Abstract
The recent outbreak of coronavirus disease 2019 (COVID-19) has gained considerable attention worldwide due to its increased potential to spread and infect the general population. COVID-19 primarily targets the human respiratory epithelium but also has neuro-invasive potential. Indeed, neuropsychiatric manifestations, such as fatigue, febrile seizures, psychiatric symptoms, and delirium, are consistently observed in COVID-19. The neurobiological basis of neuropsychiatric COVID-19 symptoms is not fully understood. However, previous evidence about systemic viral infections pointed to an ongoing neuroinflammatory response to viral antigens and proinflammatory mediators/immune cells from the periphery. Microglia cells mediate the overproduction of inflammatory cytokines, free radicals, and damage signals, culminating with neurotoxic consequences. Semi-synthetic second-generation tetracyclines, including minocycline (MINO) and doxycycline (DOXY), are safe bacteriostatic agents that have remarkable neuroprotective and anti-inflammatory properties. Promising results have been obtained in clinical trials using tetracyclines for major psychiatric disorders, such as schizophrenia and major depression. Tetracyclines can inhibit microglial reactivity and neuroinflammation by inhibiting nuclear factor kappa B (NF-kB) signaling, cyclooxygenase 2, and matrix metalloproteinases (MMPs). This drug class also has a broad profile of activity against bacteria associated with community-based pneumonia, including atypical agents. COVID-19 patients are susceptible to secondary bacterial infections, especially those on invasive ventilation. Therefore, we suggest tetracyclines’ repurposing as a potential treatment for COVID-19 neuropsychiatric manifestations. These drugs can represent a valuable multi-modal treatment for COVID-19-associated neuroinflammatory alterations based on their broad antimicrobial profile and neuroinflammation control.
Keywords: COVID-19, SARS-CoV-2 infection, Drug repurposing, Minocycline, Doxycycline, Neuroinflammation
Dear Editor,
Evidence points to the emergence of neurological and neuropsychiatric symptoms in the course of coronavirus disease 2019 (COVID-19) (Ellul et al. 2020). Most patients experience mild symptoms, such as ageusia and anosmia, early in the infection, while severely ill patients can suffer from more dramatic symptoms, such as altered consciousness, psychotic symptoms, and mood disturbances (Rogers et al. 2020; Kong et al. 2020; Helms et al. 2020). Some putative routes for neuroinvasion by coronavirus are olfactory–hematogenous route, trans-neuronal route (by using the trans-neuronal retrograde machinery), and lymphatic pathway (Iroegbu et al. 2020, for a review on this topic). Despite the evidence for the presence of the virus in the brain, some neurological and neuropsychiatric symptoms are associated with the virus-induced dysregulated immune response and “cytokine storm,” which refers to an increase of cytokine levels in a short-term period, leading to an exaggerated harmful immune response, is a hallmark of this disease (van Vuren et al. 2021).
Based on the emergency of COVID-19, there are no drug therapies developed exclusively for this infection. The antiviral remdesivir was approved for the treatment of COVID-19 in hospitalized adult and pediatric patients since it reduced patients’ recovery time (Beigel et al. 2020). Additionally, dexamethasone use resulted in lower mortality among patients hospitalized with COVID-19, receiving either invasive mechanical ventilation or oxygen alone at randomization (2020).
One crucial strategy to compensate for the absence of an effective treatment for a disease reducing cost and development time is repurposing an existing and approved drug (Ekins et al. 2020). In the context of drug repurposing, the second-generation tetracyclines, minocycline (MINO), and doxycycline (DOXY), despite being prescribed for their broad-spectrum bacteriostatic action, also present anti-inflammatory, neuroprotective, and even anti-viral effects, mainly against central nervous system (CNS) disorders (Lemaître et al. 1990; Lu et al. 2017; Balducci et al. 2019). Furthermore, these tetracyclines are semi-synthetic products with a good oral absorption profile, higher lipophilicity, a longer half-life of elimination, and suitability for intravenous administration when compared to other tetracyclines (Bastos et al. 2012). This work intends to provide a rationale for the design of clinical trials and experimental studies to explore the effects of tetracyclines for the prevention and treatment of neurological and neuropsychiatric outcomes of this potentially fatal infection that also leaves persistent sequelae.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a considerable potential to cause neuropsychiatric manifestations (Li et al. 2020). In this regard, Mao et al. 2020 reported that about 36% of SARS-CoV-2 infected patients experienced neuropsychiatric symptoms in the course of acute illness. Patients with moderate symptoms of COVID-19 commonly present headaches and less commonly may also experience dysphoria and disturbed attention associated with delirium presentation (Mao et al. 2020). On the other hand, seriously ill patients may present more severe neurologic manifestations, such as loss of consciousness, member paralysis, paresthesia/hypoesthesia, and coma (Chaudhry and Duggal 2014). These patients usually suffer from severe hypoxemia and invasive mechanical ventilation, which are associated with worse neurocognitive outcomes after recovery (Mizuguchi et al. 2007; Sasannejad et al. 2019). Regarding psychiatric symptoms, in acute illness, confusion and disturbed attention were the most prevalent symptoms, indicating delirium as the major neuropsychiatric syndrome in this stage (Ellul et al. 2020; Helms et al. 2020; Mao et al. 2020). Depression, anxiety, insomnia, emotional lability, and irritability were also found (Cheng et al. 2004; Ellul et al. 2020; Kong et al. 2020; Mao et al. 2020).
The neuro-invasive potential seems to be a common feature of coronaviruses (CoVs) (Arbour et al. 2000), leading us to infer that SARS-CoV-2 has, theoretically, a similar neuropathogenic potential. Indeed, both SARS-CoV-2 and SARS-CoV bind to the angiotensin-converting enzyme (ACE) 2 receptor to enter human cells (Bergmann et al. 2006). This receptor is expressed on neuronal and non-neuronal cells of the olfactory nerve and epithelium and likely facilitate SARS-CoV-2 brain infection through the anterograde axonal transport (Xu and Lazartigues 2020). SARS-CoV-2 can reach the brain if the virus first invades high ACE2 expressing cells in the olfactory epithelium and then passes to low ACE2 expressing mature neuronal cells to be transported along olfactory axons to the brain (Yachou et al. 2020).
Viruses can also infect endothelial cells of the blood–brain barrier (BBB), disrupting their junctions to access brain parenchyma (Spindler and Hsu 2012; Al-Obaidi et al. 2018). Once the virus invades the CNS, it is promptly recognized, through pattern recognition receptors, mainly the toll-like receptors (TLRs), by resident microglial cells, therefore inducing inflammatory mechanisms (Olson et al. 2001). This microglia activation leads to the expression of a variety of proinflammatory cytokines, chemokines, free radicals, and activated proteases, such as matrix metalloproteinases (MMPs), which together orchestrate the inflammatory response in the CNS, the so-called neuroinflammation (Woo et al. 2008; Bordt and Polster 2014; Hu et al. 2014).
Importantly, CoV neurovirulence can induce proinflammatory cytokines expression, for instance, interleukin (IL)-12, tumor necrosis factor (TNF)-α, IL-6, IL-15, and IL-1β (Li et al. 2004). This exaggerated immune response can also culminate (and even predict) with meningitis, encephalitis, meningoencephalitis, or death (Vázquez et al. 2012; Grandgirard et al. 2013). Similarly, SARS-CoV-2 infection is associated with an increased level of inflammatory mediators including cytokines and chemokines such as IL-2, IL-7, IL-10, TNF, granulocyte colony-stimulating factor (G-CSF), monocyte chemoattractant protein-1 (MCP1; also known as CCL2), macrophage inflammatory protein 1 alpha (MIP1α; also known as CCL3), CXC-chemokine ligand 10 (CXCL10), C-reactive protein, ferritin, and D-dimers (Tay et al. 2020, for a review on this topic). These proinflammatory alterations were detected in high levels in patients with severe COVID-19 (Qin et al. 2020; Yang et al. 2020). Vis-a-vis, systemic inflammation also compromises BBB function by increasing its permeability and contributing to the penetration of peripheral immune cells and cytokines into the brain (Varatharaj and Galea 2017). Contrary to the view that the brain is an immune-privileged site, brain parenchyma displays an intrinsic antiviral network. The microglia secreted antiviral cytokines, such as type I interferons (IFNs), play a central role (Drokhlyansky et al. 2017). However, not only microglia, but several neuroectodermal-derived cells, including neurons, astrocytes, and oligodendrocytes, express TLRs and reacts with IFNs to mediate the protective response against neurotrophic viruses (Farina et al. 2005; Ménager et al. 2009) (Fig. 1).
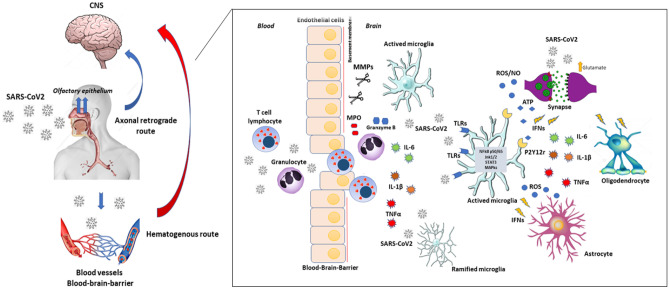
Fig. 1.
SARS-CoV-2 neuro-invasion routes, interaction with brain cells and potential underlying mechanisms for neuroinflammation. SARS-CoV-2 assesses the CNS through the hematogenous or neuronal retrograde routes. SARS-CoV-2 can infect nerve endings, such as the olfactory epithelium, and using axonal machinery, reach central structures through their interaction with host proteins such as ACE2 and TMPRSS2. The virus can also infect circulating immune cells, such as lymphocytes and macrophages, and be present in circulating serum. It can infect endothelial cells of BBB, disrupting its junctions and access brain parenchyma. Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ACE2: angiotensin I converting enzyme 2; TMPRSS2: protein transmembrane serine protease 2; BBB: blood–brain barrier. After reaching the CNS, the virus is rapidly recognized by resident microglia, which reacts to it through TLRs binding, inducing a marked antiviral pro-inflammatory response. The activation of signaling pathways, such as NF-kB, JNK1/2, and MAPkinases, orchestrates this response. It culminates with the secretion of pleiotropic pro-inflammatory cytokines (TNFα, IL-1β, and IL-6), IFNs, ROS, and nitric oxide (NO). These signals are recognized by other brain cells, such as neurons, astrocytes, and oligodendrocytes, propagating the anti-viral response and causing degenerative and pro-apoptotic effects. Activated microglia also upregulates MMPs expression, which can digest tight junctions and basement membrane proteins, contributing to BBB leakage. With BBB disruption, peripheral immune cells, such as activated T lymphocytes and granulocytes, can access brain parenchyma, also contributing to neuroinflammation and neurodegenerative changes, secreting ROS, granzymes, and pro-oxidant enzymes, such as MPO. Abbreviations: TLRs: Toll-like receptors; NFkB: nuclear factor-k beta; JNK1/2: c-Jun N-terminal kinase 1/2; MAPkinases: mitogen-activated protein kinase; TNFα: tumor necrosis factor-α, IL: interleukin; IFNs: interferons; ROS: MMPs: matrix metalloproteinases; MPO: myeloperoxidase; BBB: blood–brain barrier
Notably, inflammation and oxidative stress are underlying mechanisms of several neuropsychiatric and neurological disorders (Morris et al. 2019). Proinflammatory cytokines and some neuroactive byproducts of inflammation, such as nitric oxide (NO) and tryptophan catabolites, can mediate an increase in potent free radicals and therefore promote an apoptotic stimulus (Rath and Aggarwal 1999; Braidy et al. 2009). Thus, through inhibition of inflammation and its mediators, tetracyclines can attenuate apoptosis and oxidative stress in CNS (Scholz et al. 2015).
Accordingly, tetracyclines present marked anti-inflammatory and microglia suppressant effects that can prevent or reverse “cytokine storm” associated with SARS-CoV-2 infection. These anti-inflammatory and immunomodulatory effects seem to be independent of their antimicrobial activities (Domercq and Matute 2004) but dependent on the dose (Di Caprio et al. 2015). More specifically, MINO and DOXY are highly potent MMPs inhibitors through their strong interaction with Zn in these enzymes' catalytic site (Tilakaratne and Soory 2014). In this context, a previous study showed that murine CoVs depend on host MMPs for cell fusion and viral spread, including neuro-invasion (Phillips et al. 2017). Furthermore, MMPs, mainly MMP-9, are essential effectors for lung remodeling and destruction of the extracellular matrix, leading to damage of the endothelial basal lamina involved in adult respiratory distress syndrome (ARDS) (Chakrabarti and Patel 2005; Ong et al. 2015).
In the context of tetracyclines’ anti-inflammatory effects, elevated serum levels of IL-6 are detected in patients with severe COVID-19, implying abnormal inflammation and cytokine storm as an effective mechanism for COVID-19 lethality (Baig et al. 2020; Effenberger et al. 2020). Besides the already mentioned effects of tetracyclines on the CNS, these drugs, mainly DOXY, can reduce systemic pro-inflammatory cytokines levels in patients suffering from viral infections. In this regard, a randomized clinical trial reported a marked reduction in the mortality rate of Dengue patients treated with DOXY compared with standard therapy. Decreased IL-6 and TNFα levels were underlying mechanisms of this protective effect (Fredeking et al. 2015).
Also, tetracyclines are useful in the treatment of virus-associated encephalitis and encephalopathy. For example, MINO presents therapeutic benefits against neuro-adapted Sind-bis virus infection of mice (Darman et al. 2004), reovirus infection of mice (Richardson-Burns and Tyler 2005), simian immunodeficiency virus (SIV) infection of pigtailed macaques (Zink et al. 2005) and rhesus macaques (Ratai et al. 2010). Experimental studies showed that DOXY and MINO could induce zinc-finger antiviral protein (ZAP) expression in several mammalian cells lines and inhibit the replication of several RNA virus, such as Dengue, Ebola, HIV, Zika, and Influenza A viruses (Zhu et al. 2011; Rothan et al. 2014; Malek et al. 2020). Moreover, DOXY can directly interact with Dengue and Chikungunya (CHIKV) viral proteases, reducing virus replication and antigen detection (Yang et al. 2007; Rothan et al. 2015). Finally, a recent study based on virtual screening identified DOXY as a potential inhibitory ligand to SARS-CoV-2 PLpro. The papain-like protease (PLpro, also known as NSP3) protein of CoV plays an essential function in viral replication and host immune response modulation (Báez-Santos et al. 2015). The antiviral effects of these drugs expand their benefits against COVID-19 (Wu et al. 2020).
To date, there are ten ongoing randomized clinical trials (RCTs) registered in ClinicalTrials.gov to evaluate DOXY safety for COVID-19 treatment or prevention (mainly focused on pulmonary complications and prevention of cytokine storm). At least two of these trials were designed to evaluate the effects of DOXY oral administration in patients with confirmed COVID-19, aiming to reduce the cytokine storm and, therefore, the evolution towards a severe form of the disease (ClinicalTrials.gov Identifier: NCT04371952 and NCT04433078). Some others study the effects of DOXY in combination, for example, with ivermectin and the dietary supplements zinc, vitamins D3, and C (ClinicalTrials.gov Identifier: NCT04482686). One RCT assessed MINO (100 mg/day) efficacy for mild COVID-19 (Iranian Registry of Clinical trials—IRCT20081019001369N4).
In conclusion, based on the current knowledge about COVID-19, drugs that combine antiviral and anti-inflammatory effects and have a favorable side effects profile should be the most promising strategies (Wu et al. 2020). In this context, second-generation tetracyclines such as MINO and DOXY are not only inexpensive and widely available drugs with a safe tolerability profile but significantly fit in this profile of effects (Malek et al. 2020). Hence, second-generation tetracyclines may represent an attractive therapeutic option for COVID-19 since these drugs: i) have a potential direct anti-viral effect and inhibit viral replication; ii) alleviate the severe viral-induced systemic inflammatory response and sepsis, therefore protecting CNS cells against the harmful viral effects.
Acknowledgments
The authors thank the Brazilian Institutions CAPES, FAPEG, FUNCAP, and CNPq that partially supported this study.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Highlights
- Neuropsychiatric symptoms were reported during COVID-19.
- Coronaviruses have neuroinvasive potential and induce a potent neuroinflammatory response.
- Tetracyclines can counteract neuroinflammation caused by neurotrophic viruses.
- Tetracyclines interact with viral proteins presenting antiviral effects.
- Tetracyclines represent a potential treatment for COVID-19 neuropsychiatric symptoms.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adriano José Maia Chaves Filho, Email: adrianoafilho@hotmail.com.
Danielle S. Macedo, Email: danielle.macedo@ufc.br
References
- Al-Obaidi JMM, Bahadoran A, Wang SM, et al. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018;62:16–27. doi: 10.4149/av_2018_102. [DOI] [PubMed] [Google Scholar]
- Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by Human Respiratory Coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Santos YM, St. John SE, Mesecar AD, The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 Virus Targeting the CNS : Tissue Distribution , Host − Virus Interaction , and Proposed Neurotropic Mechanisms. 0–3. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed]
- Balducci C, Forloni G, Mancuso C, Klyubin I. Doxycycline for Alzheimer ’ s Disease : Fighting β -Amyloid Oligomers and Neuroinflammation. 2019;10:1–7. doi: 10.3389/fphar.2019.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos LFS, de Oliveira ACP, Watkins LR, et al. Tetracyclines and pain. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:225–241. doi: 10.1007/s00210-012-0727-1. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: Host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordt EA, Polster BM. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A Bipartisan affair? Free Radic Biol Med. 2014;76:34–46. doi: 10.1016/j.freeradbiomed.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Grant R, Adams S, et al. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- Chaudhry N, Duggal AK. Sepsis Associated Encephalopathy Adv Med. 2014;2014:1–16. doi: 10.1155/2014/762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SKW, Tsang JSK, Ku KH, et al. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: A series of 10 cases. Br J Psychiatry. 2004;184:359–360. doi: 10.1192/bjp.184.4.359. [DOI] [PubMed] [Google Scholar]
- Darman J, Backovic S, Dike S, et al. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J Neurosci. 2004;24:7566–7575. doi: 10.1523/JNEUROSCI.2002-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caprio R, Lembo S, Di Costanzo L, et al. Anti-Inflammatory Properties of Low and High Doxycycline Doses: An In Vitro Study. Mediators Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25:609–612. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Drokhlyansky E, Aytürk DG, Soh TK, et al. The brain parenchyma has a type I interferon response that can limit virus spread. Proc Natl Acad Sci USA. 2017;114:E95–E104. doi: 10.1073/pnas.1618157114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberger M, Grander C, Grabherr F, et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Mottin M, Ramos PRPS, et al. Déjà vu: Stimulating open drug discovery for SARS-CoV-2. Drug Discov Today. 2020;25:928–941. doi: 10.1016/j.drudis.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, et al. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Fredeking T, Zavala-Castro J, Gonzalez-Martinez P, et al. Dengue Patients Treated with Doxycycline Showed Lower Mortality Associated to a Reduction in IL-6 and TNF Levels. Recent Pat Antiinfect Drug Discov. 2015;10:51–58. doi: 10.2174/1574891x10666150410153839. [DOI] [PubMed] [Google Scholar]
- Grandgirard D, Gäumann R, Coulibaly B, et al. The causative pathogen determines the inflammatory profile in cerebrospinal fluid and outcome in patients with bacterial meningitis. Mediators Inflamm. 2013 doi: 10.1155/2013/312476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Yu F, Gong P, et al. Subneurotoxic copper(II)-induced NF-κB-dependent microglial activation is associated with mitochondrial ROS. Toxicol Appl Pharmacol. 2014;276:95–103. doi: 10.1016/j.taap.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Iroegbu JD, Ifenatuoha CW, Ijomone OM. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol Sci. 2020;41:1329–1337. doi: 10.1007/s10072-020-04469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Zheng K, Tang M, et al (2020) Prevalence and Factors Associated with Depression and Anxiety of Hospitalized Patients with COVID-19. medRxiv 2020.03.24.20043075. doi: 10.1101/2020.03.24.20043075
- Lemaître M, Guétard D, Hénin Y, et al. Protective activity of tetracycline analogs against the cytopathic effect of the human immunodeficiency viruses in CEM cells. Res Virol. 1990;141:5–16. doi: 10.1016/0923-2516(90)90052-K. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus Neurovirulence Correlates with the Ability of the Virus To Induce Proinflammatory Cytokine Signals from Astrocytes and Microglia. J Virol. 2004;78:3398–3406. doi: 10.1128/jvi.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xiao G, Luo W. Minocycline suppresses NLRP3 inflammasome activation in experimental ischemic stroke. NeuroImmunoModulation. 2017;23:230–238. doi: 10.1159/000452172. [DOI] [PubMed] [Google Scholar]
- Malek AE, Granwehr BP, Kontoyiannis DP. IDCases Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21:e00864. doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménager P, Roux P, Mégret F, et al. Toll-Like Receptor 3 (TLR3) Plays a Major Role in the Formation of Rabies Virus Negri Bodies. PLoS Pathog. 2009;5:e1000315. doi: 10.1371/journal.ppat.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand. 2007;115:45–56. doi: 10.1111/j.1600-0404.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- Morris G, Puri BK, Walker AJ, et al. Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev. 2019;107:862–882. doi: 10.1016/j.neubiorev.2019.09.025. [DOI] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct Activation of Innate and Antigen-Presenting Functions of Microglia following Infection with Theiler’s Virus. J Virol. 2001;75:9780–9789. doi: 10.1128/jvi.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CWM, Elkington PT, Brilha S, et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLOS Pathog. 2015;11:e1004917. doi: 10.1371/JOURNAL.PPAT.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, Gallagher T, Weiss SR (2017) Neurovirulent Murine Coronavirus JHM.SD Uses Cellular Zinc Metalloproteases for Virus Entry and Cell-Cell Fusion. J Virol. doi: 10.1128/jvi.01564-16 [DOI] [PMC free article] [PubMed]
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan. China: Clin Infect Dis; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratai EM, Bombardier JP, Joo CG, et al. Proton Magnetic Resonance Spectroscopy Reveals Neuroprotection by Oral Minocycline in a Nonhuman Primate Model of Accelerated NeuroAIDS. PLoS ONE. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/A:1020546615229. [DOI] [PubMed] [Google Scholar]
- Richardson-Burns SM, Tyler KL. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp Neurol. 2005;192:331–339. doi: 10.1016/j.expneurol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan HA, Bahrani H, Mohamed Z, et al. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS ONE. 2015 doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan HA, Mohamed Z, Paydar M, et al. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014 doi: 10.1007/s00705-013-1880-7. [DOI] [PubMed] [Google Scholar]
- Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23:352. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Sobotka M, Caramoy A, et al. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. 2015;12:209. doi: 10.1186/s12974-015-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler KR, Hsu TH. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012;20:282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne A, Soory M. Anti-inflammatory Actions of Adjunctive Tetracyclines and Other Agents in Periodontitis and Associated Comorbidities. Open Dent J. 2014;8:109–124. doi: 10.2174/1874210601408010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuren van EJ, Steyn SF, Brink CB et al. (2021) The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment Biomed Pharmacother 111200. 10.1016/j.biopha.2020.111200 [DOI] [PMC free article] [PubMed]
- Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Vázquez JA, Adducci MDC, Coll C, et al. Acute meningitis prognosis using cerebrospinal fluid interleukin-6 levels. J Emerg Med. 2012;43:322–327. doi: 10.1016/j.jemermed.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Woo MS, Park JS, Choi IY, et al. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Xu E Lazartigues 2020 Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus Cell Mol Neurobiol 1 10.1007/s10571-020-00915-1 [DOI] [PMC free article] [PubMed]
- Y Yachou A Idrissi El V Belapasov S Ait Benali 2020 Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients Neurol Sci 1 10.1007/s10072-020-04575-3 [DOI] [PMC free article] [PubMed]
- Yang JM, Chen YF, Tu YY, et al. Combinatorial computational approaches to identify tetracycline derivatives as flavivirus inhibitors. PLoS ONE. 2007 doi: 10.1371/journal.pone.0000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Chen G, Lv F, et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. J Am Med Assoc. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- (2020) Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed]