Abstract
Background & Aims:
We examined the frequency of and factors associated with delays in diagnosis of hepatocellular carcinoma (HCC) in a cohort of patients with cirrhosis in the Veterans Health Administration.
Methods:
In a retrospective study, we collected and analyzed data from the Veterans Health Administration’s electronic health records. We used a multivariate logistic regression model to identify factors associated with a delay in diagnosis of HCC of more than 60 days following a red flag (defined as the earliest date at which a diagnosis of HCC could have been made, based on American Association for the Study of Liver Disease 2005 guidelines). We used multivariate Cox proportional hazards model to evaluate the effects of delayed diagnosis on survival, adjusting for patient and provider characteristics.
Results:
Among 655 patients with cirrhosis and a diagnosis of HCC from 2006 through 2011, 46.9% had a delay in diagnosis of more than 60 days following a red flag for HCC. Delays in diagnosis for more than 60 days were significantly associated with lack of provider adherence to the guidelines (adjusted odds ratio [OR], 4.82; 95% CI, 3.12–7.45), a diagnostic imaging evaluation instead of only measurement of alfa fetoprotein (adjusted OR, 2.63; 95% CI, 1.09–6.24), and diagnosis as an incidental finding during examination for an unrelated medical problem (compared with an HCC-related assessment) (adjusted OR, 2.26; 95% CI, 1.09–4.67). Diagnostic delays of 60 days or more were associated with lower mortality compared to patients without a delay in diagnosis (unadjusted hazard ratio, 0.57; 95% CI, 0.47–0.68 and adjusted hazard ratio, 0.63; 95% CI, 0.50–0.78).
Conclusions:
Nearly half of veterans with cirrhosis have delays in diagnosis of HCC of 60 days or more after a red flag, defined by guidelines. Interventions are needed to improve timely follow-up of red flags for HCC and adherence to guidelines, to increase early detection of HCC.
Keywords: liver cancer, VA, surveillance, testing
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) has tripled over the last 30 years.1-4 Clinical practice guidelines advocate systematic surveillance among patients at high-risk for HCC coupled with timely follow-up of abnormal tests. Diagnostic delays can contribute to interval tumor growth and loss of eligibility for curative treatment.5,6
HCC can be difficult to diagnose in a timely manner. Providers often lack knowledge about current HCC diagnostic guidelines.7-12 Published guidelines can vary by professional organization leading to confusion among providers.7,13-18 Surveillance tests are sub-optimal, and even among patients with a known risk factor for HCC, surveillance rates are low.19-23 The evolving options for diagnostic testing, including liver biopsy, contributes to challenges in the diagnosis of HCC.24 The involvement of multiple specialties (e.g., gastroenterology, hepatology, oncology, radiology, surgery) also complicate the diagnostic process.
Our study’s aims were to 1) characterize the frequency of delays in the diagnosis of HCC in the Veterans Health Administration with cirrhosis, 2) evaluate the association between delays and patient and provider characteristics, and 3) determine the impact of diagnostic delays on survival.
MATERIALS AND METHODS
Data Source
We conducted a retrospective cohort study using data obtained from the Department of Veterans Affairs (VA) administrative data files and review of patient electronic health records (EHR). HCC diagnoses were verified by provider notes, laboratory data, imaging and pathology reports. Using structured data abstraction tools, an oncologist and internist reviewed the EHR for health services utilized in the HCC diagnostic process.
Study Population
We identified a national cohort of 10,695 patients who potentially had a HCC diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes 155.0 and 155.1) from 2006 to 2011 and follow-up that ended July 30, 2012.25 A total of 2,719 patients were then selected at random using computer-generated code for medical record review to confirm the initial diagnosis of HCC by radiology or pathology criteria according to the 2005 American Association for the Study of Liver Diseases (AASLD) guidelines, and recent VA healthcare utilization within 1 year prior to the date of HCC diagnosis (at least 1 inpatient or outpatient encounter at any VA facility).20,22,26
We excluded patients who did not have confirmed cirrhosis (ICD-9 CM codes 571.2, 571.5 or 571.6 or liver biopsy results at any time prior to or at the time of HCC diagnosis, characteristics of cirrhosis on imaging or evidence of portal hypertension by laboratory or physical exam). We also excluded patients who did not have any of the following: a liver lesion found on imaging during the study period, progress notes discussing the diagnosis of HCC, available biopsy or radiology/diagnostic information and lesion characteristics, or a HCC diagnosis at a VA facility (n=2,064). The final study population was 655 HCC patients.
Red Flag Definition
The date of red flag presentation was the earliest date when an HCC diagnosis could have been made by AASLD 2005 guideline recommendations (radiology or pathology).7,15,27-29 We defined a red flag as either: 1) liver lesion <1 cm in diameter which would need to be followed-up every 3-4 months 2) liver lesion 1-2 cm in diameter, which would need to be followed-up appropriately or be treated as HCC if applicable and 3) >2 cm in diameter with characteristic arterial vascularization or AFP>200 ng/mL, which would be treated as HCC.
Diagnostic Delay Definition
The actual HCC diagnosis date was the earliest date that either a provider documented HCC diagnosis, imaging reported HCC characteristics, or biopsy confirmed HCC.17,30,31 The date of referral or diagnostic follow-up was the first date of evaluation for further diagnostic and management activity (e.g., clinic visit with HCC-related provider, liver imaging, biopsy, or multidisciplinary tumor board meeting).30,32 We defined diagnostic delays as the difference between the dates of red flag presentation and HCC diagnosis. Based on expert recommendations and a 2013 Office of Inspector General report, we chose a cutoff for timely diagnosis as 60 days.32,33
Patient Characteristics
We collected patient-related information, clinical characteristics, and HCC risk factors. Lesion characteristics and Barcelona Clinic Liver Cancer (BCLC) HCC stage at diagnosis were also collected. Radiology reports were abstracted for contrast technique, presence of HCC features (arterial enhancement and portal venous wash out).
Factors Related to Diagnostic Delay
All variables were defined at the time of the HCC red flag presentation date. We collected patient-related factors, such as missed appointments, contraindication to diagnostic testing, or lost to follow-up. We abstracted the specialty of the provider who documented HCC diagnosis, level of doubt or confidence in the HCC diagnosis (e.g., documentation of “? HCC” or “likely HCC”), request for additional testing, method of HCC diagnosis (imaging, biopsy or AFP only), provider adherence to AASLD guidelines, and reasons for AASLD guideline non-adherence (e.g., diagnosis without guideline-based evidence, inconclusive pathology, and inappropriate tests ordered, or provider did not recognize HCC diagnosis). Diagnostic context included three situations in which HCC diagnosis was made: 1) incidental finding where a guideline-based diagnosis of HCC could be made from tests obtained during work-up for an unrelated medical problem, 2) HCC surveillance imaging, and 3) further diagnostic work-up in follow up to abnormal suspicious findings (e.g., ultrasound, liver function tests, patient-reported symptoms).
Statistical Analysis
The primary outcome was presence of a diagnostic delay for HCC defined as >60 days from the HCC red flag presentation date to HCC diagnosis date, accounting for lesion characteristics. We performed sensitivity analyses to examine different cutoffs for diagnostic delay, defined as >30 days for liver lesions >2 cm in diameter and >90 days for liver lesions <1 cm in diameter. An additional sensitivity analysis was performed to compare a subset of patients diagnosed through HCC surveillance, compared to patients who were diagnosed through diagnostic work-up evaluation. We used univariate and multivariate logistic regression model to identify patient and provider predictors that may contribute to diagnostic delays.
Kaplan-Meier survival curves were constructed to assess and compare time from HCC diagnosis to survival (death or end of study follow-up on July 30, 2012) between patients with and without a delay. We performed a sensitivity analysis to examine patients diagnosed through 2009 to allow at least 3 years of follow-up time. We also performed an additional analysis comparing survival between patients with and without delays stratified by BCLC stage and evaluated unadjusted 3- and 5-year survival to address any selection bias in our study. Multivariate Cox proportional hazards model was used to identify the effect of diagnostic delay on mortality, adjusting for patient and provider characteristics.
All analyses were conducted using STATA 14.0 (StataCorp, College Station, TX, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC). The study protocol was approved by the Baylor College of Medicine Institutional Review Board and the Michael E. DeBakey VA Research & Development Committee.
RESULTS
Patient Characteristics
In our sample of 655 confirmed HCC patients with known cirrhosis, most patients (71.5%) were between 50 to 64 years of age at the time of HCC diagnosis. Almost all patients were men (99.8%) and their racial and ethnic distribution was white (57.6%), followed by blacks (26.3%), Hispanics (14.1%) and other (2.1%) race/ethnicity. Table 1 shows a comparison of patient characteristics between those who experienced a diagnostic delay >60 days compared to those who did not experience a delay.
Table 1.
Characteristics of HCC patients (n=655)
| Variable | No delay (Diagnostic follow- up ≤60 days) (%) n=348 |
Diagnostic delay >60 days (%) n=307 |
P-value |
|---|---|---|---|
| Age at HCC diagnosis | - | - | 0.84 |
| <50 years | 10 (2.9) | 6 (2.0) | |
| 50-64 years | 247 (71.0) | 221 (72.0) | |
| 65-79 years | 70 (20.1) | 64 (20.9) | |
| ≥80 years | 21 (6.0) | 16 (5.2) | |
| Year of HCC diagnosis | - | - | <0.001 |
| 2006 | 67 (19.3) | 16 (5.3) | |
| 2007 | 53 (15.2) | 49 (16.2) | |
| 2008 | 80 (23.0) | 63 (20.9) | |
| 2009 | 80 (23.0) | 86 (28.5) | |
| 2010 | 59 (17.0) | 68 (22.5) | |
| 2011 | 9 (2.6) | 20 (6.6) | |
| Race/ethnicity | - | - | 0.39 |
| White | 203 (58.3) | 174 (56.7) | |
| Black | 94 (27.0) | 78 (25.4) | |
| Hispanic | 42 (12.1) | 50 (16.3) | |
| Other | 9 (2.6) | 5 (1.6) | |
| VA treatment facility location | - | - | 0.79 |
| Central | 57 (16.4) | 50 (16.3) | |
| East | 77 (22.1) | 78 (25.4) | |
| South | 134 (38.5) | 114 (37.1) | |
| West | 80 (23.0) | 65 (21.2) | |
| Hepatitis B (HBV) | 20 (5.8) | 13 (4.2) | 0.40 |
| Hepatitis C (HCV) | 258 (74.1) | 240 (78.2) | 0.23 |
| Diabetes mellitus | 126 (36.2) | 120 (39.1) | 0.45 |
| History of alcohol or drug abuse | 183 (52.6) | 174 (56.7) | 0.29 |
| Liver disease severity | - | - | <0.001 |
| Child-Pugh class A | 101 (29.8) | 128 (44.6) | |
| Child-Pugh class B | 178 (52.5) | 132 (46.0) | |
| Child-Pugh class C | 60 (17.7) | 27 (9.4) | |
| Barcelona staging | - | - | <0.001 |
| A | 37 (11.1) | 71 (25.7) | |
| B | 71 (21.3) | 77 (27.9) | |
| C | 138 (41.3) | 91 (33.0) | |
| D | 88 (26.4) | 37 (13.4) | |
| Lesion size | - | - | <0.001 |
| <1 cm | 20 (5.8) | 99 (32.3) | |
| 1-2 cm | 183 (52.6) | 182 (59.3) | |
| >2 cm | 145 (41.7) | 26 (15.2) | |
| Deyo-Charlson Comorbidity Index | - | - | 0.002 |
| 0 | 60 (17.5) | 29 (9.6) | |
| 1 | 145 (42.3) | 118 (38.9) | |
| ≥2 | 138 (40.2) | 156 (51.5) |
Diagnostic Characteristics
In patients who had diagnostic delays >60 days, the median time from first red flag presentation to HCC diagnosis was 6.8 months (95% CI 5.9-7.9 months). The median time from first red flag presentation to HCC diagnosis for patients with Barcelona A staging who had diagnostic delays >60 days was 7.0 months (95% CI 4.9-8.9 months), 6.6 months (95% CI 4.7-8.6 months) for patients with Barcelona B staging, 7.4 months (95% CI 5.4-9.9 months) for patients with Barcelona C staging, and 5.6 months (95% CI 3.1-7.9 months) for patients with Barcelona D staging.
Almost half of patients (47.3%) were diagnosed with HCC using imaging without biopsy; 45.6% with biopsy alone and the remaining using AFP alone (7.0%). More than half (54.2%) of patients were diagnosed with HCC through diagnostic work-up evaluation for symptoms or an abnormal finding, followed by HCC surveillance (38.5%) and <10% of patients were diagnosed with HCC incidentally. Approximately 46.9% of patients had delays >60 days from the HCC red flag presentation date to HCC diagnosis date. Almost half (45.0%) of patients who experienced a delay had a AASLD guideline non-compliant HCC diagnosis, including providers failing to recognize HCC or ordering inappropriate tests to determine HCC in 43.8% (n=85) of cases. In 17.9% of cases, contraindication to triple-phase contrast or biopsy was cited as a reason for non-adherence to AASLD guidelines. There were 263 patients (40%) referred for multidisciplinary tumor board discussion. Diagnostic characteristics for HCC patients are described further in Table 2.
Table 2.
Diagnostic follow-up characteristics of HCC patients (n=655)
| Variable | No delay (Diagnostic follow-up ≤60 days) (%) n=348 |
Diagnostic delay >60 days (%) n=307 |
P-value |
|---|---|---|---|
| Diagnostic evaluation method | - | - | <0.001 |
| Imaging | 261 (75.0) | 240 (78.2) | |
| Biopsy | 49 (14.1) | 59 (19.2) | |
| AFP only | 38 (10.9) | 8 (2.6) | |
| Diagnosis mechanism | - | - | <0.001 |
| Incidental | 20 (5.8) | 27 (8.8) | |
| Diagnostic | 229 (65.8) | 130 (42.3) | |
| Surveillance | 99 (28.5) | 150 (48.9) | |
| Provider adherence to AASLD guidelines | - | - | <0.001 |
| Yes | 292 (83.9) | 169 (55.1) | |
| No | 56 (16.1) | 138 (45.0) | |
| HCC cases non-adherent with AASLD guidelines reasoning¥ | - | - | 0.02 |
| Preliminary dx w/out evidence | 6 (10.9) | 12 (8.9) | |
| Evaluated by provider but inappropriate tests ordered or non-recognition | 29 (52.7) | 56 (41.5) | |
| Patient missed appointment or declined workup | 0 (0.0) | 5 (3.7) | |
| Contraindication to triple-phase contrast or biopsy | 14 (25.5) | 20 (14.8) | |
| Other | 6 (10.9) | 42 (31.11) | |
| Doubted diagnosis by provider | - | - | 0.06 |
| Primary care/inpatient | 11 (3.2) | 19 (6.2) | |
| GI specialist | 337 (96.8) | 288 (93.8) |
n=190
Predictors of Diagnostic Delays
In multivariate logistic regression analysis, AASLD guideline non-adherence was associated with approximately 5 times the estimated odds of HCC diagnosis delays exceeding 60 days, as compared with cases adherent with guidelines (adjusted OR 4.82; 95% CI 3.12-7.45). Imaging as the sole diagnostic evaluation method was associated with delay exceeding 60 days for HCC diagnosis (adjusted OR 2.63; 95% CI 1.09-6.24) when compared with AFP only (Table 3). Separate sensitivity analyses based on different diagnostic delay definitions (delay cutoff of >30 and >90 days) demonstrated similar findings (P>0.05). Results for our subgroup comparison of patients diagnosed through surveillance, compared to patients who were diagnosed through diagnostic work-up did not change (data not shown).
Table 3.
Correlates of diagnostic delays for HCC >60 days (n=655)
| Variable | Adjusted OR | 95% CI | P-value |
|---|---|---|---|
| Age at HCC diagnosis (ref=<50 years) | - | - | - |
| 50-64 years | 2.81 | (0.88, 8.97) | 0.08 |
| 65-79 years | 2.40 | (0.71, 8.12) | 0.16 |
| ≥80 years | 3.33 | (0.84, 13.20) | 0.09 |
| Year of HCC diagnosis (ref=2011) | - | - | - |
| 2006 | 0.15 | (0.05, 0.47) | 0.001 |
| 2007 | 0.46 | (0.16,1.26) | 0.13 |
| 2008 | 0.39 | (0.15, 1.05) | 0.06 |
| 2009 | 0.50 | (0.19, 1.33) | 0.17 |
| 2010 | 0.55 | (0.20, 1.49) | 0.24 |
| Barcelona staging (ref=A) | - | - | - |
| B | 0.86 | (0.48, 1.53) | 0.60 |
| C | 0.46 | (0.27, 0.80) | 0.005 |
| D | 0.33 | (0.18, 0.62) | 0.001 |
| Provider adherence to AASLD guidelines (ref=Yes) | - | - | - |
| No | 4.82 | (3.12, 7.45) | <0.001 |
| Doubted diagnosis by provider (ref=GI specialist) | - | - | - |
| Primary care/inpatient | 1.63 | (0.63, 4.21) | 0.31 |
| Diagnostic evaluation method (ref=AFP only) | - | - | - |
| Imaging | 2.63 | (1.09, 6.34) | 0.03 |
| Biopsy | 2.27 | (0.87, 5.91) | 0.10 |
| Diagnosis mechanism (ref=Diagnostic) | - | - | - |
| Incidental | 2.26 | (1.09, 4.67) | 0.03 |
| Surveillance | 1.89 | (1.25, 2.87) | 0.003 |
Association Between Diagnostic Delays and Mortality
Three fourths of HCC patients (75.4%) in our study population died by the end of the study period. In patients with diagnostic delays >60 days, the median overall survival was 18 months with 1-, 3-, and 5-year survival rates of 67% (95% CI 62-73%), 22% (95% CI 17-28%), and 14% (95% CI 9-21%), respectively. In patients with no delays, the median overall survival was 6 months with 1-, 3-, and 5-year survival rates of 36% (95% CI 31-41%), 15% (95% CI 11-20%), and 13% (95% CI 9-17%), respectively. Differences in survival stratified by presence of diagnostic delays were statistically significant (log-rank test P<0.001) (Figure 1).
Figure 1.
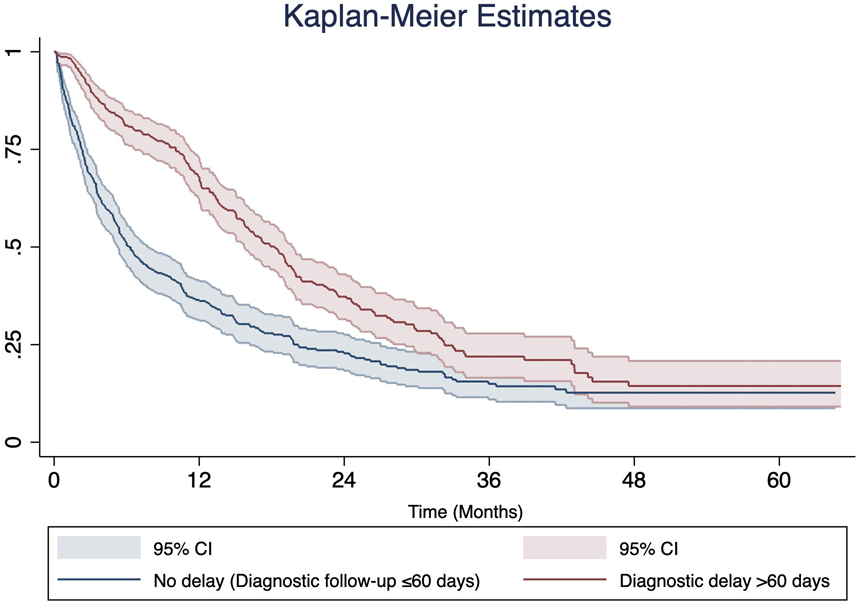
Kaplan-Meier survival estimates for 655 HCC patients stratified by the presence or absence of diagnostic delay >60 days
Unadjusted Cox model indicated diagnostic delays >60 days was associated with lower mortality compared to those without a diagnostic delay (unadjusted HR 0.57; 95% CI 0.47-0.68). In multivariate Cox proportional hazards analysis, diagnostic delays >60 days (adjusted HR 0.63; 95% CI 0.50-0.78) continued to be associated with lower mortality compared to those without a diagnostic delay (Table 4). Lower mortality was also observed in patients who were diagnosed incidentally (adjusted HR 0.46; 95% CI 0.31-0.69) or through surveillance (adjusted HR 0.53; 95% CI 0.42-0.67) compared to those with diagnostic work-up evaluation due to symptoms, and patients who received a transplant for curative treatment (adjusted HR 0.20; 95% CI 0.05-0.76) compared to resection (Table 4). In our sensitivity analysis of patients diagnosed with HCC through 2009, we found no significant differences (P>0.05).
Table 4.
Multivariate Cox proportional hazards model for association between length of diagnostic delays and mortality (n=655)
| Variable | Adjusted HR | 95% CI | P-value |
|---|---|---|---|
| Delay >60 days | 0.63 | (0.50, 0.78) | <0.001 |
| VA treatment facility location (ref=Central) | - | - | - |
| East | 0.77 | (0.57, 1.05) | 0.10 |
| South | 0.79 | (0.60, 1.05) | 0.10 |
| West | 0.72 | (0.52, 0.98) | 0.04 |
| Hepatitis C (HCV) | 1.19 | (0.95, 1.49) | 0.13 |
| Liver severity (ref=Child-Pugh class A) | - | - | - |
| Child-Pugh class B | 2.44 | (1.92, 3.09) | <0.001 |
| Child-Pugh class C | 1.37 | (0.86, 2.20) | 0.19 |
| Barcelona staging (ref=A) | - | - | - |
| B | 1.52 | (1.07, 2.17) | 0.02 |
| C | 2.19 | (1.56, 3.08) | <0.001 |
| D | 4.25 | (2.63, 6.88) | <0.001 |
| Provider adherence to AASLD guidelines (ref=Yes) | - | - | - |
| No | 1.22 | (0.97, 1.54) | 0.09 |
| Doubted diagnosis by provider (ref=GI specialist) | - | - | - |
| Primary care/inpatient | 0.65 | (0.40, 1.05) | 0.08 |
| Diagnostic evaluation method (ref=AFP only) | - | - | - |
| Imaging | 0.52 | (0.37, 0.74) | <0.001 |
| Biopsy | 0.56 | (0.38, 0.83) | 0.004 |
| Diagnosis mechanism (ref=Diagnostic) | - | - | - |
| Incidental | 0.46 | (0.31, 0.69) | <0.001 |
| Surveillance | 0.53 | (0.42, 0.67) | <0.001 |
| Curative treatment (ref=Resection) | - | - | - |
| Ablation | 0.63 | (0.30, 1.35) | 0.24 |
| Transplant | 0.20 | (0.05, 0.76) | 0.02 |
| Non-curative | 0.99 | (0.50, 1.97) | 0.98 |
| Other | 0.37 | (0.10, 1.40) | 0.14 |
| None | 2.27 | (1.12, 4.57) | 0.02 |
Additional sensitivity analyses of each BCLC tumor stage (A-D) demonstrated no significant difference in unadjusted 3- and 5-year survival for patients who had diagnostic delays compared to those without diagnostic delays (Figure 2).
Figure 2.
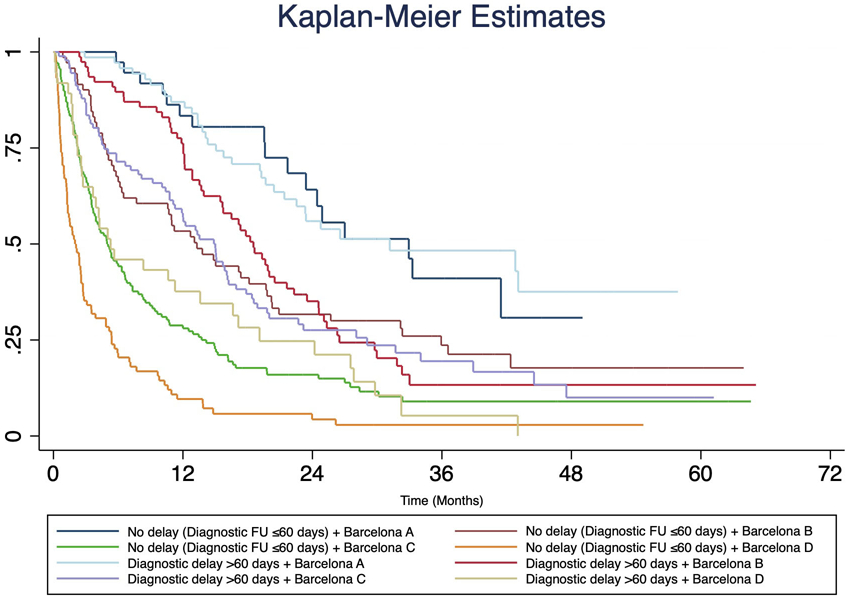
Kaplan-Meier survival estimates for 655 HCC patients with diagnostic delay >60 days and Barcelona (BCLC) staging
DISCUSSION
Nearly half of VA patients in our sample experienced diagnostic delays greater than 60 days from the HCC red flag date. This is consistent with findings from a non-VA urban hospital, where almost 40% of patients experienced a diagnostic delay.5 Previous studies have reported delays and missed opportunities of care due to both patient and provider factors.5,16,32,34,35
In our study, delays were related to patients missing or cancelling appointments, and testing issues, such as providers not ordering follow-up tests or not receiving or following-up on abnormal test results. Adherence to AASLD diagnostic guidelines for HCC was suboptimal.27,28,36,37 We found that in a third of patients, providers did not adhere to AASLD guidelines. This estimate is similar to previous studies that noted the large proportions of lack of provider adherence to AASLD guidelines for the management of liver disease, including HCC surveillance and treatment.13,17,28,35,38 This may be in part due to confusion among providers about which practice guideline to follow, as well as the complexity of HCC diagnostic imaging criteria and radiology reporting. Issues surrounding the initial diagnostic assessment (e.g. accurate and complete clinical examination and diagnostic reasoning), diagnostic test performance and interpretation (e.g. ordering appropriate lab or imaging tests, interpretation of results) and diagnostic follow-up and coordination (e.g., timely diagnostic follow-up and referral) were also reported for delays in cancer diagnosis.39
To improve HCC diagnosis in clinical settings, we recommend system level processes to improve physician education and adherence for AASLD guidelines. Interventions could include standardized educational training for physicians, establishing multi-disciplinary clinics for patients at high-risk patients for HCC, and increased utilization of HCC tumor boards in the diagnostic process. A dedicated tumor board review for HCC patients was found to be associated with increased survival.40 In our study, not all VA facilities may have had access to tumor board conferences, and therefore expansion of virtual tumor board programs may assist with diagnostic guidance. These methods would increase the knowledge-base, as well as enhance communication among physicians and subsequently lead to a timelier HCC diagnosis.
Further, introduction of the Li-RADS system standardizes the imaging diagnostic criteria and serves to improve communication between radiologists and clinicians by providing categories for level of suspicion for HCC.41 Li-RADS reporting has improved the accuracy of HCC identification by CT and MRI.42 System level processes could include system alerts for abnormal liver imaging, inclusion of guideline recommendations for further diagnostic work-up in radiology reports, and standardized radiology reporting templates for Li-RADS criteria. However, there are challenges to Li-RADS implementation. The increased number and complexity of imaging criteria for Li-RADS evaluation can be time consuming and may require a radiologist with expertise in liver imaging, which may only be available in larger, high volume centers.43-45
Patients with diagnostic delays for HCC exceeding 60 days had longer survival compared to patients without any diagnostic delays. Caution needs to be exercised in interpreting this finding. Patients with diagnostic delays may have better prognosis due to compensated cirrhosis and earlier stage. One possible explanation is that patients who presented with more advanced or worse cirrhosis severity may have received more urgent follow-up than patients with small, slow growing tumors and compensated cirrhosis. In our study, patients who had diagnostic delays had lower liver disease severity (Childs A 44.6% vs. 29.8%) and lower BCLC stage (BCLC A 25.7% vs. 11.1%) compared to patients without diagnostic delays. In favor of this argument is that almost all of the difference in survival was explained by the 1-year survival and subsequently there were no significant differences between the no delay and delay groups in the 3- and 5- year survival rates.
In addition, the slow doubling time of HCC may create a paradoxical wait time. The “waiting time paradox” where longer delays in care are associated with improved survival because of early stage at diagnosis, has been described in other cancers.46,47 Tumor biology may outweigh diagnostic delays when determining the ultimate outcome.48 If the HCC lesion has a slow rate of progression, delays in diagnosis of early stage tumors may not have a significant impact on survival.49 Given that nearly 40% of HCC can exhibit an indolent growth pattern, the waiting time paradox may also apply to a subset of patients with HCC.50
Lastly, there is no currently accepted standard definition of a diagnostic delay for HCC, although one study defined the cutoff as 60 days based on prior work, feedback from experts and guidance from the 2013 Office of Inspector General Report.32,33 A U.S. study by Patel et al. found that almost 20% of patients with HCC waited more than 3 months from presentation to diagnosis and attributed the delays to lack of provider orders on imaging.5 The traditional definition of delay for most cancers, which is generally 30-60 days, may need to be re-evaluated for HCC given the slower doubling time of HCC.32,51,52 More studies are required to examine the relevance of our finding.
To our knowledge, our study is the first to assess diagnostic delays for HCC in the setting of an integrated national health system. This allowed us to capture potential process breakdowns through the entire continuum from presentation to diagnosis. However, limited information was available in the medical record to explain reasons for delays. Future studies would need to collect qualitative data from patients and providers to better understand reasons for delays.
AASLD guidelines are still valuable because they prevent patients at high risk for bleeding due to coagulopathy and thrombocytopenia from having to undergo unnecessary biopsy. Liver cancer treatment involves multi-disciplinary coordination, and any time gained in the diagnostic process will allow for earlier entry into the process. Interventions to improve timely follow-up of red flag findings and adherence to guidelines are needed to improve early HCC detection.
Need to Know.
Background: Guidelines have defined red flags that identify patients with cirrhosis who should be examined for hepatocellular carcinoma (HCC), but it is not clear how many patients with red flags actually receive a diagnosis of HCC within 60 days of the red flag warning.
Findings: Almost half of veterans with cirrhosis have delays in diagnosis of HCC of 60 days or more after a red flag. Delays in diagnosis are associated with lack of provider adherence to the guidelines, diagnostic imaging evaluation instead of only measurement of alfa fetoprotein, and diagnosis of HCC as an incidental finding during examination for an unrelated medical problem.
Implications for patient care: Interventions are needed to improve timely follow-up of red flags for HCC and adherence to guidelines, to increase early detection of HCC.
Acknowledgments
Grant Support: This project was supported in part by the MEDVAMC Seed Award (33-140; PI: Y. Sada), the National Cancer Institute (R01 CA160738, PI: J. Davila), the facilities and resources of the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413) and the Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, United States of America. Dr. Singh is additionally supported by the Agency for Healthcare Research and Quality (R01HS27363). The views expressed in this article are those of the authors and do not necessarily represent the views of the funding institutions.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha-fetoprotein
- CAPRI
Compensation and Pension Records Interchange
- CT
computed tomography
- CDW
Corporate Data Warehouse
- HCC
Hepatocellular carcinoma
- MRI
Magnetic resonance imaging
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Footnotes
Conflicts of Interests: None of the authors have relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–823. doi:139/10/817 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. doi: 10.1002/hep.27222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016:n/a-n/a. doi: 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel N, Yopp AC, Singal AG. Diagnostic Delays Are Common Among Patients With Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2015;13(5):543–549. doi: 10.1016/j.cogdev.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. doi: 10.1111/apt.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foerster F, Galle PR. Hepatocellular carcinoma: one world, one cancer—different guidelines? HepatoBiliary Surg Nutr. 2018;7(1):41–43. doi: 10.21037/hbsn.2018.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn RS. HCC in Focus: The Role of Liver Biopsy in Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y). 2016;12(10):628–630. [PMC free article] [PubMed] [Google Scholar]
- 9.Russo FP, Imondi A, Lynch EN, Farinati F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig Liver Dis. 2018;50(7):640–646. doi: 10.1016/j.dld.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 10.Cohen GS, Black M. Multidisciplinary management of hepatocellular carcinoma: A model for therapy. J Multidiscip Healthc. 2013;6:189–195. doi: 10.2147/JMDH.S41206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):791–798.e1. doi: 10.1016/j.cgh.2014.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayo Clinic. Mayo Clinic streamlines hepatocellular carcinoma treatment. Clinical updates. https://www.mayoclinic.org/medical-professionals/clinical-updates/digestive-diseases/mayo-clinic-streamlines-hcc-treatment. Published 2015. Accessed August 28, 2018. [Google Scholar]
- 13.Leoni S, Piscaglia F, Serio I, et al. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: Experience of the Bologna Liver Oncology Group. Dig Liver Dis. 2014;46(6):549–555. doi: 10.1016/j.dld.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 14.Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407–1432. doi: 10.1002/hep.20704 [DOI] [PubMed] [Google Scholar]
- 15.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sada Y, David E, El-Serag H, Singh H, Davila J. Guideline adherence for diagnosis of liver cancer in veterans. J Clin Oncol. 2013;31. doi: 10.1200/jco.2013.31.31_suppl.89 [DOI] [Google Scholar]
- 17.Koh C, Zhao X, Samala N, Sakiani S, Liang TJ, Talwalkar JA. AASLD clinical practice guidelines: A critical review of scientific evidence and evolving recommendations. Hepatology. 2013;58(6):2142–2152. doi: 10.1002/hep.26578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francica G, Borzio M. Status of, and strategies for improving, adherence to HCC screening and surveillance. J Hepatocell Carcinoma. 2019;Volume 6:131–141. doi: 10.2147/jhc.s159269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal S, Kanwal F, Ying J, et al. Effectiveness of Surveillance for Hepatocellular Carcinoma in Clinical Practice: A United States cohort. J Hepatol. 2016;65(6):1148–1154. doi: 10.1097/OGX.0000000000000256.Prenatal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52(1):132–141. doi: 10.1002/hep.23615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwal F, El-Serag HB, Ross D. Surveillance for hepatocellular carcinoma: can we focus on the mission? Clin Gastroenterol Hepatol. 2015;13:805–807. doi: 10.1016/j.cgh.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 22.Davila JA, Henderson L, Kramer JR, et al. Utilization of Surveillance for Hepatocellular Carcinoma Among Hepatitis C Virus–Infected Veterans in the United States. Ann Intern Med. 2011;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157(1):54–64. doi: 10.1053/j.gastro.2019.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rastogi A Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24(35):4000–4013. doi: 10.3748/wjg.v24.i35.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sada Y, Hou J, Richardson P, El-Serag H, Davila J. Validation of case finding algorithms for hepatocellular cancer from administrative data and electronic health records using natural language processing. Med Care. 2016;54(2):e9–e14. doi: 10.1097/MLR.0b013e3182a30373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x [DOI] [PubMed] [Google Scholar]
- 27.Song DS, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18(3):258–267. doi: 10.3350/cmh.2012.18.3.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Song T. Hepatocellular carcinoma: Advances in diagnostic imaging. Drug Discov Ther. 2015;9(5):310–318. doi: 10.5582/ddt.2015.01058 [DOI] [PubMed] [Google Scholar]
- 30.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Befeler AS, Di Bisceglie AM. Hepatocellular Carcinoma: Diagnosis and Treatment. Gastroenterology. 2002;122(6):1609–1619. doi: 10.1053/gast.2002.33411 [DOI] [PubMed] [Google Scholar]
- 32.Murphy DR, Meyer AND, Vaghani V, et al. Development and Validation of Trigger Algorithms to Identify Delays in Diagnostic Evaluation of Gastroenterological Cancer. Clin Gastroenterol Hepatol. 2018;16(1):90–98. doi: 10.1016/j.cgh.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 33.Department of Veterans Affairs. Healthcare Inspection Quality of Care Issues. Pittsburgh, Pennsylvania; 2013. https://www.va.gov/oig/pubs/VAOIG-13-01855-336.pdf. [Google Scholar]
- 34.Graber ML, Franklin N, Gordon R. Diagnostic Error in Internal Medicine. 2005;165. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Johnson KB, Roccaro G, et al. Poor Adherence to AASLD Guidelines for Chronic Hepatitis B Management and Treatment in a Large Academic Medical Center. Am J Gastroenterol. 2014;109(6):867–875. doi: 10.1038/ajg.2014.72.Poor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of Veterans Affairs VNHCRC. Management of Hepatocellular Carcinoma ( HCC ).; 2009. http://www.hepatitis.va.gov/provider/guidelines/2009hcc.asp.
- 37.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julapalli VR, Kramer JR, El-Serag HB. Evaluation for liver transplantation: Adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transplant. 2005;11(11):1370–1378. doi: 10.1002/lt.20434 [DOI] [PubMed] [Google Scholar]
- 39.Lyratzopoulos G, Vedsted P, Singh H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br J Cancer. 2015;112(s1):S84–S91. doi: 10.1038/bjc.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a Multidisciplinary Hepatocellular Carcinoma Clinic is Associated with Improved Clinical Outcome. Ann Surg Oncol. 2014;21(4):1287–1295. doi: 10.1245/s10434-013-3413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289(3):816–830. doi: 10.1148/radiol.2018181494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy—A Systematic Review. Gastroenterology. 2019;156(4):976–986. doi: 10.1053/j.gastro.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 43.Siedlikowski ST, Kielar AZ, Ormsby EL, Bijan B, Kagay C. Implementation of LI-RADS into a radiological practice. Abdom Radiol. 2018;43(1):179–184. doi: 10.1007/s00261-017-1219-z [DOI] [PubMed] [Google Scholar]
- 44.Kim YY, Choi JY, Sirlin CB, An C, Kim MJ. Pitfalls and problems to be solved in the diagnostic CT/MRI Liver Imaging Reporting and Data System (LI-RADS). Eur Radiol. 2019;29(3):1124–1132. doi: 10.1007/s00330-018-5641-6 [DOI] [PubMed] [Google Scholar]
- 45.Clark TJ, McNeeley MF, Maki JH. Design and implementation of handheld and desktop software for the structured reporting of hepatic masses using the LI-RADS schema. Acad Radiol. 2014;21(4):491–506. doi: 10.1016/j.acra.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 46.Crawford SC, Davis JA, Siddiqui NA, De Caestecker L, Gillis CR, Hole D. The waiting time paradox: Population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. Br Med J. 2002;325(7357):196. doi: 10.1136/bmj.325.7357.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rupassara KS, Ponnusamy S, Withanage N, Milewski PJ. A paradox explained? Patients with delayed diagnosis of symptomatic colorectal cancer have good prognosis. Color Dis. 2006;8(5):423–429. doi: 10.1111/j.1463-1318.2006.00958.x [DOI] [PubMed] [Google Scholar]
- 48.Symonds RP. Rapid Response: The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002;325(196). doi: 10.1136/bmj.325.7357.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101(2009):S9–S12. doi: 10.1038/sj.bjc.6605384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi-center cohort of patients with cirrhosis. Hepatology. 2020:0–3. doi: 10.1002/hep.31159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy DR, Meyer AND, Vaghani V, et al. Electronic Triggers to Identify Delays in Follow-Up of Mammography: Harnessing the Power of Big Data in Health Care. J Am Coll Radiol. 2017;15(2):287–295. doi: 10.1016/j.jacr.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 52.Murphy DR, Meyer AND, Bhise V, et al. Computerized Triggers of Big Data to Detect Delays in Follow-up of Chest Imaging Results. Chest. 2016;150(3):613–620. doi: 10.1016/j.chest.2016.05.001 [DOI] [PubMed] [Google Scholar]


