Abstract
Despite extensive efforts by many practitioners in the field, methods for the direct α-C–H bond functionalization of unprotected alicyclic amines remain rare. A new advance in this area utilizes N-lithiated alicyclic amines. These readily accessible intermediates are converted to transient imines through the action of a simple ketone oxidant, followed by alkylation with a β-ketoacid under mild conditions to provide valuable β-amino ketones with unprecedented ease. Regioselective α′-alkylation is achieved for substrates with existing α-substituents. The method is further applicable to the convenient one-pot synthesis of polycyclic dihydroquinolones through the incorporation of a SNAr step.
Keywords: C–H bond functionalization, alicyclic amines, decarboxylative C–C bond formation, Mannich reaction, annulation
Graphical Abstract

N-lithiated alicyclic amines are converted to transient imines through the action of a simple ketone oxidant, followed by alkylation with a β-ketoacid under mild conditions to provide valuable β-amino ketones with unprecedented ease. Regioselective α′-alkylation is achieved for substrates with existing α-substituents. The method is further applicable to the convenient one-pot synthesis of polycyclic dihydroquinolones through the incorporation of a SNAr step.
Driven largely by the importance of this class of compounds in synthetic and medicinal chemistry,[1] the synthesis of substituted alicyclic amines by means of C–H bond functionalization remains a highly active area of research.[2,3] In stark contrast to the numerous advances that have been achieved with 3° or protected 2° amines, highly desirable methods for the direct synthesis of α-functionalized 2° (i.e. unprotected) alicyclic amines from their corresponding parent amines remain limited (Scheme 1).[3j,4] Moreover, α′-C–H bond functionalization of 2° alicyclic amines with an existing α-substituent is exceptionally challenging,[3j,5,6] especially with electronically activating substituents that favor functionalization at the substituted site. Leveraging the known ability of lithium amides to generate imines upon reaction with simple ketone oxidants (e.g., 1 → 2, Scheme 1),[7] we recently developed a new method for the C–H bond functionalization of unprotected alicyclic amines.[8] Specifically, transient cyclic imines 2 generated from the oxidation of lithium amides 1 engage organolithium nucleophiles to provide α-functionalized products. Regioselective α’-C–H bond functionalization of α-substituted alicyclic amines was also achieved.[8a] The scope of the nucleophile could be expanded to other organometallics such as Grignard reagents by using Lewis acids to activate the imine intermediates.[8b] However, α′-C–H bond functionalization of α-substituted amines proved to be incompatible with Lewis acid activation, hampering our efforts to further expand the scope of nucleophiles. Particularly attractive would be new methods in which α’-C–H bond functionalization is achieved with non-organometallic nucleophiles. Here we report an approach for the rapid diversification of transient imines 2 via decarboxylative alkylation with β-ketoacids 3 to provide β-aminoketones 4. In addition, utilizing o-fluoroaryl-β-ketoacids 5, polycyclic amines 6 can be obtained in a single operation via a process that involves a subsequent SNAr reaction.
Scheme 1.
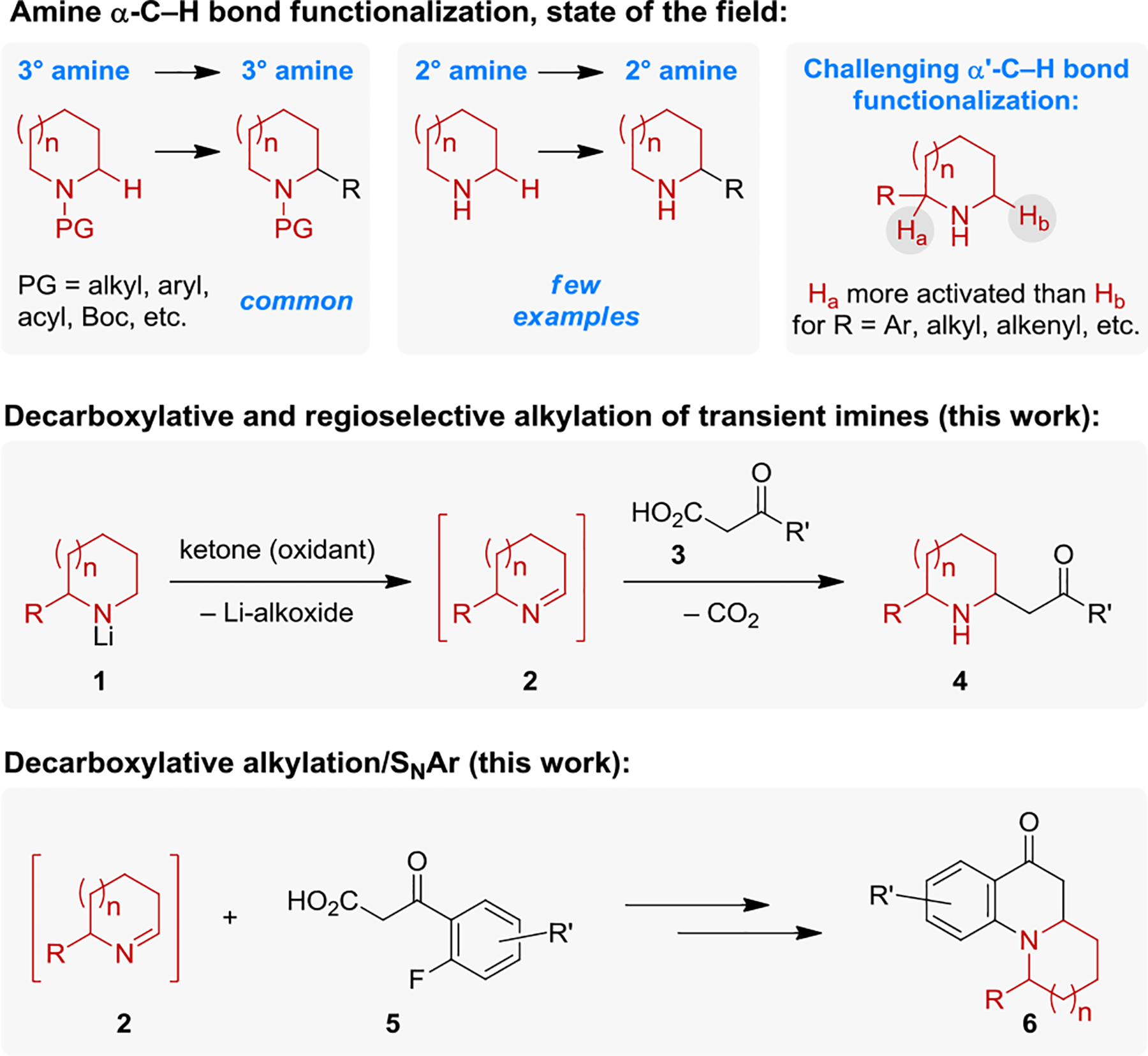
Ovefrview of methods for the C–H bond functionalization of amines and present strategy.
Mannich reactions and their decarboxylative variants employing β-keto acids represent valuable tools for the synthesis of β-amino ketones from imines.[9–11] Despite the utility of the corresponding products, the use of enolizable cyclic imines in these reactions has largely remained limited to easily accessible alicyclic imines such as 1-pyrroline and 1-piperideine.[12–15] This is likely the result of the limited availability/stability of enolizable cyclic imines, in particular chiral variants possessing a substituent in the α-position. In order to determine whether the in-situ-generation of alicyclic imines via lithium amide oxidation is compatible with the decarboxylative Mannich process, we evaluated a range of reaction conditions. Key findings with the model substrates piperidine and β-keto acid 3a are summarized in Scheme 2. Following amine deprotonation and treatment with trifluoroacetophenone to rapidly access 1-piperideine, β-keto acid 3a (1.5 equiv) was added, followed by stirring at room temperature. Desired β-amino ketone 4a was obtained in 39% yield. The yield of 4a could be increased to 50% by employing 2.5 equivalents of 3a. Considering that the Li-alkoxide resulting from the reduction of trifluoroacetophenone may interfere with the subsequent decarboxylative alkylation,16 the addition of acidic additives was explored. Indeed, small to appreciable improvements in yield were observed in all cases. Trifluoroacetic acid (TFA) outperformed acetic acid as an additive, presumably because lithium acetate is still sufficiently basic to partially impede the addition process. Under the optimized conditions, product 4a was obtained in 76% yield.
Scheme 2.
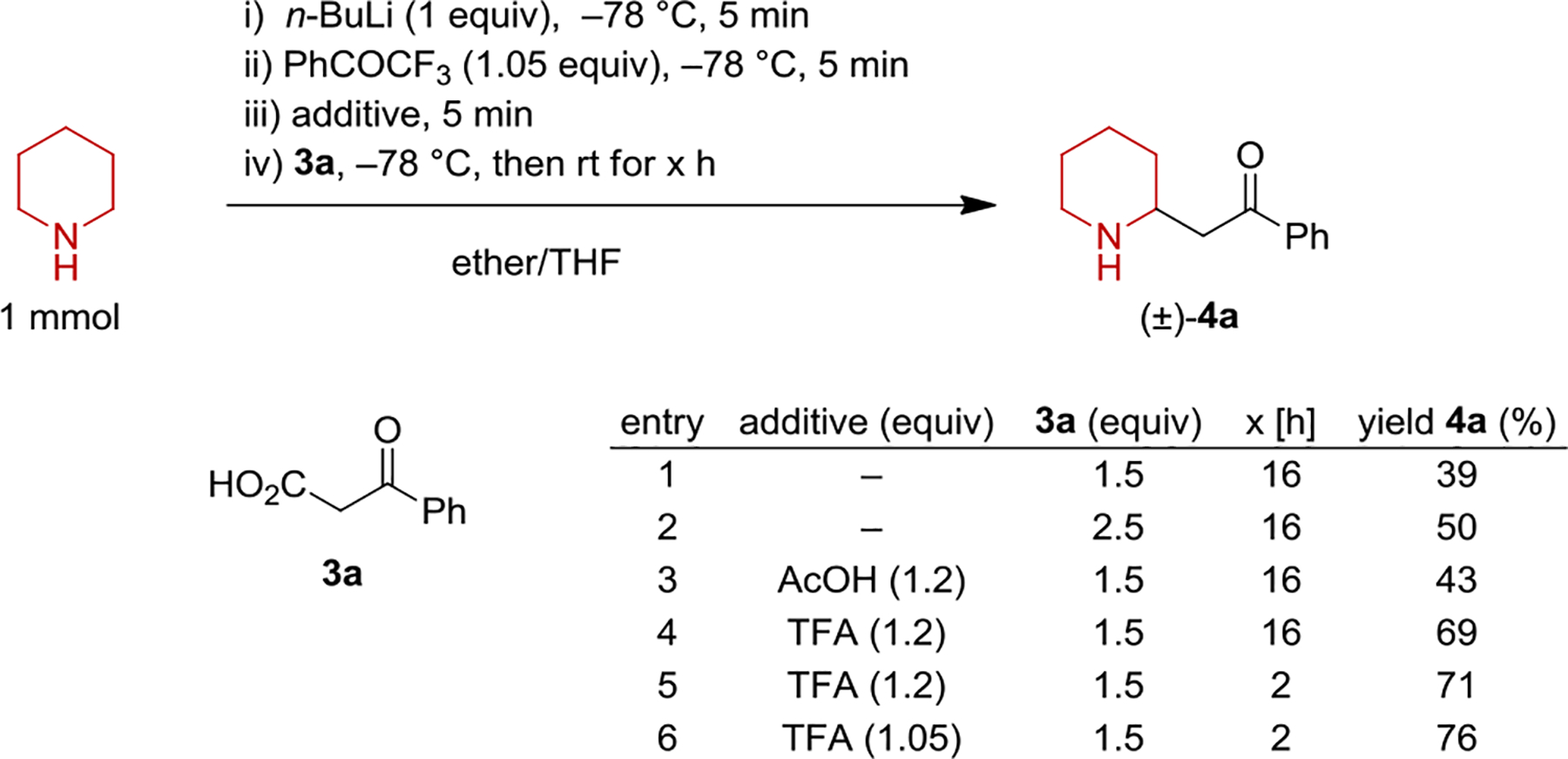
Selected optimization reactions.
The scope of the reaction was explored with a broad range of alicyclic amines and β-keto acids (Scheme 3). Different ring sizes and substitution patterns on both reaction partners were readily accommodated. Both aryl and alkyl ketones could be introduced. Synthetically useful yields of β-amino ketones 4 were obtained in most cases. Particularly useful are reactions with amines that contain α-substituents. Regioselective substitution of the α′-position was observed in all cases. Diastereoselectivities ranged from poor to excellent (vide infra). Using o-fluoroaryl-β-keto acids 5 and adding an SNAr step without the need for isolating intermediates enabled the efficient preparation of various polycyclic dihydroquinolones 6. These species, which are now available directly from their unfunctionalized amines, are rather challenging to prepare by other means.[17,18]
Scheme 3.
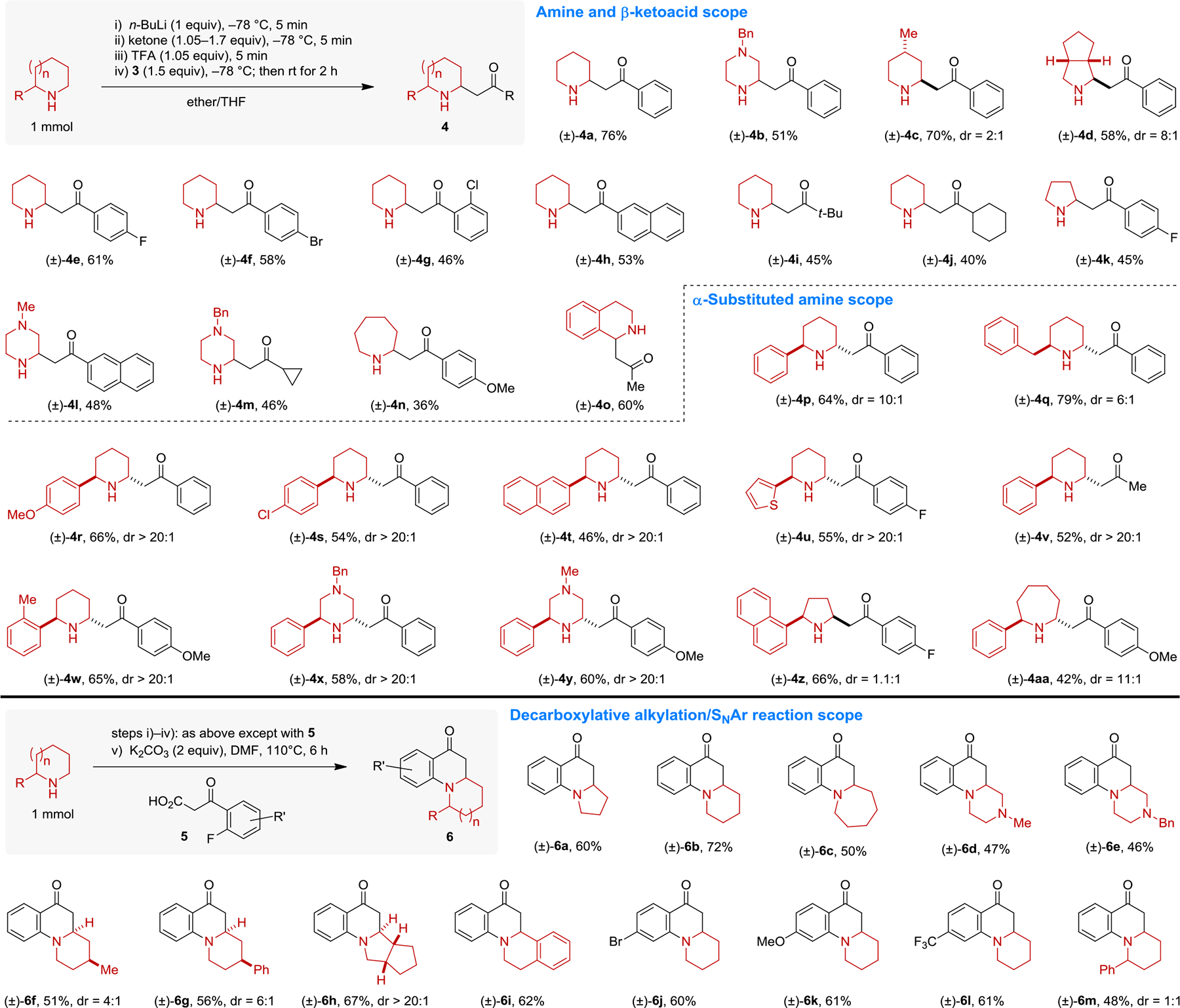
Reaction scope.
As shown in Scheme 3, products 4p–4y were obtained predominantly as trans-diastereomers. The trans-diastereomers are in fact the kinetic products of these reactions, as it has been established that the corresponding cis-isomers are thermodynamically more stable.[19] Interconversion of the diastereomers is possible, presumably via a retro-Mannich or retro-conjugate addition pathway. Indeed, simply changing the reaction conditions in the synthesis of product 4p allowed for a complete reversal of diastereoselectivity in favor of the cis-isomer (Scheme 4).
Scheme 4.

Formation of cis-product.
In summary, we have achieved the α-alkylation of unprotected alicyclic amines via a decarboxylative Mannich process involving regioselective C–H bond functionalization. Adding an SNAr step to the overall reaction sequence, this process was further extended to the synthesis of polycyclic dihydroquinolones in a single operation.
Supplementary Material
Acknowledgements
Financial support from the NIH–NIGMS (Grant R01GM101389) is gratefully acknowledged. Mass spectrometry instrumentation was supported by a grant from the NIH (S10 OD021758-01A1).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) Taylor RD, MacCoss M, Lawson ADG, J. Med. Chem 2014, 57, 5845; [DOI] [PubMed] [Google Scholar]; b) Vitaku E, Smith DT, Njardarson JT, J. Med. Chem 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- [2].a) Selected recent reviews on amine C–H bond functionalization:Campos KR, Chem. Soc. Rev 2007, 36, 1069; [DOI] [PubMed] [Google Scholar]; b) Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O, Chem. Eur. J 2010, 16, 2654; [DOI] [PubMed] [Google Scholar]; c) Yeung CS, Dong VM, Chem. Rev 2011, 111, 1215; [DOI] [PubMed] [Google Scholar]; d) Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW, Chem. Eur. J 2012, 18, 10092; [DOI] [PubMed] [Google Scholar]; e) Jones KM, Klussmann M, Synlett 2012, 23, 159; [Google Scholar]; f) Peng B, Maulide N, Chem. Eur. J 2013, 19, 13274; [DOI] [PubMed] [Google Scholar]; g) Girard SA, Knauber T, Li C-J, Angew. Chem. Int. Ed 2014, 53, 74; [DOI] [PubMed] [Google Scholar]; h) Haibach MC, Seidel D, Angew. Chem. Int. Ed 2014, 53, 5010; [DOI] [PubMed] [Google Scholar]; i) Wang L, Xiao J, Adv. Synth. Catal 2014, 356, 1137; [Google Scholar]; j) Vo C-VT, Bode JW, J. Org. Chem 2014, 79, 2809; [DOI] [PubMed] [Google Scholar]; k) Seidel D, Org. Chem. Front 2014, 1, 426; [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Qin Y, Lv J, Luo S, Tetrahedron Lett. 2014, 55, 551; [Google Scholar]; m) Seidel D, Acc. Chem. Res 2015, 48, 317; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Beatty JW, Stephenson CRJ, Acc. Chem. Res 2015, 48, 1474; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Mahato S, Jana CK, Chem. Rec 2016, 16, 1477; [DOI] [PubMed] [Google Scholar]; p) Qin Y, Zhu L, Luo S, Chem. Rev 2017, 117, 9433; [DOI] [PubMed] [Google Scholar]; q) Cheng M-X, Yang S-D, Synlett 2017, 28, 159; [Google Scholar]; r) Chu JCK, Rovis T, Angew. Chem. Int. Ed 2018, 57, 62; [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Gonnard L, Guérinot A, Cossy J, Tetrahedron 2019, 75, 145; [Google Scholar]; t) Liu S, Zhao Z, Wang Y, Chem. Eur. J 2019, 25, 2423; [DOI] [PubMed] [Google Scholar]; u) Antermite D, Bull JA, Synthesis 2019, 51, 3171; [Google Scholar]; v) Trowbridge A, Walton SM, Gaunt MJ, Chem. Rev 2020, 120, 2613. [DOI] [PubMed] [Google Scholar]
- [3].a) Selected recent examples of mechanistically diverse methods for amine C–H bond functionalization:Zhao Z, Luo Y, Liu S, Zhang L, Feng L, Wang Y, Angew. Chem. Int. Ed 2018, 57, 3792; [DOI] [PubMed] [Google Scholar]; b) Wang F, Rafiee M, Stahl SS, Angew. Chem. Int. Ed 2018, 57, 6686; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Greßies S, Klauck FJR, Kim JH, Daniliuc CG, Glorius F, Angew. Chem. Int. Ed 2018, 57, 9950; [DOI] [PubMed] [Google Scholar]; d) Griffiths RJ, Kong WC, Richards SA, Burley GA, Willis MC, Talbot EPA, Chem. Sci 2018, 9, 2295; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Idiris FIM, Majeste CE, Craven GB, Jones CR, Chem. Sci 2018, 9, 2873; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Li S-S, Lv X, Ren D, Shao C-L, Liu Q, Xiao J, Chem. Sci 2018, 9, 8253; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Maier AFG, Tussing S, Zhu H, Wicker G, Tzvetkova P, Flörke U, Daniliuc CG, Grimme S, Paradies J, Chem. Eur. J 2018, 24, 16287; [DOI] [PubMed] [Google Scholar]; h) Mori K, Isogai R, Kamei Y, Yamanaka M, Akiyama T, J. Am. Chem. Soc 2018, 140, 6203; [DOI] [PubMed] [Google Scholar]; i) Shang M, Chan JZ, Cao M, Chang Y, Wang Q, Cook B, Torker S, Wasa M, J. Am. Chem. Soc 2018, 140, 10593; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Lennox AJJ, Goes SL, Webster MP, Koolman HF, Djuric SW, Stahl SS, J. Am. Chem. Soc 2018, 140, 11227; [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Zhang J, Park S, Chang S, J. Am. Chem. Soc 2018, 140, 13209; [DOI] [PubMed] [Google Scholar]; l) Nauth AM, Schechtel E, Dören R, Tremel W, Opatz T, J. Am. Chem. Soc 2018, 140, 14169; [DOI] [PubMed] [Google Scholar]; m) Jiang H-J, Zhong X-M, Yu J, Zhang Y, Zhang X, Wu Y-D, Gong L-Z, Angew. Chem. Int. Ed 2019, 58, 1803; [DOI] [PubMed] [Google Scholar]; n) Ashley MA, Yamauchi C, Chu JCK, Otsuka S, Yorimitsu H, Rovis T, Angew. Chem. Int. Ed 2019, 58, 4002; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Guin S, Dolui P, Zhang X, Paul S, Singh VK, Pradhan S, Chandrashekar HB, Anjana SS, Paton RS, Maiti D, Angew. Chem. Int. Ed 2019, 58, 5633; [DOI] [PubMed] [Google Scholar]; p) Whitehurst WG, Blackwell JH, Hermann GN, Gaunt MJ, Angew. Chem. Int. Ed 2019, 58, 9054; [DOI] [PubMed] [Google Scholar]; q) Ma Y, Yao X, Zhang L, Ni P, Cheng R, Ye J, Angew. Chem. Int. Ed 2019, 58, 16548; [DOI] [PubMed] [Google Scholar]; r) Grainger R, Heightman TD, Ley Steven V., Lima F, Johnson CN, Chem. Sci 2019, 10, 2264; [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Vasu D, Fuentes de Arriba AL, Leitch JA, de Gombert A, Dixon DJ, Chem. Sci 2019, 10, 3401; [DOI] [PMC free article] [PubMed] [Google Scholar]; t) Asako S, Ishihara S, Hirata K, Takai K, J. Am. Chem. Soc 2019, 141, 9832; [DOI] [PubMed] [Google Scholar]; u) Lin W, Zhang K-F, Baudoin O, Nat. Catal 2019, 2, 882; [DOI] [PMC free article] [PubMed] [Google Scholar]; v) Chan JZ, Chang Y, Wasa M, Org. Lett 2019, 21, 984; [DOI] [PMC free article] [PubMed] [Google Scholar]; w) Zhou L, Shen Y-B, An X-D, Li X-J, Li S-S, Liu Q, Xiao J, Org. Lett 2019, 21, 8543; [DOI] [PubMed] [Google Scholar]; x) Kataoka M, Otawa Y, Ido N, Mori K, Org. Lett 2019, 21, 9334; [DOI] [PubMed] [Google Scholar]; y) Lee M, Adams A, Cox PB, Sanford MS, Synlett 2019, 30, 417; [DOI] [PMC free article] [PubMed] [Google Scholar]; z) Kapoor M, Chand-Thakuri P, Maxwell JM, Liu D, Zhou H, Young MC, Synlett 2019, 30, 519; [Google Scholar]; aa) Ohmatsu K, Suzuki R, Furukawa Y, Sato M, Ooi T, ACS Catal. 2020, 10, 2627; [Google Scholar]; ab) Roque JB, Kuroda Y, Jurczyk J, Xu L-P, Ham JS, Göttemann LT, Roberts CA, Adpressa D, Saurí J, Joyce LA, Musaev DG, Yeung CS, Sarpong R, ACS Catal. 2020, 10, 2929; [DOI] [PMC free article] [PubMed] [Google Scholar]; ac) Rand AW, Yin H, Xu L, Giacoboni J, Martin-Montero R, Romano C, Montgomery J, Martin R, ACS Catal. 2020, 10, 4671; [Google Scholar]; ad) Liu W, Babl T, Röther A, Reiser O, Davies HML, Chem. Eur. J 2020, 26, 4236; [DOI] [PMC free article] [PubMed] [Google Scholar]; ae) Verma P, Richter JM, Chekshin N, Qiao JX, Yu J-Q, J. Am. Chem. Soc 2020, 142, 5117; [DOI] [PMC free article] [PubMed] [Google Scholar]; af) Walker MM, Koronkiewicz B, Chen S, Houk KN, Mayer JM, Ellman JA, J. Am. Chem. Soc 2020, 142, 8194; [DOI] [PMC free article] [PubMed] [Google Scholar]; ag) Feng K, Quevedo RE, Kohrt JT, Oderinde MS, Reilly U, White MC, Nature 2020, 580, 621; [DOI] [PMC free article] [PubMed] [Google Scholar]; ah) Sarver PJ, Bacauanu V, Schultz DM, DiRocco DA, Lam Y.-h., Sherer EC, MacMillan DWC, Nat. Chem 2020, 12, 459; [DOI] [PubMed] [Google Scholar]; ai) McManus JB, Onuska NPR, Jeffreys MS, Goodwin NC, Nicewicz DA, Org. Lett 2020, 22, 679; [DOI] [PubMed] [Google Scholar]; aj) Oeschger R, Su B, Yu I, Ehinger C, Romero E, He S, Hartwig J, Science 2020, 368, 736; [DOI] [PMC free article] [PubMed] [Google Scholar]; ak) Short MA, Blackburn JM, Roizen JL, Synlett 2020, 31, 102; [DOI] [PMC free article] [PubMed] [Google Scholar]; al) Ryder ASH, Cunningham WB, Ballantyne G, Mules T, Kinsella AG, Turner-Dore J, Alder CM, Edwards LJ, McKay BSJ, Grayson MN, Cresswell AJ, Angew. Chem. Int. Ed 2020, 59, 14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Payne PR, Garcia P, Eisenberger P, Yim JCH, Schafer LL, Org. Lett 2013, 15, 2182. [DOI] [PubMed] [Google Scholar]
- [5].a) The regioselective formation of the less substituted imine from an α-substituted 2° alicyclic amine has for instance been achieved by the carefully controlled deprotonation of N-Cl prolinol derivatives and related species. Examples:Horenstein BA, Zabinski RF, Schramm VL, Tetrahedron Lett. 1993, 34, 7213; [Google Scholar]; b) Furneaux RH, Limberg G, Tyler PC, Schramm VL, Tetrahedron 1997, 53, 2915; [Google Scholar]; c) Davis BG, Maughan MAT, Chapman TM, Villard R, Courtney S, Org. Lett 2002, 4, 103; [DOI] [PubMed] [Google Scholar]; d) Maughan MAT, Davies IG, Claridge TDW, Courtney S, Hay P, Davis BG, Angew. Chem. Int. Ed 2003, 42, 3788. [DOI] [PubMed] [Google Scholar]
- [6].a) Examples of regioselective α′-C–H bond functionalization of protected 2° alicyclic amines with an existing α-substituent:Chatani N, Asaumi T, Yorimitsu S, Ikeda T, Kakiuchi F, Murai S, J. Am. Chem. Soc 2001, 123, 10935; [DOI] [PubMed] [Google Scholar]; b) Davies HML, Venkataramani C, Hansen T, Hopper DW, J. Am. Chem. Soc 2003, 125, 6462; [DOI] [PubMed] [Google Scholar]; c) Pastine SJ, Gribkov DV, Sames D, J. Am. Chem. Soc 2006, 128, 14220; [DOI] [PubMed] [Google Scholar]; d) Spangler JE, Kobayashi Y, Verma P, Wang D-H, Yu J-Q, J. Am. Chem. Soc 2015, 137, 11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) For an early review, see: (a)Majewski M, Gleave DM, J. Organomet. Chem 1994, 470, 1; Selected key contributions: [Google Scholar]; b) Wittig G, Schmidt HJ, Renner H, Chem. Ber 1962, 95, 2377; [Google Scholar]; c) Wittig G, Hesse A, Liebigs Ann. Chem 1971, 746, 149; [Google Scholar]; d) Wittig G, Hesse A, Liebigs Ann. Chem 1971, 746, 174; [Google Scholar]; e) Wittig G, Häusler G, Liebigs Ann. Chem 1971, 746, 185. [Google Scholar]
- [8].a) Chen W, Ma L, Paul A, Seidel D, Nat. Chem 2018, 10, 165; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Paul A, Seidel D, J. Am. Chem. Soc 2019, 141, 8778; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen W, Paul A, Abboud KA, Seidel D, Nat. Chem 2020, 12, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Selected recent reviews on the Mannich reaction:Karimi B, Enders D, Jafari E, Synthesis 2013, 45, 2769; [Google Scholar]; b) Saranya S, Harry NA, Krishnan KK, Anilkumar G, Asian J. Org. Chem 2018, 7, 613; [Google Scholar]; c) Cheng D-J, Shao Y-D, ChemCatChem 2019, 11, 2575; [Google Scholar]; d) Bates RW, Ko W, Barát V, Org. Biomol. Chem 2020, 18, 810. [DOI] [PubMed] [Google Scholar]
- [10].For a review on the use of β-keto acids in synthesis, see: Mao S, Chen K, Yan G, Huang D, Eur. J. Org. Chem 2020, 2020, 525. [Google Scholar]
- [11].a) Selected reviews on decarboxylative coupling reactions:Rodriguez N, Goossen LJ, Chem. Soc. Rev 2011, 40, 5030; [DOI] [PubMed] [Google Scholar]; b) Pan Y, Tan C-H, Synthesis 2011, 2011, 2044; [Google Scholar]; c) Nakamura S, Org. Biomol. Chem 2014, 12, 394; [DOI] [PubMed] [Google Scholar]; d) Xuan J, Zhang Z-G, Xiao W-J, Angew. Chem. Int. Ed 2015, 54, 15632; [DOI] [PubMed] [Google Scholar]; e) Liu P, Zhang G, Sun P, Org. Biomol. Chem 2016, 14, 10763; [DOI] [PubMed] [Google Scholar]; f) Patra T, Maiti D, Chem. Eur. J 2017, 23, 7382; [DOI] [PubMed] [Google Scholar]; g) Wei Y, Hu P, Zhang M, Su W, Chem. Rev 2017, 117, 8864; [DOI] [PubMed] [Google Scholar]; h) Rahman M, Mukherjee A, Kovalev IS, Kopchuk DS, Zyryanov GV, Tsurkan MV, Majee A, Ranu BC, Charushin VN, Chupakhin ON, Santra S, Adv. Synth. Catal 2019, 361, 2161; [Google Scholar]; i) Hyodo K, Nakamura S, Org. Biomol. Chem 2020, 18, 2781; [DOI] [PubMed] [Google Scholar]; j) McMurray L, McGuire TM, Howells RL, Synthesis 2020, 52, 1719. [Google Scholar]
- [12].a) Examples of decarboxylative Mannich reactions of acyclic and nonenolizable cyclic imines:van Tamelen EE, Baran JS, J. Am. Chem. Soc 1955, 77, 4944; [Google Scholar]; b) Ricci A, Pettersen D, Bernardi L, Fini F, Fochi M, Herrera RP, Sgarzani V, Adv. Synth. Catal 2007, 349, 1037; [Google Scholar]; c) Yin L, Kanai M, Shibasaki M, J. Am. Chem. Soc 2009, 131, 9610; [DOI] [PubMed] [Google Scholar]; d) Baudoux J, Lefebvre P, Legay R, Lasne M-C, Rouden J, Green Chem. 2010, 12, 252; [Google Scholar]; e) Abrecht S, Adam J-M, Bromberger U, Diodone R, Fettes A, Fischer R, Goeckel V, Hildbrand S, Moine G, Weber M, Org. Process Res. Dev 2011, 15, 503; [Google Scholar]; f) Pan Y, Kee CW, Jiang Z, Ma T, Zhao Y, Yang Y, Xue H, Tan C-H, Chem. Eur. J 2011, 17, 8363; [DOI] [PubMed] [Google Scholar]; g) Jiang C, Zhong F, Lu Y, Beilstein J. Org. Chem 2012, 8, 1279; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Yang C-F, Shen C, Wang J-Y, Tian S-K, Org. Lett 2012, 14, 3092; [DOI] [PubMed] [Google Scholar]; i) Yin L, Kanai M, Shibasaki M, Tetrahedron 2012, 68, 3497; [Google Scholar]; j) Böhm M, Proksch K, Mahrwald R, Eur. J. Org. Chem 2013, 2013, 1046; [Google Scholar]; k) Qian P, Dai Y, Mei H, Soloshonok VA, Han J, Pan Y, RSC Adv. 2015, 5, 26811; [Google Scholar]; l) Lahosa A, Yus M, Foubelo F, J. Org. Chem 2019, 84, 7331; [DOI] [PubMed] [Google Scholar]; m) Zhang Y, Li J-K, Zhang F-G, Ma J-A, J. Org. Chem 2020, 85, 5580; [DOI] [PubMed] [Google Scholar]; n) Zhang H, Jiang C, Tan J-P, Hu H-L, Chen Y, Ren X, Zhang H-S, Wang T, ACS Catal. 2020, 5698. [Google Scholar]
- [13].a) Examples of decarboxylative Mannich reactions of enolizable cyclic imines:Anet E, Hughes GK, Ritchie E, Nature 1949, 164, 501; [DOI] [PubMed] [Google Scholar]; b) Schöpf C, Benz G, Braun F, Hinkel H, Rokohl R, Angew. Chem 1953, 65, 161; [Google Scholar]; c) van Tamelen EE, Baran JS, J. Am. Chem. Soc 1956, 78, 2913; [Google Scholar]; d) Quick J, Oterson R, Synthesis 1976, 1976, 745; [Google Scholar]; e) Fukawa H, Terao Y, Achiwa K, Sekiya M, Chem. Lett 1982, 11, 231; [Google Scholar]; f) Zaidan RK, Evans P, Eur. J. Org. Chem 2019, 2019, 5354. [Google Scholar]
- [14].a) Examples of Mannich reactions of enolizable cyclic imines:Monaco MR, Renzi P, Scarpino Schietroma DM, Bella M, Org. Lett 2011, 13, 4546; [DOI] [PubMed] [Google Scholar]; b) Virk S, Pansare SV, Org. Lett 2019, 21, 5524. [DOI] [PubMed] [Google Scholar]
- [15].For the use of nitrones in decarboxylative Mannich reactions, see: Lisnyak VG, Lynch-Colameta T, Snyder SA, Angew. Chem. Int. Ed 2018, 57, 15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Presumably, the free β-keto acid is required in the alkylation step, with the corresponding lithium salt being less effective.
- [17].a) Selected methods for the synthesis of polycyclic dihydroquinolones involving C–H bond functionalization:Chen D-F, Han Z-Y, He Y-P, Yu J, Gong L-Z, Angew. Chem. Int. Ed 2012, 51, 12307; [DOI] [PubMed] [Google Scholar]; b) Zhang G, Wang S, Ma Y, Kong W, Wang R, Adv. Synth. Catal 2013, 355, 874; [Google Scholar]; c) Huo C, Wu M, Jia X, Xie H, Yuan Y, Tang J, J. Org. Chem 2014, 79, 9860. [DOI] [PubMed] [Google Scholar]
- [18].While full consumption of the amine starting materials was typically observed, a quantitative assessment of the mass balance is not yet possible. Competing imine-trimer formation cannot be ruled out at present and intractable polar byproducts are often formed. In case of α-substituted amine starting materials, small amounts of the regioisomeric imines were also observed. These species did not engage in the alkylation reaction and were readily separable from the target compounds.
- [19].a) Compère D, Marazano C, Das BC, J. Org. Chem 1999, 64, 4528; [Google Scholar]; b) Felpin F-X, Lebreton J, J. Org. Chem 2002, 67, 9192; [DOI] [PubMed] [Google Scholar]; c) Zheng G, Dwoskin LP, Crooks PA, J. Org. Chem 2004, 69, 8514; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ryan J, Šiaučiulis M, Gomm A, Maciá B, O’Reilly E, Caprio V, J. Am. Chem. Soc 2016, 138, 15798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


