Abstract
Vascularized Composite Allografts (VCAs) can restore fully functional anatomic units in patients with limb amputations or severe facial tissue loss. However, acute rejection of the skin is frequently observed and underscores the importance of developing tolerance induction protocols. In this study, we have characterized the skin immune system in VCAs. We demonstrate infiltration of recipient leukocytes, regardless of rejection status, and in tolerant mixed hematopoietic chimeras, the co-existence of these cells with donor leukocytes in the absence of rejection. Here we characterize the dermal T cell and epidermal Langerhans cell components of the skin immune system in our porcine model of VCA tolerance, and the kinetics of cutaneous chimerism in both of these populations in VCAs transplanted to tolerant and non-tolerant recipients, as well as in host skin. Furthermore, in biopsies from the first patient to receive a hand transplant in our program, we demonstrate the presence of recipient T cells in the skin of the transplanted limb in the absence of clinical or histological evidence of rejection.
1. Introduction
Vascularized composite allograft (VCA) transplantation achieves unprecedented restoration of function and form following complex tissue loss. The conventional immunosuppressive regimens employed for the majority of these transplants have proven effective in preventing graft loss to rejection in most compliant patients. However, this success is offset by a high incidence of acute rejection episodes, with 85% of patients experiencing at least one episode during the first year post-transplant1,2. The effects of repeated acute rejection episodes may correlate with poor long-term outcomes3, and the recent development of uncontrollable chronic rejection, leading to graft loss in several patients, reflects the insufficiency of standard immunosuppression in ensuring permanent graft survival. The induction of transplant tolerance for VCAs would both prevent acute and chronic rejection, and spare patients from life-long exposure to the risks of conventional immunosuppression.
Skin, which represents an essential component of the majority of VCAs, has long been regarded as particularly immunogenic and a stringent test of transplant tolerance protocols4,5. Attempts to induce tolerance of vascularized composite allografts have frequently resulted in split tolerance, with acceptance of musculoskeletal elements but rejection of skin or epidermis6,7. We have previously reported the induction of whole-skin VCA tolerance across MHC barriers in MGH miniature swine by simultaneous establishment of durable multi-lineage mixed hematopoietic chimerism8. In these studies, we demonstrated the early establishment of donor-specific unresponsiveness in vitro but found no evidence for either classical anergy or regulation of anti-donor responses by T regulatory cells (Tregs) in assays of circulating leukocytes, suggesting that tolerance in this model may be mediated primarily by a deletional mechanism. Furthermore, we demonstrated that stable, long-term multilineage chimerism is required for permanent immunosuppression-free VCA acceptance, as recipients that received donor HSCs but failed to engraft and contribute to long-term hematopoiesis (i.e. transient chimerism) failed to accept VCAs once immunosuppression was removed 9.
Interestingly, protocols achieving split tolerance are also accompanied by evidence of donor-specific unresponsiveness in in vitro assays of peripheral blood-derived leukocytes7, suggesting tissue-specific mechanisms operational within the skin of VCAs may contribute to outcome. The concept of a network of immunologically active cell types within skin is not new, and the skin immune system has come to be regarded as a distinct component of one’s overall immune defenses10. In recent years, the scale and complexity of the skin immune system has gained new appreciation, led by the enumeration of the T cell content of healthy human skin, which at approximately 1x106 T cells/cm2, represents twice the number found in the circulating blood volume11. Skin-resident T cells with characteristics of Tregs have also been identified12,13, and cells of a regulatory phenotype have been identified in the skin of hand transplant recipients several years post-transplant14, although the origin and significance of these cells is not clear. Furthermore, Langerhans cells, long viewed simply as the professional antigen presenting cells of the epidermis, have been demonstrated to possess both stimulatory and regulatory activity15. These findings suggest that the skin immune system is a more complex component of VCAs than previously appreciated, and that a detailed understanding of this system may be important in delineating the mechanisms operational in VCA skin tolerance.
Here we characterize the dermal T cell and epidermal Langerhans cell components of the skin immune system in our porcine model of VCA tolerance, and the kinetics of cutaneous chimerism in both these populations in VCAs transplanted to tolerant and non-tolerant recipients, as well as in host skin. Furthermore, in biopsies from the first patient to receive a hand transplant in our program, we demonstrate the presence of recipient T cells in the skin of the transplanted limb in the absence of clinical or histological evidence of rejection.
2. Materials and Methods
2.1. Study Design
This study represents an extension of our previously published studies demonstrating induction of tolerance of skin-bearing VCAs across MHC barriers in miniature swine by establishment of durable mixed chimerism8, designed to investigate the cutaneous leucocyte populations in tolerant and rejecting skin at the cellular level. In addition, the analytical techniques described were applied to samples from the first patient in our hand transplant program.
2.2. Animals
Miniature swine experiments were performed using haploidentical donor/recipient combinations selected from the MGH miniature swine herd, which are bred with defined MHC but maintained minor antigen variation16. SLAac animals positive for the allelic hematopoietic marker pig allelic antigen (PAA) were selected as donors17. Recipients were SLAad PAA negative. Animals were housed at the Transplantation Biology Research Center in accordance with the Guide for the Care and Use of Laboratory Animals18. All experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital.
2.3. Hematopoietic Cell Transplantation
Stem cell collection and recipient conditioning were performed as previously described19. Donors received 7-days of porcine IL-3 (pIL-3) and stem cell factor (pSCF), injected intramuscularly at 100ug/kg to 30kg bodyweight, 50ug/kg per additional kilogram. Mobilized HSCs were collected from peripheral blood by apheresis over three days, commencing after the 5th dose of pIL-3/pSCF. Recipients underwent conditioning with 100 cGy total body irradiation on day −2, partial T cell depletion with recombinant CD3-immunotoxin (pCD3-DT390) from day −4 to −120, and a 45 day course of Cyclosporine A (target trough 400-800ng/ml day 0-30 then taper to discontinuation). Unmodified, T cell-replete cytokine mobilized peripheral blood mononuclear cells were transplanted over 2 or 3 days following conditioning (days 0-2) as required to achieve the target dose of 15x109 cells/kg.
2.4. Vascularized Composite Allograft Transplantation
A fasciocutaneous VCA model was used21. Under general anaesthesia a skin flap of approximately 10x18cm was elevated on a vascular pedicle comprising the medial saphenous artery and veins to their junctions with the superficial femoral vessels, which were harvested to a length of 2cm to facilitate anastomosis. Flaps were harvested and flushed with 100 U/ml heparin sulphate in 0.9% normal saline. Donor vessels were anastomosed to the recipient carotid artery and internal jugular vein using standard microsurgical techniques. Warm ischemia time was approximately 60 minutes. VCA transplantation was performed simultaneously to induction of mixed chimerism within 56 hours of the first infusion of donor hematopoietic cells. Control recipients underwent VCA transplantation with neither conditioning nor maintenance immunosuppression.
2.5. Peripheral Blood and Tissue Chimerism Analysis
Peripheral blood chimerism was monitored by flow cytometry using monoclonal antibodies to PAA (1038H-10-9)17 and lineage markers to CD1 (76-7-4)22, CD3ε(898H2-6-15)23, CD4 (74-12-4)22, CD8a (76-2-11)24, CD16 (G7)25, CD25 (231.3B2)26, CD21 (11C9), CD172 (74-22-15A)22, FoxP3 (FJK-16s, eBioscience, USA) and γ/δ (MAC320, BD Bioscience, USA). Acquisition on FACSCalibur or LSRII (Becton Dickinson, USA) was followed by data analysis in FlowJo (TreeStar Inc., USA).
Cutaneous chimerism in swine was analyzed following biopsy on day 14, 30, 50 and approximately every 50 days thereafter. Biopsies of VCA and host skin were taken using 6mm biopsy punch and digested independently in 1mg/ml Dispase II (Roche, USA) for 16-24 hours at 4°C. Epidermis and dermis were then separated and dermis transferred to 1mg/ml Collagenase D (Roche, USA) for a further overnight incubation at 37°C. Epidermal samples were incubated for 30 minutes in 0.05% Trypsin (Sigma, USA) to prepare a single cell suspension of epidermal leukocytes. Following incubation in collagenase dermal samples were filtered over a 40μm filter, spun down, re-suspended in FACS media and stained. In addition to peripheral blood markers above, skin samples were stained with CD207 (Langerin, 4C7, Biolegend, USA), and MHC Class II (Class II DQ, TH16, TBRC, USA).
2.6. Histopathology and Immunohistochemistry
Biopsy samples for histopathology and immunohistochemistry were immediately placed in formalin and processed by routine histologic methods and assessed by a board certified pathologist. Immunohistochemistry was performed on formalin fixed, paraffin embedded specimens following antigen retrieval with Diva or Borg decloaking solution (Biocare Medical). Adjacent 10 μm sections were stained for CD3+ (rabbit anti-human CD3, DAKO, USA; biotinylated goat anti-rabbit IgG, Vector Labs, USA; streptavidin, Biogenix; DAB, DAKO, USA), or FoxP3+ (rat anti-FoxP3, Ebioscience, USA; rat-on-mouse HRP probe/polymer, Biocare, USA; AEC, Biocare, USA). CD3+ and FoxP3+ cells in the dermis and epidermis were quantified from images captured at 400x magnification using a DP25 camera and BX53 microscope (Olympus, USA).
2.7. Human Upper Extremity Transplantation
A male patient underwent unilateral upper extremity transplantation at MGH in October 2012 as previously reported27. Immunosuppression was induced with rabbit antithymocyte globulin, and maintained with Tacrolimus, Mycophenolate Mofetil and Prednisolone. Protocol biopsies were obtained at 6 monthly intervals post-transplant with the consent of the patient and in accordance with the Institutional Review Board protocol. At 12- and 18-month points, samples were processed for flow cytometry as described above, stained with CD3-FITC (UCHT1, BD Biosciences, USA), and HLA A1/B8 (One Lambda, USA) conjugated to Streptavidin (0.1ug/ml, BD Biosciences, USA) and acquired on LSRII (Becton Dickinson, USA). Analysis was performed using FlowJo (TreeStar Inc., USA). Samples were also processed by routine histological methods and assessed by board certified pathologist.
3. Results
3.1. T cell-replete hematopoietic cell transplantation in miniature swine establishes stable chimerism in all assessed peripheral blood lineages, including CD4+CD25+FoxP3+
Mixed chimerism across a haploidentical MHC mismatch was established in two MGH miniature swine (Figure 1A) as previously described7. The pIL3/pSCF cytokine-mobilized graft contained CD3+ T cells 19% (of which CD4+ and CD8+ single positive cells accounted for 52% and 28%, respectively and CD4+CD8+ for 3%), B Cells (CD3−CD16−) 8%, Monocytes (CD172a+CD16+SSClo) 40% and Granulocytes (CD172a+CD16+SSChi) 11% (data not shown). In comparison to previous studies, in which chimerism was monitored in broad CD3+ T cell, monocyte and granulocyte lineages, in these animals the contribution of donor-origin (PAA+) cells was followed longitudinally in 9 different lineages, including CD4+, CD8+, CD4+CD8+, γ/δ, B cells, NK cells, monocytes and granulocytes. Persistent chimerism was observed for all lineages, but chimerism did not stabilize at similar levels, with donor contribution ranging from approximately 20% of NK cells to 90% of γ/δ T cells in both animals (Figure 1B, C). Animal 21557 underwent spontaneous conversion to full donor chimerism in monocytes and granulocytes following day 50 (Figure 1C). This conversion was not associated with graft-versus-host-disease (GvHD).
Figure 1: Establishment of durable multi-lineage mixed chimerism in peripheral blood of VCA-recipient miniature swine.
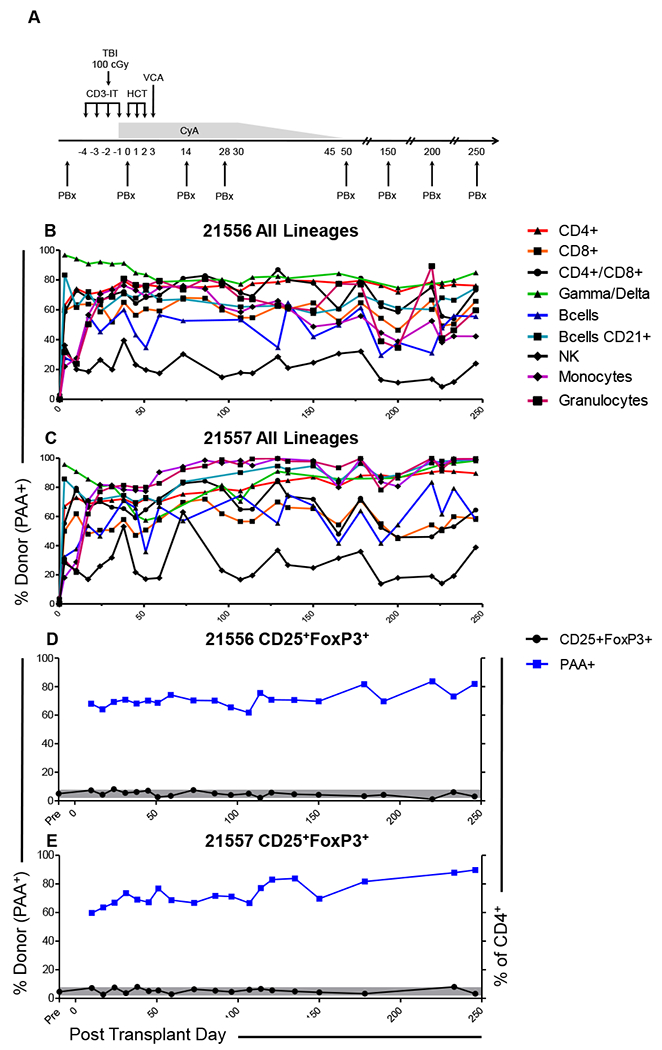
(A) Tolerance induction protocol. TBI: total body irradiation; HCT: haematopoietic cell transplantation; VCA: vascularized composite allotransplantation; CyA: Cyclosporine A; PBx: protocol biopsy. Chimerism was followed longitudinally in peripheral blood for T cell (CD4+, CD8+, CD4+CD8+, γ/δ), B cell (CD3−CD16−, CD3−CD16−CD21+), NK cell (CD3−CD16+CD172a−) and myeloid (monocyte; CD172+SSClo, granulocyte; CD172+SSChi) lineages. Data is presented as percentage donor-type (PAA+) cells in each lineage for (B) animal 21556 and (C) animal 21557. Relative abundance of cells bearing the potentially regulatory phenotype CD25+FoxP3+ as a percentage of the CD4+ population (black line), and chimerism (% PAA+, blue line) amongst this population was also followed (D, E). Grey shading indicates the range of CD25+FoxP3+ expression across the MGH miniature swine herd.
Chimerism among peripheral blood CD25+FoxP3+ cells was comparable to that observed in other T cell lineages for both animals (60-80% PAA+ of donor origin). No significant change from baseline levels was observed for cells with this potentially regulatory phenotype, either as a % of the CD4+ population (Figure 1 D, E) or in terms of absolute numbers, which failed to make a sustained recovery to pre-transplant levels in either animal (Figure S1 A, B).
3.2. VCAs undergo rapid infiltration with recipient-type T cells, which, in mixed chimeras, do not result in rejection
As previously described, chimeras accepted VCAs with no gross evidence of rejection at any point (Figure 2A), whereas control VCAs rapidly developed signs of rejection and were rejected by day 8 (Figure 2B). Once healed, VCAs on tolerant recipients were virtually indistinguishable from host skin. Histological features consistent with grade 1 rejection on the Banff scale were identified in some samples (mild perivascular infiltrate), but these were focal and transient, and non-specific causes of inflammation were considered responsible (data not shown).
Figure 2: Infiltration by recipient T cells without rejection establishes mixed chimerism within the skin of VCAs in tolerant recipients.
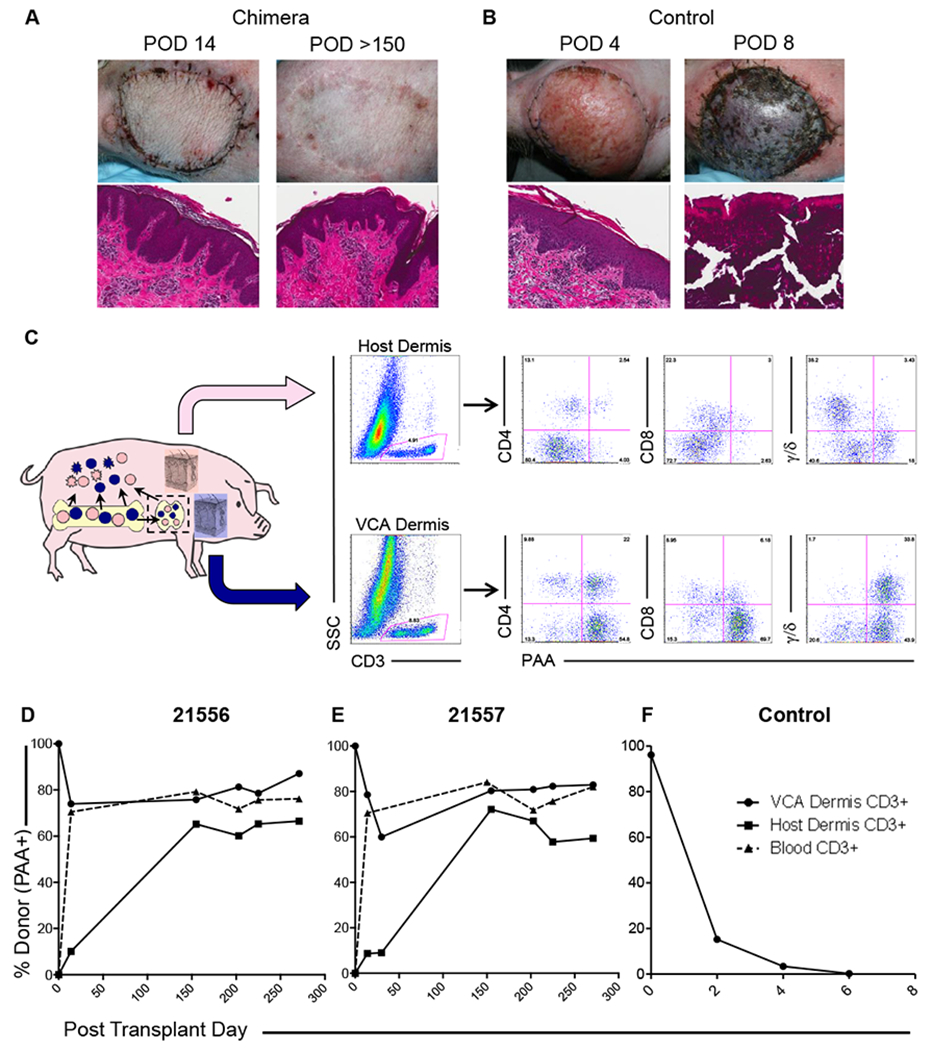
(A) Recipients of simultaneous VCA and HCT accept VCAs with neither gross nor histological evidence of rejection at any point Representative images of animal 21556. (B) Non-chimeric controls rapidly developed signs of rejection, progressing to graft loss and necrosis within 8 days. Representative images of animal 22181. (C) VCA and host skin biopsies were digested in dispase II to separate epidermal and dermal components, prior to further digestion prepare single cell suspensions of each component. Dermal CD3+ cells were analyzed by lineage and hematopoietic origin. Infiltration of recipient (PAA−) CD4+, CD8+ and γ/δ T cells was observed at the initial VCA biopsy on day 14, and found to persist at each subsequent time point. Similarly, host skin was infiltrated by donor (PAA+) T cells. Representative data from animal 21556 14 days post-transplant is shown. (D, E) Following initially rapid infiltration of VCA dermis with recipient CD3+ cells, cutaneous T cell chimerism levels equilibrated close to those observed in peripheral blood. Infiltration of donor-derived T cells in to recipient dermis was also observed and increased with time to similar levels. (F) In contrast, donor-derived T cells were rapidly and progressively lost from rejecting control VCAs as rejection progressed. Representative data from one of two control animals (22181) is shown. Host skin and peripheral blood chimerism were not analyzed in these animals, which did not undergo hematopoietic cell transplantation.
Seeking to characterize the cutaneous leukocyte populations in detail we performed independent digestion and flow cytometric analysis of epidermal and dermal components. Dermal T cells (CD3+) were analyzed for expression of CD4, CD8, γ/δ and PAA. Remarkably, both VCA and host skin dermis were found to contain chimeric populations of CD4+, CD8+ and γ/δ T cells following HSCT and VCA (Figure 2C).
Prior to transplantation, VCA dermal T cells were uniformly PAA-positive, and pre-transplant host skin was consistently PAA-negative (as were peripheral blood T cells; data not shown). However, by post-transplant day 14, the dermal CD3+ population of the VCA contained approximately 25% recipient origin (21556; 26%, 21557; 22%), demonstrating rapid infiltration of recipient T cells. In both animals, VCA dermal T cell chimerism remained relatively stable at approximately 75% donor, mirroring peripheral blood levels at each subsequent time point, and no evidence of rejection was observed. In control VCAs, donor derived T cells were rapidly lost (<1% by post-transplant day 6) and replaced with infiltrating recipient T cells, and in these animals rapid rejection occurred (Figure 2F). Surprisingly, host skin also demonstrated early infiltration of donor-derived T cells (approximately 10% donor at post-transplant day 14), equilibrating close to levels observed in VCA, and in blood by day 150 (Figure 2D, E). No evidence of skin GvHD was observed.
The possibility of local regulation within the skin of accepted VCAs, an hypothesis supported by the identification of FoxP3+ cells by immunohistochemistry (Figure 3A), could not be excluded. The specificity of Tregs and the respective contribution of donor- and host-derived Tregs remained uncertain; therefore, we sought to analyze chimerism amongst these cells in accepted VCAs. In both tolerant animals, at >250 days post-transplant, CD4+CD25+FoxP3+ cells of both donor and host origin were identified in VCA skin biopsies (Figure 3B), with levels of donor-derived cells comparable to those observed in host skin (Figure 3C), as well as the CD4+CD25+FoxP3+ population in peripheral blood (Figure 1D,E).
Figure 3: FoxP3+ cells in both tolerated VCAs and host skin demonstrate mixed chimerism.
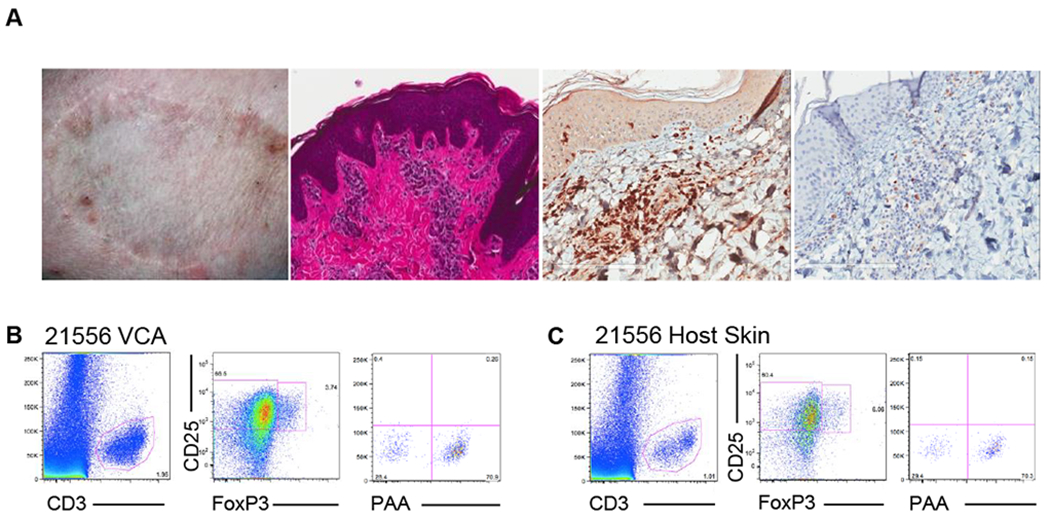
(A) FoxP3+ cells were identified within focal collections of CD3+ cells in biopsies of tolerated VCAs by immunohistochemical staining. Digestion and flow cytometric analysis of tissue samples identified cells bearing a potentially regulatory phenotype (CD25+FoxP3+) in both (B) VCA and (C) host skin. Both tissues demonstrated the presence of chimeric Treg populations, at chimerism levels comparable to those detected in peripheral blood. Representative data from animal 21556 271 days post-transplant is shown.
3.3. Host-derived epidermal Langerhans cells rapidly infiltrate epidermis of tolerant VCA
To characterize the Langerhans cell population in accepted and rejecting VCAs, we isolated CD172a+ cells from epidermis (Figure 4A). These cells were uniformly MHC Class II+, CD45+, CD1+ and greater than 98% expressed CD207 (Langerin) (Figure S2), confirming their identity as Langerhans cells. Furthermore, in animals expressing PAA, greater than 98% of this population were PAA+, allowing analysis of chimerism by differential expression of PAA and recipient MHC Class II (SLA Class IId) (Figure 4B). We observed that VCAs rapidly underwent infiltration with recipient-derived Langerhans cells (7-15% at day 14, 28-31% at day 30), whereas donor-derived Langerhans cells could not be identified in recipient skin until day 100 (Figure 4B–D). The percentage chimerism in this population in VCA and recipient skin equilibrated, at a level comparable to peripheral blood monocytes (Figure 4C, D). In animal 21557, which underwent spontaneous conversion to full donor chimerism in peripheral blood monocyte and granulocyte populations around day 50, both VCA and recipient skin Langerhans cell populations rapidly converted to 100% donor-derivation.
Figure 4: VCA tolerance is accompanied by establishment of epidermal Langerhans’ cell chimerism.
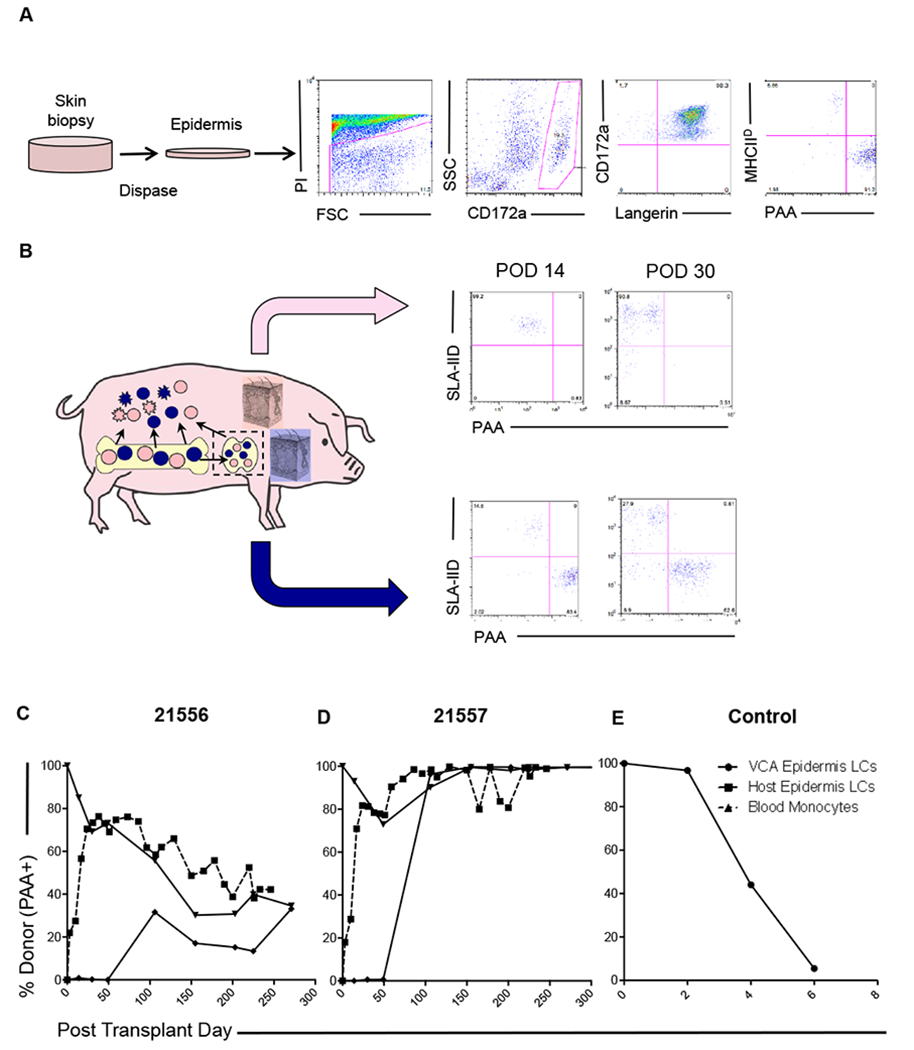
(A) Digestion of skin biopsies in dispase II facilitates independent analysis of the epidermal compartment, in which Langerhans’ cells (LCs) were identified. Chimerism was analyzed by split expression of PAA (donor) and SLA-IId (recipient). Representative data from animal 21557 day 14 is shown. (B) Chimeric VCA recipients underwent regular biopsies and parallel analysis of VCA and host skin. Recipient-derived LCs were detected early post-transplant (day 14, day 30) in VCA. Donor-derived LCs were not identified at these time points in recipient skin. Representative data from animal 21556 is shown. (C) Longitudinal analysis of epidermal LC origin demonstrated establishment of durable mixed chimerism in one recipient (21556), which equilibrated in both VCA and host skin at levels comparable to peripheral blood monocytes (dashed line). (D) In the other recipient (21557), spontaneous conversion to full donor chimerism in the myeloid lineages was detected in peripheral blood around day 50, after which time, both VCA and recipient skin epidermal LCs were found to be uniformly of donor origin. (E) In rejecting control VCAs, donor-derived LCs were progressively lost as rejection progressed, and stable chimerism was not established.
In contrast, rejecting VCAs underwent rapid dilution of resident Langerhans cells with a Class II+CD207− cell population, increasing from 5-10 % observed in donor skin pre-transplant to 55-68% by day 2, and 80-86% by day 4. Recipient-derived Langerhans cells were observed to infiltrate these rejecting VCAs from day 4 (31-55%) with near complete loss of donor contribution by day 6, immediately preceding loss of these grafts to rejection on day 7 and 8 (Figure 4E).
3.4. Recipient T cells repopulate donor skin following clinical upper extremity transplantation under triple-agent immunosuppression without evidence of rejection
To date, our reconstructive transplantation program has performed an upper extremity transplant for one patient. Neither clinical nor histological evidence of rejection was observed at any point prior to the biopsies reported herein (Figure 5A, B), and the patient remained rejection-free for over 5 years post-transplant. Consistent with this clinical status, minimal numbers of CD4 and CD8 T cells were observed on immunohistochemical staining (Figure 5C, D). However, when leukocytes were isolated for flow cytometric analysis, a significant population (52.5%) of recipient-type T cells was identified in the transplant dermis at 12 months post-transplant (Figure 5E). By 18 months, we observed almost complete replacement of the donor-type population observed by recipient cells (>90% in both CD4+ and CD8+ lineages) (data not shown).
Figure 5: Recipient T cells identified in skin of transplanted human hand without evidence of rejection.
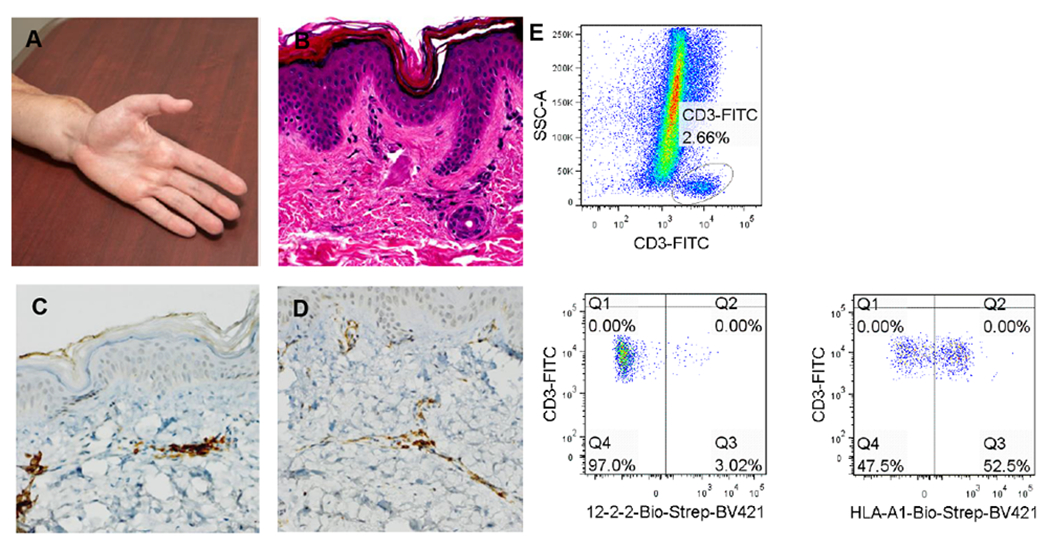
(A) Clinical photograph of transplant hand at 1 year. (B) H&E stained section of protocol biopsy obtained at 1 year – no signs of rejection are observed. Immunohistochemical staining for (C) CD4+ and (D) CD8+ at the same time point demonstrate low numbers of these cells, consistent with the absence of acute rejection. (E) Flow cytometric analysis of T cells isolated from transplanted dermis demonstrates populations of both donor (52.5%) and recipient origin (47.5%).
4. Discussion
We have previously demonstrated the induction of tolerance of VCAs across MHC barriers in the MGH miniature swine model. In contrast to studies of PBMC, which suggest neither regulation nor anergy as primary contributing factors to systemic donor-specific non-responsiveness, the observation of FoxP3+ cells within focal areas of CD3+ infiltrate in the skin of tolerated VCAs suggested that local mechanisms may contribute to the induction and maintenance of VCA skin tolerance8. Indeed, recent studies in mouse models have demonstrated a necessary role of Tregs in the maintenance of tolerance of MHC-mismatched skin and heart grafts in mixed hematopoietic chimeras 28.
Increased availability of anti-porcine antibodies facilitated more expansive analysis of peripheral blood chimerism than previously reported. The broad distribution of donor contributions across the lineages analyzed highlights the multi-lineage nature of chimerism induced by this protocol, and confirms the contribution of both donor- and host-derived elements throughout the immune system available to contribute to immune competence. Regardless of the presence of both donor- and host-derived CD25+FoxP3+ Tregs, there does not appear to be a correlation between peripheral blood Treg levels and VCA tolerance, as the percentage contribution of these cells remains stable within the normal variation observed for naïve animals in the MGH miniature swine herd, and absolute numbers did not increase above pre-transplant levels.
The skin component of VCAs is a significant target of acute rejection1,2. Recent studies have contributed to increased appreciation of the scale and complexity of the skin immune system. In human skin, resident T cells express the skin-homing addressins CLA and CCR4, and the majority are phenotypically T-effector memory (TEM) cells, as defined by lack of CD62L and CCR7 expression. Interestingly, those cells which did express these markers of central memory co-expressed the skin-homing addressins, suggesting the ability to cycle between skin and lymph node11. The presence of central memory T cells capable of patrolling between skin and lymphoid organs, combined with residence of large number of TEM cells in skin represents a formidable immune barrier and may contribute to the high incidence of skin rejection observed post-transplant, and the challenge of establishing skin tolerance. The rapid infiltration of the cutaneous component of VCAs with recipient T cells, in the absence of clinical or histological evidence of rejection in this study, demonstrates that circulating T cells are rapidly tolerized by this protocol. Furthermore, the establishment of durable T cell chimerism within both VCA and host skin, and the gradual equilibration of these chimerism levels with each other and those observed in peripheral blood is consistent with functional integration of these cells and maintenance of homeostasis in the skin immune system.
Within the recipient skin, the rapid appearance of donor-derived T cells does not induce any evidence of GvHD. This observation is remarkable, as the majority of the initial circulating donor T cells would have been transferred as part of the overall mobilized HSCT product, and thus not reflect donor-derived T cells that had undergone thymic education within the context of the recipient’s MHC (for positive and negative selection). This finding suggests that the mechanisms within the local skin microenvironment are able to prevent rejection by a T cell population not initially subject to the mechanisms of central tolerance 29.
In the current study, analysis was limited by the VCA’s tissue volume and therefore, limited cell yields were available at each time point in order to avoid compromise of VCA integrity. Subsequent refinements to our isolation protocol, and increased availability of porcine-reactive antibodies in multiple fluorochromes have addressed this issue, and future work will analyze the expression of skin homing addressins, as well as draining lymph node populations.
Recipient Langerhans cells move into the epidermis of the VCA as early as two weeks post-transplant. Langerhans cells were initially described as antigen presenting cells, found primarily within the epidermis30,31, and multiple subsequent studies have confirmed their ability to act as potent stimulators of an immune response32–35. More recently, some studies have suggested that they may act to regulate the immune response 36–38. The recent observation that under quiescent conditions human Langerhans cells activate and stimulate proliferation of skin resident Tregs, while in the presence of pathogenic antigens induce activation of TEM cells may offer an explanation for this functional dichotomy15.
The rapid appearance of recipient-derived Langerhans cells in VCA epidermis post-transplant, in comparison to the more delayed development of Langerhans cell chimerism in recipient skin, is consistent with previous descriptions of distinct populations of acute-phase and steady-state Langerhans cells39. Presumably the nonspecific inflammatory insult associated with transplantation stimulates egress of donor-derived Langerhans cells to the draining lymph node, and repopulation of the VCA epidermis with monocyte-derived precursors from blood. In contrast, the delayed appearance of donor-derived Langerhans cells in recipient skin is consistent with persistence of the long-lived, self-renewing population with only gradual contribution from donor-derived precursors in the context of prolonged mixed hematopoiesis. The role of these cells in the setting of transplantation remains to be elucidated, but the establishment of durable chimerism in both cutaneous T cells and Langerhans cells in our model may permit cognate signaling between these populations, necessary not only for immune competence but also appropriate immune homeostasis and maintenance of whole-skin tolerance. Interestingly, Langerhans cells isolated from the VCA of long-term tolerant chimeric animals stimulate proliferation of responders of both donor and host type, but not chimeric-self, in mixed lymphocyte reaction (unpublished data), suggesting that these cells are not functioning in a dominant suppressive manner. Further study of the kinetics of Langerhans cell chimerism, and the interaction of these cells with cutaneous T cells may yield insights important for the understanding of not only VCA acceptance and rejection, but also cutaneous GvHD.
In translating these techniques to the clinic, in samples from our upper extremity transplant patient, on a conventional immunosuppression regimen, substantial populations of recipient-type T cells were identified within transplanted dermis. This finding was observed uniformly in the absence of either clinical or histological evidence of rejection or general increased lymphocyte infiltration. This finding is unexpected when we consider that these T cells have not been exposed to a tolerance induction regimen. Thus, the presence of mature recipient T cells, controlled solely by chemical immunosuppression, within the transplanted skin of VCAs in the absence of rejection, represents a disconnect between the traditional clinical and histological diagnosis of rejection and the underlying immunology. Further study in additional patient samples is required to define the kinetics of cutaneous leukocyte turnover under conventional immunosuppression, and to achieve a more granular definition of the populations present, in the context of our expanding appreciation of cutaneous T cell subtypes and their functional properties40. It is also is worth noting that this technique, which is no more invasive than standard biopsy methods, and which allows the analytical power of polychromic flow cytometry to be applied, may be a useful adjunct to conventional histological techniques in monitoring and diagnosing rejection following transplantation.
In summary, we have demonstrated the rapid infiltration of VCAs with recipient T cells in the absence of gross or histological evidence of rejection, establishing cutaneous T cell chimerism and suggesting that the infiltrating cells are rapidly tolerized. Chimerism is also established among Langerhans cells, occurring more rapidly in VCAs than in recipient skin, consistent with an acute-phase response to transplantation and progressive turnover of Langerhans cell progenitors, respectively. Preliminary studies of human samples from a hand transplant recipient receiving triple agent immunosuppression also demonstrate similar influx of recipient T cells, however in this case donor T cells were observed to persist up to 1 year post-transplant, and the patient enjoyed a prolonged rejection-free period of over 5 years post-transplant.
Supplementary Material
Table 1. Summary of animals experimental animals in study of haploidentical VCA tolerance.
Including both animals reported herein and those included in previously published experiments 8.
| Animal number | Conditioning | HSCT | VCA Timing 1 | Persistent chimerism (blood) | Persistent chimerism (skin) 2 | non-responsiveness 3 | VCA survival (days) 4 | Additional Comments |
|---|---|---|---|---|---|---|---|---|
| 17468 | Yes | Yes | Delayed | Yes | N/A | Yes | >46 | Central line complications, euthanasia day 46 |
| 17469 | Yes | Yes | Delayed | Yes | N/A | Yes | >400 | - |
| 17519 | No | No | N/A | No | N/A | No | 6 | - |
| 17520 | No | No | N/A | No | N/A | No | 6 | - |
| 20311 | Yes | Yes | Delayed | Yes | N/A | Yes | >504 | Cutaneous GvHD stage 1 day 50. CyA day 76-106. Complete resolution, no reccurence. |
| 20313 | Yes | Yes | Delayed | Yes | N/A | Yes | >115 | Cutaneous GvHD stage 1 day 45, progressive to stage 3 by day 70. Relapsing-remitting course treat with CyA days 86-110, Methylprednisolone dats 71-114, 130-150, 160-179 |
| 20312 | Yes | No | Delayed | No | N/A | No | 9 | - |
| 20680 | Yes | Yes | Simultaneous | Yes | N/A | Yes | >486 | - |
| 20681 | Yes | Yes | Simultaneous | Yes | N/A | Yes | >139 | - |
| 20989 | Yes | No | Simultaneous | No | N/A | No | 79 | - |
| 21556 | Yes | Yes | Simultaneous | Yes | Yes | Yes | >395 | No clinical or histological evidence of GvHD |
| 21557 | Yes | Yes | Simultaneous | Yes | Yes | Yes | >311 | No clinical or histological evidence of GvHD |
| 22181 | No | No | N/A | No | No | No | 7 | - |
| 22183 | No | No | N/A | No | No | No | 8 | - |
VCA transplantation simulatous to induction of mixed chimerism (within 56h of first infusion of donor hematopoeitic cells) or delay 85-150 days post HSCT. N/A indicates animal received VCA with neither conditioning nor HSCT.
Skin chimerism analysis reported for first time herein.
Specific absence of anti-donor response in mixed lymphocyte and/or cell mediated lymphocytotoxicity assays utilizing peripheral blood mononuclear cells
>denotes rejection-free VCA survival at termination of experiment.
Acknowledgments
The authors wish to thank Mrs. Rebecca Brophy and Miss Kelsey Painter for secretarial and administrative support. We acknowledge support from NIH grant CO6RR020135-01 for construction of the facility used for production and maintenance of miniature swine. DAL was supported by a Novartis Scientist Scholarship from the American Association of Transplant Surgeons.
Abbreviations
- VCA
Vascularized composite allograft
- Treg
T regulatory cell
- CMV
Cytomegalovirus
- HCT
Hematopoietic cell transplantation
- GvHD
Graft versus host disease
- MGH
Massachusetts General Hospital
- pSCF
Porcine stem cell factor
- pIL-3
Porcine interleukin-3
- SLA
Swine leukocyte antigen
- CML
Cell mediated lymphocytoxicity assay
- MLR
Mixed lymphocyte reaction assay
- PAA
Pig allelic antigen
Footnotes
Disclosure
The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Petruzzo P, Lanzetta M, Dubernard JM, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2010;90(12):1590–1594. [DOI] [PubMed] [Google Scholar]
- 2.Hautz T, Zelger BG, Weissenbacher A, et al. Standardizing skin biopsy sampling to assess rejection in vascularized composite allotransplantation. Clinical Transplantation. 2013;27(2):E81–E90. [DOI] [PubMed] [Google Scholar]
- 3.Unadkat JV, Schneeberger S, Horibe EH, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am J Transplant. 2010;10(2):251–261. [DOI] [PubMed] [Google Scholar]
- 4.Murray JE. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast Reconstr Surg. 1971;47(5):425–431. [DOI] [PubMed] [Google Scholar]
- 5.Steinmuller D The enigma of skin allograft rejection. Transplant Rev. 1998;12:42. [Google Scholar]
- 6.Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75(1):25–31. [DOI] [PubMed] [Google Scholar]
- 7.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77(4):514–521. [DOI] [PubMed] [Google Scholar]
- 8.Leonard DA, Kurtz JM, Mallard C, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant. 2014;14(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leto Barone AA, Kurtz JM, Albritton A, et al. Effects of Transient Donor Chimerism on Rejection of MHC-Mismatched Vascularized Composite Allografts in Swine. Vascularized Composite Allotransplantation. 2015;2(1):1–8. [Google Scholar]
- 10.Bos JD. Skin Immune System (SIS). CRC Press; 1997. [Google Scholar]
- 11.Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176(7):4431–4439. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205(10):2221–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109(1):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eljaafari A, Badet L, Kanitakis J, et al. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttransplantation. Transplantation. 2006;82(12):1764–1768. [DOI] [PubMed] [Google Scholar]
- 15.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human Epidermal Langerhans Cells Maintain Immune Homeostasis in Skin by Activating Skin Resident Regulatory T Cells. Immunity. 2012;36(5):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunney JK, Sachs DH. Transplantation in miniature swine. V. Characterization of Ia antigens. J Immunol. 1979;122(2):623–627. [PubMed] [Google Scholar]
- 17.Fuchimoto Y, Huang C, Shimizu A, Seebach J, Arn S, Sachs DH. An allelic non-histocompatibility antigen with wide tissue distribution as a marker for chimerism in pigs. Tissue Antigens. 1999;54(1):43–52. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 2011. [Google Scholar]
- 19.Cina RA, Wikiel KJ, Lee PW, et al. Stable multilineage chimerism without graft versus host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation. 2006;81(12):1677–1685. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Duran-Struuck R, Crepeau R, et al. Development of a Diphtheria Toxin Based Antiporcine CD3 Recombinant Immunotoxin. Bioconjugate Chemistry. 2011;22(10):2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horner BM, Randolph MA, Duran-Struuck R, et al. Induction of Tolerance to an Allogeneic Skin Flap Transplant in a Preclinical Large Animal Model. Transplant Proc. 2009;41(2):539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133(1):368–375. [PubMed] [Google Scholar]
- 23.Huang CA, Lorf T, Arn JS, Koo GC, Blake T, Sachs DH. Characterization of a monoclonal anti-porcine CD3 antibody. Xenotransplantation. 1999;6(3):201–212. [DOI] [PubMed] [Google Scholar]
- 24.Jonjić S, Koszinowski UH. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J Immunol. 1984;133(2):647–652. [PubMed] [Google Scholar]
- 25.Halloran PJ, Sweeney SE, Strohmeier CM, Kim YB. Molecular cloning and identification of the porcine cytolytic trigger molecule G7 as a Fc gamma RIII alpha (CD16) homologue. J Immunol. 1994;153(6):2631–2641. [PubMed] [Google Scholar]
- 26.Denham S, Shimizu M, Bianchi ATJ, Zwart RJ, Carr MM, Parkhouse RME. Monoclonal antibodies recognising differentiation antigens on porcine B cells. Veterinary Immunology and Immunopathology. 1994;43(1–3):259–267. [DOI] [PubMed] [Google Scholar]
- 27.Eberlin KR, Leonard DA, Austen WG, et al. The volar forearm fasciocutaneous extension: a strategy to maximize vascular outflow in post-burn injury hand transplantation. Plast Reconstr Surg. 2014;134(4):731–735. [DOI] [PubMed] [Google Scholar]
- 28.Shinoda K, Akiyoshi T, Chase CM, et al. Depletion of foxp3(+) T cells abrogates tolerance of skin and heart allografts in murine mixed chimeras without the loss of mixed chimerism. Am J Transplant. 2014;14(10):2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadha R, Leonard DA, Kurtz JM, Cetrulo CL. The unique immunobiology of the skin: implications for tolerance of vascularized composite allografts. Curr Opin Organ Transplant. 2014;19(6):566–572. [DOI] [PubMed] [Google Scholar]
- 30.Stingl G, Tamaki K, Katz SI. Origin and Function of Epidermal Langerhans Cells. Immunol Rev. 1980;53(1):149–174. [DOI] [PubMed] [Google Scholar]
- 31.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978;121(5):2005–2013. [PubMed] [Google Scholar]
- 32.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. [DOI] [PubMed] [Google Scholar]
- 33.Klechevsky E, Morita R, Liu M, et al. Functional Specializations of Human Epidermal Langerhans Cells and CD14+ Dermal Dendritic Cells. Immunity. 2008;29(3):497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci USA. 2009;106(51):21795–21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furio L, Briotet I, Journeaux A, Billard H, Péguet-Navarro J. Human Langerhans Cells Are More Efficient Than CD14−CD1c+ Dermal Dendritic Cells at Priming Naive CD4+ T Cells. Journal of Investigative Dermatology. 2010;130(5):1345–1354. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31(12):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611–620. [DOI] [PubMed] [Google Scholar]
- 38.Bennett CL, Fallah-Arani F, Conlan T, et al. Langerhans cells regulate cutaneous injury by licensing CD8 effector cells recruited to the skin. Blood. 2011;117(26):7063–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seré K, Baek J-H, Ober-Blöbaum J, et al. Two Distinct Types of Langerhans Cells Populate the Skin during Steady State and Inflammation. Immunity. 2012;37(5):905–916. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7(279):279ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


