Abstract
Hepatocellular carcinoma (HCC) is a most deadly malignant disease worldwide, with no effective mechanism-based therapy available. Therefore, following the “miracle” outcomes seen in a few patients at the advanced stages of melanoma or lung cancer, the immune checkpoint inhibitors (ICIs) immediately entered clinical trials for advanced HCC patients without pre-clinical studies. Emerging data of clinical studies showed manageable toxicity and safety but limited therapeutic benefit to HCC patients, suggesting low response rate. Thus, one urgent issue is how to convert the liver tumors from cold to hot and responsive, which may rely on in-depth mechanistic studies in animal models and large scale data analysis in human patients. One ongoing approach is to design combinatorial treatment of different ICIs with other reagents and modalities. Indeed, a phase 3 clinical trial showed that combination of atezolizumab and bevacizumab achieved better overall and progression-free survival rates than sorafenib in unresectable HCC. This review highlights the value of animal models and the power of combining pre-clinical and clinical studies in efforts to improve HCC immunotherapy.
Introduction
Primary liver cancer, of which 80–90% of cases are hepatocellular carcinoma (HCC), has become a leading cause of cancer mortality and one of the most common malignancies around the globe.(1–3) HCCs are generally chemotherapy-resistant, with limited treatment options of surgical resection, liver transplantation and local ablation at the early stages.(2, 4) Sorafenib, a multi-kinase inhibitor, remains a first-line systemic drug for advanced HCCs, although it can only extend patients’ survival for several months.(5, 6) Similar low therapeutic benefits were reported for regorafenib, lenvatinib, and cabozantinib.(7–9) Numerous clinical studies that tested other pharmaceutical compounds have failed to show any significant tumor-suppressing effects in HCC.(10) The systematic failure in treating liver cancer by blocking identified oncogenic pathways is clearly due to inadequate understanding of complex mechanisms in liver tumorigenesis. Recent experimental data reported by a number of groups showed that deleting pro-oncogenic molecules in hepatocytes ironically aggravated HCC development in mice.(11) Although the underlying mechanisms remain to be elucidated, blocking hepatocyte-intrinsic oncogenic signaling apparently disturbs hepatic homeostasis and generates a tumor-promoting microenvironment in the liver. These animal data call for re-thinking of classical oncogenic signaling molecules as pharmaceutical targets in strategic design of HCC therapy.
In light of the increasing incidences of HCC unmet with efficacious therapy, it is fully understandable and appreciated that physicians started clinical phase I/II trials with immune checkpoint inhibitors (ICIs) on advanced HCC patients immediately after the astonishing success of the immunotherapy in some advanced-stage or nearly terminal melanoma or lung cancer patients. However, few preclinical studies were done in animal models prior to testing the new oncological treatment in HCC patients. This review briefly describes the short history of immunotherapy, from the pioneering work in animal models to clinical trials in solid tumors and then in liver cancer in particular. We discuss the special hepatic immunotolerance, the tumor microenvironment of HCCs, and thoughts on future design of effective combination protocols, as suggested by the most recent mouse data. Given that the initial ground-breaking work of immunotherapy was accomplished in mouse tumor models, improving its efficiency in HCC therapy will also require deeper mechanistic understanding and preclinical studies in animal models.
The original work of immunotherapy in mouse tumor models
For decades, tumor-specific immune responses have been found to be very weak and insufficient to eradicate established tumors. Tumor cells have evolved multiple mechanisms of immune evasion, aiding the development of tumor progression. However, the identification of immune-suppressive co-receptors on T lymphocytes shed lights on an intriguing prospect of enhanced anti-tumor immunity with their blockade. The cytotoxic T lymphocyte antigen-4 (CTLA-4, also known as CD152) is a member of the CD28 co-receptor family that is expressed on activated T cells and regulatory T cells (Tregs), and weakly on naïve T cells.(12) CTLA-4 binds, with higher affinity than CD28, to the CD28 ligands CD80 (B7–1) and CD86 (B7–2), leading to negative regulation of T cell activation. In 1996, Allison’s group showed that intraperitoneal injection of anti-CTLA-4 but not anti-CD28 antibody effectively inhibited tumor growth in mice subcutaneously inoculated with colon carcinoma 51BLim10 cells or Sa1N fibrosarcoma cells.(13) These results provided exciting evidence that blocking inhibitory signaling in the co-stimulatory pathway in T lymphocytes can bolster anti-tumor immunity.
PD-1 (programmed cell death protein-1) is another immune-inhibitory receptor of the CD28 family that is primarily expressed on CD8+ T cells, and also on Tregs and MDSCs (myeloid-derived suppressor cells). The primary function of the co-receptor PD-1 is to regulate peripheral immune tolerance and autoimmunity. PD-1 acts to suppress T cell activation and proliferation through interaction with its specific ligands PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), which are expressed on antigen-presenting cells, tumor cells or other cell types.(14, 15) Honjo and colleagues demonstrated that expressing PD-L1 on P815 mastocytoma cells drastically enhanced their tumorigenesis and invasiveness in vivo in syngenic mice.(16) Further, administration of anti-PD-L1 antibody significantly inhibited tumor growth in mice subcutaneously inoculated with myeloma cells.(16) These ground-breaking studies, together with other pioneering experimental data,(17) paved the way for a promising new strategy of oncological treatment by blocking immunoinhibitory signals, such as those mediated by CTLA-4 and PD-1 in T lymphocytes.
Clinical studies of ICIs in cancer patients
The original experimental data in mouse tumor models were quickly translated into clinical studies in cancer patients. The humanized anti-PD-1 antibody nivolumab was produced in mice by Medarex, and was approved as an investigational drug by the FDA in the USA in 2006. Clinical trials started in the USA in 2006 and in Japan in 2008, with the phase I results on patients with non-small cell lung cancer, melanoma and renal cell carcinoma reported in 2012.(18) Excitement grew worldwide when the new immunotherapy saved the life of a few patients at advanced stages of metastatic melanoma or kidney cancer. At the end of 2013, the journal Science selected, not without debate, cancer immunotherapy as the breakthrough of the year.
Despite remarkable results in many cancers, it has been recognized that the ICIs were not effective or only partially effective in a substantial portion of cancer patients. So far, the response rates to anti-PD-1 blockade are about 40%−70% in patients with melanoma, Hodgkin’s lymphoma, Merkel cell, microsatellite instability [MS]-high tumors, with lower responses (10–25%) observed in most other types of cancer approved by FDA for immunotherapy.(19) An additional challenge is the clinical data showing acquired resistance in patients who were initially responsive, resulting in disease recurrence.(20) Thus, one urgent issue is to improve the efficacy of immunotherapy and to turn the cold tumors to hot and responsive. In pursuit of this goal, many clinical trials are ongoing currently testing different combinations of ICIs with chemotherapy, radiotherapy, anti-VEGFA agents and chemoradiotherapy.(21) However, these efforts may reach fruition only based on deeper understanding of the underlying mechanisms of poor response or resistance.
Clinical trials in liver cancer patients
Pioneered in treating other types of solid tumors, the ICIs were soon introduced into clinical studies with HCC in multicenter trials worldwide,(22, 23) virtually in the absence of preclinical studies in animals. In 2013, Sangro and colleagues reported a pilot clinical test of the CTLA-4 antibody tremelimumab in a small cohort of 20 patients with advanced HCC developed on HCV-induced cirrhosis.(24) This phase II non-controlled, open-label, multicenter trial showed overall manageable adverse effects and signs of antitumoral and antiviral effects, which warrant further clinical investigation. Another report was released in 2017 on an open-label, non-comparative phase I/II dose escalation and expansion trial (ClinicalTrials.gov, number NCT01658878) of nivolumab (anti-PD-1 Ab) in patients with advanced HCC (CheckMate 040), led by El-Khoveiry and many collaborators worldwide.(25) Included in this prospective dose study were a total of 262 advanced HCC patients with multiple etiologies, with or without previous sorafenib treatment. This nivolumab monotherapy showed a manageable safety profile, with encouraging objective response rates and overall survival supporting its further investigation as a treatment option.
The anti-PD-1 drug pembrolizumab was investigated in a non-randomized, open-label phase II trial (KEYNOTE-224) in 104 advanced HCC patients previously treated with sorafenib.(26) This trial, led by Zhu, Finn, and many collaborators, was performed in 47 medical centers and hospitals in 10 countries. Consistent with the results of nivolumab, the clinical study with pembrolizumab also showed no severe safety and toxicity issues, with objective responses in 17% of HCC patients with HBV or HCV infection history and those with disease progression on sorafenib. The most common immune-related adverse effects were hypothyroidism, adrenal insufficiency and thyroiditis, as reported with pembrolizumab in other types of cancer. Interestingly, although combined PD-L1 expression in tumor and immune cells was predictive for response to pembrolizumab, the tumor expression alone was not. Further, 20% (6/30) patients with a lower combined PD-L1 score still exhibited a response to the anti-PD-1 therapy. (26) These results underscore the need for more refined strategies to incorporate biomarkers in ICI therapies for HCC.
Many other clinical trials for ICIs in HCC are ongoing worldwide, including two phase III (KEYNOTE-240 and KEYNOTE-394) trials in second-line advanced HCC patients, with increasing reports from new and ongoing trials expected every year. A variety of these clinical trials are designed to evaluate combined treatment strategies, with anti-CTLA-4 and anti-PD-1 or PD-L1 antibodies used in conjunction with other pharmaceuticals or physical treatment options.(27, 28) In particular, there is growing interest in ICIs combined with chemotherapy using kinase inhibitors and locoregional therapies, such as radiofrequency ablation (RFA), microwave ablation and cryoablation, as these treatments were shown to enhance tumor-specific immune responses.(29)
The special immune microenvironment in the liver
The preliminary reports of clinical trials indicated low response rates and limited therapeutic benefits of immunotherapy in HCC.(25, 26) In interrogating molecular and cellular factors that may influence the clinical outcomes, hepatic immunotolerance cannot be ignored; however, this process is not completely understood. The liver is the primary metabolic organ in the body and also plays a central role in immune regulation by receiving blood from hepatic artery and the intestines via portal vein. In addition to nutrients, the blood circulating into the liver from the gut also contains microbes, microbe-associated molecular patterns (MAMPs) and damage-associated molecular patterns (DAMPs), which are efficiently recognized, processed or removed in the unique “liver sinusoid” structure. In line with this function, the liver sinusoidal endothelial cells (LSECs) express a variety of pattern recognition receptors (PRRs) that are responsible for clearance of soluble macromolecular and colloidal wastes from the blood.(30) The liver has the largest population of resident macrophages (Kupffer cells) that also express many types of PRRs for recognition and removal of insoluble wastes by phagocytosis. Another remarkable feature of the liver is the enrichment of NK, NKT cells and TCRγδ T cells, and higher ratios of CD8+ versus CD4+ T cells, relative to the peripheral blood.
One most important immunological property of the liver is the induced status of T cell tolerance, resulting in hepatic tolerance that serves to prevent excessive immune responses to antigens absorbed from the intestine. Although the underlying mechanisms remain to be elucidated, the hepatic immunosuppressive environment is apparently maintained by orchestrated activities of naïve T cells, LSECs, dendritic cells, Kupffer cells and hepatocytes, mediated by a milieu of pro- and anti-inflammatory cytokines.(31, 32) T cell tolerance in the liver may be induced by continuous antigen presentation in the absence of co-stimulatory molecules and low CD4+ cells and in the expansion of Treg cells by IL-10 derived from Kupffer cells.(31) Although these strategies of immune suppression are critical for maintaining liver homeostasis, their potential contributions to HCC immune evasion must also be considered.
The immunological landscape of primary liver cancer
It is widely recognized that liver malignancies often develop in the context of chronic “necroinflammation” characterized by cell death, compensatory regeneration, excessive activation of hepatic stellate cells (HSCs) and fibrosis, and altered functions of infiltrated inflammatory and lymphocytic cells. In design of effective immunotherapy for HCC, two critical issues must be addressed: the strength of immunogenicity of tumor cells in the liver, and the specific mechanism(s) of immune evasion that promote HCC progression. Spontaneous tumor regression associated with systemic inflammatory responses was detected in HCC patients, suggesting at least some basal immunogenicity of liver cancer.(33) Additionally, it was estimated that more than half of HCC patients developed spontaneous immune responses against the tumor-associated antigen NY-ESO-1.(34) Percutaneous ethanol injection and radiofrequency ablation (RFA) reportedly elicited T cell infiltration into liver tumor tissue.(35, 36)
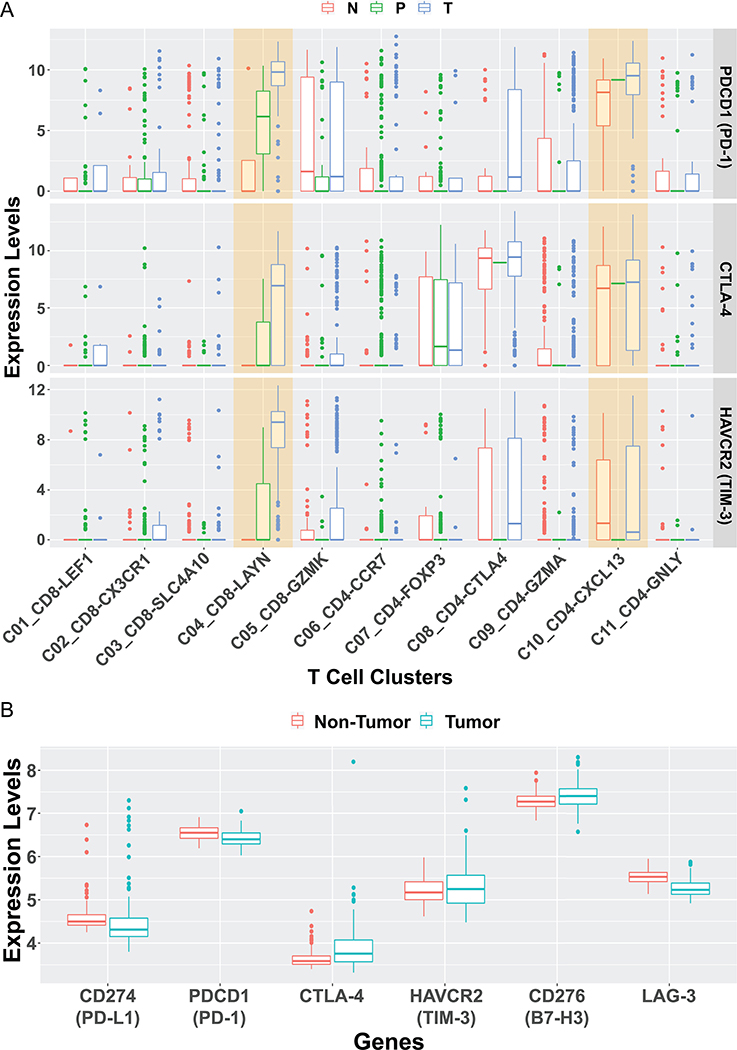
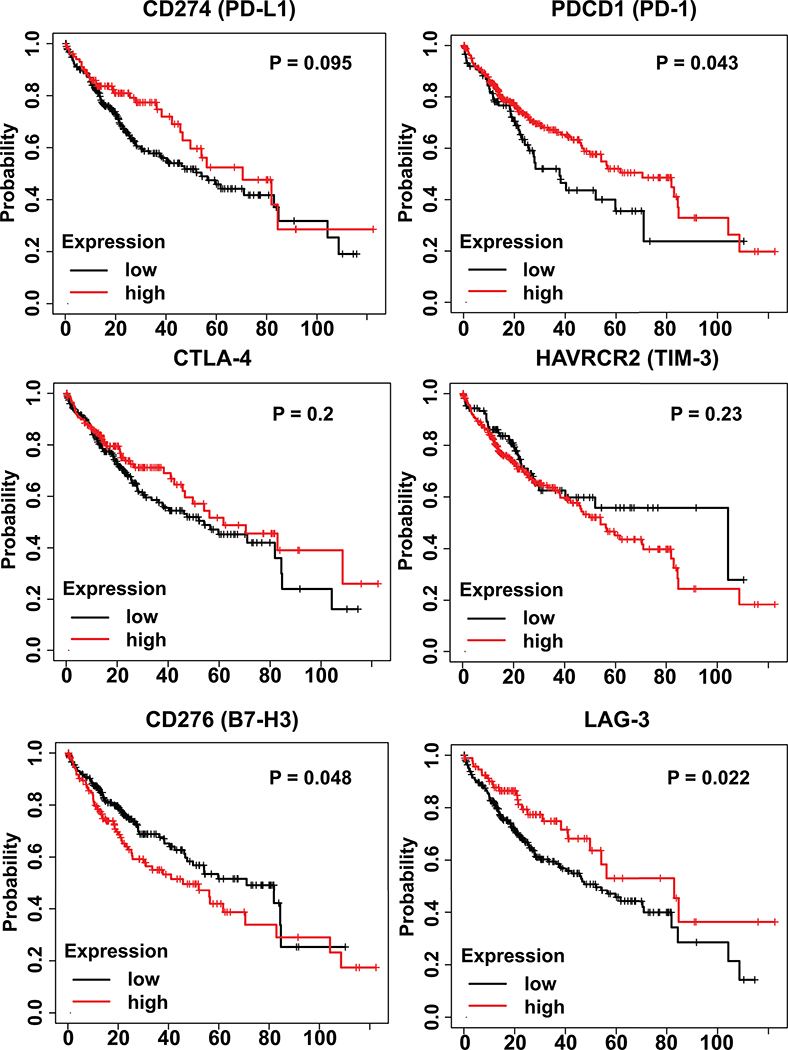
More recently, deep single-cell RNA sequencing by Zhang’s group revealed enrichment and clonal expansion of exhausted CD8+ T cells and Tregs in human HCC samples.(37) A list of both well-known and under-characterized exhaustion-related genes were also detected in the analysis, providing potential biomarkers for further dissection of HCC-infiltrating T cell functions. Of note, the cell surface membrane protein Layilin, upregulated in activated CD8 T cells and Tregs, was associated with poor prognosis and found to suppress interferon-γ production by primary CD8 T cells in vitro. Higher levels of PDCD1, CTLA-4 and HAVCR2 expression were detected in LAYN (Layilin)+CD8+ and CXCL13+CD4+ T cell subpopulations (Fig. 1A). Wang and colleagues in the TIGER-LC consortium performed a large-scale gene expression profiling in HCC/ICC patients from Thailand.(38) The microarray data was normalized with Robust Multi-array Average (RMA) and analyzed with GEO2R. No significant difference in the expression of immune-suppressive genes, including PD-L1, PD-1, CTLA4, TIM3, B7H3, and LAG3, was detected between the tumor and surrounding non-tumor tissues (Fig. 1B). We also extracted and performed bioinformatic analysis of HCC patients’ data from the TCGA database. The Kaplan-Meier plotting showed no correlation between patients’ survival and the expression levels of CD274, CTLA-4, HAVCR2 and LAG3 (Fig. 2). Whereas low expression of CD276 (B7-H3) showed modestly but significantly better survival than the high-expression group, low expression of PDCD1 (PD-1) had even worse prognosis than the high-expression group (Fig. 2).
Figure 1. The boxplot of selected immune-inhibitory genes.
A). We re-analyzed the single-cell RNA-seq data published by Zheng et al.(37) The immune-suppressive genes, such as PDCD1 (encoding PD-L1), CTLA4, and HAVCR2 (encoding TIM-3) were compared among various T cell clusters and different tissues. Their expression levels were higher in some T cell clusters from tumor tissue than adjacent normal liver tissue or peripheral blood, with similar levels detected in other clusters. As described,(37) all sequenced T cells were isolated from six treatment-naïve HCC patients and clustered into 11 subpopulations. N: adjacent normal liver tissue; P: peripheral blood; T: tumor tissue. C1: LEF1+, naïve CD8+ T cells; C2: CX3CR1+, effector memory CD8+ T cells; C3: SLC4A10+, MAIT cells; C4: LAYN+, exhausted CD8+ T cells; C5: GZMK+, CD8+ T cells; C6: CCR7+, naïve CD4+ T cells; C7: FOXP3+, peripheral Tregs; C8: CTLA4+, tumor Tregs; C9: GZMA+, CD4+ T cells; C10: CXCL13+, exhausted CD4+ T cells; C11: GNLY+, cytotoxic CD4+ T cells. To generate this plot, TPM values of each gene in all single cells the authors reported, as well as cell description, were downloaded from NCBI Gene Expression Omnibus database (GSE98638). The cells marked as “unknown” were first excluded. Then, the expression for each gene in a single cell was calculated as log(TPM+1) and displayed in boxplots. The calculation and plotting were performed using R.
B). We re-examined six immune-suppressive genes, PD-L1, PD-1, CTLA4, TIM3, B7H3, and LAG3, expressed in another patient cohort, which consisted of 151 surgically paired tumor and non-tumor samples (NCBI Gene Expression Omnibus access code GSE76297) from HCC or CCA patients from Thailand.(38) The microarray data was normalized with Robust Multi-array Average (RMA) and analyzed with GEO2R. No significant difference in the expression of these six genes was detected between the tumor and surrounding non-tumor tissues.
Figure 2. Immune-inhibitory gene expression and overall survival of HCC patients in TCGA.
The Kaplan-Meier plots were made using KM Plotter,(51) with 364 HCC patients’ RNA-seq data from the TCGA-LIHC project. The detailed method was described previously.(52) In brief, for each gene examined, all patients were divided into high- and low-expression groups by all possible cutoff values based on RNAseq data. The best performing results were shown here. Log-rank p-values were calculated and a p-value of 0.05 were considered as significant. For the six immuno-inhibitory genes examined, low expression of CD276 (encoding B7-H3) showed modestly but significantly better survival than the high-expression group. However, low expression of PDCD1 (encoding PD-1) had worse prognosis than the high-expression group. No correlations were found between expression and survival for the other genes examined.
In recent experiments, we demonstrated that additional deletion of Ptpn11/Shp2 in hepatocytes exacerbated and accelerated NASH-HCC/ICC development in Pten-deficient livers.(39) Thus, the mouse lines with hepatocyte-specific knockout of Shp2 (SKO), Pten (PKO) or both Shp2 and Pten (DKO) were characterized by increasing severity of liver tumorigenesis, representing excellent animal models to interrogate the underlying mechanisms of NASH-driven hepatocarcinogenesis.(40) However, detailed analysis of the RNA-sequencing data indicated no clear trend of immune-inhibitory gene expression profiles during the course of tumor initiation and progression in these mouse models (Fig. 3). CTNNB1 mutations and Myc overexpression are two frequently detected genetic lesions in human HCC. In mouse models, liver tumors driven by hydrodynamic injection of these oncogenes were poorly responsive to anti-PD-1 treatment.(41) Mechanistically, aberrant activation of the Wnt/β-catenin pathway led to impaired expression of chemokines, in particular CCF5, resulting in defective recruitment of dendritic cells and CD8+ T cells into the liver. These deficiencies contributed to immune escape of HCC and low response to PD-1 Ab, consistent with the observed association of β-catenin activation and resistance to anti-PD-1 therapy in human HCC.(42)
Figure 3. Expression of immune-inhibitory molecules in mouse NASH-HCC/ICC models.
As described previously,(40) RNA-sequencing was performed for liver samples of WT controls, hepatocyte-specific Pten KO (PKO), hepatocyte-specific Shp2 KO (SKO), and Pten and Shp2 double KO (DKO) mice, collected at different time points as indicated. STAR was used for sequence alignment, and Cuffdiff for FPKM calculation, to obtain normalized expression levels. No progressively increasing expressions of immune-inhibitory genes were observed during the course of tumor initiation and progression in these mouse tumor models.
Turning liver tumors from “cold” to “hot”
As discussed above, the most recent mouse and human data explain to certain extent why HCCs are in general cold tumors, poorly responsive to ICIs. Therefore, it is urgently necessary to determine whether and how the cold HCC tumors might be converted to hot. For this purpose, one rational approach is to explore treatment options based on mechanistic investigation in animal models. In experiments originally designed to dissect the communication between hepatocytes and Kupffer cells in driving hepatocarcinogenesis, our group unexpectedly found a potent tumor-inhibitory effect of polyinosinic-polycytidylic acid (polyIC) in mice.(43) polyIC is a synthetic double-stranded RNA (dsRNA) that potently activates pattern recognition receptors TLR3, RIG-I/MDA5 and protein kinase PKR.(44) This synthetic reagent has been used to induce the expression of Cre recombinase under control of the interferon-responsive Mx1 promoter.(45) polyIC administration robustly suppressed liver tumorigenesis driven by a chemical carcinogen or by hepatosteatosis in Pten-deficient liver, independent of Mx1-Cre-mediated gene deletion.(43) Furthermore, we demonstrated a potent inhibitory role of polyIC on liver cancer initiation, with no therapeutic effect on tumor progression.(43) Mechanistically, the synthetic dsRNA stimulated multiple innate immune populations including NK cells, M1 macrophages, and dendritic cells in the liver. Coordinated activation of these innate anti-tumor functions led to clearance of “high risk” transformed cells in the pre-cancer stages, resulting in prevention of liver cancer initiation.(43)
Injecting anti-PD-L1 Ab alone did not show any significant tumor-inhibitory effect in several mouse HCC models driven by oncogenes that are frequently detected in human HCCs.(46) Similar results were obtained by another group in transgenic mouse lines, suggesting low response rates to PD-L1/PD-1 blockade in HCC.(41) No significant PD-L1 expression was detected in tumor cells, surrounding hepatocytes or non-parenchymal cells during tumor progression, which would predict the low response in these animal models. The animal data are also consistent with the weak responses to ICIs observed in HCC patients. Interestingly, RNA-seq data analysis showed that polyIC stimulated hepatic expression of a variety of inflammatory and immune regulatory molecules, in particular CD274 (PD-L1) upregulation.(43) Further experiments demonstrated that polyIC-induced PD-L1 upregulation mainly in LSECs, with lower induction also detected in other non-parenchymal cells, including macrophages and dendritic cells in the liver.(46) LSECs have been recognized as a special structural and immune barrier against gut microbiota and a platform for nutrient uptake in the liver. More recent experiments demonstrated that LSECs function as a special type of antigen-presenting cell (APC) in the liver, capable of cross-presenting soluble exogenous antigens to CD8 T cells.(47) Circulating carcinoembryonic antigen was found to be taken up for cross-presentation by LSECs to induce CD8 T cell tolerance.(48)
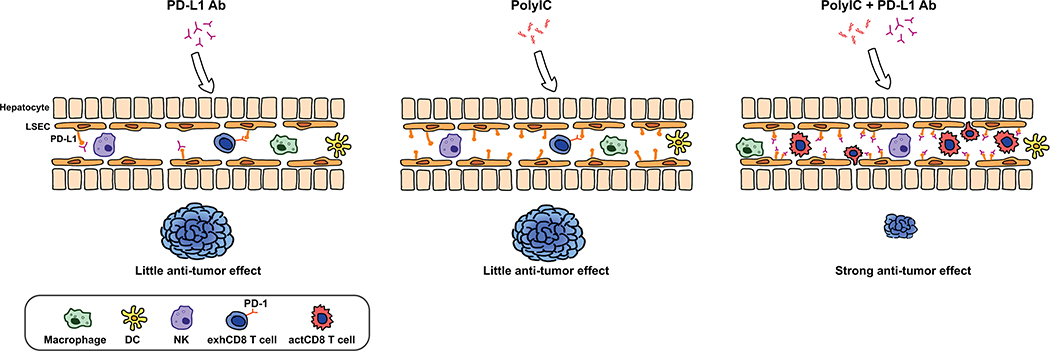
The robust induction of PD-L1 in LSECs prompted us to test a combined treatment of polyIC with PD-1/PD-L1 blockade. Indeed, the combination of polyIC and PD-L1 Ab induced a robust suppression of tumor progression in several mouse HCC models, as compared to either polyIC or PD-L1 Ab alone.(46) Importantly, this dramatic tumor-inhibitory effect was even observed when administering the two reagents at late stages of tumor progression in mice. Although the molecular basis remains to be elucidated, it is important to note the massive recruitment and infiltration of CD8+ T cells into the liver treated with the combination. We also found that polyIC-dependent induction of PD-L1 expression in LSECs was independent of tumor formation, as it was detected in both healthy and tumor-bearing livers. Thus, this approach does not rely on disruption of various immune evasion mechanisms by HCC tumors. Rather, it harnesses the unique hepatic innate immune functions, especially the specific role of LSECs in recruiting and activating cytotoxic CD8 T cells. In conclusion, these preclinical data in mice demonstrated a feasible approach to sensitize liver tumors to treatment with ICIs (Fig. 4). A combination of polyIC and PD-L1 Ab was reported to inhibit tumors grown subcutaneously from inoculated tumor cell lines.(49) However, either one of these two reagents alone also significantly inhibited subcutaneous tumor growth in that model. In contrast, we have obtained experimental results showing no growth-inhibitory effects of PD-L1 Ab on tumors grown in the liver from splenic injection of colon cancer cells, although tumors grown subcutaneously from the same cell line were suppressed by the PD-L1 Ab (Xin et al., unpublished data). Together, these results highlight the significance of interrogating HCC pathogenesis and therapy in the context of the special hepatic tumor microenvironment, using proper animal models.
Figure 4. A proposed strategy for sensitization of liver tumors to PD-1/PD-L1 blockade.
Among its many immune-regulatory functions, one prominent role of the synthetic dsRNA polyIC is to induce expression of PD-L1 on LSECs, macrophages and dendritic cells. The PD-L1 induction promotes the recruitment and infiltration of CD8 T cells, sensitizing the liver to PD-L1 Ab treatment. Indeed, a combination of polyIC and PD-L1 Ab showed a robust tumor-suppressing effect in mouse HCC models.(46) Therefore, we propose here that an effective combination immunotherapy can be developed by coordinated activation of multiple innate and adaptive immune functions, which render the cold liver tumors hot and responsive to ICIs.
Perspectives
There is no doubt that immunotherapy with ICIs has transformed oncological treatment. It is yet unclear how this paradigm-shifting strategy will benefit HCC patients, despite many ongoing clinical trials worldwide. Although the low response rates could be improved by selection of patients based on reliable biomarkers, one more transformative approach is to use additional agents to convert the low or un-responsive patients to ICI responders. Indeed, the most recent experimental data in mice has provided preclinical support for this intriguing prospect. As the proof of principle for ICI treatment was first demonstrated in mouse tumor models, we believe its improvement in HCC therapy also necessitates detailed mechanistic analysis and functional dissection of the hepatic anti-tumor immunity in animals. Combining preclinical and clinical studies will identify new immune-inhibitory molecules and pathways in liver tumorigenesis, which can become specific targets in HCC treatment. Despite extensive efforts, liver cancer research to date has not even achieved a single effective drug for liver cancer therapy, as sorafenib was originally developed for renal carcinoma and ICIs were first tested effective in other solid tumors. This disappointing situation can be changed by elucidating and harnessing special immune cell compositions, dynamics and functions in the liver to achieve a breakthrough in developing more efficacious therapeutic protocols.
Indeed, progress is being made in HCC research and therapy. While this manuscript was under review, a new report by Finn and collaborators showed that combination of atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) had better overall and progression-free survival than sorafenib in treating unresectable HCC (IMbrave 150).(50) In this global, open-label phase 3 trial (ClinicalTrials.gov number, NCT03434379), 336 patients received atezolizumab-bevacizumab treatment, with 165 patients given sorafenib. The overall survival at 12 months was 67.2% and median progression-free survival was 6.8 months in the atezolizumab-bevacizumab group, as compared to 54.6% and 4.3 months for the sorafenib group. As the first successful HCC therapy to improve survival beyond sorafenib, this study offers great promise for combination immunotherapy.
Acknowledgments
The authors wish to apologize for not citing many other important papers in this concise review article. The liver cancer research projects in the authors’ laboratory have been supported by NIH grants R01CA188506, CA236074 and CA239629.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Zhang B, Zhang Z, Huang Z, Chen Y, Chen M, Bie P, et al. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci 2018;61:660–670. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res 2014;20:2072–2079. [DOI] [PubMed] [Google Scholar]
- 11.Feng GS. Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell 2012;21:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565–594. [DOI] [PubMed] [Google Scholar]
- 13.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562–567. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020;37:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 22.Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015;62:1420–1429. [DOI] [PubMed] [Google Scholar]
- 23.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 2014;60:1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–88. [DOI] [PubMed] [Google Scholar]
- 25.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 27.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian M, Shi Y, Liu W, Fan J. Immunotherapy of hepatocellular carcinoma: strategies for combinatorial intervention. Sci China Life Sci 2019;62:1138–1143. [DOI] [PubMed] [Google Scholar]
- 29.Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol 2019;70:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology 2008;47:729–736. [DOI] [PubMed] [Google Scholar]
- 31.Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222–232. [DOI] [PubMed] [Google Scholar]
- 32.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996–1006. [DOI] [PubMed] [Google Scholar]
- 33.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015;12:681–700. [DOI] [PubMed] [Google Scholar]
- 34.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res 2004;10:4332–4341. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi K Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatogastroenterology 1998;45 Suppl 3:1254–1258. [PubMed] [Google Scholar]
- 36.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991;68:1524–1530. [DOI] [PubMed] [Google Scholar]
- 37.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017;169:1342–1356 e1316. [DOI] [PubMed] [Google Scholar]
- 38.Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwon SM, Forgues M, et al. Common Molecular Subtypes Among Asian Hepatocellular Carcinoma and Cholangiocarcinoma. Cancer Cell 2017;32:57–70 e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo X, Liao R, Hanley KL, Zhu HH, Malo KN, Hernandez C, Wei X, et al. Dual Shp2 and Pten Deficiencies Promote Non-alcoholic Steatohepatitis and Genesis of Liver Tumor-Initiating Cells. Cell Rep 2016;17:2979–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G, Luo X, Liang Y, Kaneko K, Li H, Fu XD, Feng GS. A tumorigenic index for quantitative analysis of liver cancer initiation and progression. Proc Natl Acad Sci U S A 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 2019;9:1124–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin Cancer Res 2019;25:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J, Liao R, Wang G, Yang BH, Luo X, Varki NM, Qiu SJ, et al. Preventive Inhibition of Liver Tumorigenesis by Systemic Activation of Innate Immune Functions. Cell Rep 2017;21:1870–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science 2003;300:1524–1525. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science 1995;269:1427–1429. [DOI] [PubMed] [Google Scholar]
- 46.Wen L, Xin B, Wu P, Lin CH, Peng C, Wang G, Lee J, et al. An Efficient Combination Immunotherapy for Primary Liver Cancer by Harmonized Activation of Innate and Adaptive Immunity in Mice. Hepatology 2019;69:2518–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med 2000;6:1348–1354. [DOI] [PubMed] [Google Scholar]
- 48.Hochst B, Schildberg FA, Bottcher J, Metzger C, Huss S, Turler A, Overhaus M, et al. Liver sinusoidal endothelial cells contribute to CD8 T cell tolerance toward circulating carcinoembryonic antigen in mice. Hepatology 2012;56:1924–1933. [DOI] [PubMed] [Google Scholar]
- 49.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res 2014;20:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 51.Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 2018;8:9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menyhart O, Nagy A, Gyorffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci 2018;5:181006. [DOI] [PMC free article] [PubMed] [Google Scholar]