Abstract
The coronavirus disease 19 (COVID-19) is a highly transmittable viral infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS‐CoV‐2 utilizes metallocarboxyl peptidase angiotensin receptor (ACE) 2 to gain entry into human cells. Activation of several proteases facilitates the interaction of viral spike proteins (S1) and ACE2 receptor. This leads to cleavage of host ACE2 receptors. ACE2 activity counterbalances the angiotensin II effect, its loss may lead to elevated angiotensin II levels with modulation of platelet function, size and activity.
COVID-19 disease encompasses a spectrum of systemic involvement far beyond respiratory failure alone. Several features of this disease, including the etiology of acute kidney injury (AKI) and the hypercoagulable state, remain poorly understood. Here, we show that there is a high incidence of AKI (81%) in the critically ill adults with COVID-19 in the setting of elevated D-dimer, elevated ferritin, C reactive protein (CRP) and lactate dehydrogenase (LDH) levels. Strikingly, there were unique features of platelets in these patients, including larger, more granular platelets and a higher mean platelet volume (MPV). There was a significant correlation between measured D-dimer levels and MVP; but a negative correlation between MPV and glomerular filtration rates (GFR) in critically ill cohort. Our data suggests that activated platelets may play a role in renal failure and possibly hypercoagulability status in COVID19 patients.
Keywords: COVID-19, SARS-CoV-2, AKI, hypercoagulable, Mean platelet volume, D-Dimer, renal failure
Introduction:
The coronavirus disease 19 (COVID-19) is a highly transmittable viral infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(1). On presentation, most infected individuals exhibit dry cough, dyspnea, and bilateral ground‐glass opacities on radiographic images(2). While the critical importance of respiratory derangement in COVID-19 is well appreciated, several clinical features of COVID-19 infection remain poorly understood including the increased incidence of thromboembolic disease despite being on prophylactic anticoagulation and acute renal injury requiring renal replacement therapy. For instance, based on our observation and those of others, several patients rapidly decompensate days after stabilization of oxygenation during or after cessation of mechanical ventilation. Findings of micro and macrothrombi are common in these patients (3). Others have documented cerebral ischemic infarcts in these subjects(4). Microvascular thrombosis appears to be one defining feature of novel SARS-CoV-2 infection (5). Formation of vascular thrombi in association with progressive severe endothelial injury in COVID-19 infected subjects may determine case fatality and renal dysfunction in this disease.
SARS‐CoV‐2 is single-stranded positive-sense RNA virus, containing an approximately 26–32 kilobase (kb) genome(6). Similar to other CoV, during viral entry into the host cell the spike proteins (S) on the envelope of SARS‐CoV‐2 are cleaved into S1 and S2 subunits(7). Through the S1 receptor binding domain (RBD), S1 directly binds to the peptidase domain (PD) of metallocarboxyl peptidase angiotensin receptor (ACE) 2 to gain entry into human cells (7–9). ACE receptors (ACE and ACE2) are expressed in almost all tissues and ACE2 is expressed predominantly on the alveolar epithelial type II cells and capillary endothelial cells (10,11). ACE and ACE2 regulate vascular tone, fibrinolysis and the coagulation cascade (12,13). ACE activity increases vascular tone and activates the coagulation cascade by promoting the generation of angiotensin (Ang) II. In contrast, ACE2 receptor activity decreases vascular tone and inhibits activation of the coagulation cascade by generating Ang (1–7), which activates a MAS/G protein receptor. SARS‐CoV‐2 infection leads to loss of function of ACE2 and increased angiotensin II levels (14). Activation of the renin-angiotensin–aldosterone system (RAAS) plays critical role in renal injury(15). Angiotensin II is a potent vasoconstrictor and activates the coagulation cascade by increasing platelet activation and size, inducing microvascular thrombosis (12,16).
The coagulation cascade, fibrinolytic system and the complement system closely collaborate to control a wide range of biological and pathological functions including immune responses to pathogens, cell migration and tissue hemostasis. A variety of microorganisms can trigger endothelial injury, alternative complement activation and hence activate the coagulation pathway perpetuating inflammation and organ dysfunction. We hypothesized that platelet activation through multiple mechanisms plays an important role in COVID-19 associated coagulopathy and acute kidney injury (AKI). Our data indicate significant increase incidence of AKI in critically ill patients due to COVID-19 infection. Here we show evidence that mean platelet volume (MPV) reflects platelet activation and activation of coagulation cascades and plays a major role in the development of acute renal failure.
Methods:
Study design and data collection:
We included adult patients with laboratory-confirmed COVID-19 infection admitted to the intensive care units (ICU) at Harper University Hospital in Metro Detroit between March 15 to April 20, 2020. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab. Only laboratory-confirmed cases were included. Eighty-one adults (18 years of age or older) were identified. Informed consent was waived, and researchers analyzed only deidentified (anonymized) data. Data from each institution’s electronic medical record was obtained through a research form. We obtained demographic data, information on clinical symptoms or signs at presentation, laboratory, and radiologic results. Furthermore, we reviewed the peripheral blood smears of patients during ICU admission. All laboratory tests were performed at the discretion of the treating physicians. This study was reviewed and approved by the Detroit Medical Center and the Wayne State University Institutional Review Boards.
Specimen Collection and Testing:
Clinical specimens for COVID-19 diagnostic testing were obtained in accordance with Centers for Disease Control and Prevention (CDC) guidelines. Nasal swabs were performed in all patients using a real-time RT-PCR assay developed by the CDC at the Michigan State Public Health Laboratory to detect the virus.
Laboratory data:
All laboratory data were part of routine clinical data obtained during hospitalization and ICU admission. The following data were analyzed: total white blood cell counts (WBC), absolute lymphocytes counts (ALC), granulocyte count, platelets, D- dimer, fibrinogen, prothrombin time (PT), partial prothrombin time (PTT), international normalized ratio (INR). C reactive protein (CRP), alanine aminotransferase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH); creatine kinase (CPK) and mean platelet volume (MPV). Glomerular filtration rates (GFR) were calculated based on creatinine clearance, gender and race.
Statistical analysis:
Categorical data are presented as frequency and percentage. Continuous data are presented either as box plots (WBC, LDH, CRP, D-D1, D-D2 and ferritin) or line plots with error bars between two group. Independent unpaired t-tests was used for analyses of continuous variables and the bivariate Spearman’s rank correlation coefficient was calculated to measure the relationship between the clinical variables (including AKI) and coagulation profiles. Acute kidney injury is defined as per guidelines of American society of Nephrology (17). For all analyses, p-values <0.05 were considered significant. For the GFR data, a trend analysis was performed on the data from each day for up to 8 days. Furthermore, trend analysis was performed on the daily data for GFR and MPV. Each interval represents data from every 3 day except for the last interval, which includes all data beyond 16 days. We compared the presence and absence of AKI and tested the significance of each interval. A similar analysis was applied on the daily MPV data. Statistical analyses were performed using SPSS statistical software (Version 27, Chicago, IL) and the programing language R.
Results:
During early March through April 15 we reviewed the charts of 81 subjects who were positive for COVID-19 based on the PCR assay. Among 81 subjects, 41 (51%) were female. Mean age was 60±13.8 y (range 24–95 y). Almost all subjects in our study had hypertension (95%), over half (57%) had diabetes mellitus (DM) and many had a combination of both (55%). Patients demographics are shown in Table 1. The mean body mass index (BMI) was 33±8.9 kg·m−2. Overall mortality in our study was 65%. Among study subjects, 66 patients (81%) developed acute renal injury and 49% required renal replacement therapy. As shown in Table 2, we followed a wide range of laboratory parameters, including CRP, ferritin, CPK and LDH. We also evaluated D-dimer levels at two distinct time points during the ICU stay. D-dimer 1 (D1) is the first reported result after ICU admission and D-dimer 2 (D2) was drawn approximately 5 days later. In total, 45 patients had elevated D-dimer levels on the day of ICU-admission. Therapeutic anticoagulation, either intravenous unfractionated heparin (IV-UFH) or low molecular weight heparin (Enoxaparin 1 mg/kg every 12 −24 h depending on creatinine clearance) was administered to 18 patients in this study. All other ICU patients were on pharmacologic venous thromboembolism prophylaxis with subcutaneous UFH.
Table 1.
Clinical characteristics patients
| Characteristic | All Patients (N=81) |
|---|---|
| Age (y, Mean ± SD) | 60 ±13.8 |
| BMI (Mean ± SD) | 32±11.9 |
| Gender, N (%) | |
| Female | 41 (50.9) |
| Male | 40 (49.1) |
| Race, N (%) | |
| African American | 63(78%) |
| Caucasian | 3 (3.7%) |
| Hispanic | 1(1.7%) |
| Unknown | 14 (17.3%) |
| Smoking history, N (%) | 14(6%) |
| Coexisting disorder, N (%) | |
| Hypertension | 75 (92%) |
| Diabetes | 46 (57%) |
| Chronic kidney disease | 19 (23%) |
| Cardiovascular diseases | 19 (23%) |
| Respiratory diseases | 13 (16%) |
| Cancer | 2 (2%) |
Table 2.
Initial laboratory findings
| Variable | Mean ± SD | No. | Reference ranges |
|---|---|---|---|
| White blood cell count, K/CUMM | 8.3 ± 4.5 | 81 | 3.5–10.6 |
| Platelet count, K/CUMM | 229 ± 90 | 81 | 150–450 |
| C-reactive protein, mg/L | 189.8 ± 121.4 | 81 | 0.0–5.0 |
| Ferritin, ng/mL | 1036 ± 1189 | 79 | 24–336 |
| Alanine aminotransferase, U/L | 38 ± 35 | 72 | 7–52 |
| Aspartate aminotransferase, U/L | 58 ± 53 | 73 | 13–39 |
| Alkaline phosphatase, U/L | 69 ± 29 | 61 | 45–115 |
| Lactate dehydrogenase, U/L | 569 ± 313 | 56 | 140–271 |
| Creatine phosphokinase, U/L | 565 ± 1404 | 61 | 30–223 |
| D-Dimer 1, mg/L* | 6.15 ± 8.27 | 70 | 0.0–0.50 |
| D-Dimer 2 mg/L ** | 13.5 ± 11.2 | 65 | 0.0–0.50 |
| Partial thromboplastin time, second(s) | 31.7 ± 7.9 | 58 | 23.1–33.1 |
| Prothrombin time, second(s) | 12.2 ± 3.4 | 59 | 9.4–11.7 |
| Fibrinogen, mg/dL | 482 ± 236 | 42 | 186–466 |
| Mean platelet volume, FL | 11.4 ± 1.6 | 81 | 7.3–11.4 |
D-Dimer 1: At day 1 admission
D-Dimer 2: At day 5 admission
Abbreviation: WBC: White blood cell count; CRP: C reactive protein; ALT: Alanine aminotransferase; AST: Aspartate transaminase; LDH: lactate dehydrogenase; CPK: Creatine kinase; DD1: D-Dimer 1; DD2 D-Dimer 2. PTT: Partial Prothrombin time; PT: Prothrombin time. MPV: Mean platelet volume.
There was a significant increase in D2 ranging from 0.25 to 35mg/dl with a mean±SD of 6.1453 ± 0.98 compared to D1 that was ranging from 0.9 to 35mg/dl with a mean±SD of 13.6 ± 1.38. Platelet counts varied among patients, ranging from 52,000– 482,000/μL, however, most patients had normal to high platelet levels. Only one patient had baseline thrombocytopenia with a platelet count of 52,000 μL, likely from his underlying cancer and chemotherapy. Similarly, we observed a wide variation in other laboratory values, including CRP, LDH, and ferritin as shown in Table 2. Interestingly, we observed elevated fibrinogen levels among patients with COVID-19 as shown in Table 2.
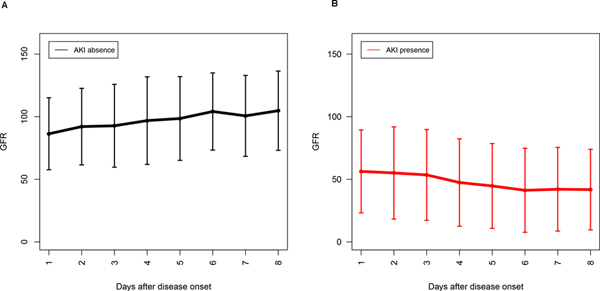
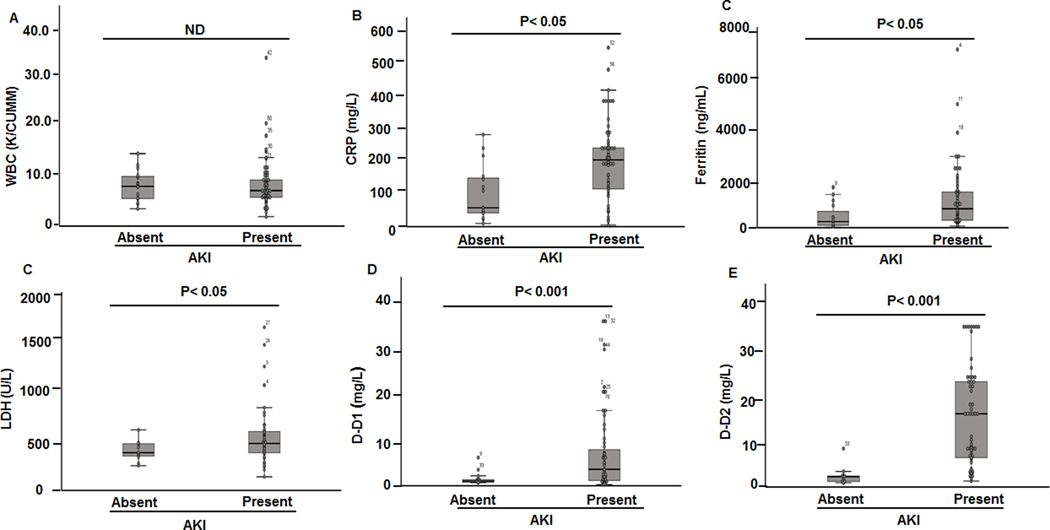
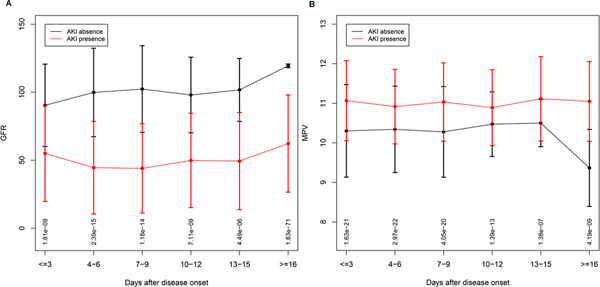
Acute renal injury (AKI) has been associated with worse outcomes in patients with COVID-19, therefore we analyzed laboratory data based on the presence or absence of AKI. There were significant differences (p value< 0.05:CI<0.095) in renal function between subjects with and without AKI. In the AKI group the mean±SD of blood urea nitrogen (BUN) was 39± 24 (mg/dL) versus 17±11 (mg/dL) in the group without AKI. The mean±SD creatinine values in AKI group was 2.3±1.9 (mg/dL) versus 1.38± 1.1 (mg/dL) in the group without AKI. Figure 1 shows the GFR trends during 8 days of ICU stay between two groups: absence of AKI (Figure 1A) and presence of AKI (Figure 1B). A Student t-test was performed to detect differences in outcome variables of selected laboratory values between the group with and without AKI. Figure 1 shows Box plots overlaid with dot plots illustrating mean±SD of the relevant laboratory data comparing subjects, who developed AKI versus, who did not. As seen in Figure 2, we found no significant differences in mean±SD of WBC (Figure 2A) between two groups. Similarly, there were no significant differences in the neutrophil and lymphocytes counts between two groups (data not shown). In contrast, we observed significant differences (p< 0.05) in CRP values (Figure 2B), ferritin (Figure 2C), and LDH (Figure 2D), between these two groups. Among patients who required renal replacement therapy, we observed a high number of subjects (65%), who exhibited circuit clotting and clotting in central lines, requiring heparin or alteplase treatment. Most striking, we found that patients, who developed AKI had significantly higher D-dimer levels upon ICU admission (D1) (Figure 2E). Interestingly, the differences in D-dimer levels were more pronounced, when we used the D-dimer 2 (D2), which was measured on average at 5th ICU day. Figure 1F shows the mean±SD of D2 between the two groups. Given the important role of platelets and prothrombin time in the activation of the coagulation pathway, we analyzed the data to identify parameters that correlate with D1and D2. Next, we performed non-parametric Spearman Correlation Test to identify laboratory parameters correlating with D-dimer levels. We used all laboratory data, including PT, PTT, fibrinogen, CRP, Hb, ferritin, platelet count, and mean platelet volume (MPV). We found a significant correlation between D1 and CRP (r=0.32; p<0.05), as well as D2 and CRP (r=0.558; p<0.01). Strikingly, we found a significant correlation of D1 and MVP (0.454; p<0.01) but we found no correlation between the number of platelets and D1. When we used D2, we found a significant correlation of D2 with ferritin (r=0.303; p< 0.05), CRP (r= 0.475; p<0.01), and MPV (r=0.420; p<0.01). Again, we found no significant correlation between D2 and PT, PTT, fibrinogen, or platelet counts. In order to further determine the kinetic of GFR variation in a time dependent manner during ICU admission, we collected the daily GFR values of all study subjects and compare the kinetics of GFR based on the presence and absence of AKI. Figure 3A shows violin plots for GFR in a time dependent manner based on presence and absence of AKI. As expected, there was a significant difference between GFRs of subjects with AKI versus no AKI, and deterioration in most patients occurred within 4–7 days of ICU stay. Similarly, most patients with AKI died despite renal replacement therapy. Only 6 patients, who showed improvement of GFR after 16 days, survived. We performed similar analysis using MPV. As shown in Figure 3B, COVID-19 subjects with AKI exhibited significantly larger platelets based on MPV. It is important to mention that all subjects with COVID-19 have higher MPV values (see normal value), however, in the AKI group the MPV was significantly higher. MVP values in our study patients did fluctuate day by day but on average, a higher MVP correlated with the presence of AKI.
Figure 1. GFR trend among COVID-19 subjects from day one to day eight of ICU- stay.
A) Mean± SD of GFR (y-axis) in COVID-19 patients without AKI from day one of admission plotted against time, showing the GFR trend. B) The trend of GFR among COVID-19 patients with AKI from day1–8 of ICU-stay. There was a significant difference between AKI and non- AKI group in terms of GFR. Trend of GFR during ICU admission among patients with COVID-19 infection for each day. Error bars plots to show the trend of GFR (y-axis) in COVID-19 patients without acute renal injury (A) and with acute renal injury (B) during ICU stay. Each error bars plot shows the data from each day. The trend line connects the means of each day, and the caps represent the SD. The GFR plot shows a continuous deterioration for patients with AKI starting from day 3, in contrast the GFR of patients without AKI shows continuous improvement.
Figure 2. Box blots of main laboratory values among COVID-19 subjects with and without renal failure.
Mean± SEM levels of altered laboratory values among COVID −19 patients who developed acute renal failure as compared to subjects who did not. A) total WBC count (C/dL). B) C reactive Protein (CRP) mg/L. C) Ferritin (ng/mL). D) Lactate dehydrogenase (LDH) (U/L). E) D-Dimer 1 (mg/L) at ICU admission. F) D-Dimer 2 (mg/L). The bottom and top of the boxes represent the 25th and 75th percentile, respectively. The top and bottom bars represent the entire stretch of the data points for the subjects, except the extreme points, which are indicated with circles (o). The hyphen indicates the median value. The y axis represents the concentrations of the laboratory values.
Figure 3. Trend of GFR and MPV during ICU stay among patients with COVID-19 infection.
A) The GFR trend presented as line plot with error bars to show GFR (y-axis) in COVID-19 patients with and without acute renal injury in time dependent manner (X-axis) during every 3 days of ICU stay. Patients with AKI (red) versus without AKI (black). B) MPV trend of patients with AKI (red) without AKI (black). Days of admission (x-axis). Each error bars plot shows the data of every 3 days. The dot represents the mean, and the cap represents the SD. The FDR p-values of each comparison are shown at the bottom of the plots. The GFR plot shows a deterioration after day 3 for patients with AKI. After 16 days, this trend showed upwards as some of the patients were either discharged or expired. The MPV plot shows a consistently higher level in AKI patients when compared to patients without AKI. After 16 days, this trend of MPV showed downwards. At each time point for GFR and MPV, the comparisons of AKI patients and without AKI were significant as shown of p-value on the bottom of each interval.
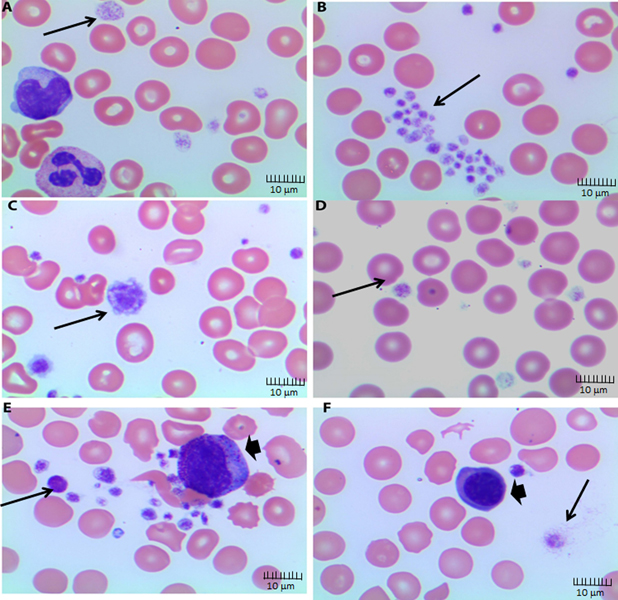
Because automated platelet counts may be unprecise, especially with varying platelets size, we reviewed patients’ peripheral blood smears, which consistently showed findings of large platelets. Figure 4 A–F shows representative blood smears of 6 different subjects. As shown in Figure 4, most patients had enlarged platelets and several patients had giant platelets (Figure 3C). Furthermore, we analyzed the data to identify correlation between laboratory data and outcome of AKI. We found that elevation of D-dimer at ICU admission or during ICU stay highly correlated with the development of AKI with a significantly lower false discovery rate (FDR) of 2.11E-09 for D-dimer 2 as compared with FDR=0.000 for the correlation of AKI with D-dimer values. This suggests rising D-Dimer levels during ICU stay may be viewed as a poor prognostic marker.
Figure 4. Peripheral blood smears of subjects with COVID-19 infection.
Peripheral blood smear showing (A) platelets with variation in granulation, shape, and size including large platelets (thin arrow), Wright-Giemsa stain, Objective 100x, immersion oil; (B) large platelets clumps (thin arrow), Wright-Giemsa stain, Objective 100x, immersion oil (C) giant platelet that is larger than a normal red blood cell and showing a pseudo-nucleus (thin arrow), Wright-Giemsa stain, Objective 100x, immersion oil; (D) platelets with variation in granulation, shape, and size, Wright-Giemsa stain, Objective 100x, immersion oil; (E) several large platelets (thin arrow) with variation in shape and size partially adherent to a myelocytes (thick arrow) (platelet satellitism), Wright-Giemsa stain, Objective 100x, immersion oil; (F) reactive plasmacytoid lymphocytes (thick arrow), Wright-Giemsa stain, Objective 100x, immersion oil.
Discussion:
Our study showed that 81% of critically ill COVID-19 patients developed AKI during their ICU course and half of these patients required renal replacement therapy. Furthermore, the mortality among the critically ill cohort of COVID-19 patients, who developed AKI was significantly higher. Previously, a large study reported the AKI incidence of 17% in critically ill patients admitted to ICU due to H1N1influenza infection(18). The incidence of AKI in bacterial sepsis and ARDS has been reported between 23– 41% during ICU stay (19,20). Our data indicates that the prevalence of AKI in COVID-19 infected individuals was substantially higher as compared to AKI incidence due to H1N1 or bacterial sepsis. In COVID-19 infection multiple mechanisms may lead to microcirculatory alterations such as endothelial injury, autonomic nervous system response, activation of the coagulation cascade and RAAS pathway.
Soon after the onset of the COVID-19 pandemic, reports of increased activation of the coagulation pathway and thrombotic events emerged (21,22). Patients may present with acute pulmonary embolism, deep venous thrombosis, acute ischemic events, or exhibit clotting of arterial and venous lines. Recent autopsy results confirmed the presence of thrombosis in many organs including lungs (23). Our data indicates that a COVID-19 infection leads to unique patterns of hypercoagulability and platelet activation play a major role in initiation of coagulation cascade. These patterns seem to be distinct to the usual DIC pattern reported in other forms of dysfunctional coagulation pathways in severe gram-negative sepsis and Acute Respiratory Distress Syndrome (ARDS)(24,25). One criteria for DIC is decreased fibrinogen levels and presence of fibrin monomers (26). In the contrary to DIC, we observed increased fibrinogen level, elevated D-dimers, but normal platelet counts in these subjects. Our data corroborates with previous reports that patients with COVID-19 have elevated fibrinogen levels. In a limited number of subjects, the level of protein C and protein S was decreased, which is consistent with consumption coagulopathy. Strikingly, we observed a unique feature of platelets in these patients, including larger, granular platelets with a higher MPV and increased frequency of large platelets clumps in the presence of normal platelet counts. This has been defined as a sign of platelet activation and indicator of increased thrombopoiesis (27). Interestingly, we found increased immature platelet fraction IPF (IPF>7) in COVID-19 infected subjects, who had evidence of elevated D-dimers and AKI. Immature platelets usually have larger size and are considered to be more reactive and increased IPF has been associated with smoking related cardiovascular disease and inflammation (28,29). Day to day variation in MVP can be related to various factors, including misinterpretation of the true platelet size due to platelets clumps, and some more activated platelets will be consumed in clot formation and not counted by automatic reader. It has been shown that larger platelets have denser α-granules, which store biologically active molecules such as GPIIb/IIIa, fibrinogen, and von Willebrand factor (vWf ) which can initiate the coagulation cascade (30). Dense granules store a variety of molecules which are secreted during platelet activation; including catecholamines, serotonin, calcium, adenosine 5′-diphosphate (ADP), and adenosine 5′-triphosphate (ATP) that involved in initiating coagulation cascade during inflammation (31). Higher MPV is also seen with DIC and it has been shown that MPV plays an important role in activation of coagulation and/or hyperfibrinolysis. If hyperfibrinolysis is the predominant feature, it is associated with remarkable bleeding rather than increased vascular thrombosis. Reductions in the levels of natural anticoagulants, such as antithrombin III and protein C are common in patients with DIC. Hyperfibrinolysis with subsequent increased bleeding is associated with an increase in the levels of cytokines which further induces plasminogen activator inhibitor I (PAI-I). In viral hemorrhagic fevers, including in Ebola and Hantavirus pulmonary syndrome, aberrant coagulation and bleeding have been reported (20,21). The infection with Ebola virus is associated with elevated PAI-I levels with increased bleeding (32), Elevated PIA-I is associated with high mortality in these patients(33). Other predominant features of Ebola virus infection are thrombocytopenia, coagulation factor deficiencies and elevated D-dimers. In contrast to viral hemorrhagic fevers, COVID-19 has not been associated with hemorrhage. Soon after the reports of activation of coagulation and increased incidence of pulmonary embolisms and DVT emerged, many centers adopted guidelines to initiate therapy with unfractionated or low molecular weight heparin in patients with COVID-19 infection. However, our observation and others suggest that some of these patients continue to be in a pro-coagulable state as indicated by clinical observations of ongoing thrombotic disease and laboratory data, for example increased in D-dimers, despite therapeutic anticoagulation(34). Experimental data in animals suggested that neither heparin nor hirudin can overcome the thrombogenic effects of Angiotensin II (Ang II). Interestingly antithrombin III could reverse the Ang II associated prothrombotic state in experimental animal model (12). It was reported by Llitjos et al that 56% of patients infected with COVID-19, who were receiving full dose anticoagulation developed VTE(35). These observations suggest a limited efficacy of conventional anticoagulant therapy in COVID-19 associated coagulopathy, warranting to evaluate the efficacy of antiplatelet drug therapy in this disease.
SARS‐CoV‐2 utilizes the novel ACE 2 receptor to gain entry into human cells (9). This process leads to shedding of host ACE2 receptor and the loss of ACE2 function (22). Because ACE2 counterbalances the deleterious effect of the RAAS, through effect of Angiotensin (1–7) (36,37), the loss of ACE2 function amplifies the deleterious effect of angiotensin II (Ang II). It has been shown that Ang II stimulation leads to increased platelet size and activation, and that aspirin does not prevent such activation (16,38). Other experimental models suggested that heparin has minimal effect on the Ang II induced platelet activation (12,38). In contrast, ACE2 activity or activation of the MAS receptor promotes antithrombotic activity and analogues of Angiotensin (1–7) prevent thrombosis formation (13,36,39). Alteration of ACE2 function and increased Ang II have been associated with chronic kidney disease and diabetes nephropathy(40). Furthermore, SARS‐CoV‐2 infection causes endothelial injury, which amplifies platelet activation. Limited available renal autopsy results of COVID-19 deceased subjects showed presence of microthrombi and fibrin thrombi in glomerular capillaries (41). Several studies have shown that hypertension, diabetes and obesity are associated with dysregulation of Ang II and ACE2(42). In both diseases increased platelet size and activation have been reported (16,43,44). It is important to note that 75% of our patients had hypertension and 58% had diabetes mellitus. The SARS‐CoV‐2 infection and the loss of function of ACE2 may potentiate the existing prothrombotic state of subjects with diabetes and hypertension. The unique features of COVID-19 infection are therefore, the loss of ACE2 function and increased Ang II activity promote severe endothelial dysfunction, platelet activation, an increased burden of microvascular thrombosis, and subsequent microcirculatory compromise in many organs including lungs, heart, CNS, and kidney associated with fatal outcome.
Acknowledgements:
This work was supported by a grant (R01HL150474 (LS) as well as the Department of Medicine, Wayne State University School of Medicine (LS).
Footnotes
Competing financial interests: None of the authors of this manuscript had any financial relationship with a commercial company.
References
- 1.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, and Favre G. Real estimates of mortality following COVID-19 infection. The Lancet Infectious Diseases [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, and Gu X. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395, 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong M, Liang X, and Wei YD (2020) Changes in Blood Coagulation in Patients with Severe Coronavirus Disease 2019 (COVID-19): a Meta-Analysis. Br J Haematol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, and Zhang H. (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. New England Journal of Medicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J-D, Sacco C, and Alexia B. (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehr AR, and Perlman S. (2015) Coronaviruses: an overview of their replication and pathogenesis in Coronaviruses, Springer; pp 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Li W, Farzan M, and Harrison SC (2005) Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309, 1864–1868 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, and Nitsche A. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, and Greenough TC (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, and van Goor H. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 203, 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tikellis C, and Thomas M. (2012) Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. International journal of peptides 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senchenkova EY, Russell J, Esmon CT, and Granger DN (2014) Roles of Coagulation and Fibrinolysis in Angiotensin II‐Enhanced Microvascular Thrombosis. Microcirculation 21, 401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraga-Silva RA, Sorg BS, Wankhede M, deDeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, and Raizada MK (2010) ACE2 activation promotes antithrombotic activity. Molecular medicine 16, 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N, Hong Y, Chen R-G, and Zhu H-M (2020) High rate of increased level of plasma Angiotensin II and its gender difference in COVID-19: an analysis of 55 hospitalized patients with COVID-19 in a single hospital, WuHan, China. medRxiv, 2020.2004.2027.20080432 [Google Scholar]
- 15.Siragy HM, and Carey RM (2010) Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. American journal of nephrology 31, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagroop I, and Mikhailidis D. (2000) Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. Journal of human hypertension 14, 581–585 [DOI] [PubMed] [Google Scholar]
- 17.Waikar SS, Curhan GC, Wald R, McCarthy EP, and Chertow GM (2006) Declining mortality in patients with acute renal failure, 1988 to 2002. Journal of the American Society of Nephrology 17, 1143–1150 [DOI] [PubMed] [Google Scholar]
- 18.Martin-Loeches I, Papiol E, Rodríguez A, Diaz E, Zaragoza R, Granada RM, Socias L, Bonastre J, Valverdú M, and Pozo JC (2011) Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection. Critical Care 15, R66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagshaw SM, George C, Bellomo R, and Committee ADM (2008) Early acute kidney injury and sepsis: a multicentre evaluation. Critical care 12, R47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, and Wenzel RP (1995) The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA : the journal of the American Medical Association 273, 117–123 [PubMed] [Google Scholar]
- 21.Di Micco P, Russo V, Carannante N, Imparato M, Rodolfi S, Cardillo G, and Lodigiani C. (2020) Clotting Factors in COVID-19: Epidemiological Association and Prognostic Values in Different Clinical Presentations in an Italian Cohort. J Clin Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Angles-Cano E, Sattler L, Mertes PM, Meziani F, and Group CT (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Puschel K, and Kluge S. (2020) Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Annals of internal medicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons J, and Pittet J-F (2015) The coagulopathy of acute sepsis. Current opinion in anaesthesiology 28, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaRosa SP, Opal SM, Utterback B, Yan SCB, Helterbrand J, Simpson AJ, Chaowagul W, White NJ, and Fisher CJ Jr (2006) Decreased protein C, protein S, and antithrombin levels are predictive of poor outcome in Gram-negative sepsis caused by Burkholderia pseudomallei. International journal of infectious diseases 10, 25–31 [DOI] [PubMed] [Google Scholar]
- 26.Venugopal A. (2014) Disseminated intravascular coagulation. Indian journal of anaesthesia 58, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park Y, Schoene N, and Harris W. (2002) Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets 13, 301–306 [DOI] [PubMed] [Google Scholar]
- 28.Schmoeller D, Picarelli MM, Paz Munhoz T, Poli de Figueiredo CE, and Staub HL (2017) Mean platelet volume and immature platelet fraction in autoimmune disorders. Frontiers in medicine 4, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardin M, Verdoia M, Negro F, Rolla R, Tonon F, and De Luca G. (2020) Impact of active smoking on the immature platelet fraction and its relationship with the extent of coronary artery disease. Eur J Clin Invest 50, e13181 [DOI] [PubMed] [Google Scholar]
- 30.Blair P, and Flaumenhaft R. (2009) Platelet α-granules: basic biology and clinical correlates. Blood reviews 23, 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Yuan Y, and Li W. (2018) Sorting machineries: how platelet-dense granules differ from α-granules. Bioscience reports 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West TE, and von Saint André-von Arnim A. (2014) Clinical presentation and management of severe Ebola virus disease. Annals of the American Thoracic Society 11, 1341–1350 [DOI] [PubMed] [Google Scholar]
- 33.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, and Spiropoulou CF (2014) Biomarker correlates of survival in pediatric patients with Ebola virus disease. Emerging infectious diseases 20, 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, and Susen S. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation [DOI] [PubMed] [Google Scholar]
- 35.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, and Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. Journal of Thrombosis and Haemostasis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabelo LA, Alenina N, and Bader M. (2011) ACE2–angiotensin-(1–7)–Mas axis and oxidative stress in cardiovascular disease. Hypertension Research 34, 154–160 [DOI] [PubMed] [Google Scholar]
- 37.Samavati L, and Uhal BD (2020) ACE2, Much More Than Just a Receptor for SARS-COV-2. Frontiers in Cellular and Infection Microbiology 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson P, Schwieler J, and Wallen N. (2000) Platelet activation during angiotensin II infusion in healthy volunteers. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis 11, 61–69 [PubMed] [Google Scholar]
- 39.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, and Zhang C. (2014) Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nature Reviews Cardiology 11, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams VR, and Scholey JW (2018) Angiotensin-converting enzyme 2 and renal disease. Current opinion in nephrology and hypertension 27, 35–41 [DOI] [PubMed] [Google Scholar]
- 41.Menter T, Haslbauer J, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, and Pargger H. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingelfinger JR (2006) ACE2: a new target for prevention of diabetic nephropathy?, Am Soc Nephrol [DOI] [PubMed] [Google Scholar]
- 43.Shah B, Sha D, Xie D, Mohler ER, and Berger JS (2012) The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diabetes care 35, 1074–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueba T, Nomura S, Inami N, Nishikawa T, Kajiwara M, Iwata R, and Yamashita K. (2010) Plasma level of platelet-derived microparticles is associated with coronary heart disease risk score in healthy men. Journal of atherosclerosis and thrombosis, 1004060217–1004060217 [DOI] [PubMed] [Google Scholar]