Abstract
Anaphylaxis is a life-threatening allergic reaction caused by cross-linking of high-affinity IgE antibodies on the surface of mast cells and basophils. Understanding the cellular mechanisms that lead to high-affinity IgE production is required to develop better therapeutics for preventing this severe reaction. A recently discovered population of “Tfh13” cells regulates the production of high-affinity IgE in mouse models of allergy and can also be found in allergic patients with IgE antibodies against food or aeroallergens. Here we describe optimized protocols for identifying Tfh13 cells in both mice and humans.
Keywords: T follicular helper cells, Tfh13 cells, intracellular cytokine staining, IL-4, IL-5, IL-13, allergy, IgE
1. Introduction
Cross-linking of high-affinity IgE on the surface of mast cells and basophils leads to the release of chemical mediators that precipitate anaphylaxis, a potentially life-threatening allergic reaction1. The increasing incidence of anaphylaxis-related hospital admissions signals an urgent need to understand the mechanisms underlying anaphylaxis and to develop better therapeutics to prevent this severe reaction2,3. The induction of IgE relies on T follicular helper (Tfh) cells, a specialized subset of CD4+ T cells that provides help to B cells in the germinal center, rather than Th2 cells4–6. Tfh cells instruct the antibody isotypes produced during a given immune response through the cytokines they secrete7. While Tfh cell-derived interleukin-4 (IL-4) is required for IgE production, it is not sufficient to induce the high-affinity, anaphylactic IgE antibodies present in allergic conditions8,9. IL-13 is required as well, which is produced by a recently identified “Tfh13” cell population9. Tfh13 cells regulate the induction of anaphylactic IgE in mouse models of allergy and are also found in patients allergic to food or aeroallergens. In addition to expressing the canonical Tfh cell markers PD-1, CXCR5, and transcription factor BCL-6, Tfh13 cells are distinguished by their co-production of the cytokines IL-4, IL-5, and IL-13 and expression of Th2 transcription factor GATA3.
The identification of Tfh13 cells by flow cytometry relies on optimized stimulation and staining conditions. Traditionally, CXCR5 has been difficult to stain on Tfh cells after the stimulation required to induce ex vivo cytokine production10. We addressed this issue by performing CXCR5 staining post-fixation with a particular antibody clone. Additionally, the increased concentration of Ca2+ in Iscove’s Modified Dulbecco’s Media (IMDM) as compared to RPMI media has been shown to enhance cytokine production by phorbol 12-myristate 13-acetate (PMA)/ionomycin-restimulated T helper cells11. While this has previously been shown to enhance production of IL-17A, IFN-γ, TNF-α, IL-10, and IL-2211, we also found IMDM to boost IL-4 and IL-13 production by Tfh13 cells, allowing for clearer identification of this rare population. Furthermore, BCL-6 is ideally stained after fixation and permeabilization with the eBioscience™ Foxp3 / Transcription Factor Staining Buffer Set; however, this method results in poor resolution of cytokine producing cells12. Therefore, our optimized Tfh13 cell staining protocol employs the BD Biosciences Fixation/Permeabilization Solution Kit to preserve cytokine staining, and overnight staining of cytokines and BCL-6 improve detection of these targets without compromising specificity. We also describe protocols for identifying Tfh13 cells by more physiologic methods of stimulation: anti-CD3/CD28 and antigen stimulation.
Analysis of peripheral blood provides the least invasive method of studying the human immune system. Although Tfh cells are predominantly located in the secondary lymphoid organs, circulating Tfh cells can be identified in the blood13,14. Circulating Tfh13 cells can either be identified using non-specific activation with PMA/ionomycin or specific activation with allergen9,15. While the latter has the advantage of resolving numbers of antigen-specific Tfh13 cells, this is only possible when the antigen is known. Protocols for both approaches are described below. Given that Tfh13 cells are a rare population in the blood, we found that isolation of CD4+ T cells from PBMCs before PMA/ionomycin activation improved their detection.
2. Identification of polyclonal and antigen-specific murine Tfh13 cells
Murine Tfh13 cells can be identified at multiple sites following the administration of relevant allergen. For instance, intranasal administration of aeroallergen Alternaria alternata induces Tfh13 cells in the mediastinal lymph nodes, while intragastric administration of food allergen peanut with cholera toxin leads to Tfh13 cell induction in mesenteric lymph nodes9. Co-administration of allergen and the model antigen 4-hydroxy-3-nitrophenylacetyl conjugated to ovalbumin (NP-OVA) allows for measurement of high-affinity NP-specific IgE antibodies as a complementary readout to Tfh13 cell induction. Polyclonal Tfh13 cells can be identified by stimulation with PMA/ionomycin or anti-CD3/CD28, while antigen-specific Tfh13 cells can be identified by NP-OVA stimulation. When performing antigen stimulation, staining for the activation marker CD154 (CD40 ligand) helps enrich for Tfh13 cells during analysis.
2A. Materials
Equipment and consumables
Feeding tubes for oral gavage
1-ml syringes
5-ml round bottom polystyrene tubes
100-μm nylon mesh filter
60-mm × 15-mm petri dishes
Frosted microscope slides
Hemocytometer
96-well round bottom TC-treated plate
Reagents
- Allergens
- Alternaria alternata extract (Greer Laboratories, Lot 322776)
- 4-hydroxy-3-nitrophenylacetyl conjugated to ovalbumin (NP-OVA) (LGC, Biosearch Technologies)
- Peanut (Western Mixers Produce & Nuts)
- Cholera toxin (List Biologicals, Lots 10165A1 & 10167A2)
- Anesthesia
- Methoxyflurane
- 30% Isoflurane
Sodium bicarbonate (AmericanBio)
Milli-Q Ultrapure water
Phosphate buffered saline (PBS) (Gibco)
Heat-inactivated fetal bovine serum (FBS) (Sigma)
Ethylenediaminetetraacetic acid (EDTA) solution, pH 8.0 (AmericanBio)
RBC Lysis Buffer (10x) (BioLegend)
Distilled water
Trypan Blue 0.4% (Gibco)
Iscove’s Modified Dulbecco’s Medium (IMDM) (Gibco)
Penicillin-streptomycin (Gibco)
L-glutamine (Gibco)
Sodium pyruvate (Gibco)
2-Mercaptoethanol (Gibco)
Phorbol 12-myristate 13-acetate (PMA) (Sigma)
Ionomycin (Sigma)
Ultra-LEAF™ Purified anti-mouse CD3ε Antibody (Clone 145–2C11, BioLegend)
Ultra-LEAF™ Purified anti-mouse CD28 Antibody (Clone 37.51, BioLegend)
Fixation/Permeabilization Solution Kit with BD GolgiPlug™ (BD Biosciences)
Mouse BD Fc Block™ (BD Biosciences)
LIVE/DEAD™ Fixable Aqua Dead Cell Stain (Life Technologies)
Table 1.
Antibody panel for polyclonal murine Tfh13 cells, without IL-5
| Step | Antigen (Clone) | Fluorophore | Dilution* | Source |
|---|---|---|---|---|
| Surface | LIVE/DEAD™ Fixable Aqua Dead Cell Stain | (AmCyan channel) | 1:1000 (1:500) | Life Technologies |
| PD-1 (RMP1–30) | Alexa Fluor® 647 | 1:200 (1:100) | BioLegend | |
| Post-fixation 1° | CD4 (RM4–5) | APC-Fire™ 750 | 1:400 | BioLegend |
| CD44 (IM7) | Brilliant Violet 605™ | 1:600 | BioLegend | |
| TCRβ (H57–597) | PerCP/Cyanine5.5 | 1:300 | BioLegend | |
| IL-4 (11B11) | PE | 1:100 | BioLegend | |
| IL-13 (eBio13A) | PE-Cyanine7 | 1:100 | eBioscience | |
| BCL-6 (K112–91) | FITC | 1:50 | BD Biosciences | |
| CXCR5 (L138D7) | Biotin | 1:300 | BioLegend | |
| Post-fixation 2° | Streptavidin | BD Horizon™ BV421 | 1:400 | BD Biosciences |
All dilutions, except those in parentheses, represent final concentrations for staining. In the steps that require serial dilution of the antibodies, the dilutions for preparing the antibody cocktail are listed in parentheses.
Table 3.
Antibody panel for antigen-specific murine Tfh13 cells
| Step | Antigen (Clone) | Fluorophore | Dilution* | Source |
|---|---|---|---|---|
| Surface | LIVE/DEAD™ Fixable Aqua Dead Cell Stain | (AmCyan channel) | 1:1000 (1:500) | Life Technologies |
| PD-1 (RMP1–30) | Alexa Fluor® 647 | 1:200 (1:100) | BioLegend | |
| Post-fixation 1° | CD4 (RM4–5) | APC-Fire™ 750 | 1:400 | BioLegend |
| CD44 (IM7) | Brilliant Violet 605™ | 1:600 | BioLegend | |
| TCRβ (H57–597) | PerCP/Cyanine5.5 | 1:300 | BioLegend | |
| IL-4 (11B11) | Brilliant Violet 421 ™ | 1:100 | BioLegend | |
| IL-13 (eBio13A) | PE | 1:100 | eBioscience | |
| CD154 (MR1) | PE-Cyanine7 | 1:100 | BioLegend | |
| CXCR5 (L138D7) | Biotin | 1:300 | BioLegend | |
| Post-fixation 2° | Streptavidin | FITC | 1:400 | BD Biosciences |
All dilutions, except those in parentheses, represent final concentrations for staining. In the steps that require serial dilution of the antibodies, the dilutions for preparing the antibody cocktail are listed in parentheses.
Reagent Setup
0.2 M sodium bicarbonate: Prepare a solution of 8.4 g sodium bicarbonate in 500 ml Milli-Q Ultrapure water.
FACS buffer: Prepare a solution of 2% (vol/vol) FBS and 1 mM EDTA in PBS.
RBC Lysis Buffer (1x): Dilute RBC Lysis Buffer (10x) 1:10 in distilled H2O.
Complete IMDM: Supplement IMDM with 10% (vol/vol) heat-inactivated FBS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 55 μM 2-mercaptoethanol.
BD Perm/Wash™ buffer (1x): Dilute BD Perm/Wash™ buffer (10x) 1:10 in distilled H2O.
2B. Methods
2B.1. Immunization and Lymph Node Collection (see Note 1)
- Immunize mice with allergen (e.g. intranasal Alternaria alternata/NP-OVA, intragastric peanut/cholera toxin)9.
- Intranasal immunization: For each mouse, prepare 10 μg Alternaria alternata extract and 25 μg NP-OVA in 50 μl PBS. Anesthetize mice with methoxyflurane using the open-drop method. Once the mice are under anesthesia and their breathing is slow and steady, administer 50 μl of antigen diluted in PBS through the nares using a pipette.
- Intragastric immunization: For each mouse, prepare 5 mg ground peanut and 10 μg cholera toxin in 200 μl 0.2 M sodium bicarbonate. Anesthetize mice with 30% isoflurane using the open-drop method. Once the mice are under anesthesia, gently gavage 200 μl of antigen diluted in 0.2 M sodium bicarbonate using a feeding tube connected to a 1-ml syringe.
Seven or eight days later, harvest draining lymph nodes (LN) (e.g. mediastinal LN for intranasal immunization, mesenteric LN for intragastric immunization). Also harvest a non-draining LN (e.g. inguinal LN) as a staining control.
2B.2. Cell Preparation
For each LN, prepare a 5-ml round bottom polystyrene tube covered with 100-μm nylon mesh filter.
In a 60-mm × 15-mm petri dish containing 1 ml FACS buffer, grind LN between two frosted microscope slides.
Rinse microscope slides and petri dish with additional 3 ml FACS buffer, and filter cell suspension into polystyrene tube.
Centrifuge the tubes for 5 min at 500 × g.
Decant the supernatant. If LN cell preparation contains red blood cells, resuspend the pellet in 500 μl 1X RBC lysis buffer. Otherwise, proceed to Step 7.
Incubate the cells in RBC lysis buffer for 1 min. Neutralize with 2 ml FACS buffer and centrifuge for 5 min at 500 × g.
Decant the supernatant and resuspend cells in FACS buffer (400 μl for mediastinal LN and inguinal LN, 1200 μl for mesenteric LN).
To count cells, take 10 μl of cells and dilute 1:10 with PBS. Then mix 10 μl of diluted cells with 10 μl of Trypan Blue (1:2). Count cells using a hemocytometer and light microscope.
Adjust the concentration of cells to 16×106 cells/ml in FACS buffer.
2B.3. Stimulation (see Notes 2 and 3)
Using 100-μm nylon mesh, filter 250 μl (4×106 cells) of each sample into a well of a 96-well round bottom plate. Leave adjacent wells empty to prevent cross-contamination of samples.
Centrifuge the plate for 3 min at 600 × g. Decant the supernatant.
- Perform one of the following methods of stimulation:
- PMA/ionomycin
- Resuspend cells in 90 μl complete IMDM (cIMDM) containing PMA (50 ng/ml) and ionomycin (1 μg/ml). Incubate the cells at 37°C with 5% CO2.
- After 1 hr, add to each well 10 μl cIMDM containing BD GolgiPlug™ (1:100), to achieve a final concentration of 1:1000 BD GolgiPlug™. Mix samples thoroughly and incubate for additional 3 hr (total stimulation time 4 hr).
- Anti-CD3/CD28
- Resuspend cells in 90 μl cIMDM containing soluble anti-CD3ε (1 μg/ml) and anti-CD28 (2 μg/ml). Incubate the cells at 37°C with 5% CO2.
- After 2 hr, add to each well 10 μl cIMDM containing BD GolgiPlug™ (1:100), to achieve a final concentration of 1:1000 BD GolgiPlug™. Mix samples thoroughly and incubate for additional 4 hr (total stimulation time 6 hr).
- Antigen
- Resuspend cells in 90 μl cIMDM containing antigen (20 μg/ml). Incubate the cells at 37°C with 5% CO2.
- After 2 hr, add to each well 10 μl cIMDM containing BD GolgiPlug™ (1:100), to achieve a final concentration of 1:1000 BD GolgiPlug™. Mix samples thoroughly and incubate for additional 10 hr (total stimulation time 12 hr).
Wash cells by adding 200 μl cold FACS buffer to each well and centrifuging plate for 3 min at 600 × g. Decant supernatant and repeat washing step. Decant supernatant.
2B.4. Surface Staining
Resuspend cells in 25 μl diluted Fc block (1:200 in cold FACS buffer). Incubate for 5 min at 4°C.
Dilute the surface antibodies in 25 μl cold FACS buffer/well at the concentration listed in Table 1 or 2 (for PMA/ionomycin- or anti-CD3/CD8-stimulated cells) or Table 3 (for antigen-stimulated cells).
Add 25 μl diluted surface antibodies mix to each well on top of the existing 25 μl diluted Fc block. Incubate for 25 min at 4°C in the dark.
Wash cells with 200 μl cold FACS buffer and centrifuge for 3 min at 600 × g. Decant supernatant and repeat washing step. Decant supernatant.
Table 2.
Antibody panel for polyclonal murine Tfh13 cells, with IL-5
| Step | Antigen (Clone) | Fluorophore | Dilution* | Source |
|---|---|---|---|---|
| Surface | LIVE/DEAD™ Fixable Aqua Dead Cell Stain | (AmCyan channel) | 1:1000 (1:500) | Life Technologies |
| PD-1 (29F.1A12) | APC-Fire™ 750 | 1:200 (1:100) | BioLegend | |
| Post-fixation 1° | CD4 (RM4–5) | PE/Dazzle™ 594 | 1:400 | BioLegend |
| CD44 (IM7) | Brilliant Violet 605™ | 1:600 | BioLegend | |
| TCRβ (H57–597) | PerCP/Cyanine5.5 | 1:300 | BioLegend | |
| IL-4 (11B11) | PE | 1:100 | BioLegend | |
| IL-5 (TRFK5) | APC | 1:150 | BioLegend | |
| IL-13 (eBio13A) | PE-Cyanine7 | 1:100 | eBioscience | |
| BCL-6 (K112–91) | FITC | 1:50 | BD Biosciences | |
| CXCR5 (L138D7) | Biotin | 1:300 | BioLegend | |
| Post-fixation 2° | Streptavidin | BD Horizon™ BV421 | 1:400 | BD Biosciences |
All dilutions, except those in parentheses, represent final concentrations for staining. In the steps that require serial dilution of the antibodies, the dilutions for preparing the antibody cocktail are listed in parentheses.
2B.5. Fixation and Permeabilization
Resuspend cells in 70 μl cold Fixation/Permeabilization solution. Incubate for 20 min at 4°C in the dark.
Wash cells with 200 μl cold 1X Perm/Wash buffer and centrifuge for 3 min at 900 × g. Decant supernatant and repeat washing step. Decant supernatant.
2B.6. Post-fixation and Intracellular Staining (see Notes 4–8)
Resuspend cells in 50 μl cold 1X Perm/Wash buffer containing post-fixation primary (1°) antibodies at the concentration listed in Table 1 or 2 (for PMA/ionomycin- or anti- CD3/CD8-stimulated cells) or Table 3 (for antigen-stimulated cells). Incubate overnight (<16 hr) at 4°C in the dark.
The next morning, wash cells with 200 μl cold 1X Perm/Wash buffer and centrifuge for 3 min at 900 × g. Decant supernatant and repeat washing step. Decant supernatant.
Resuspend cells in 50 μl cold 1X Perm/Wash buffer containing streptavidin fluorochrome conjugate (post-fixation 2°) at the concentration listed in Table 1 or 2 (for PMA/ionomycin- or anti-CD3/CD8-stimulated cells) or Table 3 (for antigen-stimulated cells). Incubate for 20 min on ice in the dark.
Wash cells with 200 μl cold 1X Perm/Wash buffer and centrifuge for 3 min at 900 × g. Decant supernatant and repeat washing step with 200 μl cold FACS buffer. Decant supernatant.
Resuspend samples in 200 μl cold FACS buffer. Keep on ice in the dark prior to flow cytometric analysis. Perform flow cytometric analysis within 2 hr.
Gating strategy is shown in Figures 1A–B and 2.
Figure 1. Identification of polyclonal murine Tfh13 cells by PMA/ionomycin stimulation.
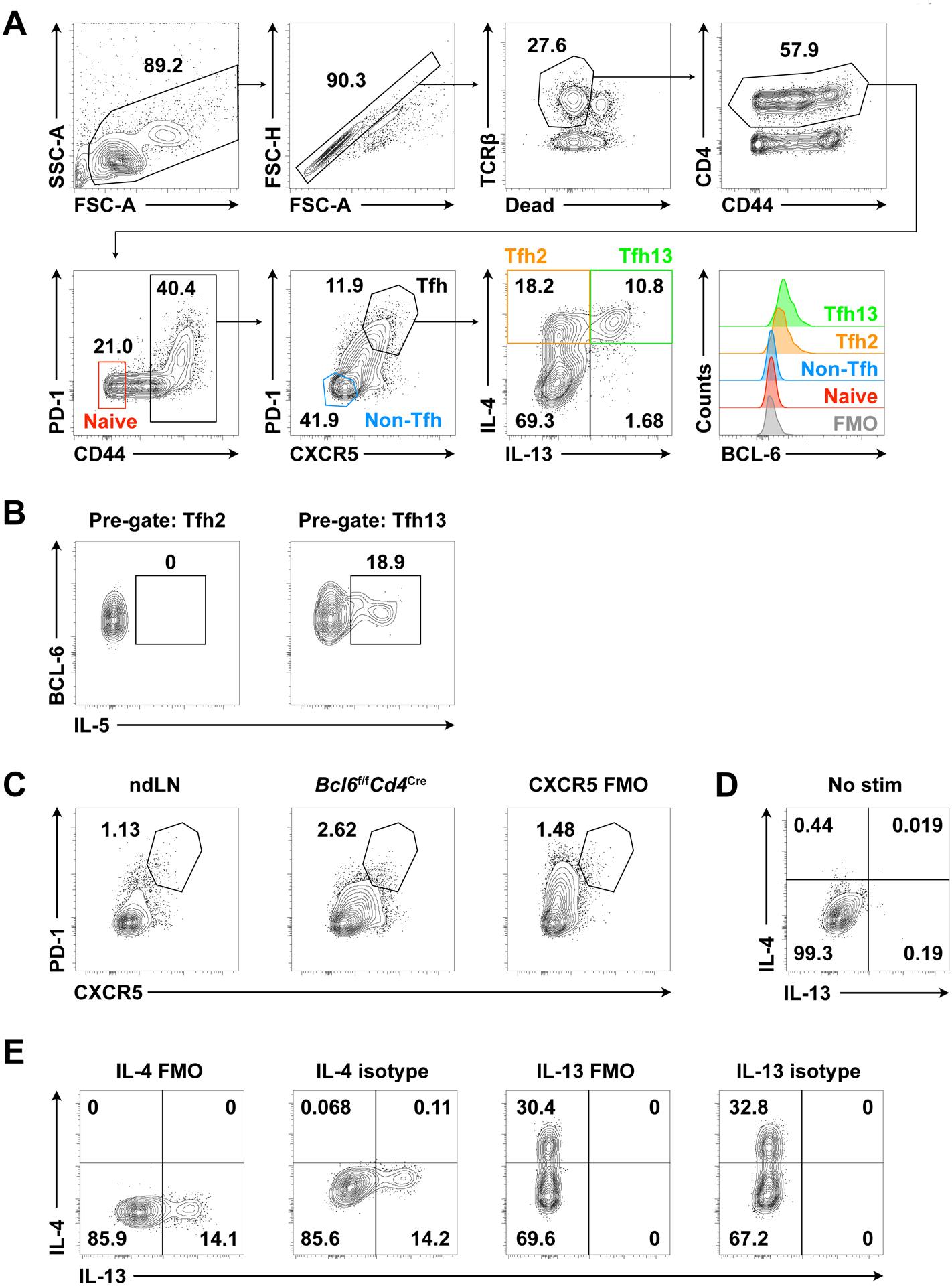
Day 7 mediastinal lymph node (medLN) cells from intranasal (i.n.) immunization with Alternaria alternata and NP-OVA followed by in vitro PMA/ionomycin stimulation. (A) Gating strategy for murine Tfh13 cells. Histogram overlay of BCL-6 expression by naïve CD4+ T cells, non-Tfh cells, Tfh2 cells, Tfh13 cells, and fluorescence minus one (FMO) control. (B) IL-5 production by Tfh2 vs Tfh13 cells. (C) PD-1 and CXCR5 staining in non-immunized non-draining lymph node (ndLN), immunized Tfh cell-deficient medLN (Bcl6f/fCd4Cre), and FMO control for CXCR5 in immunized WT medLN (CXCR5 FMO). (D) IL-4 and IL-13 intracellular staining in immunized WT medLN without stimulation. Pre-gated on Tfh cells (live CD4+ TCRβ+ CD44+ PD-1+ CXCR5+). (E) IL-4 and IL-13 FMO and isotype control staining in immunized WT medLN with stimulation. Pre-gated on Tfh cells. Samples were acquired on a BD LSR II (BD Biosciences).
Figure 2. Identification of polyclonal murine Tfh13 cells by anti-CD3/CD28 stimulation and antigen-specific Tfh13 cells by NP-OVA stimulation.
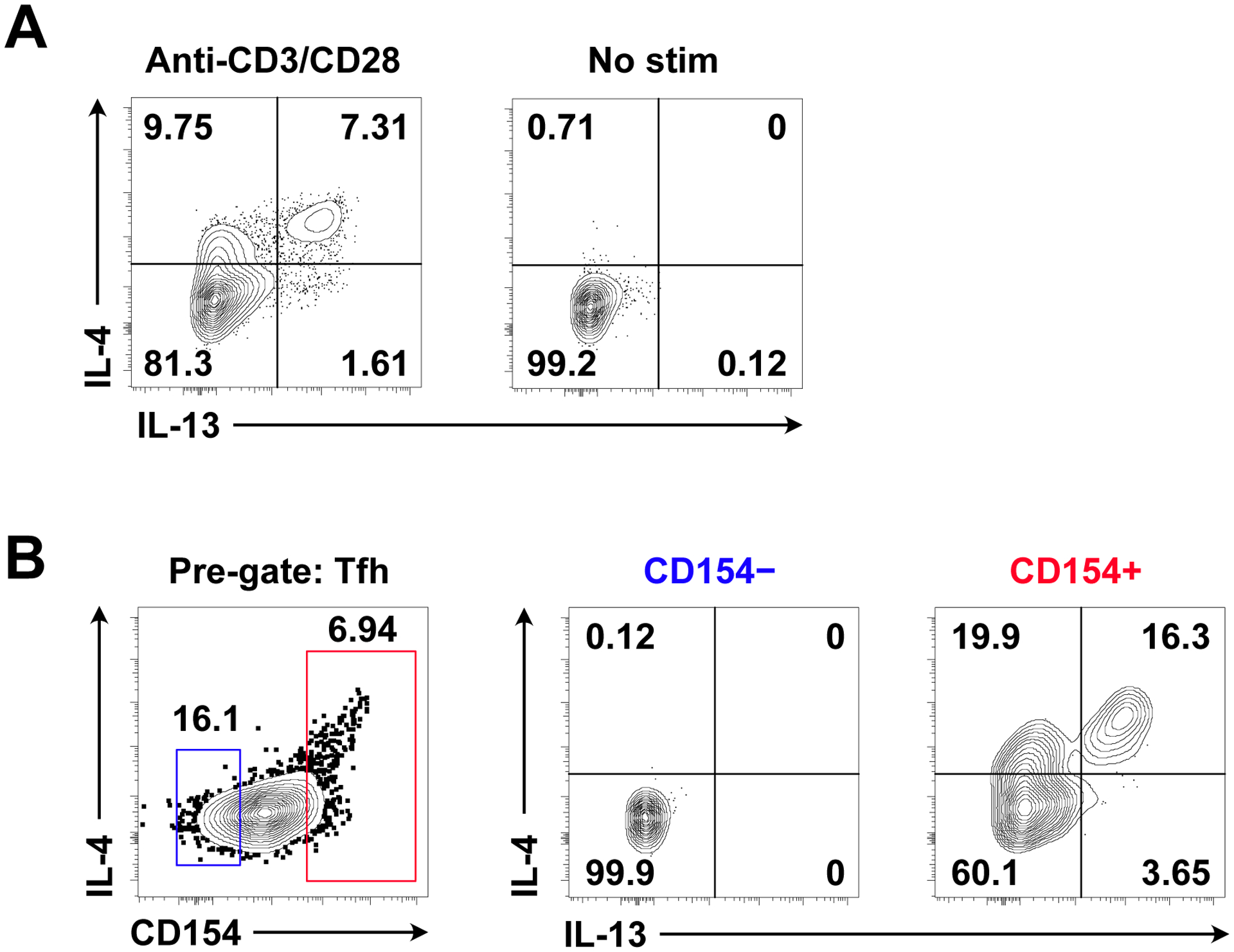
Day 7 medLN from i.n. immunization with Alternaria alternata and NP-OVA following (A) anti-CD3/CD28 or (B) NP-OVA stimulation. (A) IL-4 and IL-13 intracellular staining in anti-CD3/CD28-stimulated or non-stimulated cells. Pre-gated on Tfh cells. (B) IL-4 and IL-13 intracellular staining in CD154− versus CD154+ NP-OVA-stimulated cells. Pre-gated on Tfh cells. CD154 helps enrich for antigen-specific Tfh13 cells during analysis. Samples were acquired on a CytoFLEX (Beckman Coulter).
2C. Notes
Readers should use a method of anesthesia that is approved by their local Institutional Animal Care and Use Committee (IACUC). An alternative to using the open-drop method is to use a precision vaporizer.
Though the current protocol only describes cytokine staining for IL-4, IL-5, and IL-13, these stimulation conditions are also compatible with staining for other Tfh cell cytokines, such as IL-21, IL-17A, and IFN-γ.
Stimulation in IMDM rather than RPMI media improves detection of IL-4 and IL-13, allowing for enhanced identification of Tfh13 cells (Figure 3).
Note the particular antibody clone used for CXCR5 staining (L138D7) during this step. While clone 2G8 is more frequently used in the literature for CXCR5 staining in unstimulated cells, resolution is poor with stimulated cells.
The recommended clone for CD4 staining post-fixation is RM4–5. The other commonly used clone GK1.5 does not stain well after fixing cells. If using GK1.5, then include it in the surface staining panel.
An ideal cytokine staining control is from a parallel sample (i.e. treated in an identical manner) but genetically deficient in a particular cytokine (i.e., from an IL-13 knockout mouse). However, this is often not readily available, and so acceptable alternative controls for Tfh13 staining include cells from a non-immunized/non-draining lymph node or cells from an immunized Tfh cell-deficient mouse line (e.g. Cd4CreBcl6fl/fl) to help with Tfh cell gating (Figure 1C). Fluorescence minus one (FMO) and isotype control antibody staining as well as unstimulated cells from an immunized lymph node help with cytokine gating (Figure 1D–E).
Overnight staining of cytokines and BCL-6 improves detection of these targets without compromising specificity (Figures 1A and 1E).
For secondary staining, do not exceed 20 min of staining to prevent overstaining of CXCR5 and loss of resolution. Staining on ice further helps to reduce overstaining.
Figure 3. Enhanced cytokine production with stimulation in IMDM compared to RPMI.
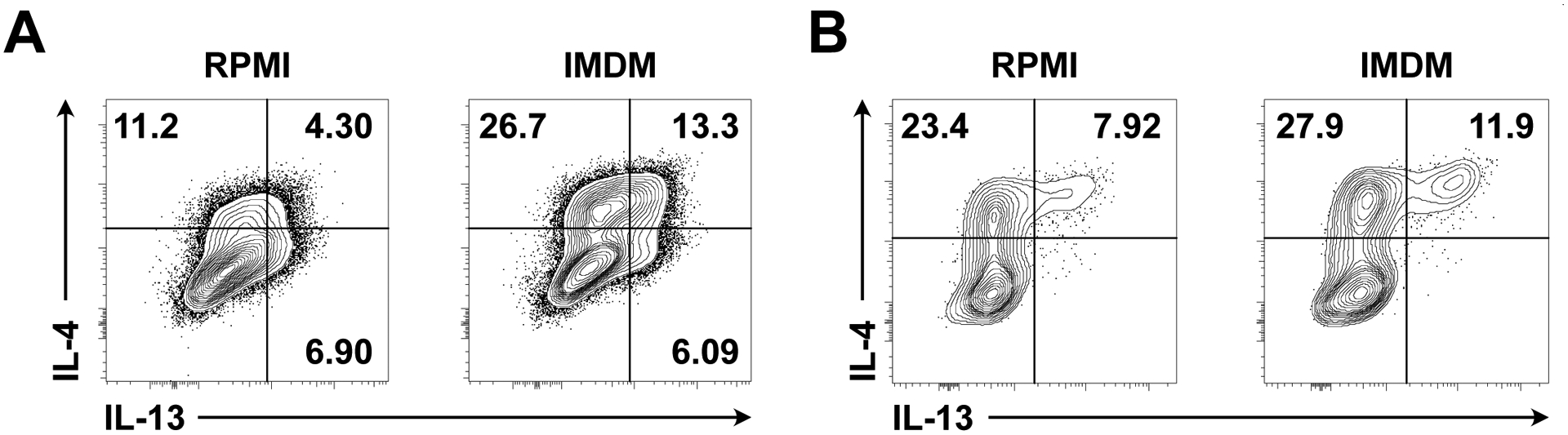
(A) Th2 cells were cultured in vitro for 3 days with plate-bound anti-CD3 (2 μg/ml), soluble anti-CD28 (2 μg/ml), human IL-2 (12.5 ng/ml), murine IL-4 (25 ng/ml), anti-IFN-γ (5 μg/ml), and anti-IL-12 (2 μg/ml). Th2 cells were re-plated on day 3 without anti-CD3 and rested for 2 additional days. On day 5 of the culture, Th2 cells were stimulated with PMA/ionomycin in complete RPMI versus IMDM. IL-4 and IL-13 intracellular staining is shown. Pre-gated on live CD4+ cells. (B) IL-4 and IL-13 intracellular staining in day 7 medLN cells from i.n. immunization with Alternaria alternata and NP-OVA. Cells were stimulated with PMA/ionomycin in complete RPMI versus IMDM. Pre-gated on Tfh cells (live CD4+ TCRβ+ CD44+ PD-1+ CXCR5+). Samples were acquired on a BD LSR II (BD Biosciences).
3. Identification of polyclonal human Tfh13 cells
When the inciting allergen is not known, human Tfh13 cells can be identified using non-specific activation with PMA/ionomycin. However, these activation conditions result in significant downregulation of surface CD4, making it difficult to identify CD4+ T cells within a bulk peripheral blood mononuclear cell (PBMC) population. To overcome this limitation, CD4+ T cells are first purified by negative selection using magnetic bead isolation. In contrast to antigen-specific activation, PMA/ionomycin activation can be performed on previously frozen PBMCs, allowing samples to be banked and later processed together to minimize batch effects.
3A. Materials
Equipment and consumables
Acid Citrate Dextrose Solution A Whole Blood Tubes (BD Biosciences, Cat #364606)
15- and 50-ml conical tubes
Hemocytometer
EasySep™ Magnet (StemCell Technologies)
5-ml polystyrene tubes (12 × 75 mm)
24- or 12-well TC-treated plate
96-well V bottom plate
Mr. Frosty™ Freezing Container (Thermo Fisher Scientific)
PolarSafe Cryogenic Storage Vials, 1 ml (Argos Technologies)
Reagents
Phosphate buffered saline (PBS) (1X) (Gibco)
Iscove’s Modified Dulbecco’s Medium (IMDM) (Gibco)
Penicillin-Streptomycin (Gibco)
L-glutamine (Gibco)
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) (Gibco)
MEM Non-essential amino acids solution (Gibco)
Sodium pyruvate (Gibco)
Heat-inactivated fetal bovine serum (FBS) (VWR)
Ethylenediaminetetraacetic acid (EDTA) solution, pH 8.0 (Gibco)
Lymphoprep™ (StemCell Technologies)
RBC Lysis Buffer (10X) (Tonbo Biosciences)
Distilled water
Trypan Blue 0.4% (Gibco)
Dimethyl sulfoxide (DMSO) (Sigma)
EasySep™ Human CD4+ T Cell Enrichment Kit (StemCell Technologies)
Phorbol 12-myristate 13-acetate (PMA) (Sigma)
Ionomycin (Sigma)
Fixation/Permeabilization Solution Kit with BD GolgiPlug™ (BD Biosciences)
FcR Blocking Reagent Human (Miltenyi)
Ghost Dye™ Violet 510 (Tonbo Biosciences)
Antibodies (see Table 4)
Table 4.
Antibody panel for polyclonal human Tfh13 cells
| Step | Antigen (Clone) | Fluorophore | Dilution* | Source |
|---|---|---|---|---|
| Live/Dead | Ghost Dye™ Violet 510 | (AmCyan channel) | 1:1000 | Tonbo Biosciences |
| Surface | CD3 (UCHT1) | PerCP/Cyanine5.5 | 1:23.5 (1:11.75) | |
| CD4 (RPA-T4) | APC-Cyanine7 | 1:33.3 (1:16.65) | ||
| CD45RA (HI100) | violetFluor™ 450 | 1:36.3 (1:18.15) | ||
| CXCR5 (RF8B2) | BD Horizon™ BB515 | 1:20 (1:10) | BD Biosciences | |
| Post-fixation | IL-4 (MP4–25D2) | PE-Cyanine7 | 1:20 | BioLegend |
| IL-13 (JES10–5A2) | APC | 1:20 | ||
| IFN-γ (4S.B3) | Alexa Fluor® 700 | 1:50 |
All dilutions, except those in parentheses, represent final concentrations for staining. In the steps that require serial dilution of the antibodies, the dilutions for preparing the antibody cocktail are listed in parentheses.
Reagent Setup
FACS buffer: Prepare a solution of 2% (vol/vol) FBS and 4 mM EDTA in PBS.
RBC Lysis Buffer (1X): Dilute RBC Lysis Buffer (10X) 1:10 in distilled H2O.
Freezing medium: Prepare a solution of 10% DMSO (vol/vol) in FBS. Store at 4°C.
Complete IMDM: Supplement IMDM with 10% (vol/vol) heat-inactivated FBS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, 10 mM HEPES, 0.1 mM non-essential amino acids, and 1 mM sodium pyruvate.
BD Perm/Wash™ buffer (1x): Dilute BD Perm/Wash™ buffer (10x) 1:10 in distilled H2O.
3B. Methods
3B.1. PBMC Isolation (see Note 1)
Make sure all the reagents are at room temperature (RT) before starting. Adjust centrifuge to 20°C. If freezing PBMCs at the end of harvest, place the freezing container at 4°C to cool it down.
Dilute 10 ml blood 1:3 in PBS + 4 mM EDTA (total volume 30 ml diluted blood).
Add 15 ml Lymphoprep to each empty 50-ml tube (use one 50-ml tube for each 10 ml undiluted blood).
Mix the diluted blood by pipetting (slow speed). Very slowly, disperse the 30 ml diluted blood on top of the Lymphoprep.
Centrifuge at 850 × g for 25 min at RT with slow brake. Keep the brakes on in all the remaining centrifugation steps.
Aspirate 15 ml of supernatant to remove platelets.
Harvest the PBMCs (white layer between the plasma and the red blood cells) using a short 10-ml serological pipette.
Transfer the PBMCs to a new 50-ml tube and fill up to 50 ml with FACS buffer.
Centrifuge at 500 × g for 10 min at RT. Aspirate supernatant.
Resuspend and mix all the pellets from a single donor in 1 ml RBC lysis buffer (1X), then add an additional 29 ml RBC lysis buffer. Mix by inverting the tube 3 times and incubate at RT for 5 min.
Fill up to 50 ml with FACS buffer.
Centrifuge at 500 × g for 8 min at RT. Aspirate the supernatant.
Resuspend the pellet in 30 ml FACS buffer to wash.
Centrifuge at 300 × g for 8 min at RT. Aspirate the supernatant.
Repeat the washing step. Aspirate the supernatant.
Resuspend the cells in the same volume of starting material (e.g. if starting with 20 ml undiluted blood, resuspend in 20 ml FACS buffer).
To count cells, mix 10 μl cells with 10 μl Trypan Blue (1:2). Count cells in duplicate using a hemocytometer and a light microscope. The expected yield is >1×106 cells/ml.
Centrifuge at 300 × g for 8 min at RT. Aspirate the supernatant.
If using samples immediately, proceed to 3B.4. If not, continue to 3B.2.
3B.2. PBMC Freezing
Resuspend cells at 5–10×106/ml in freezing medium.
Aliquot 1 ml cells per cryogenic storage vial, then transfer the vials to the pre-chilled freezing container.
Store the freezing container at −80°C overnight.
The next day, transfer the vials to freezer boxes in liquid nitrogen vapor phase for long-term storage.
3B.3. PBMC Defrosting
Warm up complete IMDM (cIMDM) at 37°C.
Thaw frozen vials of PBMCs in a 37°C water bath. Remove the vials when the freezing medium starts to turn liquid (~2 min).
With a disposable Pasteur pipette, add a few drops of warm cIMDM into the vial to thaw it completely.
Slowly transfer the cell suspension to a 15-ml tube and fill up with warm cIMDM.
Centrifuge at 300 × g for 8 min at RT.
Aspirate the supernatant and resuspend the pellet in 5 ml FACS buffer.
Count cells as described above.
Centrifuge at 300 × g for 8 min at RT. Aspirate the supernatant.
3B.4. CD4+ T Cell Isolation (see Note 2)
Prepare a suspension of cells at 5×107/ml in FACS buffer and place cells in a 5-ml polystyrene tube.
Add the EasySep Human CD4+ T cell Enrichment Cocktail at 50 μl/ml to the cell suspension and mix well.
Incubate for 10 min at RT.
Vortex the EasySep D Magnetic Particles for 30 sec.
Add the magnetic particles at 100 μl/ml to the cell suspension and mix well.
Incubate for 5 min at RT.
Bring the cell suspension to a total volume of 2.5 ml total with FACS buffer.
Mix the cells by pipetting up and down 3 times. Place the tube without cap into the EasySep Magnet.
Incubate for 5 min at RT.
Pick up the magnet with the tube inside and, in one continuous motion, invert to pour the desired fraction into a new 15-ml tube. Leave the magnet inverted for no more than 3 sec, then return to the upright position.
Centrifuge at 300 × g for 8 min at RT. Aspirate the supernatant.
3B.5. Cell Resting (see Note 3)
Resuspend the cells to 1×106 in 950 μl of cIMDM. Transfer to a well of a 24- or 12-well plate.
Rest the cells by leaving the plate in the incubator at 37°C overnight.
3B.6. Stimulation (see Notes 4–9)
For each well, prepare 50 μl cIMDM containing PMA (1 μg/ml) and ionomycin (20 μg/ml).
Add 50 μl of the stimulation mix to each well on top of the existing 950 μl cIMDM, to achieve a final concentration of 50 ng/ml PMA and 1 μg/ml ionomycin. Incubate the cells at 37°C with 5% CO2.
After 1 hr, add to each well 10 μl cIMDM containing BD GolgiPlug™ (1:10), to achieve a final concentration of 1:1000 BD GolgiPlug™.
Mix samples thoroughly and incubate the cells at 37°C for an additional 5 hr (total stimulation time 6 hr).
3B.7. Surface Staining (see Note 10)
Transfer the cells to 1.5-ml tubes.
Centrifuge at 300 × g for 8 min at RT. Aspirate the supernatant.
Resuspend the cells in 150 μl PBS and transfer to a 96-well V bottom plate.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Add 100 μl diluted Ghost Dye™ Violet 510 (1:1000 in PBS) to each well. Incubate for 10 min at RT in the dark.
Add 50 μl FACS buffer to each well.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Resuspend the cells in 100 μl FACS buffer to wash.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Add 25 μl diluted FcR block (1:5 in FACS buffer) to each well.
Incubate for 5 min at RT in the dark.
Dilute the surface antibodies in 25 μl FACS buffer/well at the concentration listed in Table 4.
Add 25 μl diluted surface antibodies mix to each well on top of the existing 25 μl diluted FcR block.
Incubate for 15 min at RT in the dark.
Add 100 μl FACS buffer to each well.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant and repeat washing step. Decant the supernatant.
3B.8. Fixation and Permeabilization
Resuspend the cells in 100 μl Fixation/Permeabilization solution. Incubate for 20 min at 4°C in the dark.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Resuspend the cells in 100 μl 1X Perm/Wash buffer to wash.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant and repeat washing step. Decant the supernatant.
Resuspend the cells in 100 μl 1X Perm/Wash buffer. Incubate overnight at 4°C in the dark.
3B.9. Post-fixation and Intracellular Staining (Note 11)
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Resuspend cells in 50 μl 1X Perm/Wash buffer containing post-fixation antibodies at the concentration listed in Table 4.
Incubate for 30 min at 4°C in the dark.
Add 100 μl 1X Perm/Wash buffer to each well.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Resuspend the cells in 100 μl 1X Perm/Wash buffer to wash.
Centrifuge at 850 × g for 4 min at RT. Decant the supernatant.
Repeat washing step. Decant the supernatant.
Resuspend the cells in 150 μl FACS buffer. Keep on ice in the dark prior to flow cytometric analysis. Perform flow cytometric analysis within 2 hr.
Gating strategy is shown in Figure 4A.
Figure 4. Identification of polyclonal human Tfh13 cells.
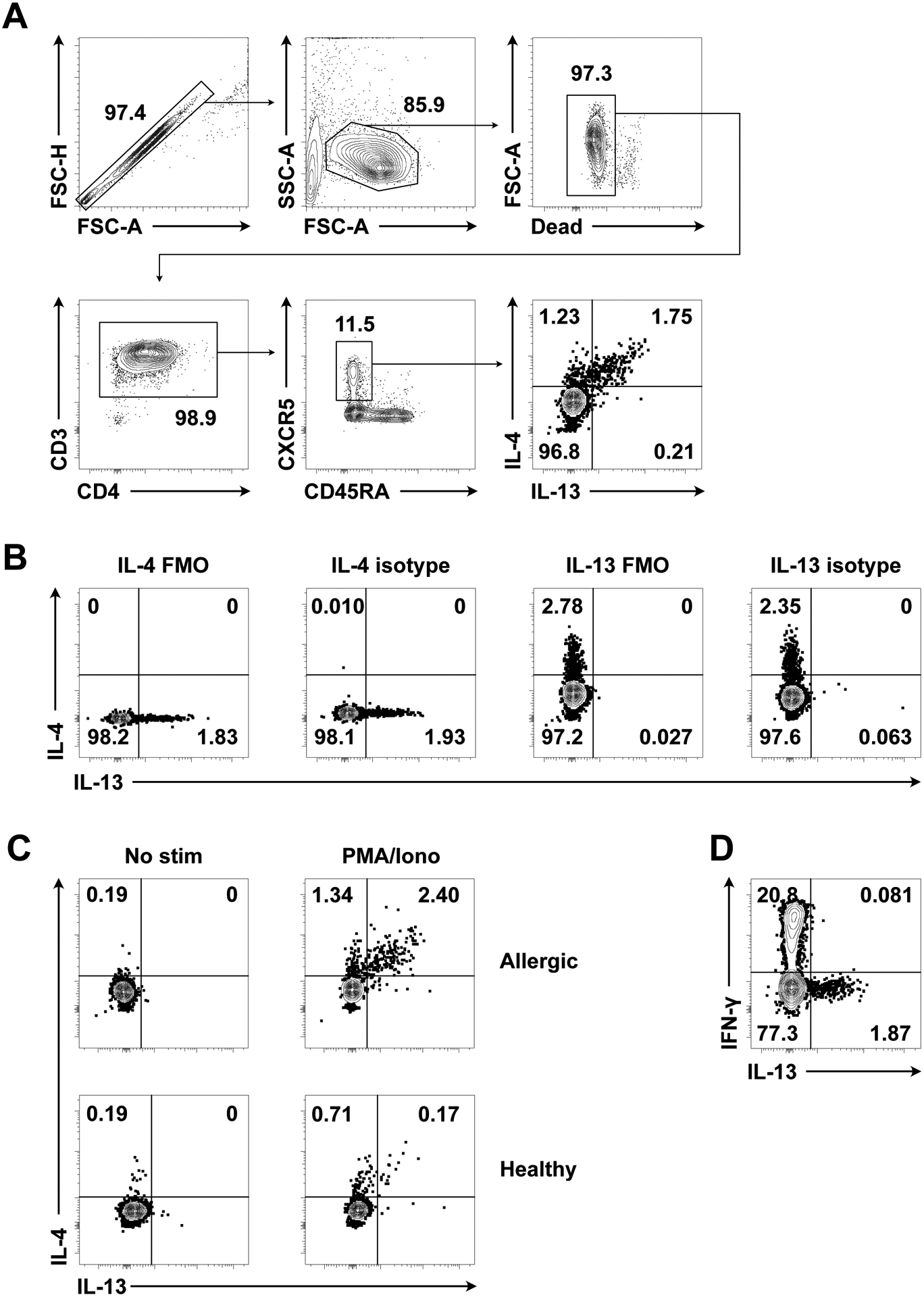
(A) Gating strategy for polyclonal human Tfh13 cells from an aeroallergen-sensitized patient after PMA/ionomycin stimulation. (B) IL-4 and IL-13 fluorescence minus one (FMO) and isotype control staining in cells from an allergic patient with stimulation. Pre-gated on circulating Tfh cells (live CD4+ CD3+ CD45RA– CXCR5+). (C) IL-4 and IL-13 intracellular staining in an allergic patient and a healthy control following no stimulation or PMA/ionomycin stimulation. Pre-gated on circulating Tfh cells. (D) IFN-γ and IL-13 intracellular staining in an allergic patient following PMA/ionomycin stimulation. Pre-gated on circulating Tfh cells. Samples were acquired on a BD FACSymphony A5 (BD Biosciences).
3C. Notes
PBMC isolation can also be performed using Ficoll-Paque PLUS (GE Healthcare). Instead of Trypan Blue, Türk’s solution (Ricca Chemical) can be used to count cells.
Isolation of CD4+ T cells improves the ease of detecting Tfh13 cells.
Resting the cells overnight before activation increases the number of cytokine-positive cells.
Fluorescence minus one (FMO) and isotype control antibody staining as well as unstimulated cells can be used to help with gating (Figure 4B–C).
We have found that GolgiPlug™ gives better cytokine staining than GolgiStop™.
PD-1 can be included in the gating strategy; however, compared to Tfh cells in lymphoid tissues, blood-circulating Tfh cells express lower levels of PD-1, so this marker can be difficult to stain.
After PMA/Ionomycin stimulation, CD4 is downregulated.
Stimulation for 6 hr promotes more cytokine-positive cells than 12 hr, due to significant cell death with prolonged stimulation.
Stimulation with PMA/Ionomycin induces increased cytokine-positive cells compared to stimulation with anti-CD3/CD28 beads.
Staining the surface markers CD3, CD4, and CXCR5 before permeabilization improves their detection.
IFN-γ can be used as a control marker for the stimulation. Tfh13 cells do not produce IFN-γ (Figure 4D).
4. Identification of peanut-specific human Tfh13 cells
When the inciting allergen is known, it is preferable to identify human Tfh13 cells by activating samples with antigen. In this system, antigen-specific cells can be identified by the upregulation of the activation marker CD154 (CD40L). For optimal staining, this protocol should be performed on freshly isolated PBMCs.
4A. Materials
Equipment and consumables
Sodium heparin tubes for blood collection (BD Biosciences, Cat #367874)
Food-grade coffee grinder
Glass funnel
Glass beaker
Grade 1 filter paper (Whatman)
Magnetic stir bar
Magnetic stirrer
Sorvall centrifuge tubes
15- and 50-ml tubes
Kimwipes (Kimberly-Clark Professional)
Hemocytometer
24-well TC-treated plate
4.5-ml Sarstedt polystyrene tubes
5-ml polystyrene tubes
Reagents
Peanut (food-grade, roasted in shell)
Acetone (Sigma)
cOmplete™ Protease Inhibitor Cocktail (Roche)
Phosphate buffered saline (PBS) (1X)
Detoxi-Gel™ Endotoxin Removing Gel (Thermo Fisher Scientific)
LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific)
Pierce™ Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific)
AIM V Medium (Gibco)
Heat-inactivated fetal bovine serum (FBS) (ATCC)
BD GolgiPlug™ (BD Biosciences)
4% Paraformaldehyde (Electron Microscopy Services)
Permeabilization Buffer (10X) (eBioscience)
Distilled water
Bovine serum albumin (BSA) (Sigma)
Ethylenediaminetetraacetic acid (EDTA)
FcR Blocking Reagent Human (Miltenyi)
LIVE/DEAD™ Fixable Aqua Dead Cell Stain (Life Technologies)
Antibodies (see Table 5)
Table 5.
Antibody panel for peanut-specific human Tfh13 cells
| Step | Antigen (Clone) | Fluorophore | Dilution* | Source |
|---|---|---|---|---|
| Live/Dead | LIVE/DEAD™ Fixable Aqua Dead Cell Stain | (AmCyan channel) | 1:1000 | Life Technologies |
| Surface | CD3 (SK7) | APC-eFluor 780 | 1:100 (1:50) | eBioscience |
| CD4 (OKT4) | Brilliant Violet 605™ | 1:100 (1:50) | BioLegend | |
| CXCR5 (RF8B2) | Alexa Fluor® 488 | 1:40 (1:20) | BD Biosciences | |
| Post-fixation | CD154 (24–31) | PE | 1:209 (1:9) | eBioscience |
| IL-4 (MP4–25D2) | Alexa Fluor® 647 | 1:104.5 (1:4.5) | BioLegend | |
| IL-13 (JES10–5A2) | BD Horizon™ V450 | 1:104.5 (1:4.5) | BD Biosciences |
All dilutions, except those in parentheses, represent final concentrations for staining. In the steps that require serial dilution of the antibodies, the dilutions for preparing the antibody cocktail are listed in parentheses.
Reagent Setup
Complete AIM V Media: Supplement AIM V medium with 2.5% (vol/vol) autologous plasma.
FACS buffer: Prepare a solution of 1% BSA and 2 mM EDTA in PBS.
Permeabilization Buffer (1X): Dilute Permeabilization Buffer (10X) (eBioscience) 1:10 in distilled H2O.
4B. Methods
4B.1. Crude Peanut Extract (CPE) Preparation (see Note 1)
Shell approximately 70 g peanut. Grind to a paste using food-grade coffee grinder.
Dissolve the paste with 200 ml cold acetone and mix well.
In a chemical hood, set up two funnels each with two layers of Grade 1 filter paper.
Pour the peanut paste/acetone mix into the funnels. Run an additional ~1.4 L (20X the volume of peanut used) cold acetone through the funnels. Discard the flow-through containing peanut fat dissolved in acetone. Dry the remaining peanut paste in the funnels overnight.
The next day, unfold the filters and collect the dry peanut powder into a clean, autoclaved beaker. Avoid the peripheral areas of yellow fat. The yield from 70 g shelled peanuts should be ~30 g dry powder.
Dissolve two Protease Inhibitor Tablets in 100 ml PBS.
Slowly add the PBS with protease inhibitor to the peanut powder until the mixture is a viscous solution (not completely liquid). This will take 50–70 ml PBS with protease inhibitor given a starting amount of 70 g shelled peanuts.
Add a magnetic stir bar into the mixture and place the beaker containing the mixture on a magnetic stirrer at room temperature (RT) on high spin. The mixture should be homogenized in ~2 hr.
Centrifuge the mixture in Sorvall tubes (4 tubes for ~100 ml) at 20,000 × g for 20 min at RT. Collect the supernatant.
Cover the opening of a 50-ml tube with a Kimwipe. Gently filter the supernatant through the Kimwipe into the tube. This should yield ~40 ml final volume.
Determine CPE protein concentration using the Coomassie (Bradford) Protein Assay Kit.
Perform serial removals of endotoxin from the filtered supernatant using Detoxi-Gel™ Endotoxin Removing Gel until the endotoxin contamination in a working concentration is below 0.5 EU/ml as measured by the LAL Chromogenic Endotoxin Quantitation Kit.
Repeat measurement of CPE protein concentration using the Coomassie (Bradford) Protein Assay Kit, as endotoxin removal may lead to loss of protein.
Run SDS-PAGE to verify the presence of major allergens. Major allergens include Ara h 1 (65 kDa), Ara h 2 (17 kDa), and Ara h 3 (14 kDa)16.
Aliquot CPE and store at −80°C.
4B.2. Plasma Isolation
Centrifuge blood tubes at 450 × g for 15 min at 22°C with no acceleration and no brake.
In a tissue culture hood, remove plasma layer and transfer to 15-ml conical tube. Use this to prepare complete AIM V media for each donor sample.
Transfer remaining blood into a 50-ml conical tube (1 50-ml tube per blood tube).
Replace plasma volume removed with equivalent volume of PBS + 2% FBS.
4B.3. PBMC Isolation
Perform PBMC isolation as described in Section 3B.1, steps 1–9.
Use 1 ml complete AIM V media to resuspend and mix all the pellets from one donor. Transfer the cells to a 15-ml conical tube. Use 10 ml complete AIM V media to rinse the 50-ml tubes for one donor and then transfer to the same 15-ml tube, which should ultimately contain cells in 11 ml media.
Centrifuge at 400 × g for 10 min at 22°C. Resuspend in 10 ml complete AIM V media.
Count cells as described in Section 3B.1, step 17. Resuspend cells at 4×106 cells/ml in complete AIM V media.
Plate 1 ml cells/well in a 24-well plate. Plate 5 wells each for CPE stimulation. Rest cells in incubator overnight.
4B.4. Stimulation (see Notes 2–4)
Add 100 μg CPE to each well and place in the incubator for 2 hr.
For each well, add 10 μl BD GolgiPlug™ diluted 1:10 in complete AIM V media, to achieve a final concentration of 1:1000 BD GolgiPlug™. Place in the incubator for an additional 4 hr (total stimulation time 6 hr).
4B.5. Surface Staining
Transfer each well of cells into a 4.5-ml conical-bottom Sarstedt polystyrene tube. Rinse each well with 2 ml PBS and transfer to tube, leading to a final volume of 3 ml per tube. Centrifuge at 500 × g for 5 min at 4°C and aspirate supernatant.
Resuspend cells in 1ml LIVE/DEAD™ Fixable Aqua Dead Cell Stain (diluted 1:1000 in PBS). Incubate for 30 min on ice in the dark.
Add 2 ml PBS and centrifuge at 500 × g for 5 min at 4°C. Aspirate supernatant.
Resuspend cells in 50 μl diluted FcR block (1:5 in FACS buffer). Incubate for 5 min at RT in the dark.
Dilute the surface antibodies in 50 μl FACS buffer/sample at the concentration listed in Table 5.
Add 50 μl diluted surface antibodies mix to each tube on top of the existing 50 μl diluted FcR block. Incubate for 30 min on ice in the dark.
Add 2 ml PBS and centrifuge at 500 × g for 5 min at 4°C. Aspirate supernatant.
4B.6. Fixation and Permeabilization
Resuspend cells in 500 μL 4% paraformaldehyde. Incubate for 5 min at RT in the dark. Vortex briefly at 1, 3, and 5 min to mix.
Add 1 ml ice-cold FACS buffer and centrifuge at 500 × g for 5 min at 4°C. Aspirate supernatant.
Wash with 1 ml 1X Permeabilization Buffer and centrifuge at 1400 × g for 10 min at 4°C. Aspirate supernatant.
Resuspend cells in 500 μL 1X Permeabilization Buffer. Incubate for 20 min at RT in the dark.
4B.7. Post-fixation and Intracellular Staining (see Note 5)
Add post-fixation staining cocktail directly to the tubes containing 500 ul of 1X Permeabilization Buffer (22.5 μl/sample) (Table 5). Incubate for 45 min on ice in the dark.
Add 2 ml 1X Permeabilization Buffer and centrifuge at 1400 × g for 10 min at 4°C. Aspirate supernatant. Repeat washing step and aspirate supernatant.
Resuspend cells in 300–500 μl FACS buffer. Keep on ice in the dark prior to flow cytometric analysis. Perform flow cytometric analysis within 1 day. Before running samples, combine duplicate samples into a 5-ml polystyrene tube.
Gating strategy is shown in Figure 5A.
Figure 5. Identification of peanut-specific human Tfh13 cells.
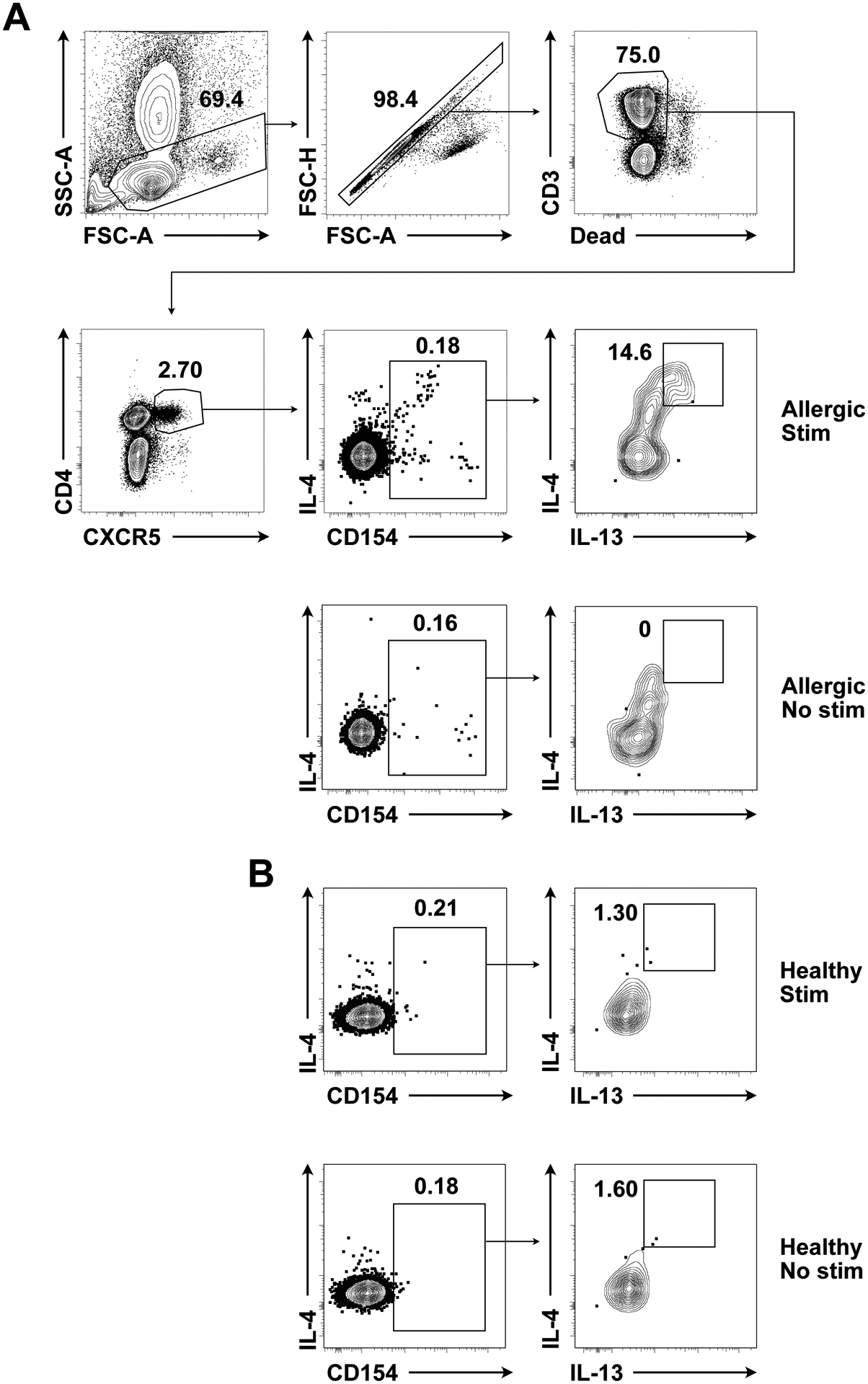
(A) Gating strategy for peanut-specific human Tfh13 cells from an allergic patient, with peanut stimulation or no stimulation. (B) IL-4 and IL-13 production by a healthy control, with peanut stimulation or no stimulation. Pre-gated on circulating Tfh cells (live CD4+ CD3+ CXCR5+). Samples were acquired on a BD LSRFortessa (BD Biosciences).
4C. Notes
Using this protocol, a large amount of crude peanut can be prepared. Alternatively, a number of peanut preparations are commercially available. For readers who do not want to perform endotoxin removal and validation, they can use LoTox™ Peanut Flour Protein with Defined Allergen Content (Indoor Biotechnologies). Stallergenes Greer sells a peanut preparation, but this formulation still requires endotoxin removal. If possible, a single batch of peanut preparation should be used for a series of experiments.
It is important to include fluorescence minus one (FMO) and isotype control antibody staining as well as unstimulated cells for setting gates (Figure 5A–B).
We have only performed this assay with peanut, however, other allergens/antigens may also work in this assay.
Do not include GolgiPlug™ on initial activation as it may affect in vitro activation of T cells by interfering with antigen processing and presentation17.
Given that circulating antigen-specific Tfh13 cells are a relatively rare population, it is important to acquire at least 8×106 events if using total PBMCs. Alternatively, fewer events can be acquired if CD4+ T cells are enriched prior to staining.
Funding:
This work was supported by T32GM007205 (JSC and SJO), Gershon/Trudeau Fellowship from Immunobiology at Yale University (UG), Robert E. Leet and Clara Guthrie Patterson Trust Mentored Research Award (TS), U24AI118644 (MCB), Food Allergy Research & Education (SCE), R01 AI136942 (SCE), R01 AI108829 (SCE), R21 AI135221 (AW), R21 AI133440 (AW), and R01 AI141609 (AW).
Abbreviations used
- BCL-6
B cell lymphoma 6
- BSA
Bovine serum albumin
- cIMDM
Complete Iscove’s Modified Dulbecco’s Media
- CPE
Crude peanut extract
- DMSO
Dimethyl sulfoxide
- EDTA
Ethylenediaminetetraacetic acid
- FACS
Fluorescence-activated cell sorting
- FBS
Fetal bovine serum
- FMO
Fluorescence minus one
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- i.n.
Intranasal
- IACUC
Institutional Animal Care and Use Committee
- IL-4
Interleukin-4
- IMDM
Iscove’s Modified Dulbecco’s Media
- LAL
Limulus amebocyte lysate
- LN
Lymph node
- medLN
Mediastinal lymph node
- ndLN
Non-draining lymph node
- NP-OVA
4-hydroxy-3-nitrophenylacetyl conjugated to ovalbumin
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate buffered saline
- PD-1
Programmed cell death protein 1
- PMA
Phorbol 12-myristate 13-acetate
- RBC
Red blood cell
- RPMI
Roswell Park Memorial Institute
- RT
Room temperature
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TC
Tissue culture
- Tfh
T follicular helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: MCB serves on the scientific advisory board of Prota Therapeutics. JSC, JG, UG, SJO, TS, SCE, and AW have no conflicts to disclose.
5. References
- 1.Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. Journal of Leukocyte Biology. 2020;107:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma L, Danoff TM, Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. Journal of Allergy and Clinical Immunology. 2014;133:1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46:1099–110. [DOI] [PubMed] [Google Scholar]
- 4.Noble A, Zhao J. Follicular helper T cells are responsible for IgE responses to Der p 1 following house dust mite sensitization in mice. Clinical & Experimental Allergy. 2016;46:1075–82. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. Journal of Allergy and Clinical Immunology. 2017;139:300–313.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, et al. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunology. 2009;10:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meli AP, Fontés G, Leung Soo C, King IL. T Follicular Helper Cell–Derived IL-4 Is Required for IgE Production during Intestinal Helminth Infection. The Journal of Immunology. 2017;199:244–52. [DOI] [PubMed] [Google Scholar]
- 9.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365:eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Xu L-L, Teuscher P, Liu H, Kaplan MH, Dent AL. An Inhibitory Role for the Transcription Factor Stat3 in Controlling IL-4 and Bcl6 Expression in Follicular Helper T Cells. J Immunol. 2015;195:2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann J, Radbruch A, Chang H-D. A Ca2+ concentration of 1.5 mM, as present in IMDM but not in RPMI, is critical for maximal response of Th cells to PMA/ionomycin. European Journal of Immunology. 2015;45:1270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jandl C, Loetsch C, King C. Cytokine Expression by T Follicular Helper Cells In: Calado DP, editor. Germinal Centers: Methods and Protocols [Internet]. New York, NY: Springer; 2017. [cited 2019 Nov 21]. p. 95–103. (Methods in Molecular Biology). Available from: 10.1007/978-1-4939-7095-7_8 [DOI] [PubMed] [Google Scholar]
- 13.Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011;34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byford E, Carr M, Piñon L, Ahearne MJ, Wagner SD. Isolation of CD4+ T-cells and Analysis of Circulating T-follicular Helper (cTfh) Cell Subsets from Peripheral Blood Using 6-color Flow Cytometry. JoVE (Journal of Visualized Experiments). 2019;e58431. [DOI] [PubMed] [Google Scholar]
- 15.Chiang D, Chen X, Jones SM, Wood RA, Sicherer SH, Burks AW, et al. Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector TH2 subsets. Journal of Allergy and Clinical Immunology. 2018;141:2107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyer K, Morrow E, Li X-M, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. Journal of Allergy and Clinical Immunology. 2001;107:1077–81. [DOI] [PubMed] [Google Scholar]
- 17.Adorini L, Ullrich SJ, Appella E, Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature. 1990;346:63–6. [DOI] [PubMed] [Google Scholar]


