Abstract
Introduction:
In hepatitis C (HCV) patients, obesity and/or diabetes may increase the risk of liver-related outcomes. We aimed to determine if diabetes and/or obesity are associated with adverse outcomes in direct acting antiviral (DAA)-treated HCV patients.
Methods:
We conducted a retrospective study of 33,003 HCV-infected, DAA-treated Veterans between 2013–2015. Body mass index was used to categorize patients into underweight (<18.5kg/m2), normal-weight (18.5 to<25kg/m2), overweight (25 to<30kg/m2), obesity I (30 to<35kg/m2) and obesity II-III (>35kg/m2). Diabetes was defined by ICD-9/10 codes in association with hemoglobin A1c>6.5% or medication prescriptions. Patients were followed from 180 days post-DAA initiation until 2/14/2019 to assess for development of cirrhosis, decompensations, hepatocellular carcinoma (HCC) and death. Multivariable Cox proportional hazards regression models were used to determine the association between diabetes and/or obesity and outcomes.
Result:
During a mean follow-up of 3 years, 10.1% patients died, 5.0% were newly diagnosed with cirrhosis, 4.7% had a decompensation and 4.0% developed HCC. Diabetes was associated with an increased risk of mortality (AHR=1.25, 95%CI 1.10–1.42), cirrhosis (AHR=1.31, 95%CI 1.16–1.48), decompensation (AHR=1.74, 95%CI 1.31–2.31), and HCC (AHR=1.32, 95%CI 1.01–1.72) among patients without baseline cirrhosis. Compared to normal-weight persons, obese persons had a higher risk of cirrhosis, but overweight and obese persons had lower risk of mortality and HCC.
Conclusion:
In this large DAA-treated Veterans cohort, pre-DAA diabetes increases mortality and liver-related events independent of SVR. Continued vigilance is warranted in patients with diabetes despite SVR. Elevated BMI categories appear to have improved outcomes, although further studies are needed to understand those associations.
Keywords: Hepatitis C, direct-acting antivirals, diabetes, obesity, fatty liver
Introduction
Hepatitis C virus (HCV) infection can lead to hepatic fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [1]. The introduction of highly effective, well-tolerated direct-acting antivirals (DAA) agents over the last 5 years has led to the eradication of chronic HCV infection, known as sustained virologic response (SVR), in unprecedented numbers of patients [2]. Although HCV eradication is associated with improved outcomes [3], patients may develop adverse liver-related morbidity even after SVR, including cirrhosis, complications of portal hypertension, and HCC [4,5]. Progression of liver disease after HCV eradication may be related to ongoing liver injury from other etiologies including obesity, diabetes and associated, unidentified non-alcoholic liver disease (NAFLD), thus mitigating the benefit from viral eradication with DAAs [6].
Consistent with these concerns, some studies suggest that patients with HCV infection who also have obesity or diabetes, have a higher risk of developing cirrhosis, decompensated cirrhosis and HCC [7]. However, little is known about the long-term effects of the presence of diabetes and obesity in the era of DAA therapy for chronic HCV. The metabolic syndrome, defined by the clustering of insulin resistance, obesity, dyslipidemia and hypertension [8], has reached epidemic proportions [9]. NAFLD, the liver manifestation of the metabolic syndrome, has increased in parallel and is now the most common cause of liver disease in the United States [10]. Obesity and diabetes, which are central features of the metabolic syndrome [11], and NAFLD likely play a role in the progression of liver-related complications in the DAA post SVR era. In light of these trends, understanding how obesity and/or diabetes affect liver disease progression in DAA-treated patients has key clinical implications and will inform the intensity of clinical monitoring, HCC surveillance, and the need for ongoing specialty care.
Accordingly, we aimed to determine whether diabetes and/or obesity are associated with mortality and adverse liver-related outcomes (i.e., development of cirrhosis, decompensated cirrhosis, and HCC) after antiviral treatment with DAAs in a national cohort of U.S. Veterans treated for HCV.
Methods
Data source
The U.S. Veterans Health Administration is the largest integrated, nationwide healthcare system in the U.S., providing healthcare to more than 8.9 million Veterans each year, at 168 Veteran Affairs (VA) Medical Centers, and 1053 outpatient clinics. Laboratory tests, demographics, comorbidities, alcohol use questionnaires, pharmacy data and clinical outcomes data were extracted from the VA’s Corporate Data Warehouse (CDW), a national data repository of VA electronic health records developed to facilitate research and quality improvement.[12] This study was approved by the Institutional Review Board of the Veterans Affairs Puget Sound Healthcare System.
Study population
We identified patients treated for chronic HCV infection with DAAs between 2013–2015 using VA pharmacy prescription data (n=38,093). We excluded patients who either died within 180 days from the start of antiviral treatment (n=51), had fewer than 180 days of available follow-up (n=52), or underwent liver transplantation prior to treatment (n=998). We also excluded 1986 patients with missing SVR data and 174 patients with missing body mass index (BMI) data. We defined SVR as a serum HCV RNA level below the lower limit of detection at least 12 weeks after completion of HCV treatment. For patients who initiated multiple DAA regimens, we analyzed only the first treatment (1358 regimens dropped), leaving 33,474 patients in the current analysis. Patients were then followed retrospectively from the time of initiation of antiviral treatment until 02/14/2019.
Definition of diabetes and obesity
The presence of type 2 diabetes, referred to as “diabetes” throughout the manuscript, was defined by the International Classification of Diseases, 9th revision (ICD-9) and ICD-10 codes for type 2 diabetes mellitus (T2DM) (ICD-9 250.00-250.92 and ICD-10 E11.00-E11.9 and E13.00-E13.9) recorded at least twice in addition to either a measurement of glycosylated hemoglobin (HbA1c) >6.5% or an active prescription of a diabetic medication over the 12 months prior to antiviral treatment [13].
BMI prior to antiviral treatment, calculated using the measured weight (kg) divided by the square of the measured height (meters), was used to categorize patients into underweight (BMI <18.5 kg/m2), normal-weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), class I obesity (BMI 30 to <35 kg/m2), class II-III obesity (BMI > 35 kg/m2). Height had a mean, median and maximum of 249, 104 and of 5677 number of days since measurement, respectively, with 81% of measurements within 1 year prior of DAA initiation. Weight had a mean, median and maximum of 49, 28 and 5466 number of days since measurement with 98% within 1 year and 90% within 6 months of DAA initiation.
Baseline patient characteristics
Age, sex, race/ethnicity, HCV DAA regimen, receipt of prior antiviral treatment, HCV genotype, and co-morbidities including hepatitis B virus (HBV) co-infection, human immunodeficiency virus (HIV) co-infection, were extracted from medical charts. Medical co-morbidities, listed in Supplemental Table 1, were included if documented at least twice before initiating antiviral treatment based on ICD-9/1CD-10 codes. The Charlson Comorbidity Index (Supplemental Table 2) was calculated using appropriate ICD-9/10 codes to capture and adjust for the overall burden of comorbidities.[14] Platelet count, total bilirubin, creatinine, albumin, aspartate aminotransferase (AST)/√alanine aminotransferase (ALT) ratio, international normalized ratio (INR), and hemoglobin values were extracted from CDW as of the time of DAA initiation or within the preceding 3 months. The Model for End Stage Liver Disease (MELD)[15], was calculated at the time of DAA treatment. Alcohol use was assessed using the AUDIT-C questionnaire, a validated screening tool for identifying unhealthy alcohol use (Supplemental Table 2). [16]
Outcomes: death and adverse liver-related outcomes
We identified outcomes that occurred during the time period starting at 180 days after DAA initiation and extending through 02/14/2019. The first 180 days after DAA initiation were excluded because DAA courses extend for 8–12 weeks with another 12 weeks or more after completion required to ascertain SVR. Outcomes included death from any etiology, development of cirrhosis, decompensated cirrhosis (defined as development of variceal bleeding, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome and hepatopulmonary syndrome), HCC, and receipt of liver transplantation. These outcomes were defined using appropriate ICD-9/ICD-10 codes that were used and validated in multiple prior studies [17] (Supplemental Table 1) recorded at least twice during follow-up in inpatient or outpatient medical records.
Statistical analysis
Cox proportional hazards regression was used to assess for associations between obesity or diabetes and overall mortality or each of the liver-related outcomes listed above, with or without adjusting for potential confounders. We decided a priori to adjust multivariable Cox proportional hazards models for the following potential confounders, which may be related to both the exposure (diabetes/obesity) and the outcomes (liver outcomes, death): SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, HCV genotype, HIV co-infection, HBV co-infection, Charlson Comorbidity Index, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, blood hemoglobin levels. We conducted additional analyses in which the presence of baseline diabetes or obesity (as defined by BMI ≥ 30 kg/m2) were adjusted for in the models. Survival analyses were stratified by the VA facility at which the antiviral treatment was administered. Patients were retrospectively followed from the date 180 days after DAA initiation until 02/14/2019 or the last day of follow-up, transplantation date or date of death, whichever occurred first.
We analyzed the entire population as well as subdividing into clinically meaningful subgroups defined by SVR or cirrhosis status. All patients were included in the analysis of mortality. However, patients who already had a specific outcome before treatment were excluded from the analysis of that outcome (e.g., patients diagnosed with cirrhosis at baseline were excluded from the analysis of cirrhosis risk).
To assess interaction terms between obesity and diabetes in their associations with any of the outcomes for any subgroups, we used a log-likelihood test of a full model (diabetes, obesity, diabetes and obesity interaction and all other predictors used in the multivariate analysis) versus a nested model (same as full model except without the interaction term).
Results
Characteristics of study population
Of the 33,474 DAA-treated patients, 29,887 (89%) achieved SVR. The most common DAA regimen was sofosbuvir/ledipasvir (58.1%) followed by paritaprevir/ritonavir/ombitasvir/dasabuvir (19.1%), sofosbuvir (±daclatasvir) (13.2%) and sofosbuvir/simeprevir (9.7%). The average age of the cohort was 61.1 years (SD 6.5 years), with the majority being men (96.8%) and non-Hispanic Whites (52.4%) (Table 1). The mean BMI was 28.2 kg/m2 (SD 5.3 kg/m2), 34.6% were obese, and 29.7% had diabetes. Nearly a quarter of the cohort had previously been treated for chronic HCV and 31.7% carried a diagnosis of cirrhosis at the start of treatment, with only a minority having at least one prior decompensation event (8.1%).
Table 1.
Baseline Characteristics of HCV-infected Veterans who Initiated DAA-only Treatment for HCV
| DIABETES | OBESITY (BMI ≥30) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total N=33.474 |
Yes N=9,849 |
No N=23,625 |
P-value* | Yes N=ll,599 |
No N=21,875 |
P-value* |
| Age, years (mean±SD) | 61.1±6.5 | 62.3±5.2 | 60.6±6.9 | < 0.001 | 60.5±6.2 | 61.4±6.6 | < 0.001 |
| Male (%) | 96.8 | 97.6 | 96.5 | < 0.001 | 96 | 97.2 | < 0.001 |
| Race/ethnicity (%) | < 0.001 | 0.15 | |||||
| White, non-Hispanic | 52.4 | 41.8 | 56.8 | 52.8 | |||
| Black, non-Hispanic | 32.7 | 42.4 | 28.7 | 32.4 | |||
| Hispanic | 5.4 | 6.4 | 5 | 5.5 | |||
| Other | 1.7 | 1.7 | 1.7 | 1.6 | |||
| Declined/missing | 7.7 | 7.7 | 7.7 | 7.7 | |||
| HCV genotype (%) | < 0.001 | 0.02 | |||||
| 1 | 83.8 | 86.2 | 82.8 | 83.2 | 84.1 | ||
| 2 | 9 | 7.9 | 9.4 | 9.6 | 8.6 | ||
| 3 | 5.1 | 4 | 5.6 | 5 | 5.2 | ||
| 4 | 0.8 | 0.8 | 0.8 | 0.7 | 0.8 | ||
| Missing | 1.3 | 1.1 | 1.4 | 1.5 | 1.2 | ||
| Prior antiviral treatment (%) | 23.4 | 27.4 | 21.7 | < 0.001 | 26.3 | 21.9 | < 0.001 |
| Diabetes (%) | 29.4 | 100.00.0 | <0.001 | 39.4 | 24.1 | < 0.001 | |
| BMI, kg/m2 (mean±SD) | 28.1±5.4 | 29.7±5.6 | 27.5±5.2 | < 0.001 | 33.9±4.1 | 25.0±2.9 | < 0.001 |
| BMI, kg/m2 (%) | < 0.001 | ||||||
| <18.5 (underweight) | 1.4 | 0.6 | 1.8 | - | - | ||
| 18.5 to<25 (normal weight) | 23.9 | 15.6 | 27.4 | - | - | ||
| 25 to <30 (overweight) | 40 | 37.4 | 41.1 | - | - | ||
| 30 to <35 (type I obesity) | 23.2 | 28.8 | 20.9 | - | - | ||
| ≥ 35 (type II & El obesity) | 11.5 | 17.6 | 8.9 | - | - | ||
| HBV co-infection (%) | 1.2 | 1.3 | 1.2 | 0.3 | 1.1 | 1.3 | 0.21 |
| HIV co-infection (%) | 4.4 | 3.9 | 4.6 | 0.01 | 2.5 | 5.4 | < 0.001 |
| Unhealthy alcohol use** (%) | 9.8 | 6.2 | 11.4 | < 0.001 | 7.8 | 10.9 | < 0.001 |
| Charlson Comorbidity Index (%) | < 0.001 | < 0.001 | |||||
| 0 | 18.2 | 13.4 | 20.3 | 18 | 18.4 | ||
| 1 | 32.9 | 9.5 | 42.7 | 30 | 34.4 | ||
| 2 | 18.2 | 26.9 | 14.5 | 19.3 | 17.5 | ||
| >2 | 30.7 | 50.3 | 22.5 | 32.6 | 29.7 | ||
| Cirrhosis (%) | 31.5 | 40.3 | 27.8 | < 0.001 | 35.4 | 29.4 | < 0.001 |
| Decompensated cirrhosis (all indications) (%) | 0.1 | 0.1 | 0.1 | 0.03 | 0.1 | 0.1 | 0.11 |
| Decompensated cirrhosis (any indication) (%) | 8.1 | 11.3 | 6.8 | < 0.001 | 9.3 | 7.5 | < 0.001 |
| Pre-treatment HCC (%) | 2.6 | 3.3 | 2.3 | < 0.001 | 2.3 | 2.7 | <0.01 |
| MELD Score ≥ 9 (%) | 29.8 | 35.6 | 27.2 | < 0.001 | 32.4 | 28.4 | < 0.001 |
| FIB-4 score ≥3.25 | 35.5 | 39.8 | 33.7 | < 0.001 | 36.5 | 35 | <0.01 |
BMI: body mass index. HBV: hepatitis B virus. HIV: human immunodeficiency virus. HCC: hepatocellular carcinoma. HCV: hepatitis C virus
P-value considered statistically significant if <0.05.
AUDIT-C ≥ 3 in women, AUDIT-C ≥ 4 in men
Compared to patients without diabetes (n=23,209), those with diabetes (n=9,794) were more likely to be older (62.3 vs 60.6, p<0.001) and non-Hispanic Black (42.4 vs 26.8, p<0.001). They were more likely to have MELD scores ≥ 9 (35.6 vs 27.3, p<0.001), have baseline cirrhosis (40.4 vs 28, p<0.001), baseline HCC (3.3 vs 2.3, p<0.001) and FIB-4 scores ≥ 3.25 (39.9 vs 33.8, p<0.001). Compared to patients without obesity (n=21,403), those with obesity (n=11,600) were younger (60.5 vs 61.4, p<0.001), and more likely to have diabetes (39.4 vs 24.4, p<0.001), MELD scores ≥ 9 (32.4 vs 28.4, p<0.001), baseline cirrhosis (35.4 vs 29.6, p<0.001) and FIB-4 scores ≥ 3.25 (36.5 vs 35.1, p<0.001).
During a mean follow-up of 3.0 years after antiviral treatment (median of 3.0 and maximum of 4.8 years), 3392 (10.1%) patients died, 1657 (5.0%) developed cirrhosis, 1569 (4.7%) developed decompensated cirrhosis and 1325 (4.0%) were diagnosed with new HCC.
Diabetes: Associations with adverse liver outcomes or mortality
Patients with SVR
Among patients who achieved SVR, mortality over an average of 3 years of follow up among patients with diabetes was 11.6%. Cirrhosis, any decompensated cirrhosis and HCC development was 5.2%, 5.4% and 3.7%, respectively.
In the multivariable model, compared to patients without diabetes, diabetes was significantly associated with overall mortality (AHR=1.15, 95% CI 1.05–1.26), development of cirrhosis (AHR=1.32, 95% CI 1.16–1.51) and decompensated cirrhosis (AHR=1.21, 95% CI 1.05–1.38) but not HCC (Table 2, Figure 1).
Table 2.
Association of baseline (i.e. pre-treatment) type 2 diabetes with mortality and liver-related outcomes by SVR (YES/NO) and presence of baseline cirrhosis (YES/NO)
| Mortality | HCC | Cirrhosis | Decompensated cirrhosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | ||
| PATIENTS WITH SVR | |||||||||||||
| No Diabetes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Diabetes | 1.49 (1.37–1.61) | 1.14 (1.04–1.25) | 1.15 (1.05–1.26) | 1.39 (1.21–1.59) | 1.07 (0.92–1.25) | 1.09 (0.93–1.28) | 1.54 (1.38–1.73) | 1.36 (1.19–1.55) | 1.32 (1.16–1.51) | 1.74 (1.55–1.96) | 1.21 (1.06–1.38) | 1.21 (1.05–1.38) | |
| PATIENTS WITH NO SVR | |||||||||||||
| No Diabetes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Diabetes | 1.27 (1.10–1.48) | 1.16 (0.98–1.37) | 1.20 (1.01–1.42) | 1.37 (1.13–1.66) | 1.18 (0.95–1.47) | 1.24 (0.99–1.56) | 1.50 (1.19–1.89) | 1.28 (0.96–1.71) | 1.20 (0.90–1.61) | 1.22 (1.00–1.48) | 0.99 (0.80–1.24) | 0.99 (0.79–1.23) | |
| PATIENTS WITH CIRRHOSIS | |||||||||||||
| No Diabetes | 1 | 1 | 1 | 1 | 1 | 1 | N/A | N/A | N/A | 1 | 1 | 1 | |
| Diabetes | 1.15 (1.04–1.26) | 1.09 (0.98–1.21) | 1.10 (0.99–1.22) | 1.05 (0.92–1.20) | 1.04 (0.90–1.20) | 1.06 (0.92–1.23) | N/A | N/A | N/A | 1.09 (0.97–1.22) | 1.04 (0.92–1.18) | 1.04 (0.92–1.18) | |
| PATIENT WITHOUT CIRRHOSIS | |||||||||||||
| No Diabetes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Diabetes | 1.54 (1.39–1.71) | 1.20 (1.06–1.36) | 1.25 (1.10–1.42) | 1.40 (1.12–1.75) | 1.23 (0.94–1.60) | 1.32 (1.01–1.72) | 1.55 (1.40–1.71) | 1.35 (1.20–1.52) | 1.31 (1.16–1.48) | 2.09 (1.67–2.62) | 1.79 (1.36–2.35) | 1.74 (1.31–2.31) | |
HR: Hazard ratio, Adj. HR: Adjusted Hazard Ratio
Adjusted for SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, HCV genotype, HIV co-infection, HBV co-infection, Charlson Comorbidity Index, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, blood hemoglobin levels
Adjusted for obesity and diabetes as well as all the characteristics listed above
Figure 1-. Forest plot of the hazard ratios for mortality and liver-related outcomes in patients with diabetes compared to patients without diabetes.
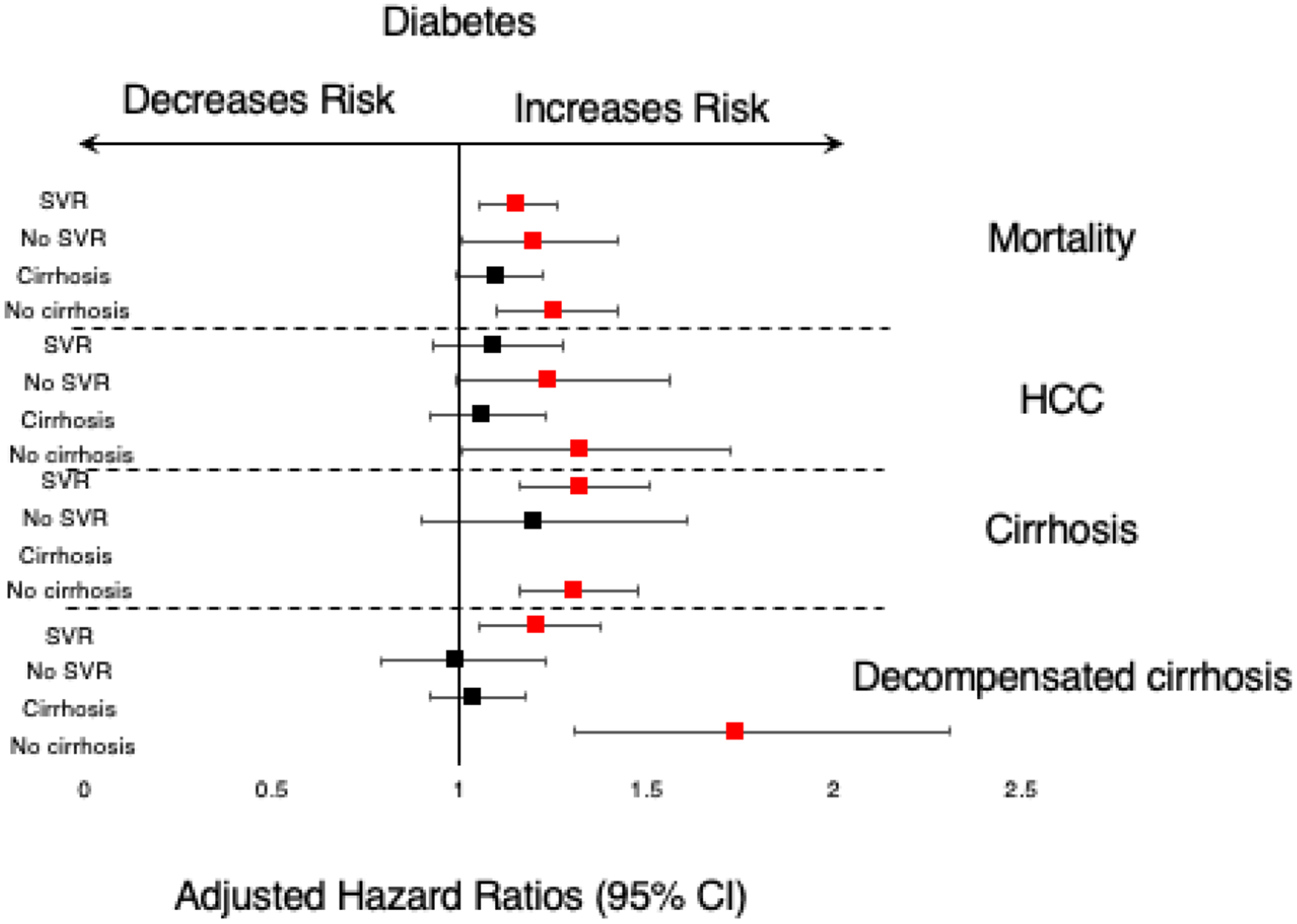
The left side of the plot demonstrates the sub-group analyses in patients with SVR, no SVR, baseline cirrhosis and without baseline cirrhosis (no cirrhosis). Outcomes are labeled on the right side of the plot.
Patients without pre-DAA cirrhosis
Among patients without baseline cirrhosis, mortality among patients with diabetes was 9.6%. Cirrhosis, any decompensated cirrhosis and HCC development was 9.6%, 2.2% and 2.0%, respectively. In the multivariable model, diabetes was significantly associated with overall mortality (AHR=1.25, 95% CI 1.10–1.42), cirrhosis (AHR=1.31, 95% CI 1.16–1.48), decompensated cirrhosis (AHR=1.74, 95% CI 1.31–2.31) and HCC development (AHR=1.32, 95% CI 1.01–1.72), compared to patients without diabetes. These findings are summarized in Table 2 and Figure 1.
We additionally assessed the associations between mortality and liver-related outcomes among patients with baseline cirrhosis and found no significant associations. Among patients who did not reach SVR, diabetes was associated with mortality (AHR=1.20, 95% CI 1.01–1.42) but not cirrhosis, decompensated cirrhosis or HCC development (Table 2, Figure 1).
Obesity: Associations with adverse liver outcomes or mortality
Patients with SVR
Among patients who achieved SVR, mortality in the overweight (BMI 25 to <30 kg/m2), obese I (BMI 30 to <35 kg/m2) and obese II-III (BMI >35 kg/m2) categories was 7.7%, 8.8% and 11.1%, respectively. Among the same groups, development of cirrhosis, any decompensated cirrhosis and HCC was 4.6%, 3.6% and 2.8% in the overweight patients and 4.6%, 3.9% and 3.2% in patients with type I obesity and 5.0%, 5.1% and 3.2% in patients with type II-III obesity, respectively.
In the multivariable model, the relationship between obesity categories and liver-related outcomes and mortality varied. For example, overweight (BMI 18.5 to <25 kg/m2) patients had a decreased risk for mortality (AHR=0.81, 95% CI 0.73–0.90) and HCC development (AHR=0.75, 95% CI 0.62–0.89) compared to normal-weight persons. The risk for morality but not HCC development was decreased in underweight patients (BMI<18.5 kg/m2) (AHR=1.62, 95% CI 1.22–2.14). The risk for mortality was also decreased for obese I (BMI 30 to <35 kg/m2) patients (AHR=0.88, 95% CI 0.78–0.99) compared to normal-weight persons (Table 3, Figure 2). Obesity type II-III (BMI > 35 kg/m2) patients also had decreased risk for HCC development (AHR=0.75, 95% CI 0.59–0.96). All BMI categories (BMI>25 kg/m2) were not significantly associated with cirrhosis or decompensated cirrhosis development.
Table 3.
Association of baseline (i.e. pre-treatment) BMI categories with mortality and liver-related outcomes by SVR (YES/NO) and presence of baseline cirrhosis (YES/NO)
| Mortality | HCC | Cirrhosis | Decompensated cirrhosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | HR | Adj. HR* | Adj. HR† | ||
| PATIENTS WITH SVR | |||||||||||||
| Underweight <18.5 | 1.72 (1.34–2.22) | 1.59 (1.20–2.10) | 1.62 (1.22–2.14) | 0.64 (0.32–1.30) | 0.66 (0.29–1.48) | 0.66 (0.29–1.50) | 0.83 (0.50–1.37) | 0.73 (0.41–1.30) | 0.76 (0.43–1.36) | 0.50 (0.23–1.05) | 0.71 (0.33–1.51) | 0.74 (0.35–1.57) | |
| Normal Weight 18.5 to<25 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overweight 25 to <30 | 0.80 (0.73–0.88) | 0.82 (0.74–0.91) | 0.81 (0.73–0.90) | 0.87 (0.74–1.04) | 0.75 (0.63–0.90) | 0.75 (0.62–0.89) | 1.13 (0.98–1.30) | 1.15 (0.99–1.34) | 1.13 (0.97–1.31) | 1.05 (0.89–1.22) | 1.03 (0.87–1.21) | 1.01 (0.86–1.20) | |
| Obese I 30 to <35 | 0.90 (0.81–1.01) | 0.90 (0.80–1.01) | 0.88 (0.78–0.99) | 1.00 (0.83–1.21) | 0.85 (0.70–1.03) | 0.84 (0.69–1.02) | 1.18 (1.01–1.38) | 1.18 (0.99–1.40) | 1.13 (0.95–1.34) | 1.13 (0.95–1.35) | 1.00 (0.83–1.20) | 0.97 (0.81–1.17) | |
| Obese II & III >35 | 1.14 (1.00–1.29) | 1.04 (0.91–1.19) | 1.01 (0.88–1.15) | 1.00 (0.79–1.26) | 0.77 (0.60–0.98) | 0.75 (0.59–0.96) | 1.41 (1.17–1.71) | 1.28 (1.04–1.57) | 1.19 (0.97–1.46) | 1.48 (1.22–1.81) | 1.07 (0.87–1.32) | 1.02 (0.83–1.26) | |
| PATIENTS WITH NO SVR | |||||||||||||
| Underweight <18.5 | 0.89 (0.44–1.81) | 0.94 (0.46–1.93) | 0.94 (0.46–1.94) | 0.85 (0.31–2.31) | 0.96 (0.35–2.63) | 0.92 (0.33–2.54) | 1.33 (0.48–3.64) | 2.10 (0.74–5.94) | 2.14 (0.76–6.06) | 0.99 (0.36–2.69) | 0.92 (0.33–2.54) | 0.92 (0.33–2.55) | |
| Normal Weight 18.5 to<25 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overweight 25 to <30 | 0.86 (0.72–1.04) | 0.83 (0.68–1.01) | 0.81 (0.66–1.00) | 1.14 (0.89–1.46) | 0.93 (0.72–1.22) | 0.91 (0.69–1.19) | 1.39 (1.02–1.90) | 1.26 (0.90–1.76) | 1.25 (0.89–1.74) | 1.14 (0.87–1.48) | 0.94 (0.71–1.26) | 0.95 (0.71–1.26) | |
| Obese I 30 to <35 | 0.82 (0.67–1.00) | 0.80 (0.64–0.99) | 0.77 (0.62–0.96) | 0.92 (0.70–1.21) | 0.73 (0.54–0.98) | 0.70 (0.52–0.95) | 1.58 (1.14–2.19) | 1.55 (1.10–2.19) | 1.51 (1.06–2.13) | 1.17 (0.89–1.55) | 0.90 (0.66–1.22) | 0.90 (0.66–1.22) | |
| Obese II & III >35 | 0.80 (0.63–1.01) | 0.74 (0.57–0.96) | 0.71 (0.55–0.93) | 0.97 (0.71–1.33) | 0.71 (0.50–1.00) | 0.67 (0.47–0.95) | 1.81 (1.25–2.62) | 1.58 (1.06–2.35) | 1.51 (1.01–2.27) | 1.65 (1.23–2.22) | 1.15 (0.83–1.58) | 1.15 (0.83–1.59) | |
| PATIENTS WITH CIRRHOSIS | |||||||||||||
| Underweight <18.5 | 1.08 (0.68–1.71) | 1.04 (0.65–1.68) | 1.05 (0.65–1.69) | 0.75 (0.35–1.60) | 0.62 (0.26–1.52) | 0.62 (0.26–1.52) | N/A | N/A | N/A | 0.53 (0.23–1.18) | 0.58 (0.26–1.32) | 0.59 (0.261.32) | |
| Normal Weight 18.5 to<25 | 1 | 1 | 1 | 1 | 1 | 1 | N/A | N/A | N/A | 1 | 1 | 1 | |
| Overweight 25 to <30 | 0.77 (0.68–0.87) | 0.80 (0.70–0.91) | 0.79 (0.70–0.91) | 0.90 (0.76–1.06) | 0.84 (0.70–1.00) | 0.83 (0.70–0.99) | N/A | N/A | N/A | 0.95 (0.82–1.10) | 0.96 (0.82–1.13) | 0.96 (0.82–1.12) | |
| Obese I 30 to <35 | 0.83 (0.72–0.95) | 0.86 (0.75–0.99) | 0.85 (0.74–0.98) | 0.96 (0.80–1.15) | 0.87 (0.71–1.05) | 0.86 (0.71–1.04) | N/A | N/A | N/A | 0.97 (0.82–1.14) | 0.93 (0.78–1.11) | 0.93 (0.78–1.11) | |
| Obese II & III >35 | 0.97 (0.84–1.13) | 0.98 (0.83–1.15) | 0.96 (0.82–1.13) | 0.87 (0.70–1.09) | 0.73 (0.58–0.92) | 0.72 (0.57–0.91) | N/A | N/A | N/A | 1.17 (0.97–1.40) | 1.01 (0.83–1.22) | 1.00 (0.82–1.21) | |
| PATIENT WITHOUT CIRRHOSIS | |||||||||||||
| Underweight <18.5 | 2.02 (1.52–2.68) | 1.71 (1.25–2.35) | 1.76 (1.29–2.41) | 0.78 (0.32–1.91) | 0.89 (0.36–2.19) | 0.92 (0.37–2.27) | 0.87 (0.56–1.37) | 0.87 (0.53–1.44) | 0.90 (0.55–1.49) | 1.28 (0.52–3.19) | 1.32 (0.52–3.31) | 1.43 (0.57–3.58) | |
| Normal Weight 18.5 to<25 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overweight 25 to <30 | 0.79 (0.70–0.90) | 0.85(0.74–0.97) | 0.83 (0.73–0.95) | 0.78 (0.61–1.01) | 0.73 (0.55–0.95) | 0.71 (0.54–0.94) | 1.15 (1.01–1.30) | 1.16 (1.01–1.33) | 1.14 (0.99–1.30) | 1.11 (0.82–1.50) | 1.17 (0.85–1.63) | 1.12 (0.81–1.56) | |
| Obese I 30 to <35 | 0.86 (0.75–0.99) | 0.91 (0.78–1.06) | 0.87 (0.75–1.01) | 0.65 (0.48–0.89) | 0.66 (0.47–0.91) | 0.63 (0.45–0.87) | 1.24 (1.08–1.43) | 1.23 (1.06–1.43) | 1.18 (1.01–1.38) | 1.15 (0.81–1.61) | 1.11 (0.76–1.61) | 1.00 (0.68–1.46) | |
| Obese II & III >35 | 1.01 (0.86–1.20) | 0.94 (0.78–1.13) | 0.88 (0.73–1.07) | 0.83 (0.57–1.21) | 0.73 (0.49–1.08) | 0.68 (0.46–1.02) | 1.50 (1.27–1.77) | 1.31 (1.10–1.57) | 1.23 (1.02–1.47) | 1.99 (1.38–2.85) | 1.58 (1.06–2.36) | 1.36 (0.90–2.04) | |
HR: Hazard Ratio, Adj. HR: Adjusted Hazard Ratio
Adjusted for SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, HCV genotype, HIV co-infection, HBV co-infection, Charlson Comorbidity Index, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, blood hemoglobin levels
Adjusted for obesity and diabetes as well as all the characteristics listed above.
Figure 2-. Forest plot of the adjusted hazard ratios for mortality and liver-related outcomes in patients with different categories of BMI.
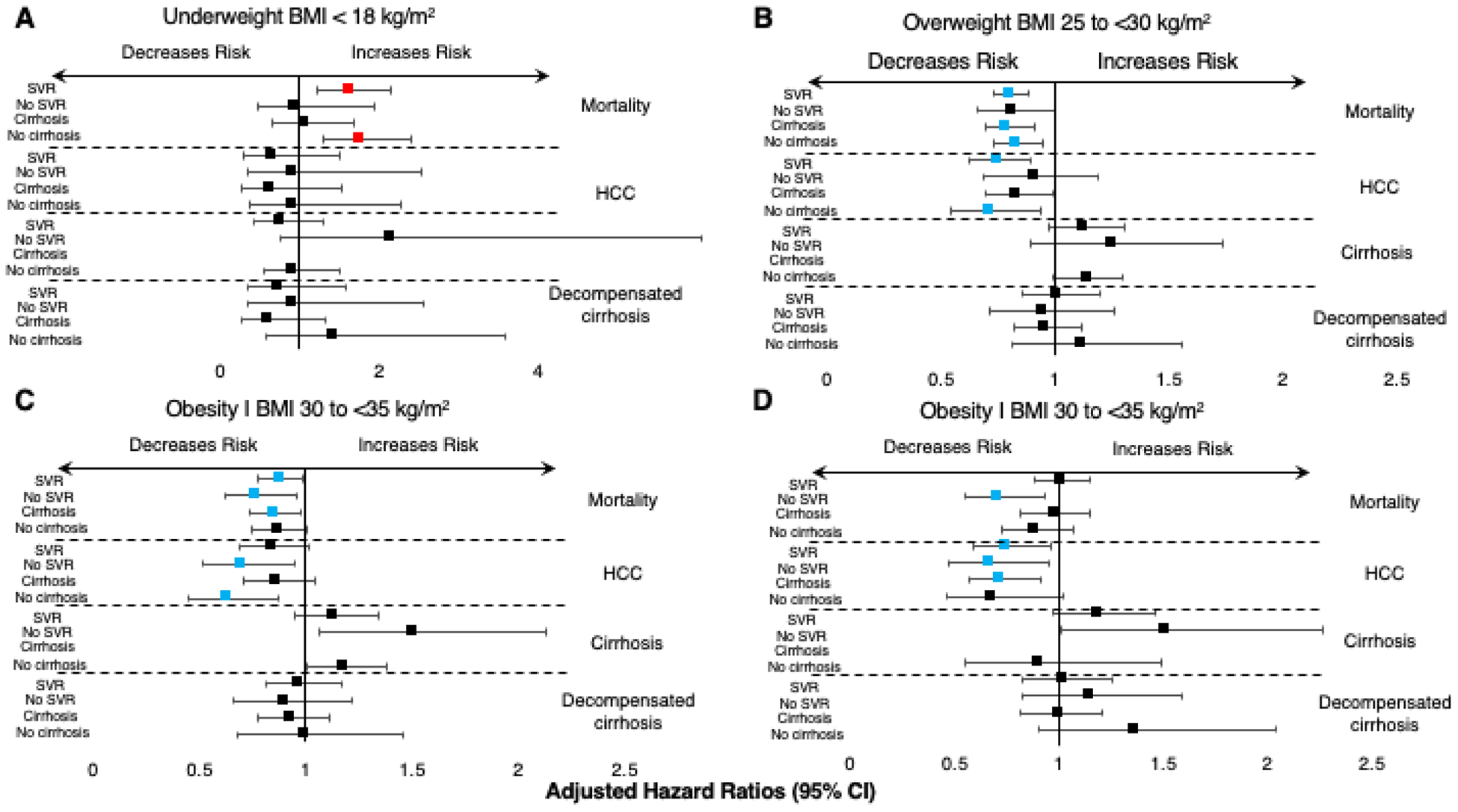
Panels A, B and C depict overweight (BMI 25–30 kg/m2), type I obesity (BMI 30–35 kg/m2) and type II-III obesity (BMI > 35 kg/m2) categories for patients (left side, top to bottom): 1. Reached SVR; 2. Did not reach SVR; 3. With baseline cirrhosis; 4. Without baseline cirrhosis, for all outcomes (labeled on the right).
Patients without pre-DAA cirrhosis
Among patients without cirrhosis at baseline, being overweight (BMI 18.5 to <25 kg/m2) was associated with overall mortality (AHR=0.83, 95% CI 0.73–0.95), compared to normal-weight persons. Underweight patients however (BMI<18.5 kg/m2), were at increased risk for mortality (AHR=1.76, 95% CI 1.29–2.41), similarly to the post SVR patients. Obesity I was inversely associated with HCC development (AHR=0.63, 95% CI 0.45–0.87). Obesity I and II-III categories were associated with increased cirrhosis (AHR=1.18, 95% CI 1.01–1.38) and decompensated cirrhosis (AHR=1.23, 95% CI 1.02–1.47), compared to normal-weight persons (Table 3).
Among patients with baseline cirrhosis, we found that overweight patients had a lower risk of overall mortality and HCC development (AHR=0.79 and AHR=0.83, respectively). Among patients who did not reach SVR, obesity I and II-III categories were associated with decreased mortality and HCC development, respectively, compared to normal-weight patients. Patients with obesity I and II had increased associations with cirrhosis development (AHR=1.51 for both) (Table 3, Figure 2).
Associations between the presence of both diabetes and obesity and adverse liver outcomes or mortality
Since diabetes and obesity are both features of the metabolic syndrome and closely linked, we sought to determine their combined effects by creating the following categories:
“no diabetes-no obesity” (the reference category)
“diabetes-no obesity” (patients with diabetes and BMI<30 kg/m2),
“obesity-no diabetes” (patients with BMI≥30 kg/m2 and no diabetes),
“obesity-diabetes” (patients with BMI≥30 kg/m2 and diabetes), and
Among patients who achieved SVR, we found that patients with diabetes alone (“diabetes-no obesity”) were at increased risk of cirrhosis (AHR=1.39, 95% CI 1.18–1.63) and decompensated cirrhosis (AHR=1.28, 95% CI 1.08–1.52) compared to patients without obesity and diabetes (“no diabetes-obesity”) (Table 4). Obesity alone (“obesity-no diabetes”) was not associated with mortality and liver-related outcomes. Patients with both diabetes and obesity (“obesity-diabetes”) were at increased risk for mortality (AHR=1.18, 95% CI 1.05–1.32) and cirrhosis development (AHR=1.41, 95% CI 1.18–1.68), compared to those without obesity or diabetes. Similarly, we found that among patients who did not have cirrhosis at baseline, diabetes alone (“diabetes-no obesity”) increased the risk of mortality (AHR=1.19, 95% CI 1.02–1.39), cirrhosis (AHR=1.39, 95% CI 1.20–1.62) and decompensated cirrhosis (AHR=2.81, 95% CI 1.55–3.06), compared to patients without obesity and diabetes (“diabetes-no obesity”). Patients with obesity alone (“obesity-no diabetes”) had an increased risk of cirrhosis development (AHR=1.16, 95% CI 1.01–1.33) but not HCC, decompensated cirrhosis or mortality. Patients with both diabetes and obesity (“obesity-diabetes”) had an increased risk of cirrhosis (AHR=1.42, 95% CI 1.22–1.67) and decompensated cirrhosis development (AHR=1.71, 95% CI 1.18–2.47) compared to those without diabetes or obesity (Table 4). We assessed for interaction terms between obesity and diabetes in their associations with any of the four outcomes in the subgroups and found no statistically significant interactions (Supplemental Table 4).
Table 4-.
Association between obesity alone, diabetes alone or both diabetes and obesity
| Mortality | HCC | Cirrhosis | Decompensated cirrhosis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | Adj. HR* | HR | Adj. HR* | HR | Adj. HR* | HR | Adj. HR* | |
| PATIENTS WITH SVR | ||||||||
| No Diabetes No Obesity** | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diabetes, No Obesity | 1.47 (1.32–1.63) | 1.12 (1.00–1.26) | 1.37 (1.14–1.64) | 1.04 (0.85–1.27) | 1.60 (1.38–1.85) | 1.39 (1.18–1.63) | 1.90 (1.62–2.21) | 1.28 (1.08–1.52) |
| Obesity BMI≥30, No Diabetes | 1.02 (0.92–1.14) | 1.03 (0.92–1.15) | 1.04 (0.87–1.25) | 0.95 (0.78–1.14) | 1.13 (0.98–1.31) | 1.10 (0.94–1.28) | 1.25 (1.07–1.47) | 1.05 (0.88–1.25) |
| Diabetes AND obesity | 1.53 (1.37–1.70) | 1.18 (1.05–1.32) | 1.44 (1.19–1.73) | 1.07 (0.87–1.31) | 1.60 (1.37–1.87) | 1.41 (1.18–1.68) | 1.84 (1.56–2.17) | 1.17 (0.98–1.40) |
| PATIENTS WITHOUT SVR | ||||||||
| No Diabetes No Obesity | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diabetes, No Obesity | 1.38 (1.14–1.68) | 1.17 (0.94–1.45) | 1.42 (1.10–1.82) | 1.21 (0.91–1.60) | 1.68 (1.23–2.31) | 1.40 (0.95–2.04) | 1.49 (1.15–1.94) | 1.03 (0.77–1.39) |
| Obesity BMI≥30, No Diabetes | 0.90 (0.74–1.09) | 0.84 (0.68–1.03) | 0.81 (0.63–1.06) | 0.72 (0.55–0.95) | 1.44 (1.08–1.90) | 1.44 (1.07–1.94) | 1.46 (1.15–1.85) | 1.07 (0.83–1.38) |
| Diabetes AND obesity | 1.09 (0.89–1.33) | 1.02 (0.82–1.27) | 1.16 (0.90–1.51) | 0.91 (0.68–1.23) | 1.72 (1.26–2.35) | 1.50 (1.04–2.16) | 1.32 (1.01–1.73) | 1.01 (0.75–1.36) |
| PATIENTS WITH CIRRHOSIS | ||||||||
| No Diabetes No Obesity | 1 | 1 | 1 | 1 | N/A | N/A | 1 | 1 |
| Diabetes, No Obesity | 1.18 (1.04–1.34) | 1.08 (0.94–1.24) | 1.00 (0.84–1.19) | 1.01 (0.83–1.21) | N/A | N/A | 1.16 (1.00–1.35) | 1.05 (0.90–1.24) |
| Obesity BMI≥30, No Diabetes | 1.04 (0.92–1.19) | 1.02 (0.89–1.17) | 0.94 (0.80–1.12) | 0.88 (0.73–1.05) | N/A | N/A | 1.15 (0.99–1.33) | 1.00 (0.85–1.17) |
| Diabetes AND obesity | 1.15 (1.01–1.31) | 1.12 (0.97–1.29) | 1.07 (0.90–1.27) | 0.98 (0.81–1.18) | N/A | N/A | 1.13 (0.96–1.32) | 1.03 (0.87–1.21) |
| PATIENTS WITHOUT CIRRHOSIS | ||||||||
| No Diabetes No Obesity | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diabetes, No Obesity | 1.46 (1.28–1.67) | 1.19 (1.02–1.39) | 1.42 (1.07–1.88) | 1.28 (0.93–1.77) | 1.62 (1.41–1.85) | 1.39 (1.20–1.62) | 2.33 (1.73–3.14) | 2.18 (1.55–3.06) |
| Obesity BMI≥30, No Diabetes | 0.88 (0.77–1.01) | 0.94 (0.81–1.09) | 0.74 (0.55–1.00) | 0.78 (0.57–1.08) | 1.21 (1.06–1.37) | 1.16 (1.01–1.33) | 1.35 (0.99–1.83) | 1.31 (0.94–1.84) |
| Diabetes AND obesity | 1.52 (1.32–1.75) | 1.17 (1.00–1.37) | 1.16 (0.84–1.60) | 1.01 (0.71–1.45) | 1.66 (1.44–1.91) | 1.42 (1.22–1.67) | 2.25 (1.65–3.08) | 1.71 (1.18–2.47) |
HR: Hazard ratio, Adj. HR: Adjusted Hazard Ratio
Adjusted for SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, HCV genotype, HIV co-infection, HBV co-infection, Charlson Comorbidity Index, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, blood hemoglobin levels
Obesity defined as BMI ≥ 30 kg/m2
Discussion
HCV eradication reduces, but does not eliminate the risk of cirrhosis, cirrhosis decompensation, HCC and mortality [3,5,18]. Determining factors associated with progressive liver disease after HCV treatment has important clinical implications, including the need for continued post-treatment monitoring for cirrhosis development and complications of cirrhosis and HCC. This large, national cohort study of US Veterans with prolonged follow up after HCV treatment had several important findings that could inform post-DAA clinical care.
First, diabetes significantly increased the risk of mortality, cirrhosis and decompensated cirrhosis even in patients who achieved SVR. Diabetes was also associated with an increased risk of HCC in patients without cirrhosis at baseline and in those without HCV SVR. Second, compared to normal weight persons, overweight and obese persons had a lower risk of mortality and HCC, in contrast to our a priori hypotheses. Third, obesity was associated with a higher risk of development of cirrhosis. Overall, these findings support the continued close monitoring and screening for liver disease progression of HCV DAA-treated patients, particularly those with diabetes at baseline.
Diabetes increases risk of mortality and liver-related outcomes
Our results show that diabetes at the time of DAA treatment for HCV presents an important diagnosis to consider as it was associated with significant increased risks for poor outcomes. These include increased risk of mortality, cirrhosis and decompensation and HCC in those without SVR or baseline cirrhosis, as well as overall mortality in those who achieved SVR. These findings have key clinical implications in the DAA era and suggest that patients with baseline diabetes constitute a population at risk for liver-related complications and should be monitored closely for the development of worsening fibrosis or cirrhosis, liver-related complications and HCC. These patients may therefore benefit from continued care of liver-subspecialists after treatment with DAAs.
Corroborating our findings, a smaller study by Hung et al. showed that diabetes was a significant risk factor for overall morality, liver-related mortality and the development of HCC in patients treated with interferon-based therapy without baseline cirrhosis (at a median follow up of 4.4 years) [19]. Similarly, Kanwal et al. also showed that in a Veteran population with virological cure of chronic HCV, diabetic patients had twice the risk of developing HCC (AHR=2.13, 95% CI 1.11–4.12), compared to those without diabetes [20].
The precise mechanism underlying this association remains unclear. For instance, diabetes is a known risk factor for cardiovascular disease, which could contribute substantially to overall mortality. Furthermore, diabetes among patients with HCV could predispose to underlying NAFLD, which drives an increased risk of worsening fibrosis, liver-related mortality and HCC. We were unable to determine cause of death and etiology of new cirrhosis diagnoses as part of this study, precluding assessment of these questions. However, we did find that if patients had cirrhosis at the start of therapy, having a diagnosis of diabetes did not impact their risk of mortality and liver-related outcomes, suggesting that cirrhosis is a stronger driver for complications compared to diabetes alone.
Others have suggested that DAA-induced HCV cure could improve insulin resistance, which may mitigate the effect of baseline diabetes on liver-related complications.[21] While we did not aim to measure insulin resistance changes with DAA-based HCV therapy, our study suggests that any improvement in insulin resistance from SVR with DAAs may not be sufficient to alter clinical outcomes, at least in the follow up time period we evaluated. As has been suggested by Garcia-Compean et al., it is conceivable that once insulin resistance has been initiated by HCV, it activates a cascade of diabetogenic mechanisms that are independent of the presence or absence of the virus [22]. Longer follow up times are needed to determine if these effects persist or resolve with time.
Obesity is associated with higher risk of cirrhosis but lower risk of HCC and overall mortality
We observed that patients with obesity had a decreased risk of mortality and HCC development after DAA treatment. There are several potential explanations for these findings. First, patients with a high BMI (considered obese by our definition) may represent a group without sarcopenia. Given that patients with sarcopenia (potentially captured by the “normal weight” reference group in our study) have poor liver-related outcomes [23], it would not be surprising to see that patients with a higher weight would have less adverse outcomes, and could potentially be “healthier”. This is consistent with the “underweight” category having increased risk of overall mortality, who may represent a group of patients with severe sarcopenia. Interestingly, the underweight category patients did not have an increased risk of HCC, suggesting that the increased mortality association is independent of HCC development. Interestingly, the underweight category patients did not have an increased risk of HCC, suggesting that the increased mortality association is independent of HCC.
Second, it could be that more severe liver disease or occult HCC could be leading to weight loss, thus driving the increased complications and mortality in the “normal weight” group. However, we are less concerned about the possibility of reverse causality, particularly due to advanced liver disease, given the adjustment for baseline cirrhosis, decompensation and MELD labs in our multivariable models. Third, confounding factors including increased use of statins or other chemopreventive agents in the obese group, could lower the risk of HCC and death in these patients [24].
Although BMI is widely used as a surrogate for obesity, it may not reflect metabolic health accurately [25], especially in the cirrhosis patient population where sarcopenia can occur with normal weight [26]. Measurement of sarcopenia, muscle mass and distribution of adipose tissue (visceral/intra-abdominal versus subcutaneous) would be needed to further ascertain this association, however this is not commonly measured in clinical practice and could therefore not be assessed as part of this study [27]. Future work to distinguish between visceral/intra-abdominal versus subcutaneous adipose tissue could help us further understand the risk of obesity in patients with cirrhosis. We therefore conclude that although obesity by standard definitions is important to understand in context of liver disease progression, it remains complex and needing further characterizations.
Limitations
While our study is strengthened by its large, national cohort of HCV patients with prolonged post-treatment follow-up, it has some limitations. First, our findings may not be generalizable to women as the majority of VA patients are male. Second, no liver histology or radiographic data were assessed to determine what percent of patients with obesity and diabetes have underlying NAFLD and/or NASH as a contributing factor. Third, given the retrospective nature of the study, residual confounders of the association between exposure and outcome may be present.
Implications and future directions
Our study has important clinical implications as it represents the largest HCV DAA-treated cohort of patients with detailed metabolic features and long-term outcomes. We found that patients cured of chronic HCV with diabetes should be followed closely for the development of any adverse outcomes. Many patients undergoing HCV treatment do not have cirrhosis at baseline and it remains unclear who deserves close monitoring and/or continued follow-up in hepatology clinic. These data suggest that patients with baseline diabetes deserve vigilant monitoring given their increased risk for new diagnosis of cirrhosis and development of liver-related complications. The specific means and frequency of follow-up in these patients remains unclear, particularly given that many non-invasive tools to monitor steatosis/fibrosis have not been validated in HCV patients who have achieved SVR [28]. Future research could assess the clinical value and cost-effectiveness of various means and frequency of post-SVR monitoring among patients with diabetes.
Supplementary Material
Acknowledgement
We would like to thank Dr. Kristin Wyatt for her statistical support and critical review of the manuscript.
Grant support
This study was funded in part by NIH/NCI grant number R01CA196692 and VA CSR&D grant number I01CX001156 to Dr. George N. Ioannou
JBN- AASLD and NIH NIDDK P30 DK 41301
JRP- NIH NIDDK P30DK41301 and Department of Veterans Affairs BX004560-01
FS- NIH T32 DK007742-2
AMM – NIH T32 DK007634
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
References
- 1.Fattovich G, Pantalena M, Zagni I et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients Am J Gastroenterol. 2002;97:2886–2895. [DOI] [PubMed] [Google Scholar]
- 2.Ioannou GN, Beste LA, Chang MF et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System Gastroenterology. 2016;151:457–471 e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma J Hepatol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]; Nahon P, Bourcier V, Layese R et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications Gastroenterology. 2017;152:142–156 e142. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou GN, Beste LA, Green PK et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients with Baseline Cirrhosis or High FIB-4 Scores Gastroenterology. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]; Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma J Hepatol. 2018;68:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapper EB, Parikh ND, Green PK et al. Reduced Incidence of Hepatic Encephalopathy and Higher Odds of Resolution Associated With Eradication of HCV Infection Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals World J Gastroenterol. 2018;24:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldt BJ, Chen W, Heathcote EJ et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus Hepatology. 2008;47:1856–1862; [DOI] [PubMed] [Google Scholar]; Ohki T, Tateishi R, Sato T et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients Clin Gastroenterol Hepatol. 2008;6:459–464; [DOI] [PubMed] [Google Scholar]; Huang YW, Yang SS, Fu SC et al. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study Hepatology. 2014;60:807–814. [DOI] [PubMed] [Google Scholar]
- 8.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome Lancet. 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 9.Loomba R, Sanyal AJ. The global NAFLD epidemic Nat Rev Gastroenterol Hepatol. 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Lindor KD. Nonalcoholic fatty liver disease Ann Epidemiol. 2007;17:863–869; [DOI] [PubMed] [Google Scholar]; Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease Hepatology. 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353–358. [DOI] [PubMed] [Google Scholar]
- 12.Fihn SD, Francis J, Clancy C et al. Insights from advanced analytics at the Veterans Health Administration Health Aff (Millwood). 2014;33:1203–1211. [DOI] [PubMed] [Google Scholar]
- 13.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data Diabetes Care. 2004;27 Suppl 2:B10–21; [DOI] [PubMed] [Google Scholar]; Hum J, Jou JH, Green PK et al. Improvement in Glycemic Control of Type 2 Diabetes After Successful Treatment of Hepatitis C Virus Diabetes Care. 2017;40:1173–1180. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation J Chronic Dis. 1987;40:373–383; [DOI] [PubMed] [Google Scholar]; Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data Medical care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M et al. A model to predict survival in patients with end-stage liver disease Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 16.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test Arch Intern Med. 1998;158:1789–1795; [DOI] [PubMed] [Google Scholar]; Bradley KA, Bush KR, Epler AJ et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population Arch Intern Med. 2003;163:821–829; [DOI] [PubMed] [Google Scholar]; Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care Alcohol Clin Exp Res. 2007;31:1208–1217. [DOI] [PubMed] [Google Scholar]
- 17.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications Clin Gastroenterol Hepatol. 2018;16:1677–1678; [DOI] [PubMed] [Google Scholar]; Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database Pharmacoepidemiol Drug Saf. 2012;21:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Li AA, Gadiparthi C et al. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016 Gastroenterology. 2018;155:1154–1163 e1153; [DOI] [PMC free article] [PubMed] [Google Scholar]; Ioannou GN, Feld JJ. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology. 2019;156:446–460 e442; [DOI] [PubMed] [Google Scholar]; Moon AM, Green PK, Rockey DC, Berry K, Ioannou GN. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding Aliment Pharmacol Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CH, Lee CM, Wang JH et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy Int J Cancer. 2011;128:2344–2352. [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents Gastroenterology. 2017;153:996–1005 e1001. [DOI] [PubMed] [Google Scholar]
- 21.Dong TS, Aby ES, Benhammou JN et al. Metabolic syndrome does not affect sustained virologic response of direct-acting antivirals while hepatitis C clearance improves hemoglobin A1c World J Hepatol. 2018;10:612–621; [DOI] [PMC free article] [PubMed] [Google Scholar]; Russo FP, Zanetto A, Gambato M et al. Hepatitis C virus eradication with direct acting antiviral improves insulin resistance J Viral Hepat. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management World J Gastroenterol. 2009;15:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis World J Gastroenterol. 2014;20:8061–8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thrift AP, Natarajan Y, Liu Y, El-Serag HB. Statin Use After Diagnosis of Hepatocellular Carcinoma Is Associated With Decreased Mortality Clin Gastroenterol Hepatol. 2019;17:2117–2125 e2113; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaplan DE, Serper MA, Mehta R et al. Effects of Hypercholesterolemia and Statin Exposure on Survival in a Large National Cohort of Patients With Cirrhosis Gastroenterology. 2019;156:1693–1706 e1612; [DOI] [PubMed] [Google Scholar]; Simon TG, Ma Y, Ludvigsson JF et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma JAMA Oncol. 2018;4:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannou GN, Weiss NS, Boyko EJ et al. Is central obesity associated with cirrhosis-related death or hospitalization? A population-based, cohort study Clin Gastroenterol Hepatol. 2005;3:67–74. [DOI] [PubMed] [Google Scholar]
- 26.Montano-Loza AJ, Meza-Junco J, Prado CM et al. Muscle wasting is associated with mortality in patients with cirrhosis Clin Gastroenterol Hepatol. 2012;10:166–173, 173 e161 [DOI] [PubMed] [Google Scholar]
- 27.Tchkonia T, Thomou T, Zhu Y et al. Mechanisms and metabolic implications of regional differences among fat depots Cell Metab. 2013;17:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anstee QM, Lawitz EJ, Alkhouri N et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


