while a dominant negative SMAD3 (dnSMAD3) decreases this reinstatement. Wang et al. (51) reported that NAc injections of the BRG1 inhibitor PF13 for 4 days decrease cue-induced reinstatement the following day. They also found that transient viral expression of BRG1 in NAc increases cue-induced reinstatement, while expression of dnSMAD3 decreases this reinstatement (51).
Other brain regions
Monsey et al. (41) reported that a single garcinol injection into lateral amygdala nucleus (LA) 1 h after a cue-exposure session decreases subsequent cue-induced reinstatement of cocaine seeking, extending the authors’ previous finding on a similar effect of systemic garcinol injections (42). These studies suggest that garcinol’s inhibitory effect on drug cue-associated memories occurs via disruption of gene transcription in LA. Shi et al. (52) reported that an BLA injection of DNMT inhibitor 5-AZA immediately, but not 6-h later, after a cue-exposure session decreases subsequent cue-induced reinstatement and cue+cocaine reinstatement of cocaine seeking. These results suggest that different amygdala subregions are involved in drug cue-associated memories that contribute to cue-induced reinstatement.
Barbier et al. (53) showed that viral-mediated knockdown of PRDM2 in dorsal medial prefrontal cortex (dmPFC), injected 1 week prior to behavioral testing, increases alcohol self-administration, aversion-resistant alcohol intake, and stress-induced reinstatement in rats. Interestingly, these behavioral phenotypes mimic those observed in rats with a history of alcohol dependence (53).
Summary
Consistent with most CPP studies, studies using the operant reinstatement model indicate that HDAC inhibition decreases relapse across drug classes. However, the inhibitory effect depends on the reinstating stimulus (cue, stress, drug priming) and the timing of HDAC inhibition (e.g., during extinction, abstinence, or memory retrieval). There is also evidence that different isoforms of HDACs in NAc play opposing roles in in reinstatement of drug seeking, suggesting that systemic effects of HDAC inhibition act through certain forms of HDACs, but not others. Inconsistent findings were observed regarding the effect of systemic injections of methyl donors and BLA injections of DNMT inhibitors on reinstatement of cocaine seeking, further supporting the complex behavioral effects of systemic epigenetic manipulations. There is also evidence for the roles of other epigenetic mechanisms, including HMTs, transcription factors, and chromatin remodelers. In addition to NAc, studies have also shown roles of epigenetic mechanisms in LA, BLA, and dmPFC in reinstatement of drug seeking for drug classes. Finally, a question for future research is whether the effect of HDAC inhibition or other epigenetic manipulations on reinstatement after extinction occurs through reconsolidation interference of drug-cue memories, consolidation of extinction memories, or both.
Incubation of drug craving
In the past 5 years, 5 studies have examined the causal roles of brain-site specific epigenetic mechanisms in incubation of cocaine and Meth craving (Table S5 and Fig. 2). In rats, incubation of drug craving has been assessed in two ways. In early studies, incubation of drug craving was assessed using a modified within-session extinction-reinstatement procedure (31) where, on different abstinence days, rats were first given 6–8 hourly extinction sessions in the absence of the drug infusion-associated discrete tone-light cue, and then tested (1-h session) for cue-induced reinstatement where lever presses led to contingent delivery of the tone-light cue (54, 55). Early studies observed reliable incubation of lever presses for both extinction responding without the cue and subsequent cue-induced reinstatement after extinction (56, 57). Consequently, most incubation of craving studies assessed incubation of craving in a single extinction session, performed on different abstinence days, where lever presses led to contingent presentations of the drug-associated discrete cues (58, 59). In the studies described below, Cannella et al. (60) used the original within-session extinction plus cue-induced reinstatement procedure, while the other studies used the single extinction session procedure, to determine epigenetic mechanisms in incubation of drug craving. The incubation of craving model may be a more suitable procedure than the classical extinction-reinstatement model to determine the role of drug-induced epigenetic changes in relapse, because repeated daily extinction training can induce extinction learning-induced epigenetic (or other brain neuroadaptation changes) that could contribute to the relapse-related behavior during testing (58, 61–64). Finally, the rat studies discussed below used extended access (6 h or more daily sessions) drug self-administration, because early studies have shown that incubation of craving is more robust after extended vs. short (2 h daily sessions) self-administration sessions (57).
Brain-site specific studies
Nucleus accumbens
Three studies focused on NAc and examined the causal role of DNA methylation and INO80 complex (a chromatin remodeler) in incubation of cocaine craving. Massart et al. (65) reported that NAc injections of RG108 (a DNA methyltransferase inhibitor) or S-adenosylmethionine (SAM, a methyl donor) decrease and increase, respectively, cocaine seeking on abstinence day 30, and that these effects last for up to abstinence day 60. Cannella et al. (60) extended these findings and examined the role of a specific DNA methyltransferase isoform in NAc, DNMT3A2, in incubation of cocaine craving. First, they reported that viral knockdown of DNMT3A2 by shRNA in NAc shell during abstinence or prior to cocaine self-administration decreases cue-induced reinstatement on abstinence day 45. In the latter condition, DNMT3A2 knockdown also decreases cue-induced reinstatement on abstinence day 1 but has no effect on cocaine self-administration. These data indicate that DNMT3A2 in NAc plays a role in cocaine relapse.
One study examined the role of a chromatin remodeler, inositol auxotroph 80 (INO80) complex in incubation of cocaine craving. Werner et al. (66) reported that transient viral expression of INO80 and an inactive form of INO80 in NAc increases and decreases, respectively, cocaine seeking on abstinence day 30. They also reported that INO80 expression in NAc is regulated by TRIM3, an E3 ubiquitin ligase, which provides evidence linking the ubiquitin-proteasome system and epigenetic modifications in the context of drug relapse.
Finally, Carpenter et al. (67) reported that CRISPR-mediated inactivation of a novel transcription factor, Nr4a1 in NAc decreases cocaine seeking in mice on abstinence day 28, but not day 1. However, the authors of this study used a short access (2-h daily sessions) self-administration procedure and cocaine seeking was lower on abstinence day 28 than on day 1 in the control groups. Thus, the epigenetic mechanism identified in this study is likely unrelated to incubation of cocaine craving.
Other brain regions
A recent study explored the role of a novel epigenetic modification, histone 3 glutamine 5 dopaminylation, H3Q5dop (Fig. 1), in ventral tegmental area (VTA) in incubation of cocaine craving in rats. Lepack et al. (68) reported that downregulating H3Q5dop in VTA by viral expression of a mutated H3 decreases cocaine seeking on abstinence day 30, accompanied by a decrease in evoked dopamine release in NAc. The authors also observed increased H3Q5dop in VTA after 30 abstinence days from heroin self-administration, suggesting that the role of H3Q5dop in VTA in relapse generalizes across drug classes.
Finally, one study explored the role of dorsal striatum (DS) in incubation of Meth craving. Li et al. (69) reported that viral overexpression of the nuclear-localized HDAC5 and viral knockdown of HDAC5 by shRNA in DS increases and decreases, respectively, Meth seeking on abstinence day 30 but not day 1. These findings support a time-dependent role of HDAC5 in DS in incubation of Meth craving. However, these results are inconsistent with previous findings on the role of NAc HDAC5 in reinstatement of cocaine seeking after extinction (see above) (47). The reasons for these different findings could be due to several factors, including the drugs (cocaine vs. Meth), self-administration procedure (extended access vs. short access), and brain regions (NAc vs. DS).
Summary
Brain-site specific studies have identified distinct epigenetic mechanisms within NAc and VTA in incubation of cocaine craving; one study also identified a causal role of HDAC5 in DS in incubation of Meth craving. The studies reviewed indicate that the role of DNMT3A2 in NAc cocaine seeking is time-independent (60). In contrast, the role of HDAC5 in DS in drug seeking is time-dependent and specific to ‘incubated’ Meth seeking after prolonged abstinence. The latter supports the notion that epigenetic mechanisms specifically contribute to relapse to drug seeking that emerge during prolonged abstinence, possibly through interacting with other abstinence-dependent neuroadaptations. Finally, Massart et al. (65), Werner et al. (66), and Lepack et al. (68) did not examine the causal role of epigenetic mechanisms on cocaine seeking during early abstinence. Therefore, it is unknown whether these epigenetic mechanisms play a time-dependent role in the development of incubation of cocaine craving.
Conclusion and future directions
Preventing relapse is major challenge in treating drug addiction. Evidence suggests that exposure to addictive drugs causes epigenetic changes in multiple brain areas, but most preclinical studies have focused on epigenetic changes observed during acute abstinence (<24 h after drug exposure) and epigenetic mechanisms that underlie psychomotor sensitization and drug reinforcement (4, 9). More recently, studies have begun to examine causal roles for epigenetic mechanisms in drug relapse, as assessed in rodent models.
Most epigenetic studies in drug relapse focused on systemic injections or NAc manipulations and demonstrated that multiple epigenetic mechanisms underlie reinstatement of drug CPP and operant responding after extinction, and incubation of drug craving. In NAc, chromatin remodeling, histone methylation, histone acetylation, transcription factors, and DNA methylation have all been shown causal roles in relapse-related behaviors. Other studies demonstrated causal roles of epigenetic mechanisms in other brain regions underlying relapse, including DS, amygdala (CeA, LA, and BLA subregions), dmPFC, and VTA. These studies suggest that epigenetic mechanisms in multiple brain regions contribute to drug relapse.
The most studied epigenetic mechanism in drug relapse is histone modifications, particularly HDACs. The general finding from these studies is that systemic and brain-site specific manipulations of HDACs, both non-specific and specific, decrease relapse to drug seeking or preference (but see (24)). Differences among studies may be due to drug classes, behavioral procedures, brain regions, and timing of HDAC manipulations. Further investigation is required to disentangle these variables.
Epigenetic mechanisms affect numerous transcriptional processes and manipulating these mechanisms likely results in nonspecific consequences. Manipulating individual epigenetic targets provides more specificity than general inhibitors, yet the downstream targets remain numerous. To improve the translational potential of this research, future studies should determine the downstream target(s) of identified epigenetic mechanisms and the causal roles of these downstream targets in relapse to drug seeking or preference. These studies will provide insight into how transcriptomic and proteomic states, beyond a single downstream target, underlie relapse and maintain relapse vulnerability during prolonged abstinence. Advancements in unbiased genome-wide and proteomic approaches have made possible the interpretation of neuroepigenomics data (70). Methods such as RNA-seq and ChIP-seq are becoming increasingly common, and some studies have already begun examining transcriptome and chromatin states during different abstinence periods (66–68, 71) and after reinstatement or relapse testing (44, 72, 73).
Finally, a question for future research is the degree to which the results from the preclinical studies reviewed here can be translated to humans. Traditionally, pharmacological agents assessed in animal models of relapse target brain mechanisms that acutely control drug seeking induced by relapse-provoking stimuli (context, cues, drug priming, stress) (31, 33, 74). In contrast, the results of the studies reviewed indicate that pharmacological manipulations of the different epigenetic enzymes do not decrease (or increase) relapse via this ‘direct’ mechanism. Instead, as discussed above, the effect of the epigenetic manipulations on relapse appears to occur ‘indirectly’ through reconsolidation interference of drug-cue memories, consolidation of extinction memories, or both. The clinical implications of these preclinical findings is that to the degree that these psychological processes are critical to human relapse, HDAC inhibitors and other epigenetic-related drugs will only be effective in humans if combined in a time-locked manner with behavioral manipulations (cue-induced memory retrieval, extinction of cue responding) that target these processes (75, 76).
Supplementary Material
Table S1. Glossary of epigenetic and physiological terms
Table S2. Summary of epigenetic changes beyond 24 h after non-contingent repeated drug administration
Table S3. Summary of epigenetic changes beyond 24 h after drug self-administration
Table S4. Epigenetic mechanisms of drug relapse assessed by conditioned place preference procedure
Table S5. Epigenetic mechanisms of operant drug relapse
Funding and Disclosure:
The authors report no biomedical financial interests or potential conflicts of interest. The write-up of the review was supported by funds to the NIDA Intramural Research Program (YS), NARSAD Young Investigator Award (XL), NIH/NIDA R00DA041350–02 (XL) and departmental startup funds (XL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We do not include the long-term effects of drug exposure on epigenetic changes in developmental and transgenerational studies, which are beyond the scope of this review.
References
- 1.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. (2005): Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 48:303–314. [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Nestler EJ (2013): Epigenetic mechanisms of drug addiction. Curr Opin Neurobiol. 23:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton PJ, Nestler EJ (2019): Epigenetics and addiction. Curr Opin Neurobiol. 59:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler EJ (2014): Epigenetic mechanisms of drug addiction. Neuropharmacology. 76 Pt B:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renthal W, Nestler EJ (2008): Epigenetic mechanisms in drug addiction. Trends Mol Med. 14:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renthal W, Nestler EJ (2009): Histone acetylation in drug addiction. Semin Cell Dev Biol. 20:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DM, Cates HM, Heller EA, Nestler EJ (2015): Regulation of chromatin states by drugs of abuse. Curr Opin Neurobiol. 30:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogge GA, Wood MA (2013): The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 38:94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robison AJ, Nestler EJ (2011): Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 12:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Sa Nogueira D, Merienne K, Befort K (2019): Neuroepigenetics and addictive behaviors: Where do we stand? Neurosci Biobehav Rev. 106:58–72. [DOI] [PubMed] [Google Scholar]
- 11.Stewart AF, Fulton SL, Maze I (2020): Epigenetics of Drug Addiction. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt WA, Barnett LW, Branch LG (1971): Relapse rates in addiction programs. J Clin Psychol. 27:455–456. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R (2011): New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 13:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny PJ (2014): Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci. 16:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith ACW, Kenny PJ (2018): MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 17:e12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MD, van der Vaart AD (2011): MicroRNAs in addiction: adaptation’s middlemen? Mol Psychiatry. 16:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P (1982): Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 243:91–105. [DOI] [PubMed] [Google Scholar]
- 18.Mueller D, Stewart J (2000): Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 115:39–47. [DOI] [PubMed] [Google Scholar]
- 19.Parker LA, McDonald RV (2000): Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacol Biochem Behav. 66:559–561. [DOI] [PubMed] [Google Scholar]
- 20.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA (2010): Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 67:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. (2013): HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 110:2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Zhang Y, Qing H, Liu M, Yang P (2010): The extinction of morphine-induced conditioned place preference by histone deacetylase inhibition. Neurosci Lett. 483:137–142. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Zhao N, Chen Y, Zhu L, Zhong Q, Liu J, et al. (2017): Sodium butyrate modulates a methamphetamine-induced conditioned place preference. J Neurosci Res. 95:1044–1052. [DOI] [PubMed] [Google Scholar]
- 24.Itzhak Y, Liddie S, Anderson KL (2013): Sodium butyrate-induced histone acetylation strengthens the expression of cocaine-associated contextual memory. Neurobiol Learn Mem. 102:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YX, Akumuo RC, Espana RA, Yan CX, Gao WJ, Li YC (2018): The histone demethylase KDM6B in the medial prefrontal cortex epigenetically regulates cocaine reward memory. Neuropharmacology. 141:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo W, Long H, Bu Q, Zhao Y, Wang H, Tian J, et al. (2019): Role of BRD4 phosphorylation in the nucleus accumbens in relapse to cocaine-seeking behavior in mice. Addict Biol.e12808. [DOI] [PubMed] [Google Scholar]
- 27.Lax E, Friedman A, Massart R, Barnea R, Abraham L, Cheishvili D, et al. (2017): PARP-1 is required for retrieval of cocaine-associated memory by binding to the promoter of a novel gene encoding a putative transposase inhibitor. Mol Psychiatry. 22:570–579. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar-Valles A, Vaissiere T, Griggs EM, Mikaelsson MA, Takacs IF, Young EJ, et al. (2014): Methamphetamine-associated memory is regulated by a writer and an eraser of permissive histone methylation. Biol Psychiatry. 76:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Lai J, Cui H, Zhu Y, Zhao B, Wang W, et al. (2015): Inhibition of histone deacetylase in the basolateral amygdala facilitates morphine context-associated memory formation in rats. J Mol Neurosci. 55:269–278. [DOI] [PubMed] [Google Scholar]
- 30.Lopez AJ, Hemstedt TJ, Jia Y, Hwang PH, Campbell RR, Kwapis JL, et al. (2019): Epigenetic regulation of immediate-early gene Nr4a2/Nurr1 in the medial habenula during reinstatement of cocaine-associated behavior. Neuropharmacology. 153:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003): The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 168:3–20. [DOI] [PubMed] [Google Scholar]
- 32.Crombag HS, Bossert JM, Koya E, Shaham Y (2008): Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 363:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016): Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 41:335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See RE (2002): Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 71:517–529. [DOI] [PubMed] [Google Scholar]
- 35.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS (2004): Extinction Training Regulates Neuroadaptive Responses to Withdrawal from Chronic Cocaine Self-Administration. Learn Mem. 11:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf ME, Ferrario CR (2010): AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 35:185–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J (2011): The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr Neuropharmacol. 9:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WS, Xu WJ, Zhu HQ, Gao L, Lai MJ, Zhang FQ, et al. (2016): Effects of histone deacetylase inhibitor sodium butyrate on heroin seeking behavior in the nucleus accumbens in rats. Brain Res. 1652:151–157. [DOI] [PubMed] [Google Scholar]
- 39.Peterson AB, Abel JM, Lynch WJ (2014): Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology. 231:1305–1314. [DOI] [PubMed] [Google Scholar]
- 40.Arndt DL, Wukitsch TJ, Garcia EJ, Cain M (2019): Histone deacetylase inhibition differentially attenuates cue-induced reinstatement: An interaction of environment and acH3K9 expression in the dorsal striatum. Behav Neurosci. 133:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monsey MS, Ruiz SG, Taylor JR (2020): Regulation of Garcinol on Histone Acetylation in the Amygdala and on the Reconsolidation of a Cocaine-Associated Memory. Front Behav Neurosci. 13:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monsey MS, Sanchez H, Taylor JR (2017): The Naturally Occurring Compound Garcinia Indica Selectively Impairs the Reconsolidation of a Cocaine-Associated Memory. Neuropsychopharmacology. 42:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castino MR, Cornish JL, Clemens KJ (2015): Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats. PLoS One. 10:e0124796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, et al. (2015): Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci. 35:8948–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitchcock LN, Raybuck JD, Wood MA, Lattal KM (2019): Effects of a histone deacetylase 3 inhibitor on extinction and reinstatement of cocaine self-administration in rats. Psychopharmacology. 236:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin JA, Werner CT, Mitra S, Zhong P, Wang ZJ, Gobira PH, et al. (2019): A novel role for the actin-binding protein drebrin in regulating opiate addiction. Nat Commun. 10:4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, et al. (2017): HDAC5 and Its Target Gene, Npas4, Function in the Nucleus Accumbens to Regulate Cocaine-Conditioned Behaviors. Neuron. 96:130–144 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson EM, Larson EB, Guzman D, Wissman AM, Neve RL, Nestler EJ, et al. (2018): Overexpression of the Histone Dimethyltransferase G9a in Nucleus Accumbens Shell Increases Cocaine Self-Administration, Stress-Induced Reinstatement, and Anxiety. J Neurosci. 38:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson EM, Sun H, Guzman D, Taniguchi M, Cowan CW, Maze I, et al. (2019): Knockdown of the histone dimethyltransferase G9a in nucleus accumbens shell decreases cocaine self-administration, stress-induced reinstatement, and anxiety. Neuropsychopharmacology. 44:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, et al. (2015): Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 18:959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang ZJ, Martin JA, Mueller LE, Caccamise A, Werner CT, Neve RL, et al. (2016): BRG1 in the Nucleus Accumbens Regulates Cocaine-Seeking Behavior. Biol Psychiatry. 80:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi HS, Luo YX, Yin X, Wu HH, Xue G, Geng XH, et al. (2015): Reconsolidation of a cocaine associated memory requires DNA methyltransferase activity in the basolateral amygdala. Sci Rep. 5:13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbier E, Johnstone AL, Khomtchouk BB, Tapocik JD, Pitcairn C, Rehman F, et al. (2017): Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol Psychiatry. 22:1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm JW, Hope BT, Wise RA, Shaham Y (2001): Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003): Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 23:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu L, Grimm JW, Dempsey J, Shaham Y (2004): Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 176:101–108. [DOI] [PubMed] [Google Scholar]
- 57.Lu L, Grimm JW, Hope BT, Shaham Y (2004): Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 47 Suppl 1:214–226. [DOI] [PubMed] [Google Scholar]
- 58.Wolf ME (2016): Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011): Neurobiology of the incubation of drug craving. Trends Neurosci. 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cannella N, Oliveira AMM, Hemstedt T, Lissek T, Buechler E, Bading H, et al. (2018): Dnmt3a2 in the Nucleus Accumbens Shell Is Required for Reinstatement of Cocaine Seeking. J Neurosci. 38:7516–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW (2010): Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 30:7984–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, et al. (2003): Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 421:70–75. [DOI] [PubMed] [Google Scholar]
- 63.Fuchs RA, Branham RK, See RE (2006): Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 26:3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaham Y, Hope BT (2005): The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 8:1437–1439. [DOI] [PubMed] [Google Scholar]
- 65.Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, et al. (2015): Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 35:8042–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Werner CT, Mitra S, Martin JA, Stewart AF, Lepack AE, Ramakrishnan A, et al. (2019): Ubiquitin-proteasomal regulation of chromatin remodeler INO80 in the nucleus accumbens mediates persistent cocaine craving. Sci Adv. 5:eaay0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carpenter MD, Hu Q, Bond AM, Lombroso SI, Czarnecki KS, Lim CJ, et al. (2020): Nr4a1 suppresses cocaine-induced behavior via epigenetic regulation of homeostatic target genes. Nat Commun. 11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, et al. (2020): Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 368:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, Carreria MB, Witonsky KR, Zeric T, Lofaro OM, Bossert JM, et al. (2018): Role of Dorsal Striatum Histone Deacetylase 5 in Incubation of Methamphetamine Craving. Biol Psychiatry. 84:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maze I, Shen L, Zhang B, Garcia BA, Shao N, Mitchell A, et al. (2014): Analytical tools and current challenges in the modern era of neuroepigenomics. Nat Neurosci. 17:1476–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cates HM, Li X, Purushothaman I, Kennedy PJ, Shen L, Shaham Y, et al. (2018): Genome-wide transcriptional profiling of central amygdala and orbitofrontal cortex during incubation of methamphetamine craving. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. (2015): Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 35:8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalivas PW, LaLumiere RT, Knackstedt L, Shen HW (2009): Glutamate transmission in addiction. Neuropharmacology. 56:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torregrossa MM, Taylor JR (2013): Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 226:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milton AL, Everitt BJ (2010): The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 31:2308–2319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Glossary of epigenetic and physiological terms
Table S2. Summary of epigenetic changes beyond 24 h after non-contingent repeated drug administration
Table S3. Summary of epigenetic changes beyond 24 h after drug self-administration
Table S4. Epigenetic mechanisms of drug relapse assessed by conditioned place preference procedure
Table S5. Epigenetic mechanisms of operant drug relapse


