Abstract
The presence of a characteristic chimeric fusion as the initiating genomic event is one defining feature of Spitz neoplasms. Characterization of specific subtypes of Spitz neoplasms allows for better recognition facilitating diagnosis. Data on clinical outcomes of the specific tumor types may help in predicting behavior. In this study we present the largest series to date on ROS1 fusion Spitz neoplasms. We present the clinical, morphologic and genomic features of 17 cases. We compared the morphologic features of these 17 cases to a cohort of 99 other non-ROS1 Spitz neoplasms to assess for features that may have high specificity for ROS1 fusions. These tumors consisted of 10 Spitz nevi and 7 Spitz tumors. None of the cases met criteria for a diagnosis of Spitz melanoma. Morphologically, the ROS1 fusion tumors of this series were characterized by a plaque-like or nodular silhouette, often densely cellular intraepidermal melanocyte proliferation, frequent pagetosis, tendency towards spindle cell cytomorphology, low grade nuclear atypia and floating nests with occasional transepidermal elimination. However, there was a significant range in microscopic appearances, including two cases with morphologic features of a desmoplastic Spitz nevus. Different binding partners to ROS1 were identified with PWWP2A and TPM3 being the most common. No case had a recurrence or metastasis. Our findings document that most ROS1 fusion Spitz neoplasms have some typical characteristic microscopic features, while a small proportion will have features overlapping with other genomic subtypes of Spitz neoplasms. Preliminary evidence suggests that they tend to be indolent or low grade neoplasms.
INTRODUCTION
The family of Spitz neoplasms is defined in the most recent edition of the World Health Organization Classification of Skin Tumors (4th edition) as a melanocytic neoplasm with a characteristic Spitz fusion or a mutation in HRAS with Spitzoid morphologic features. Recent studies have attempted to correlate specific clinical and morphologic findings in the various fusion subgroups such as ALK , NTRK1, NTRK3,MAPK, BRAF and ROS1.1–16 Genomic fusions involving the ROS1 oncogene are seen in 7 to 17% of Spitz neoplasms.17, 18 However, thus far only one study of 6 cases has described the morphologic features of ROS1 Spitz neoplasms.13
In this study, we report the clinical, histologic and molecular findings in 17 ROS1 fusion Spitz neoplasms in order to better characterize this subset of Spitz neoplasms. We compared a number of morphologic features in this set of ROS1 fusions to a control set of 99 non-ROS1 Spitz melanocytic neoplasms which have also been assessed by next generation sequencing (NGS). We describe characteristic morphologic features and report those morphologic features statistically more frequent in ROS1 Spitz compared to other subtypes of Spitz neoplasms. We also report for the first time the occurrence of ROS1 fusions in two cases of desmoplastic Spitz nevi.
MATERIALS AND METHODS
Case Selection and Genomic Sequencing
Study approval and waiver of consent for use of archived tissue were obtained through the Northwestern Institutional Review Board. The dermatopathology data base at Northwestern was searched for Spitz nevi (SN), atypical Spitz tumor (AST), and Spitz melanomas (SM) in which a ROS1 fusion was identified by NGS. We identified 8 cases matching the above criteria. The paired normal tissue were identified for Case #1, #2, #5 and #6. Additionally nine cases were contributed from the personal consultation files of KJ Busam at Memorial Sloan Kettering Cancer Center in New York. We also identified 99 cases consisting of 20 SN, 53 ST, and 26 SM. Each diagnosis was made at the time of clinical presentation based on morphology with incorporation of FISH or array CGH in select cases. The control group included 59 fusions consisting of the following genes: ALK (n = 14), MAP3K8 (n = 12), BRAF (n = 6), NTRK1 (n = 10), NTRK3 (n = 6), RET (n = 4), MET (n = 1), RASGRF (n = 1), RAF1 (n = 1), MAP3K3 (n = 1), FGFR (n = 1), ERBB4 (n = 1), PRKDC (n = 1). Additionally, there were 5 MAP3K8 truncations. Lastly there were mutations in 19 cases in the following genes: BRAF (n = 8), NRAS (n = 4), HRAS (n = 5), GNAQ (n = 1), ROS1 (n = 1). In 16 cases no known fusions or mutations were identified.
“Spitzoid” morphology was identified according to the World Health Organization Classification of Skin Tumors (4th edition) and other relevant literature. 19–22 NGS with a 1171 cancer related gene panel for DNA and a whole transcriptome sequencing on each case was performed with using the Tempus xO platform and variant-calling. 23, 24 The 1711-gene assay is validated and designed to target therapeutically actionable genes.
Tumor Classification and Clinicopathologic Features
In total there were 17 cases with ROS1 fusions. The clinical features including age, sex and site of the tumors were summarized from the medical record. Morphologic features were assessed by two board certified dermatopathologist experienced in the assessment of melanocytic tumors. The following morphologic features were evaluated: silhouette (plaque, wedge or nodular), cytology (epithelioid, spindled or both), nuclear atypia (mild, moderate or severe), pigmentation (absent, focal, or extensive), host inflammatory reaction (absent, non-brisk, or brisk), cell size (small, intermediate, large), mitotic figures per mm2, and for the absence or presence of Kamino body, maturation, ulceration, epidermal hyperplasia, plexiform growth, epithelioid sheets, pagetosis, nesting in the adnexa, and desmoplasia.
Mild nuclear atypia was defined as a slightly larger nucleus than conventional nevomelanocytes. Moderate atypia was defined as a nuclear size similar to the size of keratinocytes with a hyperchromatic nuclear membrane, visible nucleolus, and variable chromatin quality. Severe nuclear atypia was defined as a nuclear size larger than keratinocytes with a hyperchromatic nuclear membrane, prominent and/or multiple nucleoli, and coarse chromatin. For host inflammatory reaction, a brisk response was defined as a diffuse infiltration of lymphocytes across the entire base of the tumor; a non-brisk response was defined as a focal infiltration of lymphocytes that does not cover the entire base.25 For cell size, the size of melanocytes was compared to the basal keratinocytes.26 Cells about the size of basal keratinocytes were considered small, those moderately larger than basal keratinocytes were intermediate in size and cells nearly twice the size of basal keratinocytes were considered large. Clinical information including age, gender and site of tumor was also included for analysis.
Statistical Analysis
All statistical analyses were performed in R Studio v1.2.5001 to compare morphologic features across the groups Spitz neoplasms. Fisher’s exact test or Chi square test was used to compare associations in categorical variables. Student’s t-test was used to compare mean values. A p value of < 0.05 was considered statistically significant. All tests were two-sided.
Data Availability
Data Availability
Processed sequencing data (vcf files and count files) can be found through GEO Series accession number GSE142443 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142443).
RESULTS
Clinical Findings in ROS1 Fusion Spitz Neoplasms
The final diagnosis from the time of clinical care in the set of 17 ROS1 Spitz neoplasms was Spitz nevus in 10 cases and Spitz tumor in 7 cases. In none of the cases was a diagnosis of Spitz melanoma favored. The patient ages ranged from 3 to 58 with a mean age of 19 years old. There were 10 female and 7 male patients. The body site of involvement was highly variable with 4 in the head/neck region, 3 on the upper extremities, 3 on the trunk and 7 on the lower extremities. Grossly, all cases were pink to red papules. In 14 cases the clinical impression was available. In 7 cases the clinician suspected an atypical Spitz nevus and in one of these cases a dermoscopic description of radial streaming was provided. In 2 cases the clinical impression was dermatofibroma, in 2 cases it was benign nevus, in 2 cases it was pyogenic granuloma and in 1 case it was cyst.
Follow up was available for 13 of 17 cases (Table 1). The average follow up time was 23 months and ranged from 4 months to 95 months. In 12 cases the lesions were re-excised with clear margins with no evidence of recurrence. One of these 12 cases also had a sentinel lymph node biopsy (SLNB) which was negative. In one case the original biopsy was incisional and no further re-excision was performed. There was persistent tumor at a follow exam 4 months later.
Table 1.
Summary of clinical data in 16 cases of Spitz neoplasms with ROS1 fusions
| Case | Age | Gender | Location | Diagnosis | Clinical impression | Surgical Treatment | SLNB | Metastasis | Follow up | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | Right lower medial leg | Atypical Spitz tumor | Rule out atypical nevus vs Spitz nevus vs malignant melanoma; 6 x 4 mm color variated pink brown papule with radial streaming pattern at edges | Complete excision | No | No | 24 months | No |
| 2 | 37 | F | Left anterior medial thigh | Atypical Spitz tumor | 5.5 mm erythematous papule; dermatofibroma – check margins | Complete excision | No | No | 95 months | No |
| 3 | 28 | F | Left thigh | Atypical Spitz tumor | Cyst | Complete excision | No | No | 64 months | No |
| 4 | 13 | F | Left upper arm | Spitz nevus | Re-excision; rule out Spitz nevus | Complete excision | Not available | Not available | Not available | Not available |
| 5 | 20 | F | Left buttock | Spitz nevus | Intradermal nevus, rule out atypa | None | No | No | Not available | Not available |
| 6 | 6 | M | Right buttock | Spitz nevus | Changing nevus, rule out Spitz nevus | Complete excision | No | No | 35 months | No |
| 7 | 36 | F | Left shin | Atypical Spitz tumor | Melanocytic lesion, rule out atypical Spitz tumor | Not available | Not available | Not available | Not available | Not available |
| 8 | 12 | F | Right ear | Atypical Spitz tumor | None provided | Incisional biopsy without further re-excision | No | No | 4 months | Persistent tumor 4 months later |
| 9 | 15 | M | Left neck | Spitz nevus | Rule out Spitz nevus | Complete excision | No | No | 15 months | No |
| 10 | 15 | M | Right upper arm | Atypical Spitz tumor | Pyogenic granuloma versus hypertrophic scar | Complete excision | No | No | 11 months | No |
| 11 | 9 | M | Right ear | Spitz nevus | Nevus, rule out atypia | Complete excision | No | No | 10 months | No |
| 12 | 18 | M | Left mid back | Spitz nevus | Spitz nevus | Complete excision | No | No | 9 months | No |
| 13 | 17 | M | Left upper back | Desmoplastic Spitz nevus | Dermatofibroma | Complete excision | No | No | 5 months | No |
| 14 | 3 | M | Left ear | Spitz nevus | Rule out pyogenic granuloma | Complete excision | No | No | 13 months | No |
| 15 | 4 | F | Left knee | Spitz nevus | Rule out Spitz nevus | Complete excision | No | No | 13 months | No |
| 16 | 58 | F | Left arm | Atypical Spitz tumor | None provided | Complete excision | Yes, negative | No | 6 months | No |
| 17 | 6 | F | Abdomen | Desmoplastic Spitz nevus | None provided | Complete excision | No | No | Not available | No |
SLNB, sentinel lymph node biopsy; F, female; M, male;
Morphologic and Immunohistochemical Findings in ROS1 Fusions Spitz Neoplasms
The low power silhouette on the 16 ROS1 cases was mostly that of either a plaque like (n = 7) or nodular pattern (n = 7). Two cases had a wedge shaped silhouette and 1 was polypoid. In 12 cases the cytomorphology was a mixed pattern of epithelioid and spindle cells while in 4 cases there was a predominance of spindle cells. In all cases the atypia was mild or moderate with none of the cases having high grade nuclear atypia (P = 0.006) (Figure 1). This was statistically significant with ROS1 cases being less likely to have high grade nuclear atypia than the group of non-ROS1 Spitz neoplasms. The cell sizes were also all small to intermediate with none of the cases having large cells and this was also statistically significant (P = 0.001). Maturation was present in all cases and this was also statistically significant (P = 0.044). There was also a tendency for lower mitotic rate 1.3/mm2 (P = 0.001) (Table 2). Kamino bodies were also more common in this type of Spitz (8/17) than non-ROS1 Spitz (P = 0.025).
Figure 1).
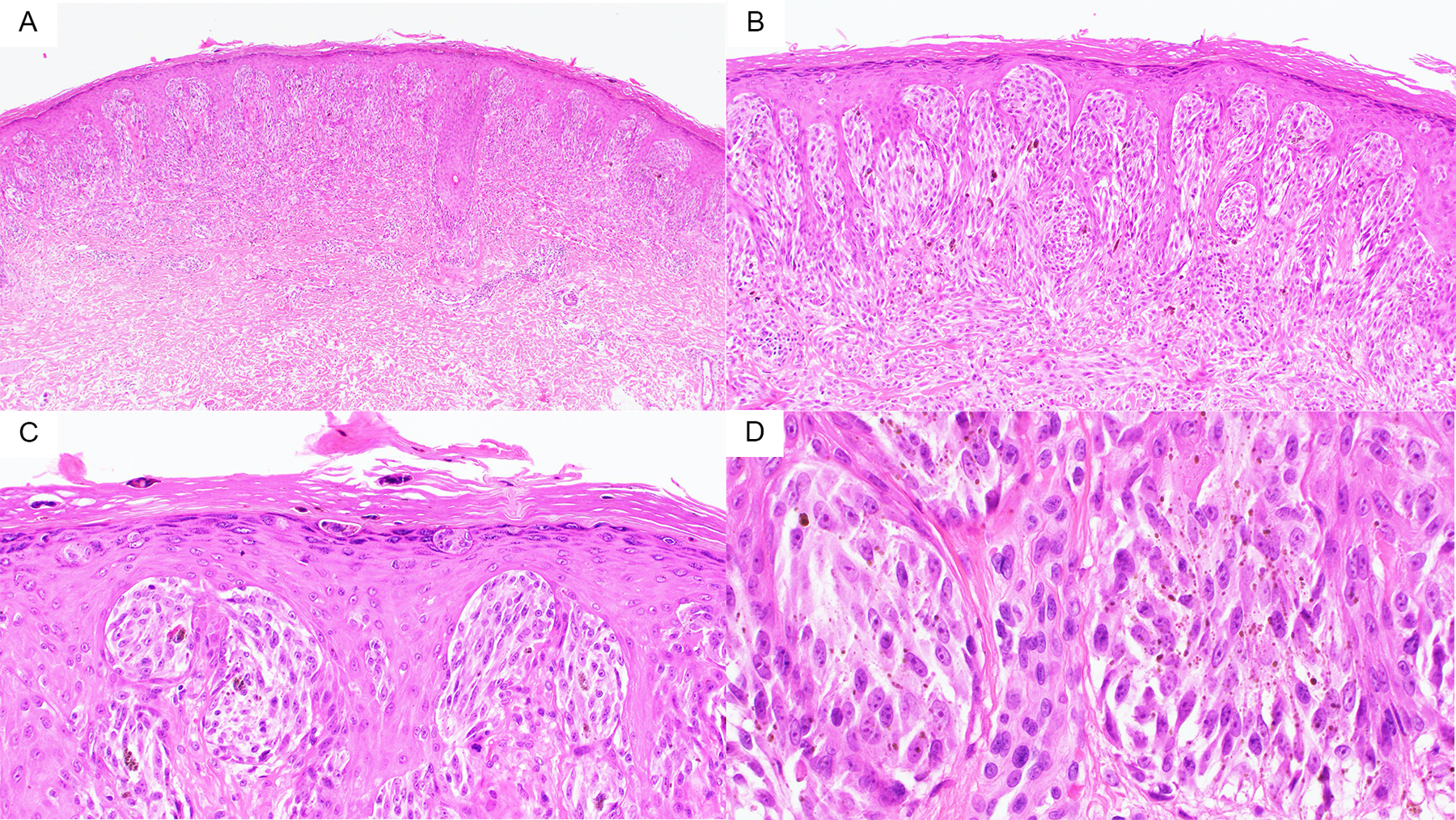
A) At 40X one can appreciate the plaque like silhouette of this ROS1 Fusion Spitz Neuvs B) At 100X the epidermal hyperplasia with a predominance of nests with spindle shaped melanocytes can be seen C) At 200X one can appreciate the transepidermal elimination of small nests into the stratum corneum. D) 400X demonstrates the Spitzoid cytomorphology with relatively low grade nuclear atypia.
Table 2.
Comparison of Clinical and Morphologic Findings in ROS1 and non-ROS1 fusion Spitz neoplasms
| All (n = 116) | Non-ROS1 vs ROS1 | |||
|---|---|---|---|---|
| Non-ROS1 (n = 99) | ROS1 (n = 17) | P | ||
| Clinical | ||||
| Age, years | 0.75 | |||
| Mean | 20.5 | 20.7 | 19.5 | |
| Range | 1–65 | 1–65 | 3–58 | |
| Gender | 0.80 | |||
| Female | 64 | 54 | 10 | |
| Male | 52 | 45 | 7 | |
| Location | 0.68 | |||
| Head/Neck | 23 | 19 | 4 | |
| Upper Extremity | 33 | 30 | 3 | |
| Trunk | 15 | 12 | 3 | |
| Lower Extremity | 45 | 38 | 7 | |
| Histologic | ||||
| Tumor Subtype | 0.003 | |||
| SN | 29 | 20 | 9 | |
| AST | 61 | 53 | 8 | |
| SM | 26 | 26 | 0 | |
| Tumor depth, mm | 0.32 | |||
| Mean | 2.04 | 1.88 | 2.93 | |
| Range | 0.25–17.0 | 0.25–12.2 | 0.40–17.0 | |
| Tumor Diameter, mm | 0.71 | |||
| Mean | 4.78 | 4.74 | 5.02 | |
| Range | 0.69–16.5 | 0.69–16.50 | 2.90–14.0 | |
| Silhouette | 0.16 | |||
| Plaque | 49 | 42 | 7 | |
| Wedge | 31 | 29 | 2 | |
| Nodular | 34 | 27 | 7 | |
| Polypoid | 2 | 1 | 1 | |
| Cytology | 0.17 | |||
| Epithelioid | 25 | 24 | 1 | |
| Spindled | 30 | 26 | 4 | |
| Both | 61 | 49 | 12 | |
| Nuclear Atypia | 0.006 | |||
| Mild | 11 | 10 | 1 | |
| Moderate | 74 | 58 | 16 | |
| Severe | 31 | 31 | 0 | |
| Kamino body | 0.025 | |||
| Absent | 89 | 80 | 9 | |
| Present | 27 | 19 | 8 | |
| Pigmentation | 0.10 | |||
| Absent | 66 | 52 | 14 | |
| Focal | 29 | 27 | 2 | |
| Extensive | 21 | 20 | 1 | |
| Maturation | 0.044 | |||
| Absent | 18 | 18 | 0 | |
| Partial | 26 | 19 | 7 | |
| Present | 72 | 62 | 10 | |
| Ulceration | 0.62 | |||
| Absent | 107 | 92 | 15 | |
| Present | 9 | 7 | 2 | |
| Inflammatory Reaction | 0.04 | |||
| Absent | 7 | 4 | 3 | |
| Non-Brisk | 68 | 57 | 11 | |
| Brisk | 41 | 38 | 3 | |
| Epidermal Hyperplasia | 0.49 | |||
| Absent | 20 | 16 | 4 | |
| Present | 96 | 83 | 13 | |
| Plexiform | 0.42 | |||
| Absent | 73 | 64 | 9 | |
| Present | 43 | 35 | 8 | |
| Epithelioid Sheet | 0.04 | |||
| Absent | 94 | 77 | 17 | |
| Present | 22 | 22 | 0 | |
| Pagetosis | 0.32 | |||
| Absent | 93 | 81 | 12 | |
| Present | 23 | 18 | 5 | |
| Cell Size | 0.001 | |||
| Small | 21 | 20 | 1 | |
| Intermediate | 65 | 49 | 16 | |
| Large | 30 | 30 | 0 | |
| Nesting Adnexa | 0.15 | |||
| Absent | 97 | 85 | 12 | |
| Present | 19 | 14 | 5 | |
| Lobulated Nests | 0.73 | |||
| Absent | 95 | 80 | 15 | |
| Present | 21 | 19 | 2 | |
| Mitotic index (per mm2) | 0.001 | |||
| Mean | 2.2 | 2.34 | 1.30 | |
| Range | 0–20 | 0–20 | 0–4 | |
Thirteen of 17 cases had overlying epidermal hyperplasia. Fourteen of 17 cases were completely amelanotic. Lobulated nests were seen in 2 cases and nesting in the adnexa in 5 cases. Five cases had notable pagetosis in the epidermis. None of these features were statistically significant compared to non-ROS1 Spitz neoplasms. Nine of 17 cases had floating nests defined as nests situated above the basal layer and in 3 cases there was transepidermal elimination of nests (Figure 1 and 2). Myxoid changes were not identified in any of the cases. Two cases were characterized by prominent stromal desmoplasia, and were morphologically best characterized as a desmoplastic Spitz nevus (Figure 3).
Figure 2).
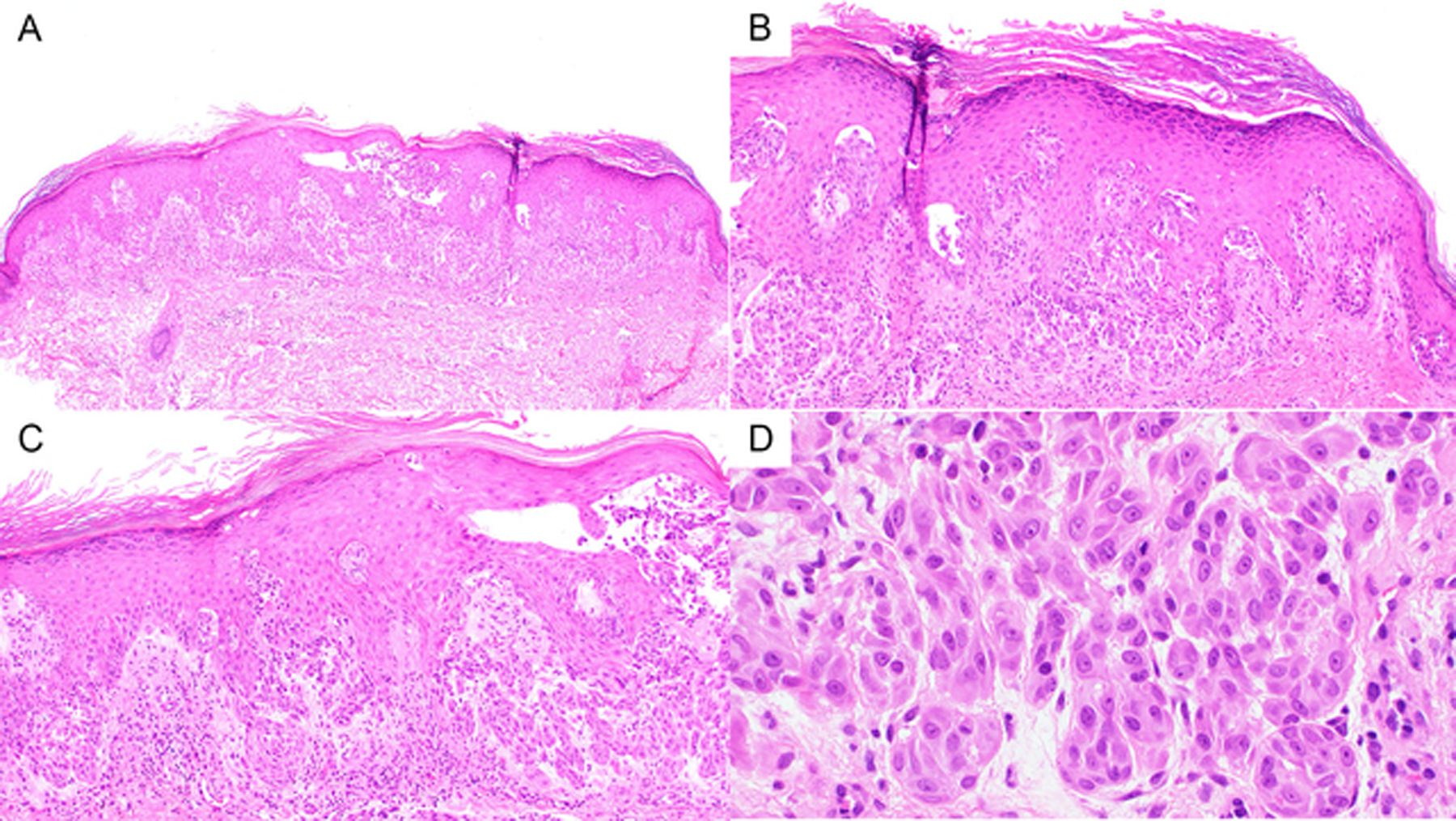
A and B) At 40x and 100X, respectively, a plaque like Spitz nevus with epidermal hyperplasia. C) At 200X one can appreciate some floating nests in the epidermis D) At 400X one can appreciate the relatively bland cytology of the Spitzoid melanocytes.
Figure 3).
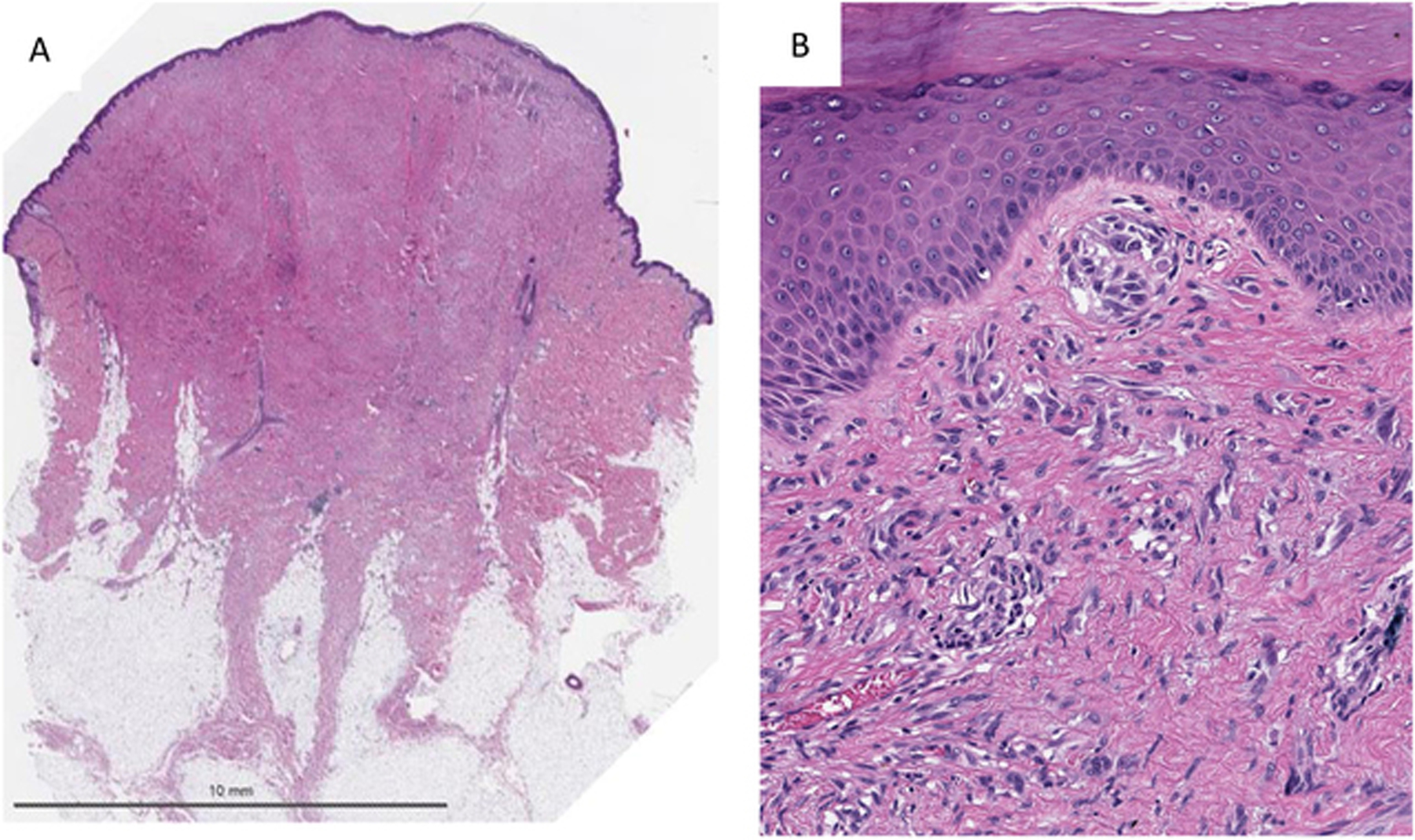
A) Low power shows a symmetric paucicellular Spitzoid neoplasm in a desmoplastic Stroma. B) Higher magnification shows small nests and individual units of Spitzoid melanocytes entrapped in a sclerotic stroma consistent with a diagnosis of desmoplastic Spitz nevus.
Immunohistochemical Staining for ROS1 was performed in 16 cases. Fifteen of the 16 cases showed strong positive staining (Figure 4). In one case only a blush staining was seen which was not convincingly positive.
Figure 4).
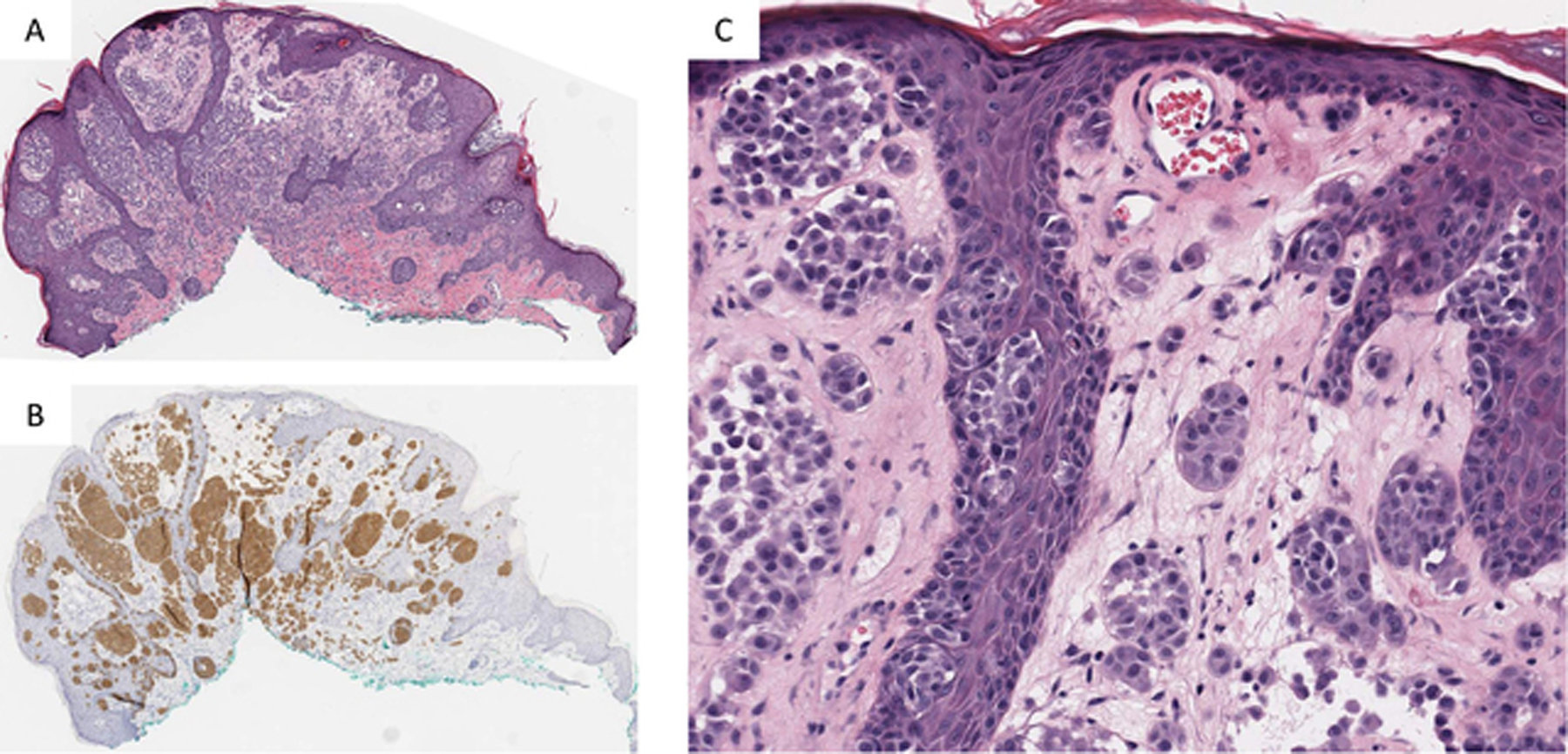
A) Low power showing plaque like silhouette of a ROS1 Fusion Spitz nevus. B) IHC staining for ROS1 shows strong and uniform staining throughout the nevus. C) Higher magnification shows nests of epithelioid and spindle shaped melanocytes with bland cytomorphology lacking significant atypia.
Genomic Findings in ROS1 Fusion Spitz Neoplasms
The fusion partner was identified in 16 of the 17 cases in the study. The most common genomic fusions among the 16 ROS1 cases were a PWWP2A-ROS1 fusion seen in 6 cases and a TPM3-ROS1 fusion also seen in 5 cases. Other recurrent fusion partners included a PPFIBP1-ROS1 fusion seen in 2 cases, and fusions partners involving MYH9-ROS1, CAPRINI1-ROS1 and MYO5A-ROS1 were each seen in 1 case (Table 3).
Table 3.
Genomic Fusions in ROS1 Spitz Neoplasms
| Case | Fusion | Copy Number Variation |
|---|---|---|
| 1 | PWWP2A-ROS1 | None identified |
| 2 | TPM3-ROS1 | Copy loss: BCL11B, CARD11, FBXO11, FGF3, FLT4, GRIN2A, HGF, MGMT, MYCN, MYOD1, NPM1, NTRK3, PLAG1, PTPRT, RET, TERT, TLX1 |
| 3 | TPM3-ROS1 | None identified |
| 4 | MYH9-ROS1 | None identified |
| 5 | PPFIBP1-ROS1 | None identified |
| 6 | MYO5A-ROS1 | Copy gain: HOXA9, JUN, MDM2 |
| 7 | TPM3-ROS1 | None identified |
| 8 | Identified by FISH Breakapart Probe | None identified |
| 9 | PWWP2A-ROS1 | None identified |
| 10 | PWWP2A-ROS1 | Copy loss: 6q.22.1* |
| 11 | PWWP2A-ROS1 | None identified |
| 12 | PPFIBP1-ROS1 | None identified |
| 13 | PWWP2A-ROS1 | None identified |
| 14 | TPM3-ROS1 | None identified |
| 15 | TPM3-ROS1 | None identified |
| 16 | CAPRIN1-ROS1 | Not assessed |
| 17 | PWWP2A-ROS1 | None identified |
Identified by SNP array
Three cases had copy number aberrations identified by NGS and SNP arrays. Two cases had copy number aberrations identified by NGS and 1 case had a copy number aberration identified by SNP array. Copy number loss of BCL11B, FGF3, CARD11, FBXO11, FLT4, GRIN2A, HGF, MGMT, MYCN, MYOD1, NPM1, NTRK3, PLAG1, PTPRT, RET, TERT and TLX1 were identified in case 2. This case was negative for copy number alterations when tested by a SNP array platform. Copy number gains of HOXA9, JUN, MDM2 were identified in case 6. Case 10 had an isolated loss at 6q.
DISCUSSION
Among two studies sequencing a large number of Spitz neoplasms the frequency of ROS1 fusions varied from 7 to 17%.17, 18 The vast majority of these cases were diagnosed as either Spitz nevus or Spitz tumor. In this study 10 were diagnosed as Spitz nevus and 7 as Spitz tumor. We did not identify any cases that met the criteria of a Spitz melanoma. In the study from Wiesner et al where kinase fusions in Spitz neoplasms were first described,17 3 of 24 ROS1 fusions were designated as Spitz melanoma, but no adverse clinical outcome was reported. This study from Wiesner et al is the larger series on ROS1 fusions but does not discuss morphologic features. Thus far there is only one study involving 6 cases of ROS1 fusions which were all designated as Spitz tumors by Donati et al which discusses morphologic features.13
While there is limited clinical outcomes information available on Spitz tumors with ROS1 fusions, among the 13 cases with follow up in this study and the 6 cases from Donati et al, there are no reported recurrences or metastases after complete excision of the primary tumors. One case in our series had a SLNB which was also negative. Thus, preliminary evidence suggests that most Spitz tumors with ROS1 fusions are likely indolent or at least in a much lower risk category compared to Spitz neoplasms with BRAF or MAP3K8 fusions which seem to constitute much of the more aggressive variants of Spitz neoplasms.9–11, 15, 27–29
We did not identify morphologic features which could allow for a definitive diagnosis of a ROS1 fusion by microscopic review alone but there were some characteristic features. This included a tendency for plaque-like or nodular silhouette without a deeply infiltrative component with a combination of epithelioid and spindle cell cytomorphology. Statistically significant features included lack of high grade cytologic atypia in all cases, lack of larger cell type, presence of maturation, frequent Kamino bodies and lower mitotic rate. These findings are consistent with the fact that all cases were diagnosed as Spitz nevus or Spitz tumor and none were thought to be Spitz melanoma.
In our cases, 13/17 had epidermal hyperplasia and 5/17 had notable epidermal pagetosis. Two cases had lobulated nests and 4 had nesting in the adnexa. None of these features were statistically significant as they can be seen in a broad spectrum of Spitz subtypes. In particular many of these features can overlap with NTRK1 fusion Spitz neoplasms. Donati et al reported transepidermal elimination of nests and myxoid changes as being present in all 6 cases. Another highly characteristic feature was floating nests seen in 9 of 17 cases with transepidermal elimination of nests in 3 cases. We did not identify significant mucinous changes though a colloidal iron was not performed. Although none of these features are totally specific, one might anticipate a ROS1 fusion in compound plaque like Spitz neoplasm with prominent intraepidermal component, Kamino bodies, with small to intermediate sized cells with low grade cytology, pagetosis and floating nests within the epidermis.
An interesting and novel observation is the detection of a ROS1 fusion in two desmoplastic Spitz nevi. This illustrates the wide spectrum of microscopic features associated with ROS1 fusions, but it also documents that the desmoplastic phenotype among Spitz nevi is not limited to HRAS aberrations. Gains of 11p (location of HRAS) and/or HRAS mutations have previously been thought to be typical of desmoplastic Spitz nevi. While they likely represent the most common aberration associated with a desmoplastic Spitz nevus, we hereby document two cases with a ROS1 kinase fusion associated with a desmoplastic phenotype.
In the 17 cases in this series, 6 different fusion partners were identified. This included PWWP2A (n = 6), TPM3 (n = 5), PPFIBP1 (n = 2), MYO5A (n = 1), CAPRINI1 (n = 1) and MYH9 (n = 1). PWWP2A was also the most frequent fusion partner in the series from Donati et al. A figure showing the chimeric protein model and the breakpoint of the fusions can be found in Figure 5. Previous in vivo studies show rising levels of phosphorylation produced by this fusion protein indicating that the ROS1 kinase is being constitutively activated.17
Figure 5).
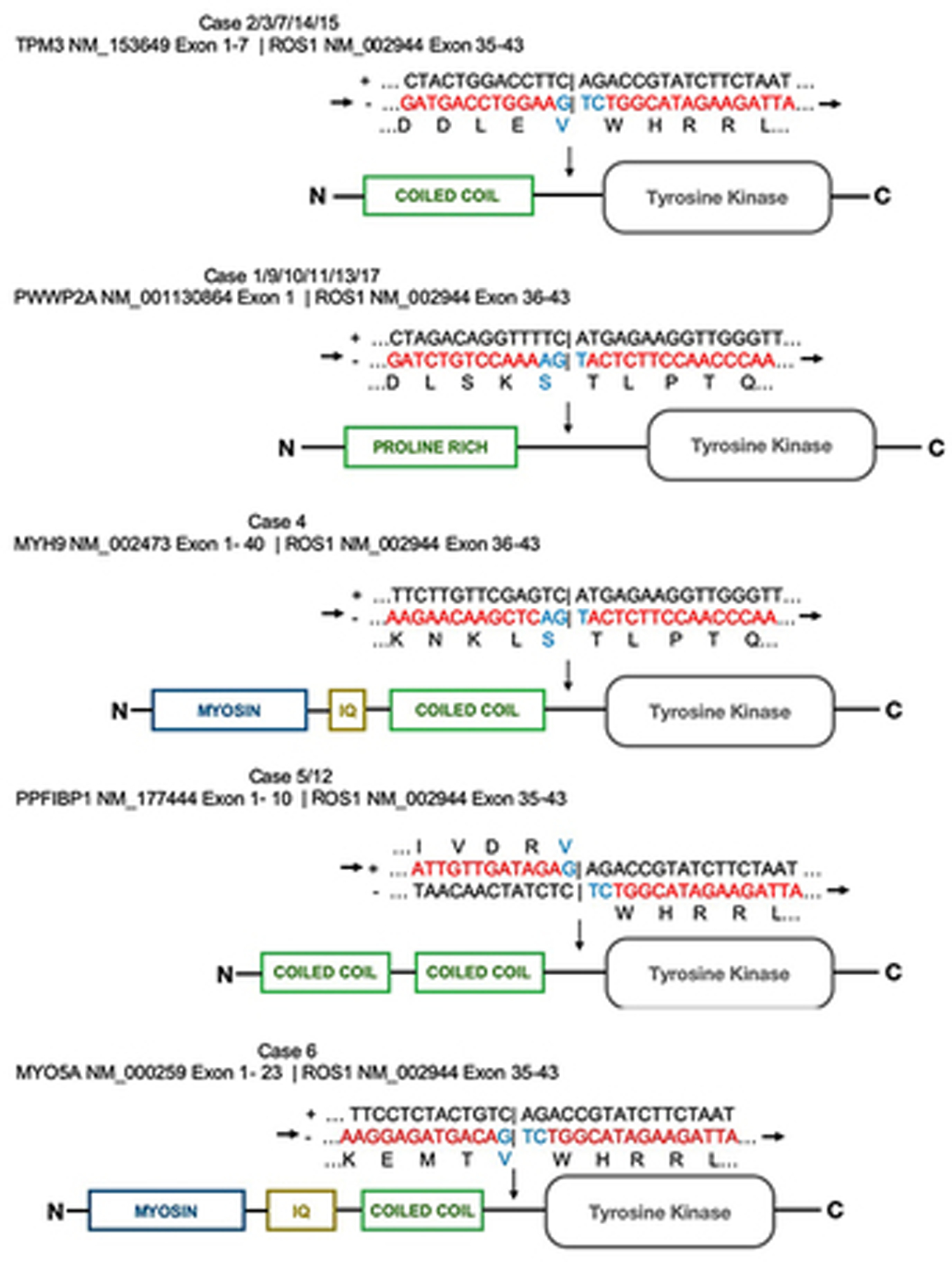
Diagrams of the chimeric fusion proteins in the ROS1 fusion Spitz neoplasms
ROS1 fusions have been identified in 9% Spitz melanomas and 1.3% in melanomas from previous studies.17, 30 There are no cases of ROS1 fusion melanoma in the TCGA database. ROS1 fusions are also seen in a subset of 1 to 2 % non small cell lung cancers. More recently ROS1 fusions were identified in 9 of 130 gliomas from an infant population.31 Also, rare cases of ROS1 fusions in angiosarcoma, thyroid and breast cancer have been reported.32–34 Interestingly in melanocytic neoplasms with ROS1 fusions the tumors seem to have an indolent clinical behavior.
In conclusion, this study describes the largest series to date on ROS1 fusion Spitz neoplasms. They seem to represent a lower grade group of tumors with generally indolent behavior. We could not find specific morphologic aberrations that were predictive of the molecular aberration but identified a number of features that were enriched in the group of ROS1 fusion tumors. They included a plaque or nodular silhouette with a cellular intraepidermal component, frequent Kamino bodies, a slight predisposition towards spindle cytology, a lower grade of cytologic atypia and floating nests/transepidermal elimination of nests. We also report for the first time the association of a desmoplastic phenotype with ROS1 fusions.
Acknowledgments
Fund sources: This work was supported by the IDP Foundation and in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
Footnotes
Conflicts of Interest: Dr. Gerami has served as a consultant for Myriad Genomics, DermTech Int., Merck and Castle Biosciences and has received honoraria for this. All other authors report no relevant conflicts of interest. This work is original and has not been previously published.
REFERENCES
- 1.Busam KJ, Kutzner H, Cerroni L, Wiesner T. Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions. Am J Surg Pathol 2014;38:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh I, de la Fouchardiere A, Pissaloux D, Mully TW, Garrido MC, Vemula SS, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraggi D, Salmaso R, Zamuner C, Munari G, Lanza C, Alaibac M et al. Prevalence of ALK gene alterations among the spectrum of plexiform spitzoid lesions. J Am Acad Dermatol 2018;79:728–735. [DOI] [PubMed] [Google Scholar]
- 4.Lee CY, Sholl LM, Zhang B, Merkel EA, Amin SM, Guitart J, et al. Atypical Spitzoid Neoplasms in Childhood: A Molecular and Outcome Study. Am J Dermatopathol 2017;39:181–6. [DOI] [PubMed] [Google Scholar]
- 5.Yeh I, Busam KJ, McCalmont TH, LeBoit PE, Pissaloux D, Alberti L, et al. Filigree-like Rete Ridges, Lobulated Nests, Rosette-like Structures, and Exaggerated Maturation Characterize Spitz Tumors With NTRK1 Fusion. Am J Surg Pathol 2019;43:737–746. [DOI] [PubMed] [Google Scholar]
- 6.Kiuru M, Jungbluth A, Kutzner H, Wiesner T, Busam KJ. Spitz Tumors: Comparison of Histological Features in Relationship to Immunohistochemical Staining for ALK and NTRK1. Int J Surg Pathol 2016;24:200–206. [DOI] [PubMed] [Google Scholar]
- 7.Yeh I, Tee MK, Botton T, Shain AH, Sparatta AJ, Gagnon A, et al. NTRK3 kinase fusions in Spitz tumours. J Pathol 2016;240:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan VL, Panah E, Zhang B, Shi K, Mohan LS, Gerami P. The role of gene fusions in melanocytic neoplasms. J Cutan Pathol. 2019;46:878–887. [DOI] [PubMed] [Google Scholar]
- 9.Quan VL, Zhang B, Mohan LS, Shi K, Isales M, Panah E, et al. Activating structural alterations in MAPK genes are distinct genetic drivers in a unique subgroup of Spitzoid neoplasms. Am J Surg Pathol. 2019;43:538–548. [DOI] [PubMed] [Google Scholar]
- 10.Houlier A, Pissaloux D, Masse I, Tirode F, Karanian M, Pincus L, et al. Melanocytic tumors with MAP3K8 fusions: report of 33 cases with morphological-genetic correlations. Mod Pathol 2020;33:846–857. [DOI] [PubMed] [Google Scholar]
- 11.Newman S, Fan L, Pribnow A, Silkov A, Rice SV, Lee S, et al. Clinical genome sequencing uncovers potentially targetable truncations and fusions of MAP3K8 in Spitzoid and other melanomas. Nat Med 2019;25:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perron E, Pissaloux D, Neub A, Hohl D, Tartar M, Mortier L, et al. Unclassified sclerosing malignant melanomas with AKAP9-BRAF gene fusion: a report of two cases and review of BRAF fusions in melanocytic tumors. Virchows Arch 2018;472:469–476. [DOI] [PubMed] [Google Scholar]
- 13.Donati M, Kastnerova L, Martinek P, Gossmann P, Sticová E, Hadravský L, et al. Spitz Tumors With ROS1 Fusions: A Clinicopathological Study of 6 Cases, Including FISH for Chromosomal Copy Number Alterations and Mutation Analysis Using Next-Generation Sequencing. Am J Dermatopathol 2020;42:92–102. [DOI] [PubMed] [Google Scholar]
- 14.Donati M, Kastnerova L, Ptakova N, Michal M, Kazakov DV. Polypoid Atypical Spitz Tumor With a Fibrosclerotic Stroma, CLIP2-BRAF Fusion, and Homozygous Loss of 9p21. Am J Dermatopathol 2020;42:204–207. [DOI] [PubMed] [Google Scholar]
- 15.Ross JS, Wang K, Chmielecki J, Gay L, Johnson A, Chudnovsky J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer 2016;138:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de la Fouchardiere A, et al. Activating MET kinase rearrangements in melanoma and Spitz tumours. Nat Commun 2015;6:7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan VL, Zhang B, Zhang Y, Mohan L, Shi K, Wagner A, et al. Integrating Next-Generation Sequencing with Morphology Improves Prognostic and Biologic Classification of Spitz Neoplasms. J Invest Dermatol 2020;140:1599–1608. [DOI] [PubMed] [Google Scholar]
- 19.Busam KJ, Gerami P, Scolyer RA. Pathology of Melanocytic Tumors. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 20.Crowson AN, Magro CM, Mihm MC. The Melanocytic Proliferations: A Comprehensive Textbook of Pigmented Lesions. Hoboken, NJ: John Wiley & Sons Inc; 2013. [Google Scholar]
- 21.Mooi W, Krausz T. Pathology of Melanocytic Disorders. BocaRaton, FL: CRC Press; 2007. [Google Scholar]
- 22.Barnhill RL, Piepkorn M, Busam KJ. Pathology of Malignant Melanoma. New York, NY: Springer; 2004. [Google Scholar]
- 23.Beaubier N, Tell R, Huether R, Bontrager M, Bush S, Parsons J, et al. Clinical validation of the Tempus xO assay. Oncotarget 2018;9:25826–25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaubier N, Tell R, Lau D, Parsons JR, Bush S, Perera J, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget 2019;10:2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busam KJ, Antonescu CR, Marghoob AA, Marghoob M, Nehal KS, Sachs DL, et al. Histologic classification of tumor-infiltrating lymphocytes in primary cutaneous malignant melanoma. A study of interobserver agreement. Am J Clin Pathol 2001;115:856–860. [DOI] [PubMed] [Google Scholar]
- 26.Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol 2013;30:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu G, Barnhill RL, Lee S, Li Y, Shao Y, Easton J, et al. The landscape of fusion transcripts in spitzoid melanoma and biologically indeterminate spitzoid tumors by RNA sequencing. Mod Pathol 2016;29:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Barnhill RL, Dummer R, Dalton J, Wu J, Pappo A, et al. TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Sci Rep. 2015;5:11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botton T, Yeh I, Nelson T, Vemula S, Sparatta A, Garrido M, et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell Melanoma Res 2013;26:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner J, Couts K, Sheren J, Saichaemchan S, Ariyawutyakorn W, Avolio I, et al. Kinase gene fusions in defined subsets of melanoma. Pigment Cell Melanoma Res 2017;30:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke M, Mackay A, Ismer B, Pickles J, Tatevossian R, Newman S, et al. Infant high grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov 2020;10:942–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritterhouse LL, Wirth LJ, Randolph GW, Sadow PM, Ross DS, Liddy W, et al. ROS1 Rearrangement in Thyroid Cancer. Thyroid 2016;26:794–797. [DOI] [PubMed] [Google Scholar]
- 33.Marks EI, Pamarthy S, Dizon D, Birnbaum A, Yakirevich E, Safran H, et al. ROS1-GOPC/FIG: a novel gene fusion in hepatic angiosarcoma. Oncotarget 2019;10:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Wang H, Fang M, Li C, Zeng Y, Wang K. ALK or ROS1-rearranged breast metastasis from lung adenocarcinoma: a report of 2 cases. Tumori 2019;105:NP67–NP71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Availability
Processed sequencing data (vcf files and count files) can be found through GEO Series accession number GSE142443 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142443).
Processed sequencing data (vcf files and count files) can be found through GEO Series accession number GSE142443 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142443).


