Abstract
Background
Home-Based Kidney Care (HBKC) is a pragmatic treatment approach that addresses patient preferences and cultural barriers to healthcare. We previously reported the results of a clinical trial of HBKC vs. usual care in a cohort of Zuni Indians in New Mexico. This study investigated the potential for differential efficacy of HBKC vs. usual care according to type 2 diabetes (T2DM) status.
Methods
We analyzed the data from all individuals who participated in a randomized clinical trial that compared HBKC to usual care among patients with CKD, and assessed whether the effect of the HBKC intervention affected the subset of patients with T2DM differently than those individuals without T2DM. We used linear regression models to estimate the effect of HBKC on improvement in Patient Activation Measure (PAM) total scores within the groups of participants defined by T2DM status, and to compare the effects between these two groups. We used generalized estimating equations (GEE) to account for household clustering.
Results
The original study enrolled 63 participants into the HBKC group, and 62 into the usual care. Ninety-eight of these individuals completed the 12-month intervention, 50 in the HBKC group and 48 in the usual care group. The present study compared the intervention effect in the 56 participants with T2DM (24 participants in the HBKC group and 32 in usual care) to the intervention effect in the 42 participants without T2DM (26 participants in the HBKC group and 16 in usual care). Those with T2DM who received the HBKC intervention experienced an average increase in PAM total scores of 16.0 points (95% Confidence Interval: 8.8 - 23.1) more than those with T2DM who were in the usual care group. For those without T2DM, the intervention had essentially no effect, with those who received the HBKC intervention having an average PAM total scores that was 1.4 points (95% C.I.: −12.4 to 9.6) lower than those who received usual care. There was a significantly different HBKC treatment effect by T2DM status (p=0.02).
Conclusion
This secondary analysis suggests that the effectiveness of this HBKC intervention on increasing patient activation is most notable among those CKD patients who also have T2DM.
Keywords: Diabetes, Chronic Kidney Disease, Zuni Indians, Patient Activation Measure, Randomized controlled trial
1. Background
Diabetes is the leading cause of advanced kidney disease worldwide (1). In the United States, chronic kidney disease (CKD) affects more than one fourth of the adult population with type 2 diabetes mellitus (T2DM) (2). The burden of CKD is greater in ethnic and racial minorities, and in rural communities where access to healthcare is limited (3). The Zuni Indians have higher rates of chronic diseases than the US population generally, with high rates of diabetes and CKD being particularly notable. The majority of Zuni Indians in New Mexico live in remote parts of the state and subsequently have limited access to health care (4). Health disparities in this population are worsened not only by challenges with access to healthcare, but also by deep-rooted cultural barriers. The Zuni Indians have experienced considerable historical and cultural trauma that has led to hesitation in seeking available health screening and healthcare (5). Fear and mistrust of healthcare providers due to the high turnover rate of medical staff at the Indian Health Services were identified as barriers within the health care system (4). Home based interventions may provide an important alternative source of care. Our study participants with T2DM and CKD often have poor access to care and treatment and thereby suffer from a host of adverse outcomes. Nearly all therapies aimed at preventing the progression of CKD in individuals with diabetes and reducing associated morbidities rely heavily on patient driven medical care including adhering to medication regimens, avoiding habits/situations that can further exacerbate the disease, and following a regimented diet. Patients’ with diabetic CKD often lack sufficient knowledge, have low levels of self-efficacy, and a poor ability to self-manage their CKD, all factors that have been shown to contribute to increased complications from CKD that often limit the potential for improved health outcomes associated with provider recommendations. Due to the multiple and ongoing needs of patients with T2DM and CKD, it is not feasible that a busy practitioner can deliver the well-rounded care that is needed for optimal outcomes in the current health care setting. The HBKC uses Community Health Representatives (CHRs) to increase access to care; aid in triaging patients to maximize available health system resources; reduce costs through patient education, screening, detection and basic care; and improve quality by contributing to patient-provider communication and continuity of care.
In a 12-month clinical trial, we found that a home-based kidney care intervention designed to address barriers to CKD care significantly improved CKD patients’ inclination to take a more active role in the management of their chronic health condition (6). Because of the high proportion of participants who had T2DM, and due to the potentially differential impact of healthcare interventions on patients according to their T2DM status, we wished to determine whether the intervention applied in the parent clinical trial influenced patients’ activation in their own healthcare differently according to T2DM status. Hence, the principal objective of this secondary analysis was to examine whether the treatment effect of the home-based kidney care intervention differed between groups of patients defined by T2DM status. We hypothesized that patients with T2DM would be more likely to experience a change in the engagement in their health care in response to the intervention than those who did not have T2DM.
2. Methods
This study is a secondary analysis of data collected for a clinical trial in patients with CKD that focused on the effectiveness of a Home-Based Kidney Care intervention on patient activation. The current study evaluates the potential that the participants’ T2DM status may modify the effectiveness of the intervention on the primary outcome. The methods of the parent trial (ClinicalTrials.gov number NCT02915029) approved under University of New Mexico Human Research Institutional Review board have already been reported (6). In brief, potential candidates from a previously established cohort were screened for relevant clinical factors (7). Of the 315 individuals screened for eligibility, 127 met the inclusion criteria of being a member of the Zuni Pueblo between the ages of 21 and 80 and having urine albumin to creatinine ratio ≥ 30 kg/m2, hemoglobin A1c ≥ 7%, or a family history of diabetes and kidney disease. All but two of the eligible individuals agreed to participate in the study, and 63 were randomized to the home-based kidney care (HBKC) intervention and 62 were assigned to the usual care control group. As more than one individual from a single household could participate in the study, and because the intervention was provided at home, randomization was performed on a household level; the 125 randomized individuals were members of 96 households that were randomized 1:1 to the two treatment arms. Neither the investigators nor the participants were blinded to the intervention because of the nature of the intervention: home-based or usual care at a local Indian Health Service clinic.
The intervention was designed to enhance a participant’s activation in his or her healthcare, and was provided to those in the HBKC treatment arm by community health workers who were members of the Zuni Pueblo and were specifically trained to deliver the intervention. The intervention provided education and care at patients’ homes, taking into consideration that patients normally feel more comfortable when receiving care in a private setting from people who are members of their communities.
The primary outcome for the randomized trial, and therefore for this secondary analysis, was a change in the total score of the Patient Activation Measure (PAM) from baseline to the end of the 12-month study period. The PAM score is from a validated tool that assesses a patient’s engagement – or activation – in his or her care (8). The scores are further categorized into 4 levels: (Level 1) believing the patient’s role is important but not taking action; (Level 2) having the confidence and knowledge necessary to take action; (Level 3) taking action to maintain and improve one’s health; and (Level 4) staying the course even under stress. PAM levels of 3 and higher were used to indicate that a patient is activated and engaged in his or her own healthcare. Secondary outcomes of interest included body mass index (BMI), blood pressure (systolic and diastolic), hemoglobin A1c, serum glucose, serum triglycerides, serum high-density lipoproteins (HDL) cholesterol, serum low-density lipoproteins (LDL) cholesterol, estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR), high sensitivity C-reactive protein (hsCRP), Morisky score, and health related quality of life assessed by the Kidney Disease Quality of Life survey (KDQOL-36) (9).
We estimated changes in PAM total scores from baseline to 12-months follow-up within four groups, defined at baseline by the combinations of treatment assignment and the presence of a T2DM diagnosis at baseline. We subsequently estimated treatment effectiveness within the T2DM and no-T2DM groups as the observed between-treatment difference within the corresponding group. We tested for the presence of a differential treatment effect by T2DM status by evaluating the statistical significance of the interaction between treatment groups and T2DM groups in the within-person changes from baseline to 12-months follow-up while accounting for baseline levels observed within each participant of each outcome. These comparisons were accomplished using generalized linear models approaches while accounting for within-household clustering with generalized estimating equations (10). We log-transformed three variables: triglycerides, urine ACR and high sensitivity CRP, to better meet statistical assumptions (11), leading to a change in the interpretation of the estimated effects from an absolute to a relative difference between the two study groups (12). We used logistic regression models to examine changes in the binary classification of whether a patient was activated, as defined by being classified into PAM Levels of 3 or 4. As with the other comparisons, we adjusted for baseline PAM level using a covariate, and accounted for within-household clustering using generalized estimating equations. We used odds ratios to summarize the treatment effect within T2DM groups, and compared the significance of the difference between T2DM groups by testing for an interaction between treatment group and T2DM status. Statistical significance was declared for two-sided p-values less than 0.05. Statistical analyses were performed using SAS/STAT® and R software packages. Trial registration is at ClinicalTrials.gov NCT02915029
3. Results
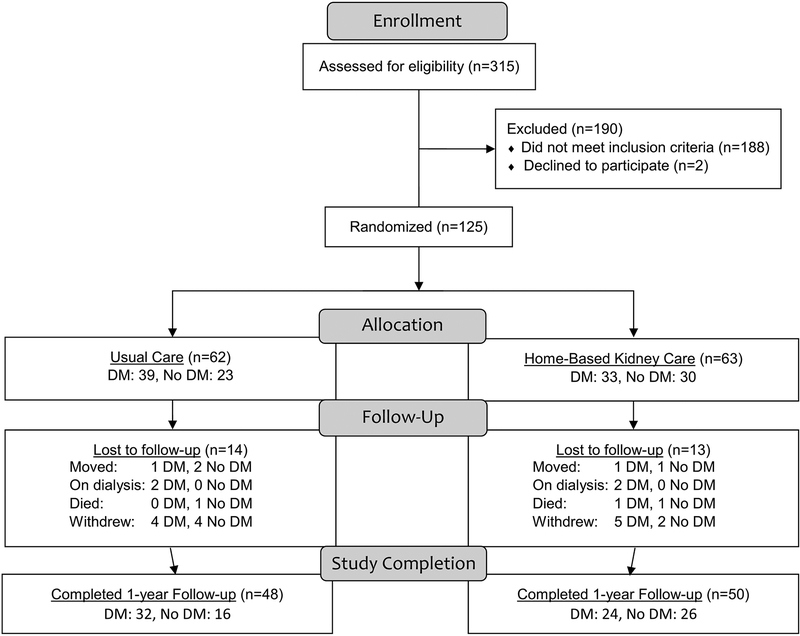
A total of 125 participants were enrolled into the original study, 63 into the HBKC intervention group and 62 into the usual care group. After 12 months, 50 individuals from the HBKC group, and 48 from the usual care group provided follow-up data. Of those completing the full 12-month study, 56 had T2DM and 42 did not. Of the 56 participants with T2DM, 24 were assigned to HBKC and 32 to usual care. Of the 42 non-T2DM participants, 26 were assigned to HBKC and 16 to usual care. The flow of participants in this study, from enrollment to randomization into the intervention and usual care groups and finally to selection for T2DM, is shown in Figure 1.
Figure 1.
Data Flowchart
Baseline characteristics of the individuals with and without T2DM in the two treatment groups are presented in Table 1. In those with T2DM, the average serum LDL cholesterol levels were higher in HBKC (mean=125 mg/dl, standard deviation [SD]=43) vs. the usual care group (mean=105 mg/dl, SD=43). Those with T2DM assigned to the HBKC group also had higher hsCRP levels (mean=10.7 mg/L, SD=13.2) than those assigned to usual care (mean=3.6 mg/L, SD=3.6). Among those without T2DM, higher hsCRP levels and lower SF-12 Mental scores were observed in the HBKC group (hsCRP: mean=4.7 mg/L, SD=4.4; SF-12 Mental: mean=47.5 points, SD=8.1) than in the usual care group (hsCRP: mean=2.2 mg/L, SD=1.4; SF-12 Mental: mean=54.3 points, SD=8.0).
Table 1.
Baseline comparisons between participants with and without diabetes in the two treatment groups.
| Diabetes | Non-diabetes | |||||
|---|---|---|---|---|---|---|
| Usual Care, n=32 | Home-Based Kidney Care, n=24 | Usual Care, n=16 | Home-Based Kidney Care, n=26 | |||
| Characteristic | Mean (±SD) or n (%a) | Mean (±SD) or n (%a) | p-valueb | Mean (±SD) or n (%a) | Mean (±SD) or n (%a) | p-valueb |
| Demographics | ||||||
| Age, yr | 50.9 (±13.0) | 47.4 (±11.2) | 0.29 | 46.8 (±10.8) | 44.5 (±10.3) | 0.50 |
| Women | 16 (50%) | 16 (66.7%) | 0.28 | 4 (25.0%) | 10 (38.5%) | 0.51 |
| High school education | 22 (68.8%) | 17 (70.8%) | 1.00 | 13 (81.3%) | 16 (61.5%) | 0.30 |
| Primary outcome measures | ||||||
| Patient activation total score | 63.0 (±11.2) | 57.3 (±19.1) | 0.20c | 67.0 (±21.6) | 64.7 (±22.8) | 0.75 |
| Patient activation level ≥ 3 | 25 (78.1%) | 17 (70.8%) | 0.55 | 14 (87.5%) | 18 (69.2%) | 0.27 |
| Secondary outcome measures | ||||||
| Body mass index, kg/m2 | 31.4 (±7.0) | 32.8 (±7.8) | 0.49 | 31.5 (±6.5) | 31.8 (±8.2) | 0.91 |
| BP, mm HG | ||||||
| Systolic | 127 (±15.5) | 128 (±12.2) | 0.66 | 137 (±18.8) | 128 (±20.0) | 0.17 |
| Diastolic | 82 (±10.5) | 80 (±12.9) | 0.65 | 89 (±14.6) | 86 (±13.3) | 0.51 |
| HbA1c, % | 8.5 (±2.3) | 9.1 (±2.6) | 0.37 | 5.6 (±0.5) | 5.9 (±1.1) | 0.30c |
| Glucose, mg/dl | 167.3 (±81.2) | 182.9 (±92.4) | 0.50 | 96.6 (±11.4) | 104.8 (±31.0) | 0.23c |
| Serum HDL cholesterol, mg/dl | 46 (±1.4) | 47 (±1.5) | 0.65c | 65 (±12.6) | 57 (±23.3) | 0.16c |
| Serum LDL cholesterol, mg/dl | 105 (±30.5) | 125 (±42.5) | 0.04 | 115 (±37.2) | 118 (±41.1) | 0.80 |
| eGFR, ml/min per 1.73 m2 | 115 (±63.7) | 149 (±63.2) | 0.05 | 156 (±67.6) | 154 (±79.0) | 0.92 |
| Urine ACR, mg/g | 1082.0 (±1631.6) | 598.3 (±1372.1) | 0.25 | 267.3 (±417.9) | 322.0 (±487.5) | 0.71 |
| hsCRP, mg/L | 3.6 (±3.6) | 10.7 (±13.2) | 0.02c | 2.2 (±1.4) | 4.7 (±4.4) | 0.01c |
| Morisky scored | 3.9 (±2.2) | 4.3 (±1.8) | 0.41 | 5.9 (±2.2) | 6.8 (±2.0) | 0.19 |
| KDQDL measures | ||||||
| SF-12 physical score | 44.9 (±8.3) | 45.2 (±9.2) | 0.93 | 45.6 (±10.4) | 46.6 (±9.3) | 0.76 |
| SF-12 mental score | 49.9 (±10.1) | 45.4 (±11.7) | 0.12 | 54.3 (±8.0) | 47.5 (±8.1) | 0.01 |
%=column percentage
p-value corresponds to the two sample independent T-test for continuous variables and the χ2 test for categorical variables.
An indication of using the Satterthwaite p-value for having the constant variance assumption violated.
Use of the Morisky Medication Adherence Scale is protected by United States copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, Department of Community Health Sciences, University of California, Los Angeles School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095–1772.
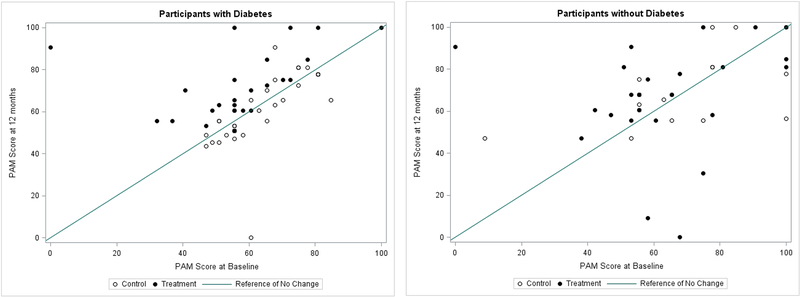
Scatterplots of the PAM scores at 12 months versus the PAM scores at baseline illustrate the changes observed over the course of the study (Figure 2). In the group of participants with T2DM, almost all individuals in the HBKC treatment group experienced an improvement in PAM score, while there was little change among those in the usual care group, from baseline to 12 months (left panel). In the group of participants without T2DM, no consistent PAM score changes were evident in either treatment group. The variation between baseline and 12-month PAM scores was considerably greater in this group than in the group of patients with T2DM (right panel). Table 2 shows the estimates of the HBKC treatment effects for the two groups of participants defined by T2DM status, and reports p-values that test the null hypothesis that there is no differential treatment effect by T2DM status. The participants with T2DM who received the HBKC intervention experienced an average increase in PAM score that was significantly higher than the average change experienced by those in the usual care group by 16.0 points (95% C.I.: 8.8 to 23.1). Those without T2DM who received the HBKC intervention exhibited a change in their average total PAM scores that was 1.4 points lower, though not significantly, than those who received usual care (95% C.I.: −12.4 to 9.6). These HBKC treatment effects were significantly different between the two groups defined by T2DM status (p=0.02). Similarly, participants in the treatment group were 8.4 times more likely to become “activated” at 12 months compared to the control group. Additionally, the odds ratios estimating the changes from baseline to follow-up in PAM activation levels for the T2DM and no-T2DM groups were 9.7 (95% C.I.: 1.7 to 54.3) and 0.8 (95% C.I.: 0.1 to 11.0), respectively. However, the difference between these two odds ratios was not statistically significant (p=0.07). The two secondary outcome measures that displayed meaningful differences between treatment groups in the initial clinical trial were BMI and hsCRP. These two secondary outcomes with significant main effects did not provide evidence of differential treatment effects by T2DM status (see Table 2).
Figure 2:
(left panel): PAM score at 12 months versus at baseline for participants with diabetes (left panel) and for participants without diabetes (right panel. A reference line of no change over time is shown, along with different plotting symbols for those in the HMKC (Treatment) and usual care (Control) groups.
Table 2.
Estimated treatment effects (HBKC mean change minus Usual Care mean change) within groups defined by baseline diabetes status, with tests for the significance of the differences in treatment effects observed in those with and without diabetes.
| Diabetes | Non-diabetes | ||
|---|---|---|---|
| Characteristic | Treatment Effect Δ a (95% C.I.) | Treatment Effect Δ a (95% C.I.) | p-valuec |
| Primary outcome measure | |||
| Patient activation total score | 16 (8.8 to 23.1) | −1.4 (−12.4 to 9.6) | 0.02 |
| Secondary outcome measures | |||
| Body mass index, kg/m2 | −1.2 (−2.2 to −0.2) | −1.2 (−2.4 to 0.0) | 0.93 |
| BP, mm HG | |||
| Systolic | −3.7 (−11.2 to 3.7) | −3.6 (−12.8 to 5.5) | 0.75 |
| Diastolic | −1.9 (−7.4 to 3.6) | −4.3 (−9.3 to 0.8) | 0.50 |
| HbA1c, % | −0.8 (−1.9 to 0.4) | −0.1 (−0.3 to 0.2) | 0.30 |
| Glucose, mg/dl | −3 (−47.5 to 41.6) | −2.2 (−11.5 to 7.0) | 0.98 |
| Serum HDL cholesterol, mg/dl | −0.1 (−7.9 to 7.7) | 2.8 (−7.0 to 12.5) | 0.39 |
| Serum LDL cholesterol, mg/dl | 4.9 (−11.2 to 21) | 3.1 (−11.4 to 17.6) | 0.96 |
| eGFR, ml/min per 1.73 m2 | 9.1 (−12.6 to 30.9) | 4.9 (−19.4 to 29.2) | 0.66 |
| Urine ACR, mg/gb | −0.5 (−1.2 to 0.3) | −0.4 (−1.2 to 0.3) | 0.22 |
| hsCRP, mg/Lb | −0.7 (−1 to −0.3) | −0.8 (−1.2 to −0.5) | 0.69 |
| KDQDL measures | |||
| SF-12 physical score | 2 (−3.2 to 7.1) | 0.7 (−3.0 to 4.5) | 0.72 |
| SF-12 mental score | 1.2 (−3.3 to 5.8) | 4.0 (−1.6 to 9.6) | 0.71 |
Estimated between-group differences of within-person changes from baseline to 12-months
Log-transformed, therefore the reported Δ represents a fold-change comparison of the HBKC group vs. the control group.
p-value corresponds to the significance of the interaction between treatment group and diabetes status.
Use of the Morisky Medication Adherence Scale is protected by United States copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, Department of Community Health Sciences, University of California, Los Angeles School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095–1772.
4. Discussion
Patient-centered care emphasizes a strong partnership between patients and clinicians to create an environment where patients gain information necessary to actively engage in decisions related to their own health. In patients with evidence of diabetes and kidney disease, understanding of basic concepts about diabetes related events including kidney function, symptoms of progressing kidney failure, and one’s own disease status is unsatisfactory, even amongst those actively engaged in endocrinology and nephrology specialist care. Health literacy such as numeracy skills can promote a greater level of self-efficacy, leading to increased adherence to management particularly in diabetes patients (13, 14). Further, low self-engagement in CKD patients is associated with poor clinical outcome. This relationship is worsened with diabetes (15). The intervention of interest in this work addresses preexisting cultural barriers to increase patient engagement in their own healthcare.
Although a high proportion of the participants in the randomized controlled trial had T2DM, the evaluation of the primary results of the intervention did not shed light on the effect of the intervention on the CKD patients with T2DM. We were therefore interested in learning whether the intervention was effective for these patients, even when it was not uniquely designed for intervening in this specific disease. The effect of the HBKC intervention that we had observed in the parent clinical trial was completely described by its effectiveness among individuals with T2DM. The HBKC intervention group had PAM scores that increased on average by 16.0 points (95% C.I.: 8.8 to 23.1) among the participants with T2DM. Those without T2DM who received the HBKC intervention exhibited an average change in total PAM scores that was actually lower, by 1.4 points, than the average change observed among those receiving usual care (95% C.I.: −12.4 to 9.6). This secondary analysis confirms that the intervention had a significant impact on improving the activation of T2DM patients in the management of their own health care. In fact, the magnitude of the effect was even higher in those with T2DM than what was observed in the parent study, suggesting that the intervention may be more effective for CKD patients with T2DM than for those without T2DM. We did not see statistically significant improvements in clinical indicators in response to the intervention in either the T2DM or nondiabetic groups as we did in the primary analysis. This may be attributable to reduced power to detect these changes in the subsets defined by the presence or absence of T2DM. Nevertheless, results of the primary intervention suggest that greater patient activation may ultimately lead to better clinical outcomes. Larger validation studies are currently on going to evaluate this hypothesis in four indigenous communities in those with T2DM.
The observed intervention effect on CKD patients with T2DM could be explained in multiple ways. Diabetes is effectively managed with potentially modifiable diabetes care factors such as model of care and patient behavior as opposed to non-modifiable diabetes care factors such as demographic characteristics and clinical comorbidities (16–19). People with both T1DM and T2DM are more and more expected to be active patients that is to be in charge of their own health and the healthcare they receive. The delivery of appropriate care is of particular importance in obtaining successful T2DM outcomes, and most healthcare providers agree on the form that this care should take (19). In contrast, research regarding the most effective model of care for CKD management is still ongoing (20, 21). The results from this study supports that there may be differences in the most effective model of care for CKD patients who do not have diabetes compared to those presenting with T2DM. This contrast may be heightened in our cohort due to the cultural and socioeconomic barriers that exist in rural New Mexico.
Previous research has found that increased patient activation is associated with improved outcome in many chronic conditions (22–24). In particular, in 2020 meta-analysis of ten randomized controlled trial estimating the effect of patient activation on T2DM management in 3728 total patients, Almutairi et al. found that patient activation lead to improved glycemic control measured by reduction of HbA1c level (25). They highlighted physical activity, healthy diet and foot care as mode of improved outcome. More activated patients with diabetes and pre-diabetes had better outcomes than less activated patients did. More activated patients without diabetes or pre-diabetes were less likely to develop pre-diabetes over a three-year period (26). Miller et al evaluated the effects of the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) program on patient activation in adults living with T2DM. Findings from this study showed that the structured DESMOND program effectively improved patient activation in individuals with T2D in a routine, real-world environment. Overall, more than half of all participants exhibited a clinically significant improvement of at least 5 points in PAM score. Furthermore, the proportion of people scoring in the highest level of activation (PAM level 4, 72.5–100) almost doubled from pre to post-DESMOND (27). Many other studies showed a graduated association between activation stage and frequent attendance at primary care for chronic conditions including cancer. Furthermore, patient activation reported as independently associated with lower health care consultation rates among people with a cardiovascular condition (28). The reported association of PAM with cancer patient experiences from diagnosis to survivorship suggested that less activated cancer patients were more vulnerable to poor experiences and outcomes (29).
Although our study showed higher patient engagement, we did not observe statistically significant clinical outcomes such as reduced HbA1c level. Our study length may not have been long enough to capture the long-term effects of patient activation given the historical background and geographical uniqueness in our cohort. While the change in clinical measures may take time, the strength in the design of our intervention lies in the culturally sensitive delivery of care that increases adherence to a healthy diet and physical activity identified as mode of improved outcome.
Strengths of this post-hoc analysis include the study population, which is at high risk of progressive CKD and has a high prevalence of T2DM, and the use of CHRs trusted by the community to deliver the intervention. Although the specific approach used to engage the Zuni Indians in their healthcare may not be relevant in another community that does not share the same culture and beliefs, the concept of culturally-relevant interventions may be generalizable. Limitations include the small sample size, which was further accentuated in the present study by comparing differential effects of the intervention in two subsets of the clinical trial participants, those with and without T2DM. Currently, we are addressing this limitation by studying the role of PAM in diabetes and CKD care in a larger study of four indigenous communities. Another limitation is that this was a post-hoc analysis of a clinical trial designed and powered to address a different and simpler research question. Nevertheless, a statistically significant difference in intervention effects was observed between those with and without T2DM, suggesting that the awareness of the risks posed by CKD and the willingness to actively engage in their own healthcare may be greater in those who know they have a chronic disease that predisposes them to CKD.
5. Conclusion
Our study addresses two critical needs for patients with diabetic CKD: additional knowledge about self-care to prevent diabetic CKD and reduce the impact of the disease on quality of life, and prevention tools and techniques that are culturally relevant, appropriate, and accessible (30). This secondary analysis of the HBKC study demonstrated that behavioral and lifestyle educational reinforcement through alternate weekly home visits by the CHRs with quarterly group sessions was an effective means of providing care to Zuni patients with T2DM and CKD who may otherwise avoid diagnosis and treatment due to stigmatization. The CHR-led HBKC model provided the additional care necessary to bolster patient levels of disease-specific knowledge, self-efficacy, and diabetes and CKD self-management, enabling the patients to more effectively carry out the recommendations that they received during the home visit as compared to patients who received clinic-based usual care. In conclusion, the community efforts to increase awareness and understanding of kidney disease, its risks, and care may complement health-system based strategies in the fight against kidney disease, particularly among those with diabetes.
Highlights.
The burden of chronic kidney disease is greater in ethnic and racial minorities and in rural communities where access to healthcare is limited.
The Patient Activation Measure is a validated scoring tool that assesses patient’s ability to effectively participate in his or her own healthcare.
In settings where healthcare is limited, home-based intervention for chronic kidney disease provided by trained community health representatives improves participant activation in their own health and healthcare relative to standard clinical practice.
Home-based kidney care increased the Patient Activation Measure score in Zuni Indians with diabetes and chronic kidney disease relative to usual care.
The efficacy of this intervention vs. usual care was greater in patients with diabetes than in those without diabetes.
Acknowledgements
We thank Dr. Kathleen Colleran and Dr. Bruce Struminger for their role in the design and conduct of the study. We also thank the tribal stakeholders, including the Zuni tribal Governor and his council members, as well as the tribal advisory panel members who contributed to the logistics of study-related activities. Finally, we sincerely thank the Zuni people for welcoming us into their lives. Individual de-identified participant data shared according to the policies of the Patient-Centered Outcomes Research Institute (PCORI) as described at https://www.pcori.org/research-results/2013/reducing-health-disparity-chronic-kidney-disease-zuni-indians.
This research was supported by Patient-Centered Outcomes Research Institute (PCORI) award AD-12-11-5532 and AD-1511-33553 (to V.O.S.) and the Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases. V.O.S is also supported by R01 DK119199-A1 grant from NIH and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103451.
Abbreviations
- CHR
Community Health Representative
- CKD
Chronic kidney disease
- HBKC
Home-based kidney care
- PAM
Patient activation measure
- RCT
Randomized controlled trial
- T2DM
Type 2 diabetes mellitus
Footnotes
Conflict of interest
None of the authors listed has conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The Global Epidemiology of Diabetes and Kidney Disease. Adv Chronic Kidney Dis. 2018;25(2):121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014. JAMA. 2016. August 9;316(6):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews DC, Bello AK, Saadi G, World Kidney Day Steering Committee. Burden, Access, and Disparities in Kidney Disease. Am J Hypertens. 2019. 16;32(4):433–9. [DOI] [PubMed] [Google Scholar]
- 4.Shah VO, Ghahate DM, Bobelu J, Sandy P, Newman S, Helitzer DL, et al. Identifying barriers to healthcare to reduce health disparity in Zuni Indians using focus group conducted by community health workers. Clin Transl Sci. 2014. February;7(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitbeck LB, Adams GW, Hoyt DR, Chen X. Conceptualizing and measuring historical trauma among American Indian people. Am J Community Psychol. 2004. June;33(3–4):119–30. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RG, Pankratz VS, Ghahate DM, Bobelu J, Faber T, Shah VO. Home-Based Kidney Care, Patient Activation, and Risk Factors for CKD Progression in Zuni Indians: A Randomized, Controlled Clinical Trial. CJASN. 2018. December 7;13(12):1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacCluer JW, Scavini M, Shah VO, Cole SA, Laston SL, Voruganti VS, et al. Heritability of measures of kidney disease among Zuni Indians: the Zuni Kidney Project. Am J Kidney Dis. 2010. August;56(2):289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Educ Couns. 2005. January;56(1):28–34. [DOI] [PubMed] [Google Scholar]
- 9.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008. May;10(5):348–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988. December;44(4):1049–60. [PubMed] [Google Scholar]
- 11.Biswas A, Datta S, Fine JP, Segal MR. Statistical Advances in the Biomedical Sciences: Clinical Trials, Epidemiology, Survival Analysis, and Bioinformatics. John Wiley & Sons; 2007. 624 p. [Google Scholar]
- 12.StatNews #83 Interpreting Coefficients in Regression with Log-Transformed Variables [Article Online], 2012. Available from: https://www.yumpu.com/en/document/read/51760991/statnews-83-interpreting-coefficients-in-regression-with-log-Accessed 7 July 2020
- 13.Huang Y-M, Shiyanbola OO, Chan H-Y. A path model linking health literacy, medication self-efficacy, medication adherence, and glycemic control. Patient Educ Couns. 2018;101(11):1906–13. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y-M, Shiyanbola OO, Chan H-Y, Smith PD. Patient factors associated with diabetes medication adherence at different health literacy levels: a cross-sectional study at a family medicine clinic. Postgraduate Medicine. 2020. April 1;0(0):1–9. [DOI] [PubMed] [Google Scholar]
- 15.Schrauben SJ, Hsu JY, Rosas SE, Jaar BG, Zhang X, Deo R, et al. CKD Self-management: Phenotypes and Associations With Clinical Outcomes. Am J Kidney Dis. 2018;72(3):360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiyanbola OO, Walbrandt Pigarelli DL, Unni EJ, Smith PD, Maurer MA, Huang Y-M. Design and rationale of a mixed methods randomized control trial: ADdressing Health literacy, bEliefs, adheRence and self-Efficacy (ADHERE) program to improve diabetes outcomes. Contemp Clin Trials Commun. 2019. June;14:100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y-M, Shiyanbola OO, Smith PD. Association of health literacy and medication self-efficacy with medication adherence and diabetes control. Patient Prefer Adherence. 2018;12:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiyanbola OO, Unni E, Huang Y-M, Lanier C. The association of health literacy with illness perceptions, medication beliefs, and medication adherence among individuals with type 2 diabetes. Res Social Adm Pharm. 2018;14(9):824–30. [DOI] [PubMed] [Google Scholar]
- 19.Lauffenburger JC, Lewey J, Jan S, Lee J, Ghazinouri R, Choudhry NK. Association of Potentially Modifiable Diabetes Care Factors With Glycemic Control in Patients With Insulin-Treated Type 2 Diabetes. JAMA Netw Open. 2020. January 3;3(1):e1919645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicoll R, Robertson L, Gemmell E, Sharma P, Black C, Marks A. Models of care for chronic kidney disease: A systematic review. Nephrology (Carlton). 2018. May;23(5):389–96. [DOI] [PubMed] [Google Scholar]
- 21.Smekal MD, Tam-Tham H, Finlay J, Donald M, Thomas C, Weaver RG, et al. Patient and provider experience and perspectives of a risk-based approach to multidisciplinary chronic kidney disease care: a mixed methods study. BMC Nephrol. 2019. 29;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes EL, Long MD, Kappelman MD, Martin CF, Sandler RS. High Patient Activation Is Associated With Remission in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019. June 18;25(7):1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronin RM, Dorner TL, Utrankar A, Allen W, Rodeghier M, Kassim AA, et al. Increased Patient Activation Is Associated with Fewer Emergency Room Visits and Hospitalizations for Pain in Adults with Sickle Cell Disease. Pain Med. 2019. 01;20(8):1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney RL, Lemon SC, Person SD, Pagoto SL, Saczynski JS. The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: a systematic review. Patient Educ Couns. 2015. May;98(5):545–52. [DOI] [PubMed] [Google Scholar]
- 25.Almutairi N, Hosseinzadeh H, Gopaldasani V. The effectiveness of patient activation intervention on type 2 diabetes mellitus glycemic control and self-management behaviors: A systematic review of RCTs. Prim Care Diabetes. 2020;14(1):12–20. [DOI] [PubMed] [Google Scholar]
- 26.Sacksa Rebecca M., Greene Jessica, Hibbard Judith, Overton Valerie, Parrotta Carmen D. Does patient activation predict the course of type 2 diabetes? A longitudinal study. Patient Education and Counseling 100 (2017) 1268–1275. [DOI] [PubMed] [Google Scholar]
- 27.Miller Venus M., Davies Melanie J., Christopher Etherton-Beer Sophie McGough, Schofield Deborah, Jensen Jessica F., Watson Natasha. Increasing patient activation through diabetes self-management education: Outcomes of DESMOND in regional Western Australia. Patient Education and Counseling 103 (2020) 848–853. [DOI] [PubMed] [Google Scholar]
- 28.Donald Maria, Ware Robert S., Ieva Z Ozolins Nelufa Begum, Crowther Ruth, Bain Christopher. The role of patient activation in frequent attendance at primary care: A population-based study of people with chronic disease. Patient Education and Counseling 83 (2011) 217–221. [DOI] [PubMed] [Google Scholar]
- 29.Hibbarda Judith H., Mahoney Eldon, Sonet Ellen. Does patient activation level affect the cancer patient journey? Patient Education and Counseling Volume 100, Issue 7, July 2017, Pages 1276–1279. [DOI] [PubMed] [Google Scholar]
- 30.Shah Vallabh, Carroll Casey, Mals Ryan, Ghahate Donica, Bobelu Jeanette, Sandy Phillip, Colleran Kathleen, Schrader Ronald, Faber Thomas, Burge Mark. A Home-based Educational Intervention Improves Patient Activation Measures and Diabetes Health indicators Among Zuni Indians. PLOS 1 May 2015, 10(5): e0125820. [DOI] [PMC free article] [PubMed] [Google Scholar]