Abstract
Background:
Merkel cell carcinoma (MCC) guidelines derive from melanoma and do not recommend baseline cross-sectional imaging for most patients. However, MCC is more likely to have metastasized at diagnosis than melanoma.
Objective:
To determine how often baseline imaging identifies clinically occult MCC in patients with newly diagnosed disease with and without palpable nodal involvement.
Methods:
Analysis of 584 patients with MCC with a cutaneous primary tumor, baseline imaging, no evident distant metastases, and sufficient staging data.
Results:
Among 492 patients with clinically uninvolved regional nodes, 13.2% had disease upstaged by imaging (8.9% in regional nodes, 4.3% in distant sites). Among 92 patients with clinically involved regional nodes, 10.8% had disease upstaged to distant metastatic disease. Large (>4 cm) and small (<1 cm) primary tumors were both frequently upstaged (29.4% and 7.8%, respectively). Patients who underwent positron emission tomography–computed tomography more often had disease upstaged (16.8% of 352), than those with computed tomography alone (6.9% of 231; P = .0006).
Limitations:
This was a retrospective study.
Conclusions:
In patients with clinically node-negative disease, baseline imaging showed occult metastatic MCC at a higher rate than reported for melanoma (13.2% vs <1%). Although imaging is already recommended for patients with clinically node-positive MCC, these data suggest that baseline imaging is also indicated for patients with clinically node-negative MCC because upstaging is frequent and markedly alters management and prognosis.
Keywords: baseline imaging, clinical guidelines, CT, distant metastasis, MCC, melanoma, Merkel cell carcinoma, nodal metastasis, nonmelanoma skin cancer, occult disease, PET-CT, scans, sentinel lymph node biopsy, skin cancer, SLNB, staging
CAPSULE SUMMARY
• For 1 in 8 patients with Merkel cell carcinoma (MCC) with nonpalpable regional nodes, baseline imaging shows occult metastatic disease, markedly altering management and prognosis. In contrast, scans of patients with node-negative melanoma are rarely beneficial (<1%).
• Baseline imaging frequently changes management in patients with clinically node-negative as well as node-positive MCC.
Merkel cell carcinoma (MCC) is a neuroendocrine skin cancer with an incidence of approximately 2835 cases/year in the United States and a rising burden worldwide.1–3 Typically, MCC appears as a nonspecific red/purple or skin-colored asymptomatic nodule.4 This falsely reassuring presentation results in frequent delay of diagnosis, which, when combined with the fast-growing nature of MCC, often results in regional or distant spread at presentation.5 Until 2017, there were no effective therapies for metastatic MCC. However, with the approval of immune checkpoint inhibitors that target PD-L1 (avelumab)6,7 and PD-1 (pembrolizumab and nivolumab),8–10 the prognosis of metastatic MCC has dramatically improved.9,11 Furthermore, there is emerging evidence that immune checkpoint inhibitors are more effective when tumor burden is lower, providing further impetus for early identification of metastatic disease. Indeed, several trials are currently enrolling to test whether adjuvant immunotherapy is indicated for patients who present with high-risk disease.12,13
Malignant melanoma is approximately 35 times more common than MCC.1,2 Thus, many MCC recommendations are based on melanoma. These include the role of imaging in baseline staging. Specifically, the National Comprehensive Cancer Network (NCCN) and the Society for Surgical Oncology/American Board of Internal Medicine’s Choosing Wisely campaign both strongly recommend against baseline cross-sectional imaging (scans) for patients presenting with localized melanoma without physical examination evidence for lymph node involvement.14–16 This recommendation is due to data suggesting that fewer than 1% of patients with melanoma with localized disease have disease upstaged by baseline imaging, as well as a high rate of false positive scan results that lead to unnecessary worry and procedures.17,18 Analogous to melanoma, current NCCN MCC guidelines do not recommend routine baseline imaging for patients presenting with clinically localized disease (clinical evidence level: expert consensus).19 However, compared to melanoma, national registry data show that MCC has a 3-fold higher chance of having spread at diagnosis to regional and distant sites (Fig 1),20 suggesting that melanoma-derived recommendations may not be appropriate for MCC. Furthermore, 4 small studies21–24 of staging by [18F]-fluorodeoxyglucose positron emission tomography–computed tomography (PET-CT), ranging from 18 to 102 patients, have suggested that in contrast to melanoma, baseline imaging may often affect treatment and management in patients with MCC. We therefore used our MCC registry (containing >1,400 patients) to evaluate the potential utility of baseline imaging for patients presenting with MCC without clinically evident distant metastatic spread.
Fig 1.
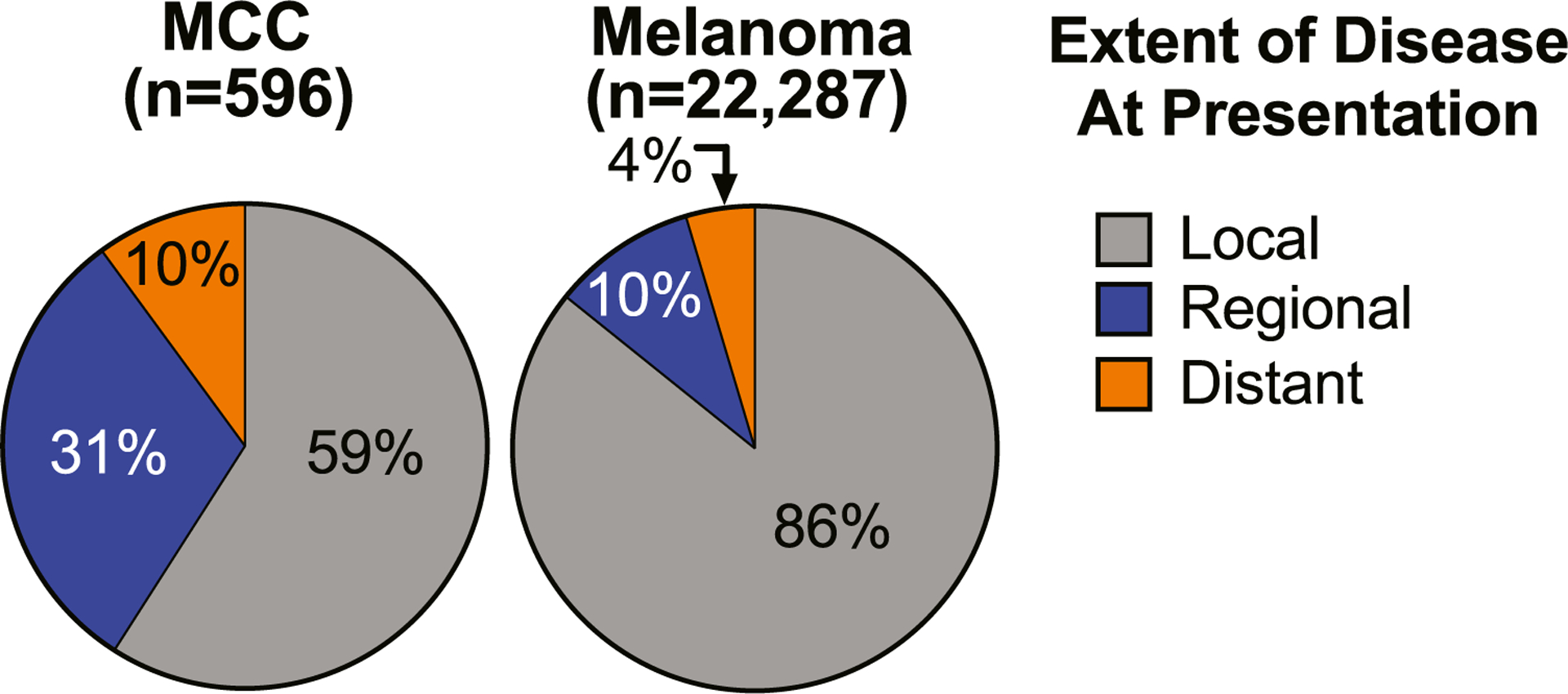
MCC and melanoma: frequency of regional or distant metastasis at presentation. Data were extracted from SEER; all cases of MCC (n = 596) and melanoma (n = 22,287) were diagnosed in the year 2016 and reported to SEER with sufficient staging information. MCC has clinically and statistically significantly higher rates of both regional and distant spread at presentation (P <.0001). MCC, Merkel cell carcinoma; SEER, Surveillance, Epidemiology and End Results Registry.
METHODS
MCC registry
This cohort of patients with MCC was identified from a Seattle-based repository.4,25,26 All patients with pathologically confirmed MCC enrolled in the repository before the data cutoff date of December 17, 2018, were considered for inclusion (n = 1,439) (Fig 2). All studies were performed with Fred Hutchinson institutional review board approval (no. 6585).
Fig 2.
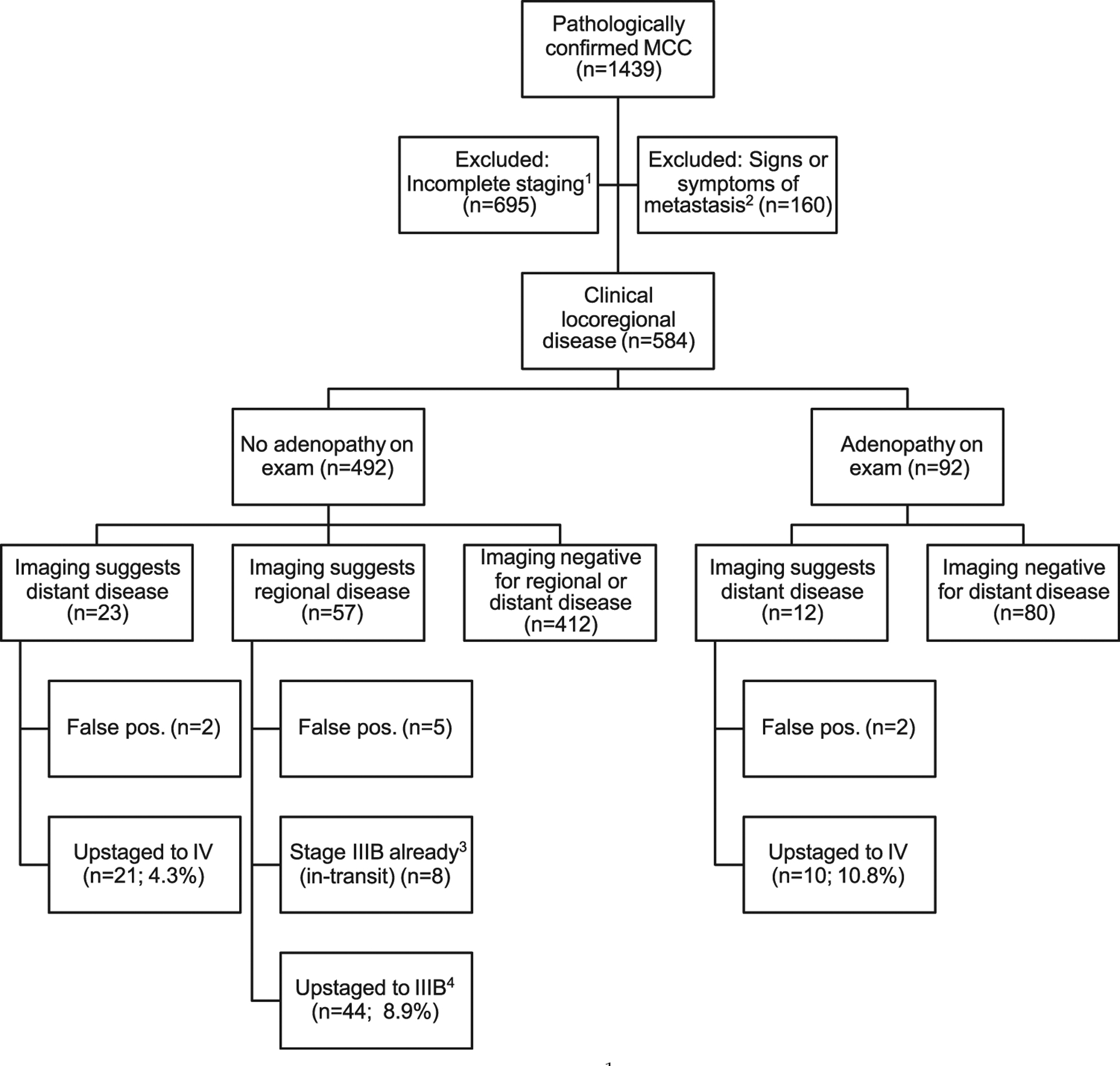
Selection diagram for patients with MCC. 1Patients excluded from the present study because of insufficient staging data, of whom n = 210 did not receive baseline imaging.2Patients excluded from the present study for whom imaging studies would be routinely indicated (unknown primary lesion or signs and symptoms of metastatic spread of disease).3Patient staging is unaffected by imaging because of the presence of in transit lesion. 4Disease upstaged to IIIB (n = 39 to p-IIIB by surgical pathologic evaluation, n = 5 to c-IIIB by scan only). MCC, Merkel cell carcinoma; pos, positive.
MCC baseline imaging analysis set
Patients were included in the analysis cohort (Fig 2) if they had a cutaneous primary tumor, n o symptoms of distant metastasis, baseline imaging as part of the diagnostic workup (this has been routinely performed at our center since 2010), and sufficient data for MCC staging (American Joint Committee on Cancer, eighth edition). Patients were excluded if presenting with MCC of an unknown primary lesion, metastatic lesions on examination, or metastatic symptoms, because these patients would routinely undergo imaging. Patients with insufficient staging information or those who did not undergo baseline imaging were also excluded. The final analysis set included 584 patients. Patients were diagnosed with MCC between the years 1980 and 2018. Age at diagnosis ranged from 11 to 98 years.
Radiologic imaging
Baseline imaging was defined as cross-sectional imaging (CT, PET-CT, or magnetic resonance imaging) of at least the chest-abdomen-pelvis and draining node bed obtained within 3 months of pathologic documentation of MCC. Imaging findings were considered to be true positive if evidence for previous clinically unappreciated regional or distant metastatic spread was either confirmed pathologically or treated presumptively (separately delineated in Fig 2). Imaging was considered to be false positive if imaging was suggestive of regional or distant metastatic spread but subsequent pathologic evaluation of the involved areas showed no MCC. The report of the clinical radiologist was used to determine imaging node status (scans were not reread by central radiology) to reflect real-world use. Incidental findings (adrenal adenomas, thyroid nodules, etc) that were not read as possibly or probably related to MCC were not counted as false positive findings.
Statistical analysis
Statistical analyses were performed with Stata software (StataCorp, College Station, TX), and figures were generated using GraphPad Prism software (GraphPad, San Diego, CA). A P value of .05 was established a priori to be the threshold for statistical significance, and 2-sided P values were used for all comparisons. Distributions of continuous variables were compared with the t test (unpaired, with Welch correction), and contingency tables were evaluated with Fisher’s exact test (2×2 tables) or chi-square analyses (all others).
Surveillance, Epidemiology, and End Results registry
Deidentified, descriptive, population-based registry data regarding the extent of disease at presentation for MCC and melanoma (Fig 1) were extracted from the Surveillance, Epidemiology and End Results registry (SEER 18 Research Registry) for all incident cases of MCC and melanoma in 2016 with associated local-regional-distant staging information.20 Data were extracted on May 2, 2019.
RESULTS
Patients presenting with localized MCC on examination frequently have disease upstaged by imaging
A total of 492 patients presented with a cutaneous MCC primary lesion, no palpable lymph node enlargement, and no signs or symptoms of disseminated MCC and underwent baseline imaging (Fig 2). From this cohort of patients with clinically localized disease, 65 (13.2%) patients had disease upstaged by imaging, with 44 (8.9%) cases changed to stage IIIB (radiographic nodal involvement) and 21 (4.3%) cases changed to stage IV (distant metastatic involvement) (Table I and Fig 3). Thus, the number of patients presenting with localized MCC needed to image (number needed to image [NNI]) to upstage disease in 1 patient is 8, and the NNI to upstage disease in 1 patient to distant metastatic disease is 24. There were no major differences in sex, age, or immune suppression status between upstaged and nonupstaged individuals (Table I). However, the primary site was significantly associated with radiographic upstaging (P = .01), with individuals presenting with tumors on the trunk most likely to be upstaged (Table I). As expected, patients presenting with a larger primary tumor diameter were more likely to be upstaged by imaging (P<.001) (Fig 3 and Table I). However, there was no apparent cutpoint below which imaging was uninformative. Specifically, even for the smallest tumor size category (1 cm) with nonpalpable lymph nodes, scans upstaged disease in 7.8% of patients (NNI = 13) (Fig 3). Therefore, there is clinical utility of baseline imaging for all sizes of MCC primary tumors.
Table I.
Baseline characteristics
| Characteristics | p | No radiographic evidence of spread (n = 427) | Radiographically identified regional or distant metastasis (n = 65) |
|---|---|---|---|
| Patients with nonpalpable lymph nodes | |||
| Sex, n (%) | .21 | ||
| Male (n = 313) | 267 (85.3) | 46 (14.7) | |
| Female (n = 179) | 160 (89.4) | 19 (10.6) | |
| Age at diagnosis, y | .096 | ||
| Median (range) | 68 (11–95) | 69 (45–86) | |
| Primary tumor size, cm | .0013 | ||
| Median (range) | 1.5 (0.05–10) | 2.5 (0.5–10) | |
| Primary tumor site, n (%) | .011 | ||
| Head and neck (n = 187) | 160 (85.6) | 27 (14.4) | |
| Trunk (n = 70) | 54 (77.1) | 16 (22.9) | |
| Extremity (n = 235) | 213 (90.6) | 22 (9.4) | |
| Immune suppression, n (%) | .84 | ||
| Yes (n = 65) | 56 (86.2) | 9 (13.8) | |
| No (n = 427) | 371 (86.9) | 56 (13.1) | |
| Imaging modality, n (%) | .0005 | ||
| PET-CT (n = 306) | 253 (82.7) | 53 (17.3) | |
| CT only (n = 186) | 174 (93.5) | 12 (6.5) | |
| No radiographic evidence of spread (n = 82), n (%) | Radiographically identified regional or distant metastasis (n = 10), n (%) | ||
| Patients with palpable lymph nodes | |||
| Sex, n (%) | .59 | ||
| Male (n = 73) | 66 (90.4) | 7 (9.6) | |
| Female (n = 19) | 16 (84.2) | 3 (15.8) | |
| Age at diagnosis, y | .56 | ||
| Median (range) | 64 (21–98) | 67 (52–85) | |
| Primary tumor size, cm | .27 | ||
| Median (range) | 2.0 (0.2–8.3) | 3.3 (1–9) | |
| Primary tumor site, n (%) | .056 | ||
| Head and neck (n = 33) | 32 (97.0) | 1 (3.0) | |
| Trunk (n = 18) | 17 (94.4) | 1 (5.6) | |
| Extremity (n = 41) | 33 (80.5) | 8 (19.5) | |
| Immune suppression, n (%) | .17 | ||
| Yes (n = 21) | 17 (81.0) | 4 (19.0) | |
| No (n = 71) | 65 (91.6) | 6 (8.4) | |
| Imaging modality, n (%) | .74 | ||
| PET-CT (n = 46) | 40 (87.0) | 6 (13.0) | |
| CT or MRI only (n = 46) | 42 (91.3) | 4 (8.7) |
CT, Computed tomography; MRI, magnetic resonance imaging; PET-CT, positron emission tomography–computed tomography.
Fig 3.
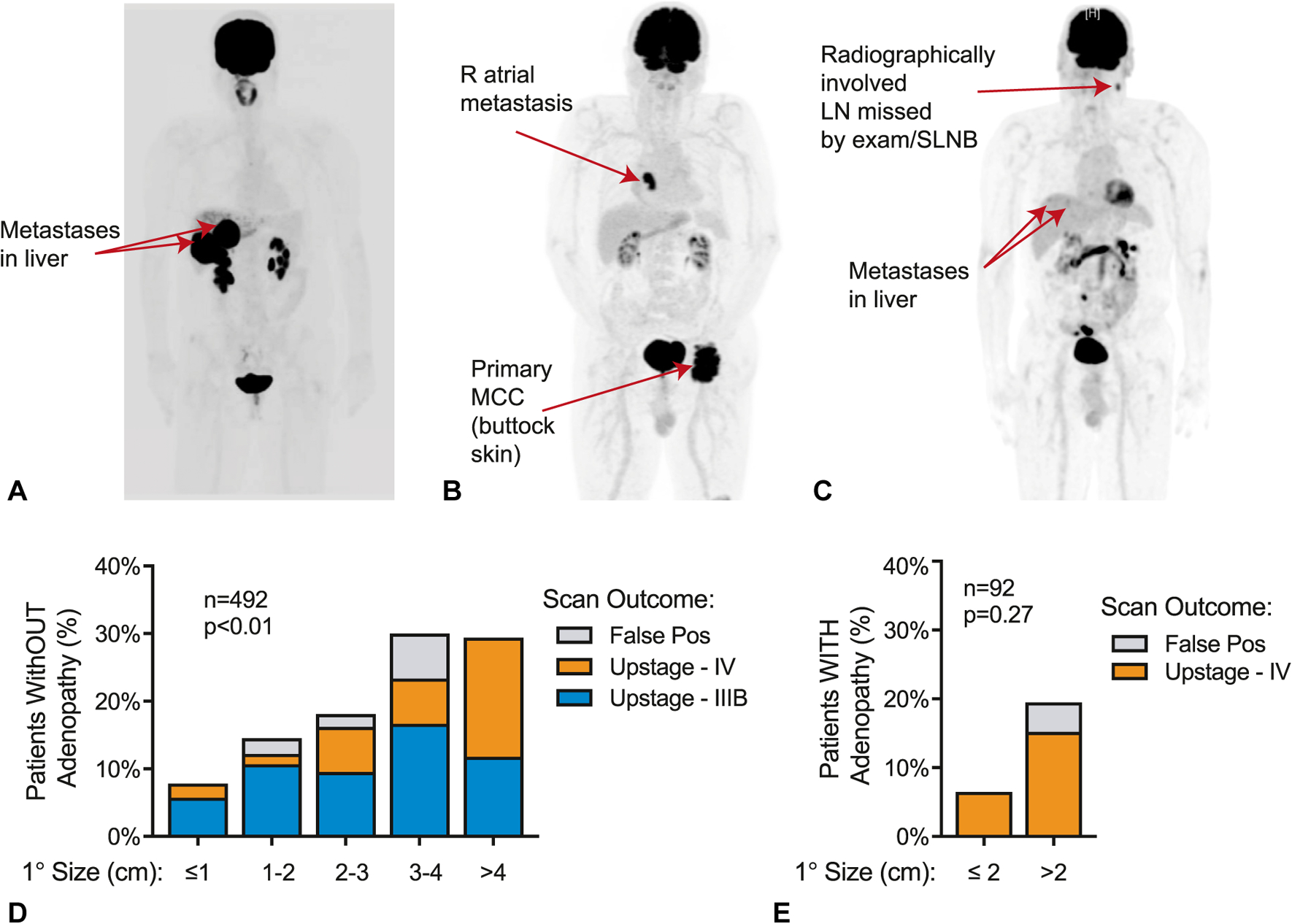
The clinical utility of baseline imaging in MCC. A–C, Three representative patients for whom baseline imaging revealed asymptomatic distant metastatic disease that was not appreciated on medical history or physical examination. Metastases were subsequently biopsy proven. A, A 55-year-old woman who presented with a 1-cm MCC primary tumor on the left medial aspect of the chest (resected before imaging); PET-CT revealed multiple hepatic metastases. B, A 85-year-old man presenting with a 10-cm primary tumor on the left buttock found to have a right atrial metastasis. C, A 74-year-old man who presented with a 1-cm primary tumor on the left temple and underwent SLNB with involvement of sentinel nodes. Subsequent PET-CT revealed additional involved regional lymph nodes (not sampled in the SLNB procedure) and distant hepatic metastases. D, Utility of baseline imaging in patients with MCC presenting without adenopathy on physical examination. Overall, 65 of 492 patients (13.2%) were found to have previously unappreciated nodal or distant metastatic spread on baseline imaging, and 7 of 492 patients (1.4%) had false positive imaging. P = .0013 for trend by primary (1°) tumor size. E, Utility of baseline imaging in patients with MCC presenting with adenopathy on physical examination. Overall, 10 of 92 patients (10.8%) were found to have previously unappreciated distant metastatic disease, and 2 of 92 (2.2%) patients had false positive imaging. P = .27 (not significant) for trend by primary tumor size. LN, Lymph node; MCC, Merkel cell carcinoma; PET-CT, positron emission tomography–computed tomography; Pos, positive; SLNB, sentinel lymph node biopsy.
Given the propensity for MCC to have delayed diagnosis, we investigated whether delay to diagnosis might be associated with higher risk of disease being upstaged by imaging. Although the median interval from lesion appearance to biopsy was slightly longer for patients with upstaged disease (median, 115 d; range, 0–3708 d; n = 59) than for those with nonupstaged disease (median, 84 d; range, 0–3132 d; n = 403), this did not reach statistical significance (P = .18). Importantly, there were multiple patients with radiographic upstaging whose lesions were biopsied within 2 weeks of lesion appearance, suggesting that there is no early detection window that would preclude the need for radiographic evaluation.
Patients with MCC presenting with clinically palpable lymph nodes often have distant metastases detectable by imaging
A total of 92 patients presented with cutaneous MCC and suspected regional involvement based on palpable lymph nodes, without signs or symptoms of distant metastatic spread. Of these, 10 (10.8%) were found to have distant metastatic spread that was later biopsy confirmed; their MCC was thus radiographically upstaged to stage IV (Table I and Fig 3). Although there were trends that suggested increased upstaging in patients with larger tumors, immune suppression, or tumors on an extremity, none of these relationships reached statistical significance (Table I). The NNI to upstage MCC in patients presenting with palpable lymph nodes and suspected regional disease was 10.
Imaging has a high positive predictive value for MCC spread
One concern with baseline imaging is the potential for false positives resulting in unnecessary workup. In our cohort, 94% of patients (79 of 84) whose scans suggested upstaging underwent pathologic evaluation of the detected lesion, thus allowing direct determination of the positive predictive value (PPV) of scans in such patients.
The PPV of a scan finding suggestive of MCC spread for pathologically proven MCC was very high, at 88.6% (70 of 79, all of whom underwent pathologic confirmation). In the overall cohort, only 1.5% of patients (9 of 584) who underwent imaging had radiographic suggestion of MCC spread/upstaging that was later disproven pathologically. Thus, the false positive rate was 1 in every 65 patients.
Imaging does not replace the need for sentinel lymph node biopsy in MCC
A total of 412 patients presented with a cutaneous MCC primary lesion, no palpable lymph node enlargement, and no signs or symptoms of disseminated MCC and had no evidence of spread on baseline imaging (Table I). Of these, 126 (30.6%) had a positive node found on surgical pathologic nodal evaluation, primarily by sentinel lymph node biopsy (SLNB) (Fig 4). Of note, the denominator includes all patients, not just those who underwent SLNB, to account for the possible confounding variable of those with positive SLNB being more likely to undergo scans. Including the none–SLNB-evaluated patients reduces the rate of sentinel lymph node positivity. Even with this, nearly 1 in 3 patients had a positive SLNB despite negative imaging and physical examination and thus had disease upstaged to pathologic stage IIIA based solely on their node biopsy data.
Fig 4.
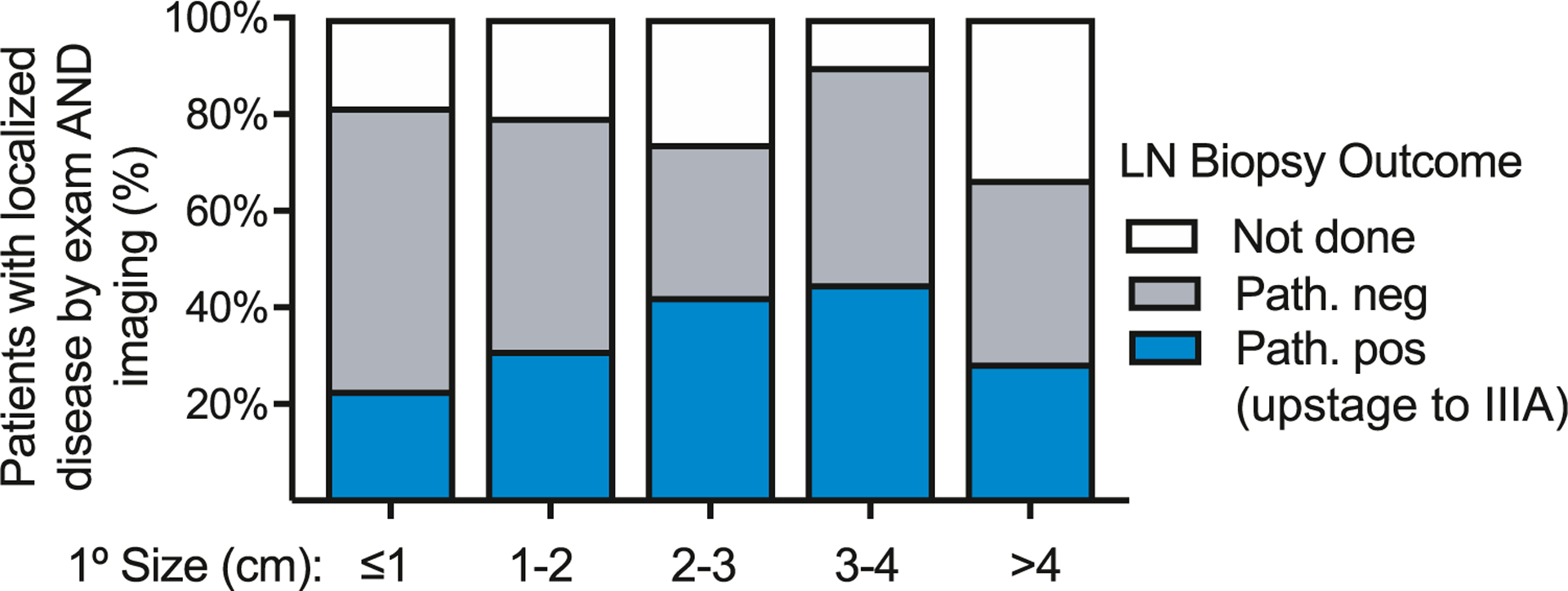
The utility of lymph node biopsy in patients with MCC with clinically localized disease by both examination and baseline imaging. Outcomes of pathologic nodal evaluation are shown for patients with clinically localized disease by both examination/history and baseline imaging (n = 412). Not done indicates that pathologic nodal evaluation was not performed. (These patients are included in the analysis set to avoid falsely elevating the rate of SLNB utility by clinician bias toward performing SLNB in higher-risk clinical scenarios.) LN, Lymph node; MCC, Merkel cell carcinoma; neg, negative; Path, pathologic nodal evaluation; pos, positive; SLNB, sentinel lymph node biopsy.
PET-CT appears more sensitive than CT alone
A total of 352 patients underwent baseline PET-CT imaging, whereas 231 underwent CT alone (Tables I and II). Overall, 16.8% of patients who underwent PET-CT imaging had disease upstaged compared to 6.9% of those who received CT only (P = .0006).
DISCUSSION
MCC is a skin cancer of increasing clinical impact.1,27,28 Although the field of MCC has benefited from advances in melanoma, particularly the broad use of SLNB29–31 and the advent of PD-1 pathway-–based immunotherapy,8,9,32–34 there are important differences in MCC biology and clinical behavior that sometimes require different management. One major contrast lies in the metastatic potential of the diseases: MCC is 3 times more likely to spread and recur than melanoma.20 Therefore, melanoma-derived imaging recommendations may not be appropriate. Currently, NCCN guidelines for melanoma14 indicate imaging only with documented nodal involvement (4% to 10% of patients with disease upstaged)35–38 and do not recommend imaging for clinically localized disease (<1% of patients with disease upstaged).17 MCC imaging guidelines currently reflect melanoma guidelines.19 However, 2 prior retrospective studies of 1821 and 6122 patients with newly diagnosed MCC and 2 prior prospective studies of 10223 and 5824 patients with MCC have all suggested that, unlike melanoma, baseline imaging findings for MCC are frequently positive and may have clinical utility. We thus sought to use our detailed registry to evaluate the potential utility of baseline imaging in MCC.
Patients who present with clinically localized MCC represent approximately 65%39,40 of all MCC cases and are not currently recommended to undergo baseline imaging by NCCN guidelines.19 Here, we report that among a large cohort of these patients with MCC (n = 492), rates of radiographic upstaging were far higher than reported for melanoma (<1%)17 and at a clinically important frequency (13.2%, or 1 in 8 patients overall, with 8.9% to stage IIIB and 4.3% to stage IV). Upstaging MCC to stage IIIB is important because this significantly alters clinical management, prognostication,39 and trial eligibility. Furthermore, upstaging to stage IV has a dramatic impact on the appropriate next steps for treatment (generally, systemic immunotherapy as opposed to surgery and radiation).19 Therefore, baseline imaging should ideally be completed before surgical lymph node evaluation and definitive therapy in patients with clinically node-negative disease to determine treatment based on the actual extent of disease. Because of the increased sensitivity of PET-CT as compared to CT alone in the present cohort (as well as in prior studies21,22,41), PET-CT appears to be superior for baseline imaging in MCC. However, this will need to be more formally evaluated in future studies. For MCC, based on pathologic confirmation of scan findings, baseline imaging in our cohort had a low rate of false positivity (<2%) and a high PPV (88.6%). These findings are importantly different from melanoma. We believe our data support a change in MCC management to include baseline cross-sectional imaging for nearly all patients with MCC, even those presenting with clinically localized disease.
Among patients who have clinically node-negative disease, our findings support the continued utility of surgical pathologic nodal evaluation even if baseline imaging is performed and scan findings are negative. Of patients who presented with a cutaneous MCC primary lesion, no palpable lymph node enlargement, and no signs or symptoms of disseminated MCC, 30.6% had nodal involvement (primarily via SLNB) despite no evidence of spread on baseline imaging. These findings are consistent with prior studies and current NCCN recommendations that SLNB is an important prognostic tool for most patients with MCC, even in the absence of concerning findings on baseline imaging.19,30,42
For patients who present with palpable adenopathy, these findings provide support for current guidelines suggesting the benefit of baseline cross-sectional imaging for this population.19 Among patients with palpable disease in regional lymph nodes, more than 1 in 10 (10.8%) had distant metastatic MCC appreciable on scans. Therefore, in most cases, imaging should precede the initiation of definitive management such as wide local excision or node dissection.
Our study had limitations. First, imaging modalities were heterogeneous, and the NNI would likely be lower (baseline imaging benefit higher) had our study been restricted to PET-CT.22–24 Furthermore, central nervous system imaging was infrequent, and asymptomatic brain metastases may have been missed, although they are uncommon in MCC.43,44 Second, although the NNI for baseline imaging in this cohort compares favorably to many other cancer settings where scans are routinely recommended, we did not specifically perform cost-benefit analyses to determine the economic benefit or risk of scans; this is an area that should be pursued in future studies. Third, many patients included in this study received their treatment at a tertiary referral center, and therefore this cohort may not be fully representative of the MCC population more broadly. Fourth, this study was retrospective in nature. Performing large prospective imaging studies in MCC is challenging because of the low incidence of MCC combined with the need for rapid workup and treatment initiation. Although retrospective, this study included several features to minimize bias: 1) the large number of patients on whom baseline imaging information was assessed (more than 5-fold larger than any previously reported study, to our knowledge) helps ensure more representative findings; 2) detailed clinical and pathologic information allowed determination of both true positive and false positive scan rates; 3) patients for whom a clinician would already typically order imaging were excluded (eg, patients with an unknown primary tumor or symptoms of metastasis).
Here, we report that baseline imaging frequently detects clinically occult metastatic disease in patients with MCC, including those with only localized disease as assessed by physical examination. The present study of 584 patients more than doubles the total number of informative MCC cases reported in the literature (239 patients were previously reported across 4 studies21–24 that address this topic) and presents findings that are consistent with the prior reports. In aggregate, these studies uniformly support the benefit of routinely including baseline imaging in MCC management (unless age or comorbidities suggest that only palliative care is appropriate) before the initiation of definitive locoregional therapy.
Funding sources:
Supported in part by the National Institutes of Health/National Cancer Institute (grants P30 CA015704, 1P01CA-225517-01A1, and T32CA009515), Fred Hutchinson Cancer Research Center Integrated Immunotherapy Research Core, Society for Immunotherapy of Cancer SITC-Merck fellowship, and the MCC Patient Gift Fund at the University of Washington. The funding agencies did not participate in design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosure:
Dr Akaike has received grant support from Nihon Medi-Physics Co, Ltd.
Dr Bhatia has received grant support from Bristol Myers Squibb, Merck, Novartis, EMD Serono, Oncosec, Immune Design, and NantKwest, as well as honoraria (for advisory board participation) from Bristol Myers Squibb, EMD-Serono and Sanofi-Genzyme. Dr Paulson has received grant support from SITC-Merck, Bluebird Bioscience, and EMD Serono. Dr Nghiem reports receiving grant support from EMD Serono and Bristol Myers Squibb as well as honoraria from Merck and EMD-Serono. Authors Singh and Alexander and Drs Lewis, McEvoy, Byrd, and Behnia have no conflicts of interest to declare.
Abbreviations used:
- MCC
Merkel cell carcinoma
- NCCN
National Comprehensive Cancer Network
- NNI
number needed to image
- PET-CT
positron emission tomography–computed tomography
- PPV
positive predictive value
- SLNB
sentinel lymph node biopsy
Footnotes
IRB approval status:
Reviewed and approved by the Fred Hutchinson Cancer Research Center IRB (approval no. 6585).
REFERENCES
- 1.Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stang A, Becker JC, Nghiem P, Ferlay J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: an international assessment. Eur J Cancer. 2018;94:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465–473. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman HL, Hunger M, Hennessy M, Schlichting M, Bharmal M. Nonprogression with avelumab treatment associated with gains in quality of life in metastatic Merkel cell carcinoma. Future Oncol. 2017;14:255–266. [DOI] [PubMed] [Google Scholar]
- 8.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 Trial. J Clin Oncol. 2020;38:2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angelo SP, Hunger M, Brohl AS, et al. Early objective response to avelumab treatment is associated with improved overall survival in patients with metastatic Merkel cell carcinoma. Cancer Immunol Immunother. 2019;68(4):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adjuvant avelumab in Merkel cell cancer (ADAM). National Institutes of Health. 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03271372. Accessed May 23, 2020.
- 13.Pembrolizumab compared to standard of care observation in treating patients with completely resected stage I-III Merkel cell cancer, STAMP study. National Institutes of Health. 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT03712605. [DOI] [PubMed]
- 14.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(4):367–402. [DOI] [PubMed] [Google Scholar]
- 15.Choosing wisely. ABIM Foundation. Available at: https://www.choosingwisely.org.
- 16.Saletti P, Sanna P, Gabutti L, Ghielmini M. Choosing wisely in oncology: necessity and obstacles. ESMO Open. 2018;3(5): e000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007;110(5):1107–1114. [DOI] [PubMed] [Google Scholar]
- 18.Mohr P, Eggermont AM, Hauschild A, Buzaid A. Staging of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi14–vi21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Merkel cell carcinoma (version 1.2020). 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. Accessed May 23, 2020.
- 20.Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014;150(7):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George A, Girault S, Testard A, et al. The impact of (18)F-FDG-PET/CT on Merkel cell carcinoma management: a retrospective study of 66 scans from a single institution. Nucl Med Commun. 2014;35(3):282–290. [DOI] [PubMed] [Google Scholar]
- 22.Hawryluk EB, O’Regan KN, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68(4):592–599. [DOI] [PubMed] [Google Scholar]
- 23.Siva S, Byrne K, Seel M, et al. 18F-FDG PET provides high-impact and powerful prognostic stratification in the staging of Merkel cell carcinoma: a 15-year institutional experience. J Nucl Med. 2013;54(8):1223–1229. [DOI] [PubMed] [Google Scholar]
- 24.Poulsen M, Macfarlane D, Veness M, et al. Prospective analysis of the utility of 18-FDG PET in Merkel cell carcinoma of the skin: a Trans Tasman Radiation Oncology Group Study, TROG 09:03. J Med Imaging Radiat Oncol. 2018;62(3):412–419. [DOI] [PubMed] [Google Scholar]
- 25.Tseng YD, Parvathaneni U. Primary radiation therapy for Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 2018; 100(1):14. [DOI] [PubMed] [Google Scholar]
- 26.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulson KG, Bhatia S. Advances in immunotherapy for metastatic Merkel cell carcinoma: a clinician’s guide. J Natl Compr Canc Netw. 2018;16(6):782–790. [DOI] [PubMed] [Google Scholar]
- 28.Becker JC, Stang A, Hausen AZ, et al. Epidemiology, biology and therapy of Merkel cell carcinoma: conclusions from the EU project IMMOMEC. Cancer Immunol Immunother. 2018;67(3): 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger ME, McMasters KM. ASO author reflections: the sentinel lymph node in melanoma: now more important than ever. Ann Surg Oncol. 2018;25(Suppl 3):906–907. [DOI] [PubMed] [Google Scholar]
- 30.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006; 142(6):685–690. [DOI] [PubMed] [Google Scholar]
- 31.Angeles CV, Wong SL. Importance of sentinel lymph node biopsy in Merkel cell carcinoma. J Oncol Pract. 2016;12(7):647–648. [DOI] [PubMed] [Google Scholar]
- 32.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulson KG, Lahman M, Chapuis AG, Brownell I. Immunotherapy for skin cancer. Int Immunol. 2019;31:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzaid AC, Tinoco L, Ross MI, Legha SS, Benjamin RS. Role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol. 1995;13(8): 2104–2108. [DOI] [PubMed] [Google Scholar]
- 36.Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11(4):638–643. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TM, Fader DJ, Chang AE, et al. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol. 1997;4(5):396–402. [DOI] [PubMed] [Google Scholar]
- 38.Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4(3):252–258. [DOI] [PubMed] [Google Scholar]
- 39.Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Concannon R, Larcos GS, Veness M. The impact of (18)F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: results from Westmead Hospital, Sydney, Australia. J Am Acad Dermatol. 2010;62(1):76–84. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Larcos G, Howle J, Veness M. Lack of clinical impact of (18) F-fluorodeoxyglucose positron emission tomography with simultaneous computed tomography for stage I and II Merkel cell carcinoma with concurrent sentinel lymph node biopsy staging: a single institutional experience from Westmead Hospital, Sydney. Australas J Dermatol. 2017;58(2):99–105. [DOI] [PubMed] [Google Scholar]
- 43.Kouzmina M, Koljonen V, Leikola J, Bohling T, Lantto E. Frequency and locations of systemic metastases in Merkel cell carcinoma by imaging. Acta Radiol Open. 2017;6(3):2058460117700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis CW, Qazi J, Hippe DS, et al. Patterns of distant metastases in 215 Merkel cell carcinoma patients: implications for prognosis and surveillance. Cancer Med. 2020;9(4):1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]


