Abstract
Pregnancy-associated myocardial infarction (PAMI) is a primary contributor to maternal cardiovascular morbidity and mortality. Specific attention to the etiology of myocardial infarction (MI), diagnostic evaluation, treatment strategies, and post-event care is necessary when treating women with PAMI. This review summarizes the current knowledge, consensus statements, and essential nuances.
Keywords: acute coronary syndrome, myocardial infarction, pregnancy, spontaneous coronary artery dissection
Introduction and Definition of Pregnancy-Associated Myocardial Infarction (PAMI)
In contrast to the overall global decline of maternal mortality, the United States rates continue to rise.1, 2 PAMI accounts for over 20% of maternal cardiac deaths.1 PAMI is defined as MI3 during pregnancy or the postpartum period. Formal postpartum definitions vary in the literature, typically ranging from 6 to 12 weeks with some authors suggesting a period of ≥12 months.4
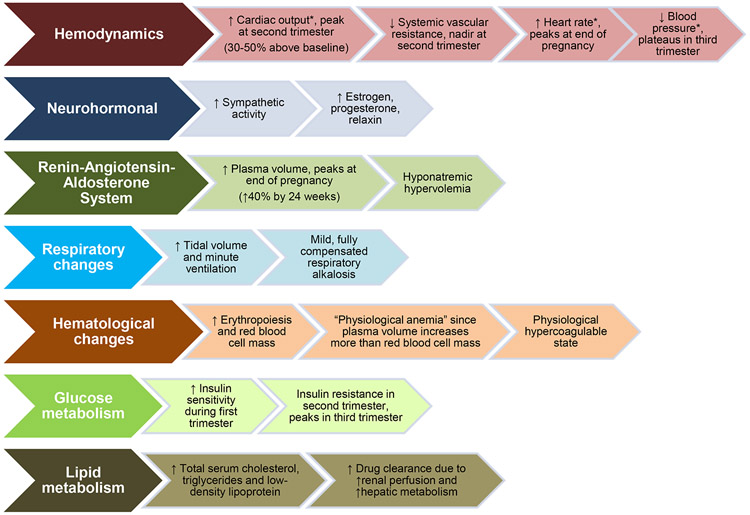
Nearly half of maternal deaths occur within the first day of delivery, and 66% of deaths occur within the first week.5 Many of the dramatic hemodynamic and physiologic changes of pregnancy(Figure 16-8) and early postpartum period return to normal by 6-12 weeks postpartum.6, 7 Pregnancy-associated hypercoagulability decreases by 6 weeks and normalizes around 12 weeks.9 Generally, the time period after miscarriage, termination, or stillbirth is considered postpartum, although the associated changes may be of a different magnitude and duration than those following a full-term pregnancy.
Figure 1.
Hemodynamic, physiologic and metabolic changes of pregnancy.
*Cardiac output, heart rate, and blood pressure all increase substantially during labor and delivery
Incidence and Prevalence of PAMI
PAMI occurs in 2.8-8.1 per 100,000 deliveries10-12 which is 4-fold higher than the MI occurrence among non-pregnant, reproductive-aged women.10 The incidence of PAMI is increasing and may relate to improved case detection and greater numbers of older women with underlying cardiovascular risk factors becoming pregnant. The case fatality rate in contemporary studies is estimated at 5%12 and is higher than MI fatality rates among non-pregnant women of reproductive age.13
One of the most consistently reported risk factors for PAMI is age >35 years.10, 11, 14 Other risk factors include cigarette smoking, hypertension, diabetes, and hyperlipidemia but are less common in women with PAMI compared to MI not associated with pregnancy.10, 11, 14, 15 This observation is consistent with the high prevalence of non-atherosclerotic etiologies of PAMI, such as spontaneous coronary artery dissection (SCAD). Less common risk factors include preeclampsia,11 multiple gestation,16 thrombophilia, blood transfusions, and postpartum infections.10 Black women have higher rates of PAMI, likely mediated by a higher prevalence of other coexistent risk factors.10, 11
A meta-analysis of population-based, international studies found that the incidence of PAMI is highest in the antepartum period,13 whereas a US PAMI population-based study observed most cases occurring postpartum.12 In 150 case reports of women with PAMI, 24% were diagnosed in the 3rd trimester and 47% were postpartum.14 The majority(75%) presented with ST-segment elevation MI(STEMI) and 24% had severely reduced left ventricular ejection fraction(LVEF) ≤30%, with complications of cardiogenic shock(38%) and ventricular arrhythmias(12%).14 In a registry study of 54 women with PAMI due to SCAD, 54% occurred within the first week postpartum. Women with pregnancy-associated SCAD were more likely to present with STEMI, left main and/or multivessel SCAD, and reduced LVEF compared to women with SCAD not associated with pregnancy.17
Presentation and Diagnosis of PAMI
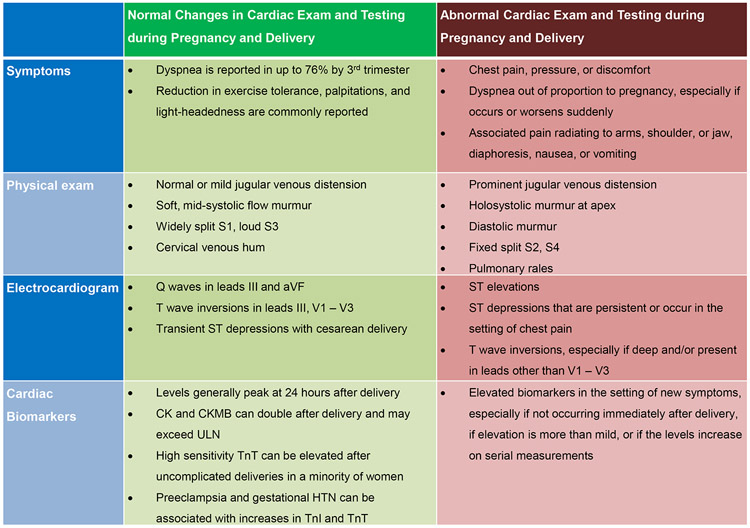
PAMI should be suspected in pregnant or postpartum women presenting with cardiac arrest, acute onset of angina or dyspnea, ischemic changes on electrocardiogram(ECG), and/or elevated cardiac biomarkers.18, 19,20 Changes in symptoms, physical exam, ECG, and biomarkers may occur during normal pregnancies and are important to distinguish from those indicative of PAMI(Figure 218, 21-25). For instance, dyspnea is common in late pregnancy, but associated orthopnea or chest discomfort should prompt a cardiovascular evaluation. Normal pregnancies are associated with ECG abnormalities including left axis deviation, inferior Q-waves, and T-wave inversions. Transient ST-depression can occur in healthy women during cesarean section delivery under regional anesthesia.18, 21
Figure 2.
Normal and abnormal symptoms, exam, and testing in pregnancy and delivery.
CK=creatine kinase; ULN=upper limit of normal; TnT=troponin T; TnI=troponin I
After uncomplicated labor and delivery, creatine kinase and creatine kinase-MB can exceed the upper limit of normal.22 Cardiac troponin I(cTnI) and troponin T(cTnT) levels usually remain within normal limits after delivery, although high sensitivity cTnT may be elevated immediately postpartum in up to 4% of asymptomatic women.22, 23 Preeclampsia and gestational hypertension are associated with abnormal cTnT and cTnI levels after delivery.24 Therefore, clinical suspicion should be based on presentation and symptoms, interval changes in biomarkers, and regional wall motion abnormalities on echocardiography.
Etiologies of PAMI
PAMI may be caused by either obstructive or nonobstructive lesions in the coronary arteries. Patients with MI with normal or non-obstructive (<50% stenosis) coronary arteries are considered as having MI with nonobstructive coronary arteries(MINOCA).26 Etiologies of MINOCA overlap with etiologies of obstructive MI as discussed below and include coronary plaque disruption with thrombosis and spontaneous thrombolysis; coronary vasospasm with resolution prior to angiography; coronary embolism; SCAD; and microvascular dysfunction.3, 26 The algorithm for work-up of MINOCA26 can be applied to patients with PAMI; however, studies such as cardiac magnetic resonance imaging and coronary function assessment should be considered after delivery.
Plaque rupture and/or erosion with atherothrombosis:
Obstructive, unstable atherosclerotic coronary artery disease represents the underlying mechanism in roughly one-third of PAMI.14, 27 This occurs due to rupture of the thin fibrous cap of a lipoprotein-rich plaque with exposure of the necrotic core and coronary thrombosis formation.28, 29 Coronary thrombus may also form in response to plaque erosion in which disrupted endothelium exposes plaque predominantly composed of smooth muscle cells and proteoglycans.29, 30
SCAD:
Although the exact prevalence is uncertain,31 SCAD is increasingly recognized as likely causing at least one-third of PAMI.14, 17 SCAD is due to a hematoma ±an intimal tear of the coronary arteries associated with an obstruction of flow that is not attributed to atherosclerotic, iatrogenic or traumatic causes.32, 33 Intracoronary thrombus is typically absent.34 Patients with SCAD commonly have fibromuscular dysplasia(FMD) or other arteriopathies such as dilatation, dissection, and aneurysms.32, 33
In-situ thrombosis or embolus:
Pregnancy is a physiologic, hypercoagulable state that may lead to in-situ intracoronary thrombus or embolism to the coronary arteries.8, 16, 35 Predisposing conditions include Kawasaki’s disease,18 systemic inflammatory conditions, autoimmune disorders such as antiphospholipid antibody syndrome, tumors, malignancy, atrial fibrillation, cardiomyopathy, and valvular heart disease.36 Since half of patients with thrombosis during pregnancy have an underlying thrombophilia and thrombophilia is a risk factor for PAMI, testing for thrombophilia if there is no other predisposition for thrombosis beyond pregnancy is advisable.10, 37
Coronary vasospasm:
Coronary vasospasm is challenging to diagnose as the etiology of PAMI, but it has been observed to cause STEMI during pregnancy14, 38 and was noted in 2% of a PAMI series.14 Prolonged vasospasm may contribute to intracoronary thrombus formation.38
Differential Diagnosis:
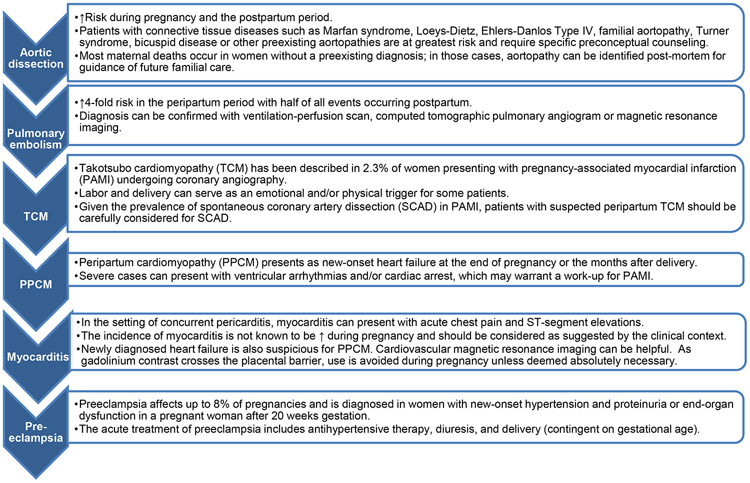
A number of serious conditions may have a similar clinical presentation as PAMI occasionally with abnormal ECG and biomarker elevation(Figure 313, 18, 39-46).
Figure 3.
The differential diagnosis of pregnancy-associated myocardial infarction.
Initial Management of PAMI
Expeditious diagnosis and treatment of an acute PAMI is of utmost importance due to the high maternal and fetal mortality. Appropriate therapy focused on maternal outcomes will also increase fetal survival, and thus, the maternal condition should dictate clinical management. A multidisciplinary “Pregnancy Heart Team” consisting of expertise from cardiology, obstetrics and/or maternal fetal medicine, anesthesiology and, depending on the clinical situation, cardiothoracic surgery, neonatology, and critical care may facilitate collective decision-making.18
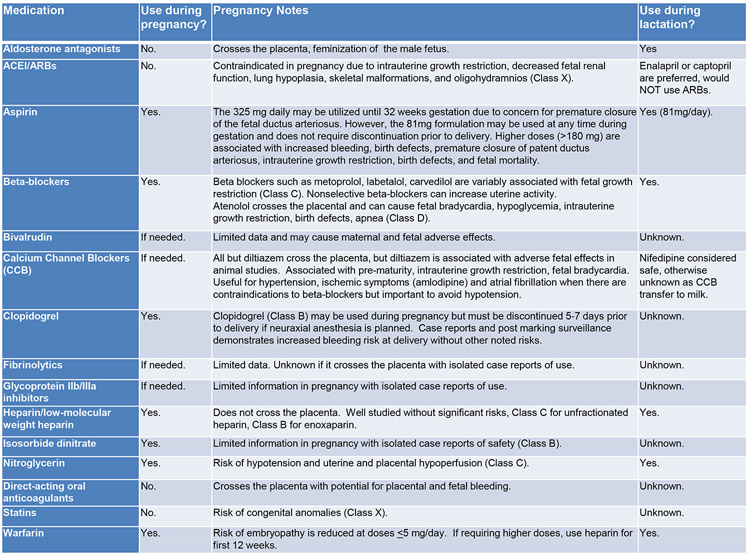
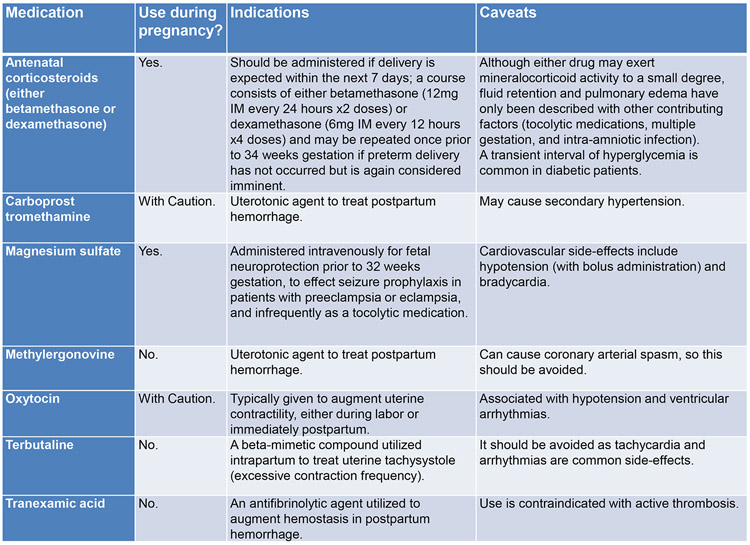
Importantly, standard of care for the nonpregnant woman with MI should be standard of care for the pregnant woman47-50 with modifications as indicated. This includes using aspirin, heparin, clopidogrel, and nitrates(Figure 451-53). Full dose aspirin(325 mg) may be used until 32 weeks gestation, and 81 mg may be used any time during gestation. Heparin is the preferred anticoagulant since it does not cross the placenta and safety during pregnancy is established.51 Clopidogrel is the preferred P2Y12 inhibitor in pregnancy, and the more potent options should be avoided or used with caution.51 Due to the absence of safety data, glycoprotein IIb/IIIa inhibitors generally should be avoided or used with caution. Dual antiplatelet therapy(DAPT) is reasonable if a percutaneous coronary intervention(PCI) with intracoronary stenting is planned. Single agent aspirin may be considered such as in PAMI due to SCAD32, although this is based on expert consensus,32 and data demonstrating benefit versus harm is lacking. Beta-blockers are generally considered safe in pregnancy, but use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers must be delayed until after delivery due to risks to the fetus.51 Nitrates can alleviate discomfort or resolve/diagnose coronary vasospasm but should be used prudently to avoid maternal hypotension and placental hypoperfusion.14 As with any MI, primary PCI is preferred to fibrinolytic therapy if it can be performed in a timely fashion. This may be particularly true in the pregnant women, given the multiple etiologies of MI, the increased bleeding risks, and the relative contraindication to fibrinolytic therapy.27 Nonetheless, successful utilization of thrombolytics in pregnancy, particularly for treatment of pulmonary embolism, has been reported.54
Figure 4.
Cardiac medications and safety during pregnancy and lactation.
ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin II receptor blocker
Note that the prior FDA Classification System is used in the above table, although it has now been transitioned to a different classification system.
Diagnostic Coronary Angiography in PAMI
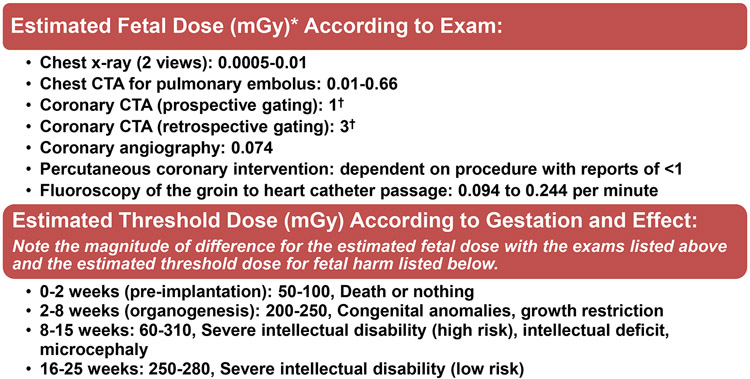
Ionizing radiation from coronary angiography (and PCI) is considered an acceptable risk in pregnancy when the procedure is otherwise indicated (Figure 555-57). Fetal radiation effects are unlikely to occur for dose levels below 50 millisieverts(mSv). Given that the fetal radiation dose associated with coronary angiography is expected to be less than 1mSv, the risk of radiation injury to the fetus is likely negligible in comparison with the natural incidence of congenital abnormalities.55-57 Standard practice methods to minimize patient (and fetal) radiation dose include short fluoroscopy time, decreased frame rates, and minimization of cineangiography.
Figure 5.
Radiation safety: estimated fetal dose according to exam and threshold dose according to gestation.
CTA=computed tomography angiography; mGy=milligray.
*The amount of energy deposited per kilogram of tissue is measured in mGy as reported above whereas the amount of energy deposited per kilogram of tissue normalized for biological effectiveness is measured in milliSieverts. Annual average background radiation is 1.1-2.5 mGy.
†These coronary CTA estimates likely represent the high end of fetal radiation exposure. Since the fetus is outside of the primary beam, scatter is the primary contributor of radiation and therefore expected to be low. Fetal exposure for chest imaging increases with gestation due to the fetus being closer to the primary beam. Larger patients require higher peak kilovoltage and tube current thereby increasing radiation exposure. Variation in the imaging techniques such as scan mode and pulsing window (e.g., the narrowest pulsing window leads to the lowest dose) can be optimized to reduce the total radiation exposure.
Interventionalists should be prepared for procedural complications. In one review, 5/129(3.8%) women with PAMI had coronary dissection during angiography.14 It is unclear if these were all iatrogenic dissections, if some were exacerbations of unidentified SCAD, or if this risk would be similar in a population-based study. It is also possible for patients to decompensate or arrest due to evolving ischemia during coronary angiography. If coronary angiography is being performed at a viable gestational age(≥23 weeks), administration of antenatal corticosteroids and notification of a “standby” obstetrical team able to perform emergent resuscitative cesarean delivery in the event of maternal decompensation should be considered. Continuous fetal heart rate monitoring during the procedure should be performed only if immediate cesarean delivery for refractory non-reassuring fetal heart rate pattern is feasible.
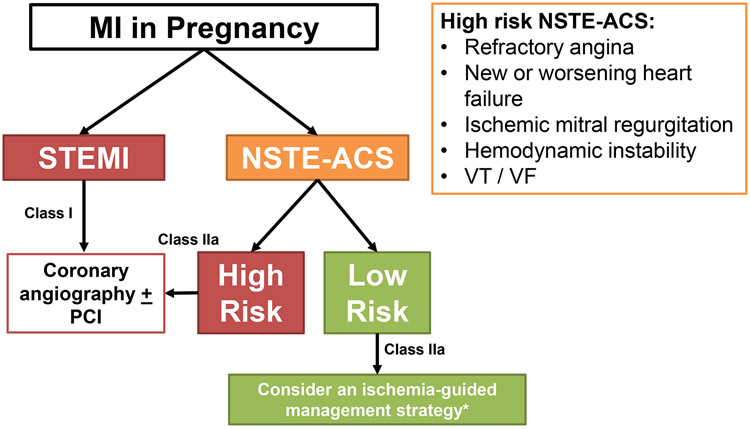
In the non-ST-elevation-acute coronary syndrome (NSTE-ACS) guidelines, an ischemia-guided management strategy without routine diagnostic coronary angiography has been suggested for patients with low risk (assessed by risk scores such as the TIMI and GRACE scores)47; this recommendation is based on data from non-pregnant patients with MI. The most recent European Society of Cardiology consensus suggests consideration for management without invasive angiography of low-risk NSTE-ACS patients with PAMI(Figure 6).18, 48 However, the risk scores do not incorporate the excess risk associated with the pregnant/postpartum state nor have they been validated in a PAMI population. Coronary CTA is a noninvasive, alternative diagnostic modality to consider in those who are low-risk, although limitations include the possibility of inconclusive findings due to limited resolution or motion artifact.58 Identifying the etiology of MI informs the future care of a PAMI patient, even if revascularization is not performed. Therefore, invasive diagnostic coronary angiography should be considered unless the anticipated risks outweigh the perceived benefits in a low-risk patient, such as someone with resolved symptoms and negative or mildly elevated cardiac biomarkers.
Figure 6.
Management strategy for pregnancy-associated myocardial infarction.
* An ischemia-guided management strategy without routine invasive coronary angiography may be appropriate in select, low-risk patients with NSTE-ACS, such as a patient with resolved symptoms or negative/mildly elevated cardiac biomarkers. Recommend caution with use of the NSTE-ACS risk scores as they do not incorporate the risk of pregnancy and have not been validated in a PAMI population.
MI=myocardial infarction; NSTE-ACS=non-ST-elevation acute coronary syndrome;
PCI=percutaneous coronary intervention; STEMI=ST-elevation myocardial infarction;
VF=ventricular fibrillation; VT=ventricular tachycardia
Coronary Revascularization in PAMI
Coronary revascularization should be reserved for women with the greatest likelihood to derive benefit, which varies considerably based on the timing of MI with respect to labor and delivery, clinical status, coronary anatomy, and the mechanism of infarction. Consequently, management must be individualized. PCI is recommended for STEMI59 or NSTEMI with favorable coronary anatomy and high-risk features, including refractory angina despite medical therapy, hemodynamic instability, sustained ventricular arrhythmias, clinical signs of heart failure, or ischemic mitral regurgitation.
The mechanism of PAMI is a key determinant of the benefit of PCI. When obstructive, unstable atherosclerotic coronary artery disease is present, PCI with stent implantation is ideal. In patients with flow-limiting coronary thromboembolism in the absence of atherosclerotic stenosis, aspiration thrombectomy ± balloon angioplasty with antithrombotic therapy may be sufficient to achieve coronary revascularization, and stent implantation may be deferred.60 Among patients with MINOCA, PCI is not indicated and treatment is determined by the underlying etiology.26
PCI for SCAD is associated with reduced rates of technical success and significant risk of procedural complications.32, 34, 61 Deep guide catheter engagement during coronary cannulation and high-pressure contrast injections can propagate dissections and expand infarction. Coronary guidewires may be unintentionally positioned in a false lumen or dissection plane where stent deployment would be a potentially devastating complication. Therefore, in cases of SCAD where the patient is hemodynamically stable with satisfactory coronary flow, conservative management is generally preferred.32 The coronaries often heal, although initial inpatient monitoring is recommended due to risk of interval worsening of SCAD requiring revascularization.62 Unfortunately, most SCAD PAMI patients have severe clinical presentations including STEMI, left main or multi-vessel SCAD, reduced LVEF, and hemodynamic instability.17, 19, 63 In these patients, emergent PCI or CABG may be necessary.
Novel PCI techniques may reduce the risks of coronary intervention for SCAD. When there is uncertainty regarding coronary guidewire position, intracoronary imaging may prevent balloon angioplasty or stent deployment within the false lumen. One group reported successful antegrade dissection re-entry to treat a SCAD occlusion when the wire was subintimal on intracoronary images.64 Stenting segments of healthy vessel proximal and distal to the dissection has been a suggested strategy to reduce propagation. Some have suggested cutting balloon angioplasty to achieve vessel fenestration and decompression of intramural hematoma prior to stent implantation or as a stand-alone therapy.65 Since there are no prospective studies comparing PCI techniques in SCAD, procedural considerations are based on expert opinion and institutional experience.
Evidence supporting the safety of PCI in pregnancy consists of case series of bare metal coronary stent(BMS) implantation.14, 27 Although drug-eluting stents(DES) have been used in pregnancy without apparent harm to the fetus,14, 57, 66 there are no large series to support the safety of this approach. Nonetheless, DES confer reduced risks of MI and repeat revascularization in non-pregnant adults compared with BMS,67 and in the absence of any evidence of fetal harm, DES use would be preferred. Due to the usual requirement of >3months of DAPT after DES implantation, stent selection may depend on the timing of MI with respect to anticipated delivery and the associated bleeding risks. In general, DES are preferred in the first two trimesters, while BMS or DES may be reasonable in the third trimester.
Current recommendations indicate that DAPT should be continued for a year following MI.47, 49 However, pregnant patients may need to interrupt DAPT with cessation of Plavix at about 7 days prior to neuraxial anesthesia. If such interruption is necessary earlier than 3 months after DES placement, short-acting intravenous glycoprotein IIb/IIIa inhibitors such as epitifibatide have been suggested as a bridge.68,66, 69 However, the safety of epitifibatide or intravenous P2Y12 inhibitors such as cangrelor is unknown in pregnancy; therefore, these should avoided when possible and only cautiously considered when weighing options in unique situations. Alternatives to be discussed by the multidisciplinary Pregnancy Heart Team include a shortened timeframe for DAPT interruption prior to neuraxial anesthesia or an elective cesarean section under general anesthesia despite DAPT. Such decisions depend on the nuances of each patient and should be individualized.
CABG may be considered in patients with an indication for coronary revascularization who are poor candidates for PCI based on guidelines for non-pregnant adults.70 In patients with SCAD, CABG should be considered when PCI is considered to confer excess risk, such as left main or two-vessel SCAD, or as bailout therapy in cases of PCI failure.32 Arterial grafts optimize durability of revascularization and are often preferred for those with atherosclerotic MI.71 In those with SCAD, venous or arterial grafts may occlude or become atretic after native vessel healing and restoration of competitive flow.34 While uncommon, FMD may affect the left internal mammillary artery(LIMA).72 Therefore, decision-making regarding grafts among those with SCAD require a case-by-case assessment and may depend on factors such as timeliness of graft harvesting.
Cardiogenic Shock, Congestive Heart Failure, and Cardiac Arrest
In a report of 150 women with PAMI, 38% developed shock or congestive heart failure.14 In these high risk patients with hemodynamic instability, mechanical circulatory support with intra-aortic balloon pumps, percutaneous ventricular assist devices and extracorporeal membrane oxygenators have been used during pregnancy73-75 and are considered life-saving measures.
Women with PAMI may also present with or decompensate to cardiac arrest. If cardiac arrest occurs, prompt, high-quality cardiopulmonary resuscitation(CPR) is of most benefit to the mother and fetus.20 Patients should be supine on a firm backboard with CPR performed according to the standard protocol. No medication substitutions or dose modifications are required. Recommendations specific to pregnancy include continuous manual left uterine displacement to offload aortocaval compression during CPR, intravenous access in a vein above the diaphragm to ensure that therapy is not obstructed by the gravid uterus, and full preparation to conduct an immediate resuscitative cesarean delivery if return of spontaneous circulation is not achieved after 4 minutes of resuscitation. Maternal CPR should be continued with minimal interruptions during the preparation and performance of a cesarean delivery. Cesarean delivery should be performed at the site of arrest if possible to best optimize the mother’s condition.20
Delivery after PAMI
Presuming hemodynamic stability, timing of delivery may be predicated by gestational age at initial event, current maternal cardiac status and anticipated degree of interim recovery, availability of key personnel and resources, and future anticoagulation requirements. Practice guidelines recommend postponing delivery for at least 2 weeks after PAMI, but this recommendation is based on expert opinion18 due to literature suggesting a high rate of maternal mortality with early delivery.27 Time and circumstance permitting, a multidisciplinary Pregnancy Heart Team should devise a detailed plan with contingencies for management of pregnancy and delivery.18
In the absence of specific antepartum maternal or fetal complications, delivery is undertaken for standard obstetric indications: either a gestational age of 39-40 weeks is elected or spontaneous labor is awaited. Existing data in the congenital heart disease population suggests that elective cesarean delivery does not appear to confer either obstetrical or cardiovascular benefit,76 and vaginal delivery is preferred.12, 18, 77
Certain obstetric medications should be avoided in women with PAMI(Figure 778). With regard to intrapartum analgesia, a neuraxial anesthetic can be used if the patient is on baby aspirin alone but not on DAPT. The P2Y12 inhibitor should be stopped 7 days prior if safe. An intrapartum “cardiac protocol” may include 1)early administration of neuraxial anesthesia; 2)arterial catheter placement for direct blood pressure monitoring; 3)meticulous recording of fluid status; 4)continuous telemetry monitoring (as applicable); 5)delayed Valsalva maneuvers in the second stage of labor to optimize cardiac output and continuous cardiac venous return, with operative vaginal delivery (forceps, vacuum, other assistive devices) reserved exclusively for standard indications; 6)avoidance of excessive blood loss.
Figure 7.
Obstetric medication considerations in women with pregnancy-associated myocardial infarction.
For patients who are not candidates for or decline regional anesthesia, intravenous opioid analgesics (fentanyl, meperidine, morphine) represent a “second-line” option, recognizing none confer similar hemodynamic benefits to neuraxial anesthesia. Minimal data currently exists regarding safety of inhaled nitrous oxide, which has mixed efficacy for pain reduction.79 Nitrous oxide is associated with myocardial depression and transient pulmonary hypertension, although a higher risk of postoperative cardiac events was not observed in the non-pregnant surgical population.79, 80 In anticipation of significant physiologic intravascular-extravascular fluid redistribution, patients with PAMI are usually monitored in an intensive care unit for the initial 24-48 hours postpartum.
Acute Management of Post-Partum MI
Acute management of MI in the postpartum period is more straightforward than antepartum MI as fetal risks are no longer pertinent. Maternal bleeding risks may persist early after delivery but are expected to decline with time. Considerations for coronary revascularization for postpartum MI are similar to those in non-pregnant women.81, 82
Breastfeeding after PAMI
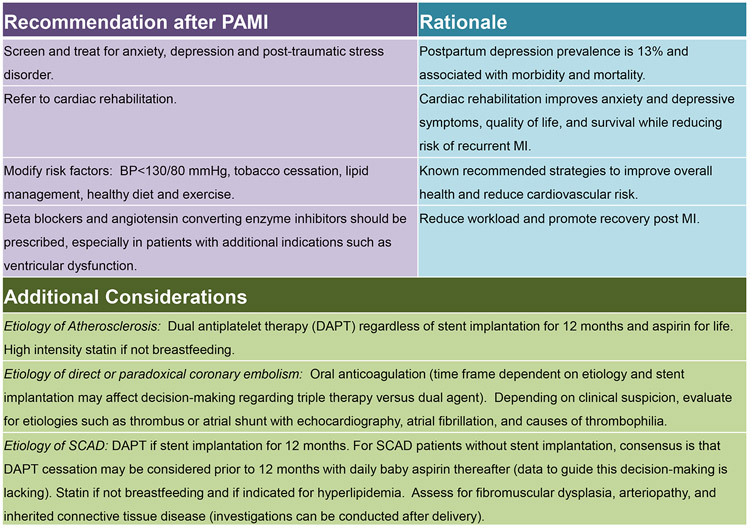
General recommendations regarding lactation should be tailored to individual patient, although most medications cross into breast milk(Figure 4). If further radiographic studies utilizing iodinated contrast are planned, lactation does not need to be interrupted, since less than 1% is excreted into breast milk.55 Other post-PAMI management strategies are outlined in Figure 830, 32, 33, 49, 50, 83.
Figure 8.
Outpatient management considerations after pregnancy-associated myocardial infarction.
Future Pregnancies
Advisability of pregnancy following PAMI is contingent on the primary etiology and degree of left ventricular dysfunction. Two small series from the 1990s describe maternal complication rates of 71-100% with fetal growth restriction a common sequelae.84, 85 A more contemporary series found 10% recurrence rate of significant cardiac complications with mortality rate up to 23% and increased frequencies of postpartum hemorrhage and fetal/neonatal demise.86 None of the major cardiac risk stratification strategies(CARPREG I/II, ZAHARA, modified WHO) index PAMI specifically, although severe systemic ventricular dysfunction(LVEF<30%) is listed as a contraindication to pregnancy in the modified WHO classification. The European Society of Cardiology consensus indicates that pregnancy may be considered without clinical evidence of persistent left ventricular dysfunction or ischemia, with a recommendation to delay conception for 12 months following MI.18 If the previous MI was due to SCAD, the consensus has been to recommend against future pregnancy, cognizant there may be a role for individualized counseling.32
Conclusion
PAMI represents a unique patient population for which careful consideration of etiologies, thoughtful and expedient decision-making for treatment, and attentive outpatient care is necessary. These measures, along with the input from a multidisciplinary Pregnancy Heart Team, are pertinent for individualizing care with guidance from published series, consensus documents, and prior experience.
Acknowledgements
The authors would like to acknowledge Ms. Jenny Cummins for her secretarial support and Kenneth Fetterly, Ph.D. for his expertise on radiation safety.
Sources of Funding: The work of Dr. Tweet and Dr. Lewey is supported by the Building Interdisciplinary Careers in Women’s Health (BIRCWH) NIH HD65987 and HD85848, respectively. The work of Dr. Smilowitz is supported by NIH KL2 TR001446 from the National Center for Advancing Translational Sciences.
Footnotes
Disclosures: The authors have no disclosures to report.
References
- 1.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Updated February 4, 2020 Accessed April 23, 2020. [Google Scholar]
- 2.Creanga AA, Syverson C, Seed K and Callaghan WM. Pregnancy-Related Mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA and White HD. Fourth Universal Definition of Myocardial Infarction. J Am Coll Cardiol. 2018;72:2231. [DOI] [PubMed] [Google Scholar]
- 4.Vijayaraghavan R, Verma S, Gupta N and Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation. 2014;130:1915–20. [DOI] [PubMed] [Google Scholar]
- 5.Postnatal Care for Mothers and Newborns. Highlights from the World Health Organization 2013 Guidelines. https://www.who.int/maternal_child_adolescent/publications/WHO-MCA-PNC-2014-Briefer_A4.pdf. Updated April, 2015 Accessed April 23, 2020. [Google Scholar]
- 6.Ouzounian JG and Elkayam U. Physiologic Changes During Normal Pregnancy and Delivery. Cardiol Clin. 2012;30:317–329. [DOI] [PubMed] [Google Scholar]
- 7.Sanghavi M and Rutherford JD. Cardiovascular Physiology of Pregnancy. Circulation. 2014;130:1003–1008. [DOI] [PubMed] [Google Scholar]
- 8.Soma-Pillay P, Nelson-Piercy C, Tolppanen H and Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB and Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK and Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564–71. [DOI] [PubMed] [Google Scholar]
- 11.Ladner HE, Danielsen B and Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480–4. [DOI] [PubMed] [Google Scholar]
- 12.Smilowitz NR, Gupta N, Guo Y, Zhong J, Weinberg CR, Reynolds HR and Bangalore S. Acute Myocardial Infarction During Pregnancy and the Puerperium in the United States. Mayo Clin Proc. 2018;93:1404–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson P, Narous M, Firoz T, Chou D, Barreix M, Say L and James M. Incidence of myocardial infarction in pregnancy: a systematic review and meta-analysis of population-based studies. Eur Heart J Qual Care Clin Outcomes. 2017;3:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A and Roth A. Pregnancy-associated acute myocardial infarction: A review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702. [DOI] [PubMed] [Google Scholar]
- 15.Leifheit-Limson EC, D'Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, Krumholz HM and Lichtman JH. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction: The VIRGO Study. J Am Coll Cardiol. 2015;66:1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush N, Nelson-Piercy C, Spark P, Kurinczuk JJ, Brocklehurst P and Knight M. Myocardial infarction in pregnancy and postpartum in the UK. Eur J Prev Cardiol. 2013;20:12–20. [DOI] [PubMed] [Google Scholar]
- 17.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH and Best PJM. Spontaneous Coronary Artery Dissection Associated With Pregnancy. J Am Coll Cardiol. 2017;70:426–435. [DOI] [PubMed] [Google Scholar]
- 18.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Lung B, Johnson MR, Kintscher U, Kranke P, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 19.Havakuk O, Goland S, Mehra A and Elkayam U. Pregnancy and the Risk of Spontaneous Coronary Artery Dissection: An Analysis of 120 Contemporary Cases. Circ Cardiovasc Interv. 2017;10:e004941. [DOI] [PubMed] [Google Scholar]
- 20.Jeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, Katz VL, Lapinsky SE, Einav S, Warnes CA, et al. Cardiac Arrest in Pregnancy: A Scientific Statement From the American Heart Association. Circulation. 2015;132:1747–73. [DOI] [PubMed] [Google Scholar]
- 21.Mathew JP, Fleisher LA, Rinehouse JA, Sevarino FB, Sinatra RS, Nelson AH, Prokop EK and Rosenbaum SH. ST segment depression during labor and delivery. Anesthesiology. 1992;77:635–41. [DOI] [PubMed] [Google Scholar]
- 22.Shivvers SA, Wians FH Jr., Keffer JH and Ramin SM. Maternal cardiac troponin I levels during normal labor and delivery. Am J Obstet Gynecol. 1999;180:122. [DOI] [PubMed] [Google Scholar]
- 23.Smith R, Silversides C, Downey K, Newton G and Macarthur A. Assessing the incidence of peripartum subclinical myocardial ischemia using the troponin T assay: an observational pilot study. Int J Obstet Anesth. 2015;24:30–4. [DOI] [PubMed] [Google Scholar]
- 24.Lau ES and Sarma A. The Role of Cardiac Biomarkers in Pregnancy. Curr Treat Options Cardiovasc Med. 2017;19:49. [DOI] [PubMed] [Google Scholar]
- 25.Elkayam and Gleicher, eds.Cardiac Problems in Pregnancy:Diagnosis and Management of Maternal and Fetal Heart Disease,Third Edition.New York:Wiley-Liss,Inc;1998. [Google Scholar]
- 26.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 27.Roth A and Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–80. [DOI] [PubMed] [Google Scholar]
- 28.Bentzon JF, Otsuka F, Virmani R and Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. [DOI] [PubMed] [Google Scholar]
- 29.Falk E, Nakano M, Bentzon JF, Finn AV and Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;34:719–28. [DOI] [PubMed] [Google Scholar]
- 30.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE and Wenger NK. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133:916–47. [DOI] [PubMed] [Google Scholar]
- 31.Krittanawong C and Yue B. The Difficulty in Identifying Pregnancy-Associated Coronary Artery Dissection Using Nationwide Inpatient Databases. J Am Coll Cardiol. 2018;71:468. [DOI] [PubMed] [Google Scholar]
- 32.Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adlam D, Alfonso F, Maas A, Vrints C and Writing C. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr., Hayes SN and Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–86. [DOI] [PubMed] [Google Scholar]
- 35.Mittal SR. Acute myocardial infarction in a 20 year pregnant female with prosthetic mitral valve. J Assoc Physicians India. 2013;61:664–5. [PubMed] [Google Scholar]
- 36.Gulati R, Behfar A, Narula J, Kanwar A, Lerman A, Cooper L and Singh M. Acute Myocardial Infarction in Young Individuals. Mayo Clin Proc. 2020;95:136–156. [DOI] [PubMed] [Google Scholar]
- 37.Battinelli EM, Marshall A and Connors JM. The role of thrombophilia in pregnancy. Thrombosis. 2013;2013:516420–516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iadanza A, Del Pasqua A, Barbati R, Carrera A, Gentilini R, Favilli R and Pierli C. Acute ST elevation myocardial infarction in pregnancy due to coronary vasospasm: A case report and review of literature. Int J Cardiol. 2007;115:81–85. [DOI] [PubMed] [Google Scholar]
- 39.ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. 2018;132:e1–e17. [DOI] [PubMed] [Google Scholar]
- 40.Simpson EL, Lawrenson RA, Nightingale AL and Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108:56–60. [DOI] [PubMed] [Google Scholar]
- 41.Oindi FM, Sequeira E, Sequeira HR and Mutiso SK. Takotsubo cardiomyopathy in pregnancy: a case report and literature review. BMC Pregnancy and Childbirth. 2019;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hausvater A, Smilowitz NR, Saw J, Sherrid M, Ali T, Espinosa D, Mersha R, DeFonte M and Reynolds HR. Spontaneous Coronary Artery Dissection in Patients With a Provisional Diagnosis of Takotsubo Syndrome. J Am Heart Assoc. 2019;8:e013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48, 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 44.Bird ST, Gelperin K, Sahin L, Bleich KB, Fazio-Eynullayeva E, Woods C, Radden E, Greene P, McCloskey C, Johnson T, et al. First-trimester exposure to gadolinium-based contrast agents: A utilization study of 4.6 million U.S. pregnancies. Radiology. 2019;293:193–200. [DOI] [PubMed] [Google Scholar]
- 45.Mervak BM, Altun E, McGinty KA, Hyslop WB, Semelka RC and Burke LM. MRI in pregnancy: Indications and practical considerations. J Magn Reson Imaging. 2019;49:621–631. [DOI] [PubMed] [Google Scholar]
- 46.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 47.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non ST-Elevation Acute Coronary Syndromes. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, et al. ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction. Circulation. 2007;116:e148–e304. [DOI] [PubMed] [Google Scholar]
- 49.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 50.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Circulation. 2016;133:1135–1147.26490017 [Google Scholar]
- 51.Halpern DG, Weinberg CR, Pinnelas R, Mehta-Lee S, Economy KE and Valente AM. Use of Medication for Cardiovascular Disease During Pregnancy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:457–476. [DOI] [PubMed] [Google Scholar]
- 52.Garey J, Lavigne S, Lione A, Lusskin S, Nichols G and Scialli A. Reprotox. https://www.reprotox.org/. Accessed April 23, 2020.
- 53.ACOG Practice Bulletin No. 742: Postpartum pain management. Obstet Gynecol. 2018;132:e35–43. [DOI] [PubMed] [Google Scholar]
- 54.Sousa Gomes M, Guimaraes M and Montenegro N. Thrombolysis in pregnancy: a literature review. J Matern Fetal Neonatal Med. 2019;32:2418–2428. [DOI] [PubMed] [Google Scholar]
- 55.Jain C ACOG Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol. 2019;133:186. [DOI] [PubMed] [Google Scholar]
- 56.Colletti PM, Lee KH and Elkayam U. Cardiovascular Imaging of the Pregnant Patient. AJR Am J Roentgenol. 2013;200:515–521. [DOI] [PubMed] [Google Scholar]
- 57.Kuba K, Wolfe D, Schoenfeld AH and Bortnick AE. Percutaneous Coronary Intervention in Pregnancy: Modeling of the Fetal Absorbed Dose. Case Rep Obstet Gynecol;2019:8410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tweet MS, Gulati R, Williamson EE, Vrtiska TJ and Hayes SN. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc Imaging. 2016;9:436–50. [DOI] [PubMed] [Google Scholar]
- 59.O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. [DOI] [PubMed] [Google Scholar]
- 60.Sakai K, Inoue K and Nobuyoshi M. Aspiration thrombectomy of a massive thrombotic embolus in acute myocardial infarction caused by coronary embolism. Int Heart J. 2007;48:387–392. [DOI] [PubMed] [Google Scholar]
- 61.Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A and Mancini GBJ. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 62.Waterbury TM, Tweet MS, Hayes SN, Eleid MF, Bell MR, Lerman A, Singh M, Best PJM, Lewis BR, Rihal CS, et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circ Cardiovasc Interv. 2018;11:e006772. [DOI] [PubMed] [Google Scholar]
- 63.Lobo AS, Cantu SM, Sharkey SW, Grey EZ, Storey K, Witt D, Benson G, Garberich RF, Kubota Y, Bairey Merz CN and Henry TD. Revascularization in Patients With Spontaneous Coronary Artery Dissection and ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2019;74:1290–1300. [DOI] [PubMed] [Google Scholar]
- 64.Daniels D, Nishi T, Tremmel JA. Antegrade Dissection Re-Entry After Subintimal Wiring of an Occluded Vessel From Spontaneous Coronary Artery Dissection. JACC Case Reports. 2020;2:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yumoto K, Sasaki H, Aoki H and Kato K. Successful Treatment of Spontaneous Coronary Artery Dissection With Cutting Balloon Angioplasty as Evaluated With Optical Coherence Tomography. JACC: Cardiovascular Interventions. 2014;7:817–819. [DOI] [PubMed] [Google Scholar]
- 66.Al-Aqeedi RF and Al-Nabti AD. Drug-eluting stent implantation for acute myocardial infarction during pregnancy with use of glycoprotein IIb/IIIa inhibitor, aspirin and clopidogrel. J Invasive Cardiol. 2008;20:E146–9. [PubMed] [Google Scholar]
- 67.Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, Kaiser C, Remkes W, Raber L, de Belder A, et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503–2510. [DOI] [PubMed] [Google Scholar]
- 68.Ben Morrison T, Horst BM, Brown MJ, Bell MR and Daniels PR. Bridging with glycoprotein IIb/IIIa inhibitors for periprocedural management of antiplatelet therapy in patients with drug eluting stents. Catheter Cardiovasc Interv. 2012;79:575–82. [DOI] [PubMed] [Google Scholar]
- 69.Bauer ME, Bauer ST, Rabbani AB and Mhyre JM. Peripartum management of dual antiplatelet therapy and neuraxial labor analgesia after bare metal stent insertion for acute myocardial infarction. Anesth Analg. 2012;115:613–5. [DOI] [PubMed] [Google Scholar]
- 70.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 71.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM Jr., et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–735. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez Lengua CA, Jacobi A and Hecht HS. Spontaneous LIMA graft dissection in a patient with fibromuscular dysplasia. Eur Heart J Cardiovasc Imaging. 2015;16:1373. [DOI] [PubMed] [Google Scholar]
- 73.Mayr A, Lederer W, Mortl M, Margreiter J, Hoi M, Hasibeder W and Mutz N. Successful treatment of severe myocardial failure after postpartum haemorrhage with the use of an intra-aortic balloon pump. Intensive Care Med. 1999;25:223–5. [DOI] [PubMed] [Google Scholar]
- 74.Pacheco LD, Saade GR and Hankins GDV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42:21–25. [DOI] [PubMed] [Google Scholar]
- 75.Desai N, Chaudhry K and Aji J. Impella left ventricular assist device in cardiac arrest after spinal anaesthesia for caesarean section. BMJ Case Rep. 2015;bcr2015211958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Hagen IM, Roos-Hesselink JW, Ruys TP, Merz WM, Goland S, Gabriel H, Lelonek M, Trojnarska O, Al Mahmeed WA, Balint HO, et al. Pregnancy in Women With a Mechanical Heart Valve: Data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132:132–42. [DOI] [PubMed] [Google Scholar]
- 77.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG and Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–72. [DOI] [PubMed] [Google Scholar]
- 78.ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 79.Klomp T, van Poppel M, Jones L, Lazet J, Di Nisio M and Lagro-Janssen ALM. Inhaled analgesia for pain management in labour. Cochrane Database of Syst Rev. 2012;9:CD009351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myles PS, Leslie K, Chan MTV, Forbes A, Peyton PJ, Paech MJ, Beattie WS, Sessler DI, Devereaux PJ, Silbert B, Schricker T and Wallace S. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. The Lancet. 2014;384:1446–1454. [DOI] [PubMed] [Google Scholar]
- 81.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 82.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 83.Raphael CE, Heit JA, Reeder GS, Bois MC, Maleszewski JJ, Tilbury RT and Holmes DR. Coronary embolus: An underappreciated cause of acute coronary syndromes. JACC: Cardiovascular Interventions. 2018;11:172–180. [DOI] [PubMed] [Google Scholar]
- 84.Frenkel Y BG, Reisin L, Rath S, Mashiach S, Battler A. . Pregnancy after myocardial infarction: are we playing safe? Obstet Gynecol. 1991;77:822–5. [PubMed] [Google Scholar]
- 85.Vinatier DVS, Depret-Mosser S, Dufour P, Prolongeau JF, Monnier JC, Decoulx E, Theeten G. Pregnancy after myocardial infarction. Eur J Obstet Gynecol Reprod Biol 1994;56:89–93. [DOI] [PubMed] [Google Scholar]
- 86.Burchill LJ, Lameijer H, Roos-Hesselink JW, Grewal J, Ruys TPE, Kulikowski JD, Burchill LA, Oudijk MA, Wald RM, Colman JM, et al. Pregnancy risks in women with pre-existing coronary artery disease, or following acute coronary syndrome. Heart. 2015;101:525. [DOI] [PubMed] [Google Scholar]