Abstract
Cancer is a disease of aging and, as the world’s population ages, the number of older persons with cancer is increasing and will make up a growing share of the oncology population in virtually every country. Despite this, older patients remain vastly underrepresented in research that sets the standards for cancer treatments. Consequently, most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients, and effective strategies to improve clinical trial participation of older adults with cancer remain sparse. This systematic review evaluated published studies regarding barriers to participation and interventions to improve participation of older adults in cancer trials. The quality of the available evidence was low and, despite a literature describing multifaceted barriers, only one intervention study aimed to increase enrollment of older adults in trials. Our findings starkly amplify the paucity of evidence-based effective strategies to improve participation of this underrepresented population in cancer trials. Within these limitations, we provide our opinion on how the current cancer research infrastructure must be modified to accommodate the needs of older patients. We offer several underutilized solutions to expand clinical trials to include older adults with cancer. However, as currently constructed, these recommendations alone will not solve the evidence gap in geriatric oncology, and efforts are needed to meet older and frail adults where they are by expanding clinical trials designed specifically for this population and leveraging real-world data.
Keywords: clinical trials, older adults, oncology, patient selection, patient participation, practice patterns
INTRODUCTION
Patients 70 years of age or older represent 42% of the overall cancer population.1–3 However, older patients are vastly underrepresented in clinical trials that set the standards for the efficacy and safety of cancer treatments.4–6 Only 24% of participants in trials registered with the US Food & Drug Administration (FDA) are 70 years or older,4–6 and fewer than 10% of patients in this age group participate in National Cancer Institute (NCI)-sponsored clinical trials.7–14 Even when older adults are enrolled in cancer trials, they typically have fewer functional impairments or co-morbid conditions15 than the average older patient treated in clinical practice.9, 13, 14, 16 Consequently, most of what we know about the risks and benefits of cancer therapeutics is based on clinical trials conducted in younger, healthier patients,4, 17 leading to systematic differences in treatment and disparities in health outcomes between older and younger patients with cancer.18–31
Although common barriers to enrollment of older patients in oncology clinical trials have been the subject of frequent inquiry, the participation of this population, particularly those 70 years of age and older and/or with poor health, has not changed substantially over time.20, 32–35 Several studies have described the barriers as complex and multifaceted, often involving a combination of system, physician, and patient factors.31, 36–41 Specific efforts to improve the clinical trial enrollment of older patients with cancer have included a physician-directed educational intervention,42 focused committees, policy statements,43–47 and the development of a limited number of trials dedicated to older patients.48–53 However, few studies54, 55 have synthesized this research. A clear understanding of barriers to clinical trial enrollment and interventions tested is needed to develop new, effective strategies to facilitate the inclusion of older adults in cancer clinical trials.
Beyond prior literature reviews,55–58 which are limited to broad overviews of the evidence, only one systematic review by Townsley and colleagues54 focused on the barriers that impede accrual of older patients with cancer. This systematic review, based on studies published from 1994 to 2004, was performed prior to the adoption of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), which is the standardized framework for conducting and reporting systematic reviews,59 and did not evaluate the quality of the evidence. Furthermore, the review focused on studies that assessed the barriers to clinical trial participation for older adults and did not assess intervention studies or efforts to remove these barriers, which is necessary to inform future efforts.
To advance knowledge based on the existing evidence and to address the limitations of the previous reviews, we conducted a systematic review focused on evaluating two questions: (1) What barriers hinder participation of older adults in cancer clinical trials? and (2) What interventions influence and improve their participation beyond trials designed specifically for their age group? Our goal was to synthesize prior research, which we hypothesized would be highly heterogeneous, under a uniform framework that can inform the development of new, evidence-based trial recruitment strategies for older adults with cancer and guide policy choices about how to direct future research and resources.
METHODS
Search Strategy
We conducted and reported this systematic review according to prespecified criteria60 outlined by the PRISMA guidelines.59 The study protocol was registered with PROSPERO (number CRD42018085677). One investigator (AL), a health information specialist, searched six databases: PubMed MEDLINE, Ovid MEDLINE, Embase, Scopus, PsycINFO, and the Cochrane Library. There were no specified date, age, sex, or language restrictions. The coverage dates for this review began from each database’s inception (MEDLINE, 1946; Embase, 1947; Scopus, 1966; PsycINFO, 1806; and Cochrane Library, 1995) and ended on January 15, 2019. The search strategy contained four core components, linked using the AND operator: (1) clinical trials (e.g., therapeutic research, human experimentation); (2) participation or recruitment (e.g., eligibility, patient selection, patient participation); (3) older adults (e.g., elderly, geriatric, aging); and (4) cancer (e.g., neoplasms, malignancies, chemotherapy). Controlled vocabulary (i.e., Medical Subject Headings [MeSH] terms) and keywords were identified for each of the four core components. The search was developed initially for PubMed and then adapted for each of the other five databases by mapping the search terms to additional controlled vocabulary and subject heading terminology. Search terms were reviewed by an independent health information specialist (consultant) at an outside institution to ensure that the search strategy was relevant and comprehensive. Full details of all search terms can be found in the appendix (pp 8–12).
Reference lists from previous reviews and key articles retrieved were also examined for relevant studies. In addition, reviewers with expertise in geriatric oncology from the Cancer and Aging Research Group (CARG)47, 61, 62 were invited to nominate additional publications for possible inclusion.
Study Selection
Duplicate articles were removed in EndNote (version X9). Remaining articles were exported into a reference management software (Covidence; Veritas Health Innovation Ltd) for study selection. Titles and abstracts of studies were independently screened for eligibility by two reviewers (KG, SP). Disagreements were adjudicated by a third reviewer (MSS). All studies deemed eligible by title and abstract screening underwent a full-text review by two independent reviewers (SP, JL) using the same criteria. Discussion or involvement of a third reviewer (MSS) was used to address discordant eligibility ratings. Studies were eligible for inclusion if they: (1) were published in English, (2) had full text available, (3) were empirical, peer-reviewed experimental, quasi-experimental, or observational studies (i.e., not reviews, letters, case series, or conference proceedings), (4) evaluated barriers to participation and/or interventions to improve participation of older adults in oncology clinical trials, and (5) focused on patients age ≥65 with cancer. Studies were excluded if they: (1) described the problem (i.e., reported underrepresentation) but did not examine the reasons for low enrollment of older adults, (2) reported interventions associated with improving enrollment of the general cancer population but did not examine how these interventions increase representation of older patients with cancer, or (3) reported a specific therapeutic trial for older adults with cancer (i.e., trials purposely designed for older patients).
Data Extraction
A standardized template, adapted from the Cochrane Collaboration,63 was used to extract data on study characteristics (year of publication, authors, journal, geographic location, funding source), study question (aims, design, duration, participants, cohort eligibility and size, study measures), results (outcomes, key findings), and authors’ stated conclusions. Two paired reviewers (SP, JL) independently extracted this information from each study and resolved any disagreements through discussion.
To structure data synthesis, we used the Accrual to Clinical Trials (ACT) framework,64 in which the majority of reasons for low enrollment in clinical trials can be categorized as system, provider, patient, or caregiver factors. Two reviewers (ARW, JL) independently coded barriers and/or interventions identified from the studies using thematic content analysis.65–67 Discordant coding was discussed and adjudicated by consensus.
Risk of Bias Assessment
Appraisal of study quality was performed using study quality assessment tools from the National Heart, Lung, and Blood Institute (NHLBI), which are specifically tailored to the design of each study and include items for evaluating potential flaws in study methods or implementation (e.g., source of bias, confounding, study power).68 For each item in the assessments, quality reviewers could select “yes,” “no,” or “cannot determine/not reported/not applicable.” Based on their responses, the reviewers then rated individual studies as being of “good,” “fair,” or “poor” quality. A “good” study is considered to have the least risk of bias, and results are considered valid. A “fair” study is susceptible to some bias deemed not sufficient to invalidate its results. A “poor” rating indicates significant risk of bias. The quality rating for each study was independently assessed by two reviewers (ARW, JL), with any disagreements subsequently resolved through discussion and involvement of a third reviewer (MSS).
RESULTS
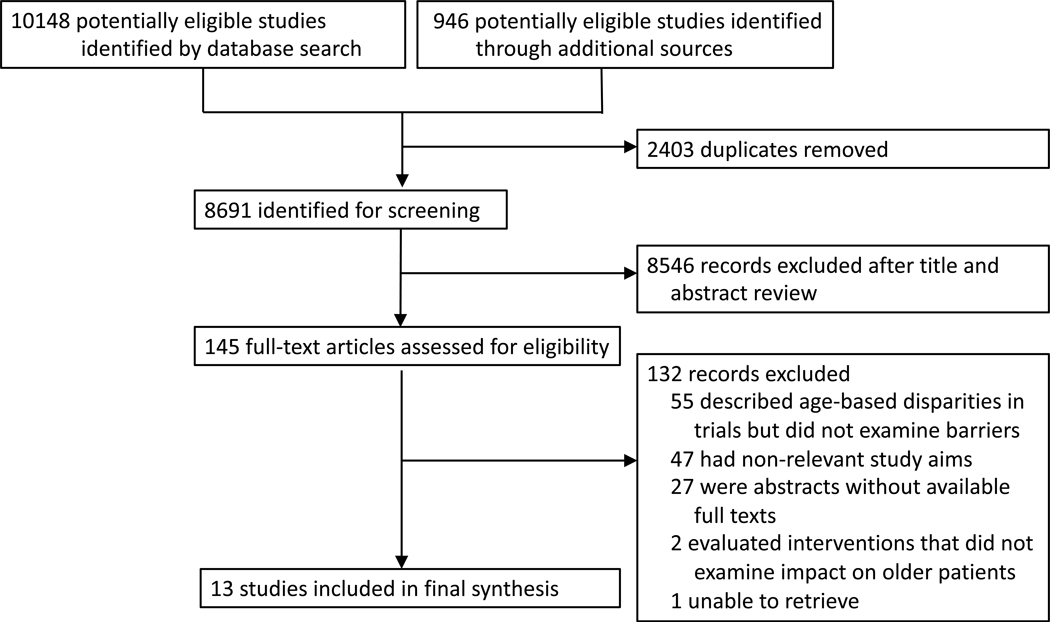
A total of 10,148 articles were identified from the six database searches, and 946 additional articles were identified from reference lists. After removing duplicate publications, 8,691 articles were screened for eligibility. Of the 145 studies eligible for full-text review, 13 met the inclusion criteria (Figure 1). The appendix (pp 2–3) summarizes the study characteristics, designs, and findings. Given the limited size and marked heterogeneity of the evidence base, a quantitative synthesis (meta-analysis) of the identified studies was not possible, and our analysis focused on a qualitative synthesis.
Figure 1.
PRISMA Flow Diagram
Study Characteristics
Table 1 summarizes the characteristics of the studies included in the evidence synthesis. The majority of studies (12 [92%] of 13)31, 36–38, 69–76 were observational studies (9 cross-sectional,36–38, 71–76 two cohort,31, 70 and one case-control69) and evaluated only barriers. Only one interventional randomized controlled trial (RCT)42 met the final inclusion criteria. Six studies31, 38, 73–76 were published in 2010 or later, and two36, 69 were published before 2004. Most studies (8 [62%] of 13)31, 36, 38, 42, 69, 71, 72, 75 were based in the USA. Solid tumor malignancies were the most prevalent cancer type assessed, being the focus of 11 studies;31, 36, 37, 69–76 only three (23%) of 13 studies included patients with hematologic malignancies.37, 72, 74 Five studies sampled patients,37, 70, 72, 74, 76 four sampled providers,36, 38, 73, 75 and three sampled both patients and providers.31, 69, 71 The interventional RCT was targeted for providers.42 No studies sampled caregivers.
Table 1.
Characteristics of the 13 studies included in this systematic review
| Characteristic | Number of studies (N=13) | References |
|---|---|---|
| Year published | ||
| Before 2004 | 2 (15%) | 36, 69 |
| 2004 to 2009 | 5 (38%) | 37, 42, 70–72 |
| After 2010 | 6 (46%) | 31, 38, 73–76 |
| Country of origin | ||
| USA | 8 (62%) | 31, 36, 38, 42, 69, 71, 72, 75 |
| Canada | 2 (15%) | 37, 70 |
| Netherlands | 1 (8%) | 73 |
| Ireland | 1 (8%) | 74 |
| Germany | 1 (8%) | 76 |
| Minimum age used to define older adults | ||
| 65 | 11 (85%) | 31, 36, 38, 42, 69–74, 76 |
| 70 | 2 (15%) | 37, 75 |
| Study population | ||
| Provider | 5 (38%) | 36, 38, 42, 73, 75 |
| Patients | 5 (38%) | 37, 70, 72, 74, 76 |
| Both | 3 (23%) | 31, 69, 71 |
| Sample source | ||
| Multiple institutions | 9 (69%) | 31, 36, 42, 69, 71–73, 75, 76 |
| Single institution | 3 (23%) | 37, 70, 74 |
| Population-based | 1 (8%) | 38 |
| Study design | ||
| Intervention | 1 (8%) | 42 |
| Observation | 12 (92%) | 31, 36–38, 69–76 |
| Cross-Sectional | 9 (69%) | 36–38, 71–76 |
| Surveys | 11(85%) | 31, 36–38, 69, 71–76 |
| Qualitative analyses | 4 (31%) | 37, 69, 70, 75 |
| Cohort | 2 (15%) | 31, 70 |
| Case-Control | 1 (8%) | 69 |
| Cancer type | ||
| Solid | 11 (85%) | 31, 36, 37, 69–76 |
| Breast | 6 (46%) | 31, 36, 37, 69, 73, 74 |
| Colon | 2 (15%) | 37, 75 |
| Lung | 1 (8%) | 37 |
| Prostate | 1 (8%) | 37 |
| Hematological | 3 (23%) | 37, 72, 74 |
| All types | 2 (15%) | 38, 42 |
Studies assessing barriers to older adult participation in cancer clinical trials
Twenty-three subcategories of barriers were identified (Table 2) across the 12 observational studies. Using the ACT framework, barriers were categorized as system, provider, patient, and caregiver factors.
Table 2.
Identified barriers to clinical trial participation of older adults with cancer
| Kornblith (2002) | Kemeny (2003) | Moore (2004) | Townsley (2006) | Basche (2008) | Puts (2009) | Javid (2012) | Hamaker (2013) | Ayodel (2016) | McCleary (2018) | Prieske (2018) | Freedman (2018) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| System | Eligibility criteria | ● | ● | ● | ● | ● | ● | ||||||
| Consent form language | ● | ● | ● | ||||||||||
| Trial availability | ● | ● | |||||||||||
| Provider | Concern for toxicity | ● | ● | ● | ● | ● | ● | ● | |||||
| Concern for patient age | ● | ● | ● | ● | ● | ||||||||
| Time/burden | ● | ● | ● | ● | |||||||||
| Preference for another treatment | ● | ● | ● | ||||||||||
| Lack of personnel | ● | ● | ● | ||||||||||
| Preference against research in general | ● | ● | ● | ● | ● | ||||||||
| Unaware of available trials | ● | ● | |||||||||||
| Patient | Knowledge | ● | ● | ● | ● | ● | ● | ||||||
| Transportation | ● | ● | ● | ● | ● | ● | |||||||
| Time/burden | ● | ● | ● | ● | ● | ● | |||||||
| Concern about efficacy and toxicity | ● | ● | ● | ● | ● | ● | |||||||
| Against experimentation | ● | ● | ● | ● | ● | ||||||||
| Treatment preferences | ● | ● | ● | ● | |||||||||
| Finances | ● | ● | ● | ● | |||||||||
| Age (e.g., believing they are too old) | ● | ● | |||||||||||
| Emotional burden | ● | ● | |||||||||||
| Care-giver | Preferences | ● | ● | ● | ● | ||||||||
| Burden | ● | ● | |||||||||||
Six (50%) of 12 observational studies31, 36, 38, 71, 73, 75 reported system barriers. All six (100%) of these studies31, 36, 38, 71, 73, 75 reported stringent eligibility criteria as a major barrier. Other system barriers noted were language used in consent forms31, 38, 73 and appropriate trial availability.38, 71
Nine (75%) of 12 observational studies31, 36–38, 69, 71, 73–75 reported provider barriers. Seven (78%) of those nine studies reported that providers are reluctant to enroll older adults due to risk of increased toxicity, including concerns due to patient multi-morbidities and potential toxicity profiles of investigational treatments.31, 36, 38, 69, 71, 73, 75 Five (56%) of nine studies found that providers were hesitant on the basis of patients’ age alone.31, 36, 38, 73, 75 Other provider barriers included time demands,31, 36, 73, 75 lack of personnel,31, 38, 73 preferences for another treatment,36, 69, 73 provider bias against research in general,31, 36, 37, 74, 75 lack of awareness of available trials,36, 69 and provider discomfort with randomization.31, 75
Ten (83%) of 12 observational studies reported patient barriers.31, 36–38, 69, 70, 72–74, 76 Six (60%) of those 10 studies reported limitations due to patient knowledge,31, 36–38, 74, 76 transportation issues,31, 36, 38, 72, 74, 76 time demands or burden associated with trials,31, 38, 70, 72, 73, 76 patient concerns about efficacy and toxicity of investigational drugs,31, 37, 70, 72, 74, 76 and concerns with experimentation.31, 36, 69, 72, 73 Other identified barriers were patients’ treatment preferences,31, 36, 69, 73 concerns about financial coverage,31, 36, 38, 72 age (e.g., patient believes he/she is too old),37, 74 and emotional burden.38, 70
Although four (33%) of the 12 studies31, 36–38 reported caregiver barriers, none sampled caregivers directly. In all four studies, physicians and patients reported that barriers include caregivers’ concerns, 31, 36–38 and in two studies (50%), caregiver burden.31, 36
Studies assessing strategies to improve older adult participation in cancer clinical trials
Only one RCT42 was identified. Published in 2005, this cluster-randomized study (N=125 institutions) examined whether a physician-directed geriatric educational intervention could increase the accrual of older patients (age ≥ 65 years) to NCI-sponsored cancer treatment trials. The educational intervention consisted of an educational symposium, geriatric oncology educational materials, a list of available protocols, monthly e-mail and mail reminders, and a case discussion seminar. Fifty-three institutions were randomly assigned to receive the educational intervention, and 72 institutions were assigned as controls, receiving standard educational information.
The study found that the intervention did not significantly improve accrual of older patients. Before the intervention, the overall percentages of older patients accrued to phase II and III treatment protocols reported was 40% among the intervention arm compared with 36% in control arm (P=0.40). During the first and second years post-intervention, the percentage of older patients in clinical trials in the intervention and control arms were 36% versus 32% (P=0.35) and 31% versus 31% (P=0.83), respectively.
Quality of the Evidence
Risk of bias was assessed using NHLBI study quality assessment tools (appendix pp 13–14). Of the 12 observational studies, three were rated as having low risk of bias (“good” quality),69, 70, 72 eight were rated as having uncertain risk of bias (“fair” quality),31, 36–38, 71, 73, 74, 76 and one was rated as having high risk of bias (“poor” quality).75 The RCT42 was rated as having uncertain risk of bias (“fair”) based on study design factors, such as unclear adherence to the intervention and lack of blinding and a reported power calculation.
DISCUSSION
This systematic review identified 13 relevant empirical studies, including 12 observational studies examining barriers hindering participation of older adults in cancer clinical trials and one (negative) RCT aiming to increase enrollment of older adults in trials.31, 36–38, 42, 69–76 Our findings starkly amplify the paucity of high-quality evidence that uniformly and comprehensively defines the barriers in various care settings, with even more limited research on interventions to address these barriers. Consequently, effective strategies to improve participation of older adults in cancer clinical trials remain woefully underdeveloped.
Our systematic review findings underscore the complex, burdensome, and structural impediments that effectively exclude older and frail patients with cancer from clinical trials. To address these, the current research infrastructure must be modified to accommodate the needs of older patients and, if their inclusion cannot be operationalized, we must determine new ways to meet older adults “where they are” rather than where they “should be” to fit the current structure. Instead of the standard approach to cancer trials, we offer the following underutilized solutions to expand clinical trials to include older adults with cancer (Table 3).
Table 3.
Recommendations to expand the inclusion of older adults in cancer clinical trials
| Overarching Solutions | Specific Strategies | Examples for Implementation |
|---|---|---|
| Operational Modifications to the Current Cancer Research Infrastructure | Geriatricize Trial Design | • Design trials specifically for older adults (e.g., single-arm phase II A171601) • Extended trial design (no precedent) • Adaptative design (e.g., phase III CALGB 49007) • Prospective cohort design (e.g., TLC study) • Post-marketing surveillance cohorts/registries (e.g., NRMI Genentech study) • Embedded study (e.g., A041202, EA2186) • Concurrent differential dosing trials (e.g., FOCUS2, GO2) |
| Measure Relevant Endpoints | • Composite endpoints (e.g., Overall Treatment Utility [OTU] or ‘therapeutic success,’ which combines efficacy, toxicity, and patient compliance) • Treatment failure–free survival (TFFS) • Time to treatment failure (TTF) • Patient-reported toxicity (e.g., PRO-CTACE) • Aging-related measures (e.g., single or multiple domains of GA and other measures to capture function or cognition) • Quality of life-related measures (e.g., PROMIS, EORTC, Q-TWIST) • Was It Worth It (WIWI) |
|
| Broaden (Further) Eligibility Criteria | • Use measures of function (e.g., gait speed) or other evaluations of biological age rather than performance status • Incorporate standardized, objective measures of multimorbidity, such as the Charlson Comorbidity Index (consider a hierarchy of comorbid conditions) • Engage (early) with patient advocates, geriatricians, or geriatric oncologists |
|
| Address Site/Stakeholder-Specific Barriers | • Avoid shotgun, ‘one size fits all’ approach • Evaluate specific site and stakeholder barriers • Develop multilevel, tailored interventions to meet unique needs |
|
| Expand the Reach of Cancer and Aging Research Beyond Standard Trials | Design Pragmatic Clinical Trials | • Consider cluster-randomized trials (e.g., COACH trial) • Expand to community-practices (e.g., NCORP) |
| Leverage Real-World Data | • Use EHRs, tumor registries, claims data, and other sources • Link cancer (e.g., SEER) and aging data (e.g., HRS) |
|
Abbreviations: CALGB=Cancer and Leukemia Group B; TLC=Thinking and Living with Cancer Study; NRMI=National Registry of Myocardial Infarction; PROMIS= Patient Reported Outcome Measurement Information System; EORTC= European Organization for Research and Treatment of Cancer; Q-TWIST= Quality-adjusted Time Without Symptoms and Toxicity; COACH=Communicating About Aging and Cancer Health (COACH) clinical trial NCORP=NCI Community Oncology Research Program, PROs = patient-reported outcomes, EHR = electronic health record, SEER=Surveillance, Epidemiology, End-result; HRS=Health and Retirement Study
Operational Modifications to the Current Cancer Research Infrastructure
There are several ways to modify trial designs to accommodate the needs of older adults. The CARG, in collaboration with the National Institute on Aging (NIA) and the NCI, held a series of conferences funded by a U13 grant to identify and address gaps in knowledge in the care of older adults with cancer. The group has published several white papers, including one focused on how to modify clinical trials for older adults with cancer.77 Here, we highlight several of these recommendations and how they have been incorporated into current trials.
Design Trials Specific to Older Adults.
Clinical trials can specifically focus on older adults and address questions that are most pertinent to the geriatric oncology population. An example of this is the Cancer and Leukemia Group B (CALGB) 49907 phase III RCT which compared standard adjuvant polychemotherapy with monochemotherapy in patients age 65 and older with breast cancer.48 Similarly, single-arm phase II studies can be designed specifically for older adults. The Alliance A171601 trial is a single-arm, open-label, phase II study assessing the tolerability of palbociclib in patients 70 years and older with metastatic breast cancer.78 This study design is advantageous because it incorporates standard of care practices (using FDA-approved drugs), captures adverse events in a population that was underrepresented in the registration trials, and advances our understanding of tolerability (how treatment impacts aging and quality of life) as well as age-related changes in the pharmacology of cancer treatment.
Modify Trial Design to Collect More Data on Older Adults.
Clinical trials can be adapted to collect more evidence on older adults through extended and adaptive designs. Extended trial design allows for the addition of a cohort of older patients to the treatment arm that was shown to be superior in an RCT. Adaptive trial design allows for modification of a trial design as the study proceeds, based on interim data analysis.43 In CALGB 49907, for example, an adaptive Bayesian design was used, which allowed for interim analysis of the accumulated data at a specified time point. At this time point, the treatment effect in one of the treatment arms satisfied a predefined futility boundary, and as a result, accrual to that arm was terminated. Using this approach, accrual to the other treatment arm can be continued until the planned total sample size is reached. This study design is advantageous because of the potential for a smaller sample size requirement if the underperforming study arms are eliminated after interim data analysis and overcomes the costly and lengthy limitations of large trials.
Leverage Population Cohort Studies.
Prospective cohort studies can be used to answer commonly posed questions in geriatric oncology regarding the feasibility, dosing, and toxicity of a selected regimen.24, 79, 80 This can be used to add data if the older adults cannot be included in the pivotal clinical trials. There are many examples of cohort studies in geriatric oncology. Several cohort studies were utilized to develop clinical risk prediction models, such as the CARG Chemotherapy Toxicity Score and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) Score.20, 34, 35 Similarly, longitudinal cohort studies can provide important insights on the late-term effects of cancer treatment on aging in older cancer survivors, as has been done in the Thinking and Living with Cancer (TLC) Study.81
Establish Post-marketing Surveillance Studies.
Post-marketing surveillance studies use cohort designs to longitudinally monitor populations underrepresented or not studied in the registration trials. This may be an important opportunity for advancing the evidence base in geriatric oncology. Investigators and treatment centers should partner with pharmaceutical companies for post-marketing surveillance of the efficacy and toxicities of cancer drugs in the older and frailer population. A successful example of this is the National Registry of Myocardial Infarction (NRMI), a Genentech-funded study with more than 2.2 million patients with cardiovascular disease across 1,600 hospitals.82 Similar registries may be developed in geriatric oncology.
Embed Biological or Functional Age Evaluation in Trials.
An embedded study (i.e., correlative or ancillary study) can be used to identify the characteristics of patients at high risk for toxicity and evaluate the toxicity profile of new drugs. An example is Alliance A041202, which embeds a comprehensive geriatric assessment (GA) within the schema of a phase III trial evaluating the efficacy of ibrutinib, alone or in combination with rituximab, relative to chemoimmunotherapy in patients 65 years or older with untreated chronic lymphocytic leukemia.83 Similar trials have utilized this approach, including ECOG EA2186. These companion sub-studies are important to fill critical knowledge gaps in the care of older adults and can identify specific aging measures that may predict overall survival and treatment-related mortality for this population.
Conduct Concurrent Differential Dosing Trials for Older Adults.
Concurrent differential dosing trials can fill the dearth of information regarding the optimal dose and schedule for cancer therapeutics for the geriatric population. Providers have many concerns about the patient risk of treatment toxicity in older and frail patients and their willingness to deliver the full chemotherapy dose with the first cycle of treatment may be influenced by age-related vulnerabilities, particularly if the treatment goal is palliation. Studies such as the Fluorouracil, Oxaliplatin, and CPT-11 [irinotecan]: Use and Sequencing 2 (FOCUS2) trial51 for older and frail adults with metastatic colorectal cancer used a reduced first dosing of treatment, then allowed providers to escalate to the standard dosage if the patient tolerated treatment well. Another example is the GO2 phase III trial which examined dose de-escalation arms in older patients with advanced gastroesophageal cancer.84 Efforts should be directed to conduct dose de-escalation and dose titration studies to examine optimal strategies that improve treatment tolerability without compromising efficacy in this population.
Measure Relevant Endpoints.
Sponsors and investigators should carefully consider “what” endpoints matter to older patients. Cancer clinical trials are often well poised to collect a narrow set of cancer-specific endpoints (e.g., response rate, survival, toxicity) in order to demonstrate drug safety and efficacy. Most of these studies use sub-analyses based on chronological age to determine toxicity in the geriatric population. However, given the heterogeneity in the health status of older adults and the strong evidence that chronological age alone does not adequately characterize health status in this population,85–87 there is a need for greater attention to patient-specific endpoints that measure clinical and biological aging-related consequences of cancer treatment.88 Understanding how a drug impacts outcomes, such as function or cognition,89 is essential information needed by clinicians and patients to make informed treatment decisions.90 Furthermore, broader endpoints tailored specifically for the geriatric oncology population, such as co-primary or composite measures of tolerability, treatment efficacy, and GA endpoints/patient-reported outcomes (e.g., Overall Treatment Utility), are needed to capture what is most important to older adults.91–95
Broaden (Further) Eligibility Criteria.
Our findings, consistent with prior literature,9, 31, 46, 71, 96, 97 highlight that narrow eligibility criteria remain a major barrier to older adult access to available trials. Recent efforts to address this problem include the “Inclusion Across the Lifespan Policy” from the National Institutes of Health (NIH) and several publications from the American Society of Clinical Oncology (ASCO), Friends of Cancer Research, and the FDA working groups that recommended changes to the most commonly used exclusion criteria.15, 43, 98, 99 However, concrete steps to implement these recommendations are needed. For example, efforts to establish a hierarchy of comorbid conditions and which ones could be acceptable for clinical trial criteria are needed to provide guidance for investigators and sponsors. Furthermore, use of measures of function (e.g., gait speed) or other evaluations of biological age, rather than performance status (which has been demonstrated as a suboptimal measure of function in older adults), is recommended for increasing inclusion of older adults in trials. Incorporating standardized, objective measures of multimorbidity, such as the Charlson Comorbidity Index, can also be used to establish criteria for older adult inclusion. Sponsors and investigators should engage with patient advocates, geriatricians, or geriatric oncologists to better understand the needs of older adults when designing oncology trials.44, 100
Advance Regulatory and Policy Efforts.
Since the 1980s, the FDA has made a concerted effort to encourage enrollment of patients age 65 and older in registration clinical trials.101–103 Under FDA regulations, new drug applications must include efficacy and safety data presented by age, gender, and racial subgroups and, when appropriate, other subgroups of the population of patients treated, such as patients with renal failure. Specific information pertinent to the drug’s experience in older adults is contained in the “Geriatric Use” subsection of the package inserts of approved products.104 However, for many newly approved cancer treatments, there is inadequate prescribing information about the efficacy and safety data for patients older than 65 or 75, and, consequently, sparse data or conclusions can be drawn from the product labeling.105 Recognizing this, the FDA partnered with ASCO in 2017 to conduct the first public workshop on geriatric oncology.44 Building on discussions from the workshop, in 2020, the agency published the first oncology-specific guidance for including an adequate representation of older adults, specifically those over age of 75, in registration trials.106 These are important first steps and highlight the agency’s leadership and willingness to work on this important issue. However, the current guidance functions as recommendations, not requirements. Future efforts are needed at the regulatory and policy level to translate these recommendations into action. For example, efforts to work with sponsors during the planning process for new drug applications can highlight incentives for companies to enroll older adults, including the potential for broader label indications and the possibility that clinicians may use treatments in larger patient populations if this evidence is collected. Additionally, post-marketing commitments for companies, where appropriate, may be another approach to obtain more data on older adults in registration trials.
Evaluate and Address Site/Stakeholder-Specific Barriers.
Beyond the structural barriers, efforts should be directed to identify site-, provider-, patient-, and caregiver-specific barriers, such as those highlighted in this review. It is unlikely that there is a ‘one size fits all’ approach to addressing site and stakeholder barriers. Knowledge of specific barriers is therefore useful to develop tailored strategies and may be more effective than attempting to develop generic strategies for global barriers that may not be relevant to a heterogenous population.107
Our findings, consistent with others, highlight that practical impediments, such as lack of access, insurance constraints, inconvenience, and cost, limit older patient participation in cancer clinical trials.54, 72, 108 Tailored approaches to overcome these barriers are needed. To help reduce burden of travel, for example, strategies using innovation such as telehealth may reduce the number of in-person visits required for a study.109, 110 Alternatively, travel assistance programs, launched through partnership with organizations, such as the American Cancer Society, and companies, such as Uber, can provide logistical and financial support for patients, which may be helpful to facilitate research participation.111 The FDA also recently updated its guidance on payment and reimbursement of research participants to clarify that reimbursement for travel expenses and associated costs, such as airfare, parking, and lodging, are not considered “undue influence” and are “generally acceptable.”112
In addition to addressing practical barriers, nonpractical psychosocial barriers, such as knowledge gaps and negative attitudes among both patients and their caregivers cannot be ignored. Efforts to increase older adult and caregiver engagement, to provide clarification of patient preferences and values, may allow individuals to be better prepared to consider participation in a clinical trial, if presented as a treatment option. Education about clinical trials and the importance of participation in research will improve knowledge, attitudes, and preparation for decision-making about enrollment in clinical trials.
Engage Referring Providers in the Clinical Trial Process.
Referring providers play an important role in facilitating patient access to clinical trials. Referring providers often introduce the concept of clinical trials to their patients and refer patients to oncologists who participate in clinical trials. This may be of particular significance in the older patient population, where studies have shown that lack of primary provider support or a reluctance to travel to university centers where trials are most often conducted are key deterrents to clinical trial participation.54, 72, 113 Educating referring providers, such as primary care providers or local community oncologists, is thus an important yet overlooked mechanism for increased accrual of older adults to cancer clinical trials. Building relationships with referring providers may promote a research-oriented culture that can facilitate older adult participation in clinical trials.
Expanding the Reach of Cancer and Aging Research Beyond Standard Clinical Trials
Design Pragmatic Clinical Trials.
Merely increasing the enrollment of older adults in efficacy trials as currently constructed will not remediate the evidence gap in geriatric oncology, and designing pragmatic studies, dedicated specifically to the older population, is a promising solution. Many older adults have health conditions or other limitations that preclude enrollment in most randomized controlled trials, and despite aggressive efforts to broaden the eligibility criteria, it is not realistic for these patients to participate in efficacy or early-phase studies, which must be rigorously controlled and constrained.43, 44 However, pragmatic, older adult-specific trials could examine whether these novel treatments can be broadly implemented, if approved. Moreover, these studies can evaluate whether the risks and benefits of new treatments apply to a more demographically, socioeconomically, and clinically diverse patient population, including less fit and even frail older adults who may not have otherwise been eligible for the efficacy study.
To facilitate the development and implementation of these trials, collaboration between patient advocates, geriatricians, and oncologists should take place to ensure that these studies are amenable to the participation of older and/or frail patients and that the endpoints measured meet their needs.44, 91, 114 Furthermore, efforts should be made to ensure that these pragmatic trials are open in community settings, where the vast majority of older patients are treated.115 As our findings highlight, older adults may face more challenges than younger patients with travel, caregiver support, and other logistics associated with trial participation. Infrastructures such as the NCI Community Oncology Research Program (NCORP), a national network designed to open participation of NCI-approved studies at community-based practices, should be leveraged to support a larger and more diverse patient population, accelerate accrual, and increase generalizability of trial findings.116, 117 One successful example of this is the Improving Communication in Older Cancer Patients and Their Caregivers (COACH) study (NCT02054741), a cluster-randomized clinical trial of community oncology practices within the University of Rochester NCORP that examined whether a GA summary with recommendations to oncologists can reduce toxicities and improved communication in patients age 70 years and older with advanced cancer.118 Future efforts are needed to increase design and conduct of geriatric-specific pragmatic trials through partnership with NCORP, NCI’s National Clinical Trials Network (NCTN), and the national infrastructure for geriatric oncology research through CARG, supported by the NIA.119–124 Our hope is that increased conduct of pragmatic trials designed for older adults in diverse health care settings will represent the seeds of a more inclusive clinical trial system to improve the evidence base for treating cancer in older adults, especially those who are frail or have comorbidities.
Leverage Real-World Data.
We should expand our use of real-world data, which includes higher numbers of older patients, to fill the evidence gap on older adults with cancer due to the gaps in the trials. Real-world data can be retrospectively analyzed from multiple sources of large population-based observational cohorts. For example, investigators can link cancer data from SEER-Medicare with geriatric information from aging databases such as the Health and Retirement Study125 to conduct epidemiology and health services research. Alternatively, real-word data from electronic health records (EHRs) or other health information technology databases that combine data from multiple EHRs across multiple practices (e.g., CancerLinQ or Flatiron Health) may help fill the evidence gap.126–128
Geriatric oncology researchers should work with other stakeholders to develop a framework for using real-world data in clinical research and to establish the benefits and limitations of these new data. Many of these databases remain limited because they fail to capture measures of the GA domains, and future efforts are needed for improved collection and integration of functional or biological age (GA data) as standard elements into EHRs and other large population-based cohort studies. An example of this is the ongoing Life and Longevity After Cancer Study, a cancer survivor cohort embedded within the Women’s Health Initiative, which collects both cancer and aging measures to fill knowledge gaps regarding how cancer and its treatment impacts the aging process.129
Limitations and Strengths
Our study has limitations. First, to maintain our focus on barriers and interventions, we excluded studies that described age-based gaps in clinical trials or that examined interventions in the general adult population, which may have provided additional insight. We also excluded interventions that could improve the evidence base for treating older adults, such as dedicated trials designed specifically for older patients, as our focus was on strategies aimed at improving clinical trial enrollment, not the evidence base per se. Second, owing to the heterogeneity of barrier and intervention studies, a meta-analysis could not be conducted, and our analysis was limited to a qualitative synthesis of the data. Third, most of the studies included were observational in nature and therefore vulnerable to the effects of confounding. We tried to mitigate these effects by assessing and reporting risk of bias. Finally, we categorized the barriers as system, provider, patient, and caregiver factors using the ACT framework; however, many of these factors are interrelated, and the barriers are more complex than can be conceptualized in a single uniform model.
Despite these limitations, our systematic review also has several strengths. First, to our knowledge, this is the first systematic review to synthesize the literature on barriers to older adult participation in cancer clinical trials and strategies to overcome them. Despite our comprehensive search for interventional studies on this topic, we found only one interventional trial—a sobering fact that underscores the need for further work in this area. Second, this study extends previous knowledge by including research published after 2004, conducting a quality assessment of the evidence, and reporting the findings according to PRISMA guidelines. Third, incorporating these studies into a unified framework enabled the identification of gaps and opportunities in the design and implementation of interventions to facilitate older adult participation in cancer research. We offer solutions building on findings from this review, prior position papers,43, 44 and ongoing dialogues among stakeholders in the CARG,47 FDA, NIH,130 Society of International Geriatric Oncology (SIOG),131 American Geriatrics Society (AGS),132 and ASCO.43, 44 Finally, our findings and recommendations can guide future policy choices on how to direct research and resources aimed at improving the health and wellbeing of older adults with cancer.
As the world’s population ages, older adults with cancer will make up a growing share of the oncology population in virtually every country. Hence, the lack of evidence to treat older adults is relevant to all providers of cancer patients. Our paper is therefore a “Call to Action” across disciplines: All oncologists and primary care providers, not just geriatric oncologists, need to encourage their older patients to participate in clinical trials. This is a crucial time to rigorously evaluate the barriers to clinical trial participation in the geriatric population, and it is imperative for the healthcare system to address these issues in order to ensure that all patients with cancer receive the highest-quality, evidence-based care.
CONCLUSION
Our findings emphasize the complex, multifaceted barriers to enrolling older adults in cancer clinical trials. Building on this, we offer specific recommendations on increasing the enrollment of older adults in existing clinical trials. However, as currently constructed, we believe this alone will not solve the evidence gap in geriatric oncology, and efforts are needed to expand clinical trials designed specifically for this population and leverage real-world data.
Supplementary Material
Acknowledgments:
We dedicate this work in honor of Dr. Arti Hurria, founder of the Cancer and Aging Research Group, who tragically passed away prior to the drafting of this manuscript. Arti’s dream was to improve outcomes for older adults—the current study exemplifies her vision, and we feel these results will bring us one step closer to realizing her dream and positively impact future efforts to address the evidence gap in geriatric oncology.
We also acknowledge Ms. Joy Eskander and Ms. Noel Vargas for their assistance with the data analysis and Dr. Kerin K. Higa for editorial assistance.
Funding/Support: This work was supported by the National Institute on Aging (NIA R03AG064377), the National Cancer Institute (NCI K12CA001727), the Waisman Innovation Fund, and Circle 1500.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Conflict of interest Disclosures:
Dr. Kimmick reports personal fees and non-financial support from Eisai, Boehringer Ingelheim, and Genomic Health; personal fees from Agendia; non-financial support from Seattle Genetics, Novartis, and Pfizer; grants from Bionovo, PUMA, and Roche, outside the submitted work.
Dr. Sedrak reports grants from National Institute on Aging (NIA R03AG064377), National Cancer Institute (NCI K12CA001727), Waisman Innovation Fund, and Circle 1500, during the conduct of the study; grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation, outside the submitted work;
Dr. Wildes reports research funding from Janssen and consulting fees from Carevive Systems and Seattle Genetics.
The remaining authors report no conflicts of interest.
REFERENCES
- 1.Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 2.Ferlay JE, Lam F, Colombet M, et al. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 3.Berger NA, Savvides P, Koroukian SM, et al. Cancer in the elderly. Trans Am Clin Climatol Assoc. 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 4.Singh H, Kanapuru B, Smith C, et al. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the US Food and Drug Administration. J Clin Oncol. 2017;35. [Google Scholar]
- 5.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. [DOI] [PubMed] [Google Scholar]
- 6.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30:2036–2038. [DOI] [PubMed] [Google Scholar]
- 7.Trimble EL, Carter CL, Cain D, Freidlin B, Ungerleider RS, Friedman MA. Representation of older patients in cancer treatment trials. Cancer. 1994;74:2208–2214. [DOI] [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr., , Albain KS Underrepresentation of patients 65 years of age or older in cancer-treatment trials. The New England journal of medicine. 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. [DOI] [PubMed] [Google Scholar]
- 10.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- 11.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Annals of surgical oncology. 2007;14:3328–3334. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Soares HP, Balducci L, Djulbegovic B, National Cancer I. Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25:1272–1276. [DOI] [PubMed] [Google Scholar]
- 13.Ludmir EB, Mainwaring W, Lin TA, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludmir EB, Subbiah IM, Mainwaring W, et al. Decreasing incidence of upper age restriction enrollment criteria among cancer clinical trials. Journal of Geriatric Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard MA, Clayton JA, Lauer MS. Inclusion across the lifespan: NIH policy for clinical research. JAMA. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–1623. [DOI] [PubMed] [Google Scholar]
- 17.Singh H, Beaver JA, Kim G, Pazdur R. Enrollment of older adults on oncology trials: An FDA perspective. Journal of Geriatric Oncology. 2017;8:149–150. [DOI] [PubMed] [Google Scholar]
- 18.Quipourt V, Jooste V, Cottet V, Faivre J, Bouvier AM. Comorbidities alone do not explain the undertreatment of colorectal cancer in older adults: a French population-based study. Journal of the American Geriatrics Society. 2011;59:694–698. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill CB, Baxi SS, Atoria CL, et al. Treatment-related toxicities in older adults with head and neck cancer: A population-based analysis. Cancer. 2015;121:2083–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonadonna G, Valagussa P. Dose-Response Effect of Adjuvant Chemotherapy in Breast-Cancer. New Engl J Med. 1981;304:10–15. [DOI] [PubMed] [Google Scholar]
- 22.Berry MF, Worni M, Pietrobon R, D’Amico TA, Akushevich I. Variability in the Treatment of Elderly Patients with Stage IIIA (N2) Non-Small-Cell Lung Cancer. J Thorac Oncol. 2013;8:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurria A, Wong FL, Villaluna D, et al. Role of Age and Health in Treatment Recommendations for Older Adults With Breast Cancer: The Perspective of Oncologists and Primary Care Providers. J Clin Oncol. 2008;26:5386–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast Cancer Adjuvant Chemotherapy Decisions in Older Women: The Role of Patient Preference and Interactions With Physicians. J Clin Oncol. 2010;28:3146–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. Journal of the National Cancer Institute. 2001;93:850–857. [DOI] [PubMed] [Google Scholar]
- 26.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. New Engl J Med. 2001;345:1091–1097. [DOI] [PubMed] [Google Scholar]
- 27.Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. Journal of the American Geriatrics Society. 2004;52:1681–1687. [DOI] [PubMed] [Google Scholar]
- 28.Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: A nationwide study. J Clin Oncol. 2004;22:4302–4311. [DOI] [PubMed] [Google Scholar]
- 29.Cress RD, O’Malley CD, Leiserowitz GS, Campleman SL. Patterns of chemotherapy use for women with ovarian cancer: A population-based study. J Clin Oncol. 2003;21:1530–1535. [DOI] [PubMed] [Google Scholar]
- 30.Merchant TE, McCormick B, Yahalom J, Borgen P. The influence of older age on breast cancer treatment decisions and outcome. Int J Radiat Oncol. 1996;34:565–570. [DOI] [PubMed] [Google Scholar]
- 31.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). The oncologist. 2012;17:1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. [DOI] [PubMed] [Google Scholar]
- 33.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. [DOI] [PubMed] [Google Scholar]
- 34.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 36.Kornblith AB, Kemeny M, Peterson BL, et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002;95:989–996. [DOI] [PubMed] [Google Scholar]
- 37.Townsley CA, Chan KK, Pond GR, Marquez C, Siu LL, Straus SE. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC cancer. 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman RA, Dockter TJ, Lafky JM, et al. Promoting Accrual of Older Patients with Cancer to Clinical Trials: An Alliance for Clinical Trials in Oncology Member Survey (A171602). The oncologist. 2018;23:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedrak MS, Mohile SG, Sun V, et al. Barriers to clinical trial enrollment of older adults with cancer: A qualitative study of the perceptions of community and academic oncologists. Journal of Geriatric Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu T. Educational initiatives in geriatric oncology – Who, why, and how? Journal of Geriatric Oncology. 2016;7:390–396. [DOI] [PubMed] [Google Scholar]
- 41.Kimmick G. Clinical trial accrual in older cancer patients: The most important steps are the first ones. Journal of Geriatric Oncology. 2016;7:158–161. [DOI] [PubMed] [Google Scholar]
- 42.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23:2201–2207. [DOI] [PubMed] [Google Scholar]
- 43.Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33:3826–3833. [DOI] [PubMed] [Google Scholar]
- 44.Levit LA, Singh H, Klepin HD, Hurria A. Expanding the Evidence Base in Geriatric Oncology: Action Items From an FDA-ASCO Workshop. JNCI: Journal of the National Cancer Institute. 2018;110:1163–1170. [DOI] [PubMed] [Google Scholar]
- 45.Hurria A, Cohen HJ, Extermann M. Geriatric Oncology Research in the Cooperative Groups: A Report of a SIOG Special Meeting. Journal of geriatric oncology. 2010;1:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtman SM, Harvey RD, Smit MAD, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35. [DOI] [PubMed] [Google Scholar]
- 47.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. The New England journal of medicine. 2009;360:2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy Plus Tamoxifen With or Without Irradiation in Women Age 70 Years or Older With Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus Tamoxifen with or without Irradiation in Women 70 Years of Age or Older with Early Breast Cancer. The New England journal of medicine. 2004;351:971–977. [DOI] [PubMed] [Google Scholar]
- 51.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. The Lancet. 2011;377:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corre R, Greillier L, Caër HL, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non–Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. 2016;34:1476–1483. [DOI] [PubMed] [Google Scholar]
- 53.Hurria A, Soto-Perez-de-Celis E, Blanchard S, et al. A Phase II Trial of Older Adults With Metastatic Breast Cancer Receiving nab-Paclitaxel: Melding the Fields of Geriatrics and Oncology. Clinical Breast Cancer. 2019;19:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. [DOI] [PubMed] [Google Scholar]
- 55.Hurria A. Clinical trials in older adults with cancer: past and future. Oncology (Williston Park, NY). 2007;21:351–358; discussion 363–354, 367. [PubMed] [Google Scholar]
- 56.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer control : journal of the Moffitt Cancer Center. 2014;21:209–214. [DOI] [PubMed] [Google Scholar]
- 57.Payne JK, Hendrix CC. Clinical trial recruitment challenges with older adults with cancer. Applied nursing research : ANR. 2010;23:233–237. [DOI] [PubMed] [Google Scholar]
- 58.Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: An examination of barriers and solutions. Vaccine. 2010;28:901–906. [DOI] [PubMed] [Google Scholar]
- 59.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097-e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Booth A. PROSPERO’s progress and activities 2012/13. Syst Rev. 2013;2:111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohile SG, Hurria A, Cohen HJ, et al. Improving the quality of survivorship for older adults with cancer. Cancer. 2016;122:2459–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. Journal of geriatric oncology. 2016;7:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. [Google Scholar]
- 64.Paskett ED, Katz ML, DeGraffinreid CR, Tatum CM. Participation in cancer trials: recruitment of underserved populations. Clinical advances in hematology & oncology : H&O. 2003;1:607–613. [PubMed] [Google Scholar]
- 65.Tobin GA, Begley CM. Methodological rigour within a qualitative framework. Journal of advanced nursing. 2004;48:388–396. [DOI] [PubMed] [Google Scholar]
- 66.Maxwell JA. Qualitative Research Design: An Interactive Approach. Thousand Oaks, California: SAGE Publications, Inc; 2013. [Google Scholar]
- 67.Ritchie J, Lewis J. Qualitative research practice: A guide for social science students and researchers. London: Sage; 2003. [Google Scholar]
- 68.Perrone F, Jommi C, Di Maio M, et al. The association of financial difficulties with clinical outcomes in cancer patients: secondary analysis of 16 academic prospective clinical trials conducted in Italy. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27:2224–2229. [DOI] [PubMed] [Google Scholar]
- 69.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. [DOI] [PubMed] [Google Scholar]
- 70.Puts MTE, Monette J, Girre V, et al. Participation of older newly-diagnosed cancer patients in an observational prospective pilot study: an example of recruitment and retention. BMC cancer. 2009;9:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore DH, Kauderer JT, Bell J, Curtin JP, Van Le L. An assessment of age and other factors influencing protocol versus alternative treatments for patients with epithelial ovarian cancer referred to member institutions: a Gynecologic Oncology Group study. Gynecologic oncology. 2004;94:368–374. [DOI] [PubMed] [Google Scholar]
- 72.Basche M, Baron AE, Eckhardt SG, et al. Barriers to enrollment of elderly adults in early-phase cancer clinical trials. J Oncol Pract. 2008;4:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamaker ME, Seynaeve C, Nortier JW, et al. Slow accrual of elderly patients with metastatic breast cancer in the Dutch multicentre OMEGA study. Breast (Edinburgh, Scotland). 2013;22:556–559. [DOI] [PubMed] [Google Scholar]
- 74.Ayodele O, Akhtar M, Konenko A, et al. Comparing attitudes of younger and older patients towards cancer clinical trials. J Geriatr Oncol. 2016;7:162–168. [DOI] [PubMed] [Google Scholar]
- 75.McCleary NJ, Hubbard J, Mahoney MR, et al. Challenges of conducting a prospective clinical trial for older patients: Lessons learned from NCCTG N0949 (alliance). J Geriatr Oncol. 2018;9:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prieske K, Trillsch F, Oskay-Ozcelik G, et al. Participation of elderly gynecological cancer patients in clinical trials. Archives of gynecology and obstetrics. 2018;298:797–804. [DOI] [PubMed] [Google Scholar]
- 77.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32:2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Identifier NCT03633331, Palbociclib and Letrozole or Fulvestrant in Treating Patients With Estrogen Receptor Positive, HER2 Negative Metastatic Breast Cancer. Bethesda, MD: U.S. National Library of Medicine; 2018. [Google Scholar]
- 79.Kaplan HG, Malmgren JA, Atwood MK. Adjuvant chemotherapy and differential invasive breast cancer specific survival in elderly women. Journal of Geriatric Oncology. 2013;4:148–156. [DOI] [PubMed] [Google Scholar]
- 80.Mandelblatt JS, Faul LA, Luta G, et al. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30:2609–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mandelblatt JS, Zhai W, Ahn J, et al. Symptom burden among older breast cancer survivors: The Thinking and Living With Cancer (TLC) study. Cancer. 2020;126:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibson CM. NRMI and current treatment patterns for ST-elevation myocardial infarction. American heart journal. 2004;148:S29–33. [DOI] [PubMed] [Google Scholar]
- 83.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. 2018;379:2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall PS, Swinson D, Waters JS, et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. 2019;37:4006–4006. [Google Scholar]
- 85.Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in Healthy Aging. The Journals of Gerontology: Series A. 2013;69:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2014;12:82–126. [DOI] [PubMed] [Google Scholar]
- 87.Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. The Lancet Oncology. 2018;19:e305–e316. [DOI] [PubMed] [Google Scholar]
- 88.Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. 2019;111:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What Every Oncologist Should Know About Geriatric Assessment for Older Patients With Cancer: Young International Society of Geriatric Oncology Position Paper. Journal of oncology practice. 2018;14:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohile SG, Magnuson A, Pandya C, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. Journal of the National Comprehensive Cancer Network. 2018;16:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Association of Community Cancer Centers. Multidisciplinary approaches to caring for older adults with cancer. Association of Community Cancer Centers; 2019. [Google Scholar]
- 92.Handforth C, Hall P, Marshall H, Seymour M. Overall treatment utility: A novel outcome measure to convey the balance of benefits and harms from cancer treatment. Journal of Geriatric Oncology. 2013;4:S49. [Google Scholar]
- 93.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society Of Geriatric Oncology position article. J Clin Oncol. 2013;31:3711–3718. [DOI] [PubMed] [Google Scholar]
- 96.Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility Criteria of Randomized Controlled Trials Published in High-Impact General Medical JournalsA Systematic Sampling Review. JAMA. 2007;297:1233–1240. [DOI] [PubMed] [Google Scholar]
- 97.Bellera C, Praud D, Petit-Monéger A, McKelvie-Sebileau P, Soubeyran P, Mathoulin-Pélissier S. Barriers to inclusion of older adults in randomised controlled clinical trials on Non-Hodgkin’s lymphoma: A systematic review. Cancer Treatment Reviews. 2013;39:812–817. [DOI] [PubMed] [Google Scholar]
- 98.Health NIo. Inclusion Across the Lifespan: June 1–2, 2017 Workshop Summary National Institutes of Health; Available at: https://report.nih.gov/UploadDocs/NIH%20Inclusion%20Across%20the%20Lifespan%20Workshop%20Summary%20Report%20_FINAL_508.pdf. Accessed August 26, 2018. [Google Scholar]
- 99.Kim ES, Bruinooge SS, Roberts S, et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patient-Centered Outcomes Research Institute. PCORI Methodology Standards. 2017. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards. Accessed April 22, 2020.
- 101.U.S Department of Health and Human Services, Food and Drug Administration. E7 Studies in Support of Special Populations: Geriatrics 08/24/2018. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e7-studies-support-special-populations-geriatrics. Accessed July 20, 2020.
- 102.U.S Department of Health and Human Services, Food and Drug Administration. Study of Drugs Likely to be used in the Elderly. 5/5/2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/study-drugs-likely-be-used-elderly. Accessed July 30 2020, 2020.
- 103.U.S Department of Health and Human Services, Food and Drug Administration. E7 Studies in Support of Special Populations; Geriatrics; Questions and Answers 8/24/2018. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e7-studies-support-special-populations-geriatrics-questions-and-answers. Accessed July 30, 2020.
- 104.U.S Department of Health and Human Services, Food and Drug Administration. Content and Format for Geriatric Labeling 05/07/2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-and-format-geriatric-labeling. Accessed July 30, 2020.
- 105.Hinshaw T, Kapusnik-Uner J, Zarowitz B, Matuszewski K. Identifying knowledge gaps in the labeling of medications for geriatric patients. P T. 2013;38:535–540. [PMC free article] [PubMed] [Google Scholar]
- 106.U.S Department of Health and Human Services, Food and Drug Administration. Inclusion of Older Adults in Cancer Clinical Trials 03/05/2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/inclusion-older-adults-cancer-clinical-trials. Accessed July 30, 2020.
- 107.Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. Journal of oncology practice. 2013;9:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lara PN Jr., , Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. [DOI] [PubMed] [Google Scholar]
- 109.Clark JM, Heifetz LJ, Palmer D, Brown LM, Cooke DT, David EA. Telehelath allows for clinical trial participation and multimodality therapy in rural patient with stage 4 non-small cell lung cancer.. Cancer treatment and research communications. 2016;9:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DiGiovanni G, Mousaw K, Lloyd T, et al. Development of a telehealth geriatric assessment model in response to the COVID-19 pandemic. J Geriatr Oncol. 2020;11:761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.American Cancer Society. American Cancer Society Transportation. American Cancer Society Available at: https://www.cancer.org/treatment/support-programs-and-services/patient-transportation.html. Accessed July 30, 2020.
- 112.U.S Department of Health and Human Services, Food and Drug Administration. Payment and Reimbursement to Research Subjects Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/payment-and-reimbursement-research-subjects. Accessed July 30, 2020.
- 113.Penberthy L, Brown R, Wilson-Genderson M, Dahman B, Ginder G, Siminoff LA. Barriers to therapeutic clinical trials enrollment: differences between African-American and white cancer patients identified at the time of eligibility assessment. Clinical Trials. 2012;9:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hurria A, Mohile SG, Dale W. Research priorities in geriatric oncology: addressing the needs of an aging population. J Natl Compr Canc Netw. 2012;10:286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong AR, Sun V, George K, et al. Barriers to Participation in Therapeutic Clinical Trials as Perceived by Community Oncologists. JCO oncology practice. 2020:Jop1900662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Printz C. NCI launches program for community-based clinical research: NCORP replaces 2 previous programs. Cancer. 2014;120:3097–3098. [DOI] [PubMed] [Google Scholar]
- 117.Geiger AM, O’Mara AM, McCaskill-Stevens WJ, Adjei B, Tuovenin P, Castro KM. Evolution of Cancer Care Delivery Research in the NCI Community Oncology Research Program. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mohile SG, Mohamed MR, Culakova E, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). 2020. [Google Scholar]
- 119.Koll TT, Magnuson A, Dale W, et al. Developing a clinical and biological measures of aging core: Cancer and Aging Research Group infrastructure. Journal of Geriatric Oncology. 2020;11:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elias R, Loh KP, Targia V, et al. Behavioral, psychological, and supportive care interventions in geriatric oncology: The Cancer and Aging Research Group infrastructure core. Journal of Geriatric Oncology. 2020;11:347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wong ML, Lichtman SM, Morrow GR, et al. Geriatric oncology health services research: Cancer and Aging Research Group infrastructure core. Journal of Geriatric Oncology. 2020;11:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sedrak MS, Li D, Walter LC, et al. Cores for geriatric oncology infrastructure in the Cancer and Aging Research Group: Biostatistics, epidemiology, and research design (the analytics core). Journal of Geriatric Oncology. 2020;11:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Subbiah IM, Lundebjerg N, Appleby J, Wallach G, Beilenson J, Dale W. Development of a strategic plan for the dissemination and communication of aging research through the Cancer and Aging Research Group (CARG) infrastructure grant. Journal of Geriatric Oncology. 2020;11:359–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anand M, Magnuson A, Patil A, et al. Developing sustainable national infrastructure supporting high-impact research to improve the care of older adults with cancer: A Delphi investigation of geriatric oncology experts. Journal of Geriatric Oncology. 2020;11:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fisher GG, Ryan LH. Overview of the Health and Retirement Study and Introduction to the Special Issue. Work, Aging and Retirement. 2017;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Griffith SD, Tucker M, Bowser B, et al. Generating Real-World Tumor Burden Endpoints from Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv Ther. 2019;36:2122–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sledge GW Jr., Miller RS, Hauser R. CancerLinQ and the future of cancer care. Am Soc Clin Oncol Educ Book. 2013:430–434. [DOI] [PubMed] [Google Scholar]
- 128.Miller RS, Wong JL. Using oncology real-world evidence for quality improvement and discovery: the case for ASCO’s CancerLinQ. 2018;14:5–8. [DOI] [PubMed] [Google Scholar]
- 129.Paskett ED, Caan BJ, Johnson L, et al. The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: Description and Baseline Characteristics of Participants. 2018;27:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oncology Center of Excellence, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration. Inclusion of Older Adults in Cancer Clinical Trials, Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration; 2020. [Google Scholar]
- 131.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaughan CP, Dale W, Allore HG, et al. AGS Report on Engagement Related to the NIH Inclusion Across the Lifespan Policy. Journal of the American Geriatrics Society. 2019;67:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.