Abstract
Background
Tumor biomarkers (TBMs) reflect disease burden and correlate with survival for small bowel neuroendocrine tumors (SBNETs). This study sought to determine the performance of chromogranin A (CgA), pancreastatin (PST), neurokinin A (NKA), and serotonin (5HT) during follow-up assessment of resected SBNETs.
Methods
An institutional database identified patients undergoing surgery for SBNETs. Tumor biomarker levels were assessed as categorical (normal vs elevated) and continuous variables for association with progression-free survival (PFS) and overall survival (OS) via the Kaplan-Meier method with Cox multivariable models adjusted for confounders. Sensitivity, specificity, and predictive values of TBM levels in identifying imaging-confirmed progression were calculated.
Results
In 218 patients (44 % female, 92 % node+, 73 % metastatic, 97 % G1 or G2), higher levels of CgA, PST, NKA, and 5HT correlated with higher-grade and metastatic disease at presentation (p < 0.05). Elevated pre- and postoperative CgA, PST, and NKA correlated with lower PFS and OS (p < 0.05; median follow-up period, 49.6 months). Normal CgA, PST, and NKA were present in respectively 20.3 %, 16.9 %, and 72.6 % of the patients with progression, whereas elevated levels were present in respectively 69.5 %, 24.8 %, and 1.3 % of the patients without progression. Using TBMs to determine progression showed superiority of PST (78.9 % accuracy) over CgA (63.3 % accuracy) or CgA and PST together (60.3 % accuracy).
Conclusion
Although specific for progression, NKA was rarely elevated, limiting its usefulness. Pre- and postoperative PST and CgA correlated with disease burden and survival, with PST providing better discrimination of outcomes. During the follow-up period, use of PST most accurately detected progression. These results suggest that PST should replace CgA for SBNET surveillance.
Introduction
Small bowel neuroendocrine tumors (SBNETs) are increasing in incidence and currently are the most common tumors of the small intestine.1,2 The utility of tumor biomarkers (TBMs) in diagnosing and monitoring SBNETs remains a contested issue. Biomarkers may be clinically valuable because they can provide information regarding individual prognosis, response to treatment, and recurrence or progression, which inform clinical decision-making and treatment. Although imaging and treatment of SBNETs have evolved greatly in the past decade, guidelines regarding tumor biomarkers remain largely unchanged. Expert consensus guidelines recommend evaluating chromogranin A (CgA) in surveillance regimens for recurrence or progression.3–5 Introduced as a TBM for NETs in the 1980s,6 CgA remains one of the most consistently monitored TBMs in NETs.
Despite its widespread use, limitations of CgA include low sensitivity and specificity, 7 lack of standardization between assays,8 and lack of consensus regarding reference ranges. In detecting imaging-confirmed disease progression, CgA has a reported sensitivity of 71 % and a reported specificity 50 %.9 The lack of specificity is due to a high rate of false positives because CgA is released from normal neuroendocrine tissue10 and elevated with some medications and diseases such as non-neuroendocrine neoplasias, congestive heart failure, and inflammatory diseases.7,9,11 Renal dysfunction can cause a rise of CgA levels to those seen in NETs, with worsening dysfunction leading to higher CgA levels12. Proton-pump inhibitor (PPI) use is a common cause of elevated CgA levels, and even a short course of PPIs can cause a 2.5-fold increase in CgA levels.13
Other TBMs used to monitor patients with SBNETs include neurokinin A (NKA), serotonin (5HT), and pancreastatin (PST). Neuron-specific enolase is less commonly monitored because it is highly variable, provides little prognostic information, and has lower sensitivity and specificity in determining recurrence.14 Pancreastatin, a cleavage product of CgA,10,15 is of interest as a higher-quality biomarker because it is more sensitive and specific than CgA in detecting progression and predicting survival.16–20
This study sought to compare the prognostic value, sensitivity, and specificity of CgA, PST, NKA, and 5-HT in the follow-up evaluation of surgically treated patients with SBNETs.
METHODS
In this single-institution, retrospective study, clinical data from all patients undergoing surgical resection for duodenal, jejunal, and ileal SBNETs between 1999 and 2019 were analyzed under an institutional review board-approved protocol. Demographic data, survival outcomes from date of surgery, and TBM levels (CgA, PST, NKA, and 5HT) from before and after surgery, as well as during long-term follow-up evaluation, were recorded. Data for 98 patients in a previous report were updated.16
A single surgeon performed all the procedures for cure or palliation of disease. Most operations involved resection of the primary tumor and mesenteric nodes with or without retroperitoneal nodes, hepatic cytoreduction where possible, and cholecystectomy in most cases.16,21,22 Patients with metastatic disease were treated intraoperatively with an octreotide drip at 100 μg/h, which was weaned during 24 h postoperatively. Tumor grade was determined by the Ki-67 proliferation index in whole sections from tumors.
Patients generally were followed by TBM measurement and computed tomography (CT) scan every 6 months for the first 2 years postoperatively, with the frequency of further follow-up assessment determined by disease status (usually continuing every 6 months for patients with progression or persistent disease). Biochemical response was defined as a reduction in biomarker levels of 50 % or more from the preoperative level.17,21,23 Progression was recorded on the date that imaging showed new or increasing size of metastatic lesions or on the date of death from any cause.
The normal ranges for markers were per laboratory reference ranges as follows: CgA (<95 ng/mL), 5HT (<200 ng/mL) (both CgA and 5HT from ARUP Laboratories, Salt Lake City, UT, USA), PST (<135 pg/mL), and NKA (≤40 pg/mL) (both PST and NKA from Interscience Institute, Inglewood, CA, USA).
Progression-free survival (PFS) and overall survival (OS) were calculated by the Kaplan-Meier method. Tumor biomarkers were assessed for association with PFS and OS, as both categorical (normal vs elevated above the reference range) and continuous variables, with Cox multivariable models adjusted for confounders.24 Continuous laboratory values were log-transformed to account for skew. Values reported as less than a lower limit of detection were assigned a value half the range (e.g. <10 was recorded as 5).
The median follow-up was determined using the reverse Kaplan-Meier method.25 Sensitivity, specificity, and predictive values of TBM levels in identifying imaging-confirmed progression were calculated. Calculations used TBM values at the last follow-up visit for patients without progression. Fisher’s exact test or Wilcoxon rank-sum test was used to compare patient characteristics. Paired (i.e., pre- and postsurgery) laboratory values were compared via Wilcoxon sign-rank tests, with significance set at a p value lower than 0.05. Analyses were performed using R version 3.6.2 (Vienna, Austria).
RESULTS
Surgical resection of SBNETs was performed for 218 patients, and 95 (44 %) of these patients were women. The median age at surgery was 62 years (range, 28–84 years), and most of the patients were in their sixth or seventh decade (interquartile range [IQR], 54.7–70.7 years). Most of the patients had nodal involvement (92 %) and distant metastasis (73 %) at the time of surgery. The highest grade from any tumor source (primary, node, or liver metastases) was recorded for 212 patients. Grade 1 (G1) tumors were present in 52.4 %, G2 in 44.3 %, and G3 in 3.3 % of the patients.
The median follow-up period was 49.6 months (95 % confidence interval [CI], 42.3–59.6 months). During this time, 101 of the patients progressed, and 57 patients died. The median PFS from surgery was 49.2 months (95 % CI, 33.6–82.8 months), and the median OS was 118.8 months (95 % CI, 90–160.8 months).
Tumor Biomarker Levels
The different tumor biomarkers had varying rates of preoperative elevation above their reference ranges. Only 35 of 163 patients had elevated NKA (21.5 %), whereas PST, CgA, and 5HT were elevated in respectively 73.9 %, 77.3, % and 86.3 % of the patients.
To determine correlation of TBMs with known indicators of aggressive disease, preoperative CgA, PST, NKA, and 5HT were tested for univariate association with tumor grade as well as nodal and distant metastases (Table 1). The patients with G1 disease had a lower median preoperative PST than the patients with G2 or G3 disease (p < 0.01). Lower median PST values also were associated with negative nodal status (p = 0.04) and absence of distant metastasis (p < 0.01). In comparison, the median CgA levels did not differ between the patients with and without nodal metastasis (p = 0.7). Lower CgA levels were associated with lower grade and absence of metastasis (p < 0.01 for both). Like PST, lower NKA and 5HT levels were associated with lower-grade disease, negative nodal status, and no distant metastases. These results suggest that PST, NKA, and 5HT levels correlate with grade and extent of disease at presentation.
TABLE 1.
Association between preoperative tumor biomarker levels and clinicopathologic characteristics of SBNET diseasea
| PST (n = 199) | CgA (n = 198) | NKA (n = 163) | 5HT (n = 205) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (pg/mL) | p Value | Median (ng/mL) | p Value | Median (pg/mL) | p Value | Median (ng/mL) | p Value | ||
| Grade | G1 (n = 111) | 259 | <0.01 | 161 | <0.01 | 14.5 | <0.01 | 852 | <0.01 |
| G2 or G3 (n = 101) | 473 | 369 | 23.0 | 1147 | |||||
| Nodal status | Negative (n = 18) | 170 | 0.04 | 200 | 0.7 | 5.0 | 0.02 | 322 | 0.045 |
| Positive (n = 197) | 392 | 234 | 20.0 | 1016 | |||||
| Metastasis | No (n=57) | 95.5 | <0.01 | 97.0 | <0.01 | 11.5 | <0.01 | 395 | <0.01 |
| Yes (n = 158) | 564 | 289 | 22.0 | 1152 | |||||
SBNET, small bowel neuroendocrine tumor; PST, pancreastatin; CgA, chromogranin A; NKA, neurokinin A; 5HT, serotonin
Bold indicates statistical significance (p < 0.05). Higher levels of PST, NKA, and 5HT were associated with increased tumor grade, node-positivity, and metastases. Higher levels of CgA were associated with increased grade and metastases, but not positive nodal status.
Tumor Biomarker Levels and Survival
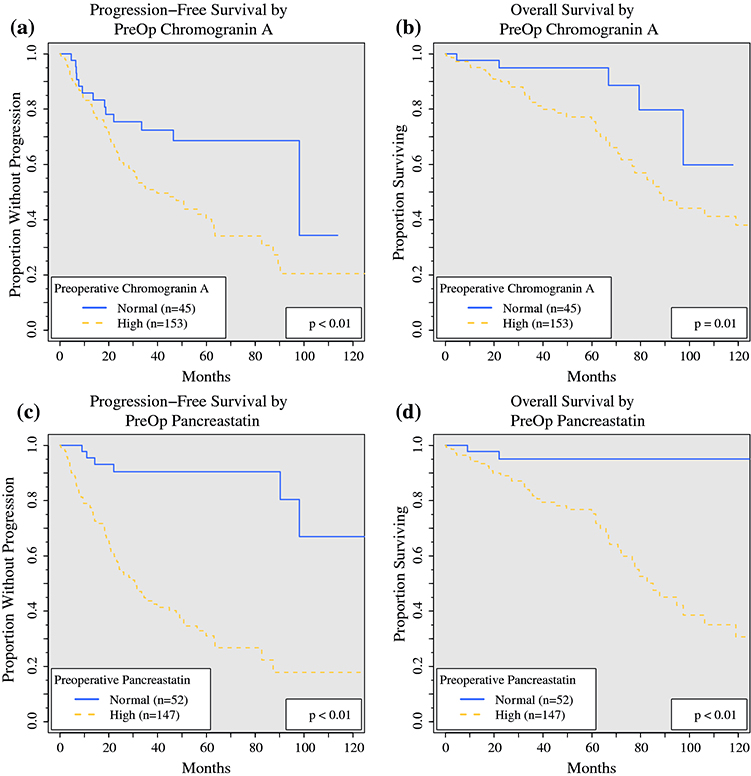
To determine the prognostic value of tumor biomarkers, PST, CgA, NKA, and 5HT were tested as categorical variables (elevated above reference range vs normal) for univariate association with PFS and OS (Table 2, Fig. 1). Elevated preoperative PST was significantly associated with lower PFS (median, 31.0 vs 141.1 months; p < 0.01) and OS (median, 85.1 months vs not reached; p < 0.01). Elevated preoperative CgA also was associated with lower PFS (median, 39.7 vs 98.1 months; p < 0.01) and OS (median, 87.8 months vs not reached; p = 0.01). Elevated NKA was associated with lower PFS and OS, but elevated 5HT was not associated with a statistically significant difference in PFS or OS.
TABLE 2.
Association of preoperative tumor biomarker levels with progression-free survival (PFS) and overall survival (OS)a
| PST p Value | CgA p Value | NKA p Value | 5HT p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median PFS (months) | Normal preop | 141.1 (n = 52) | <0.01 | 98.1 (n = 45) | 0.01 | 63.5 (n = 128) | <0.01 | 87.4 (n = 28) | 0.3 |
| Elevated preop | 31.0 (n = 147) | 39.7 (n = 153) | 20.6 (n = 35) | 50.7 (n = 177) | |||||
| Median OS (months) | Normal preop | NR (n = 52) | <0.01 | NR (n = 45) | 0.01 | 94.8 (n = 128) | <0.01 | NR (n = 28) | 0.3 |
| Elevated preop | 85.1 (n = 147) | 87.8 (n = 153) | 61.6 (n = 35) | 106.2 (n = 177) | |||||
PST, pancreastatin; CgA, chromogranin A; NKA, neurokinin A; 5HT, serotonin; preop, preoperative; NR not reached
Bold indicates statistical significance (p < 0.05). Elevated preoperative PST, CgA, and NKA were associated with lower median PFS and OS. Elevated 5HT was not associated with decreased PFS or OS.
FIG. 1.
Progression-free and overall survival based on preoperative CgA and PST levels. The survival of patients with normal preoperative levels (blue solid line) are compared with those of patients with high levels (elevated above reference range, red dashed lines). The patients with elevated preoperative CgA had decreased (a) PFS (median PFS, 39.7 vs 98.1 months; p < 0.01) and (b) OS (median OS, 87.8 vs not reached; p = 0.01), and those with elevated postoperative PST also had decreased (c) PFS (median PFS, 31.0 vs 141.1 months; p < 0.01) and (d) OS (median OS, 85.1 months vs not reached, p < 0.01). CgA, chromogranin A; PST, pancreastatin; PFS, progression-free survival; OS, overall survival
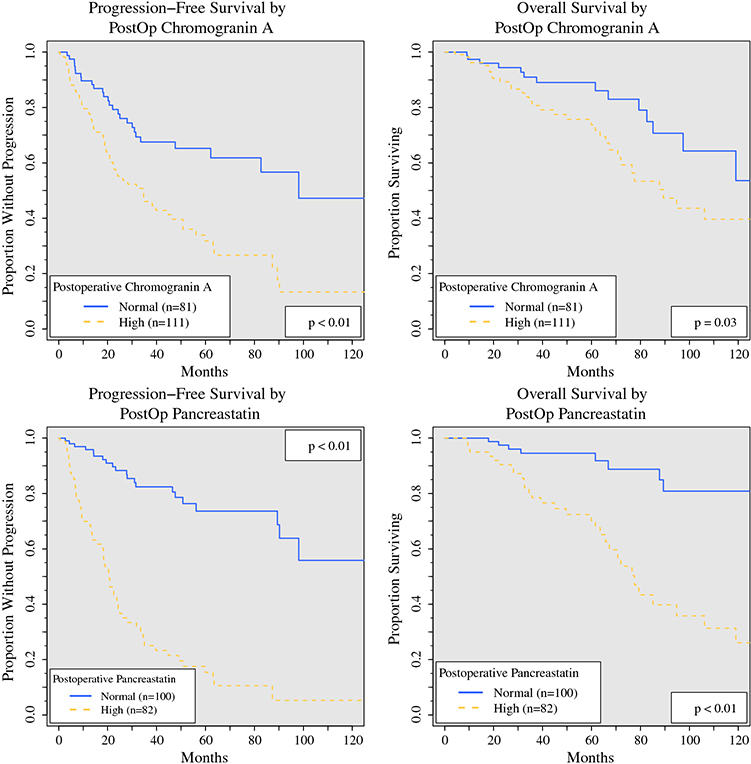
Postoperative laboratory values were similarly tested as categorical variables for univariate association with PFS or OS (Table 3, Fig. 2). The median time between surgery and postoperative evaluation of TBMs was 3.6 months (IQR, 1.8–6.1 months). Biomarker levels were less commonly elevated after surgery. Postoperative PST, CgA, NKA, and 5HT were elevated above reference ranges in respectively 45.0 %, 57.8 %, 12.0 %, and 74.1 % of the patients. The postoperative levels of TBMs were significantly lower than the preoperative levels (p < 0.01 for all). Among the patients with high preoperative TBM levels, the median postoperative values were 60.3 % (PST) to 36.4 % (NKA) lower, whereas 8.8 % (PST) to 14.4 % (CgA) of the patients’ postoperative TBM levels were higher than their preoperative levels. Elevated postoperative PST was associated with significantly lower PFS (median, 20.6 vs 130.9 months; p < 0.01) and OS (median, 77.6 months vs not reached; p < 0.01). Elevated postoperative CgA also was associated with lower PFS (median, 34.5 vs 98.1 months; p < 0.01) and OS (median, 89.4 vs 125.4 months; p = 0.03). These results indicate that elevated PST, CgA, and NKA levels are associated with worse PFS and OS.
TABLE 3.
Association between postoperative tumor biomarker levels and progression-free survival (PFS) and overall survival (OS)a
| PST | CgA | NKA | 5HT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median PFS (months) | Normal postop | 130.9 (n = 100) | <0.01 | 98.1 (n = 81) | <0.01 | 89.4 (n = 113) | <0.01 | 62.1 (n = 50) | 0.2 |
| Elevated postop | 20.6 (n = 82) | 34.5 (n = 111) | 9.7 (n = 15) | 39.7 (n = 143) | |||||
| Median OS (months) | Normal postop | NR (n = 100) | <0.01 | 125.4 (n = 81) | 0.03 | 106.2 (n = 113) | <0.01 | NR (n = 50) | 0.1 |
| Elevated postop | 77.6 (n = 82) | 89.4 (n = 111) | 31.8 (n = 15) | 106.2 (n = 143) | |||||
PST, pancreastatin; CgA, chromogranin A; NKA, neurokinin A; 5HT, serotonin; postop, postoperative
Bold indicates statistical significance (p < 0.05). Elevated postoperative PST, CgA, and NKA were associated lower median PFS and OS. Elevated 5HT was not associated with decreased PFS or OS.
FIG. 2.
Progression-free and overall survival based on postoperative CgA and PST levels. The survival of patients with normal postoperative levels (blue solid line) are compared with those of patients with high levels (elevated above reference range, red dashed lines). Patients with elevated postoperative CgA had decreased (a) PFS (median PFS, 34.5 vs 98.1 months; p < 0.01) and (b) OS (median OS, 89.4 vs 125.4 months; p = 0.03), and those with elevated postoperative PST also had decreased (c) PFS (median PFS, 20.6 vs 130.9 months; p < 0.01) and (d) OS (median OS, 77.6 months vs not reached; p < 0.01). CgA, chromogranin A; PST, pancreastatin; PFS, progression-free survival; OS, overall survival
In addition to the classification of TBM as elevated or normal, the degree of TBM elevation also may provide prognostic information. To determine the association between the degree of TBM elevation and the risk of progression or death, TBMs were analyzed as continuous variables. The tumor biomarkers were log2-transformed due to skew, and hazard ratios (HRs) reflect the change in risk associated with a doubling of the TBM level. For every doubling of preoperative PST, the risk of progression increased by more than 40 % (HR, 1.41; 95 % CI, 1.28–1.55; p < 0.01), and the risk of death increased by 50 % (HR, 1.50; 95 % CI, 1.31–1.73; p < 0.01). The HRs were similar for postoperative PST (progression HR, 1.4; 95 % CI, 1.29–1.52) and death (HR, 1.48; 95 % CI, 1.31–1.55) (p < 0.01 for both).
Preoperatively, the HR for progression was 1.28 (95 % CI, 1.18–1.39) for CgA, 1.27 (95 % CI, 1.12–1.44) for NKA, and 1.29 (95 % CI, 1.10–1.52) for serotonin (5HT) (p < 0.01 for all). Preoperatively, the HR for death was 1.27 (95 % CI, 1.14–1.41) for CgA, 1.37 (95 % CI, 1.14–1.65) for NKA, and1.33 (95 % CI, 1.05–1.70; p = 0.02) for serotonin (5HT) (p < 0.01 unless otherwise specified).
Postoperatively, the HR for progression was 1.28 (95 % CI, 1.17–1.39) for CgA, 1.28 (95 % CI, 1.13–1.46) for NKA, and 1.44 (95 % CI, 1.23–1.67) for serotonin (5HT) (p < 0.01 for all). Posoperatively, the HR for death was 1.37 (95 % CI, 1.21–1.54) for doubling of CgA, 1.41 (95 % CI, 1.17–1.69) for doubling of NKA, and 1.45 (95 % CI, 1.18–1.78) for doubling of serotonin (5HT) (p < 0.01 for all). These findings suggest that higher elevations of pre- and postoperative PST, CgA, NKA, and 5HT are associated with increased risk of progression and death. Each TBM then was analyzed individually as a continuous variable in separate multivariable models. After correction for age, metastases, node-positive disease, and grade, the higher levels of preoperative CgA, PST, and NKA remained independently associated significantly with diminished OS (CgA. p = 0.01; PST and NKA, p < 0.01) and PFS (p < 0.01 for all). Preoperative 5HT was not independently predictive of either PFS or OS in this model. We sought to compare TBMs in a unified multivariable model.
However, all TBMs could not be simultaneously included in a valid model due to insufficient events and collinearity of information. As a result, we created an optimal multivariable model using reverse stepwise selection (Table 4). With this procedure, we determined which TBM carried the most predictive power by including all TBMs in a model and then subtracting the biomarker with the greatest p value until only biomarkers significantly associated with the outcome remained. Models were created for PFS and OS using pre- and postoperative TBM levels. In each case, after stepwise selection, only PST remained in the model (p values <0.01 for all PST), suggesting that addition of other biomarkers did not provide significantly improved survival information beyond that carried by PST.
TABLE 4.
Stepwise-selected multivariable models to determine independently significant variables associated with progression-free survival (PFS) and overall survival (OS)a
| Preoperative model (n = 194) | PFS | OS | ||
|---|---|---|---|---|
| Clinical feature | HR (CI) | p Value | HR (CI) | p Value |
| Age (per year) | 1.01 (1.00−1.04) | 0.09 | 1.05 (1.01−1.71) | <0.01 |
| Node-positive | 1.05 (0.37−2.99) | 0.9 | 5.50 (0.69−43.8) | 0.1 |
| Metastasis present | 4.21 (1.67−10.6) | <0.01 | 4.09 (0.93−17.9) | 0.06 |
| Low grade | 0.54 (0.34−0.84) | <0.01 | 0.37 (0.19−0.69) | <0.01 |
| Log2-preop PST | 1.35 (1.20−1.51) | <0.01 | 1.43 (1.19−1.71) | <0.01 |
| Postop model (n = 176) | ||||
| Age (per year) | 1.02 (1.00−1.04) | 0.03 | 1.04 (1.01−1.08) | <0.01 |
| Node-positive | 0.82 (0.38−1.78) | 0.6 | 2.16 (0.59−7.86) | 0.2 |
| Metastasis present | 2.23 (1.07−4.63) | 0.03 | 1.33 (0.43−4.12) | 0.6 |
| Low grade | 0.54 (0.34−0.86) | 0.01 | 0.41 (0.21−0.81) | <0.01 |
| Log2-postop PST | 1.40 (1.27−1.54) | <0.01 | 1.48 (1.28−1.71) | <0.01 |
HR, hazard ratio; CI, confidence interval; preop, preoperative; postop, postoperative; PST, pancreastatin
Bold indicates statistical significance (p < 0.05). Tumor biomarker levels were log2-transformed due to skew. Hazard ratios thus reflect the risk associated with each doubling of pancreastatin levels.
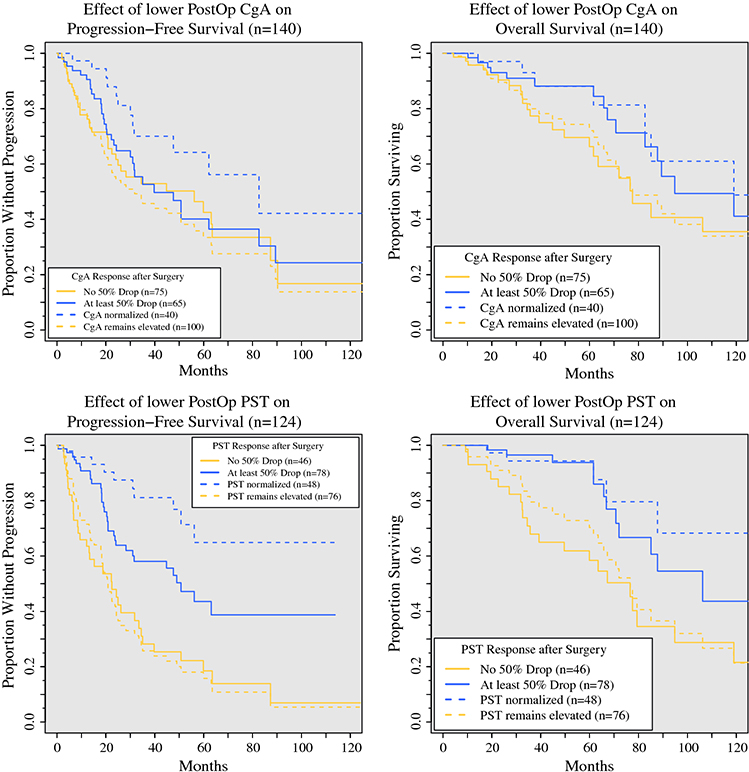
Having determined that pre- and postoperative PST and CgA provide prognostic information, we sought to determine whether the normalization of PST and CgA after surgical treatment provides prognostic information. The patients with elevated preoperative PST (n = 124) and CgA (n = 140) were considered (Fig. 3). These patients were stratified by postoperative TBM levels (normal vs continued elevation), and OS and PFS were determined. The patients whose PST normalized postoperatively (n = 48) had a significantly longer OS (median not reached vs 76.6 months; p < 0.01), PFS (median not reached vs 20.9 months; p < 0.01), 5-year OS (94 % vs 70 %; p < 0.01), and 5-year PFS (65 % vs 16 %; p < 0.01).<AQ6> Normalization of CgA predicted longer PFS (median, 82.7 vs 31.8 months; p < 0.01), but not OS (median, 119.0 vs 77.6 months; p = 0.10).
FIG. 3.
Progression-free and overall survival of patients with preoperatively elevated CgA and PST levels, as determined by biochemical response (postoperative level <50 % of preoperative level, solid lines) or normalization of levels (dashed lines). Red lines designate patients whose postoperative levels remained elevated or who did not achieve biochemical response, whereas blue lines show those with normalized levels or those achieving a biochemical response. a PFS improved with normalization of CgA (median PFS, 82.7 vs 31.8 months; p < 0.01), but not a biochemical response in CgA (5-year PFS, 40 vs 43 %; p = 0.60). b OS did not improve with normalization of CgA (median OS, 119.0 vs 77.6 months; p = 0.10) or a biochemical response (5-year OS, 88 vs 66 %; p = 0.10). c PFS improved with normalization of PST (5-year PFS, 65 vs 16 %; p < 0.01) and a biochemical response (5-year PFS, 44 vs 19 %; p < 0.01). d OS improved with normalization of PST (5-year OS, 94 vs 70 %; p < 0.01) and a biochemical response (5-year OS, 94 vs 58 %; p < 0.01). CgA, chromogranin A; PST, pancreastatin; PFS, progression-free survival; OS, overall survival
Most of the patients with elevated preoperative PST failed to normalize postoperatively, but the majority still had substantial drops in postoperative PST. Biochemical response has been defined previously as a postoperative decrease in TBM level by at least 50 %.21,23 To determine whether a biochemical response in PST predicted improved survival, we considered the patients who had elevated preoperative PST and experienced a biochemical response postoperatively (n = 78). These patients had a significantly longer 5-year OS than the patients who did not normalize or experience a biochemical response (94 % vs 58 %; p < 0.01). A large difference also was observed in the 5-year PFS between the patients with and without a biochemical response (44 % vs 19 %; p < 0.01). This demonstrated that even if PST does not normalize after surgery, a reduction of 50 % or more portends improved survival. Biochemical response in CgA was less informative. Among the 65 patients with a biochemical response in CgA, neither PFS nor OS differed from those for the 75 patients without a response (5-year PFS, 40 % vs 43 %; p = 0.6; 5-year OS, 88 % vs 66 %; p = 0.1).
The sensitivities and specificities of TBMs in predicting imaging-confirmed progression at any time during the follow-up period were calculated. Elevated postoperative PST was more sensitive (83.1 %) and specific (65.7 %) in determining disease progression than CgA (sensitivity, 76.7 %; specificity, 52.3 %), although the difference did not reach statistical significance. Although very specific (98.7 %), NKA was insensitive (27.4 %) for determining progression. By comparison, PST was less likely to be falsely elevated than CgA (false-positive rate, 24.8 % vs. 69.5 %, p < 0.01). The rates of falsely normal markers were similar for PST and CgA (false-negative rate, 16.9 % vs 20.3 %; p = 0.35). The overall accuracy of PST was higher (78.9 %), whereas the accuracy of CgA was 63.3 % (p < 0.01). Using both PST and CgA to determine progression resulted in lower accuracy than using either alone (60.3 %). Thus, PST more accurately identified progression than CgA alone or even CgA and PST together.
DISCUSSION
This study found that higher PST levels correlate with more advanced SBNET disease. Elevated pre- and postoperative PST levels independently predicted decreased OS and PFS for the patients who underwent surgical cytoreduction. For the patients who had elevated preoperative PST, postoperative normalization or biochemical response predicted longer OS and PFS than for the patients whose PST levels failed to normalize or respond biochemically.
Postoperative normalization of CgA predicted improved PFS, but not OS. For detecting progression, PST was more sensitive and specific, with lower false-positive and false-negative rates than CgA. Of the biomarkers evaluated, PST was the most accurate, classifying patients accurately as they progressed more often than CgA alone or CgA and PST. Elevated 5HT did not correlate with survival, and NKA was too seldom elevated to be useful for most patients. Thus, although its overall accuracy of about 80 % for detecting progression was insufficient for PST to be recommended as the sole determinant of progression, particularly when imaging is excellent and remains the gold standard, the association of higher PST with more advanced disease and worse outcomes makes consistently increasing PST a reasonable criterion for therapy escalation. Additionally, for clinical trial inclusion, the large difference in 5-year PFS between the patients with and those without elevated PST suggests that consideration of patients’ PST levels could identify high-risk patients most likely to benefit from and demonstrate the effects of novel therapies.
Although the levels of PST, CgA, and NKA correlated with survival outcomes after adjustment for grade, nodal status, and age, CgA and NKA were less informative than PST, and neither were independently associated with survival after consideration of PST.
In the multivariable model, node-positive status was not independently associated significantly with PFS or OS, and the presence of distant metastasis was not associated with OS. This likely was due to the high rate of metastases in our patient group (92 % had nodal and 73 % had liver metastases), and to the fact that most of the patients who had distant metastases also had positive nodes, rendering these factors less informative in the multivariable analysis. This finding also demonstrated the power of PST as an indicator of disease extent and survival because metastases and node-positive status are not significant after consideration of PST.
The advantages of PST as a tumor biomarker may derive from its smaller structure and more specific function. It is a post-translational proteolytic peptide product of CgA, a larger prohomone found throughout the neuroendocrine and central nervous systems.10,15,26 The CgA-derived peptides have many functions, including regulation of metabolism, catecholamine release, calcium levels, blood pressure, reproductive hormones, and immune response to infections.11 As such, CgA is elevated in benign and malignant endocrine diseases, cardiovascular disease, renal insufficiency, auto-immune disorders, gastritis, and non-neuroendocrine neoplasias, including cancers of the pancreas, lung, prostate, ovary, and breast.11 The role of PST is more specific in regulating metabolism, and its circulating plasma levels are 100-fold lower than CgA levels.27,28 The levels of PST are not affected by PPI use, which is significant because 20 % of patients with gastroenteropancreatic NETs take PPIs.9,29 The radioimmunoassay for PST is specific for its carboxy-terminal, with minimal cross-reactivity to CgA, and the two assays are comparable in price.16,27 As a biomarker, PST is thus more informative and less plagued by the imprecision of CgA.
The limitations of this study were its retrospective nature and the small number of deaths, which limited the ability to calculate OS and to include all TBMs in the multivariable model. Additionally, we did not routinely check 24-h urinary 5-hydroxyindoleacetic acid (5-HIAA) levels, which is a longstanding practice in many centers. A 24-h urine collection is more cumbersome and less practical than blood tests. We did not routinely require that our patients avoid serotonin-rich foods before blood testing, which limits the utility of 5HT.
The subjects in this study were unique in that most of them presented with advanced disease, all underwent resection of primary tumor and regional lymph nodes, and hepatic cytoreduction was performed at a high rate. The high rate of progression allowed us to study the value of changing marker levels in relation to progression.
Another strength of this study was the direct comparison of multiple commonly tested TBMs in a large sample of patients with long follow-up times. Our results confirmed previous findings that PST is more sensitive and specific than CgA in detecting progression, and that elevated PST levels portend shorter survival in surgically treated SBNETs.19,20 Other studies have found that PST also predicts survival for patients treated with somatostatin analogue therapy and transarterial chemoembolization.17,30
Not all centers routinely follow TBMs.4 Some centers question the accuracy of TBMs and their role in clinical decision-making. Other centers advocate assays targeting circulating tumor mRNA, such as the NETest (Wren Laboratories, Branford, CT, USA), as a more accurate alternative to monoanalyte biomarkers. The NETest is a multianalyte algorithmic real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) test for 51 NET marker genes.31,32
A recent publication reported the the NETest is about 85 % accurate in identifying progression.33 Our study found that PST has a comparable accuracy of 78.8 % in differentiating stable from progressive disease. The NETest is not widely used, and to date has not been recommended in various follow-up guidelines.34 The NETest is more expensive than a PST assay (≥$500 vs $225 per test), although the exact price for the former has not been determined to date. Other biomarkers, such as circulating tumor cells and miRNA assays, are being studied but do not yet have practical clinical applications.35
The current study suggests that monoanalyte TBMs still have value in clinical decision-making, and it may not be time to abandon them. The National Comprehensive Cancer Network (NCCN) guidelines recommend routinely checking CgA levels in surveillance of SBNET patients, but our results support routine evaluation of PST instead of CgA in SBNET surveillance. Levels of PST reflect the extent of disease at presentation and provide more substantial prognostic information than CgA. Tumor biomarkers are a useful adjunct to imaging (CT, MRI, and/or 68Ga-DOTATATE), and we use these two complementary methods to help confirm progression at our clinic. If progression occurs during current therapy (or observation), it generally is our practice to consider changing therapy. In many instances, progression may appear minimal or equivocal on imaging, and correlating the degree of change on scans with trends in TBMs can be extremely helpful in deciding when to increase the dose of somatostatin analogues, initiate everolimus, pursue embolotherapy, or treat with peptide receptor radionuclide therapy. Patients with elevated postoperative PST levels and those who do not achieve significant biochemical response after tumor resection/cytoreduction also are identified as at increased risk of progression, and therefore may benefit from increased monitoring or earlier consideration of additional therapies.
ACKNOWLEDGMENTS
This work was supported by NIH grants (no. T32CA148062 [C.G.T., A.J.S.], T32CA078586 [S.K.S.]), Specialized Programs of Research Excellence grant no. P50 CA174521-01 (J.R.H., A.M.B., J.S.D., T.M.O.), and CTSA program grant no. UL1TR002537.
Footnotes
CONFLICT OF INTEREST Chandrikha Chandrasekharan, MBBS serves on the advisory board of Lexicon. The remaining authors have no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw. 2018;16:693–702. [DOI] [PubMed] [Google Scholar]
- 4.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas. 2017;46:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Öberg K, Couvelard A, Delle Fave G, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology. 2017;105:201–11. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986;314:1145–51. [DOI] [PubMed] [Google Scholar]
- 7.Marotta V, Nuzzo V, Ferrara T, et al. Limitations of chromogranin A in clinical practice. Biomarkers. 2012;17:186–91. [DOI] [PubMed] [Google Scholar]
- 8.Stridsberg M, Eriksson B, Oberg K, Janson ET. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol. 2003;177:337–41. [DOI] [PubMed] [Google Scholar]
- 9.Vezzosi D, Walter T, Laplanche A, et al. Chromogranin A measurement in metastatic well-differentiated gastroenteropancreatic neuroendocrine carcinoma: screening for false positives and a prospective follow-up study. Int J Biol Markers. 2011;26:94–101. [DOI] [PubMed] [Google Scholar]
- 10.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–49. [DOI] [PubMed] [Google Scholar]
- 11.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A: biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–43. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao RJ, Mezger MS, O’Connor DT. Chromogranin A in uremia: progressive retention of immunoreactive fragments. Kidney Int. 1990;37:955–64. [DOI] [PubMed] [Google Scholar]
- 13.Pregun I, Herszenyi L, Juhasz M, et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion. 2011;84:22–8. [DOI] [PubMed] [Google Scholar]
- 14.Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26:791–802. [DOI] [PubMed] [Google Scholar]
- 15.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–8. [DOI] [PubMed] [Google Scholar]
- 16.Sherman SK, Maxwell JE, O’Dorisio MS, O’Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21:2971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strosberg D, Schneider EB, Onesti J, et al. Prognostic impact of serum pancreastatin following chemoembolization for neuroendocrine tumors. Ann Surg Oncol. 2018;25:3613–20. [DOI] [PubMed] [Google Scholar]
- 18.Khan TM, Garg M, Warner RR, Uhr JH, Divino CM. Elevated serum pancreastatin is an indicator of hepatic metastasis in patients with small bowel neuroendocrine tumors. Pancreas. 2016;45:1032–5. [DOI] [PubMed] [Google Scholar]
- 19.Woltering EA, Beyer DT, Thiagarajan R, et al. Elevated plasma pancreastatin, but not chromogranin A, predicts survival in neuroendocrine tumors of the duodenum. J Am Coll Surg. 2016;222:534–42. [DOI] [PubMed] [Google Scholar]
- 20.Woltering EA, Voros BA, Beyer DT, et al. Plasma pancreastatin predicts the outcome of surgical cytoreduction in neuroendocrine tumors of the small bowel. Pancreas. 2019;48:356–62. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: what is the optimal strategy? Surgery. 2016; 159:320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keck KJ, Maxwell JE, Utria AF, et al. The Distal predilection of small bowel neuroendocrine tumors. Ann Surg Oncol. 2018;25:3207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 25.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- 26.Konecki DS, Benedum UM, Gerdes HH, Huttner WB. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987;262:17026–30. [PubMed] [Google Scholar]
- 27.O’Dorisio TM, Krutzik SR, Woltering EA, et al. Development of a highly sensitive and specific carboxy-terminal human pancreastatin assay to monitor neuroendocrine tumor behavior. Pancreas. 2010;39:611–6. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Margalet V, Gonzalez-Yanes C, Najib S, Santos-Alvarez J. Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. RegulPept. 2010;161:8–14. [DOI] [PubMed] [Google Scholar]
- 29.Raines D, Chester M, Diebold AE, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas. 2012;41:508–11. [DOI] [PubMed] [Google Scholar]
- 30.Stronge RL, Turner GB, Johnston BT, et al. A rapid rise in circulating pancreastatin in response to somatostatin analogue therapy is associated with poor survival in patients with neuroendocrine tumours. Ann Clin Biochem. 2008;45:560–6. [DOI] [PubMed] [Google Scholar]
- 31.Modlin IM, Kidd M, Malczewska A, et al. The NETest: the clinical utility of multigene blood analysis in the diagnosis and management of neuroendocrine tumors. Endocrinol Metab Clin North Am. 2018;47:485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Öberg K, Califano A, Strosberg JR, et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol. 2020;31:202–12. [DOI] [PubMed] [Google Scholar]
- 34.Öberg K, Krenning E, Sundin A, et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect. 2016;5:174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modlin IM, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: from monoanalytes to transcripts and algorithms. Best Pract Res Clin Endocrinol Metab. 2016;30:59–77. [DOI] [PubMed] [Google Scholar]