Abstract
Background:
Smoking is a risk factor for fracture, but the mechanism by which smoking increases fracture risk is unclear.
Methods:
Musculoskeletal health was compared with dual x-ray absorptiometry (DXA), high resolution peripheral quantitative computed tomography (HR-pQCT), trabecular bone score (TBS), and vertebral fracture assessment (VFA) in current and past smokers and non-smokers from a multi-ethnic study of adults ≥ age 65. Skeletal indices were adjusted for age and weight.
Results:
Participants (n=311) were mean age (±SD) 76.1±6.5 years, mostly female (66.0%) and non-white (32.7% black/39.4% mixed race/26.3% white). Mean pack-years was 34.6±20.4. In men (n=106), weight and BMI were lower (both p<0.05) in current vs. past smokers. Male smokers consumed half the calcium of never and past smokers. BMD by DXA did not differ by smoking status at any skeletal site in either sex. Current male smokers had 13.5-15.3% lower TBS vs. never and past smokers (both p<0.05). By HR-pQCT, trabecular (Tb) volumetric BMD was 26.6-30.3% lower and trabeculae were fewer, thinner and more widely spaced in male current vs. past and never smokers at the radius (all p<0.05). Cortical indices did not differ. Tibial results were similar, but stiffness was also 17.5-22.2% lower in male current vs. past and never smokers (both p<0.05). In women, HR-pQCT Tb indices did not differ, but cortical porosity was almost twice as high in current vs. never smokers at the radius and 50% higher at the tibia (both p<0.05).
Conclusions:
In summary, current smoking is associated with trabecular deterioration at the spine and peripheral skeleton in men, while women have cortical deficits. Smoking may have sex-specific skeletal effects. The consistent association with current, but not past smoking, suggests the effects of tobacco use may be reversible with smoking cessation.
Keywords: tobacco, microstructure, skeletal, fracture
Introduction
Smoking is the leading cause of preventable death. While smoking is recognized to affect the cardiovascular and respiratory systems, it has pervasive effects on health, including the musculoskeletal system. Numerous studies indicate smoking is a risk factor for fracture. Smoking not only increases the risk for all types of fracture, but hip fracture risk in particular is higher among smokers than non-smokers and the increased risk may escalate with aging [1-4]. Further, when fracture occurs, smoking increases the risk of poor fracture healing and non-union, further increasing the morbidity associated with fractures among smokers [5].
The mechanism by which smoking increases fracture risk has not been fully elucidated, though multiple factors may contribute. While smokers tend to have lower body weight compared to their non-smoking counterparts due to the appetite suppressive effects of nicotine, the increased risk of fracture persists independently of low weight [6]. Muscle mass and function may also be reduced by smoking, but few studies have comprehensively evaluated this [7, 8]. In vitro studies also suggest direct effects of smoking and/or nicotine on osteoblasts and osteoclasts that decrease bone formation while increasing resorption [9-13].
While the effects of smoking on bone remodeling would be expected to reduce bone mineral density (BMD), studies are inconsistent with regard to the effect of smoking on BMD, as measured by DXA. Some, but not all studies, indicate smoking is associated with low BMD or greater rates of age-related bone loss, particularly in post-menopausal women [1, 2, 14-16]. The risk of fracture, however, exceeds the reduction in BMD suggesting that other aspects of bone quality or non-skeletal factors may impact risk [17]. Few studies have investigated the effect of smoking on skeletal microstructure by HR-pQCT. Studies in both older and younger men show trabecular deficits predominate in active male smokers compared to non-smokers [18, 19]. In contrast, a recent HR-pQCT study in women aged 64 or younger indicated cortical effects including higher cortical porosity, lower cortical volumetric BMD, and reduced stiffness [20].
No HR-pQCT studies have evaluated both men and women, and it remains unclear if the conflicting HR-pQCT results in men and women represent sex-specific effects of smoking or differences due to enrollment criteria or other study-specific factors. Further, there are no HR-pQCT data available in older women (>65 years), though this group is at the highest risk for fracture and smoking may compound risk. Moreover, it is unclear whether the effects of past smoking have sustained effects on skeletal microstructure. The purpose of this analysis was to assess the effects of current and past smoking on skeletal microstructure, mechanical competence, muscle mass and function, as well as fall and vertebral fracture frequency in older men and women utilizing multiple advanced skeletal assessment techniques including HR-pQCT, finite element analysis, DXA, VFA and TBS.
Methods
Design
This is a cross-sectional analysis comparing skeletal health in elderly adult smokers and non-smokers who were participating in a population–based cohort study of aging. The Columbia University Irving Medical Center (CUIMC) Institutional Review Board approved this study. All participants provided written informed consent.
Study Population
The Washington Heights Hamilton Heights Inwood Community Aging Project (WHICAP) is an NIH-funded community-based prospective cohort study of aging among >5,900 elderly, African-American, Caribbean Hispanic, and Caucasian urban-dwelling residents (age >65) living in Northern Manhattan. The design and recruitment for the study have previously been reported [21]. Briefly, a probability sample of Medicare recipients, age ≥65 without dementia from 3 zip codes in Northern Manhattan was invited to participate. The original cohort was recruited beginning in 1992 and enriched with further recruitment from 1999-2010 and again from 2009 to present. Returning participants were invited to this ancillary study assessing bone health. Those who agreed to participate underwent evaluation with DXA, HR-pQCT, a dynamometer for grip strength and a questionnaire regarding their health and fracture history. Participants were enrolled between 1/2019 and 3/2020.
DXA
Areal BMD and body composition were measured with a QDR Discovery or Horizon instrument (Hologic Inc, Waltham, MA, USA). BMD measurements were obtained at the lumbar spine (LS; L1–L4), femoral neck (FN), and 1/3 radius. T-scores were obtained using the manufacturer's Caucasian reference norms. Participants were scanned at all three skeletal sites unless hardware precluded the analysis of BMD at a given site, in which case the site(s) with hardware were omitted. We excluded vertebra with hardware or other artifacts from the analysis of BMD at the spine. In vivo precision, determined according to the standard method at this facility, is 1.28% at the LS, 1.36% at the hip, and 0.70% for the distal radius (1/3 site)[22]. Subtotal (excluding head) fat and lean mass were obtained and expressed as percentages. Body composition data were available in 255.
Spine TBS and Lateral VFA was calculated from subjects’ spine DXA image using TBS iNsight software as previously described (version 3.0.3; Medimaps, Geneva, Switzerland) [23]. TBS was available in 306 participants. Lateral VFA was acquired from T4 to L5. Participants were categorized as having VF(s) in the imaged spine based on an International Society for Clinical Densitometry (ISCD)-certified densitometrist’s reading of the interpretable image using the Genant semi-quantitative method: mild, moderate and severe compression fractures were defined as a 20-25%, 26-40% or >40% reduction in vertebral height, respectively [24]. The Genant visual semi-quantitative method is the current recommended clinical technique for diagnosing vertebral fracture with VFA. VFA was available in 281 and in the remainder could not be obtained or interpreted due to poor visualization/hardware.
HR-pQCT
HR-pQCT was performed with an XtremeCT II scanner (Scanco Medical, Brüttisellen, Switzerland) which uses a microfocus x-ray source (68 kVp voltage, 900 μA current, 43 sec integration time) scanning a region 10.2 mm long along the axis of the long bone resulting in VOI of 60.7 μm isotropic voxel size. The non-dominant distal radius and tibia were scanned unless there was a contraindication (prior fracture or metal implant), in which case the contralateral limb was scanned. Region of Interest was defined on a 2-D scout view by placing a reference line at the endplate: proximal endplate for radius and distal endplate for tibia. Images were acquired using a relative offset from the reference line; radius scans at 4% of limb length and tibia at 7.3%. A single highly trained operator acquired and analyzed all scans. Scans were scored for motion on a scale of 1-5 and scans with motion score >3 were excluded from analysis. We used the manufacturer’s standard method to filter and binarize the HR-pQCT images. An automated segmentation algorithm was used to segment the cortical and trabecular regions. We assessed standard HR-pQCT morphological microstructure outcomes, including area; density - total, trabecular (Tb) and cortical (Ct) volumetric BMD (vBMD); microstructure - trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp), cortical thickness (Ct.Th), and cortical porosity (Ct.Po). In vivo short-term reproducibility (CV) for HR-pQCT measures at our center is between 0-5% for all measures except Ct.Po.
FEA
Bone strength was estimated from the HR-pQCT images using micro-finite element analysis (μFEA) based on a voxel conversion approach. We simulated a uniaxial compression on each radius and tibia model up to 1% strain using a homogeneous Young's modulus of 10 GPa and Poisson's ratio of 0.3. We used μFEA solver provided by the manufacturer (Scanco Medical FE-software v1.13, Scanco Medical, Brüttisellen, Switzerland) to solve the models. We estimated whole bone stiffness (N/mm). FEA was available in 246 participants at the tibia and 230 at the radius.
Grip strength
Grip strength was assessed using a hand-held dynamometer with maximum force using the participant’s dominant hand. Three trials were performed in 289 participants. An average score was recorded for each participant based on all three trials.
Questionnaire and Clinical evaluation
Information regarding past medical history, lifestyle, and medications was collected by questionnaire. Fall recall was assessed by questionnaire by asking participants if they had fallen in the last 12 months and the number of falls they sustained. Daily dietary calcium and vitamin D intake was assessed with a validated standardized food frequency questionnaire as previously described [25]. Physical activity was assessed with the physical activity scale for the elderly (PASE) [26]. Self-rated health was assessed on a scale of 1-5 corresponding to poor, fair, good, very good and excellent. Weight and height were measured by balance beam and a wall-mounted, calibrated Harpenden stadiometer, respectively.
Statistics
Descriptive statistics were expressed as means and standard errors or absolute numbers or percentages. Between-group differences in continuous demographic and skeletal indices were evaluated stratified by sex with general linear models (GLM) with Scheffé’s adjustment for multiple comparisons. Adjusted analyses were controlled for age and weight using GLM and were considered the primary analysis and results were expressed as mean ±standard error of the mean (SEM). Fisher’s exact test was used to assess differences in categorical variables. Correlations were assessed with pearson and spearman correlation. SAS version 9.4 (Cary, NC) was used for all analyses. A two-tailed p-value <0.05 was considered statistically significant.
Results
Three hundred and eleven participants were enrolled. Eighteen were active smokers, 118 former smokers and 175 never smokers. The majority of the cohort was non-Caucasian: 32.7% black, 39.4% mixed race, 26.3% white and 1.6% Asian, Pacific Islander/Native Hawaiian or American Indian. Participants were mean age 76.1±6.0 years and mostly female (66.0%). Current smokers had a mean smoking exposure of 34.6±20.4 pack-years (Table 1). Former smokers had ceased smoking on average 32.3±15.7 years prior to enrollment. As shown in Table 1, male current smokers had 16.8% lower weight compared to past smokers but did not differ from never smokers (both p<0.05). BMI was also lower in male current smokers compared to past and never smokers (Table 1). Daily total calcium intake was about 50% lower in male current smokers compared to never and past smokers (both p<0.05). Male smokers did not differ from non-smokers or past smokers in age, height, daily vitamin D intake, alcohol use, exercise, number of falls in the last 12 months, self-rated health, number of historical clinical fractures, use of osteoporosis medications or frequency of diabetes. As shown in Table 1, among women, the distribution of racial and ethnic groups differed between smokers and non-smokers. Female never smokers were also shorter compared to past smokers, but did not differ in other demographic or clinical covariates (Table 1).
Table 1.
Demographics and Clinical Characteristics
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Current N=8 |
Never N=52 |
Past N=46 |
p | Current N=10 |
Never N=123 |
Past N=72 |
p | |
| Age (years) | 73.2±2.1 | 75.3±0.8 | 76.4±0.9 | 0.34 | 72.2±1.9 | 76.7±0.5 | 76.3±0.7 | 0.09 |
| Race | ||||||||
| American Indian | 0 | 0 | 0 | 0.14 | 0 | 0.8 | 0 | 0.008 |
| Asian | 0 | 0 | 0 | 0 | 0 | 2.8 | ||
| Black | 50.0 | 23.1 | 30.4 | 50.0 | 26.8 | 47.2 | ||
| Hawaiian/Pacific Islander | 0 | 0 | 0 | 0 | 0 | 1.4 | ||
| White | 12.5 | 46.2 | 26.1 | 10.0 | 22.0 | 22.2 | ||
| Mixed | 37.5 | 30.8 | 43.5 | 40.0 | 50.4 | 26.4 | ||
| Ethnicity (% Hispanic) | 50.0 | 30.8 | 45.7 | 0.23 | 40 | 57.7 | 36.1 | 0.01 |
| Weight (pounds) | 154.8±10.9 | 175.2±4.3 | 186.2±4.6 | 0.02a | 171.6±10.8 | 154.0±3.1 | 164.3±4.0 | 0.06 |
| Height (inches) | 66.7±1.0 | 67.3±0.4 | 67.5±0.4 | 0.77 | 61.3±0.9 | 61.6±0.2 | 62.6±0.3 | 0.03 b |
| BMI (kg/m2) | 24.5±1.5 | 27.2±0.6 | 28.6±0.6 | 0.02a | 31.9±1.8 | 28.5±0.5 | 29.4±0.7 | 0.16 |
| Physical activity | 89.4±17.1 | 92.3±6.7 | 79.1±7.1 | 0.40 | 99.6±13.3 | 82.9±3.8 | 87.2±4.9 | 0.43 |
| Calcium intake (mg/day) | 601±190 | 1115±74 | 1236±79 | 0.01c | 995±187 | 1262±53 | 1177±70 | 0.30 |
| Vitamin D intake (IU/day) | 1145±422 | 1334±165 | 1093±176 | 0.60 | 624±553 | 1512±158 | 1577±206 | 0.27 |
| Drinks per week | 0.5±2.4 | 3.1±0.9 | 2.9±1.0 | 0.59 | 1.4±1.1 | 1.0±0.3 | 1.7±0.4 | 0.46 |
| Pack-years | 30.7±7.8 | 30.2±4.1 | 37.7±6.2 | 13.9±1.8 | ||||
| % with fall in last year | 12.5 | 17.3 | 21.7 | 0.86 | 10.0 | 34.2 | 33.3 | 0.32 |
| Falls in last year | 1.0±2.2 | 2.3±0.7 | 1.8±0.7 | 0.78 | 2.0±2.6 | 1.9±0.4 | 2.0±0.5 | 0.99 |
| Self-rated health | 3.1±0.3 | 2.4±0.1 | 2.6±0.1 | 0.14 | 3.4±0.3 | 2.9±0.1 | 3.1±0.1 | 0.14 |
| % with fracture | 50.0 | 75.7 | 43.5 | 0.41 | 30.0 | 43.1 | 47.2 | 0.83 |
| Number of Historical Fractures |
1.5±0.9 | 1.7±0.3 | 1.9±0.4 | 0.92 | 1.3±0.6 | 1.6±0.1 | 1.5±0.2 | 0.72 |
| Current Osteoporosis treatment |
0.0 | 3.9 | 0.0 | 0.57 | 10.0 | 14.6 | 5.6 | 0.14 |
| Diabetes | 37.5 | 19.2 | 32.6 | 0.24 | 40.0 | 30.1 | 31.9 | 0.78 |
Values represent mean±SEM or percentages; a:p<0.05 current vs. past; b:p<0.05 never vs. past; c:p<0.05 current vs. never and past
On average, T-scores in male smokers were in the osteopenic range at the femoral neck and radius, but normal at the spine. Before adjustment for age and weight, areal BMD by DXA was lower in male smokers compared to never and prior smokers at the lumbar spine and femoral neck (Table 2). However, these differences were attenuated after adjusting for age and weight. As shown in Table 2, age- and weight-adjusted BMD by DXA did not differ by smoking status at any skeletal site in men or women. Current male smokers had TBS values in the degraded range on average, whereas non-smokers and past smokers had average values in the partially degraded range. In male smokers, age- and weight-adjusted spine TBS was 13.5 and 15.3% lower compared to never and past smokers (both p<0.05), but never and past smokers did not differ from each other. Mean TBS values were in the degraded range in all the female groups. Before adjustment for covariates, TBS was lower in past female smokers compared to never smokers but this difference was attenuated and no longer significant after adjusting for age and weight. There were no differences in the frequency of vertebral fractures by smoking status in either sex as assessed by VFA. Before adjusting for age and weight, male past smokers had lower lean mass and higher fat mass, but there were no significant differences in body composition after adjusting for age and weight in men and no differences before or after adjusting for covariates in women (Table 2). There were no differences in grip strength before or after adjusting for age and weight in either sex.
Table 2.
Areal BMD and Body Composition by DXA, Trabecular Bone score and Grip Strength
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current | Never | Past | p | p adjusted |
Current | Never | Past | p | p adjusted |
|
| LS T-score | −1.0±0.6 | 0.0±0.3 | 0.9±0.3 | 0.01c | 0.18 | −0.8±0.6 | −0.6±0.2 | −0.5±0.2 | 0.86 | 0.84 |
| FN T-score | −1.5±0.3 | −1.2±0.1 | −0.6±0.1 | 0.01c | 0.11 | −1.0±0.4 | −1.5±0.1 | −1.3±0.1 | 0.24 | 0.91 |
| 1/3 radius T- score |
−1.9±0.6 | −1.3±0.3 | −0.7±0.3 | 0.13 | 0.36 | −0.3±0.5 | −1.2±0.1 | −1.1 ±0.2 | 0.27 | 0.62 |
| TBS | 1.120±0.048 | 1.256±0.019 | 1.265±0.020 | 0.02a | 0.002a | 1.114±0.041 | 1.198±0.012 | 1.156±0.015 | 0.03b | 0.12 |
| % fracture (VFA) | 12.5 | 12.8 | 12.2 | 1.0 | 0.95 | 11.1 | 14.6 | 15.2 | 1.0 | 0.89 |
| Lean mass (%) | 76.6±2.6 | 73.1±1.0 | 69.3±1.0 | 0.005c | 0.18 | 60.1±2.3 | 61.0±0.6 | 60.5±0.8 | 0.85 | 0.82 |
| Fat mass (%) | 23.4±2.6 | 26.9±1.0 | 30.7±1.0 | 0.005c | 0.18 | 39.9±2.3 | 39.0±0.6 | 39.5±0.8 | 0.85 | 0.82 |
| Grip strength | 20.6±3.4 | 26.7±1.2 | 28.8±1.3 | 0.07 | 0.20 | 17.1 ±1.7 | 15.7±0.5 | 17.7±0.7 | 0.06 | 0.11 |
Values represent mean±SEM or percentages; a:p<0.05 current vs. past and never; b: p<0.05 never vs. past; c: p<0.05 past vs. current and never; p-adjusted: p-values after adjusting for age and weight
Differences in skeletal microstructure at the radius and tibia by HR-pQCT are shown in Table 3. Trabecular vBMD at the radius was 27.7 and 31.0% lower in current male smokers compared to past and never smokers respectively, but past and never smokers did not differ from each other. These differences remained significant after adjusting for age and weight (all p<0.05). Consistent with these findings trabecular number was lower and trabecular spacing was higher before and after adjusting for covariates in current male smokers compared to never and past smokers, but the latter two groups did not differ from each other. Age- and weight-adjusted trabecular thickness was 6.2% lower in current vs. never smokers. There were no differences in cortical indices or stiffness at the radius in men before or after adjusting for covariates.
Table 3.
Volumetric BMD, Microstructure and Estimated Bone Strength by HR-pQCT at the Distal Radius and Tibia
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current | Never | Past | p | p adjusted |
Current | Never | Past | p | p adjusted |
|
| Distal Radius (4%) | ||||||||||
| Tb.vBMD (mgHA/ccm) | 113.3±11.7 | 164.1±4.7 | 156.8±5.3 | 0.005a | 0.008a | 151.7±12.6 | 127.7±3.7 | 128.8±4.7 | 0.19 | 0.30 |
| Ct.vBMD (mgHA/ccm) | 818.3±27.1 | 815.9±11.0 | 826.8±12.4 | 0.80 | 0.78 | 797.5±23.1 | 802.2±6.7 | 793.0±8.5 | 0.70 | 0.36 |
| Tb.N (1/mm) | 1.203±0.069 | 1.419±0.028 | 1.400±0.031 | 0.02a | 0.04a | 1.319±0.080 | 1.261±0.023 | 1.266±0.030 | 0.78 | 0.88 |
| Tb.Th (mm) | 0.224±0.005 | 0.240±0.002 | 0.235±0.002 | 0.01b | 0.01b | 0.235±0.008 | 0.227±0.002 | 0.227±0.003 | 0.54 | 0.50 |
| Tb.Sp (mm) | 0.828±0.04 | 0.678±0.02 | 0.680±0.02 | 0.005a | 0.01a | 0.738±0.08 | 0.807±0.02 | 0.780±0.03 | 0.57 | 0.97 |
| Ct.Th (mm) | 0.888±0.08 | 0.974±0.03 | 0.957±0.04 | 0.57 | 0.63 | 0.849±0.06 | 0.767±0.02 | 0.782±0.02 | 0.39 | 0.73 |
| Ct.Po (%) | 1.3±0.3 | 1.4±0.1 | 1.4±0.1 | 0.81 | 0.86 | 1.8±0.2 | 1.1±0.07 | 1.2±0.09 | 0.009a | 0.007a |
| Stiffness (kN/mm) | 58.0±6.8 | 71.8±3.0 | 74.5±3.6 | 0.10 | 0.25 | 52.1±5.6 | 44.6±1.5 | 47.9±1.8 | 0.20 | 0.64 |
| Distal Tibia (7.3%) | ||||||||||
| Tb.vBMD (mgHA/ccm) | 122.5±12.6 | 175.9±5.1 | 166.3±5.6 | 0.007a | 0.002a | 156.3±12.7 | 145.2±3.7 | 147.9±4.9 | 0.67 | 0.92 |
| Ct.vBMD (mgHA/ccm) | 826.8±27.4 | 828.4±11.1 | 832.2±12.2 | 0.97 | 0.83 | 771.6±24.5 | 794.4±7.2 | 782.6±9.4 | 0.47 | 0.13 |
| Tb.N (1/mm) | 1.132±0.075 | 1.288±0.030 | 1.317±0.034 | 0.09 | 0.39 | 1.251±0.076 | 1.231±0.022 | 1.206±0.029 | 0.73 | 0.54 |
| Tb.Th (mm) | 0.244±0.007 | 0.263±0.003 | 0.255±0.003 | 0.02b | 0.02d | 0.257±0.006 | 0.250±0.002 | 0.253±0.002 | 0.37 | 0.48 |
| Tb.Sp (mm) | 0.882±0.05 | 0.761±0.02 | 0.742±0.02 | 0.04a | 0.19 | 0.804±0.07 | 0.817±0.02 | 0.840±0.03 | 0.75 | 0.56 |
| Ct.Th (mm) | 1.319±0.105 | 1.556±0.043 | 1.442±0.047 | 0.05d | 0.03e | 1.206±0.091 | 1.161±0.027 | 1.197±0.035 | 0.67 | 0.96 |
| Ct.Po (%) | 4.2±0.6 | 4.3±0.2 | 3.7±0.3 | 0.31 | 0.21 | 4.9±0.5 | 3.8±0.1 | 4.2±0.2 | 0.03b | 0.03b |
| Stiffness (kN/mm) | 156.9±15.8 | 221.2±7.0 | 217.0±8.1 | 0.002a | 0.004a | 155.2±12.9 | 142.3±3.5 | 155.9±4.4 | 0.05 | 0.28 |
Values represent mean±SEM or percentages; a:p<0.05 current vs. past and never; b: p<0.05 current vs. never; c:p<0.05 all groups differ from each other; d:p<0.05 never vs. current and past; e: p<0.05 never vs. past smokers; p-adjusted: p-values after adjusting for age and weight
There were similar trabecular patterns at the tibia in men. Male current smokers had 19.7 and 26.0% lower age- and weight-adjusted trabecular vBMD compared to past and never smokers respectively, but the latter two groups did not differ from each other. Trabecular thickness was higher in never smokers compared to both past and current smokers after adjusting for age and weight. Cortical thickness was also 9.6% lower in past versus never smokers after adjusting for age and weight. These differences resulted in 17.5 and 22.2% lower age and weight-adjusted stiffness in current smokers compared to past and never smokers respectively, but the latter two groups did not differ from each other. Representative HR-pQCT images from a male current and never smoker are shown in Figure 1.
Figure 1:
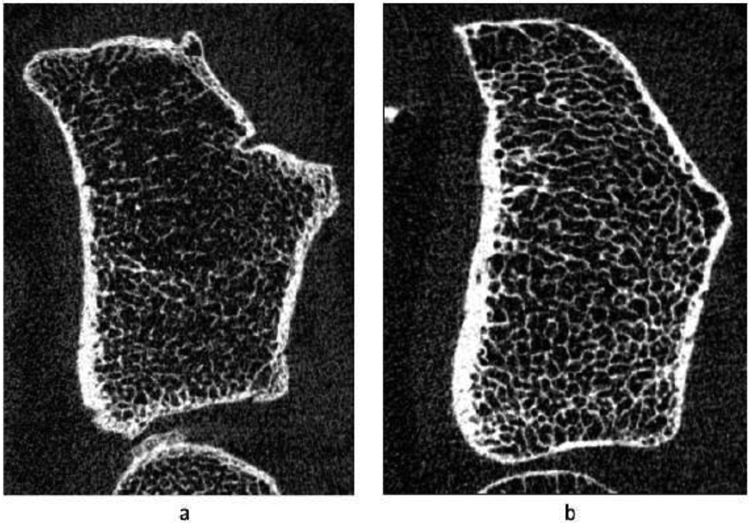
Gray-scale image of a cross-sectional slice from HR-pQCT scans showing bone microstructure at the distal radius (4%) in a) male smoker and b) never smoker
In women, there were no differences in trabecular indices before or after adjusting for age and weight at either the radius or tibia (Table 3). Age- and weight-adjusted cortical porosity was 50% and 80% higher in current smokers compared with past and never smokers respectively at the radius. Similarly, age- and weight-adjusted cortical porosity was 28.9% higher in current versus never smokers at the tibia. There were no differences in stiffness at the radius or tibia in women after adjusting for age and weight. There were no significant associations between pack-years and HRpQCT or FEA skeletal indices at any site (data not shown). Representative HR-pQCT images from a female current and never smoker are shown in Figure 2.
Figure 2:
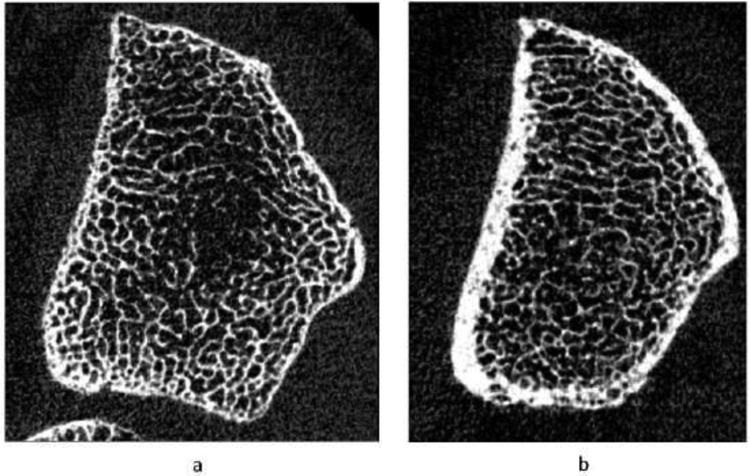
Gray-scale image of a cross-sectional slice from HR-pQCT scans showing bone microstructure at the distal radius (4%) in a) female smoker and b) never smoker
Discussion
In this study, we have shown that current smoking is associated with skeletal microstructural deterioration in older men and women. The pattern of deterioration differed by sex, however. In men, trabecular deficits predominated and were evident at the spine, radius and tibia, leading to reduced mechanical competence in the latter. Whereas in women, cortical porosity was increased in the peripheral skeleton. To our knowledge, this is the first HR-pQCT study to assess the effect of smoking on skeletal microstructure in both sexes and the only study to include older women (≥age 65). Our results are generally consistent with a recent study in young women (< age 65) that indicated female currents smokers had higher cortical porosity [20]. Our results extend findings to the age group most at risk for fracture. In addition, however Johnson et al. showed smokers had other cortical deficits including lower cortical vBMD and lower stiffness, whereas our study did not. [20]. The reasons for these differences are unclear but the study by Johnson et al. was larger and included only moderate to heavy smokers.
Our results indicating lower trabecular density and thickness in the peripheral skeleton in older men are similar to those of a recent HR-pQCT study in young men < age 25 [27]. Rudang et al. reported lower trabecular bone volume fraction at the tibia and lower trabecular thickness at the radius and tibia in smokers [27]. Similarly, a study in older men showed lower trabecular volumetric density, lower trabecular number, and more heterogenous trabecular network, but no cortical deficits [18]. Our study further extends findings to the central skeleton by showing that TBS is in the degraded range at the lumbar spine in older male smokers. Studies in young men using a lower resolution modality, pQCT, have shown both cortical and trabecular effects [16]. These inconsistencies may be due to the inability of pQCT to accurately segment the cortical and trabecular compartments.
Overall, the analogous results in younger vs. older individuals suggests that the effect of smoking may be similar regardless of age. The reasons for the sexual dimorphism in skeletal response to smoking is unclear and, unfortunately, cannot be addressed by our study. It is possible that the marked effects of estrogen deficiency after menopause in older women mask a smaller effect of smoking on the trabecular skeleton. Consistent with this, mean TBS values in women in our study were in the degraded range regardless of smoking, whereas older men who were not current smokers had mean TBS values that were partially degraded. Our results build on a growing literature that indicates a potential interaction between smoking and sex upon skeletal microstructure. A study in young men indicated an interaction between estradiol levels and the effect of smoking on bone [16]. The negative effects of smoking were attenuated by higher estradiol levels in this study.
Unfortunately, our study cannot completely address the mechanism by which microstructure is affected by smoking. Other work implicates several direct and indirect mechanisms causing bone loss in smokers. Low weight in smokers has been hypothesized to decrease mechanical loading and osteogenesis, though our results were independent of weight. Additionally, reduced vitamin D levels have been reported in smokers in several studies leading to impaired calcium absorption, which may be compounded by reduced calcium intake, the latter of which was seen in our study [28, 29]. Other studies suggest smokers have high serum cortisol and lower sex steroid levels compared to non-smokers [30-32]. Some work has suggested that, in part, reduced bone mass may be secondary to increased alcohol intake, reduced physical activity or other lifestyle factors in smokers, though these factors did not differ in our study [17]. There is also evidence for a direct effect of smoking and/or nicotine on osteoblasts and osteoclasts. Nicotine has been shown to reduce osteoblast development and also affect skeletal angiogenesis [33]. Smokers have also been found to have lower osteoprotegerin levels compared to non-smokers, which would be expected to increase osteoclastogenesis [34, 35].
Regardless of the mechanism, the magnitude of the effect of smoking on skeletal microstructure was large – in men there was a 20-30% reduction in trabecular density and stiffness in smokers vs. never smokers while in women cortical porosity was almost twice as high at the radius and 50% higher at the tibia. The effect sizes are similar or greater than that seen in studies comparing those with and without fracture, suggesting that these differences are clinically meaningful and would be expected to result in an increased risk of fracture [36, 37].
Our results suggest that imaging modalities that assess microstructure (HR-pQCT and TBS) may be more sensitive than measurement of areal BMD by DXA in detecting the effects of smoking upon the skeleton. We did not find any differences in age- and weight adjusted areal BMD in smokers of either sex. This may have important implications for clinical practice. DXA may underestimate risk of fracture in smokers. In this regard, the fracture risk assessment tool, FRAX, which does account for the higher risk of fracture in current smokers vs. non-smokers at the same BMD, may be useful. Our results indicate TBS, alone or incorporated into the FRAX estimate, may be a helpful adjunct as well.
Male smokers in our study had lower weight compared to past smokers. Calcium intake was also lower in current smokers compared to both never and past smokers. These findings are not unexpected as those who quit smoking often gain weight as a result of increased caloric intake and/or decreased metabolic energy expenditure [38-40]. Smoking decreases appetite, which might affect the consumption of calcium rich foods leading to lower dietary calcium intake. It is unlikely these differences in anthropometric and lifestyle factors accounted for the microstructural differences observed because differences in these covariates were less pronounced between current and never smokers than current and past smokers. Further, we adjusted for differences in weight between groups. Otherwise, the groups were fairly similar in terms of other measured covariates, though we cannot completely eliminate the possibility that smokers had other differences affecting skeletal health for which we did not account.
We found deteriorated skeletal microstructure tended to be present in current, but not past smokers compared to never smokers, though there were some site-specific differences. At the radius, never and past smokers had similar values with regard to trabecular and cortical parameters. Some effects of past smoking were evident at the tibia where past smokers had worse cortical and trabecular thickness compared to never smokers. Though confirmation by a longitudinal study is needed, the results suggest that smoking cessation may result in improvement in some microstructure parameters to levels that are similar to never smokers. Few studies have assessed the effect of smoking cessation on skeletal health. One study showed that risk for hip fracture declines after smoking cessation but the benefit is not observed for up to 10 years [41]. Consistent with our results, a study using DXA indicated no difference in BMD between former and never smokers [42]. Similarly, an HR-pQCT study in men indicated no difference in skeletal microstructure in former and never smokers [18].
Among women, smokers tended to be more likely to be Black compared to never smokers. Given the sample size, we did not assess the effects of smoking stratified by race. Because other studies have found that Black women tend to have lower cortical porosity compared to white women, the greater frequency of Black women in the current smokers’ group may suggest that we might have seen more marked differences in cortical porosity if the groups were more balanced in their racial distributions [43].
We did not find a difference in physical function, falls, historical fractures or vertebral fractures as assessed by VFA in smokers and never smokers. These results are generally consistent with prior work. The study of osteoporotic fractures found current smokers had worse physical function except for grip strength [44]. Though there is limited data, active smoking has been associated with sarcopenia in one study [7]. We did not find this to be the case. Similar to our study, others have not consistently shown an increased risk for falls in smokers [17, 45]. Some longitudinal studies have shown an increased risk of vertebral fracture in smokers [2, 3, 46]. This pattern was not observed in our cross-sectional VFA data. In some cases, VFA cannot visualize the upper thoracic spine well. Thus, it is possible that upper thoracic fractures were not seen.
Our study has several limitations, First, the number of current smokers was small. We may have found effects on other microstructural indices with a larger sample size. The overall percentage of smokers in our study, however, is similar to the frequency (~8%) in seniors in the United States [47]. Secondly, smokers may have differences in a number of measured and unmeasured health related factors. While we controlled for age and weight, the sample size precludes controlling for other lifestyle differences. Our study was cross-sectional and we cannot determine if similar findings in former smokers would be seen in longitudinal studies of smoking cessation.
Our study also has several strengths. We assessed both men and women, as well as current and former smokers, in the age range most at risk for fracture. We also utilized multiple modalities to assess several aspects of musculoskeletal health including areal BMD, TBS, skeletal microstructure by HR-pQCT, mechanical competence, body composition, physical function and falls, and vertebral fracture assessment. Participants were enrolled from a racially diverse population-based cohort, which limits selection bias and increases the generalizability of these findings. Finally, we assessed HR-pQCT using a relative offset to ensure same region of interest was assessed across the cohort to account for differences in height and limb length. We believe this analysis provides further insight into sex-specific effects of smoking in older adults as well as the potential skeletal benefits of smoking cessation.
In summary, current smoking is associated with trabecular skeletal microstructural deficits at the spine and peripheral skeleton in men, while women have cortical deficits in the peripheral skeleton. Our results indicate smoking may have sex-specific microstructural effects. Given similar BMD by DXA in smokers and non-smokers, evaluation of areal BMD may not fully reveal underlying skeletal pathology due to smoking. This implies that assessment using other modalities may be important for fully assessing fracture risk in smokers. The less consistent association between past smoking and microstructural deterioration suggests the effects of tobacco use on the skeleton could be reversible with smoking cessation.
Acknowledgments
Funding: R01AR071986, PO1AG07232, R01AG037212, RF1AG054023
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose
References
- 1.Law MR and Hackshaw AK, A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ, 1997. 315(7112): p. 841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward KD and Klesges RC, A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int, 2001. 68(5): p. 259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestergaard P and Mosekilde L, Fracture risk associated with smoking: a meta-analysis. J Intern Med, 2003. 254(6): p. 572–83. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, et al. , Smoking and fracture risk: a meta-analysis. Osteoporos Int, 2005. 16(2): p. 155–62. [DOI] [PubMed] [Google Scholar]
- 5.Scolaro JA, et al. , Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am, 2014. 96(8): p. 674–81. [DOI] [PubMed] [Google Scholar]
- 6.Mineur YS, et al. , Nicotine decreases food intake through activation of POMC neurons. Science, 2011. 332(6035): p. 1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szulc P, et al. , Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr, 2004. 80(2): p. 496–503. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher PC and Hirdes JP, Risk factor for accidental injuries within senior citizens' homes: analysis of the Canadian Survey on Ageing and Independence. J Gerontol Nurs, 2005. 31(2): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 9.Yan C, Avadhani NG, and Iqbal J, The effects of smoke carcinogens on bone. Curr Osteoporos Rep, 2011. 9(4): p. 202–9. [DOI] [PubMed] [Google Scholar]
- 10.Walker LM, et al. , Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone, 2001. 28(6): p. 603–8. [DOI] [PubMed] [Google Scholar]
- 11.Ho YC, et al. , Up-regulation of osteolytic mediators in human osteosarcoma cells stimulated with nicotine. J Periodontal Res, 2009. 44(6): p. 760–6. [DOI] [PubMed] [Google Scholar]
- 12.Rothem DE, et al. , Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab, 2009. 27(5): p. 555–61. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal J, et al. , Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci U S A, 2013. 110(27): p. 11115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Law MR, et al. , Cigarette smoking, sex hormones and bone density in women. Eur J Epidemiol, 1997. 13(5): p. 553–8. [DOI] [PubMed] [Google Scholar]
- 15.Hu JF, et al. , Bone density and lifestyle characteristics in premenopausal and postmenopausal Chinese women. Osteoporos Int, 1994. 4(6): p. 288–97. [DOI] [PubMed] [Google Scholar]
- 16.Taes Y, et al. , Early smoking is associated with peak bone mass and prevalent fractures in young, healthy men. J Bone Miner Res, 2010. 25(2): p. 379–87. [DOI] [PubMed] [Google Scholar]
- 17.Cusano NE, Skeletal Effects of Smoking. Curr Osteoporos Rep, 2015. 13(5): p. 302–9. [DOI] [PubMed] [Google Scholar]
- 18.Szulc P, et al. , Poor trabecular microarchitecture in male current smokers: the cross-sectional STRAMBO study. Calcif Tissue Int, 2011. 89(4): p. 303–11. [DOI] [PubMed] [Google Scholar]
- 19.Lorentzon M, et al. , Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab, 2007. 92(2): p. 497–503. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JE and Troy KL, Moderate-to-heavy smoking in women is potentially associated with compromised cortical porosity and stiffness at the distal radius. Arch Osteoporos, 2018. 13(1): p. 89. [DOI] [PubMed] [Google Scholar]
- 21.Tang MX, et al. , Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 2001. 56(1): p. 49–56. [DOI] [PubMed] [Google Scholar]
- 22.Bonnick SL, et al. , Importance of precision in bone density measurements. J Clin Densitom, 2001. 4(2): p. 105–10. [DOI] [PubMed] [Google Scholar]
- 23.Hans D, et al. , Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom, 2011. 14(3): p. 302–12. [DOI] [PubMed] [Google Scholar]
- 24.Genant HK, et al. , Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res, 1993. 8(9): p. 1137–48. [DOI] [PubMed] [Google Scholar]
- 25.Walker MD, et al. , Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res, 2009. 24(12): p. 1953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washburn RA, et al. , The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol, 1993. 46(2): p. 153–62. [DOI] [PubMed] [Google Scholar]
- 27.Rudang R, et al. , Smoking is associated with impaired bone mass development in young adult men: a 5-year longitudinal study. J Bone Miner Res, 2012. 27(10): p. 2189–97. [DOI] [PubMed] [Google Scholar]
- 28.Brot C, Jorgensen NR, and Sorensen OH, The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr, 1999. 53(12): p. 920–6. [DOI] [PubMed] [Google Scholar]
- 29.Need AG, et al. , Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int, 2002. 13(1): p. 83–8. [DOI] [PubMed] [Google Scholar]
- 30.Seyler LE Jr., et al. , The effects of smoking on ACTH and cortisol secretion. Life Sci, 1984. 34(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 31.Field AE, et al. , The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab, 1994. 79(5): p. 1310–6. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri RL, Gochberg J, and Ryan KJ, Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest, 1986. 77(6): p. 1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, et al. , Uncoupled angiogenesis and osteogenesis in nicotine-compromised bone healing. J Bone Miner Res, 2010. 25(6): p. 1305–13. [DOI] [PubMed] [Google Scholar]
- 34.Tang TH, Fitzsimmons TR, and Bartold PM, Effect of smoking on concentrations of receptor activator of nuclear factor kappa B ligand and osteoprotegerin in human gingival crevicular fluid. J Clin Periodontol, 2009. 36(9): p. 713–8. [DOI] [PubMed] [Google Scholar]
- 35.Lappin DF, et al. , Effect of smoking on serum RANKL and OPG in sex, age and clinically matched supportive-therapy periodontitis patients. J Clin Periodontol, 2007. 34(4): p. 271–7. [DOI] [PubMed] [Google Scholar]
- 36.Stein EM, et al. , Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res, 2014. 29(5): p. 1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundh D, et al. , Increased Cortical Porosity in Older Men With Fracture. J Bone Miner Res, 2015. 30(9): p. 1692–700. [DOI] [PubMed] [Google Scholar]
- 38.Aubin HJ, et al. , Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ, 2012. 345: p. e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, et al. , Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality. N Engl J Med, 2018. 379(7): p. 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris KK, Zopey M, and Friedman TC, Metabolic effects of smoking cessation. Nat Rev Endocrinol, 2016. 12(11): p. 684. [DOI] [PubMed] [Google Scholar]
- 41.Cornuz J, et al. , Smoking, smoking cessation, and risk of hip fracture in women. Am J Med, 1999. 106(3): p. 311–4. [DOI] [PubMed] [Google Scholar]
- 42.Gerdhem P and Obrant KJ, Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int, 2002. 13(12): p. 932–6. [DOI] [PubMed] [Google Scholar]
- 43.Putman MS, et al. , Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res, 2013. 28(10): p. 2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson HD, et al. , Smoking, alcohol, and neuromuscular and physical function of older women. Study of Osteoporotic Fractures Research Group. JAMA, 1994. 272(23): p. 1825–31. [DOI] [PubMed] [Google Scholar]
- 45.Faulkner KA, et al. , Lifestyle predicts falls independent of physical risk factors. Osteoporos Int, 2009. 20(12): p. 2025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jutberger H, et al. , Smoking predicts incident fractures in elderly men: Mr OS Sweden. J Bone Miner Res, 2010. 25(5): p. 1010–6. [DOI] [PubMed] [Google Scholar]
- 47.Current Cigarette Smoking Among Adults in the United States. 2018. [cited 2020 6/4/2020]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.


