Abstract
Background/Purpose:
We sought to test select properties of a novel, expandable bioadhesive composite that allows for enhanced adhesion control in liquid environments.
Methods:
Rabbit fetuses (n=23) underwent surgical creation of spina bifida on gestational day 22–25 (term 32–33 days). Defects were immediately covered with a two-component tough adhesive consisting of a hydrogel made of a double network of ionically crosslinked alginate and covalently crosslinked polyacrylamide linked to a bridging chitosan polymer adhesive. Animals were euthanized prior to term for different analyses, including hydraulic pressure testing.
Results:
Hydrogels remained adherent in 70% (16/23) of the recovered fetuses and in all of the last 14 fetuses as the procedure was optimized. Adherent hydrogels showed a median two-fold (IQR:1.7–2.4) increase in area at euthanasia, with defect coverage confirmed by ultrasound and histology. The median maximum pressure to repair failure was 15mmHg (IQR:7.8–55.3), exceeding reported neonatal cerebrospinal fluid pressures.
Conclusions:
This novel bioadhesive composite allows for selective, stable attachment of an alginate-polyacrylamide hydrogel to specific areas of the spina bifida defect in a fetal rabbit model, while the hydrogel expands with the defect over time. It could become a valuable alternative for the prenatal repair of spina bifida and possibly other congenital anomalies.
Keywords: Bioadhesive, Tough adhesive, Biocomposite, Wound repair, Surgical coverage, Fetal surgery
1. Introduction
The sub-optimal characteristics of biological grafts or synthetic materials available for repair of certain tissue defects limit the ability to achieve long-term coverage. Specific procedural needs may also pose additional limitations. In this study, we investigated a recently described bioinert, inordinately tough composite bioadhesive as a means to overcome some of these obstacles [1]. This material is biologically-derived and mimics certain features of the highly adherent mucus secreted by Arion subfuscus, a terrestrial pulmonate gastropod mollusk of the Arionidae family. It consists of a dual interpenetrating network of ions, proteins, and sugars that provide mechanical toughness and adhesive strength [2–5]. It also includes energy-dissipating alginate hydrogels combined with highly elastic covalently crosslinked acrylamide gels to generate a tough hydrogel network with robust mechanical properties and unprecedented adhesion [6].
While this material could potentially have various applications, in this study we specifically tested our hypothesis that it could overcome the peculiar challenges posed by fetal tissue repair, such as continuous exposure to amniotic fluid and need for defect expansion over time. More specifically, we chose a fetal spina bifida model, in which the prevention of tethering of the spinal cord to the covering patch is an additional requirement.
2. Methods
This study was approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee under protocol #19–03–3876R.
2.1. Composite Bioadhesive Structure and Stability Testing
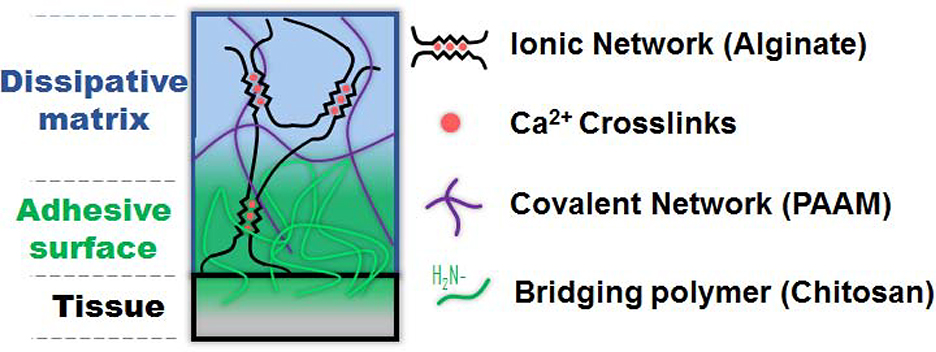
The two-component bioadhesive used consisted of a tough hydrogel made of a double network of ionically crosslinked alginate combined with a covalently crosslinked polyacrylamide (PAAM), with a bridging chitosan polymer acting as the adhesive [1] (Figure 1). This “system” allows for controlled adhesion of the composite exclusively where the hydrogel, adhesive, and tissue contact each other. Adhesion occurs through a combination of electrostatic interactions, covalent bonds, and physical interpenetration conferring an enhanced degree of adhesion control. In spina bifida, this allows for adhesion solely at the periphery of the defect, reducing the risk of cord tethering.
Figure 1:
Diagram depicting the composite adhesive system as it is applied to fetal tissue. The top component represents the hydrogel made of a dissipative matrix consisting of ionically (calcium-based) crosslinked alginate and covalently crosslinked polyacrylamide (PAAM). The middle layer represents the bridging chitosan polymer adhesive.
To assess hydrogel stability in vitro, the hydrogels were punched into disks (diameter = 8mm, thickness = 1.5mm), which were washed briefly in Hank’s Balanced Salt Solution containing 1.5mM CaCl2 (Gibco, Thermofisher Scientific, Waltham, MA) for 30 minutes followed by submersion in commercially available human amniotic fluid (ZenBio, Research Triangle Park, NC). Hydrogel samples were incubated within the amniotic fluid in a sealed environment at 37°C for 7 days. After incubation, samples were washed briefly in water, frozen at −20°C, and lyophilized. After lyophilization, specimen dry weights were recorded and compared to the dry weights of samples that had not been submerged in amniotic fluid to assess for hydrogel degradation after amniotic fluid exposure. Tested samples were not used for the in vivo experiments.
2.2. Surgical Spina Bifida Defect Creation and Coverage
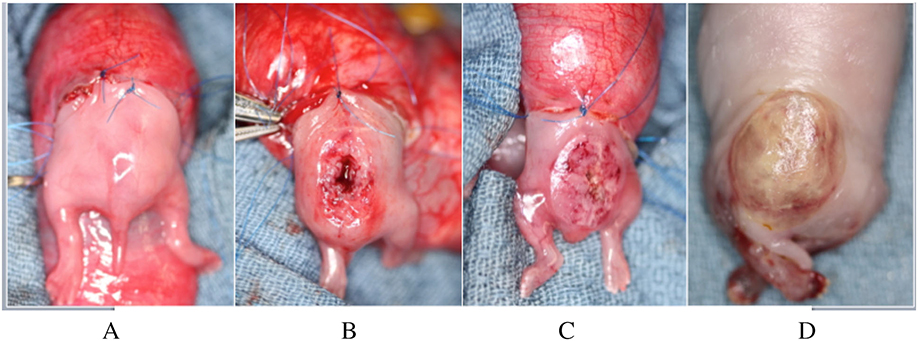
Time-dated pregnant New Zealand does were housed individually under standard light/dark cycling conditions and fed a normal diet ad libitum. Surgery was performed on gestational day 22–25 (E22–25; term= E32–33). They were anesthetized with 1.0–5.0% isoflurane (Abbot Laboratories, North Chicago, IL) in 60–100% oxygen and given 20mg/kg of intravenous cefazolin prior to the incision. Under standard sterile technique, a midline laparotomy was performed and the bicornuate uterus was exposed. Depending upon the total number of fetuses present per uterine horn, 1–2 fetuses per doe at the most distal aspect of either horn underwent surgical creation of a spina bifida defect. First, a purse-string was placed through the myometrium and gestational membranes using 5–0 Prolene suture (Ethicon, Somerville, NJ); a hysterotomy was then made within the area of the purse-string. Both the hind limbs and rump of the fetus were exteriorized such that the dorsal lumbosacral spine was accessible through the hysterotomy while the remainder of the fetus remained inside the amniotic cavity with no tension on the umbilical cord (Figure 2A–C). A spina bifida defect was created as previously described [7]. Briefly, a midline incision was performed over the lumbar area to divide the fetal skin and paraspinal muscles, which were sharply resected to expose the underlying lumbar vertebrae. A four-level lumbar laminectomy was performed beginning uniformly at the level of the fetal pelvic crest, resulting in exposure of the spinal cord (Figure 2B). The dura mater was not uniformly opened.
Figure 2:
Gross views of: A) the exposed hindlimbs and rump of a rabbit fetus prior to creation of a spina bifida defect; B) completed spina bifida defect; C) completed application of the composite adhesive system covering the defect; D) adherent composite covering the spina bifida defect after euthanasia.
After its creation, the defect was immediately covered with the dual-component tough bioadhesive system (Figure 2C). The volume of chitosan polymer applied was increased in the last 14 fetal procedures, in order to optimize adhesion of the composite. Uniform pressure was steadily applied onto the composite for seven minutes to ensure firm adhesion. This time was a conservative estimate based upon previous data showing a direct relationship between adhesion energy and duration of initial pressure application [1]; it is possible that lower application times are safe, which could be assessed in future experiments. The size of the hydrogel in two dimensions (length and width) was measured at time of placement and its area was calculated using the formula for area in ellipses. The fetus was returned to the amniotic cavity. The purse-string was tied to close the hysterotomy. The uterus was returned to the abdomen, and the laparotomy was closed in three layers. Does were allowed to recover and proceed to term.
2.3. Defect Coverage Analyses
All does were euthanized prior to term at post-operative day 5–8 using Fatal-Plus™ solution (Vortech, Dearborn, MI). The fetuses were procured via a post-mortem c-section. Fetuses with evidence of recent demise were also obtained for testing. Persistent adhesion of the hydrogel to the spina bifida defect, or lack thereof, was examined by gross inspection. The area of adherent hydrogels was calculated again as described above.
A subset of fetuses that had not undergone surgical defect creation in utero was also procured at time of euthanasia to serve as a comparison group for ultrasound analysis and mechanical testing. These subjects, referred to as “time-zero” fetuses (as opposed to the “operated” fetuses described above), underwent post-euthanasia creation of a spina bifida defect with identical composite patch placement as described intra-operatively, immediately followed by ultrasound and mechanical testing.
Ultrasound screening of defect coverage was performed with a high-frequency 3-D ultrasound machine (Vevo3100, Visualsonics, Toronto, Canada). Hydraulic pressure testing was performed via a 22-gauge needle inserted into the space between the exposed spinal cord and the hydrogel covering it. Semi-rigid tubing connected the needle to a syringe pump (PHD 2000 Dual Syringe Pump, Harvard Apparatus, Holliston, MA), which applied increasing pressure into the defect cavity by pumping saline at a rate of 2 mL/min while pressure was recorded as a function of time. The maximum pressure to repair failure, defined as hydrogel leak/detachment, rupture, or dissection of the pumped saline into the surrounding cutaneous tissue, was recorded for each animal.
A subset of operated fetuses with adherent hydrogels at time of euthanasia underwent histological analysis. Specimens were fixed in 10% neutral buffered formalin at room temperature for at least 72 hours. Histology was performed on fixed paraffin-embedded specimens stained with hematoxylin–eosin (H&E).
2.4. Statistical Analysis
Hydrogel dry weights in vitro were found to have a normal distribution using the Shapiro-Wilk normality test. A Student’s t-test was therefore used to compare the dry weights of hydrogels with and without amniotic fluid exposure. Hydraulic pressure data were found not to have a normal distribution on the Shapiro-Wilk test. Therefore, non-parametric Mann-Whitney U testing was performed to compare these results between operated and time-zero fetuses, as well as between recently demised and viable operated fetuses. Mann-Whitney U testing was also used to compare the differences in hydrogel area from the time of surgery to euthanasia. A p-value threshold of ≤0.05 was used to establish statistical significance. Statistical analysis was performed using SPSS version 24.0 (IBM Corporation, Armonk, NY).
3. Results
The median hydrogel dry weight one week after amniotic fluid exposure in vitro was 14.3mg (IQR: 13.9–15.2) compared to 15.6mg (IQR: 15.6–15.7) in hydrogels without amniotic fluid exposure. This difference was not statistically significant (p=0.06), although only 5 samples in each exposure group underwent that comparison.
There were no maternal complications post-operatively. However, 2/16 does delivered 4 operated fetuses vaginally one day before term on E31. Overall, 16/23 (69.6%) of the fetuses with spina bifida had hydrogels that were still adherent at procurement (Figure 2D). None of the 4 operated fetuses delivered vaginally had adherent hydrogels after delivery. Therefore, the sustained adhesion rate in the animals undergoing procurement via c-section was 84.2% (16/19). After optimization of the procedure with a higher volume of chitosan adhesive applied, 100% (14/14) of the operated fetuses had adherent hydrogels at euthanasia. Tethering of the spinal cord to the covering patch was not grossly observed in any animal.
Dimensional hydrogel measurements were performed in 15 fetuses with adherent hydrogels at euthanasia. The median area of the implanted hydrogel patch at time of defect creation was 117.8 mm2 (IQR: 91.9–141.4). At euthanasia, the median area of the implanted hydrogel patch was 268.6 mm2 (IQR: 183.8–311.0), showing a median 2.0-fold (IQR: 1.7–2.4) increase after implantation. There was no significant difference in the increase in hydrogel area between viable and recently demised fetuses (p=1.0).
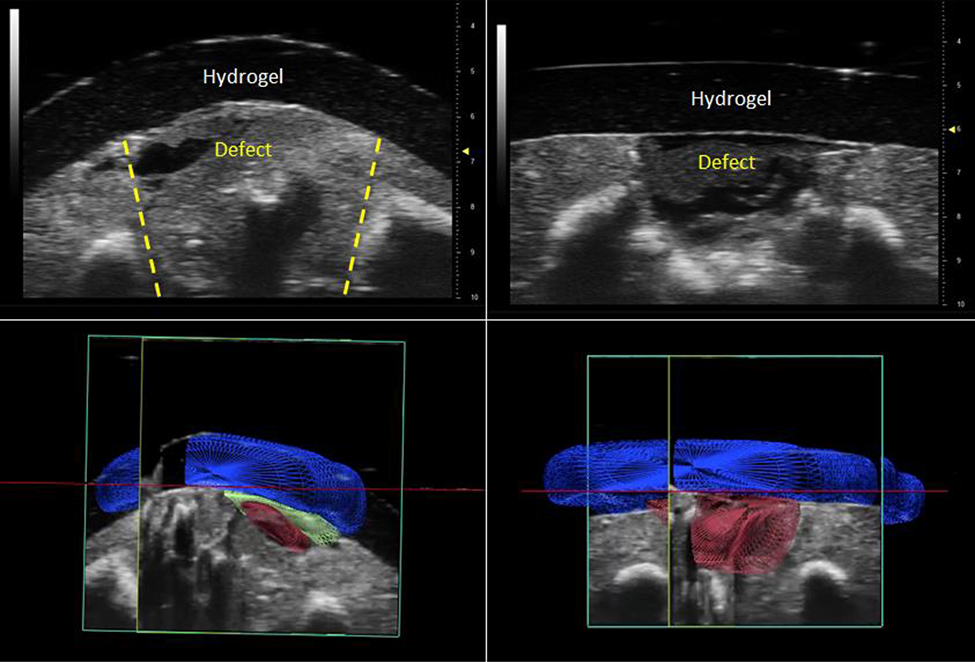
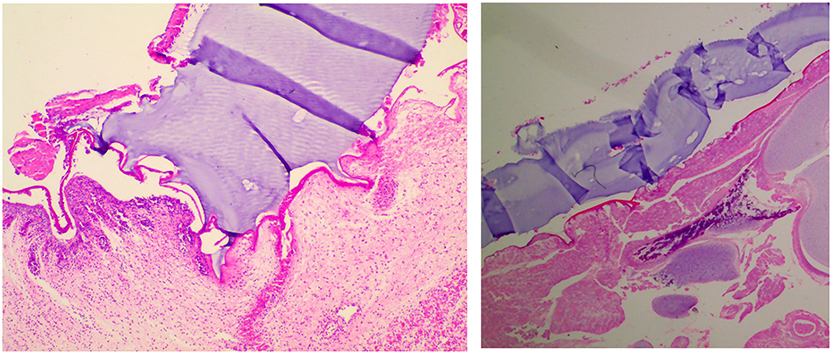
Defect coverage with an adherent hydrogel was confirmed using high-frequency ultrasound in 4 operated fetuses and 4 time-zero fetuses (Figure 3). Histological analysis was performed in 7 operated fetuses showing the adherent hydrogels appearing to interdigitate into the surrounding host tissue, yet not tethering to the spinal cord (Figure 4). In select histology samples, a layer of thin, rudimentary neoskin was noticed partially covering the defect underneath the hydrogel, apparently originating from adjacent normal skin.
Figure 3:
High-frequency ultrasound was used to demonstrate adhesion between the hydrogel and fetal tissue in both operated (2D: upper left; 3D: lower left) and time-zero (2D: upper right, 3D: lower right) fetuses after euthanasia. In the 3D ultrasound images, 3D renderings of different structures were created based upon relative echogenicity as follows: the hypoechoic hydrogel is rendered in blue, the hypoechoic defect is rendered in red, and a layer of hyperechoic tissue interface between the hydrogel and defect is rendered in green.
Figure 4:
Representative histological images showing: (left) integration of the hydrogel (purple), chitosan bioadhesive (bright pink), and fetal tissue, with interdigitation of the hydrogel-adhesive composite into the fetal tissue; (right) lack of adhesion of the hydrogel to the underlying flattened spinal cord typical of spina bifida. (H&E, 100x).
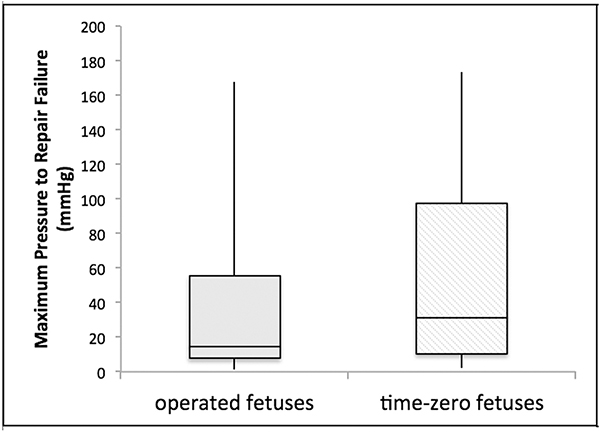
Hydraulic pressure testing could be performed in 12 operated fetuses with adherent hydrogels at time of euthanasia and in 10 time-zero fetuses. The results are summarized in Figure 5. The median maximal pressure to repair failure was 15.0 mmHg (IQR: 7.8–55.3) in operated fetuses and 31.2 mmHg (IQR: 10.4–97.4) in time-zero fetuses. These differences were not statistically significant (p=0.5). Within the operated fetus cohort, there was no significant difference between viable (n=5; median 41.8 mmHg; IQR 12.2–106.0) and recently demised fetuses (n=7; median 10.1 mmHg; IQR: 2.5–24.1) (p=0.2).
Figure 5:
Maximum pressure to repair failure on hydraulic testing in operated (median 15.0 mmHg; IQR: 7.8–55.3) and time-zero (median 31.2 mmHg; IQR: 10.4–97.4) fetuses. These differences were not statistically significant (p=0.5).
4. Discussion
The fetal environment is among the most demanding for preservation of non-primary tissue coverage. The repaired area grows rapidly and is constantly bathed by a liquid largely derived from urine. In this study, we covered a prenatal spina bifida defect with a tough composite bioadhesive that proved stable over time within amniotic fluid both in vitro and in vivo. Tissue adhesion was maintained throughout gestation in the majority of operated fetuses and in all animals after optimization of the bridging chitosan polymer application. Our inclusion of recently demised animals in the analyses had the potential to negatively bias the results, yet it did not, suggesting a strong chemical adhesion between the components of the composite and even “dead” tissue. Furthermore, the median maximum pressures to repair failure that we observed exceeded reported cerebrospinal fluid (CSF) pressures both in rabbit (range: 0.7–3.2 mmHg) [8] and human (range: 0–5.7 mmHg) [9] neonates. This suggests that the adherent composite could potentially withstand physiologic CSF pressures, thus reducing the risk of a CSF leak and the associated pervasive Chiari-II malformation [10, 11]. Future studies should further investigate if this bioadhesive is able to reduce rates of hindbrain herniation, which was not a goal of the present work.
Our decision to use this particular material was informed by previous studies in other applications. In postnatal small and large animal models, this composite has been shown to support adhesion energies up to 200 mmHg to heart, skin, and liver tissue despite the presence of blood products and dynamic movement, while maintaining good tissue biocompatibility [1]. Based on these unique properties, there are a variety of other potential clinical applications for this composite, for example in the containment of hollow viscous leaks/fistulas and as an adjuvant hemostatic agent. The latter has already been described in a rodent liver laceration model [1]. Furthermore, previous data have demonstrated excellent biocompatibility with no direct effects on cell viability and minimal inflammatory response [1, 12, 13]. Although the biocompatibility of this composite within the fetal environment was not directly evaluated in this study, we saw no evidence of inflammation on histology. Further investigation of that aspect in the fetal setting will be justified at eventual pre-clinical stages.
In addition, when compared to single-component materials previously reported for the prenatal repair of experimental spina bifida which are applied unselectively to the entire defect, such as reverse thermal gels or blended polymer films [14, 15], there are inherent advantages to the present hydrogel’s dual-component architecture. Specifically, adhesion occurs exclusively where the hydrogel, chitosan polymer, and tissue contact, allowing for a targeted and controlled tissue bond. In our case, this was at the periphery of the spina bifida defect. Further, the hydrogel component is otherwise biologically inert and does not interact with tissue in the absence of the chitosan adhesive. This feature lowers the risk of tethering of the exposed spinal cord to the overlying hydrogel, which is known to worsen neurological outcomes [16]. While spinal cord tethering was not a pre-defined outcome measure in this study, there was no obvious gross evidence of cord tethering after euthanasia in any specimen.
We included a control group of “time-zero” fetuses submitted to hydraulic pressure testing in order to examine whether both the adhesive and mechanical properties of the composite would show evidence of deterioration over time in vivo, despite the short gestational time in rabbits. Our results showing no significant difference in median maximum pressure to repair failure in these two groups suggest these properties were stable over time. Nonetheless, the interpretation of these mechanical results was limited by the sizeable variation within both groups. This variation may have reflected differences in the types of repair failure observed, such as hydrogel leak/detachment at its interface with the host tissue or dissection of the pumped saline into the surrounding cutaneous tissue. Of note, none of the hydrogels sustained failure in the form of structural rupture or complete detachment from the underlying tissue, further supporting the strength, stability, and durability of its adhesion properties.
On histology, a layer of thin, rudimentary skin appearing to derive from normal skin at the periphery of the defect was visible underneath the hydrogel in a few specimens. Neo-tissue formation suggests that the adherent hydrogels may promote some degree of wound healing, although this was not systematically investigated. This is consistent with prior studies using other patch systems that also showed the development of neo-tissue under cellulose films and silicone-based patches [17, 18].
While this composite bioadhesive was delivered exclusively via open surgery in this study, a videofetoscopic approach seems well within the realm of possibilities, with only minor modifications required to introduce each component into the uterus. The hydrogel component is very pliable and highly resistant to tear, which would allow for it to be rolled around an instrument and inserted via a port site into the uterus. The chitosan, already in liquid form, could also be delivered via injection through a port site to the periphery of the defect.
Although the rabbit is considered a large animal model, it cannot replicate all the biomechanical forces inherent to larger models such as sheep, or those present in human fetuses. In addition, the rabbit model is limited by a short gestation (32–33 days) and our inability to reliably survive the animals postnatally, hindering longer-term evaluation of composite stability and adhesion, as well as neurological outcomes. However, previous studies of this composite in other applications suggest long-term preservation of the bioadhesive toughness for up to 16 weeks (unpublished data, manuscript under review). Studies using larger animal models with longer gestation, such as sheep, would be required prior to eventual clinical translation. Nonetheless, this study was a necessary step to determine whether further scrutiny of this strategy aiming at eventual clinical application is warranted. The present data would indicate that it is.
Despite these considerations, we conclude that this novel composite bioadhesive allows for selective and stable attachment of an alginate-polyacrylamide hydrogel matrix to specific areas of the spina bifida defect in a fetal rabbit model, while the matrix expands with the defect over time. It could become a valuable alternative for the prenatal repair of spina bifida and possibly other congenital anomalies.
Acknowledgement
This work was funded by the Kevin and Kate McCarey Fund for Surgical Research at Boston Children’s Hospital, the National Institute on Aging of the NIH (F32AG057135) and The Wyss Institute for Biologically Inspired Engineering at Harvard University. The funding sources had no role in study design, data collection or analysis, writing of this report, or the decision to submit this article for publication.
Footnotes
Declarations of Interest
There are two issued US patents (9,387,276 and 10,383,980) and pending patent applications for this composite, on which one or two of the authors are inventors.
Type of Study: N/A (Animal and Laboratory Study)
Level of Evidence: N/A (Animal and Laboratory Study)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li J, Celiz AD, Yang J, et al. Tough adhesives for diverse wet surfaces. Science 2017;357 (6349):378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun M, Menges M, Opoku F, et al. The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue. J Exp Biol 2013;216 (Pt 8):1475–83. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw A, Salt M, Bell A, et al. Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs. J Exp Biol 2011;214 (Pt 10):1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlicki JM, Pease LB, Pierce CM, et al. The effect of molluscan glue proteins on gel mechanics. J Exp Biol 2004;207 (Pt 7):1127–35. [DOI] [PubMed] [Google Scholar]
- 5.Wilks AM, Rabice SR, Garbacz HS, et al. Double-network gels and the toughness of terrestrial slug glue. J Exp Biol 2015;218 (Pt 19):3128–37. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter 2014;10 (5):672–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Housley HT, Graf JL, Lipshultz GS, et al. Creation of myelomeningocele in the fetal rabbit. Fetal Diagn Ther 2000;15 (5):275–9. [DOI] [PubMed] [Google Scholar]
- 8.Greene CS, Jr., Lorenzo AV, Hornig G, et al. The lowering of cerebral spinal fluid and brain interstitial pressure of preterm and term rabbits by furosemide. Z Kinderchir 1985;40 Suppl 1 5–8. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser AM and Whitelaw AG. Normal cerebrospinal fluid pressure in the newborn. Neuropediatrics 1986;17 (2):100–2. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard S, Davey MG, Rintoul NE, et al. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg 2003;38 (3):451–8; discussion 451–8. [DOI] [PubMed] [Google Scholar]
- 11.Danzer E, Finkel RS, Rintoul NE, et al. Reversal of hindbrain herniation after maternal-fetal surgery for myelomeningocele subsequently impacts on brain stem function. Neuropediatrics 2008;39 (6):359–62. [DOI] [PubMed] [Google Scholar]
- 12.Blacklow SO, Li J, Freedman BR, et al. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci Adv 2019;5 (7):eaaw3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darnell MC, Sun JY, Mehta M, et al. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013;34 (33):8042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardill J, Williams SM, Shabeka U, et al. An Injectable Reverse Thermal Gel for Minimally Invasive Coverage of Mouse Myelomeningocele. J Surg Res 2019;235 227–236. [DOI] [PubMed] [Google Scholar]
- 15.Tatu R, Oria M, Pulliam S, et al. Using poly(l-lactic acid) and poly(varepsilon-caprolactone) blends to fabricate self-expanding, watertight and biodegradable surgical patches for potential fetoscopic myelomeningocele repair. J Biomed Mater Res B Appl Biomater 2019;107 (2):295–305. [DOI] [PubMed] [Google Scholar]
- 16.Bauer DF, Beier AD, Nikas DC, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on the Management of Patients With Myelomeningocele: Whether Prenatal or Postnatal Closure Affects Future Ambulatory Status. Neurosurgery 2019;85 (3):E409–e411. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez e Oliveira Rde C, Valente PR, Abou-Jamra RC, et al. Biosynthetic cellulose induces the formation of a neoduramater following pre-natal correction of meningomyelocele in fetal sheep. Acta Cir Bras 2007;22 (3):174–81. [DOI] [PubMed] [Google Scholar]
- 18.Fontecha CG, Peiro JL, Aguirre M, et al. Inert patch with bioadhesive for gentle fetal surgery of myelomeningocele in a sheep model. Eur J Obstet Gynecol Reprod Biol 2009;146 (2):174–9. [DOI] [PubMed] [Google Scholar]